Abstract
Gallic acid (3,4,5-trihydroxybenzoic acid), is a natural product found in various foods and herbs that are well known as powerful antioxidants. Our previous report demonstrated that it inhibits mast cell-derived inflammatory allergic reactions by blocking histamine release and pro-inflammatory cytokine expression. In this report, various amide analogs of gallic acid have been synthesized by introducing different amines through carbodiimide-mediated amide coupling and Pd/C-catalyzed hydrogenation. These compounds showed a modest to high inhibitory effect on histamine release and pro-inflammatory cytokine expression. Among them, the amide bearing (S)-phenylglycine methyl ester 3d was found to be more active than natural gallic acid. Further optimization yielded several (S)- and (R)-phenylglycine analogs that inhibited histamine release in vitro. Our findings suggest that some gallamides could be used as a treatment for allergic inflammatory diseases.
Keywords: gallic acid, allergic inflammation, histamine, pro-inflammatory cytokine
1. Introduction
Gallic acid (3,4,5-trihydroxybenzoic acid), a polyphenol natural product obtained from various herbs, is known to have diverse biological effects such as anti-oxidation, anti-inflammation, and anti-cancer. In a previous research, Shin and coworkers demonstrated that gallic acid inhibits mast cell-mediated inflammatory allergic reactions by blocking histamine release and pro-inflammatory cytokine expression [1].
The prevalence rate of allergic diseases has been rising globally for more than 50 years. Approximately 50% of children are sensitized to common allergens [2]. Allergic inflammation is classified into three phases: early-phase, late-phase, and chronic allergic inflammation. In early-phase reactions, histamine, a major factor in the allergic response, is released from mast cells and induces vasodilation, increases vascular permeability, and recruits leukocytes. Repetitive allergen exposure alters organ function by affecting structural cells and increases production of cytokines resulting in chronic allergic inflammation [3].
Mast cells play key roles in immunoglobulin E (IgE)-mediated allergic reactions through secretion of preformed or newly synthesized mediators such as histamine, lipid-derived mediators, chemokines, cytokines, and growth factors [4]. The signaling pathway of mast cells triggered by antigen cross-linking of IgE bound to FcεRI has been previously described. Stimulation of FcεRI increases degranulation, production of lipid-derived mediators, and expression of cytokines [5]. Therefore, suppression of histamine and pro-inflammatory cytokine release might be an appropriate therapeutic target to reduce allergic inflammation. Human mast cells (HMC-1) are known as an appropriate model for studying allergic reactions characterized by release of histamine and expression of pro-inflammatory cytokines [6,7].
2. Results and Discussion
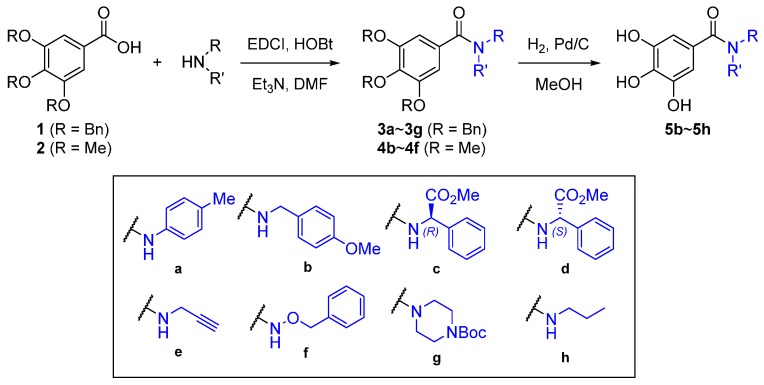
To increase the efficacy of gallic acid, gallic acid analogs were designed and synthesized by the amidation of gallic acid with various amines (Figure 1) [8]. In addition, 3,4,5-trimethoxybenzamides and 3,4,5-trisbenzyloxybenzamides were synthesized in a similar fashion.
Figure 1.
Gallic acid and its amide analogs.
Our synthesis of 3,4,5-trihydroxybenzamides commenced with formation of 3,4,5-tris(benzyloxy)benzoic acid amides using some selected amines, followed by hydrogenolysis of all benzyl groups. 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDCI) was chosen as a carboxyl-activating agent for the amide coupling with amines such as p-toluidine, p-methoxybenzylamine, (R)- and (S)-phenylglycine methyl ester, propargyl amine, benzyloxyamine and Boc-piperazine. Thus, we obtained the amides 3a~3g from the respective amines, followed by hydrogenolysis under H2, Pd/C to obtain the resulting 3,4,5-trihydroxybenzamides 5b~5h in good yield. Similarly, 3,4,5-trimethoxybenzamides 4b~4f were prepared from 3,4,5-trimethoxybenzoic acid (Scheme 1). Compounds 4a, 4e and 5h were previously reported in the literature [9,10,11,12,13,14].
Scheme 1.
Synthesis of 3,4,5-trisbenzyloxy-, 3,4,5-trimethoxyl- and 3,4,5-trihydroxybenzamides.
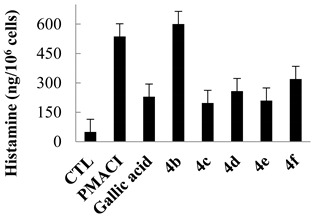
The release of histamine via degranulation is a key characteristic of activated mast cells, and HMC-1 cells stimulated with phorbol 12-myristate 13-acetate and the calcium ionophore A23187 (PMACI) also released a high level of histamine. Gallic acid, a known inhibitor of histamine release, was used as a positive control. Pretreatment with variously modified compounds (10 μM) differently inhibited the level of histamine released by PMACI-stimulated HMC-1 cells as shown in Table 1 and Table 2. Since the p-toluidine analog 3a showed the weakest inhibition rate (8.9% at 10 µM), neither the trimethoxybenzamide nor trihydroxybenzamide of p-toluidine 4a and 5a was evaluated further. The gallamides with (R)-phenylglycine methyl ester 3c and 4c were more potent than (S)-enantiomers 3d and 4d. Unfortunately, reduction of trisbenzyloxy to trihydoxybenzamides 5c and 5d decreased the inhibition rate (24% and 36% at 10 µM). In contrast, p-methoxybenzylamine derivatives 3b and 5b exhibited a lower inhibition rate than did gallic acid. Trimethoxybenzamide 4b enhanced the histamine release by HMC-1 cells rather than having an inhibitory effect; consequently, 4b may trigger an allergic reaction. The N-propargylamide analog 3e and N-propylamide 5h, which was obtained by reduction of 3e, exhibited a lower histamine inhibition rate (39.4% and 31.2% at 10 µM) than did gallic acid. However, the trimethoxybenzamide analog 4e showed a slightly increased inhibitory activity (67% at 10 µM) compared with that of gallic acid. Compared with gallic acid, 3,4,5-trihydroxybenzamide analogs did not have an improved effect on histamine inhibition in vitro; however, several compounds such as 3c, 3d, 3g, 4c, 4d, 4e, and 5b exhibited similar or better inhibitory effects on histamine release.
Table 1.
Inhibitory activity of 3,4,5-trisbenzyloxy- and 3,4,5-trihydroxybenzamide analogs (10 µM) on histamine release in HMC-1 cells.
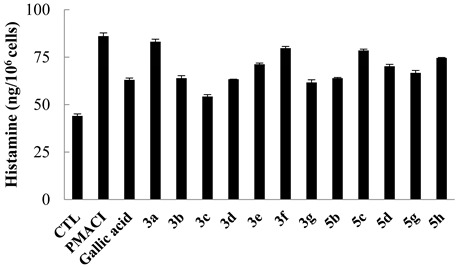
| Compound | % Inhibition |
|---|---|
| Gallic acid | 58.9 |
| 3a | 8.9 |
| 3b | 48.3 |
| 3c | 74.3 |
| 3d | 54.9 |
| 3e | 39.4 |
| 3f | 20.9 |
| 3g | 59.9 |
| 5b | 52.1 |
| 5c | 24.3 |
| 5d | 35.8 |
| 5g | 41.9 |
| 5h | 31.2 |
Table 2.
Inhibitory activity of 3,4,5-trimethoxylamides (10 µM) on histamine release in HMC-1 cells.
| Compound | % Inhibition |
|---|---|
| Gallic acid | 63.0 |
| 4b | −12.9 |
| 4c | 69.6 |
| 4d | 57.2 |
| 4e | 67.0 |
| 4f | 44.5 |
Cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-6 are released by mast cells and are more important in chronic-phase allergic reactions. TNF-α play a key role in immunity by activating NF-κB and regulating immune cells. IL-6 maintains CD4+ T cell survival and promotes Th2 modulation; moreover, local accumulation of IL-6 produced by mast cells is known to induce passive cutaneous anaphylaxis (PCA) reaction. Therefore, the inhibition of pro-inflammatory cytokines is also a key strategy to treat allergic inflammation. To determine whether gallic acid and its amide analogs can inhibit inflammation, the expression of pro-inflammatory cytokines such as TNF-α and IL-6 was evaluated by real-time PCR. Some compounds showed different inhibitory effects on the gene expression of TNF-α and IL-6. As shown in Table 3, 3d and 5d significantly reduced the gene expression of TNF-α, and five compounds—3c, 3d, 3e, 3g, and 5d—reduced expression of IL-6.
Table 3.
Gene expression of pro-inflammatory cytokines. (Values are related to β-actin) * significant inhibition of cytokines.
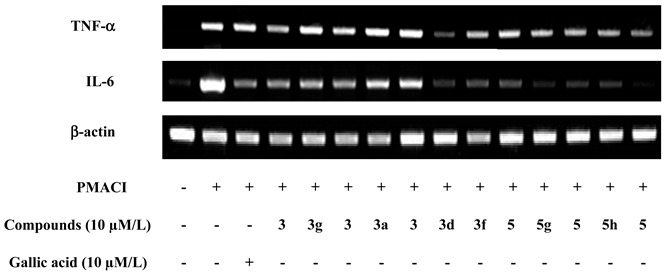
| PMACI | Compound (10 µM) | TNF-α | IL-6 |
|---|---|---|---|
| - | - | 0.35 ± 0.03 | 0.46 ± 0.06 |
| + | - | 0.71 ± 0.06 | 1.05 ± 0.14 |
| + | Gallic acid | 0.59 ± 0.02 * | 0.67 ± 0.02 * |
| + | 3a | 0.93 ± 0.22 | 0.84 ± 0.11 |
| + | 3b | 1.10 ± 0.03 | 0.81 ± 0.11 |
| + | 3c | 0.88 ± 0.07 | 0.67 ± 0.05 * |
| + | 3d | 0.46 ± 0.04 * | 0.71 ± 0.08 * |
| + | 3e | 0.65 ± 0.12 | 0.59 ± 0.09 * |
| + | 3f | 0.67 ± 0.12 | 0.85 ± 0.16 |
| + | 3g | 0.85 ± 0.08 | 0.68 ± 0.04 * |
| + | 5b | 0.96 ± 0.22 | 0.80 ± 0.19 |
| + | 5c | 0.81 ± 0.20 | 0.81 ± 0.09 |
| + | 5d | 0.50 ± 0.07 * | 0.45 ± 0.09 * |
| + | 5g | 0.92 ± 0.16 | 0.83 ± 0.08 |
| + | 5h | 0.88 ± 0.11 | 0.81 ± 0.17 |
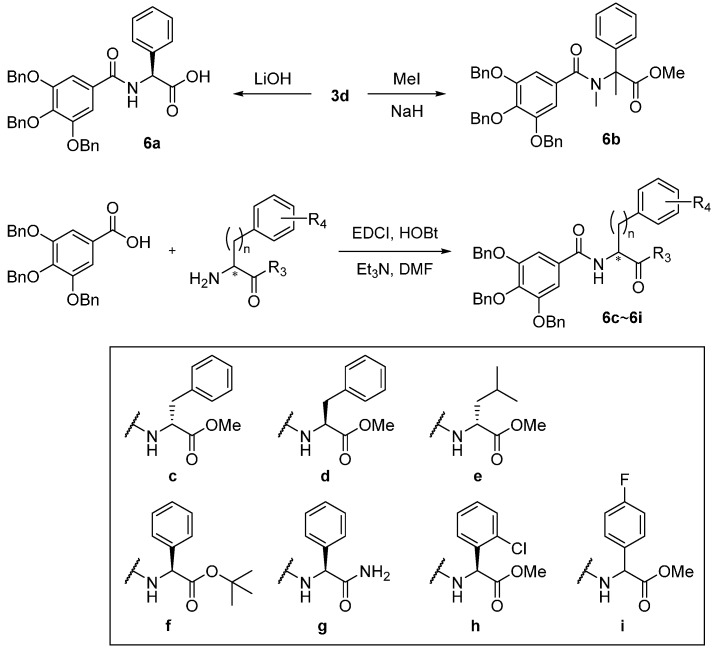
Combined with the result of the inhibitory effect on histamine release, 3c and 3d significantly inhibited both histamine release and expression of TNF-α and IL-6. Due to the inhibitory effect on both histamine and pro-inflammatory cytokine release, 3c and 3d were targeted for more modifications in order to improve their biological activity and drug-like properties. In addition, the analogs derived from 3c and 3d could enable us to elucidate structure-activity relationships and modes of action (Scheme 2). Thus, the methyl ester 3d was hydrolyzed by LiOH in H2O/THF to furnish the carboxylic acid 6a. In a second modification, 3d was methylated with two equivalents of MeI in the presence of NaH at 0 °C to furnish the tertiary amide 6b. Various gallamides 6e~6i were prepared by EDCI-mediated coupling with (S)-leucine methyl ester, (R)- and (S)-phenylalanine methyl ester, (S)-phenylalanine tert-butyl ester, (S)-2-amino-2-phenylacetamide, (S)-(+)-2-chloro-phenylglycine methyl ester and (±)-4-fluorophenylglycine methyl ester.
Scheme 2.
Synthesis of the second-generation gallamide analogs.
As shown in Table 4, we evaluated the inhibitory activity of the second-generation gallamide analogs at 10 nM on histamine release. We found that 3d and some derivatives markedly inhibited histamine release even at quite low concentrations. The carboxylic acid 6a and the dimethylated amide 6b of 3d exhibited a similar inhibitory activity on histamine release as did 3d. Additionally, (R)- and (S)-phenylalanine methyl esters 6c and 6d had a lower inhibition rate than did 3d. Similar to 4b, 6f and 6h increased the histamine concentration released by HMC-1 cells instead of inhibiting it. The incorporation of an electron-withdrawing group, such as chloro (compound 6h) and fluoro (compound 6i), at the aromatic ring of 3d was not tolerated. Interestingly, leucine amide 6e showed even better inhibitory activity than the initial compound 3d.
Table 4.
Inhibitory activity of 3d and its analogs (10 nM) on histamine release in RBL-2H3 cells.
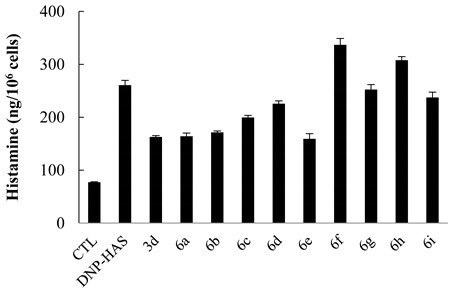
| Compound | % Inhibition |
|---|---|
| 3d | 53 |
| 6a | 52 |
| 6b | 48 |
| 6c | 32 |
| 6d | 18 |
| 6e | 55 |
| 6f | −42 |
| 6g | 4 |
| 6h | −26 |
| 6i | 11 |
3. Conclusions
It was well established that gallic acid inhibits mast cell-derived inflammatory allergic reactions by blocking histamine release and pro-inflammatory cytokine expression. However, the structure of gallic acid is too small and its hydrophilic polyphenol groups affect metabolism; therefore, it needs to be converted to drug-like compounds. In order to solve these problems, new gallamide analogs were synthesized. Fifteen of 17 compounds could inhibit histamine release; compounds 3c and 3d showed greatest inhibitory activity on release of both histamine and pro-inflammatory cytokines such as TNF-α and IL-6. The results give insight into the mechanism by which 3d exerts anti-allergic and anti-inflammatory activity on mast cells, and suggests that 3d might be considered as a therapeutic candidate for mast cell-mediated allergic inflammatory diseases.
4. Materials and Methods
4.1. General Information
All starting materials and reagents were obtained from commercial suppliers and were used without further purification. Air and moisture sensitive reactions were performed under an argon atmosphere. Flash column chromatography was performed using silica gel 60 (230–400 mesh, Merck, Darmstadt, Germany) with the indicated solvents. Thin-layer chromatography was performed using 0.25 mm silica gel plates (Merck, Darmstadt, Germany). 1H- (600 MHz) and 13C-NMR (150 MHz) spectra were recorded on an AVANCE III System 600 MHz spectrometer (Bruker, Billerica, MA, USA) as solutions in CDCl3, DMSO-d6 or methanol-d4. High-resolution mass spectra (HRMS) were obtained on JMS-700 instrument (JEOL, Akishima, Tokyo, Japan) with electrospray ionization. Spectral data of 3a~6i are available in Supplementary Materials.
4.2. Synthesis of Gallamide Analogs
3,4,5-Tris(benzyloxy)-N-p-tolylbenzamide (3a). To a DMF solution (2 mL) of 3,4,5-tris(benzyloxy)benzoic acid (110 mg, 0.25 mmol), p-toluidine (32 mg, 0.3 mmol), EDCI (60 mg, 0.4 mmol), HOBt (4 mg, 30 μmol) and TEA (87 μL, 0.50 mmol) were added. After stirring overnight, the reaction mixture was diluted with ethyl acetate and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:2) to generate pure 3a (111 mg, 80%). 1H-NMR (CDCl3) δ 7.46 (d, J = 8.3 Hz, 2H), 7.44–7.32 (m, 12H), 7.29–7.25 (m, 5H), 7.17 (d, J = 8.1 Hz, 3H), 7.12 (s, 2H), 5.15 (s, 4H), 5.12 (s, 2H), 2.34 (s, 3H); 13C-NMR (CDCl3) δ 165.41, 152.95, 141.52, 137.50, 136.74, 135.48, 134.32, 130.59, 129.71, 128.72, 128.70, 128.35, 128.20, 128.12, 127.69, 120.29, 107.13, 75.32, 71.57, 21.05; HRMS (EI) m/z calcd. for 529.2253; found 529.2252.
3,4,5-Tris(benzyloxy)-N-(4-methoxybenzyl)benzamide (3b). To a DMF solution (2 mL) of 3,4,5-tris(benzyloxy)benzoic acid (110 mg, 0.25 mmol), 4-methoxybenzylamine (56 mg, 0.28 mmol), EDCI (115 mg, 0.60 mmol), HOBt (4.0 mg, 30 μmol) and TEA (90 μL, 0.50 mmol) were added. After stirring overnight, the reaction mixture was diluted with ethyl acetate and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:3) to generate pure 3b (98 mg, 70%). 1H-NMR (CDCl3) δ 7.21~7.41(m, 15H), 7.05 (s, 2H), 6.90 (m, 2H), 5.11 (s, 4H), 5.08 (s,2H), 4.53 (d, 2H), 3.81 (s, 3H); 13C-NMR (CDCl3) δ 136.6, 129.3, 128.5, 128.5, 128.2, 128.0, 127.5, 114.1,107.0, 71.5, 29.7; HRMS (EI) m/z calcd. for 559.2359; found 559.2361.
(R)-Methyl 2-phenyl-2-(3,4,5-tris(benzyloxy)benzamido)acetate (3c). To a DMF solution (2 mL) of 3,4,5-tris(benzyloxy)benzoic acid (110 mg, 0.25 mmol), (R)-2-Phenylglycine methyl ester hydrochloride (61 mg, 0.3 mmol), EDCI (59 mg, 0.4 mmol), HOBt (3.8 mg, 30 μmol) and TEA (90 μL, 0.50 mmol) were added. After stirring overnight, the reaction mixture was diluted with ethyl acetate and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:3) to yield pure 3c (115 mg, 78%). 1H-NMR (CDCl3) δ 7.42–7.23 (m, 23H), 7.11 (s, 2H), 6.98 (d, J = 6.9 Hz, 1H), 5.70 (d, J = 6.9 Hz, 1H), 5.11 (s, 4H), 5.09 (s, 2H), 3.77 (s, 3H); 13C-NMR (CDCl3) δ 171.83, 166.43, 153.10, 141.92, 137.71, 136.97, 136.78, 129.37, 129.20, 128.98, 128.92, 128.89, 128.54, 128.37, 128.31, 127.92, 127.69, 107.50, 75.49, 71.79, 57.25, 53.28; HRMS (EI) m/z calcd. for 587.2308; found 587.2307.
(S)-Methyl 2-phenyl-2-(3,4,5-tris(benzyloxy)benzamido)acetate (3d). To a DMF solution (2 mL) of 3,4,5-tris(benzyloxy)benzoic acid (110 mg, 0.25 mmol), (S)-2-phenylglycine methyl ester hydrochloride (56 mg, 0.28 mmol), EDCI (115 mg, 0.60 mmol), HOBt (4.0 mg, 30 μmol) and TEA (90 μL, 0.50 mmol) were added. After stirring for 3 h, the reaction mixture was diluted with ethyl acetate and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:3) to yield pure 3d (127 mg, 86%). 1H-NMR (CDCl3) δ 7.41–7.32 (m, 17H), 7.27–7.22 (m, 3H), 7.12 (s, 2H), 7.06 (d, J = 6.8 Hz, 1H), 5.69 (d, J = 6.9 Hz, 1H), 5.08 (s, 2H), 5.08 (s, 4H), 3.75 (s, 3H); 13C-NMR (CDCl3) δ 171.59, 166.21, 152.83, 141.64, 137.48, 136.73, 136.52, 129.10, 128.91, 128.71, 128.66, 128.63, 128.28, 128.11, 128.05, 127.67, 127.48, 107.23, 75.23, 71.50, 57.03, 53.01; HRMS (EI) m/z calcd. for 587.2308; found 587.2307.
3,4,5-Tris(benzyloxy)-N-(prop-2-yn-1-yl)benzamide (3e). To a DMF solution (4 mL) of 3,4,5-tris-(benzyloxy)benzoic acid (220 mg, 0.50 mmol), propargylamine (33 mg, 0.60 mmol), EDCI (115 mg, 0.60 mmol), HOBt (7.0 mg, 50 μmol) and TEA (90 μL, 0.50 mmol) were added. After stirring for 24 h, the reaction mixture was diluted with ethyl acetate and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:3) to produce the amide 3e (212 mg, 89%). 1H-NMR (CDCl3) δ 7.41–7.21 (m, 15H), 7.08 (s, 2H), 6.40 (s, 1H), 5.07 (s, 2H), 5.06 (s, 4H), 4.17 (dd, J = 5.2, 2.5 Hz, 2H), 2.24 (t, J = 2.5 Hz, 1H); 13C-NMR (CDCl3) δ 166.90, 152.87, 141.48, 137.46, 136.68, 129.22, 128.65, 128.29, 128.15, 128.07, 127.64, 107.05, 79.63, 75.24, 71.92, 71.46, 29.94; HRMS (EI) m/z calcd. for 477.1940; found 477.1942.
N,3,4,5-Tetrakis(benzyloxy)benzamide (3f). To a DMF solution (2 mL) of 3,4,5-tris(benzyloxy)benzoic acid (1) (110 mg, 0.25 mmol), O-benzylhydroxylamine hydrochloride (48 mg, 0.3 mmol), EDCI (58 mg, 0.3 mmol), HOBt (4 mg, 30 μmol) and TEA (90 μL, 0.50 mmol) were added. After stirring overnight, the reaction mixture was diluted with ethyl acetate and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:2) to generate pure 3f (102 mg, 74%). 1H-NMR (CDCl3) δ 7.42–7.20 (m, 21H), 6.95 (s, 2H), 5.06 (s, 2H), 5.03 (s, 4H), 4.97 (s, 2H); 13C-NMR (CDCl3) δ 166.20, 152.80, 141.56, 137.35, 136.51, 135.30, 129.39, 128.84, 128.66, 128.56, 128.22, 128.07, 128.00, 127.54, 127.11, 106.83, 78.35, 75.17, 71.27; HRMS (EI) m/z calcd. for 545.2202; found 545.2205.
tert-Butyl 4-(3,4,5-tris(benzyloxy)benzoyl)piperazine-1-carboxylate (3g). To a DMF solution (2 mL) of 3,4,5-tris(benzyloxy)benzoic acid (110 mg, 0.25 mmol), 1-Boc-piperazine (56 mg, 0.3 mmol), EDCI (115 mg, 0.60 mmol), HOBt (5.0 mg, 40 μmol) and TEA (90 μL, 0.50 mmol) were added. After stirring overnight, the reaction mixture was diluted with ethyl acetate and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:3) to generate pure 3g (103 mg, 68%). 1H-NMR (CDCl3) δ 7.44–7.25 (m, 15H), 6.65 (s, 2H), 5.11 (s, 4H), 5.10 (s, 2H), 3.91–2.81 (m, 8H), 1.47 (s, 9H); 13C-NMR (CDCl3) δ 170.07, 154.52, 152.68, 139.79, 137.55, 136.70, 130.42, 128.60, 128.26, 128.23, 128.03, 127.98, 127.38, 107.24, 80.29, 75.23, 71.18, 28.43; HRMS (EI) m/z calcd. for 608.2886; found 608.2888.
3,4,5-Trihydroxy-N-(4-methoxybenzyl)benzamide (4b). To a CH2Cl2 solution (3 mL) of 3,4,5-trimethoxybenzoic acid (100 mg, 0.471 mmol), 4-Methoxybenzylamine (78 mg, 0.566 mmol), EDCI (117 mg, 0.613 mmol), HOBt (70 mg, 0.46 mmol) and TEA (0.31 mL, 1.78 mmol) were added. After stirring overnight, the reaction mixture was diluted with ethyl acetate and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 2:1) to generate 85 mg of 4b (yield 54%).1H-NMR (CDCl3) δ 7.23 (d, J = 8.6 Hz, 2H), 7.01 (s, 2H), 6.83 (d, J = 8.6 Hz, 2H), 6.67 (t, J = 5.1 Hz, 1H), 4.51 (d, J = 5.7 Hz, 2H), 3.84 (s, 3H), 3.82 (s, 6H), 3.77 (s, 3H); 13C-NMR (CDCl3) δ 167.26, 159.31, 153.39, 141.09, 130.61, 130.11, 129.54, 114.34, 104.66, 61.16, 56.52, 55.57, 43.94.; HRMS (EI) m/z calcd. for 289.0950; found 289.0948.
(R)-Methyl 2-phenyl-2-(3,4,5-trimethoxybenzamido)acetate (4c). To a CH2Cl2 solution (3 mL) of 3,4,5-trimethoxybenzoic acid (100 mg, 0.471 mmol), (R)-(−)-2-phenylglycine methyl ester hydrochloride (114 mg, 0.56 mmol), EDCI (117 mg, 0.613 mmol), HOBt (70 mg, 0.46 mmol) and TEA (0.24 mL, 1.41 mmol) were added. After stirring overnight, the reaction mixture was diluted with CH2Cl2 and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:1) to generate 85 mg of 4c (yield 50%). 1H-NMR (CDCl3) δ 7.46–7.33 (m, 5H), 7.13 (d, J = 6.8 Hz, 1H), 7.05 (s, 2H), 5.74 (d, J = 6.9 Hz, 1H), 3.89 (s, 6H), 3.88 (s, 3H), 3.78 (s, 3H); 13C-NMR (CDCl3) δ 171.69, 166.37, 153.30, 141.40, 136.57, 129.15, 129.02, 128.75, 127.51, 104.72, 61.02, 57.10, 56.47, 53.06; HRMS (EI) m/z calcd. for 359.1369; found 359.1370.
(S)-Methyl 2-phenyl-2-(3,4,5-trimethoxybenzamido)acetate (4d). To a CH2Cl2 solution (3 mL) of 3,4,5-trimethoxybenzoic acid (100 mg, 0.471 mmol), (S)-(−)-2-phenylglycine methyl ester hydrochloride (110 mg, 0.66 mmol), EDCI (210 mg, 1.09 mmol), HOBt (70 mg, 0.46 mmol) and TEA (0.24 mL, 1.41 mmol) were added. After stirring overnight, the reaction mixture was diluted with CH2Cl2 and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:1) to produce 90 mg of 4d (yield 53%). 1H-NMR (CDCl3) δ 7.43–7.40 (m, 2H), 7.37–7.30 (m, 3H), 7.19 (d, J = 6.9 Hz, 1H), 7.04 (s, 2H), 5.72 (d, J = 6.9 Hz, 1H), 3.86 (s, 9H), 3.76 (s, 3H); 13C-NMR (CDCl3) δ 171.65, 166.35, 153.24, 141.33, 136.51, 129.08, 128.95, 128.69, 127.50, 104.69, 60.96, 57.07, 56.40, 52.99; HRMS (EI) m/z calcd. for 359.1369; found 359.1370.
3,4,5-Trimethoxy-N-(prop-2-yn-1-yl)benzamide (4e). To a CH2Cl2 solution (3 mL) of 3,4,5-trimethoxybenzoic acid (100 mg, 0.471 mmol), propargylamine (31 mg, 0.56 mmol), EDCI (250 mg, 1.32 mmol), HOBt (30 mg, 0.20 mmol) and TEA (0.3 mL, 1.71 mmol) were added. After stirring overnight, the reaction mixture was diluted with CH2Cl2 and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:1) to generate 88 mg of 4e (yield 52%). 1H- NMR (CDCl3) δ 7.01 (s, 2H), 6.40 (s, 1H), 4.23 (dd, J = 5.3, 2.6 Hz, 2H), 3.88 (s, 6H), 3.87 (s, 3H), 2.28 (s, 1H); 13C-NMR (CDCl3) δ 166.81, 153.11, 141.02, 129.04, 104.35, 79.40, 71.77, 60.83, 56.23, 56.11, 29.78; HRMS (EI) m/z calcd. for 317.1263; found 317.1265.
N-(benzyloxy)-3,4,5-trimethoxybenzamide (4f). To a CH2Cl2 solution (3 mL) of 3,4,5-trimethoxybenzoic acid (100 mg, 0.471 mmol), O-benzylhydroxylamine hydrochloride (83 mg, 0.52 mmol), EDCI (253 mg, 1.32 mmol), HOBt (24 mg, 0.15 mmol) and TEA (0.3 mL, 1.71 mmol) were added. After stirring overnight, the reaction mixture was diluted with CH2Cl2 and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:1) to generate 115 mg of 4f (yield 77%). 1H- NMR (CDCl3) δ 9.01 (s, 1H), 7.46–7.29 (m, 5H), 6.90 (s, 2H), 5.00 (s, 2H), 3.84 (s, 3H), 3.81 (s, 6H); 13C-NMR (CDCl3) δ 166.33, 153.31, 141.31, 135.32, 129.46, 128.91, 128.70, 127.26, 104.54, 78.45, 60.99, 56.32; HRMS (EI) m/z calcd. for 317.1263; found 317.1265.
3,4,5-Trihydroxy-N-(4-methoxybenzyl)benzamide (5b). A 10 mL round-bottom flask was charged with 3b (30 mg, 0.05 mmol), MeOH (3 mL), and a Teflon-coated magnetic stirring bar, 10% Pd/C (10 mg) were added, producing a black slurry. The flask was equipped with a hydrogen balloon attached to a stainless steel needle. The slurry was left to stir under hydrogen atmosphere. After 24 h, the hydrogen atmosphere of the flask was purged with nitrogen. The mixture was then filtered through a pad of Celite to give a dark green solution. This solution was concentrated to give 5b (14 mg, 90%). 1H-NMR (CD3OD) δ 7.24 (d, J = 8.7 Hz, 2H), 6.86 (d, J = 8.8 Hz, 4H), 4.44 (s, 2H), 3.76 (s, 3H); 13C- NMR (CD3OD) δ 170.20, 160.14, 146.50, 137.93, 132.29, 129.58, 126.05, 114.69, 107.69, 55.51, 43.73; HRMS (EI) m/z calcd. for 289.0950; found 289.0948.
(R)-Methyl 2-phenyl-2-(3,4,5-trihydroxybenzamido)acetate (5c). A 10 mL round-bottom flask was charged with 3a (89 mg, 0.15 mmol), MeOH (3 mL), and a Teflon-coated magnetic stirring bar. 10% Pd/C (12 mg) was added to the resulting solution, producing a black slurry. The flask was equipped with a hydrogen balloon attached to a stainless steel needle. The slurry was left to stir under hydrogen atmosphere. After 24 h, the hydrogen atmosphere of the flask was purged with nitrogen. The mixture was then filtered through a pad of Celite to give a dark green solution. This solution was concentrated to give 5c (43 mg, 88%). 1H-NMR (CD3OD) δ 7.34~7.43 (m, 5H), 6.89 (s, 2H), 5.63 (s, 1H), 3.71 (s, 3H); 13C-NMR (CD3OD) δ171.49, 168.75, 145.21, 137.04, 136.23, 128.48, 128.13, 127.51, 123.96, 106.76, 57.30, 51.75; HRMS (EI) m/z calcd. for 317.0899; found 317.0900.
(S)-Methyl 2-phenyl-2-(3,4,5-trihydroxybenzamido)acetate (5d). A 10 mL round-bottom flask was charged with 4a (60 mg, 0.1 mmol), MeOH (3 mL), and a Teflon-coated magnetic stirring bar. 10% Pd/C (5 mg) was added to the resulting solution, producing a black slurry. The flask was equipped with a hydrogen balloon attached to a stainless steel needle. The slurry was left to stir under hydrogen atmosphere. After 24 h, the hydrogen atmosphere of the flask was purged with nitrogen. The mixture was then filtered through a pad of Celite to give a dark green solution. This solution was concentrated to give 5d (30 mg, 92%) 1H-NMR (CD3OD) δ 7.28~7.41 (m, 5H), 6.86 (s, 2H), 5.60 (s, 1H), 3.68 (s, 3H); 13C-NMR (CD3OD) δ 171.50, 168.75, 145.24, 137.07, 136.25, 128.47, 128.12, 127.51, 123.96, 106.76, 57.30, 51.70; HRMS (EI) m/z calcd. for 317.0899; found 317.0900.
tert-Butyl 4-(3,4,5-trihydroxybenzoyl)piperazine-1-carboxylate (5g). A 10 mL round-bottom flask was charged with 7a (40 mg, 0.06 mmol), MeOH (3 mL), and a Teflon-coated magnetic stirring bar. 10% Pd/C (10 mg) was added to the resulting solution, producing a black slurry. The flask was equipped with a hydrogen balloon attached to a stainless steel needle. The slurry was left to stir under hydrogen atmosphere. After 24 h, the hydrogen atmosphere of the flask was purged with nitrogen. The mixture was then filtered through a pad of Celite to give a dark green solution. This solution was concentrated to give 5g (20 mg, 91%). 1H-NMR (CD3OD) δ 6.36 (s, 2H), 3.64–3.29 (m, 8H), 1.38 (s, 9H); 13C-NMR (CD3OD) δ 173.19, 156.18, 146.93, 136.53, 126.39, 107.80, 107.60, 81.60, 28.58; HRMS (EI) m/z calcd. for 338.1478; found 338.1477.
3,4,5-Trihydroxy-N-(prop-2-ynyl)benzamide (5h). A 10 mL round-bottom flask was charged with 5a (20 mg, 0.04 mmol), MeOH (3 mL), and a Teflon-coated magnetic stirring bar. 10% Pd/C (5 mg) was added to the resulting solution, producing a black slurry. The flask was equipped with a hydrogen balloon attached to a stainless steel needle. The slurry was left to stir under hydrogen atmosphere. After 24 h, the hydrogen atmosphere of the flask was purged with nitrogen. The mixture was then filtered through a pad of Celite to give a dark green solution. This solution was concentrated and purified by Biotage® SNAP Ultra C18 reversed phase cartridge ( from 10 to 90% ACN with 0.1% TFA) to give 5h (6 mg, 67%). 1H-NMR (CD3OD) δ 6.83 (s, 2H), 3.26 (t, J = 7.2 Hz, 2H), 1.59 (dd, J = 14.5, 7.3 Hz, 2H), 0.95 (t, J = 7.4 Hz, 3H); 13C-NMR (CD3OD) δ 170.59, 146.62, 137.95, 126.41, 107.73, 58.32, 42.67, 23.79, 18.36, 11.75; HRMS (EI) m/z calcd. for 211.0845; found 211.0842.
(S)-2-Phenyl-2-(3,4,5-tris(benzyloxy)benzamido)acetic acid (6a). The 3c (150 mg, 0.26 mmol) was suspended in THF (3 mL) in a 25-mL round-bottomed flask. In a separate flask, lithium hydroxide (16 mg) was dissolved in deionized water (1 mL). Both mixtures were chilled to 4 °C and combined to form a turbid white mixture. After 1 h of stirring, the mixture had become homogeneous. After 24 h, 1 mL of 1 M HCl was added, and the mixture was allowed to warm to room temperature. Following the addition of brine, the mixture was extracted four times with EtOAc, and the combined organic layers were evaporated. This yielded 6a as white powder (80 mg, 55%). 1H-NMR (CD3OD) δ 7.90 (s, 1H), 7.51 (dd, J = 8.1, 0.9 Hz, 2H), 7.46–7.44 (m, 3H), 7.40–7.29 (m, 13H), 7.25–7.14 (m, 5H), 5.46 (s, 1H), 5.13 (s, 4H), 5.01 (s, 2H); 13C-NMR (CD3OD) δ 174.62, 168.22, 154.02, 141.95, 141.24, 138.78, 138.32, 131.00, 129.74, 129.54, 129.33, 129.16, 129.06, 129.02, 128.92, 128.60, 128.36, 107.94, 76.19, 72.28, 61.10; HRMS (EI) m/z calcd. for 573.2151; found 573.2150.
Methyl 2-phenyl-2-(3,4,5-tris(benzyloxy)-N-methylbenzamido) propanoate (6b). Under a N2 atmosphere, to a solution of (S)-methyl 2-phenyl-2-(3,4,5-tris(benzyloxy)benzamido)acetate (200 mg, 0.34 mmol) in DMF (2.5 mL), NaH (20 mg, 60% purity, 0.51 mmol) was added slowly at 0 °C. After 30 min, iodomethane (0.042 mL, 0.48 mmol) was added slowly. The solution was stirred for 10 min at room temperature, after which the reaction was quenched by adding an excess amount of saturated NH4Cl aqueous solution, followed by extraction with ethyl acetate. The organic phase was washed with brine and then dried over anhydrous MgSO4. After the solution was filtered and the solvent was evaporated under vacuum, the residue was subjected to silica gel column chromatography using 25% EtOAc in hexane to give 6b (30 mg, 15%). H-NMR (CDCl3) δ 7.62–7.51 (m, 2H), 7.46–7.18 (m, 19H), 6.87–6.79 (m, 2H), 5.18–5.07 (m, 6H), 3.66 (s, 3H), 2.38 (s, 3H), 1.96 (s, 3H); 13C-NMR (CDCl3) δ 172.9, 172.1, 152.6, 140.3, 137.8, 137.5, 136.8, 131.0, 128.6, 128.5, 128.4, 128.3, 128.2, 127.9 (2), 127.4, 108.0, 75.2, 66.9, 52.6, 35.9, 18.6. HRMS (EI) m/z calcd. for 615.2621; found 615.2619.
Methyl (3,4,5-tris(benzyloxy)benzoyl)-d-phenylalaninate (6c). To a DMF solution (10 mL) of 3,4,5-tris(benzyloxy)benzoic acid (408 mg, 0.92 mmol), d-phenylalanine methyl ester hydrochloride (200 mg, 0.92 mmol), EDCI (196 mg, 1.02 mmol), HOBt (71 mg, 0.46 mmol) and TEA (0.30 mL, 2.04 mmol) were added. After stirring overnight, the reaction mixture was diluted with ethyl acetate and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:3) to generate pure 6c (220 mg, 40%). 1H-NMR (CDCl3) δ 7.25–7.41 (m, 18H), 7.11 (dd, 2H, J = 1.8, 7.8 Hz), 7.00 (s, 2H), 6.37 (d, 1H), 5.09 (s, 4H), 5.08 (s, 2H), 5.02–5.05 (m, 1H), 3.78 (s, 3H), 3.23 (qd, 2H, J = 7.8, 24.6 Hz); 13C- NMR (CDCl3) δ 172.0, 166.4, 152.7, 141.4, 137.4, 136.6, 135.8, 129.3, 129.2, 128.6, 128.5, 128.0, 127.9, 127.5, 127.2, 106.8, 75.1, 71.3, 53.5, 52.4, 37.8; HRMS (EI) m/z calcd. for 601.2464; found 601.2463.
Methyl (3,4,5-tris(benzyloxy)benzoyl)-l-phenylalaninate (6d). To a DMF solution (10 mL) of 3,4,5-tris(benzyloxy)benzoic acid (517 mg, 1.17 mmol), l-phenylalanine methyl ester hydrochloride (229 mg, 1.06 mmol), EDCI (230 mg, 1.20 mmol), HOBt (80 mg, 0.52 mmol) and TEA (0.37 mL, 2.09 mmol) were added. After stirring overnight, the reaction mixture was diluted with ethyl acetate and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:3) to generate pure 6d (420 mg, 66%). 1H-NMR (CDCl3) δ 7.25–7.41 (m, 18H), 7.11 (dd, 2H, J = 1.8, 7.8 Hz), 7.00 (s, 2H), 6.37 (d, 1H), 5.09 (s, 4H), 5.08 (s, 2H), 5.02–5.05 (m, 1H), 3.78 (s, 3H), 3.23 (qd, 2H, J = 7.8, 24.6 Hz); 13C- NMR (CDCl3) δ 172.0, 166.4, 152.7, 141.4, 137.4, 136.6, 135.8, 129.3, 129.2, 128.6, 128.5, 128.0, 127.9, 127.5, 127.2, 106.8, 75.1, 71.3, 53.5, 52.4, 37.8; HRMS (EI) m/z calcd. for 601.2464; found 601.2466.
Methyl (3,4,5-tris(benzyloxy)benzoyl)leucinate (6e). To a CH2Cl2 solution (3 mL) of 3,4,5-tris(benzyloxy)benzoic acid (240 mg, 0.54 mmol), l-leucine methyl ester hydrochloride (180 mg, 1.06 mmol), EDCI (190 mg, 1.00 mmol), HOBt (30 mg, 0.19 mmol) and TEA (0.14 mL, 0.85 mmol) were added. After stirring overnight, the reaction mixture was diluted with ethyl acetate and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:5) to generate 120 mg of 6e (yield 47%). 1H-NMR (CDCl3) δ 7.44–7.17 (m, 15H), 7.10 (s, 2H), 6.93 (d, J = 8.1 Hz, 1H), 4.85–4.79 (m, 1H), 3.74 (s, 3H), 1.77–1.56 (m, 3H), 0.96 (dd, J = 6.3, 2.7 Hz, 6H); 3C-NMR (CDCl3) δ 174.61, 166.67, 152.75, 141.35, 137.56, 136.85, 128.91, 128.62, 128.52, 128.21, 127.98, 127.92, 127.77, 106.89, 75.18, 71.32, 52.48, 51.36, 41.37, 25.06, 23.03, 21.85; HRMS (EI) m/z calcd. for 567.2421; found 567.2466.
tert-Butyl (S)-2-phenyl-2-(3,4,5-tris(benzyloxy)benzamido)acetate (6f). To a CH2Cl2 solution (3 mL) of 3,4,5-tris(benzyloxy)benzoic acid (180 mg, 0.40 mmol), l-Phenylalanine tert-butyl ester hydrochloride (100 mg, 0.41 mmol), EDCI (90 mg, 0.47 mmol), HOBt (30 mg, 0.19 mmol) and TEA (0.14 mL, 0.85 mmol) were added. After stirring overnight, the reaction mixture was diluted with ethyl acetate and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:3) to generate 110 mg of 6f (yield 45%). 1H-NMR (CDCl3) δ 7.46–7.27 (m, 19H), 7.21 (t, J = 6.1 Hz, 1H), 7.19 (s, 2H), 5.65 (d, J = 7.0 Hz, 1H), 5.12 (s, 6H), 1.46 (s, 9H); 13C-NMR (CDCl3) δ 170.22, 165.96, 152.79, 141.48, 137.49, 137.32, 136.74, 129.18, 128.82, 128.63, 128.58, 128.27, 128.24, 128.05, 127.99, 127.66, 127.26, 107.13, 82.84, 75.18, 71.42, 57.40, 27.90; HRMS (EI) m/z calcd. for 629.2777; found 629.2774.
(S)-N-(2-Amino-2-oxo-1-phenylethyl)-3,4,5-tris(benzyloxy)benzamide (6g). To a CH2Cl2 solution (3 mL) of 3,4,5-tris(benzyloxy)benzoic acid (390 mg, 0.88 mmol), (S)-2-amino-2-phenylacetamide (180 mg, 1.06 mmol), EDCI (190 mg, 1.00 mmol), HOBt (70 mg, 0.46 mmol) and TEA (0.31 mL, 1.78 mmol) were added. After stirring overnight, the reaction mixture was diluted with ethyl acetate and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:1) to generate 302 mg of 6g (yield 57%). 1H-NMR (DMSO-d6) δ 8.75 (d, 1H, J = 7.8 Hz), 7.72 (s, 1H), 7.51 (d, 2H, J = 7.2 Hz), 7.46 (d, 4H, J = 7.2 Hz), 7.23–7.40 (m, 17H), 5.66 (d, 1H, J = 7.8 Hz), 5.17 (s, 4H), 4.99 (s, 2H); 13C- NMR (DMSO-d6) δ 172.26, 165.79, 152.37, 139.21, 137.36, 129.60, 128.84, 128.69, 128.63, 128.47, 128.33, 128.26, 128.12, 127.99, 127.95, 107.35, 74.65, 70.83, 60.18; HRMS (EI) m/z calcd. for 572.2311; found 572.2312.
Methyl 2-(2-chlorophenyl)-2-(3,4,5-tris(benzyloxy)benzamido)acetate (6h). To a CH2Cl2 solution (3 mL) of 3,4,5-tris(benzyloxy)benzoic acid (286 mg, 0.65 mmol), (S)-(+)-2-chlorophenylglycine methyl ester hydrochloride (130 mg, 0.65 mmol), EDCI (137 mg, 0.71 mmol), HOBt (50 mg, 0.33 mmol) and TEA (0.34 mL, 1.9 mmol) were added. After stirring overnight, the reaction mixture was diluted with ethyl acetate and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:3) to generate 30 mg of 6h (yield 8%). 1H-NMR (CDCl3) δ 7.46–7.26 (m, 18H), 7.13 (d, J = 6.9 Hz, 1H), 7.10 (s, 2H), 6.01 (d, J = 7.0 Hz, 1H), 5.10 (d, J = 6.6 Hz, 4H), 5.09 (s, 2H), 3.77 (d, J = 8.4 Hz, 3H); 13C-NMR (CDCl3) δ 142.80, 138.02, 124.72, 113.56, 109.35, 108.60, 106.68, 105.53, 102.65, 102.19, 101.79, 100.75, 100.56, 100.54, 100.18, 100.00, 99.94, 99.53, 99.30, 79.13, 47.12, 43.37, 27.29, 25.14; HRMS (EI) m/z calcd. for 621.1918; found 621.1917.
Methyl 2-(4-fluorophenyl)-2-(3,4,5-tris(benzyloxy)benzamido)acetate (6i). To a CH2Cl2 solution (3 mL) of 3,4,5-tris(benzyloxy)benzoic acid (390 mg, 0.88 mmol), methyl amino(4-fluorophenyl)acetate hydrochloride (240 mg, 1.09 mmol), EDCI (310 mg, 1.62 mmol), HOBt (70 mg, 0.52 mmol) and TEA (0.57 mL, 3.2 mmol) were added. After stirring overnight, the reaction mixture was diluted with ethyl acetate and washed with water and brine, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate/n-hexane = 1:3) to generate 320 mg of 6i (yield 48%). 1H-NMR (CDCl3) δ 7.46–7.29 (m, 15H), 7.29–7.27 (m, 2H), 7.13–7.08 (m, 2H), 7.08–6.99 (m, 2H), 6.99–6.91 (m, 1H), 5.69 (d, J = 6.7 Hz, 1H), 5.16–5.12 (m, 4H), 5.10 (s, 2H), 3.79 (s, 3H); 13C-NMR (CDCl3) δ 171.37, 166.04, 163.59, 161.95, 152.82, 141.73, 137.35, 136.62, 132.46, 129.12, 129.06, 128.73, 128.59, 128.23, 128.08, 128.01, 127.60, 116.06, 115.92, 107.20, 75.18, 71.52, 56.20, 53.07; HRMS (EI) m/z calcd. for 605.2214; found 605.2217.
4.3. Determination of the Mast Cell Degranulation
4.3.1. Histamine Assay Use PMACl in HMC-1 Cells
To determine the mast cell degranulation, level of histamine in culture media was measured. HMC-1 cells were grown in Iscove’s modified Dulbecco’s medium (GIBCO, Grand Island, NY, USA) supplemented with 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin G, 100 mg/mL streptomycin, and 250 ng/mL amphotericin B at 37 °C in 5% CO2 incubator. The passage ranging 4–8 of HMC-1 cells (5 × 105/well in 24-well plates) were pretreated with or without drugs for 1 h and then stimulated with 4 nM of phorbol 12-mystate 13-acetate (PMA, Sigma-Aldrich, St Louis, MO, USA) and 1 μM of calcium ionophore A23187 (CI, Sigma-Aldrich) for 8 h. The cells were separated from the media by centrifugation at 150 g for 5 min at 4 °C. To separate histamine from serum and media, 0.1 N HCl and 60% perchloric acid were added. After centrifugation, the supernatant fluid transferred to effendorf tube containing 5 N NaOH, 5 M NaCl, and n-butanol and then vortexed. The organic phase was gathered, shaken with 0.1 N HCl and n-haptane, and then centrifuged. The histamine in the aqueous phase is assayed using the o-phthaldialdehyde spectrofluorometric procedure as previously described [6].
4.3.2. Histamine Assay Use DNP-Human Serum Albumin in RBL-2H3 Cells
To evaluate mast cell degranulation, the level of histamine in culture media was measured. RBL-2H3 cells (5 × 105/well in 24-well plates) were sensitized with anti-DNP IgE (50 ng/mL). After incubation overnight, cells were pretreated with or without compound for 1 h and then stimulated with DNP-HAS (100 ng/mL) for 4 h. The cells were separated from the media by centrifugation at 150 g for 5 min at 4 °C. To separate histamine from serum and media, 0.1 N HCl and 60% perchloric acid were added. After centrifugation, the supernatant fluid was transferred to an Eppendorf tube containing 5 N NaOH, 5 M NaCl, and n-butanol, then vortexed. The organic phase was gathered, shaken with 0.1 N HCl and n-haptane, then centrifuged. The histamine in the aqueous phase was assayed using the o-phthaldialdehyde spectrofluorometric procedure as previously described [6].
4.4. RNA Extraction and Real-Time PCR
Prior to isolation of total cellular RNA, HMC-1 cells (5 × 105/well in 24-well plates) were pretreated with or without compound for 1 h and then stimulated with PMA (40 nM) and CI (1 μM) for 1 h. RNAiso Plus reagent (Takara Bio Inc., Shiga, Japan) was used to extract total RNA, in accordance with the manufacturer’s protocol. Complementary DNA (cDNA) was synthesized from 2 μg of total RNA using the Maxime RT-Pre Mix Kit (iNtRON Biotechnology, Daejeon, Korea). Quantitative real-time PCR was carried out using the Thermal Cycler Dice TP850 (Takara Bio Inc.) according to the manufacturer’s protocol. The 25 μL reaction mixture was composed as follows: 1.5 μL of cDNA (150 ng), 1 μL of each of the forward and reverse primers (0.4 μM), 12.5 μL of SYBR Premix Ex Taq (Takara Bio Inc.), and 9 μL of D2O [15].
4.5. Statistical Analysis
Each data represents the mean ± SE of 3 independent experiments. Statistical analyses were performed using Prism5 (GraphPad Software, San Diego, CA, USA), and treatment effects were analyzed using a one-way ANOVA followed by Dunnett’s test. A value of p < 0.05 was used to indicate a significant difference.
Acknowledgments
This research was supported by grants from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI14C1135) and the National Research Foundation of Korea grant funded by the Korean government (grant number: NRF-2016R1D1A1A09917300).
Supplementary Materials
The following are available online, Figures S1–S52: Spectral data.
Author Contributions
T.Y.S., S.H.K. and S.Y.S. conceived and designed the experiments; X.F. and I.G.J. performed the experiments; X.F. and I.G.J. analyzed the data; X.F. contributed reagents/materials/analysis tools; X.F. and I.G.J. wrote the paper.
Conflicts of Interest
The authors declare the following competing financial interest(s): I.G.J., T.Y.S., S.H.K. and S.Y.S. are named on a patent application disclosing the novel compounds described here. The authors declare no other conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References and Notes
- 1.Kim S.-H., Jun C.D., Suk K., Choi B.J., Lim H., Park S., Lee S.H., Shin H.Y., Kim D.K., Shin T.Y. Gallic acid inhibits histamine release and pro-inflammatory cytokine production in mast cells. Toxicol. Sci. 2006;91:123–131. doi: 10.1093/toxsci/kfj063. [DOI] [PubMed] [Google Scholar]
- 2.Akdis C.A., Agache I. Global Atlas of Allergy. European Academy of Allergy and Clinical Immunology; Zurich, Sitzerland: 2014. [Google Scholar]
- 3.Galli S.J., Tsai M., Piliponsky A.M. The development of allergic inflammation. Nature. 2008;454:445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Metz M., Maurer M. Mast cells–key effector cells in immune responses. Trends Immunol. 2007;28:234–241. doi: 10.1016/j.it.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Galli S.J., Tsai M. IgE and mast cells in allergic disease. Nat. Med. 2012;18:693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Je I.-G., Kim H.-H., Park P.-H., Kwon T.K., Seo S.-Y., Shin T.-Y., Kim S.-H. SG-HQ2 inhibits mast cell-mediated allergic inflammation through suppression of histamine release and pro-inflammatory cytokines. Exp. Biol. Med. 2015;240:631–638. doi: 10.1177/1535370214555663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim H.-H., Kim S.-W., Kim D.-S., Oh H.-M., Rho M.-C., Kim S.-H. Vigna angularis inhibits mast cell-mediated allergic inflammation. Int. J. Mol. Med. 2013;32:736–742. doi: 10.3892/ijmm.2013.1430. [DOI] [PubMed] [Google Scholar]
- 8.Cho S.J., Roh J.S., Sun W.S., Kim S.-H., Park K.D. N-Benzylbenzamides: A new class of potent tyrosinase inhibitors. Bioorg. Med. Chem. Lett. 2006;16:2682–2684. doi: 10.1016/j.bmcl.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 9.For 4a, Pandey K., Muthyala M.K., Choudhary S., Kumar A. Imidazolium salt-supported Mukaiyama reagent: An efficient condensation reagent for amide bond formation. RSC Adv. 2015;5:13797–13804.
- 10.For 4a, Saeed A., Simpson J. Unusual C–H···π interactions in the structure of 3,4,5-trimethoxy-N-p-tolylbenzamide. J. Chem. Crystallogr. 2013;43:51–57.
- 11.For 4e, Hashmi A.S.K., Jaimes M.C.B., Schuster A.M., Rominger F. From propargylic amides to functionalized oxazoles: Domino gold catalysis/oxidation by dioxygen. J. Org. Chem. 2012;77:6394–6408. doi: 10.1021/jo301288w.
- 12.For 4e, Tran H.-A., Kitov P.I., Paszkiewicz E., Sadowska J.M., Bundle D.R. Multifunctional multivalency: A focused library of polymeric cholera toxin antagonists. Org. Biomol. Chem. 2011;9:3658–3671. doi: 10.1039/c0ob01089h.
- 13.For 4e, Upadhyaya K., Hamidullah, Singh K., Arun A., Shukla M., Srivastava N., Ashraf R., Sharma A., Mahar R., Shukla S.K., et al. Identification of gallic acid based glycoconjugates as a novel tubulin polymerization inhibitors. Org. Biomol. Chem. 2016;14:1338–1358. doi: 10.1039/c5ob02113h.
- 14.For 5h, Lipeeva A.V., Pokrovsky M.A., Baev D.S., Shakirov M.M., Bagryanskaya I.Y., Tolstikova T.G., Shults E.E. Synthesis of 1H-1, 2, 3-triazole linked aryl (arylamidomethyl)–dihydrofurocoumarin hybrids and analysis of their cytotoxicity. Eur. J. Med. Chem. 2015;100:119–128. doi: 10.1016/j.ejmech.2015.05.016.
- 15.Bae Y., Lee S., Kim S.-H. Chrysin suppresses mast cell-mediated allergic inflammation: Involvement of calcium, caspase-1 and nuclear factor-κB. Toxicol. Appl. Pharmacol. 2011;254:56–64. doi: 10.1016/j.taap.2011.04.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.