Abstract
This study evaluated the effect of 4 weeks of low-load resistance exercise with blood flow restriction (BFRE) on increasing strength in comparison with high-load resistance exercise (HLE), and assessed changes in blood, vascular and neural function. Healthy adults performed leg extension BFRE or HLE 3 days/week at 30% and 80% of strength, respectively. During BFRE, a cuff on the upper leg was inflated to 30% above systolic blood pressure. Strength, pulse-wave velocity (PWV), ankle-brachial index (ABI), prothrombin time (PT) and nerve conduction (NC) were measured before and after training. Markers of coagulation (fibrinogen and D-dimer), fibrinolysis [tissue plasminogen activator (tPA)] and inflammation [high sensitivity C-reactive protein (hsCRP)] were measured in response to the first and last exercise bouts. Strength increased 8% with BFRE and 13% with HLE (P < 0.01). No changes in PWV, ABI, PT or NC were observed following training for either group (P > 0.05). tPA antigen increased 30–40% immediately following acute bouts of BFRE and HLE (P = 0.01). No changes were observed in fibrinogen, D-dimer or hsCRP (P > 0.05). These findings indicate that both protocols increase the strength without altering nerve or vascular function, and that a single bout of both protocols increases fibrinolytic activity without altering selected markers of coagulation or inflammation in healthy individuals.
Keywords: vascular occlusion, KAATSU, muscle, exercise
One of the most common and effective methods for increasing both muscle strength and mass is via high-load resistance exercise (HLE) training (loads > 80% of maximal strength) (Campos et al., 2002; Kraemer et al., 2002). However, not everyone can perform HLE. For example, high mechanical loading of a joint or muscle is frequently contraindicated in injured and post-surgical individuals with compromised musculotendinous integrity. Over the past decade, mounting evidence suggests that performing low-load exercise coupled with modest blood flow restriction to the exercising muscles serves as a potent stimulus for increasing muscle strength and mass (Shinohara et al., 1998; Takarada et al., 2000, 2002, 2004; Nicholas et al., 2001; Abe et al., 2006; Madarame et al., 2008) [for a review, see (Clark & Manini, 2009)]. While these studies support its general efficacy in increasing muscle performance in younger and older healthy humans, there have been few empirical studies evaluating aspects related to the relative safety of blood flow-restricted resistance exercise (BFRE).
In recent years, BFRE has become popular in Japan (referred to as KAATSU Training), and Nakajima et al. (2006) recently surveyed 105 facilities that use BFRE training in a variety of different populations to examine the incidence and occurrence observed of the side effects of the training. In total, over 30 000 BFRE training sessions were reported with the most frequent occurring side effects being subcutaneous bruising at the location of the cuff (13.1%), numbness (1.3%) and lightheadedness (0.3%). Additionally, a few cases of venous thrombosis (0.06%) were reported, although this incidence rate appears to actually be lower than that reported for the general Asian population (~0.2–0.26%) (Klatsky et al., 2000), and an acute bout of BFRE has been reported not altering prothrombin time (PT) or markers of coagulation, but rather to stimulate the fibrinolytic system (Nakajima et al., 2007).
In general, based on the aforementioned side effects as well as physiological reasoning, some of the primary concerns regarding BFRE are those associated with training-induced alterations in blood and vascular function as well as potential changes in the peripheral nervous system. For example, the reported numbness with BFRE likely results due to pressure being applied to the peripheral nerve resulting in ischemia and causing nerve conduction blockage (Lundborg, 1988). However, it is possible that the numbness and acute nerve conduction alterations do not result in any long-term mal-adaptations as the side effects are typically rapidly reversible following the removal of the stimuli (Lundborg, 1988). Additionally, resistance exercise training alone (without blood flow restriction) has been reported to increase peripheral arterial stiffness (Collier et al., 2008), although contradictory findings of no exercise-induced change do exist (Cortez-Cooper et al., 2005; Casey et al., 2007; Yoshizawa et al., 2009). Mechanical compression of vessels has been shown to induce structural damage in animals (Risberg et al., 1988; Margovsky et al., 1997). Ergo, there is theoretical concern that there could be an interactive effect of resistance training coupled with repetitive mechanical compression of the vasculature with regard to peripheral vascular function.
Therefore, the purpose of this study was to investigate the effect of 4 weeks of low-load BFRE knee extension training and HLE knee extension training on blood, vascular and neural function. We chose to compare the changes associated with low-load BFRE to a standard HLE regimen to increase the practical utility of our findings. Specifically, we assessed the training-induced changes in pulse wave velocity (PWV; an index of arterial stiffness), ankle-brachial index (ABI), nerve conduction velocity (NCV) and PT (global measure of the integrity of the extrinsic and final common pathways of the procoagulant cascade). Additionally, we assessed the acute exercise-induced effects on fibrinolytic [tissue plasminogen activator (tPA) antigen] and coagulation responses (fibrinogen and D-dimer), as well as acute inflammation [high-sensitivity C-reactive protein (hsCRP)]. We also measured isometric knee extension muscle strength to compare the relative effectiveness of the two exercise protocols in enhancing muscular strength. Based on the current literature, indicating BFRE and HLE training induces adaptations in muscle performance in the absence of significantly large numbers of adverse events, we hypothesized that the exercise training regimens would increase muscle strength without significantly altering any of the relative safety outcome measures.
Methods
General description of the study design
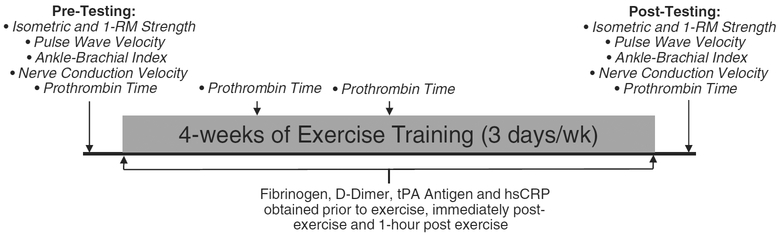
A total of 16 young, healthy adults completed this study. All subjects underwent 4 weeks of bilateral knee extension resistance exercise training 3 days/week. Nine repetition maximum BFRE training at an intensity of 30% repetition maximum (RM) strength with a tourniquet compression of 1.3 times their resting brachial systolic blood pressure (SBP), while seven subjects performed HLE at an intensity of 80% 1-RM strength. Subjects were randomized into the respective groups. Before the first day of training and 2–3 days after the last day of training, the subject’s isometric muscle strength, PWV, ABI, PT and NCV were assessed. We evaluated these outcomes several days after the last exercise session to allow for the potential for acute effects of a single bout to be minimized. Basal PT was also measured after the first and second weeks of training to monitor changes in the global blood clotting status of subjects. Additionally, we obtained venous blood samples on the first and last day of exercise training. These samples were obtained before exercise, immediately after exercise and 1-h post-exercise and analyzed for concentrations of tPA antigen, fibrinogen, D-dimer and hsCRP. The testing timeline is illustrated in Fig. 1.
Fig. 1.
Timeline of the assessment of the outcome measures.
Subjects
Seventeen individuals (15 men, 2 women) between 18 and 30 years enrolled in the study, but one subject dropped out following randomization to the HLE group due to reasons unrelated to the research study. Sixteen subjects were randomized to either the BFRE training protocol (n 5 9: 8 men, 1 woman; 23.7 ± 1.4 years, 70.2 ± 1.7 cm, 76.7±4.9 kg), or the HLE training protocol (n = 7: 6 men, 1 woman; 24.3±1.8 years, 69.1 ± 0.9 cm, 75.4 ± 3.3 kg). Because the relative health risks associated with BFRE have been poorly defined, we chose to take a very conservative approach when recruiting subjects and as such only enrolled young apparently, healthy individuals with no history of previous chronic illness and those who had not had acute illness with 3 months. Specifically, subjects were excluded if they had an ABI of <0.95, a resting SBP exceeding 140 mmHg or a diastolic blood pressure exceeding 90 mmHg, a body mass index ≥ 30, any orthopedic, neurologic or cardiovascular diseases or limitations, were currently taking any medications or supplements (including hormonal contraceptives), had a history or family history of blood clotting, reported smoking in the last 6 months, or who had participated in systematic resistance training (≥ 1 day/week over the past year) or currently participated in other types of regular exercise sessions (>60 min/week) as assessed by interviews and questionnaires before enrollment. This study and all methods were approved by the Ohio University Institutional Review Board, and all subjects provided informed consent before participation.
Resistance training protocols
Subjects were randomly assigned to either the HLE or BFRE training groups. The HLE training protocol conformed to the protocol recommended by the American College of Sports Medicine (Kraemer et al., 2002). Subjects performed three sets of dynamic (isotonic) bilateral knee extension exercise at 80% of 1 RM on a knee extension dynamometer (MedX, Ocala, Florida, USA) to volitional failure. Subjects performed between 8 and 12 repetitions with 90-s rest between each set (2-s concentric and 2-s eccentric action). The weights were progressively increased to maintain this range of repetitions to failure per set.
The BFRE protocol consisted of the same knee extension exercise as described above (isotonic, 2-s concentric and 2-s eccentric action, three sets with 90-s of rest between sets). However, subjects in this group performed the exercise at an intensity of 30% 1 RM while a pneumatic pressure cuff (6 × 83 cm SC5 tourniquet cuff inflated via an E20 Rapid Cuff Inflator, D.E. Hokanson Inc., Bellevue, Washington, USA) located on the proximal thigh was inflated to 130% above the resting brachial SBP (Cook et al., 2007). This device continually maintains a constant pressure; thus, the pressure was the same throughout the entire exercise protocol. While the pressure cuff was inflated above resting SBP, it should be noted that the compressive pressure experienced at the artery is generally attenuated as there is a disassociation between tourniquet pressure and underlying soft-tissue pressure, especially in the lower extremities (e.g., the tissue pressure is lower than the tourniquet pressure reflected in the underlying tissue as they vary inversely with the circumference of the thigh) (Shaw & Murray, 1982). This cuff pressure has been shown previously to reduce superficial femoral artery blood flow by ~ 50% (from 370 ± 71 mL/min at rest to 195±70 mL/min following BFRE) (Takano et al., 2005). Additionally, because leg venous pressure is very low (Groothuis et al., 2008), the cuff pressure also likely restricted a portion of venous return. Subjects performed contractions to volitional failure and as training progressed the weight was adjusted to maintain this repetition range between 30 and 50 repetitions (the range of repetitions that subjects performed at this given exercise intensity before the start of training). The pressure cuff was inflated throughout the exercise session and released upon the completion of the last set. Before the exercise bouts, both training groups performed 15 repetitions of the knee extension exercise at 30% 1-RM intensity to warm up.
One RM was defined as the quantity of mass (kg) that the subject could lift no more than once. Subjects were required to lift a load from 108 to 181 of full knee extension. The experimenter chose the initial weight, and then the weight was increased until the subject could not perform the task through the entire range of motion. One RM was determined within 2 kg or less, and the 1-RM testing usually required five to six attempts. The last weight lifted successfully over the full range of motion was considered to be the 1 RM. Subjects were given a 1-min rest between each attempt.
Blood sampling and storage
Samples of blood were obtained on the first and last days of exercise training to assess acute and chronic effects of BFRE and HL exercise on markers of inflammation, blood coagulation and fibrinolysis. Subjects were instructed to fast for 12 h before the blood sampling, and to avoid alcohol for 24 h before sampling. Samples were collected at the same time of day (between 7:00 and 10:00 hours) for all subjects to minimize diurnal variation in analytes, and upon arriving at the laboratory, the subjects were instructed to rest quietly in a seated position for 10 min to minimize postural effects and fluid shifts on blood variables. Venous blood samples were obtained from the antecubital vein immediately before exercise, approximately 3 min after exercise and 1 h after exercise. Blood was collected in tubes containing a citrate solution (Biopool; Ventura, California, USA) for the determination of fibrinolytic variables, sodium citrate (Becton Dickson; Franklin Lakes, New Jersey, USA) for coagulation analytes, untreated tubes for inflammatory markers and EDTA tubes (Becton Dickson) for the determination of hemoglobin and hematocrit. During the 1-h rest period between blood draws, the subject remained seated and was given water ad libitum up to 1000 mL, but no other food or liquids were allowed. Tubes were inverted to mix upon sampling, iced until they were subsequently centrifuged at 2300–2800 revolutions per minute for 20 min, serum and plasma was separated, aliquoted and then stored at −70 C until analyses were performed. The EDTA-treated whole blood was used to determine hemoglobin (Hbg Pro; ITC Medical; San Francisco, California, USA) and hematocrit (microhematocrit method, Micro MB; IEC; Pittsburg, Pennsylvania, USA) in triplicate for the correction of plasma volume shifts using the method of Dill and Costill (1974). All analyte concentrations are reported as plasma volume-adjusted concentrations.
Outcome measures
Isometric muscle strength
During the assessment of isometric strength, subjects were seated in a knee extension dynamometer (MedX), which allowed for strict control of the hip and knee joint angles. The backrest was adjusted to seat the subject at a hip joint angle of 100° from flexion, and a seat belt was secured to prevent any movement of the hip joint. Forces were measured using a force transducer (model U1 T, HBM Inc., Marlborough, Massachusetts, USA; sensitivity of 0.002 V/N), amplified and recorded at 1000 Hz using a 16-bit data acquisition card (MP150, BioPac Systems Inc., Santa Barbara, California, USA). The exerted force was displayed on a 43 cm computer monitor (AppleVision, Apple Computer Inc., Cupertino, California, USA) located 1 m directly in front of the subject. During testing, subjects crossed their hands across their lap and gradually increased force production over the first second, and then exerted a maximum effort for 2–3 s. During this test, the knee joint angle was set at 60° from extension. Three maximal leg extension contractions were performed with a 1–2-min rest period between efforts. If subjects continually recorded more force with increasing trials, or if the trials were not within 5% of each other, additional trials were performed until a plateau was reached. Strong verbal encouragement was provided by the investigators. The trial consisting of the highest value was considered the MVC force.
PWV
An increase in arterial stiffness is an important determinant of cardiovascular risk (Blacher et al., 1999). As an indirect measure of peripheral arterial stiffness, we calculated the PWV between the common femoral (~2 cm proximal to the bifurcation) and posterior tibial artery of the ankle. While subjects were supine, the distance from the femoral to the posterior tibial recording sites were measured as straight lines between these points on the body surface with a tape measure. Next, pressure waveforms were recorded at these sites simultaneously using two Doppler probes with analog outputs (MD6, D.E. Hokanson Inc.). The pulse wave signals were sampled at 1000 Hz (Biopac MP150 Systems, Goleta, California, USA) and subsequently smoothed by a 10-point running average that allowed for a clear identification of the pulse wave foot. Peripheral PWV was calculated by visually determining the delay between the appearances of the pulse wave foot in the two sites and divided by the travel distance. The investigator performing the analyses were blinded to the subject group and time condition. A total of 10 pulse contours were averaged for each session.
ABI
ABI is a measure of the fall in blood pressure in the arteries supplying the legs and is commonly used to detect peripheral vascular disease (Sacks et al., 2003). To quantify ABI, the subject was positioned in a supine position, and the brachial and ankle SBP’s were obtained from both sides of the body using a Doppler probe (MD6, D.E. Hokanson Inc.). Here, the brachial SBP was assessed along with the SBP in the posterior tibial artery of the ankle, and the ankle pressure was divided by the brachial pressure and expressed as an index. The normal range of ABI’s in young healthy adults is 1.02–1.26 (Male et al., 2007), although the cutoff for pathological indications of peripheral vascular disease is typically 0.90 (NHBLI, 2008).
PT
PT is a measure of the integrity of the extrinsic and final common pathways of the procoagulant cascade (Kamal et al., 2007). The PT represents the time, in seconds, for plasma to clot after the addition of calcium and an activator of the extrinsic pathway (thromboplastin), and as such changes in clotting factors within these pathways result in an altered PT (Kamal et al., 2007). PT was evaluated before and after training as well as after the first and second weeks of training. To assess PT, a fingerstick blood sample (~15 μL) was analyzed amperometrically after activation of theȈ coagulation with human recombinant thromboplastin (CoaguCheck XS, Roche Diagnostics, Basel, Switzerland). PT was expressed as the international normalized ratio (INR). Normal lab values for PT when expressed as the INR are from 0.8–1.2.
NCV
Nerve conduction of the left leg was assessed by evaluating the latency response of the H-reflex, which is a spinal reflex response resultant from electrical nerve stimulation. We utilized techniques described previously by our group (Clark et al., 2007), which involves stimulating the tibial nerve (Grass S88, Quincy, Massachusetts, USA) and recording the soleus electromyogram signal with surface electrodes at a sampling rate of 100 kHz (MP150, BioPac Systems). The reflex latency represents the time required for sensory Ia conduction from the stimulus site (popliteal fossa) to the spinal cord, synaptic delay at the motorneuron and the efferent motor nerve conduction to the recording site (soleus). As such, this measure represents a global index of both sensory and motor NCV, and this method has been shown to be very accurate in detecting marginal conduction changes (Troni et al., 1983). To account for between subject differences in body stature, the reflex latency time was divided by leg length (Troni et al., 1983).
Fibrinogen
Fibrinogen is a soluble glycoprotein that is a precursor to the fibrin monomer, the primary fibrillar protein involved in clot formation. Serum levels of fibrinogen were measured using an ELISA kit with an intra-assay coefficient of variation of 6.0% (Human Fibrinogen ELISA Kit, ICL Inc.; Newberg, Oregon, USA).
D-dimer
D-dimer is a protein fragment present in the blood after a blood clot is degraded through fibrinolytic pathways. Plasma levels of D-dimer were measured using an ELISA kit with an intra-assay coefficient of variation of 9.4% (TintElize DDimer, Trinity Biotech, Bray, Co Wicklow, Ireland).
tPA antigen
tPA is a fibrinolytic protein that catalyzes the conversion of plasminogen to plasmin. Plasma levels of the tPA antigen were measured using an ELISA kit with intra-assay coefficients of variation of 4.9% (TintElize tPA, Trinity Biotech).
High-sensitivity C-reactive protein
hsCRP is an acute phase inflammation protein. The concentration of CRP was determined utilizing a high-sensitivity ELISA kit with an intra-assay coefficient of variation of 5.2% (MP Biomedicals, Orangeburg, New York, USA).
Statistical analysis
Repeated measures analysis of variance (RM-ANOVA) procedures were performed to determine the effect of “exercise mode” (between-subjects factor: BFRE and HLE) and “training state” (within-subjects factor: pre-training and post-training) on the respective dependent variables. For the blood samples obtained immediately following an acute bout, a third within-subjects variable was included. For all analyses, a preset α-level of significance equal to 0.05 was required for statistical significance, and significant main effects and/or interaction terms were followed up with Sidak post hoc tests. Effect sizes (here, referring to the partial 2, which represents the proportion of the total variation attributable to the factor, separating other factors from the total non-error variation) are also reported as an additional statistical parameter to aid in the interpretation of the findings. The SPSS statistical package (version 10.0, Chicago, Illinois, USA) was used for data analysis. Data are presented as means ± SE, unless otherwise stated.
Results
Muscle strength
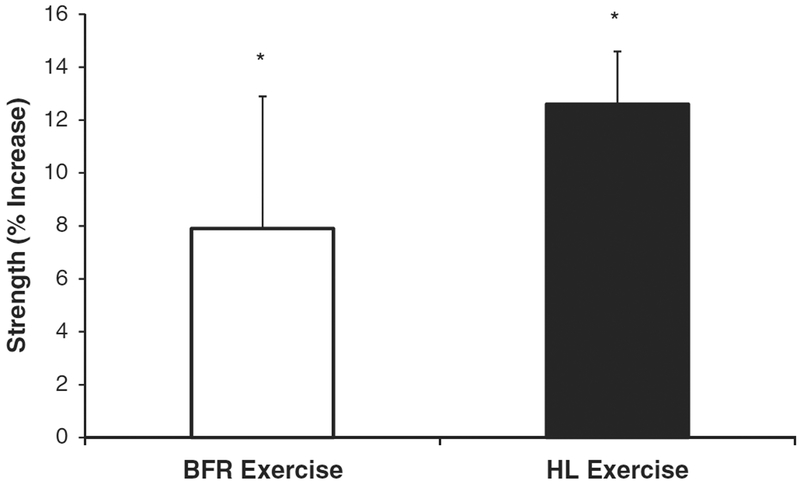
A training state main effect for resistance training increasing isometric strength was observed (P<0.01; ES = 0.42), indicating that both BFRE and HLE increased isometric strength. We did not observe a significant difference between the two exercise protocols for the magnitude of strength increase (training state × exercise mode interaction P = 0.28; ES = 0.09). On average, isometric strength increased ~ 8% in the BFRE training group (1103.4 ± 104.1 to 1173.8 ±90.8N), and ~13% in the HLE training group (1273.5 ± 125.1 to 1424.6 ± 130.7 N) (Fig. 2).
Fig. 2.
Changes in isometric strength following 4 weeks of low-load blood flow restricted (BFR) resistance exercise and high-load (HL) resistance exercise. Isometric strength increased significantly for both groups. *Significantly different than pre-training levels (P ≤ 0.05).
PWV
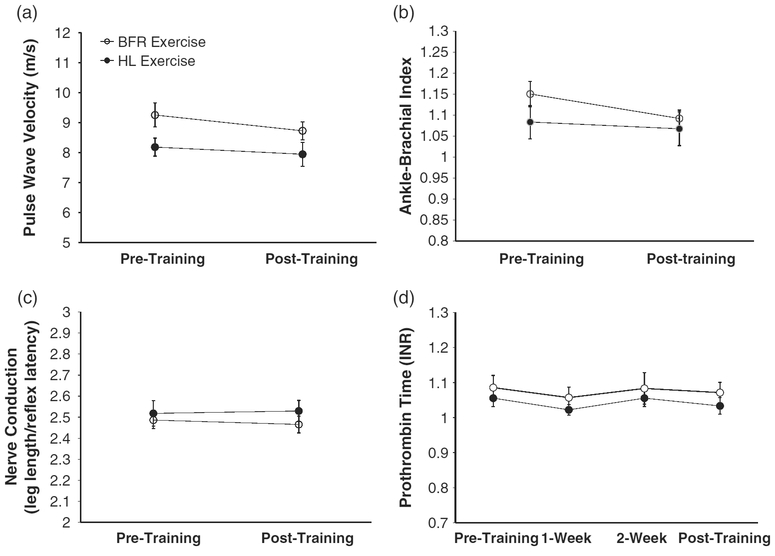
PWV did not change following training in either group (training state main effect P = 0.12, ES = 0.16; training state × exercise mode interaction P = 0.54, ES = 0.03) (Fig. 3(a); BFRE group before and after training: 9.26 ± 0.39 vs 8.73 ± 0.32 cm/s; HLE group before and after training: 8.19 ± 0.27 vs 7.94 ± 0.40 cm/s)
Fig. 3.
No changes were observed following 4 weeks of low-load blood flow restricted (BFR) resistance exercise training and high-load (HL) resistance exercise training with respect to pulse wave velocity (a), ankle-brachial index (b), nerve conduction velocity (c) and prothrombin time (d).
ABI
There were no differences between the left and right side ABI’s and thus these were averaged for analysis and presentation. ABI did not change following training in either group (training state main effect P = 0.17, ES = 0.13; training state × exercise mode interaction P = 0.43, ES = 0.05) (Fig. 3(b); BFRE group before and after training: 1.15 ± 0.03 vs 1.09 ± 0.02; HLE group before and after training: 1.08 ± 0.04 vs 1.07 ± 0.04).
PT
PT did not change following training in either group (training state main effect P = 0.27, ES = 0.10; training state × exercise mode interaction P = 0.99, ES = 0.00) (Fig. 3(c); BFRE group before training, and after 1-, 2- and 4-weeks of training: 1.06 ± 0.02, 1.02 ± 0.02, 1.06 ± 0.02 1.03 ± 0.02, INR; HLE group before training, and after 1, 2- and 4-weeks of training: 1.09 ± 0.03, 1.06 ± 0.03, 1.08 ± 0.04 and 1.07 ± 0.03 INR). With regard to individual changes in PT, none of the subjects exhibited PT readings outside of the normal range on any occasion.
Nerve conduction
NCV did not change following training in either group (training state main effect P = 0.83, ES = 0.01;training state × exercise mode interaction P = 0.51, ES = 0.04) (Fig. 3(d); BFRE group before and after training: 32.73 ± 0.87 vs 33.06 ± 1.11 msec; HLE group before and after training: 32.11 ± 0.06 vs 31.94 ± 0.29 msec).
Fibrinogen
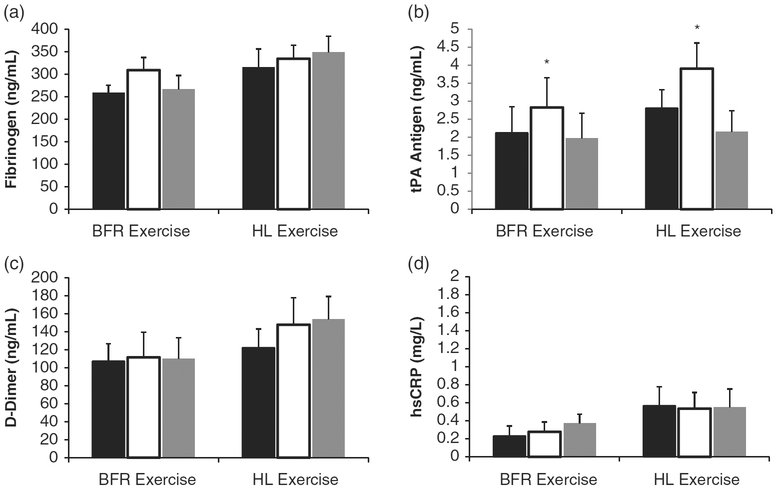
A single bout of exercise did not change fibrinogen levels after the first exercise session (acute response P = 0.14, ES0.14; acute response × group P = 0.25, ES = 0.10), nor was there any chronic training-induced effects (acute response × training state interaction P = 0.09, ES = 0.17; acute response training state × group interaction P = 0.92, ES = 0.01). Data representing the acute changes in fibrinogen levels, averaged across samples from the first and last day of training are illustrated in Fig. 4(a) (BFRE group at rest, immediately post-exercise and 1-h post-exercise: 259.3 ± 22.4 to 309.4 ± 30.4 to 266.3 ± 38.6 ng/mL; HLE group at rest, immediately post-exercise and 1-h post-exercise: 316.1 ± 31.8 to 334.8 ± 30.3 to 348.8 ± 32.4 ng/mL).
Fig. 4.
Changes in concentrations of fibrinogen (a), tissue plasminogen activator (tPA) antigen (b), D-dimer (c) and high-sensitivity C-reactive protein (hsCRP) (d) under resting conditions (before exercise; black bars), immediately following (open bars) and 1-h following (gray bars) a single bout of blood flow-restricted (BFR) exercise and high-load (HL) exercise. tPA activity was increased immediately following BFR and HL exercise (*P = 0.01). No significant changes were observed for fibrinogen, D-dimer or CRP. No significant interaction terms were observed from the data obtained from the first and last days of training; thus, data have been averaged for clarity.
tPA antigen
An acute response main effect for resistance training increasing tPA antigen immediately following a single exercise session was observed (P = 0.01; ES = 0.33), indicating that when data were averaged across samples from the first and last day of training that BFRE and HLE increased tPA. We did not observe a significant difference between the two exercise protocols for the magnitude of tPA activity increase (acute response ×; exercise mode interaction P = 0.48; ES = 0.06) nor did we observe a chronic training-induced effect (acute response ×; training state interaction P = 0.85, ES = 0.01; acute response ×; training state ×; group interaction P = 0.60, ES =0.04). On average, tPA activity increased 33% in the BFRE training group immediately post-exercise (rest, immediately post-exercise and 1-h post-exercise: 2.12 ± 0.74 to 2.83 ± 0.82 to 1.97 ± 0.60 ng/mL), and 39% in the HLE training group (rest, immediately post-exercise and 1-h post-exercise: 2.81 ± 0.51 to 3.91 ± 0.71 to 2.16 ± 0.58 ng/mL) (Fig. 4(b)).
D-dimer
A single bout of exercise did not change D-Dimer levels after the first exercise session (acute response P = 0.43, ES = 0.05; acute response × group P = 0.41, ES = 0.05), nor was there any chronic training-induced effects (acute response × training state interaction P = 0.80, ES = 0.02; acute response × training state group interaction P = 0.94, ES = 0.01). Data representing the acute changes in D-Dimer levels, averaged across samples from the first and last day of training are illustrated in Fig. 4(c) (BFRE group at rest, immediately post-exercise and 1-h post-exercise: 107.3 ± 19.5 to 111.5 ± 28.0 to 109.7 ± 23.7 ng/mL; HLE group at rest, immediately post-expertise and 1-h post-expertise : 122.2 ± 20.8 to 147.8 ± 30.3 to 153.8 ± 25.4 ng/mL).
hsCRP
A single bout of exercise did not change hsCRP levels after the first exercise session (acute response P = 0.24, ES = 0.10; acute response × group P = 0.47, ES = 0.05), nor was there any chronic training-induced effects (acute response × training state interaction P = 0.11, ES = 0.15; acute response × training state × group interaction P = 0.21, ES = 0.11). Data representing the acute changes in hsCRP levels, averaged across samples from the first and last day of training are illustrated in Fig. 4(d) (BFRE group at rest, immediately post-exercise and 1-h post-exercise: 0.23 ± 0.08 to 0.28 ± 0.09 to 0.37 ± 0.18 mg/L; HLE group at rest, immediately post-exercise and 1-h post-exercise: 0.53 ± 0.18 to 0.54 ± 0.14 to 0.55 ± 0.18 mg/L).
Discussion
The purposes of this study were to assess the efficacy of low-load BFRE training in increasing muscular strength, and determine if 4 weeks of low-load BFRE training alters blood, vascular and/or nervous system properties in young, healthy individuals. Because we were interested in comparing the changes in these outcome measures with the commonly performed and recommended exercise prescription for increasing muscle strength (as opposed to simply comparing it with low-load exercise without restriction), we compared BFRE with HLE training. We did not observe any changes in PWV, ABI, PT or NCV following training in either the BFRE or HLE training groups. Additionally, we did not observe any changes in fibrinogen, D-dimer or hsCRP following a single bout of the respective exercise. Both BFRE and HLE training increased isometric strength to a similar extent. Collectively, these results indicate that both short-duration BFRE and HLE resistance training is effective at increasing strength. Additionally, these findings indicate that BFRE and HLE of short duration in young, healthy individuals does not negatively alter several clinical assessments of peripheral vascular stiffness, peripheral nerve conduction and blood clotting function.
To date, most BFRE training studies have compared the relative effects of low-load BFRE with low-load exercise without blood flow restriction (Shinohara et al., 1998; Takarada et al., 2000, 2002, 2004; Moore et al., 2004; Sumide et al. in press). These studies suggest that low-load exercise with blood flow restriction increases muscle strength more than the same exercise without restriction (Shinohara et al., 1998; Takarada et al., 2000, 2002, 2004; Moore et al., 2004; Sumide et al. in press). Our findings of BFRE inducing similar, but somewhat less mean increases in strength when compared with HLE without restriction is consistent with the findings from Takarada et al. (2000) who reported that 16 weeks of low/moderate-load (50% 1-RM) BFR exercise with the elbow flexors increased isokinetic muscle strength by ~ 18%, whereas high-load (80% 1-RM) resistance exercise increased strength by ~ 22% (Takarada et al., 2000). It should be noted that, Ȉ while the two protocols increased strength similarly, it is possible that our sample size was too small to detect differences in strength gains between the two protocols. It is difficult to know whether the mechanisms for the increased strength were the same for the two exercise regimens. In general, skeletal muscle hypertrophy is not observed with short-duration HLE resistance training (Young et al., 1983; Narici et al., 1989; Higbie et al., 1996; Hakkinen et al., 1998; Akima et al., 1999), and it has long been suggested that much of the strength increase in the earlier phases of HLE resistance training is due to neural adaptations (Moritani & deVries, 1979). Conversely, as little as 3 weeks of treadmill walking with BFRE has been reported to increase muscle size (Abe et al., 2006). Thus, it is possible that the physiologic mechanisms underlying our observed increases in strength with both protocols may differ, although further study is required to investigate this postulate.
While several studies have been conducted on the efficacy of BFRE (Shinohara et al., 1998; Takarada et al., 2000, 2002, 2004; Abe et al., 2006; Madarame et al., 2008; Sumide et al., in press), as well as the mechanisms of BFRE (Fujita et al., 2007; Drummond et al., 2008), few experimental studies have evaluated markers of relative safety (Takano et al., 2005; Nakajima et al., 2006, 2007). Among the studies that have evaluated the BFRE effects on health-risk indicators, the majority of these have examined the acute effects following a single exercise session (Takano et al., 2005; Nakajima et al., 2007). In the present study, we examined the effect of 4 weeks of BFRE training on several common indicators of vascular health, blood clotting function and nerve conduction, as well as the acute effects on markers of blood coagulation, fibrinolysis and inflammation. The only change observed in the present study in these variables was an increase in tPA activity immediately following a single bout of both BFRE and HLE. This observation suggests that both BFRE and HLE acutely increase fibrinolytic activity.
While theoretical concerns about the safety of BFRE exist, no studies have reported severe adverse reaction to acute or chronic bouts of BFRE, although case and clinical reports do exist (Sato, 2005; Nakajima et al., 2006). Regarding the theoretical risk associated with blood clotting, we observed that both BFRE and HLE acutely increased tPA antigen immediately following a single bout of exercise, suggesting that an acute bout of these exercises enhances fibrinolytic potential without elevating the thrombolytic potential (based on no changes in fibrinogen or D-dimer). This finding is consistent with those of Nakajima et al. (2007) who reported that an acute bout of BFRE increases tPA antigen without altering plasminogen activator inhibitor-1 (PAI-1; the principal inhibitor of tPA) or D-dimer (Nakajima et al., 2007). It has long been known that vascular compressions alone actually appears to stimulate the fibrinolytic system without the elevation of the coagulation cascade (Holemans, 1963; Hozknecht et al., 1969; Robertson et al., 1972; Shaper et al., 1975; Stegnar & Pentek, 1993). Resistance exercise has also been shown to stimulate the fibrinolytic system (deJong et al., 2006). As such, it is difficult to know whether the increased tPA antigen we observed in association with BFRE was due to vascular compression, resistance exercise, or a relative combination of the two. We also measured the effect of chronic training on PT, which is among the most commonly ordered coagulation tests clinically and is a global measure of the integrity of the extrinsic and final common pathways of the procoagulant pathway (Kamal et al., 2007). Our observation of the exercise regimens not altering basal PT following exercise training suggests that the global clotting function was not altered with chronic training.
We obtained indices of changes in vascular function (PWV and ABI), and observed that neither exercise-training modes significantly altered these parameters. Collier et al. (2008) have recently reported that 4 weeks of HLE resistance training increases peripheral PWV, although there are several studies reporting no effects of resistance exercise training on PWV (Cortez-Cooper et al., 2005; Casey et al., 2007; Yoshizawa et al., 2009) and it is possible that the aforementioned studies findings may have been influenced by their study participants being pre- and stage-1 hypertensives who performed whole-body resistance exercise training. Thus, it is possible that our discrepant findings are due to differences in the study population and/or the specific resistance exercise training protocol. Additionally, we assessed sensory motor nerve conduction, as individuals performing BFRE occasionally report acute numbness (Nakajima et al., 2006). We did not observe any significant slowing of nerve conduction through the spinal pathway. This finding is not overly surprising as the duration of the vascular compression is of a relatively short duration (~10–15 min), and considerably longer durations of compression associated with surgery are rapidly reversible and rarely result in nerve damage (Lundborg, 1988).
Study limitations
While this study evaluated the effect of BFRE and HLE resistance training on measures of muscle strength, blood, vascular and/or nervous system function and observed reasonably positive outcomes (i.e., increased strength without changes in several risk factor outcome measures), we must caution these findings based on the subject samples enrolled in the project. Specifically, these findings must be taken within the context of the population studied (young, healthy, adults on no medications who were predominantly male) and not be extended to those of higher risks populations (i.e., elderly, post-surgery, etc.). Even within the framework of young, healthy individuals, these findings must be delimited to protocols of short duration, as it is unknown whether different outcomes would occur with a longer duration training protocol. Additionally, determining the “relative safety” of a given intervention requires a detailed study of a whole host of potential outcomes. As such, our observations must be restricted to the respective dependent variables assessed in the present study, and further research on other potentially altered physiological properties is warranted to more definitively assess the effects of BFRE training. For example, if we had measured certain markers that are more likely to respond to acute inflammation (e.g. IL-6), or other markers of clotting formation and breakdown (e.g., PAI-1), we may have observed different findings and thus drawn different conclusions.
Another limitation of the present study is related to the applied cuff pressure. Our cuff pressure was set at 30% above resting SBP; however, due to the dis-association between tourniquet pressure, underlying soft-tissue pressure and limb circumference (Shaw & Murray, 1982), it is likely that our cuff pressure did not uniformly restrict blood flow similarly across all subjects. This lack of control may have caused a differential effect between subjects. We chose our cuff pressure based on the current literature, and the observation that a bout of BFRE with this cuff pressure resulted in more muscle fatigue when compared with a higher cuff pressure (Cook et al., 2007). However, it is suggested that future investigations measure either the degree of restriction or muscle tissue oxygenation imposed with BFRE to give a better mechanistic understanding of the physiological effects.
Perspectives
Over the past decade, mounting evidence suggests that performing low-load exercise coupled with a modest blood flow restriction to the exercising muscles serves as a potent stimulus for increasing muscle strength and mass (Shinohara et al., 1998; Takarada et al., 2000, 2002, 2004; Nicholas et al., 2001; Abe et al., 2006; Madarame et al., 2008) [for a review, see (Clark & Manini, 2009)]. While these studies support its general efficacy in increasing muscle performance in younger and older healthy humans, there have been few empirical studies evaluating aspects related to the relative safety of BFRE. In the present study, we investigated the effect of 4 weeks of low-intensity BFRE knee extension training and HLE knee extension training on blood, vascular and neural function. We observed an increase in isometric strength with 4 weeks of BFRE and HLE, with neither of the training protocols altering the outcome variables associated with vascular function, blood clotting function or peripheral nerve conduction. Future research is needed to determine the effects on clinically based outcome variables and to determine whether BFRE is a potential rehabilitation strategy for patients contraindicated for high mechanical loading and undergoing rehabilitation.
Acknowledgements
We would like to thank Darren P. Casey, PhD of the Department of Anesthesiology at the Mayo Clinic for the critical review and feedback on a draft of this paper.
Funding: This study was supported in part by an OURC grant from the Ohio University to B. C. Clark. T. M. Manini was, in part, supported by the University of Florida, Claude D. Pepper Center (P30AG028740).
References
- Abe T, Kearns CF, Sato Y. Muscle size and strength are increased following walk training with restricted venous blood flow from the leg muscle, Kaatsu-walk training. J Appl Physiol 2006: 100: 1460–1466. [DOI] [PubMed] [Google Scholar]
- Akima H, Takahashi H, Kuno SY, Masuda K, Masuda T, Shimojo H, Anno I, Itai Y, Katsuta S. Early phase adaptations of muscle use and strength to isokinetic training. Med Sci Sports Exerc 1999: 31: 588–594. [DOI] [PubMed] [Google Scholar]
- Blacher J, Asmar R, Djane S, London GM, Safar ME. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension 1999: 33: 1111–1117. [DOI] [PubMed] [Google Scholar]
- Campos GE, Luecke TJ, Wendeln HK, Toma K, Hagerman FC, Murray TF, Ragg KE, Ratamess NA, Kraemer WJ, Staron RS. Muscular adaptations in response to three different resistance-training regimens: specificity of repetition maximum training zones. Eur J Appl Physiol 2002: 88: 50–60. [DOI] [PubMed] [Google Scholar]
- Casey DP, Beck DT, Braith RW. Exp Biol Med 2007: 9: 1228–1235. [DOI] [PubMed] [Google Scholar]
- Clark BC, Cook SB, Ploutz-Snyder LL. Reliability of techniques to assess human neuromuscular function in vivo. J Electromyogr Kinesiol 2007: 17: 90–101. [DOI] [PubMed] [Google Scholar]
- Clark BC, Manini TM. Blood flow restricted exercise and skeletal muscle health. Exerc Sport Sci Rev 2009: 37: 78–85. [DOI] [PubMed] [Google Scholar]
- Collier SR, Kanaley JA, Carhart R Jr., Frechette V, Tobin MM, Hall AK, Luckenbaugh AN, Fernhall B. Effect of 4 weeks of aerobic or resistance exercise training on arterial stiffness, blood flow and blood pressure in pre- and stage-1 hypertensives . J Hum Hypertens 2008: 22 (10): 678–686. [DOI] [PubMed] [Google Scholar]
- Cook SB, Clark BC, Ploutz-Snyder LL. Effects of exercise load and blood-flow restriction on skeletal muscle function. Med Sci Sports Exerc 2007: 39: 1708–1713. [DOI] [PubMed] [Google Scholar]
- Cortez-Cooper MY, DeVan AE, Anton MM, Farrar RP, Beckwith KA, Todd JS, Tanaka H. Effects of high intensity resistance training on arterial stiffness and wave reflection in women. Am J Hypertens 2005: 18: 930–934. [DOI] [PubMed] [Google Scholar]
- deJong AT, Womack CJ, Perrine JA, Franklin BA. Hemostatic responses to resistance training in patients with coronary artery disease. J Cardiopulm Rehabil 2006: 26: 80–83. [DOI] [PubMed] [Google Scholar]
- Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 1974: 37: 247–248. [DOI] [PubMed] [Google Scholar]
- Drummond MJ, Fujita S, Takashi A, Dreyer HC, Volpi E, Rasmussen BB. Human muscle gene expression following resistance exercise and blood flow restriction. Med Sci Sports Exerc 2008: 40: 691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita S, Abe T, Drummond MJ, Cadenas JG, Dreyer HC, Sato Y, Volpi E, Rasmussen BB. Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J Appl Physiol 2007: 103: 903–910. [DOI] [PubMed] [Google Scholar]
- Groothuis JT, Poelkens F, Wouters CW, Kooijman M, Hopman MT. Leg intravenous pressure during head-up tilt. J Appl Physiol 2008: 105: 811–815. [DOI] [PubMed] [Google Scholar]
- Hakkinen K, Newton RU, Gordon SE, McCormick M, Volek JS, Nindl BC, Gotshalk LA, Campbell WW, Evans WJ, Hakkinen A, Humphries BJ, Kraemer WJ. Changes in muscle morphology, electromyographic activity, and force production characteristics during progressive strength training in young and older men. J Gerontol A Biol Sci Med Sci 1998: 53: B415–B423. [DOI] [PubMed] [Google Scholar]
- Higbie EJ, Cureton KJ, Warren GL III, Prior BM. Effects of concentric and eccentric training on muscle strength, cross-sectional area, and neural activation. J Appl Physiol 1996: 81: 2173–2181. [DOI] [PubMed] [Google Scholar]
- Holemans R Increase in fibrinolytic activity by venous occlusion. J Appl Physiol 1963: 18: 1123–1129. [DOI] [PubMed] [Google Scholar]
- Hozknecht F, Spottl F, Braunsteiner H. Enhancement of fibrinolysis by venous occlusion. Results of long-term thrombelastography and activator determination. Thromb Diath Haemorrh 1969: 21: 304–310. [PubMed] [Google Scholar]
- Kamal AH, Tefferi A, Pruthi RK. How to interpret and pursue an abnormal prothrombin time, activated partial thromboplastin time, and bleeding time in adults. Mayo Clin Proc 2007: 82: 864–873. [DOI] [PubMed] [Google Scholar]
- Klatsky AL, Armstrong MA, Poggi J. Risk of pulmonary embolism and/or deep venous thrombosis in Asian-Americans. Am J Cardiol 2000: 85: 1334–1337. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Adams K, Cafarelli E, Dudley GA, Dooly C, Feigenbaum MS, Fleck SJ, Franklin B, Fry AC, Hoffman JR, Newton RU, Potteiger J, Stone MH, Ratamess NA, Triplett-McBride T. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults . Med Sci Sports Exerc 2002: 34: 364–380. [DOI] [PubMed] [Google Scholar]
- Lundborg G Nerve injury and repair. Edinburgh: Churchill Linvingstone, 1988. [Google Scholar]
- Madarame H, Neya M, Ochi E, Nakazato K, Sato Y, Ishii N. Cross-transfer effects of resistance training with blood flow restriction. Med Sci Sports Exerc 2008: 40: 258–263. [DOI] [PubMed] [Google Scholar]
- Margovsky AI, Lord RS, Chambers AJ. The effect of arterial clamp duration on endothelial injury: an experimental study. Aust N Z J Surg 1997: 67: 448–451. [DOI] [PubMed] [Google Scholar]
- Male S, Coull A, Murphy-Black T. Preliminary study to investigate the normal range of ankle brachial pressure index in young adults. J Clin Nurs 2007: 16(10): 1878–1885. [DOI] [PubMed] [Google Scholar]
- Moore DR, Burgomaster KA, Schofield LM, Gibala MJ, Sale DG, Phillips SM. Neuromuscular adaptations in human muscle following low intensity resistance training with vascular occlusion. Eur J Appl Physiol 2004: 92: 399–406. [DOI] [PubMed] [Google Scholar]
- Moritani T, deVries HA. Neural factors versus hypertrophy in the time course of muscle strength gain. Am J Phys Med 1979: 58: 115–130. [PubMed] [Google Scholar]
- Nakajima T, Kurano M, Iida H, Takano H, Oonuma H, Morita T, Meguro K, Sato Y, Nagata T. Use and safety of KAATSU training: results of a national survey. Int J KAATSU Training Res 2006: 2: 5–13 [Google Scholar]
- Nakajima T, Takano H, Kurano M, Iida H, Kubota N, Yasuda T, Kato M, Meguro K, Sato Y, Yamazaki Y, Kawashima S, Ohshima H, Tachibana S, Nagata T, Abe T, Ishii N, Moria T. Effects of KAATSU training on haemostasis in healthy subjects. Int J KAATSU Training Res 2007: 3: 11–20. [Google Scholar]
- Narici MV, Roi GS, Landoni L, Minetti AE, Cerretelli P. Changes in force, cross-sectional area and neural activation during strength training and detraining of the human quadriceps. Eur J Appl Physiol Occup Physiol 1989: 59: 310–319. [DOI] [PubMed] [Google Scholar]
- NHBLI. Peripheral arterial disease, 2008. Available at http://www.nhlbi.nih.gov/health/dci/Diseases/pad/pad_all.html (accessed 28 January 2010).
- Nicholas SJ, Tyler TF, McHugh MP, Gleim GW. The effect on leg strength of tourniquet use during anterior cruciate ligament reconstruction: a prospective randomized study. Arthroscopy 2001: 17: 603–607. [DOI] [PubMed] [Google Scholar]
- Risberg B, Bylock A, Romanus M. Endothelial fibrinolysis and ultrastructure following graded mechanical trauma. Acta Chir Scand 1988: 154: 353–358. [PubMed] [Google Scholar]
- Robertson BR, Pandolfi M, Nilsson IM. “Fibrinolytic capacity” in healthy volunteers as estimated from effect of venous occlusion of arms. Acta Chir Scand 1972: 138: 429–436. [PubMed] [Google Scholar]
- Sacks D, Bakal CW, Beatty PT, Becker GJ, Cardella JF, Raabe RD, Wiener HM, Lewis CA. Position statement on the use of the ankle brachial index in the evaluation of patients with peripheral vascular disease. A consensus statement developed by the Standards Division of the Society of lnterventional Radiology . J Vasc lnterv Radiol 2003: 14(9 Part 2): S389. [DOI] [PubMed] [Google Scholar]
- Sato Y The history and future of KAATSU training. Int J Kaatsu Training Res 2005: 1: 1–5. [Google Scholar]
- Shaper AG, Marsh NA, Patel I, Kater F. Response of fibrinolytic activity to venous occlusion. Br Med J 1975: 3: 571–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JA, Murray DG. The relationship between tourniquet pressure and underlying soft-tissue pressure in the thigh. J Bone Jt Surg Am 1982: 64: 1148–1152. [PubMed] [Google Scholar]
- Shinohara M, Kouzaki M, Yoshihisa T, Fukunaga T. Efficacy of tourniquet ischemia for strength training with low resistance. Eur J Appl Physiol Occup Physiol 1998: 77: 189–191. [DOI] [PubMed] [Google Scholar]
- Stegnar M, Pentek M. Fibrinolytic response to venous occlusion in healthy subjects: relationship to age, gender, body weight, blood lipids and insulin. Thromb Res 1993: 69: 81–92. [DOI] [PubMed] [Google Scholar]
- Sumide T, Sakuraba K, Sawaki K, Ohmura H, Tamura Y. Effect of resistance exercise training combined with relatively low vascular occlusion. J Sci Med Sport, in press. [DOI] [PubMed] [Google Scholar]
- Takano H, Morita T, Iida H, Asada K, Kato M, Uno K, Hirose K, Matsumoto A, Takenaka K, Hirata Y, Eto F, Nagai R, Sato Y, Nakajima T. Hemodynamic and hormonal responses to a short-term low-intensity resistance exercise with the reduction of muscle blood flow. Eur J Appl Physiol 2005: 95: 65–73. [DOI] [PubMed] [Google Scholar]
- Takarada Y, Sato Y, Ishii N. Effects of resistance exercise combined with vascular occlusion on muscle function in athletes. Eur J Appl Physiol 2002: 86: 308–314. [DOI] [PubMed] [Google Scholar]
- Takarada Y, Takazawa H, Sato Y, Takebayashi S, Tanaka Y, Ishii N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol 2000: 88: 2097–2106. [DOI] [PubMed] [Google Scholar]
- Takarada Y, Tsuruta T, Ishii N. Cooperative effects of exercise and occlusive stimuli on muscular function in low-intensity resistance exercise with moderate vascular occlusion. Jpn J Physiol 2004: 54: 585–592. [DOI] [PubMed] [Google Scholar]
- Troni W, Cantello R, Rainero E. The use of the H reflex in serial evaluation of nerve conduction velocity. Electroencephalogr Clin Neurophysiol 1983: 55: 82–90. [DOI] [PubMed] [Google Scholar]
- Yoshizawa M, Maeda S, Miyaki A, Misono M, Saito Y, Tanabe K, Kuno S, Ajisaka R. Effect of 12 weeks of moderate-intensity resistance training on arterial stiffness: a randomised controlled trial in women aged 32–59 years. Br J Sports Med 2009: 43: 615–618. [DOI] [PubMed] [Google Scholar]
- Young A, Stokes M, Round JM, Edwards RH. The effect of high-resistance training on the strength and cross-sectional area of the human quadriceps. Eur J Clin Invest 1983: 13: 411–417. [DOI] [PubMed] [Google Scholar]