Abstract
Luculia plants are famed ornamentals with sweetly fragrant flowers. Luculia yunnanensis Hu is an endemic plant from Yunnan Province, China. Headspace-solid phase microextraction-gas chromatography-mass spectrometry (HS-SPME-GC-MS) was used to identify the volatile organic compounds (VOCs) of the different flower development stages of L. yunnanensis for the evaluation of floral volatile polymorphism. The results showed that a total of 40 compounds were identified at four different stages. The main aroma-active compounds were 3-carene, α-cubebene, α-copaene, δ-cadinene, and isoledene. Floral scent emission had the tendency to ascend first and descend in succession, reaching its peak level at the initial-flowering stage. The richest diversity of floral volatiles was detected at the full-flowering stage. Principal component analysis (PCA) indicated that the composition and its relative content of floral scent differed at the whole flower development stage. In comparison with the other two species of Luculia (L. pinceana and L. gratissima), the composition and its relative content of floral scent were also different among the tree species.
Keywords: Luculia, volatile organic compound, SPME-GC-MS, floral scent, flower development
1. Introduction
The genus of Luculia Sweet comprises small trees or shrubs belonging to the Rubiaceae family (Trib. Cinchoneae). It has about five species in the world, mainly distributed in Southeastern Asia. There are three species distributed in China; i.e., L. pinceana Hooker, L. gratissima (Wallich) Sweet and L. yunnanensis Hu. Among them, L. yunnanensis is the unique one, endemic to Yunnan Province and naturally confined to open slopes and secondary shrubby woodland on limestone mountains at an altitude between 1200 and 3200 m [1]. Luculia species can be easily recognized by the compact and long-term blooming inflorescences consisting of white, pink to red, and sweetly fragrant flowers with extremely long corolla tubes [2]. L. yunnanensis distinguishes itself from its allied species by the surface of inflorescence axes, the hypanthium portion of the calyx, and its fruit covered by densely tomentose pubescences. Moreover, it is difficult to find wild populations of L. yunnanensis, as its appropriate habitats have been fragmented by human activities [1,3].
Plants synthesize a wide variety of volatile organic compounds (VOCs) that facilitate interactions with their lived environment: attracting pollinators, protecting flowers from harmful insects, and communicating with other plants. The floral scent compositions of L. pinceana and L. gratissima have been reported [4,5]. The main components of floral scent are paeonol and γ-murolene in L. pinceana and L. gratissima, respectively. Therefore, detecting the VOCs of L. yunnanensis could not only give clues to its conservation biology, but also provide reference to the development of L. yunnanensis as an ornamental with fragrant scent in the future.
Though the species of family Rubiaceae are not common aromatic plants, Luculia plants have a promising prospect as an essential oil source. Components and proportions of VOCs may vary at different flower developmental stages. Therefore, it is essential to investigate the VOCs polymorphism of different stages of flower development for determination of the suitable period for L. yunnanensis flower harvest.
Headspace solid phase micro-extraction (HS-SPME) followed by capillary gas-chromatography mass-spectrometry (GC-MS) has high reproducibility under the same test conditions, and is currently a widely used technique for flower volatile analysis [6,7]. In this paper, we investigated the floral volatiles in L. yunnanensis using HS-SPME coupled with GC-MS to evaluate the volatile polymorphism of different flower development stages, which provide guidance for the evaluation of flower scent quality and the generation of fragrant L. yunnanensis for future breeding programs and the function of floral fragrance.
2. Results and Discussion
2.1. Identification of Scent Components
The volatiles of four different stages of L. yunnanensis flower development (Figure 1) are given in Table 1, with the compounds listed in order of their retention time (RT) in the DB-5MS column. A total of forty VOCs were detected in L. yunnanensis flower development. Among these volatiles, 22 compounds were identified in four different stages. The main aroma-active compounds were identified at 3-carene, α-cubebene, α-copaene, δ-cadinene, and isoledene; these compounds might dominate the fragrance of L. yunnanensis. For instance, 3-carene smells like lemon or resin, α-cubebene smells like herb or wax, α-copaene smells like wood or spice, δ-cadinene smells like thyme and medicine or wood [8]. Together, the volatile compounds form the delightful fragrance of the L. yunnanensis flower.
Figure 1.
Different development stages of L. yunnanensis and its habitat. (I) bud stage; (II) initial-flowering stage; (III) full-flowering stage; (IV) end-flower stage; (V) habitat of L. yunnanensis.
Table 1.
Volatile compounds identified in four different stages of L. yunnanensis flower development using headspace solid phase micro-extraction (HS-SPME) coupled with gas-chromatography mass-spectrometry (GC-MS) (HS-SPME-GC-MS). (I) bud stage; (II) initial-flowering stage; (III) full-flowering stage; and (IV) end-flower stage.
| Peak | RT | LRI | LRI * | Compounds | CAS # | Relative Content (%) ± SD | |||
|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | ||||||
| Monoterpenes | |||||||||
| 1 | 8.35 | 903 | 929 | (1S)-2,6,6-Trimethylbicyclo[3.1.1]hept-2-ene | 7785-26-4 | 0b | 0b | 0.25 ± 0.06a | 00b |
| 2 | 8.72 | 916 | 910 | Santolina triene | 2153-66-4 | 0b | 0.01 ± 0.01b | 0.16 ± 0.08a | 00b |
| 3 | 9.45 | 943 | 941 | -α-Pinene | 80-56-8 | 0b | 0b | 0.99 ± 0.20a | 00b |
| 4 | 9.81 | 957 | 966 | β-Pinene | 127-91-3 | 0b | 0.09 ± 0.01b | 0.07 ± 0.04b | 0.42 ± 0.10a |
| 7 | 11.32 | 1011 | 1011 | 3-Carene | 13466-78-9 | 3.36 ± 0.44c | 10.72 ± 2.20b | 9.17 ± 1.65bc | 29.18 ± 4.53a |
| 8 | 12.63 | 1059 | 1056 | trans-β-Ocimene | 3779-61-1 | 0.88 ± 0.10b | 0.61 ± 0.13b | 0.99 ± 0.49b | 1.86 ± 0.04a |
| 11 | 13.81 | 1102 | 1127 | α-Campholenal | 91819-58-8 | 0b | 0b | 0.60 ± 0.30a | 00b |
| 12 | 14.37 | 1124 | - | α-Santoline alcohol | 90823-36-2 | 0b | 0b | 0.35 ± 0.18a | 0b |
| 13 | 14.84 | 1142 | 1137 | Limonene oxide | 1195-92-2 | 0.20 ± 0.14a | 0a | 0.28 ± 0.19a | 0a |
| Aliphatics | |||||||||
| 5 | 10.64 | 987 | 987 | Methyl heptenone | 110-93-0 | 0.68 ± 0.26a | 0b | 0b | 0b |
| 6 | 11.14 | 1005 | - | Furan, 2,3-dihydro-4-(1-methylethyl)- | 34314-84-6 | 3.27 ± 0.89a | 0.33 ± 0.02b | 0.17 ± 0.03b | 1.07 ± 0.05b |
| 9 | 13.46 | 1089 | 1130 | 2,6-Dimethyl-1,3,5,7-octatetraene | 460-01-5 | 0b | 0b | 0.29 ± 0.08a | 0b |
| 15 | 19.67 | 1340 | 1373 | Propanoic acid, 2-methyl-, 2-ethyl-3-hydroxyhexyl ester | 74367-31-0 | 1.12 ± 0.13a | 0.25 ± 0.05b | 0.22 ± 0.11b | 1.16 ± 0.14a |
| 40 | 28.08 | 1828 | 1827 | Isopropyl myristate | 110-27-0 | 0b | 0b | 0.18 ± 0.06b | 1.51 ± 0.15a |
| Benzenoids | |||||||||
| 10 | 13.71 | 1098 | 1107 | Phenylethyl alcohol | 60-12-8 | 0b | 0.10 ± 0.02a | 0b | 0b |
| 14 | 18.31 | 1295 | - | 6,7-Dimethyl-1,2,3,5,8,8a -hexahydronaphthalene | 107914-92-1 | 0b | 0.14 ± 0.03a | 0.17 ± 0.09a | 0b |
| 22 | 22.45 | 1433 | 1451 | Paeonol | 552-41-0 | 0b | 0b | 12.73 ± 2.43a | 0b |
| 36 | 24.99 | 1550 | 1550 | Benzene, 1,2,3-trimethoxy-5-(2-propenyl)- | 487-11-6 | 0c | 0.41 ± 0.03a | 0c | 0.13 ± 0.07b |
| Sesquiterpenes | |||||||||
| 16 | 19.87 | 1346 | 1346 | α-Cubebene | 17699-14-8 | 13.39 ± 0.63a | 10.24 ± 1.68b | 8.68 ± 1.09b | 7.52 ± 1.02b |
| 17 | 20.34 | 1361 | 1365 | α-Copaene | 3856-25-5 | 16.59 ± 3.43a | 17.92 ± 2.73a | 9.30 ± 0.73b | 7.45 ± 0.49b |
| 18 | 21.13 | 1387 | 1377 | Isoledene | 95910-36-4 | 9.79 ± 1.21a | 8.67 ± 0.65a | 8.33 ± 1.94a | 7.19 ± 1.40a |
| 19 | 21.52 | 1399 | 1400 | Caryophyllene | 87-44-5 | 4.23 ± 0.49a | 2.05 ± 0.12b | 1.19 ± 0.26b | 3.76 ± 0.69a |
| 20 | 21.87 | 1412 | 1421 | β-Ylangene | 20479-06-5 | 0.60 ± 0.01a | 0.34 ± 0.07b | 0.08 ± 0.03c | 0.36 ± 0.02b |
| 21 | 22.22 | 1425 | 1438 | α-Guaiene | 3691-12-1 | 0b | 0.12 ± 0.03a | 0.04 ± 0.02b | 0b |
| 23 | 22.68 | 1441 | 1450 | cis-Muurola-3,5-diene | Not available | 3.43 ± 0.36ab | 3.76 ± 0.50ab | 4.48 ± 1.17a | 2.61 ± 0.48b |
| 24 | 22.81 | 1447 | 1446 | Humulene | 6753-98-6 | 3.89 ± 0.68b | 1.70 ± 0.21c | 3.42 ± 1.29bc | 9.69 ± 0.49a |
| 25 | 23.01 | 1454 | 1458 | β-Gurjunene | 17334-55-3 | 0b | 0.23 ± 0.07a | 0.04 ± 0.02a | 0b |
| 26 | 23.32 | 1466 | 1474 | β-Cadinene | 523-47-7 | 2.27 ± 0.06a | 1.63 ± 0.20a | 2.42 ± 0.64a | 1.61 ± 0.55a |
| 27 | 23.42 | 1469 | 1475 | Naphthalene,1,2,4a,5,6,8a -hexahydro-4,7-dimethyl-1-(1-methylethyl)- | 483-75-0 | 0b | 0.66 ± 0.08a | 0.08 ± 0.05b | 0.77 ± 0.01a |
| 28 | 23.56 | 1474 | 1474 | γ-Muurolene | 30021-74-0 | 3.15 ± 0.33b | 4.50 ± 0.77a | 1.79 ± 0.48c | 1.95 ± 0.22bc |
| 29 | 23.84 | 1485 | 1515 | Cubebol | 23445-02-5 | 4.36 ± 0.42a | 3.89 ± 0.64a | 3.85 ± 0.41a | 2.52 ± 0.41b |
| 30 | 23.97 | 1490 | 1484 | -Aristolene | 6831-16-9 | 1.05 ± 0.14a | 0.83 ± 0.06ab | 1.01 ± 0.41a | 0.42 ± 0.02b |
| 31 | 24.07 | 1493 | 1500 | (E,E)-α-Farnesene | 502-61-4 | 0.42 ± 0.02b | 1.36 ± 0.26b | 4.65 ± 1.13a | 1.21 ± 0.07b |
| 32 | 24.29 | 1503 | 1494 | 4-epi-Cubebol | Not available | 4.24 ± 0.51b | 9.06 ± 1.41a | 4.67 ± 1.48b | 2.90 ± 0.73b |
| 33 | 24.44 | 1514 | 1519 | δ-Cadinene | 483-76-1 | 14.66 ± 2.85a | 12.68 ± 2.56a | 13.07 ± 2.79a | 9.16 ± 0.77a |
| 34 | 24.62 | 1525 | 1528 | Cadine-1,4-diene | 16728-99-7 | 5.74 ± 0.05ab | 6.13 ± 0.73a | 4.47 ± 1.86ab | 3.13 ± 0.25b |
| 35 | 24.87 | 1542 | 1542 | α-Calacorene | 21391-99-1 | 0c | 0.32 ± 0.06a | 0.18 ± 0.04b | 0c |
| 37 | 25.68 | 1596 | 1596 | Guaiol | 489-86-1 | 1.09 ± 0.14a | 0.24 ± 0.07b | 0.30 ± 0.04b | 1.11 ± 0.27a |
| 38 | 25.82 | 1608 | 1630 | α-Acorenol | 28296-85-7 | 0.72 ± 0.09a | 0.30 ± 0.04b | 0.33 ± 0.05b | 0.73 ± 0.15a |
| 39 | 26.32 | 1652 | 1647 | Cubenol | 21284-22-0 | 0.81 ± 0.01a | 0.71 ± 0.02a | 1.00 ± 0.43a | 0.58 ± 0.01a |
| Monoterpenes | 4.50 ± 0.41c (9.11%) | 11.43 ± 2.31bc (20.21%) | 12.86 ± 2.93b (22.78%) | 31.46 ± 4.46a (14.18%) | |||||
| Aliphatics | 5.07 ± 0.75a (14.79%) | 0.58 ± 0.08c (13.79%) | 0.86 ± 0.25c (29.07%) | 3.74 ± 0.33b (8.82%) | |||||
| Benzenoids | 0b | 0.65 ± 0.07b (10.77%) | 12.90 ± 2.35a (18.22%) | 0.13 ± 0.07b (53.85%) | |||||
| Sesquiterpenes | 90.43 ± 1.17a (1.29%) | 87.34 ± 2.46a (2.82%) | 73.38 ± 5.20b (7.09%) | 64.67 ± 4.09b (6.32%) | |||||
Values, expressed as mean ± SD of triplicate measurements, with different letter (a–c) in the same row indicating significant difference according to Tukey’s test (p < 0.05). The data in brackets are coefficients of variation. RT: retention time; LRI: linear retention index; LRI *: linear rentention index taken from NIST Standard Reference Database 69 [15]; CAS #: chemical abstracts service registry number.
As we all know, floral scent often serves as an olfactory signal for the attraction of insects. For example, bees can find the location of flowers under the guidance of linalool or 2-phenylethanol [9]; and indole for flies [10]; phenylacetaldehyde, 2-phenylethanol, oxoisophorone, linalool, and linalool oxide for butterflies [11], etc. Most visitors successfully transfer the pollen to conspecific flowers as pollinators [12]. To survive biotic stresses, many plant species have developed elaborate mechanisms to protect themselves against insects (e.g., emitting floral VOCs). (E,E)-α-Farnesene emitted from the flowers of Prorhinotermes canalifrons functioned as an alarm pheromone [13]. Besides (E,E)-α-farnesene, trans-β-ocimene (which can elicit strong antennal responses in butterfly [14]) also appeared in L. yunnanensis. Our preliminary results showed that the visiting insects of L. yunnanensis flowers in the field included Apis florae, Bombus sp., Lucillia sp., etc. (Figure 2).
Figure 2.
Flowers of L. yunnanensis visited by different insects.
In addition, some compounds of floral VOCs had a good effect on resisting bacteria and fungus [7,16,17]. Amongst the floral scent composition of L. yunnanensis, phenylethyl alcohol has antifungal activity against Botrytis cinerea [18]; limonene oxide has antibacterial activities against Agrobacterium tumefaciens, Erwinia ananas, Erwinia chrysanthemi, and Enterobacter cloacae [19]; paeonol has potential acaricide activity for the control of Dermatophagoides pteronyssinus and Dermatophagoides farina [16]. From this point of view, L. yunnanensis might be qualified as an indoor fragrant houseplant. Recent studies have shown that plants can rapidly alter their own floral volatile production in response to floral volatile cues from their neighbors in flower opening [20,21]. This specific mechanism can increase the rate of pollination and mating by increasing floral volatile emission to attract more pollinators. The relationship between the floral VOCs of L. yunnanensis and its environment needs further research in the future.
2.2. Changes of Scent Emission in Four Different Stages
L. yunnanensis flowers were selected on the basis of their botanical characteristics to evaluate floral volatile polymorphisms according to different development stages: bud stage, initial-flowering stage, full-flowering stage, and end-flowering stage. The floral scents are orchestrated during the flower development. Table 1 and Figure 3 show the distinct changes in scent composition and concentration across flowering stages. Scent components were emitted drastically at the initial-flowering stage. The highest diversity of floral volatiles was detected at the full-flowering stage. In most plants (e.g., citrus flowers [22], Vanda Mimi Palmer [23], Cananga odorata [24], Ocimum citriodorum [25], Penstemon digitalis [26], and Hosta flowers [27]), the amount of scent emission and the diversity of floral volatiles were sharply increased at the stage of full bloom and decreased highly after full bloom. By contrast, the patterns of scent emission across L. yunnanensis flowering stages are different from one other.
Figure 3.
Total ionic chromatogram of scent components emitted from the flowers of L. yunnanensis in different stages. (I) bud stage; (II) initial-flowering stage; (III) full-flowering stage; and (IV) end-flower stage.
As for the bud stage, 24 volatile compounds were identified, and the most abundant compounds were α-copaene (16.59%), δ-cadinene (14.66%), α-cubebene (13.39%), and isoledene (9.79%). For the initial-flowering stage, 31 volatile compounds were identified, and the most abundant compounds were α-copaene (17.92%), δ-cadinene (12.68%), 3-carene (10.72%), and α-cubebene (10.24%). For the full-flowering stage, 37 volatile compounds were identified, and the most abundant compounds were δ-cadinene (13.07%), paeonol (12.73%), α-copaene (9.30%), and 3-carene (9.17%). In the end-flowering stage, 26 volatile compounds were identified, and the abundant compounds were 3-carene (29.18%), humulene (9.69%), and δ-cadinene (9.16%).
The mean Bray–Curtis similarity index range was 64.29–81.40% (Table 2). The bud stage was more similar to the initial-flowering stage (BCS = 81.40%) than to the full-flowering stage (BCS = 71.88%), and was largely dissimilar to the end-flowering stage (BCS = 63.71%). Across all flower-life stages, the end-flowering stage was largely dissimilar to other three stages. Regarding the comparison among the studied stages, 22 volatiles among the total volatile constituents notably existed in all life-flower stages. The results showed high similarity among the four stages, although several constitutes (α-pinene, α-campholenal, phenylethyl alcohol, paeonol, etc.) were exclusively identified in different stages.
Table 2.
The Bray–Curtis similarity values (%) among stages of flower development of L. yunnanensis. (I) bud stage; (II) initial-flowering stage; (III) full-flowering stage; and (IV) end-flower stage.
| I | II | III | IV | |
|---|---|---|---|---|
| I | 100 | |||
| II | 81.40 | 100 | ||
| III | 71.88 | 76.30 | 100 | |
| IV | 63.71 | 65.67 | 64.29 | 100 |
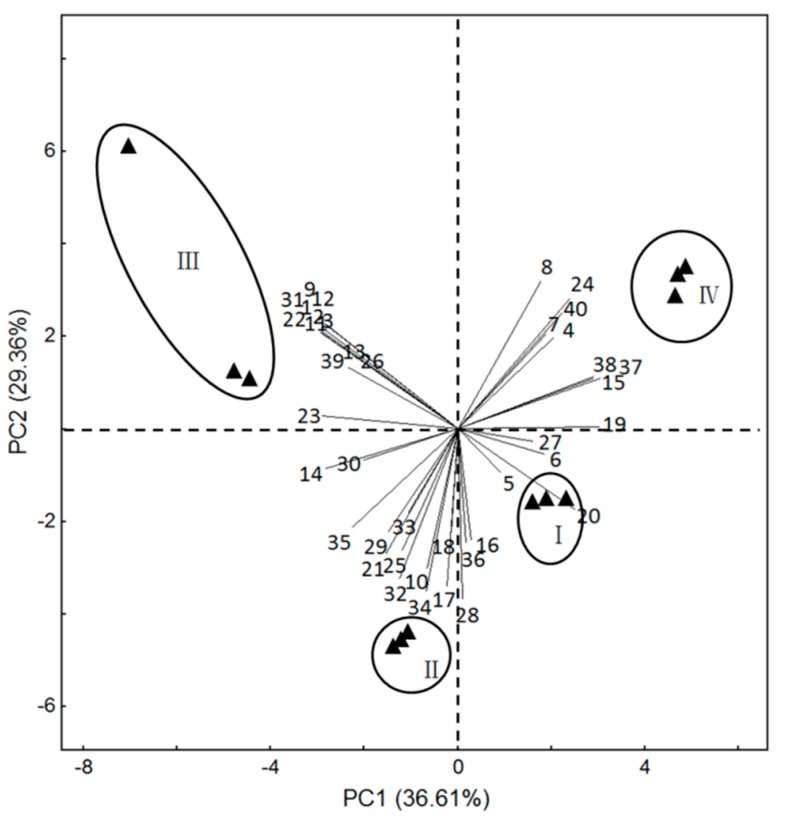
To identify which volatiles contributed the most to the differences among the four flower stages, the data on 40 volatile compounds identified in L. yunnanensis at whole life-flower scale were analyzed by using principal component analysis (PCA). The first two components of PCA explained 36.61% and 29.36% of the variation, explaining ~66% of combined variance (Figure 4). The volatiles that had high positive scores on PC 1 included propanoic acid, 2-methyl-, 2-ethyl-3-hydroxyhexyl ester, caryophyllene, α-acorenol, guaiol, and β-ylangene, which were highly positively related to the bud stage and the initial-flowering stage. Volatiles with high positive scores on PC 2 comprised trans-β-ocimene, humulene, isopropyl myristate, α-campholenal, and α-santoline alcohol, which were positively correlated with the full-flowering stage and initial-flowering stage.
Figure 4.
Principal component plot (PC1 vs. PC2 plots) for L. yunnanensis at different stages of growth, showing correlations with volatiles (numbers correspond to those in Table 1). (I) bud stage; (II) initial-flowering stage; (III) full-flowering stage; (IV) end-flower stage.
2.3. Variation in Volatile Compounds from Three Species of Luculia
Sesquiterpenes were the most abundant amongst floral scent compounds, the content of which reached as high as 64% in L. yunnanensis and L. gratissima [5], in the floral scent of L. pinceana were benzenoids [4]. By PCA analysis, the floral scent showed differences among Luculia (the wide-open flower of L. gratissima, the full-flowering stage of L. pinceana, and the four stages of L. yunnanensis) (Figure 5). L. gratissima occupied 10 special compounds, such as octanoic acid, ethyl ester, β-bourbonene, trans-isoeugenol, etc. L. pinceana had 13 special compounds, such as cyclosativene, limonene, (Z)-verbenol, etc. L. yunnanensis possessed 17 special compounds, such as β-pinene, phenylethyl alcohol, limonene oxide, etc. Previous research indicated that significant differences in floral scent composition were found in closely-related species [28], which is consistent with our study.
Figure 5.
Principal component plots (PC1 vs. PC2 plots) for the volatiles of the full-flowering stage of L. gratissima, the full-flowering stage of L. pinceana, and the different flower stages of L. yunnanensis. (I) bud stage; (II) initial-flowering stage; (III) full-flowering stage; (IV) end-flower stage.
Reports have revealed that some characterized compounds were usually rich in the early stage of flower development; for example, linalool dominated the samples from younger-stage inflorescences in Protea species [29], and 1,8-cineole and (Z)-3-hexenol were only identified from essential oil in the bud stage of Rosa canina [30]. These characterized compounds could make the young organs healthier [31]. However, these compounds did not appear in L. yunnanensis or L. pinceana, and this might be because the content of these volatile compounds in a single flower was too low.
3. Materials and Methods
3.1. Plant Materials
Nine fresh early flowering inflorescences of three L. yunnanensis plants (separate distance among plants more than 100 m, three inflorescences per plant) were collected from Nujiang Lisu Autonomous Prefecture, Yunnan Province (26°21′ N; 98°49′ E) during its flourishing florescence on 9 November, 2016, and were inserted into deionized water before being transported to the Research Institute of Resources Insects, Chinese Academy of Forestry (RIRICAF) in Kunming. Subsequently, the inflorescences were preserved at 25 ± 1 °C. The flowers were classified into four groups according to their botanical characteristics (Figure 1) [4]: (I) bud stage: buds complete closed; (II) initial-flowering stage: semi-open petal; (III) full-flowering stage: completely open petals, observable yellow pistils and stamens; and (IV) end-flowering stage: petals and calyxes withered, stamens turned brown. Three replicates (three flowers from three inflorescences per replicate) were randomly conducted from different plants, and the results are means of three tests, including nine flowers.
3.2. Method
Volatile compounds of a complete flower were monitored using solid-phase microextraction (SPME). A total of five L. yunnanensis individuals were randomly selected and sampled for scent collection between 8:00 and 11:00; the method of scent collection was described by Li et al. [7]. The methods of scent collection and HS-SPME-GC-MS were consistent with the methods of L. pinceana [4] and L. gratissima [5]. Sampling by HS-SPME was performed by 100 µm polydimethylsiloxane (PDMS) SPME fiber. After the equilibration time (15 min), the fiber was exposed to the headspace of the capped glass vial (20 mL) to absorb volatile compounds for 40 min. The empty capped vial was used as the blank control.
A gas chromatograph-mass spectrometer (TRACE GC Ultra/ITQ900, Thermo Fisher Scientific, Inc., Waltham, MA, USA) coupled with a DB-5MS capillary column (5% diphenyl cross-linked 95% dimethylpolysiloxane, 30 m × 0.25 mm i.d. × 0.25 µm film thickness. Agilent J & W Scientific, Folsom, CA, USA) were used for the GC-MS analysis. The oven temperature was programmed at 40 °C for 2 min, increasing at a rate of 6 °C/min to 130 °C and then increasing at 15 °C/min to 280 °C for 5 min. The injector was performed in splitless mode for 1 min. Helium was the carrier gas at a flow rate of 1.0 mL/min. The mass spectrometer was operated in electron impact mode at an ionization voltage of 70 eV and ion source temperature of 250 °C, and the scan range was 50–650 amu.
3.3. Data Analysis
The GC-MS data were processed using the TF Xcalibur 2.1.0 software. Component identification was carried out using NIST 2008 mass spectral database and confirmed by the comparison of their linear retention index (LRI) with published data [15]. Identification of individual components could be confirmed by the comparison of both mass spectrum and GC retention data with those of authentic standards [32]. A series of n-alkane standards (C6-C19) (Accu Standard, New Haven, CT, USA) were analyzed under the same conditions to obtain LRI values for the volatile compounds. Peak areas were normalized as percentage and used to determine the relative amounts of the volatiles.
3.4. Statistical Analysis
The data were analyzed by one-way analysis of variance (ANOVA). Principal component analysis (PCA) and Bray–Curtis similarity (BCS) were carried out using PC-ORD for Windows (Version 5.0; MjM Software, Gleneden Beach, OR, USA).
4. Conclusions
HS-SPME coupled with GC-MS was adapted to determine the composition and content of floral scent emitted from different flower development stages of L. yunnanensis. The present investigation showed that 40 VOCs were identified in whole life flower-stages, and sesquiterpenes were the most abundant floral scent compounds. The amount of floral scent emission had the tendency to first ascend and descend in succession, reaching its peak level at the initial-flowering stage. However, the highest diversity of floral volatiles was detected at the full-flowering stage. Compared with the other two species of Luculia (L. pinceana and L. gratissima), the composition and relative content of floral scent were also different at four stages of the tree species. Furthermore, L. yunnanensis has a promising prospect for development as an essential oil source and for future breeding programs and cultivation, owing to the main compounds of the floral scent.
Acknowledgments
We thank Hua Zheng for assistance in GC-MS analysis and Haixia Wu for improving the English of this manuscript. This work was financially supported by Special Fund for Forest Scientific Research in the Public Welfare (201404705), Technology Innovation Talent Project of Yunnan Province (2016HB007) and the Fundamental Research Funds for the Central Non-profit Research Institution of CAF (riricaf201003M).
Author Contributions
Yuying Li, Hong Ma and Zhenghong Li conceived and designed the experiments; Yuying Li performed the experiment and wrote the manuscript; Youming Wan and Zhenghai Sun analyzed the data; Taiqiang Li edited the manuscript; Xiongfang Liu, Xiuxian Liu, Rui He and Yan Ma contributed to materials collection. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Ma H., Wang L., Wan Y.M., Li H.Z., Li Z.H., Liu X.X., Liang N., Li W.J. A set of novel microsatellite markers developed for Luculia yunnanensis (Rubiaceae), an endangered plant endemic to yunnan, china. Int. J. Mol. Sci. 2012;13:534–539. doi: 10.3390/ijms13010534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen T., Charlotte M.T. Flora of China. Volume 19. Science Press; Beijing, China: 2011. Luculia; pp. 214–215. [Google Scholar]
- 3.Wan Y., Li Z., Ma H., Liu X. The effects of different treatment condition on seed germination characteristics of Luculia yunnanensis. J. Anhui Agric. Sci. 2010;4:61. [Google Scholar]
- 4.Li Y., Ma H., Wan Y., Li T., Liu X., Sun Z., Li Z. Volatile organic compounds emissions from Luculia pinceana flower and its changes at different stages of flower development. Molecules. 2016;21:531. doi: 10.3390/molecules21040531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin W., Lin S. Floral scent composition in Luculia gratissima (Wallich) Sweet analyzed by HS-SPME-GC-MS. J. Essent. Oil Bear. Plants. 2016;7:1801–1806. doi: 10.1080/0972060X.2016.1238783. [DOI] [Google Scholar]
- 6.Pistelli L., Noccioli C., D’Angiolillo F., Pistelli L. Composition of volatile in micropropagated and field grown aromatic plants from Tuscany Islands. Acta Biochim. Pol. 2013;60:43–50. [PubMed] [Google Scholar]
- 7.Sun H., Zhang T., Fan Q., Qi X., Zhang F., Fang W., Jiang J., Chen F., Chen S. Identification of floral scent in Chrysanthemum cultivars and wild relatives by Gas Chromatography-Mass Spectrometry. Molecules. 2015;20:5346–5359. doi: 10.3390/molecules20045346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flavornet and Human Odor Space. [(accessed on 22 May 2017)]; Available online: http://www.flavornet.org/flavornet.html.
- 9.Dotterl S., Vater M., Rupp T., Held A. Ozone differentially affects perception of plant volatiles in western honey bees. J. Chem. Ecol. 2016;42:486–489. doi: 10.1007/s10886-016-0717-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zito P., Dötterl S., Sajeva M. Floral volatiles in a sapromyiophilous plant and their importance in attracting house fly pollinators. J. Chem. Ecol. 2015;41:340–349. doi: 10.1007/s10886-015-0568-8. [DOI] [PubMed] [Google Scholar]
- 11.Andersson S., Nilsson L.A., Groth I., Bergstrom G. Floral scents in butterfly-pollinated plants: Possible convergence in chemical composition. Bot. J. Linn. Soc. 2002;140:129–153. doi: 10.1046/j.1095-8339.2002.00068.x. [DOI] [Google Scholar]
- 12.Cruz R.M., Martins C.F. Pollinators of Richardia grandiflora (Rubiaceae): An important ruderal species for bees. Neotropical Entomol. 2015;44:21–29. doi: 10.1007/s13744-014-0252-7. [DOI] [PubMed] [Google Scholar]
- 13.Sobotník J., Hanus R., Kalinová B., Piskorski R., Cvacka J., Bourguignon T., Roisin Y. (E,E)-α-farnesene, an alarm pheromone of the termite Prorhinotermes canalifrons. J. Chem. Ecol. 2008;34:478–486. doi: 10.1007/s10886-008-9450-2. [DOI] [PubMed] [Google Scholar]
- 14.Andersson S., Dobson H.E.M. Antennal responses to floral scents in the butterfly Heliconius melpomene. J. Chem. Ecol. 2003;29:2319–2330. doi: 10.1023/A:1026278531806. [DOI] [PubMed] [Google Scholar]
- 15.NIST Standard Reference Database. [(accessed on 22 May 2017)]; Available online: http://webbook.nist.gov/chemistry.
- 16.Kim H.K., Tak JHAhn Y.J. Acaricidal activity of Paeonia suffruticosa root bark-derived compounds against Dermatophagoides farinae and Dermatophagoides pteronyssinus (Acari: Pyroglyphidae) J. Agric. Food Chem. 2005;52:7857–7861. doi: 10.1021/jf048708a. [DOI] [PubMed] [Google Scholar]
- 17.Ren J.R., Yang L.N., Wang Y., Yao H.J. Chemical profile of floral scent at different flower developmental stages of rose de rescht (Rosa damascena Mill.) cultivated in Beijing. J. Essent. Oil Bear. Plants. 2016;19:433–443. doi: 10.1080/0972060X.2014.890081. [DOI] [Google Scholar]
- 18.Mo E.K., Chang K.S. Phenylethyl alcohol (PEA) application slows fungal growth and maintains aroma in strawberry. Postharvest Biol. Technol. 2007;45:234–239. doi: 10.1016/j.postharvbio.2007.02.005. [DOI] [Google Scholar]
- 19.Kotan R., Kordali S., Cakir A. Screening of antibacterial activities of twenty-one oxygenated monoterpenes. Zeitschrift Naturforschung C. 2014;62:507–513. doi: 10.1515/znc-2007-7-808. [DOI] [PubMed] [Google Scholar]
- 20.Caruso C.M., Parachnowitsch A.L. Do plants eavesdrop on floral scent signals? Trends Plant Sci. 2016;21:9–15. doi: 10.1016/j.tplants.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Ninkovic V., Markovic D., Dahlin I. Decoding neighbour volatiles in preparation for future competition and implications for tritrophic interactions. Perspect. Plant Ecol. Evol. Syst. 2016;23:11–17. doi: 10.1016/j.ppees.2016.09.005. [DOI] [Google Scholar]
- 22.Azam M., Song M., Fan F., Zhang B., Xu Y., Xu C., Chen K. Comparative analysis of flower volatiles from nine citrus at three blooming stages. Int. J. Mol. Sci. 2013;14:22346–22367. doi: 10.3390/ijms141122346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohd-Hairul A.R., Namasivayam P., Lian G.E.C., Abdullah J.O. Terpenoid, benzenoid, and phenylpropanoid compounds in the floral scent of Vanda Mimi Palmer. J. Plant Biol. 2010;53:358–366. doi: 10.1007/s12374-010-9123-x. [DOI] [Google Scholar]
- 24.Qin X.W., Hao C.Y., He S.Z., Wu G., Tan L.H., Xu F., Hu R.S. Volatile organic compound emissions from different stages of Cananga odorata flower development. Molecules. 2014;19:8965–8980. doi: 10.3390/molecules19078965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Kateb H., Mottram D.S. The relationship between growth stages and aroma composition of lemon basil Ocimum citriodorum. Vis. Food Chem. 2014;152:440–446. doi: 10.1016/j.foodchem.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Burdon R.C., Raguso R.A., Kessler A., Parachnowitsch A.L. Spatiotemporal floral scent variation of Penstemon digitalis. J. Chem. Ecol. 2015;41:641–650. doi: 10.1007/s10886-015-0599-1. [DOI] [PubMed] [Google Scholar]
- 27.Liu Q., Sun G., Wang S., Lin Q., Zhang J., Li X. Analysis of the variation in scent components of Hosta flowers by HS-SPME and GC-MS. Sci. Hortic. 2014;175:57–67. doi: 10.1016/j.scienta.2014.06.001. [DOI] [Google Scholar]
- 28.Jurgens A., Dotterl S., Liede-Schumann S., Meve U. Floral scent composition in early diverging taxa of Asclepiadoideae, and Secamonoideae (Apocynaceae) S. Afr. J. Bot. 2010;76:749–761. doi: 10.1016/j.sajb.2010.08.013. [DOI] [Google Scholar]
- 29.Steenhuisen S.L., Raguso R.A., Jurgens A., Johnson S.D. Variation in scent emission among floral parts and inflorescence developmental stages in beetle-pollinated Protea species (Proteaceae) S. Afr. J. Bot. 2010;76:779–787. doi: 10.1016/j.sajb.2010.08.008. [DOI] [Google Scholar]
- 30.Hosni K., Zahed N., Chrif R., Brahim N.B., Kallel M., Sebei H. Volatile oil constituents of Rosa canina L.: Differences related to developmental stages and floral organs. Plant Biosyst. 2011;145:627–634. doi: 10.1080/11263504.2011.586378. [DOI] [Google Scholar]
- 31.Flamini G., Cioni P.L. Odour gradients and patterns in volatile emission of different plant parts and developing fruits of grapefruit (Citrus paradisi L.) Food Chem. 2010;120:984–992. doi: 10.1016/j.foodchem.2009.11.037. [DOI] [Google Scholar]
- 32.Feulner M., Schuhwerk F., Dotterl S. Floral scent analysis in Hieracium subgenus Pilosella and its taxonomical implications. Flora. 2009;204:495–505. doi: 10.1016/j.flora.2008.06.003. [DOI] [Google Scholar]