Abstract
The development of a novel repellent plays an important role in the integrated control of Blattella germanica. A series of novel hydronopylformamides derivatives were synthesized from a naturally occurring compound (-)-β-pinene. The structures of these hydronopylformamides derivatives were characterized by Fourier transform infrared spectroscopy (FTIR), nuclear magnetic resonance spectroscopy (1H-NMR and 13C-NMR), and electron impact mass spectrometry (EI-MS). Repellency of these hydronopylformamides derivatives against Blattella germanica was evaluated by the using petri dish arena method. The results showed that four derivatives (compounds 8a, 8b, 8c and 8e) exhibited repellency against Blattella germanica at a concentration of 20 mg/mL. Compound 8a was the most active compound among these derivatives, where the repelling ratios of compound 8a against Blattella germanica were 66.10%, 50.46%, 48.26%, at concentrations of 20 mg/mL, 10 mg/mL, and 5 mg/mL, respectively. In addition, compound 8a showed better repellency than the traditional insect repellent N, N-diethyl-3-methylbenzamide (DEET), which indicated that compound 8a had a good application prospect in the prevention of Blattella germanica. This research hopes to promote the value-added utilization of (-)-β-pinene and the development of novel German cockroach repellents.
Keywords: β-pinene, synthesis, Blattella germanica, repellent
1. Introduction
Blattella germanica is a common household insect pest worldwide, which may disseminate many serious human diseases [1,2]. Therefore, the control of Blattella germanica is of great importance. Currently, the control of Blattella germanica populations depend on continued application of insecticides. Although the application of insecticides is efficient, the increasing resistance of Blattella germanica to insecticides seriously threatens the ongoing management of Blattella germanica [3]. Furthermore, the negative effects of insecticides in the indoor environment have also caused much concern. Hence, these issues have highlighted the need for the development of an integrated strategy for the control of Blattella germanica. Insect repellents, which would discourage Blattella germanica and let them fly or crawl away, were regarded as a good option for the integrated control of Blattella germanica.
Naturally occurring compounds are ideal raw materials for the development of insect repellents as they are safer and pose less risk to the environment [4]. Many reports have described the repellency of naturally occurring compounds against cockroaches [5,6,7,8]; however, the repellency and duration times of naturally occurring compounds were usually unsatisfactory. To improve the repellency and duration times, the chemical modification of naturally occurring compounds was a feasible method. The preparation of novel repellents by modifying naturally occurring compounds has attracted much attention in recent years.
(-)-β-pinene is a main component of turpentine, which is an essential oil obtained from live trees, mainly pines. Previous research has found that (-)-β-pinene exhibited a mild repellency against Blattella germanica, and that some synthetic derivatives of β-pinene exerted better repellency [9]. These findings indicated that novel repellent candidates might be screened out from the derivatives of (-)-β-pinene. In order to screen novel repellent candidates against Blattella germanica, a series of novel (-)-β-pinene derivatives were designed and synthesized, and the repellency of these derivatives against Blattella germanica was evaluated in this research.
2. Results and Discussion
2.1. Chemistry
Since N,N-diethyl-m-toluamide (DEET, Figure 1) was discovered and used as insect repellent, the repellency of amides was the subject of constant attention. DEET was synthesized in 1953, and by far it was and still is, the most widely used insect repellent [10]. However, some toxic effects of DEET have since been disclosed, such as underlying neurological disorders, neurotoxicity, allergic reactions, etc. [11,12,13,14]. Hence, the development of safer amide repellents is important and still in high demand.
Figure 1.
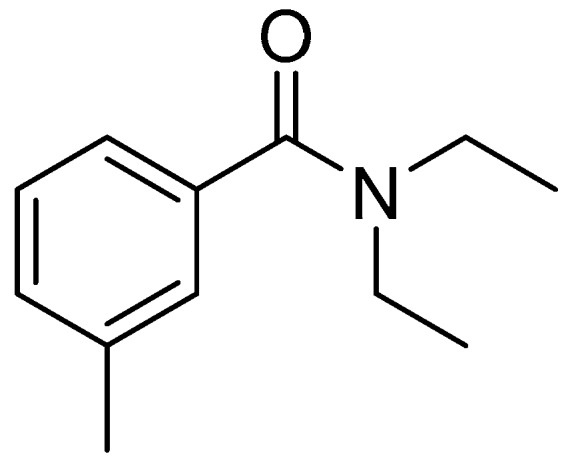
Molecular structure of N, N-diethyl-m-toluamide (DEET).
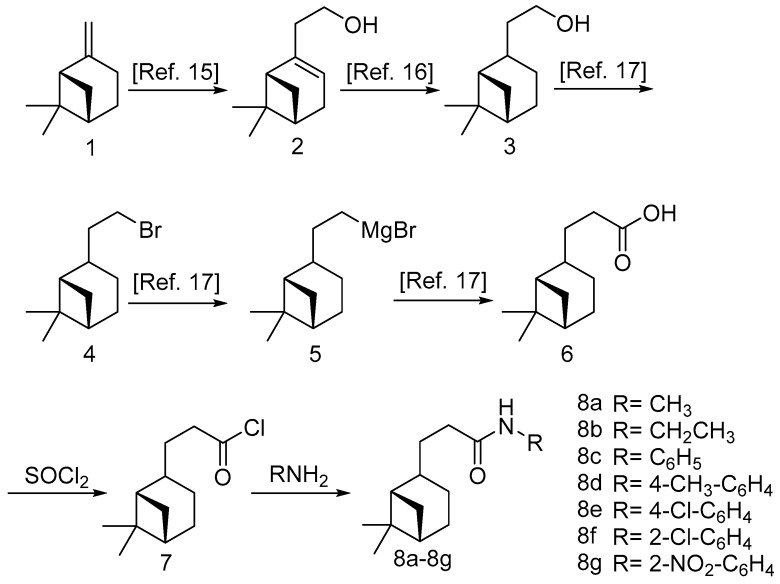
In this work, a series of amides derivatives of (-)-β-pinene were synthesized (Figure 2). (-)-β-pinene was first transferred into nopol via a Prins reaction [15], and then hydronopol was obtained by the hydrogenation of nopol [16]. After a Grignard reaction, hydronopol was converted to hydronopyl formic acid [17]. Hydronopylformamides were prepared by the amidation reactions of a series of amines and the acyl chloride of hydronopyl formic acid. Generally, amides are typically synthesized from the union of carboxylic acids and amines; however, it is usually necessary to activate the carboxylic acids in advance as the carboxylic acids and amines do not spontaneously combine at mild conditions. There are many reagents that can be used to activate the carboxylic acids, such as carbodiimides, salts of 1H-benzotriazole, acid halides, etc. [18]; nevertheless, the most common acid halide, thionyl chloride, was selected as the activator in this work due to its high-efficiency, low-cost and post-treated convenience.
Figure 2.
Synthesis routine of hydronopylformamides 8a–8g.
The structures of these hydronopylformamides were characterized by Fourier transform infrared spectroscopy (FTIR), nuclear magnetic resonance spectroscopy (1H-NMR and 13C-NMR), and electron impact mass spectrometry (EI-MS) Taking compound 8e as an example, in the IR spectrum, the absorption band at 3276 cm−1 and 3240 cm−1 was assigned to the -NH group; the absorption bands at 2983–2858 cm−1 were attributed to -CH2-, and -CH-; the absorption bands at 1655 cm−1 and 1595–1491 cm−1 represented the existence of -C=O and aromatic ring; the absorption bands at 1540 was assigned to C-N; and the absorption band at 834 cm−1 was attributed to 1,4-disubstituted benzene. In the 1H-NMR spectrum of compound 8e, the signal appearing as a singlet at about 7.68 ppm was assigned to the proton of the acyl amino group. The signals attributed to four aromatic protons of benzene rings were observed at 7.47 ppm and 7.25 ppm. Other signals were assigned to the protons in the pinane skeleton. In the 13C-NMR spectrum of compound 8e, the characteristic C=O peaks related to the acyl amino group were observed at 171.92 ppm; the signals of aromatic carbons appeared at 136.55–121.07 ppm; and the characteristic C-Cl peaks related to the benzene ring were observed at 129.02 ppm. The signals of aliphatic groups appeared between 46.12 ppm and 22.13 ppm. In the EI-MS spectrum of compound 8e, the signals at 305 [M+] and 307 [M+ + 2] indicated that the molecular weight of 8e was 305. These results showed that the characterization data were in full agreement with the proposed structures.
2.2. Biological Activity
The repellency of hydronopylformamides at the concentration of 20 mg/mL against Blattella germanica are listed in Table 1. From this, it can be seen that compounds 8a, 8b, 8c, and 8e exhibited repellency against Blattella germanica, and compound 8a with a repelling rate of 67.06% against Blattella germanica was the most active compound. Compounds 8d, 8f, and 8g did not show any repellency against Blattella germanica; furthermore, compound 8g exhibited slight attractive activity against Blattella germanica.
Table 1.
Repellency of hydronopylformamides against Blattella germanica at a concentration of 20 mg/mL.
| Compounds | R | Melting Point | Repelling Rate ± Standard Error (%) |
|---|---|---|---|
| 8a | CH3 | 38.0–38.5 | 67.06 ± 3.07 |
| 8b | CH2CH3 | 41.5–42.2 | 38.89 ± 3.61 |
| 8c | C6H5 | 84.7–85.7 | 41.19 ± 6.05 |
| 8d | 4-CH3-C6H4 | 125.1–126.3 | −1.83 ± 5.18 |
| 8e | 4-Cl-C6H4 | 123.2–124.2 | 8.45 ± 7.81 |
| 8f | 2-Cl-C6H4 | 108.8–109.4 | −5.48 ± 9.97 |
| 8g | 2-NO2-C6H4 | 54.3–55.3 | −23.59 ± 6.67 |
| Ethanol | - | - | 0.39 ± 1.18 |
| Acetone | - | - | 0.71 ± 1.42 |
| DEET | - | −33.0 | 54.77 ± 6.61 |
The influence of substituent groups and melting point of these hydronopylformamides on their biological activity was also discussed. As shown in Table 1, compound 8a (with a methyl group in the N atom) exhibited the best repellency; however, other derivatives with bigger substituent groups in the N atom presented weaker repellency, and these results revealed that for the hydronopylformamides, a smaller substituent group in the N atom was more beneficial for repellency. When the melting points of hydronopylformamides were lower than 100 °C, the hydronopylformamides exhibited biological activity either as repellency (compounds 8a, 8b and 8c) or attractive activity (compound 8g). However, when the melting points of hydronopylformamides were higher than 100 °C, the hydronopylformamides showed weak biological activity.
Furthermore, the repellency of compound 8a and DEET against Blattella germanica at three different concentrations were tested, and the results are summarized in Table 2. Table 2 shows that the repellency of compound 8a was better than that of DEET at all tested concentrations, including 20 mg/mL, 10 mg/mL, and 5 mg/mL. It is generally known that DEET is a broad-spectrum repellent that serves an effective defensive role against many insects, such as cockroaches, mosquitos, flies, etc. [10]. In this research, compound 8a exhibited better repellency than DEET against the cockroach Blattella germanica, which indicated that compound 8a was a good application prospect in the prevention of Blattella germanica. Further research is required to evaluate the repellency of compound 8a against more cockroach species.
Table 2.
Repellency of compound 8a and DEET against Blattella germanica at three different concentrations.
| Compounds | Repelling Rate ± Standard Error (%) | ||
|---|---|---|---|
| 20 mg/mL | 10 mg/mL | 5 mg/mL | |
| 8a | 67.06 ± 3.07 | 50.46 ± 5.07 | 48.26 ± 5.18 |
| DEET | 54.77 ± 6.61 | 34.02 ± 4.41 | 29.56 ± 6.48 |
3. Materials and Methods
3.1. General
(-)-β-pinene was purchased from the spice company Jiangxi Jishui Hongda Natural Perfume Co., Ltd., Ji’an, China, and other reagents were of reagent grade. Melting points were determined using X-4B melting point apparatus (Shanghai Precision & Scientific Instrument Co., Ltd., Shanghai, China) and were uncorrected. IR spectra were recorded on a Nicolet IR6700 FT-IR spectrometer (Nicolet, Waltham, MA, USA). 1H-NMR and 13C-NMR spectra were recorded on a Bruker AVANCE 400 NMR spectrometer (Bruker, Karlsruhe, Germany) at 400 MHz and 100 MHz, respectively, using CDCl3 as the solvent and TMS as the internal standard. EI-MS were recorded on a Clarus 600C GC/MS spectrometer (PerkinElmer, Waltham, MA, USA). The purity of compounds was detected by Fuli GC9790 gas chromatography (Zhejiang Fuli analysis instrument Co., Ltd., Wenling, China). Blattella germanica were obtained from Jiangxi Hilltop Chemical Industrial Co., Ltd., Zhangshu, China.
3.2. Synthesis
3.2.1. Synthesis of Nopol (2), Hydronopol (3), Hydronopyl Bromide (4), Hydronopyl Formic Acid (6)
Based on previous studies described in References [15,16,17], nopol (2), hydronopol (3), hydronopyl bromide (4), and hydronopyl formic acid (6) were prepared.
3.2.2. Synthesis of Hydronopylformamides (8a–8g) (Taking Compound 8e as An Example)
Thionyl chloride (1.785 g, 15 mmol) was slowly added to a solution of hydronopyl formic acid (1.962 g, 10 mmol) in anhydrous methylene chloride (10 mL), and stirred at 50 °C for 3 h. Next, the mixture was concentrated under reduced pressure to remove the solvent and excess thionyl chloride. The residue was dissolved in dried methylene chloride (10 mL), and added dropwise into a solution of 4-chloroaniline (1.914 g, 15 mmol) and triethylamine (1.518 g, 15 mmol) in anhydrous methylene chloride (10 mL), and the mixture was stirred at 50 °C until the reaction was complete (as per the thin-layer chromatography and gas chromatography analysis). The mixture was cooled, diluted with 80 mL methylene chloride, and treated with 20 mL 5% aqueous solution of HCl, washed with a saturated aqueous solution of sodium chloride and dried over anhydrous sodium sulfate, after which the solvent was removed under reduced pressure. Further purification was accomplished by silica gel chromatography to give pure products 8e.
The structures of these hydronopylformamides derivatives were characterized by Fourier transform infrared spectroscopy (FTIR), nuclear magnetic resonance spectroscopy (1H-NMR and 13C-NMR), and electron impact mass spectrometry (EI-MS), and the spectrums were included in the Supplementary Materials available online.
3-((1S,5S)-6,6-Dimethylbicyclo[3.1.1]heptan-2-yl)-N-methylpropanamide (compound 8a). White solid, m.p. 38.0–38.5 °C, yield 70.4%, GC purity 97.5%; IR ν (cm−1): 3294, 2982, 2936, 2986, 2861, 1645, 1561, 1468, 1383, 1366; 1H-NMR (400 MHz, CDCl3) δ (ppm): 5.65 (1H, br), 2.78 (3H, s), 2.29 (1H, m), 2.17–2.10 (2H, m), 1.91–1.82 (6H, m), 1.69 (2H, m), 1.43 (1H, m), 1.15 (3H, s), 0.98 (3H, s), 0.83 (1H, d, J = 8 Hz); 13C-NMR (100 MHz, CDCl3) δ (ppm): 173.969, 46.100, 41.356, 41.127, 38.629, 35.238, 33.630, 33.371, 28.110, 26.330, 26.243, 23.226, 22.139; MS m/z (%RA): 53(14), 55(32), 58(57), 67(30), 69(24), 73(100), 74(21), 77(13), 79(22), 81(24), 82(20), 83(13), 86(71), 87(10), 91(12), 93(15), 95(13), 107(8), 109(7), 112(6), 121(11), 123(6), 127(15), 128(16), 135(6), 136(17), 137(28), 138(4), 140(13), 153(6), 154(4), 166(15), 178(7), 180(2), 194(4), 209(4), 210(0.6).
3-((1S,5S)-6,6-Dimethylbicyclo[3.1.1]heptan-2-yl)-N-ethylpropanamide (compound 8b). Light yellow solid, m.p. 41.5–42.2 °C, yield 61.0%, GC purity 99.1%; IR ν (cm−1): 3285, 2976, 2934, 2901, 2864, 1643, 1553, 1468, 1383, 1366; 1H-NMR (400 MHz, CDCl3) δ (ppm): 5.72 (1H, s), 2.28 (1H, m), 2.16~2.05 (2H, m), 1.92–1.79 (6H, m), 1.69 (2H, m), 1.43 (1H, m), 1.14 (3H, s), 1.10 (3H, t), 0.97 (3H, s), 0.82 (1H, d, J = 8 Hz); 13C-NMR (100 MHz, CDCl3) δ (ppm): 173.139, 46.089, 41.346, 41.144, 38.594, 35.358, 34.198, 33.612, 33.336, 28.075, 26.302, 23.183, 22.115, 14.815; MS m/z (%RA): 53(10), 55(28), 67(26), 69(19), 72(60), 77(11), 79(17), 81(19), 82(13), 83(11), 87(100), 88(15), 91(10), 93(11), 95(10), 100(56), 101(7), 107(6), 109(6), 121(7), 123(5), 126(4), 136(7), 137(19), 141(12), 142(9), 154(12), 167(3), 168(3), 178(4), 180(12), 208(3), 223(5), 224(0.8).
3-((1S,5S)-6,6-Dimethylbicyclo[3.1.1]heptan-2-yl)-N-phenylpropanamide (compound 8c). White solid, m.p. 84.7–85.7 °C, yield 67.9%, GC purity 97.7%; IR ν (cm−1): 3285, 3253, 2986, 2972, 2915, 2878, 2857, 1658, 1598, 1499, 1550, 1466, 1358, 754, 691; 1H-NMR (400 MHz, CDCl3) δ (ppm): 7.56 (1H, br), 7.54 (2H, d), 7.29 (2H, t), 7.09 (1H, t), 2.35–2.30 (3H, m), 1.97–1.79 (8H, m), 1.49–1.45 (1H, m), 1.17 (3H, s), 1.00 (3H, s), 0.86 (1H, d, J = 12 Hz); 13C-NMR (100 MHz, CDCl3) δ (ppm): 171.771, 138.011, 128.870, 124.453, 119.785, 46.148, 41.363, 41.057, 38.642, 36.330, 33.633, 33.193, 28.101, 26.321, 23.224, 22.134; MS m/z (%RA): 53(6), 55(15), 67(12), 69(11), 77(15), 79(10), 81(8), 92(34), 93(100), 94(12), 95(6), 107(4), 109(2), 120(5), 134(20), 135(66), 136(8), 137(5), 148(24), 189(2), 202(3), 228(2), 271(6), 272(1.1).
3-((1S,5S)-6,6-Dimethylbicyclo[3.1.1]heptan-2-yl)-N-(p-tolyl)propanamide (compound 8d). White solid, m.p. 125.1–126.3 °C, yield 58.9%, GC purity 98.5%; IR ν (cm−1): 3278, 3242, 2985, 2938, 2902, 2858, 1650, 1601, 1511, 1540, 1459, 1381, 1366, 816; 1H-NMR (400 MHz, CDCl3) δ (ppm): 7.61 (1H, s), 7.40 (2H, d, J = 8 Hz), 7.09 (2H, d, J = 8 Hz), 2.36–2.26 (3H, m), 2.30 (3H, s), 1.96–1.76 (8H, m), 1.48~1.42 (1H, m), 1.18 (3H, s), 1.00 (3H, s), 0.85 (1H, d, J = 8 Hz); 13C-NMR (100 MHz, CDCl3) δ (ppm): 171.726, 135.443, 133.580, 129.309, 119.900, 46.122, 41.324, 41.032, 38.612, 36.227, 33.625, 33.220, 28.875, 26.309, 23.204, 22.091; MS m/z (%RA): 53(5), 55(12), 67(9), 69(9), 77(10), 79(13), 81(6), 91(12), 93(8), 95(4), 105(16), 106(52), 107(100), 108(13), 120(3), 134(4), 135(3), 137(2), 148(11), 149(62), 150(5), 162(19), 163(2), 216(3), 242(2), 285(7), 286(1.2).
N-(4-Chlorophenyl)-3-((1S,5S)-6,6-dimethylbicyclo[3.1.1]heptan-2-yl)propanamide (compound 8e). White solid, m.p. 123.2–124.2 °C, yield 60.2%, GC purity 99.9%; IR ν (cm−1): 3276, 3240, 2983, 2939, 2905, 2858, 1655, 1595, 1491, 1540, 1467, 1398, 1360, 834, 1092; 1H-NMR (400 MHz, CDCl3) δ (ppm): 7.68 (1H, s), 7.47 (2H, d, J = 8 Hz), 7.25 (2H, d, J = 8 Hz), 2.37–2.26 (3H, m), 1.95–1.77 (8H, m), 1.47–1.41 (1H, m), 1.17 (3H, s), 0.99 (3H, s), 0.85 (1H, d, J = 8 Hz); 13C-NMR (100 MHz, CDCl3) δ (ppm): 171.919, 136.549, 129.018, 128.874, 121.073, 46.124, 41.319, 41.051, 38.638, 33.636, 33.132, 28.092, 26.299, 23.229, 22.132; MS m/z (%RA): 53(7), 55(22), 67(17), 69(17), 77(8), 79(13), 81(13), 82(7), 91(15), 93(12), 95(9), 105(3), 107(6), 121(5), 123(3), 126(25), 127(100), 128(15), 129(29), 130(3), 137(7), 168(7), 169(49), 170(6), 171(16), 182(16), 184(6), 223(2), 225(0.6), 262(2), 264(1), 305(4.4), 306(0.7), 307(1.6), 308(0.3).
N-(2-Chlorophenyl)-3-((1S,5S)-6,6-dimethylbicyclo[3.1.1]heptan-2-yl)propanamide (compound 8f). White solid, m.p. 108.8–109.4 °C, yield 64.2%, GC purity 99.2%; IR ν (cm−1): 3230, 2977, 2934, 2909, 2874, 1657, 1585, 1533, 1482, 1532, 1441, 1383, 1365, 1063, 756; 1H-NMR (400 MHz, CDCl3) δ (ppm): 8.38 (1H, d, J = 8 Hz), 7.64 (1H, s), 7.35 (1H, m), 7.26 (1H, m), 7.02 (1H, m), 2.44–2.31 (3H, m), 2.06–1.81 (8H, m), 1.53–1.47 (1H, m), 1.19 (3H, s), 1.03 (3H, s), 0.88 (1H, d, J = 8 Hz); 13C-NMR (100 MHz, CDCl3) δ (ppm): 171.484, 134.617, 128.868, 127.673, 124.578, 121.532, 46.087, 41.385, 40.911, 38.676, 36.532, 33.600, 33.060, 28.116, 26.319, 23.235, 22.156; MS m/z (%RA): 53(9), 55(27), 67(20), 69(21), 77(11), 79(17), 81(16), 82(9), 83(6), 91(12), 93(18), 95(11), 99(5), 105(3), 107(9), 119(3), 126(8), 127(100), 128(10), 129(35), 130(3), 134(68), 135(9), 136(8), 137(10), 148(3), 154(4), 169(46), 171(15), 182(22), 184(7), 224(3), 236(3), 250(1), 262(2), 264(2), 270(2), 305(5.1), 306(1), 307(1.7), 308(0.3).
3-((1S,5S)-6,6-Dimethylbicyclo[3.1.1]heptan-2-yl)-N-(2-nitrophenyl)propanamide (compound 8g). Yellow solid, m.p. 54.3–55.3 °C, yield 57.0%, GC purity 98.8%; IR ν (cm−1): 3242, 2977, 2939, 2910, 2856, 1663, 1608, 1592, 1491, 1538, 1518, 1466, 1365, 1346, 779; 1H-NMR (400 MHz, CDCl3) δ (ppm): 10.37 (1H, s), 8.78 (1H, d, J = 8 Hz), 8.19 (1H, d, J = 8 Hz), 7.62 (1H, m), 7.15 (1H, m), 2.47–2.32 (3H, m), 2.00–1.84 (8H, m), 1.49 (1H, m), 1.17 (3H, s), 1.02 (3H, s), 0.87 (1H, d, J = 8 Hz); 13C-NMR (100 MHz, CDCl3) δ (ppm): 172.373, 136.038, 135.927, 134.970, 125.642, 122.937, 122.021, 45.967, 41.292, 40.809, 38.630, 37.215, 33.539, 32.831, 28.051, 26.051, 23.197, 22.080; MS m/z (%RA): 53(25), 54(14), 55(100), 65(23), 67(76), 68(11), 69(72), 77(31), 78(12), 79(67), 80(20), 81(65), 82(48), 83(13), 90(15), 91(46), 92(31), 93(69), 94(11), 95(43), 107(5), 119(35), 120(7), 121(34), 122(38), 123(33), 124(8), 131(15), 132(20), 133(43), 134(16), 135(29), 136(10), 137(8), 138(73), 139(15), 145(11), 146(9), 147(15), 148(19), 149(10), 150(12), 151(18), 159(5), 161(5), 163(7), 172(9), 173(10), 177(6), 186(11), 193(13), 199(5), 211(3), 225(5), 239(5), 281(19), 282(3), 298(3), 316(1.4), 317(0.2).
3.3. Biological Assay
Repellency of novel hydronopylformamides against Blattella germanica was evaluated by the methods reported in the literatures [19]. Seven hydronopylformamides and DEET were dissolved in ethanol or acetone to prepare solutions with an initial concentration of 20 mg/mL. A piece of round filter paper (15.0 cm) was cut in half, one half was treated with 1.5 mL of the tested compound solution, and the other half was treated with 1.5 mL of ethanol or acetone. The two halves of the filter paper were dried for 5 min before being placed into a 15 cm petri dish arena. The walls of the petri dish were treated in advance with a mixture of Vaseline and paroline to prevent the insect from escaping. One cockroach at a time was introduced and left to acclimatize for 5 min. After 5 minutes, the number of seconds the cockroach spent on the treated or untreated side out of a total of 300 s (5 min) was timed with two stopwatches. The filter paper halves and cockroaches were only used once. Each test was replicated 10 times. All tests were run under overhead florescent lighting at an ambient temperature (20–25 °C) and humidity (50–70% RH). Repellency was calculated as per Equation (1).
| Repelling ratio = (T1 − T2)/300 × 100% | (1) |
where T1 was the number of seconds spent on the untreated side; and T2 was the number of seconds spent on the treated side.
4. Conclusions
The repellency of seven novel hydronopylformamides derivatives of (-)-β-pinene (compound 8a–8g) was evaluated against Blattella germanica. The results showed that compounds 8a, 8b, 8c and 8e exhibited repellency against Blattella germanica, and compound 8a was the most active. Furthermore, the repellency of compound 8a was better than that of DEET at all tested concentrations, including 20 mg/mL, 10 mg/mL, and 5 mg/mL. This research hopes to contribute to the value-added utilization of β-pinene, and the development of novel German cockroach repellents.
Acknowledgments
This work is supported by the National Key Research and Development Program of China during the 13th Five-Year Plan Period (2016YFD0600801), the National Natural Science Foundation of China (31660178), and the Major Disciplines Academic and Technical Leader Training Program of Jiangxi Province (20133BCB22004). The authors are grateful to the Collaborative Innovation Center of Jiangxi Typical Trees Cultivation and Utilization, and the Jiangxi provincial key laboratory for bamboo germplasm resources, laboratory utilization, and support with experimental instruments. The authors thank Pimiao Jia and Meilin Deng, staff at the Jiangxi Hilltop Chemical Industrial Co. Ltd., for their kind help on repellency evaluation. The authors also appreciate the assistance of Weiqing Zheng (staff member at the Nanchang Center for Disease Control and Prevention).
Supplementary Materials
The following are available online: FTIR, NMR and GC-MS spectrums of 8a–8g.
Author Contributions
All authors conceived and designed the experiments; Z.W. contributed reagents/materials/analysis tools, conceived of the project and directed the experiments; S.L., Y.L. and Z.X. performed the experiments; all authors analyzed the data; and S.L. wrote the paper. All authors gave their approval to the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 8a–8g are available from the authors.
References
- 1.Nasirian H. Contamination of cockroaches (Insecta: Blattaria) to medically fungi: A systematic review and meta-analysis. J. Med. Mycol. 2017 doi: 10.1016/j.mycmed.2017.04.012. in press. [DOI] [PubMed] [Google Scholar]
- 2.Akinjogunla O.J., Odeyemi A.T., Udoinyang E.P. Cockroaches (Periplaneta americana and Blattella germanica): Reservoirs of multi drug resistant (MDR) bacteria in Uyo, Akwa Ibom State. Sci. J. Biol. Sci. 2012;1:19–30. [Google Scholar]
- 3.Naqqash M.N., Gökçe A., Bakhsh A., Salim M. Insecticide resistance and its molecular basis in urban insect pests. Parasitol. Res. 2016;115:1363–1373. doi: 10.1007/s00436-015-4898-9. [DOI] [PubMed] [Google Scholar]
- 4.Mann R.S., Kaufman P.E. Natural product pesticides: Their development, delivery and use against insect vectors. Mini-Rev. Org. Chem. 2012;9:185–202. doi: 10.2174/157019312800604733. [DOI] [Google Scholar]
- 5.Omara S.M., Al-Ghamdi K.M., Mahmoud M.A., Sharawi S.E. Repellency and fumigant toxicity of clove and sesame oils against American cockroach (Periplaneta americana (L.) Afr. J. Biotechnol. 2013;12:963–970. [Google Scholar]
- 6.Chao L.X., Qiyong L., Han C., Zhi L.Q., Yao J.S., Long L.Z. Evaluation of contact toxicity and repellency of the essential oil of pogostemon cablin leaves and its constituents against Blattella germanica (Blattodae: Blattelidae) J. Med. Entomol. 2015;52:86–92. doi: 10.1093/jme/tju003. [DOI] [PubMed] [Google Scholar]
- 7.Zibaee I., Khorram P.B., Hamoni M. Evaluation of repellent activity of two essential oils and their mixed formulation against cockroaches (Dictyoptera: Blattidae, Blattellidae) in Iran. J. Entomol. Zool. Stud. 2016;4:106–113. [Google Scholar]
- 8.Adzharia H.N., Sook S.Y. Extraction and chemical compositions of Ginger (Zingiber Officinale Roscoe) essential oils as a cockroach repellent. Aust. J. Basic Appl. Sci. 2017;11:1–8. [Google Scholar]
- 9.Han Z.J., Wang Z.D., Jiang Z.K., Qian W.H., Chen J.Z., Zheng W.Q. Repellent activity evaluation of terpenoids against German cockroaches (in Chinese) Chin. J. Hyg. Insect. Equip. 2012;18:290–295. [Google Scholar]
- 10.Katz T.M., Miller J.H., Hebert A.A. Insect repellents: Historical perspectives and new developments. J. Am. Acad. Dermatol. 2008;58:865–871. doi: 10.1016/j.jaad.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Osimitz T.G., Murphy J.V., Fell L.A., Page B. Adverse events associated with the use of insect repellents containing N,N-diethyl-m-toluamide (DEET) Regul. Toxicol. Pharm. 2010;56:93–99. doi: 10.1016/j.yrtph.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Corbel V., Stankiewicz M., Pennetier C., Fournier D., Stojan J., Girard E., Dimitrov M., Molgó J., Hougard J.M., Lapied B. Evidence for inhibition of cholinesterases in insect and mammalian nervous systems by the insect repellent DEET. BMC Biol. 2009;7:47. doi: 10.1186/1741-7007-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swale D.R., Sun B., Tong F., Bloomquist J.R. Neurotoxicity and mode of action of N,N-diethyl-meta-toluamide (DEET) PLoS ONE. 2014;9:e103713. doi: 10.1371/journal.pone.0103713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McHenry M., Lacuesta G. Severe allergic reaction to diethyltoluamide (DEET) containing insect repellent. Allergy Asthma Clin. Immunol. 2014;10:a30. doi: 10.1186/1710-1492-10-S2-A30. [DOI] [Google Scholar]
- 15.Costa V.V., Bayahia H., Kozhevnikova E.F., Gusevskaya E.V., Kozhevnikov I.V. Highly active and recyclable metal oxide catalysts for the Prins condensation of biorenewable feedstocks. Chem. Cat Chem. 2014;6:2134–2139. doi: 10.1002/cctc.201402160. [DOI] [Google Scholar]
- 16.Zhao L.H., Xiao Z.Q., Chen J.Z., Wang Z.D., Fan G.R., Chen S.X. Synthesis and structural analysis of hydronopol and its halides (in Chinese) Chem. Ind. For. Prod. 2012;32:39–42. [Google Scholar]
- 17.Liu Y., Xiao Z.Q., Lu P.Y., Chen J.Z., Wang Z.D., Fan G.R. Synthesis and structural analysis of hydronopyl formic acid and its esters (in Chinese) Chem. Ind. For. Prod. 2013;33:57–61. [Google Scholar]
- 18.Valeur E., Bradley M. Amide bond formation: Beyond the myth of coupling reagents. Chem. Soc. Rev. 2009;38:606–631. doi: 10.1039/B701677H. [DOI] [PubMed] [Google Scholar]
- 19.Liu Z.L., Yu M., Li X.M., Wan T., Chu S.S. Repellent activity of eight essential oils of Chinese medicinal herbs to Blattella germanica L. Rec. Nat. Prod. 2011;5:176–183. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.