Abstract
The mechanisms of the anabolic effect of parathyroid hormone (PTH) in bone are not fully defined. The bone anabolic effects of PTH require fibroblast growth factor 2 (FGF2) as well as Wnt signaling and FGF2 modulates Wnt signaling in osteoblasts. In vivo PTH administration differentially modulated Wnt signaling in bones of wild type (WT) and in mice that Fgf2 was knocked out (Fgf2KO). PTH increased Wnt10b mRNA and protein in WT but not in KO mice. Wnt antagonist SOST mRNA and protein was significantly higher in KO group. However, PTH decreased Sost mRNA significantly in WT as well as in Fgf2KO mice, but to a lesser extent in Fgf2KO. Dickhopf 2 (DKK2) is critical for osteoblast mineralization. PTH increased Dkk2 mRNA in WT mice but the response was impaired in Fgf2KO mice. PTH significantly increased Lrp5 mRNA and phosphorylation of Lrp6 in WT but the increase was markedly attenuated in Fgf2KO mice. PTH increased β-catenin expression and Wnt/β-catenin transcriptional activity significantly in WT but not in Fgf2KO mice. These data suggest that the impaired bone anabolic response to PTH in Fgf2KO mice is partially mediated by attenuated Wnt signaling.
Keywords: PTH, FGF2, Wnt signaling, Bone
Highlights
-
•
In vivo PTH administration differentially modulated Wnt signaling in bones of WT and Fgf2KO mice.
-
•
PTH treatment increased WNT10b and DKK2 expression in WT mice but the increase was blunted in Fgf2KO mice
-
•
PTH increased Lrp5 mRNA and phosphorylation of Lrp6 in WT but the increase was markedly attenuated in Fgf2KO mice.
-
•
PTH treatment increased β-catenin protein level and Wnt/β-catenin transcriptional activity in WT but not in Fgf2KO mice
-
•
The impaired bone anabolic response to PTH in Fgf2KO mice is partially mediated by attenuated Wnt signaling.
1. Introduction
Osteoporosis is a disease characterized by low bone mass and a deterioration in the micro-architecture of bone tissue, which leads to bone fractures (Holroyd et al., 2008). In year 2025 an estimated 44 million Americans are threatened by osteoporosis and the cost for osteoporosis-related fractures is predicted to be $25.3 billion (NOF, 2013). Therefore, osteoporosis is an enormous health and economic problem. Parathyroid hormone (PTH) is currently the only anabolic agent for treatment of osteoporosis in the U.S. Since 2002, when the FDA approved intermittent PTH administration, great progress has been made in understanding how intermittent PTH treatment mediates its anabolic bone response. However, the detailed mechanisms of PTH actions are not fully defined.
We previously showed that maximal bone anabolic effects of PTH require fibroblast growth factor 2 (FGF2). PTH induced FGF2 and FGF receptor mRNA expression in osteoblast cells (Hurley et al., 1999). In addition, PTH treatment increased serum FGF2 in osteoporotic subjects together with enhanced bone formation (Hurley et al., 2005). However, the anabolic response of PTH on bone formation in mice was impaired in the absence of endogenous FGF2 (Hurley et al., 2006) (Fei et al., 2011b). These data suggest that endogenous FGF2 is required for maximal bone anabolic response of PTH.
FGF2 is one member of the FGF family. It is expressed in osteoblasts and stored in the extracellular matrix (Ornitz and Marie, 2015). FGF2 stimulates osteoblast precursor proliferation (Fei and Hurley, 2012; Hurley et al., 2002). Although continuous administration of FGF2 decreases osteoblast differentiation markers, intermittent FGF2 treatment stimulates osteoblast differentiation and bone formation in vitro and in vivo (Hurley et al., 2002; Montero et al., 2000). Fgf2KO mice further reveal the importance of FGF2 in bone. There is markedly reduced plate-like trabecular structures and loss of connecting rods of trabecular bone in the absence of endogenous FGF2 (Montero et al., 2000). Interestingly, we observed that FGF2 expression decreased in osteoblasts from aged subjects compared to cells from young subjects (Hurley et al., 2016). Clinical trials demonstrate that local application of FGF2 stimulates periodontal regeneration (Kitamura et al., 2011) and accelerates healing of tibial shaft fractures (Kawaguchi et al., 2010). These data support that FGF2 positively regulates osteoblast differentiation and bone formation.
Bone anabolic response of PTH also requires the Wnt signaling pathway (Fei and Hurley, 2012; Jilka, 2007). Wnt signaling can occur either through the non-canonical pathway or the canonical Wnt/β-catenin signaling. Non-canonical pathway includes the Wnt/calcium pathway and the Wnt/planar cell polarity pathway (Piters et al., 2008). The canonical Wnt/β-catenin pathway is well studied in bone. To initiate signaling, ligand Wnt bind to receptor Lrp5/Lrp6 and Frizzled. This binding will block the destruction complex including kinase glycogen synthase kinase 3β (GSK3β), therefore, β-catenin will be stabilized and accumulate in the nucleus, where it binds to transcription factor T cell specific transcription factor and activate downstream target genes. Wnt signaling is regulated by Wnt antagonists, such as sclerostin (the gene coding sclerostin is Sost) and dickkopfs (DKKs) (Kamiya et al., 2008; Kawano and Kypta, 2003).
Extensive studies support that PTH treatment regulates genes of the Wnt signaling family (Kulkarni et al., 2005; Li et al., 2007; Onyia et al., 2005; Qin et al., 2003). We previously reported that FGF2 stimulation of osteoblast differentiation is partially through modulation of the Wnt/β-catenin signaling pathway (Fei et al., 2011a). We hypothesize that the impaired bone anabolic response of PTH in Fgf2KO mice is mediated by attenuated Wnt signaling. In the present study, we examined acute effects of PTH treatment (single injection, 20 μg/kg body weight) on Wnt signaling. We observed attenuated Wnt/β-catenin signaling in bones of Fgf2KO mice, which may contribute to the impaired bone anabolic response to PTH.
2. Materials and methods
2.1. Generation of 3.6Col1GFPsaphTg/Tg; Fgf2−/− mice
To generate mice in which osteoblast lineage cells are labeled with green fluorescent and Fgf2 gene is globally knocked out, 3.6Col1GFPsaphTg/Tg mice on a FVB/N genetic background previously made in our lab (Xiao et al., 2009) was crossed with Fgf2 null mice on a black swiss 129Sv genetic background (Zhou et al., 1998). 3.6Col1GFPsaphTg/Tg; Fgf2+/− × 3.6Col1GFPsaphTg/Tg; Fgf2+/− breeding pairs were maintained in the transgenic facility in the Center for Comparative Medicine at the UConn Health to generate 3.6Col1GFPsaphTg/Tg; Fgf2+/+ (WT) and 3.6Col1GFPsaphTg/Tg; Fgf2−/− (Fgf2KO) mice used in this study. Three months-old WT and Fgf2KO littermate female mice were used in the present study unless otherwise specified. These mice are on a mixed black swiss 129Sv/FVB/N genetic background and osteoblast lineage cells are labeled with green fluorescence sapphire. Mice genotype was performed using primers as previously described (Montero et al., 2000). Mice were sacrificed by CO2 narcosis and cervical dislocation. The UConn Health Institutional Animal Care and Use Committee approved all animal protocols.
2.2. PTH injection
Three independent experiments were performed using three-month old female mice. Mice were weighed and injected subcutaneously with vehicle (Veh, 0.001 M HCl in PBS with 1 mg/ml BSA) or 20 μg/kg body weight human PTH (1–34) (Bachem, Torrance, CA, USA). Eight hours after injection, mice were sacrificed by CO2 narcosis and cervical dislocation. Tibiae were dissected, epiphyses were removed and the remaining bones were snap frozen in liquid nitrogen for RNA or protein extraction.
2.3. RNA isolation and quantitative real-time PCR analysis
Total RNA was extracted from whole tibia (including bone and bone marrow) utilizing TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacture's protocol. Three microgram RNA was reverse transcribed with a commercial kit (Clontech, CA, USA). Quantitative real-time PCR (qPCR) was carried out using the QuantiTect™ SYBR Green PCR kit by a MyiQ™ instrument (Bio-Rad, Laboratories Inc. Hercules, CA, USA). Glyceraldehyde 3‑phosphate dehydrogenase (GAPDH) was utilized as an internal control for each sample. Relative mRNA expression was calculated using a formula reported previously (Pfaffl, 2001). mRNA was normalized to Gapdh mRNA level and expressed as the fold-change relative to the first sample for each experimental group. The mouse specific primers used are shown in Table 1.
Table 1.
Primers used for quantitative real time-PCR.
| Gene | Forward | Reverse |
|---|---|---|
| Gapdh | 5′-CAGTGCCAGCCTCGTCCCGTAGA-3′ | 5′-CTGCAAATGGCAGCCCTGGTGAC-3′ |
| β-Catenin | 5′-TGCTGAAGGTGCTGTCTGTC-3′ | 5′-GCTGCACTAGAGTCCCAAGG-3′ |
| Dkk1 | 5′-GGGAGTTCTCTATGAGGGCG-3′ | 5′-AAGGGTAGGGCTGGTAGTTG-3′ |
| Dkk2 | 5′-CTGGGATGGCAGAATCTAGG-3′ | 5′-AATCCAGGTTTCCATCATGC-3′ |
| Lrp5 | 5′-ACCCGCTGGACAAGTTCATC-3′ | 5′-TCTGGGCTCAGGCTTTGG-3′ |
| Lrp6 | 5′-GGTGTCAAAGAAGCCTCTGC-3′ | 5′-ACCTCAATGCGATTTGTTCC-3′ |
| Sost | 5′-GGAATGATGCCACAGAGGTCAT-3′ | 5′-CCCGGTTCATGGTCTGGTT-3′ |
| Wnt3a | 5′-CTCCTCTCGGATACCTCTTAGTG-3′ | 5′-CCAAGGACCACCAGATCGG-3′ |
| Wnt10b | 5′-TTCTCTCGGGATTTCTTGGATTC-3′ | 5′-TGCACTTCCGCTTCAGGTTTTC-3′ |
2.4. Protein extraction and western blot
Whole tibiae (including bone and bone marrow) were broken down using polytron homogenizer and protein extracts were harvested in RIPA buffer (Cell Signaling, MA, USA). Protein concentrations were measured with BCA protein assay reagent (Pierce, IL, USA). Equal amounts of protein were fractioned on SDS-PAGE gel and transferred onto a PVDF membrane (Bio-Rad, CA, USA). Membranes were blocked for 1 h with 5% non-fat dry milk, and then incubated overnight at 4 °C with antibody against Wnt10b (4μg/ml, catalog number: ab70816, Abcam, MA, USA), pLRP6 antibody (1:1000, catalog number: 2568s, Cell Signaling Technology, MA, USA), LRP6 (1:500, catalog number: sc-15399, Santa Cruz, CA, USA), inactive GSK3β (1:1000, catalog number: 9336, Cell Signaling Technology, MA, USA), total GSK3β (1:1000, catalog number: 9315, Cell Signaling Technology, MA, USA), SOST (1:5000, AF1589, R&D System, MN, USA), active β-catenin (1:1000, catalog number: 8841, Cell Signaling Technology, MA, USA), total β-catenin (1:500, sc-7199, Santa Cruz, CA, USA), or Actin (1:10000, sc-47778, Santa Cruz, CA, USA). Membranes were incubated with anti-rabbit secondary antibody (1:10000, catalog number: 7074s, Cell Signaling Technology, MA, USA) or anti-goat secondary antibody (1:10000, catalog number: sc-2020, Santa Cruz, CA, USA) at room temperature for 1 h. Blots were developed with Super Signal® West Dura Extended Duration Substrate (Thermo Scientific, Rockford, IL, USA). Western blot bands were analyzed by NIH Image (version 1.61; NIH, Bethesda, Maryland, USA).
2.5. Immunofluorescence staining
For histological analysis, femurs were immediately fixed in 4% PFA at 4 °C for 48 h. Each sample was embedded in Shandon Cryomatrix (Thermo Electron Corporation, PA, USA). Frozen samples were cut into 6-μm sections on Cryofilm type IIC (Choung et al., 2001). The sections were washed in 1× PBS/1%FBS and permeabilized with 0.25% Triton X-100 in 1× PBS/1%FBS for 10 min. After rinsing with 1× PBS/1%FBS, the sections were stained with anti-SOST antibody (1:40, catalog number: AF1589, R&D System, MN, USA), anti-DKK2 antibody (1:50, sc-25517, Santa Cruz, CA, USA) or anti-phospho-LRP6 (pLRP6, Ser1490) antibody (1:50, catalog number: bs-3253R, Bioss, Woburn, MA, USA) overnight at 4 °C. After washing with 1xPBS/1%FBS three times, sections were incubated with donkey anti-goat Alexa Flour (1:200, A-11058, ThermoFisher SCIENTIFIC, Rockford, IL, USA) or Texa Red goat anti-rabbit (1:1000, T2767, Invitrogen, Carlsbad, CA, USA) at room temperature for 1 h. Sections were counter-stained for nuclei with DAPI, mounted with PBS/glycerol (1:1) and imaged using fluorescent microscopy on Zeiss Axioplan microscopy.
2.6. Luciferase assay
Primary calvarial OBs from 3 days old WT and Fgf2KO male and female mice were plated at 30,000 cells/well/300 μl in 48-well plate in DMEM with 10% FBS and 1% penicillin/streptomycin (P/S). At 70–90% confluence, cells were transfected with the TOP-flash reporter construct using X-tremeGENE HP DNA Transfection Reagent (0.75 μl X-tremeGENE HP DAN Transfection Reagent/500 ng of TOP-flash DNA/5 ng of cytomegalovirus 5′-regulatory region DNA) according to manufacturer's instructions (Roche). Co-transfection with a construct containing the cytomegalovirus 5′-regulatory region driving the β‑galactosidase gene was used to control for transfection efficiency. 36 h post transfection cell were treated with Vehicle or 10 nM PTH for 3 h or 8 h, washed twice with PBS, and harvested in a reporter lysis buffer (Promega). Luciferase and β‑galactosidase activities were measured using an Optocomp luminometer (MGM Instruments, Hamden, CT). Luciferase activity was corrected for β‑galactosidase activity to control for transfection efficiency. FOP-flash plasmid was used as negative controls.
2.7. Statistical analysis
Results are expressed as the means ± standard error (SE). ANOVA followed by Least Significant Difference (LSD) for Post Hoc Multiple Comparisons was used. SPSS software was used for statistical analysis, and the results were considered significantly different at p < 0.05.
3. Results
3.1. PTH treatment increased Wnt10b mRNA and protein expression in bones of WT but not Fgf2KO mice
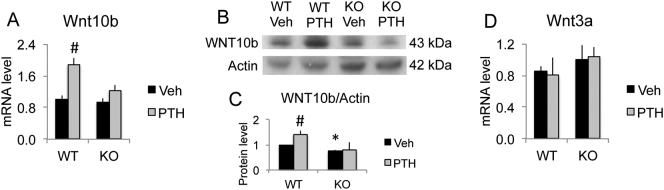
Ligand Wnt10b has been demonstrated to positively regulate osteoblast differentiation and bone formation. Eight hours after PTH injection, we observed a significant increase of Wnt10b mRNA expression in WT mice but no significant change in Fgf2KO mice (Fig. 1A). Correspondingly, PTH treatment induced a marked increase of Wnt10b protein expression in WT mice. However, no increase of expression in Wnt10b protein after PTH treatment was observed in the absence of endogenous FGF2 (Fig. 1B & C). Notably, although no significant reduction of Wnt10b mRNA expression in Fgf2KO mice compared to WT mice at the age of three-month (Fig. 1A), there is a reduction of Wnt10b protein in Fgf2KO mice (Fig. 1C). Consistent with our observation that Fgf2KO mice develop severe low bone mass phenotype with aging (Montero et al., 2000), we previously reported that Fgf2KO mice display significant reduction in Wnt10 mRNA expression and a marked decrease of protein levels at the age of eight-month (Fei et al., 2011a). We also examined effect of PTH on the mRNA expression of Wnt ligand Wnt3a. We did not observe a significant difference induced by PTH treatment in either genotype (Fig. 1D).
Fig. 1.
PTH differentially regulated Wnt 10b in bones of WT and Fgf2KO mice. Three-month old female mice were treated with PTH (20 μg/kg body weight) or vehicle for 8 h. Left tibia was harvest for RNA extraction and right tibia was harvest for protein extraction. (A) Wnt10b mRNA expression by qPCR, n = 6–11 mice/group. (B) Wnt10b protein expression by western blot and (C) quantification, n = 3 mice/group. (C) Wnt3a mRNA expression by qPCR, n = 3 mice/group. Data presented are Mean ± SE. *: WT-Veh vs. Fgf2KO-Veh p < 0.05; #: compared with corresponding Veh p < 0.05 by ANOVA.
3.2. SOST mRNA and protein expression was significantly higher in bones of Fgf2KO mice at basal level and was reduced significantly after PTH treatment
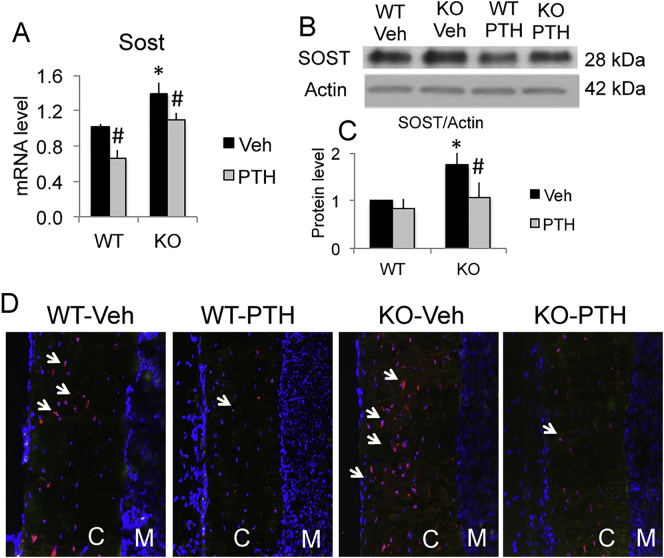
Wnt antagonist SOST is a negative regulator of Wnt signaling as well as of bone formation. We observed a significant increase of Sost mRNA (Fig. 2A) and a marked increase of SOST protein expression at basal level in Fgf2KO mice (Fig. 2B, C), which may contribute to the reduced bone formation in Fgf2KO mice. PTH administration decreased Sost mRNA expression in both genotypes. PTH markedly reduced SOST protein expression in whole bone in Fgf2KO mice as shown by Western blots (Fig. 2B & C) and in osteocytes of cortical bone by immunofluorescent staining (Fig. 2D).
Fig. 2.
SOST mRNA and protein were higher in Fgf2KO mice at basal level and reduced after PTH treatment. Three-month old female mice were treated with PTH (20 μg/kg body weight) or vehicle for 8 h. Left tibia was harvest for RNA extraction and right tibia was harvest for protein extraction. Femurs were used for IF. (A) SOST mRNA by qPCR, n = 6–11 mice/group. (B) SOST protein expression by Western blot and (C) quantification, n = 3 mice/group. (D) SOST protein in osteocyte of cortical bone by IF (arrows). SOST: red; DAPI: blue. C: cortical bone; M: bone marrow. Data presented are Mean ± SE. *: WT-Veh vs. Fgf2KO-Veh p < 0.05; #: compared with corresponding Veh p < 0.05 by ANOVA. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. PTH increased Dkk2 and Dkk1 mRNA in bones of WT but not in Fgf2KO mice
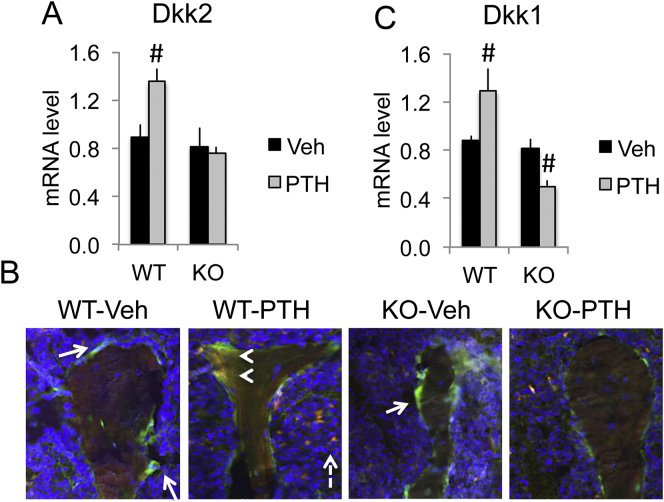
Besides functioning as a Wnt antagonist, DKK2 has been shown to be essential for osteoblast terminal differentiation and mineralization. Here, we observed PTH in vivo treatment increased Dkk2 mRNA and protein expression in WT mice (Fig. 3A & B). In contrast, PTH induced increase of Dkk2 mRNA and protein was blunted in Fgf2KO mice (Fig. 3A & B). To our knowledge, this is the first report about PTH induction of DKK2 expression in bone. We also examined Dkk1, an inhibitor of Wnt signal in bones of WT and Fgf2KO with and without PTH treatment. As shown in Fig. 3C, there is no difference in Dkk1 mRNA between WT and Fgf2KO mice at the basal level. PTH treatment significantly increased Dkk1 mRNA in WT mice, but significantly decreased Dkk1 mRNA in Fgf2KO mice.
Fig. 3.
PTH differentially regulated Dkk2 and Dkk1 mRNA in bones of WT and Fgf2KO mice. Three-month old female mice were treated with PTH (20 μg/kg body weight) or vehicle for 8 h. Left tibia was harvest for RNA extraction. (A) Dkk2 mRNA expression by qPCR, n = 6–11 mice/group. (B) (E) IF staining of DKK2. Osteoblasts on trabecular surface were label with green fluorescence sapphire (arrows). DKK2 was labeled with red fluorescence (dashed arrows). As noted, PTH markedly increased DKK2 in osteoblasts of WT mice but the increase was attenuated in the Fgf2KO mice (yellow, arrow heads). (C) Dkk1 mRNA expression by qPCR, n = 6–11 mice/group. Data presented are Mean ± SE. *: WT-Veh vs. Fgf2KO-Veh p < 0.05; #: compared with corresponding Veh p < 0.05 by ANOVA. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. PTH markedly increased pLRP6 in bones of WT mice but the increase was attenuated in Fgf2KO mice
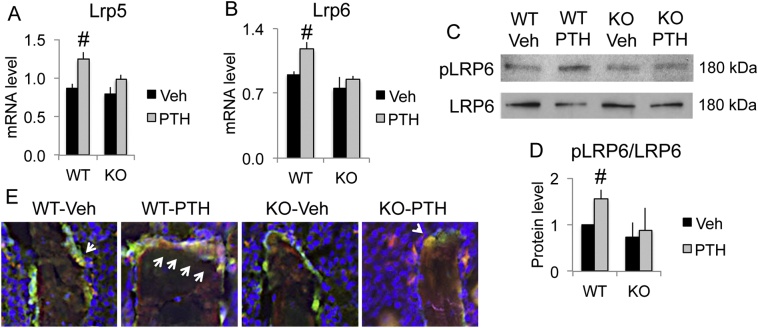
Wnt ligand needs to bind to Wnt receptor Lrp5 and Lrp6 to initiate Wnt signaling. Interestingly, we observed that PTH treatment significantly increased mRNA expression of Lrp5 (Fig. 4A) and Lrp6 (Fig. 4B) in WT mice; however, no significant increase was observed in Fgf2KO mice. Phosphorylation at serine 1490 of LRP6 is essential for activation of Wnt/β-catenin signaling. As shown by Western blot (Fig. 4C & D) PTH markedly increased pLRP6 in WT mice but the increase was attenuated in the Fgf2KO mice. Immunofluorescent staining (Fig. 4E) showed osteoblasts with green fluorescence on the trabecular surface. pLRP6 was labeled with red fluorescence. As noted, PTH markedly increased pLRP6 in osteoblasts of WT mice but the increase was attenuated in the Fgf2KO mice (arrows).
Fig. 4.
PTH markedly increased pLRP6 in bones of WT mice but the increase was attenuated in Fgf2KO mice. Three-month old female mice were treated with PTH (20 μg/kg body weight) or vehicle for 8 h. Left tibia was harvest for RNA extraction and right tibia was harvest for protein extraction. Femurs were used for IF. (A) Lrp5 and (B) Lrp6 mRNA expression by qPCR, n = 6–11 mice/group. Western blot analysis of (C) pLRP6 vs. total LRP6 and (D) quantification, n = 3 mice/group. Data presented are Mean ± SE. *: WT-Veh vs. Fgf2KO-Veh p < 0.05; #: compared with corresponding Veh p < 0.05 by ANOVA. (E) IF staining of pLRP6. Osteoblasts on trabecular surface were label with green fluorescence sapphire. pLRP6 was labeled with red fluorescence. As noted, PTH markedly increased pLRP6 in osteoblasts of WT mice but the increase was attenuated in the KO mice (arrows). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
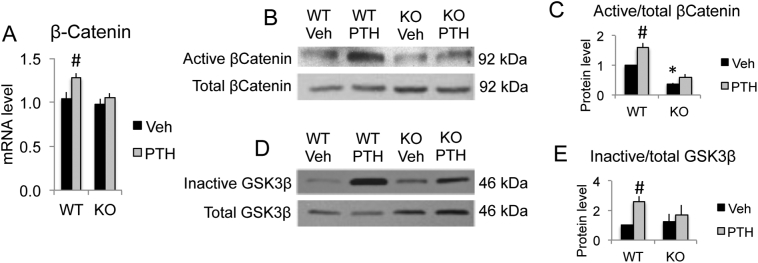
3.5. PTH treatment increased β-catenin mRNA and protein expression in bones of WT but not in Fgf2KO mice
β-Catenin is the key signaling factor of Wnt signaling. PTH in vivo treatment induced a significant increase in expression of β-catenin mRNA (Fig. 5A) in WT mice. However, no significant increase was observed in Fgf2KO mice (Fig. 5A). Correspondingly, PTH stimulation markedly increased active β-catenin protein in WT but the increase was greatly blocked in Fgf2KO Mice (Fig. 5B, C). PTH stimulation of β-catenin protein level is partially due to protein synthesis and protein stabilization as we observed that there is a 58.4% increase of active β-catenin protein while 22.2% increase of β-catenin mRNA induction by PTH treatment compared to vehicle group in WT mice. PTH stimulation of β-catenin protein level in WT mice is largely due to protein stabilization as there is significant increase in inactive GSK3β protein expression (Fig. 5D & E).
Fig. 5.
PTH differentially regulated β-catenin expression in bones of WT and Fgf2KO mice. Three-month old female mice were treated with PTH (20 μg/kg body weight) or vehicle for 8 h. Left tibia was harvest for RNA extraction and right tibia was harvest for protein extraction. (A) β-Catenin mRNA expression by qPCR, n = 6–11 mice/group. (B) Active vs. total β-catenin protein expression by Western blot and (C) quantification, n = 3 mice/group. (D) Inactive vs. total GSK3β protein expression by Western blot and (E) quantification, n = 3 mice/group. Data presented are Mean ± SE. *: WT-Veh vs. Fgf2KO-Veh p < 0.05; #: compared with corresponding Veh p < 0.05 by ANOVA.
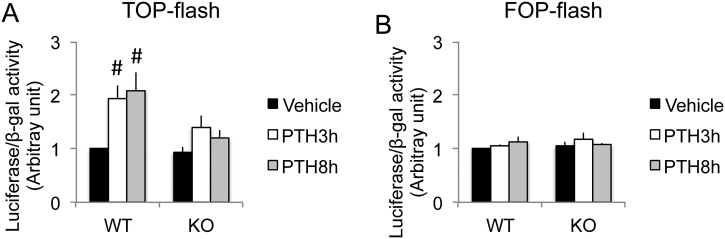
3.6. PTH stimulated Wnt/β-catenin transcriptional activity in primary calvarial osteoblasts from WT but not Fgf2KO mice
To further determine the crosstalk between Wnt/β-catenin signaling and FGF2 signaling in the action of PTH in bone, we examined Wnt/β-catenin transcriptional activity in primary calvarial osteoblasts from both genotypes using TOPflash luciferase assay. FOPflash was used as negative controls. In the TOPflash construct, transcription of luciferase is driven by seven copies of TCF/LEF binding sites and is specifically controlled by Wnt/β-catenin signaling (Korinek et al., 1997). PTH increased TOPflash luciferase activity significantly at both treatment duration of 3-h and 8-h in WT osteoblasts only (Fig. 6A). The increase of TOPflash luciferase activity by PTH, however, was markedly blocked in Fgf2KO primary calvarial osteoblasts. At all time points that were examined, there was no difference in negative control FOPflash luciferase activity in both genotypes. (Fig. 6B).
Fig. 6.
Effect of PTH treatment on Wnt/β-catenin transcriptional activity in primary calvarial osteoblasts of WT and Fgf2KO mice. Primary calvarial osteoblasts from 3 days old WT and Fgf2KO mice were plated at 30,000 cells/well/300 μl in 48-well plate in DMEM +10% FBS + 1% P/S. At the confluence of 70–90% confluence, cells were transfected with (A) TOPflash luciferase reporter or (B) FOPflash luciferase reporter and beta-galactosidase (BetaGal) for 36 h, then the cells were treated with Vehicle or 10 nM PTH for 3 h or 8 h. TOPflash luciferase activity or TOPflash luciferase activity was normalized to betaGal and the ratio in WT vehicle group was set to one. FOPflash was used as negative controls. Data are pool results from three independent experiments. Data presented are Mean ± SE. #: compared with corresponding Veh p < 0.05 by ANOVA.
4. Discussion
In the present study, in the in vivo study using female mice we found that 1) PTH treatment significantly increased ligand Wnt10b mRNA expression and markedly increased protein expression in WT mice but the increase was attenuated in Fgf2KO mice; 2) Wnt antagonist Sost mRNA and protein expression was increased significantly in Fgf2KO mice at basal level; PTH treatment markedly reduced Sost mRNA in both genotypes. 3) PTH treatment markedly increased Dkk2 mRNA expression in WT mice but the increase was blunted in Fgf2KO mice; 4) PTH induced a significant increase of Lrp5 mRNA and increased Lrp6 mRNA in WT mice but caused no increase in Fgf2KO mice; PTH markedly increased pLRP6 in WT mice but the increase was blocked in Fgf2KO mice; 5) PTH treatment increased β-catenin mRNA and protein level. In addition, in vitro cell culture using calvarial OBs from both male and female mice showed that PTH increased Wnt/β-catenin transcriptional activity significantly in WT but the increase was markedly attenuated in Fgf2KO mice. These data suggest that attenuated Wnt signaling may contribute to the impaired anabolic effects of PTH in bone in the absence of endogenous FGF2.
FGF2 stimulates osteoblast differentiation and bone formation in vitro as well as in vivo (Hurley et al., 2002). In contrast, disruption of the Fgf2 gene results in decreased bone mass and bone formation (Montero et al., 2000). PTH intermittent treatment increased serum FGF2 in osteoporotic patients together with enhanced bone formation (Hurley et al., 2005). In addition, PTH induced Fgf2 mRNA and FGFR expression in murine osteoblast cells (Hurley et al., 1999). However, the anabolic response of PTH on bone is greatly impaired in the absence of endogenous FGF2 (Hurley et al., 2006). Intermittent PTH treatment (80 μg/kg) for four weeks significantly increased parameters of bone formation in male WT mice, but the changes in Fgf2KO mice on a black swiss/129Sv genetic background was much smaller and not significant (Hurley et al., 2006). This impaired bone anabolic response of PTH in the absence of endogenous FGF2 in osteoblast lineage was further confirmed in another genetic background 3.6Col1GFPsaph/black swiss/129Sv/FVB/N even with a lower dose of PTH (40μg/kg/day for two weeks) (Fei et al., 2011b). These data strongly support that maximal anabolic response of PTH requires FGF2.
The potential mechanisms by which FGF2 mediates the anabolic actions of PTH in bone involve transcription factor Runx2 and ATF4. We previously demonstrated that PTH treatment increased Runx2 protein expression and nuclear accumulation only in in WT but not in Fgf2KO osteoblasts (Sabbieti et al., 2009). PTH regulation of Runx2 is mediated by the activation of cAMP response element binding proteins (Sabbieti et al., 2009). Our previous study reported that the impaired bone anabolic effect of PTH in Fgf2KO mice is in part mediated by another osteoblast transcription factor ATF4. PTH treatment in vivo enhanced Atf4 mRNA and protein expression together with increased bone formation in WT mice (Fei et al., 2011b). In contrast, the increase of ATF4 expression as well as bone formation stimulated by PTH was attenuated in Fgf2KO mice (Fei et al., 2011b). These data support that FGF2 is important in PTH effects on osteoblast differentiation, which is mediated by transcription factor Runx2 and ATF4.
We previously reported that FGF2 stimulation of osteoblast differentiation and bone formation is mediated by modulation of the Wnt signaling pathway (Fei et al., 2011a). Fgf2 deletion results in significant reduction of canonical Wnt gene expression of Wnt10b, Lrp5 and β-catenin mRNA during osteoblast differentiation. On the other hand, administration of exogenous FGF2 in Fgf2KO bone marrow stromal cells promoted β-catenin nuclear accumulation and partially rescued the phenotype of decreased mineralization. We further showed that FGF2 modulation of Wnt signaling may be through GSK3β and DKK2 (Fei et al., 2011a).
In the present study, we examined effect of short term PTH treatment on Wnt signaling in WT mice and Fgf2KO mice. PTH treatment induced a significant increase of Wnt10b mRNA and protein expression in WT mice. However, no significant change was observed in the absence of endogenous FGF2 after PTH treatment. Wnt10b is a canonical Wnt/β-catenin ligand and it promotes osteoblast differentiation and bone formation. Overexpression of Wnt10b in mature osteoblasts (Bennett et al., 2007) enhances osteoblast differentiation and increases bone formation. However, deletion of Wnt10b results in reduced osteoblast differentiation and decreased bone formation (Bennett et al., 2007; Stevens et al., 2010). These data indicate that increased Wnt10b expression induced by PTH treatment may contribute to the increased bone formation in WT mice. However, no significant change was observed in the absence of FGF2 after PTH treatment, which may lead to the impaired bone anabolic response to PTH in Fgf2KO mice. These data support that FGF2 is important in the effect of PTH induction of Wnt10b in bone.
Multiple cells including osteoblasts and adipocytes are responsible for the changes in the gene expression of the whole bone to PTH treatment (Fei and Hurley, 2012; Jilka, 2007). Interestingly, Wnt10b promotes osteoblastogenesis but suppresses adipogenesis (Bennett et al., 2005; Cawthorn et al., 2012). We also observed increased bone marrow fat accumulation in Fgf2KO mice and this bone fat accumulation phenotype progresses along with aging (Xiao et al., 2010). We are in the process of investigating whether PTH treatment is able to reduce bone marrow fat accumulation in Fgf2KO mice and whether FGF2 is required for PTH to reduce bone marrow fat accumulation.
Another finding was that in bone Wnt antagonist Sost (the gene encoding protein sclerostin/SOST) mRNA expression and SOST protein expression was increased significantly in the absence of endogenous FGF2. Increased SOST will attenuate Wnt/β-catenin signaling, thereby leading to reduced osteoblast differentiation and bone formation in Fgf2KO mice. Notably, PTH treatment greatly reduced Sost mRNA expression in WT, which is consistent with previous findings (Bellido et al., 2005; Keller and Kneissel, 2005).
Interestingly, another Wnt antagonist Dkk2 mRNA and protein expression was markedly increased after PTH treatment in WT mice but the increase was blunted in Fgf2KO mice. To our knowledge, this is the first report of induction of DKK2 in bone by intermittent PTH treatment. DKK2 is essential for osteoblast terminal differentiation and Dkk2 knockout mice are osteopenic (Li et al., 2005). In addition, bone matrix of Dkk2 knockout mice is poorly mineralized (Li et al., 2005). The increase of DKK2 expression stimulated by PTH in WT mice may promote osteoblast differentiation and increase bone formation. These data further support the essential function of DKK2 in bone formation and suggest that DKK2 is a novel mediator of the anabolic response of PTH in bone. A previous study reported that DKK2 promotes angiogenesis in rodent and human endothelial cells (Min et al., 2011). Vascular interaction during bone formation is very important (Towler, 2011). Intermittent PTH treatment has been shown to relocate bone marrow blood vessels closer to bone-forming sites (Prisby et al., 2011). It would be very interesting to examine whether DKK2 promotes angiogenesis during bone formation and whether DKK2 is essential for intermittent PTH promoting bone marrow blood vessels closer to bone forming sites.
Although Dkk1 is an endogenous inhibitor of Wnt signaling and suppresses bone formation in vivo (Bovolenta et al., 2008). However, it has been reported that low-dose PTH (10 μg/kg/day) increased the expression of Dkk1 in both bone marrow stromal cells (BMSCs) and osteoblasts/osteocytes (Saidak et al., 2014). Interestingly, we also found that low-dose PTH increased the expression of Dkk1 in bones of WT mice. This may be due to the secondary response from the activation of Wnt signaling which is known to increase Dkk1 expression (Chamorro et al., 2005).
We also observed that PTH treatment significantly increased Lrp5 mRNA expression in WT mice, however, no significant change was observed in the Fgf2KO mice. Induction of Lrp5 gene expression has been reported in rats and also in cell lines (Kulkarni et al., 2005). There was a marked increase of Lrp6 after PTH treatment in WT mice but no change was observed in Fgf2KO mice. Both Wnt co-receptor Lrp5 and Lrp6 are essential for bone homeostasis. Loss of function mutations of Lrp5 (Gong et al., 2001) and Lrp6 (Mani et al., 2007) associated with low bone mass. In contrast, gain of function mutations associated with high bone mass (Ai et al., 2005; Boyden et al., 2002; Van Wesenbeeck et al., 2003). Interestingly, PTH induced similar increases in bone mass in Lrp5 knockout mice compared to wild type mice (Iwaniec et al., 2007; Sawakami et al., 2006). One potential explanation is that Lrp6 is able to compensate for Lrp5 in Lrp5−/− mice. One needs to note that receptor Lrp5 and Lrp6 seem to have distinct role in bone since Lrp6+/−; Lrp5−/− mice have significant lower BMD than Lrp6+/+; lrp5−/− mice. Lrp6 has been reported to form a complex with PTH receptor (Wan et al., 2008). Knock down of Lrp6 with siRNA reduced PTH-induced β-catenin stabilization (Wan et al., 2008). Furthermore, phosphorylation at serine 1490 has been shown to be essential for activation of Wnt/β-catenin signaling (Tamai et al., 2004; Wan et al., 2008). Our data of increased pLRP6 stimulated by PTH is consistent with previous report (Wan et al., 2008). Binding of PTH to its receptor PTH1R induced association of LRP6 and this formation of ternary complex promoted phosphorylation of LRP6 (Wan et al., 2008). Importantly, the increase of pLRP6 by PTH was greatly blocked in the absence of endogenous FGF2 (Fig. 4C & D), which may contribute to the impaired bone response to PTH. It would be interesting to investigate whether Lrp6 co-receptor alone is sufficient for PTH anabolic response in bone. Recent study show that under physiological conditions, both Lrp5 and Lrp6 are required to respond to Wnt ligands to active Wnt/β-catenin signaling in mammary epithelial cells and fibroblasts (Goel et al., 2012). More studies are needed to fully understand the function of Wnt co-receptors in the anabolic bone response to PTH.
The key factor of Wnt signaling, β-catenin positively regulates bone formation and is essential for PTH signaling in bone. PTH treatment induced a significant increase of β-catenin mRNA and protein expression and Wnt/β-catenin transcriptional activity in WT mice but no marked difference was observed in the absence of endogenous FGF2. Rapid stimulation of β-catenin induced by PTH injection was also observed in a rat model (Wan et al., 2008). We previously conducted in vitro mechanistic studies and reported a significant reduction of β-catenin mRNA and a marked decrease of β-catenin protein expression in Fgf2KO bone marrow stromal cell cultures compared to WT cultures (Fei et al., 2011a). Reduced Wnt/β-catenin signaling contributes to the decreased osteoblast differentiation and reduced bone formation in Fgf2KO mice. The potential mechanisms by which PTH regulates β-catenin involves Smad3 (Tobimatsu et al., 2006) and protein kinase A (Wan et al., 2008). We previously showed that GSK3β is involved in FGF2 modulation of Wnt/β-catenin signaling (Fei et al., 2011a). We propose that increased β-catenin signaling promotes bone formation, while the attenuated Wnt/β-catenin signaling leads to the impaired bone anabolic response to PTH in Fgf2KO mice.
Although in vivo studies have established an essential role of FGF2 and PTH in bone formation, the mechanism of FGF2 and PTH action in OBs appears to be complex, involving direct osteoblast-intrinsic effects and indirect effects of PTH on other cells/tissues. Our in vitro study using calvarial osteoblast isolated from WT and Fgf2KO mice showed that endogenous FGF2 is important in PTH effects on OB proliferation, differentiation and apoptosis (Sabbieti et al., 2009), that PTH increased Wnt/β-catenin transcriptional activity significantly in WT but the increase was markedly attenuated in Fgf2KO mice, indicating that the anabolic PTH effect is dependent, at least in part on FGF2 expression in OBs. However, it is possible that PTH could act on other cells/tissues to produce intermediates that could then be responsible for some of the effects observed in the current study since acute response of serum calcium and phosphate level to PTH has been observed (Okazaki et al., 2008) and PTH is known to regulate bone formation through it's effect on other cells rather than OBs. It is known that PTH regulates serum calcium through its effects on bone, kidney, and the intestine; reduces the reabsorption of phosphate from the proximal tubule of the kidney; and increases the activity of 1‑α‑hydroxylase enzyme, which converts 25‑hydroxycholecalciferol, the major circulating form of inactive vitamin D, into 1,25‑dihydroxycholecalciferol, the active form of vitamin D, in the kidney.
One of the limitation of the study is that most of the in vivo data is relative to female mice. However, our previously published work showed impaired bone anabolic response to 4-week PTH treatment in both male and female (Hurley et al., 2006). In addition, using BMSCs from male mice we showed that the impaired bone anabolic effect of PTH in the absence of endogenous FGF2 is partially due to reduced ATF4 expression (Fei et al., 2011b). These studies suggest that endogenous FGF2 is critically important in PTH anabolic effects on bone.
In summary, data in the present study support our hypothesis that the impaired anabolic response to PTH in the absence of endogenous FGF2 is due in part to attenuated Wnt signaling. Multiple components of the Wnt signaling including Wnt10b, DKK2, SOST, Lrp6, GSK3β and β-catenin are altered by FGF2 deficiency. Maximal anabolic response of PTH on bone requires both FGF2 and Wnt signaling (Fei and Hurley, 2012). As the only anabolic agent for osteoporosis treatment available in the US, PTH has been in clinical use for >10 years (Capriani et al., 2012). Since PTH treatment has high cost, safety concerns and other limitations, there remains a clinical need to develop better bone anabolic agents. Both FGF2 and Wnt/β-catenin signaling are under active clinical study. Particularly, FGF2 stimulates periodontal regeneration (Kitamura et al., 2011) and accelerates healing of tibial shaft fractures (Kawaguchi et al., 2010) in patients. Although SOST antibody is a very promising new drug to promote bone formation and it is already in clinical trial phase 3 program (ClinicalTrials.gov, Accessed at July 2013), our current study of PTH crosstalk with FGF2 and Wnt signaling may contribute to new therapeutic strategies for skeletal diseases with low bone formation.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Transparency document
Transparency document.
Acknowledgements
This study was supported in part by the National Institutes of Health (NIH/NIAMS, 9R01AR072985-05A1).
Footnotes
The Transparency document associated with this article can be found, in online version.
References
- Ai M., Holmen S.L., Van Hul W., Williams B.O., Warman M.L. Reduced affinity to and inhibition by DKK1 form a common mechanism by which high bone mass-associated missense mutations in LRP5 affect canonical Wnt signaling. Mol. Cell. Biol. 2005;25:4946–4955. doi: 10.1128/MCB.25.12.4946-4955.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellido T., Ali A.A., Gubrij I., Plotkin L.I., Fu Q., O'Brien C.A., Manolagas S.C., Jilka R.L. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology. 2005;146:4577–4583. doi: 10.1210/en.2005-0239. [DOI] [PubMed] [Google Scholar]
- Bennett C.N., L. K., Wright W.S., Suva L.J., Lane T.F., Hankenson K.D., OA MacDougald. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc. Natl. Acad. Sci. U. S. A. 2005;102:3324–3329. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C.N., O. H., Ma Y.L., Zeng Q., Gerin I., Sousa K.M., Lane T.F., Krishnan V., Hankenson K.D., MacDougald O.A. Wnt10b increases postnatal bone formation by enhancing osteoblast differentiation. J. Bone Miner. Res. 2007;22:1924–1932. doi: 10.1359/jbmr.070810. [DOI] [PubMed] [Google Scholar]
- Bovolenta P., Esteve P., Ruiz J.M., Cisneros E., Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J. Cell Sci. 2008;121:737–746. doi: 10.1242/jcs.026096. [DOI] [PubMed] [Google Scholar]
- Boyden L.M., Mao J., Belsky J., Mitzner L., Farhi A., Mitnick M.A., Wu D., Insogna K., Rp L. High bone density due to a mutation in LDL-receptor-related protein 5. N. Engl. J. Med. 2002;346:1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- Capriani C., Irani D., Bilezikian J.P. Safety of osteoanabolic therapy: a decade of experience. J. Bone Miner. Res. 2012;27:2419–2428. doi: 10.1002/jbmr.1800. [DOI] [PubMed] [Google Scholar]
- Cawthorn W.P., Bree A.J., Yao Y., Du B., Hemati N., Martinez-Santibañez G., MacDougald O.A. Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a β-catenin-dependent mechanism. Bone. 2012;50:477–489. doi: 10.1016/j.bone.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro M.N., Schwartz D.R., Vonica A., Brivanlou A.H., Cho K.R., Varmus H.E. FGF-20 and DKK1 are transcriptional targets of beta-catenin and FGF-20 is implicated in cancer and development. EMBO J. 2005;24:73–84. doi: 10.1038/sj.emboj.7600460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choung M.-G., Baek I.-Y., Kang S.-T., Han W.-Y., Shin D.-C., Moon H.-P., Kang K.-H. Isolation and determination of anthocyanins in seed coats of black soybean (Glycine max (L.) Merr.) J. Agric. Food Chem. 2001;49:5848–5851. doi: 10.1021/jf010550w. ( ClinicalTrials.gov. Accessed at July 2013. http://clinicaltrials.gov/) [DOI] [PubMed] [Google Scholar]
- Fei Y., Hurley M.M. Role of fibroblast growth factor 2 and wnt signaling in anabolic effects of parathyroid hormone on bone formation. J. Cell. Physiol. 2012;227:3539–3545. doi: 10.1002/jcp.24075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei Y., Xiao L., Doetschman T., Coffin D.J., Hurley M.M. Fibroblast growth factor 2 stimulation of osteoblast differentiation and bone formation is mediated by modulation of the Wnt signaling pathway. J. Biol. Chem. 2011;286:40575–40583. doi: 10.1074/jbc.M111.274910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei Y., Xiao L., Hurley M.M. The impaired bone anabolic effect of PTH in the absence of endogenous FGF2 is partially due to reduced ATF4 expression. Biochem. Biophys. Res. Commun. 2011;412:160–164. doi: 10.1016/j.bbrc.2011.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel S., Chin E.N., Fakhraldeen S.A., Berry S.M., Beebe D.J., Alexander C.M. Both LRP5 and LRP6 receptors are required to respond to physiological Wnt ligands in mammary epithelial cells and fibroblasts. J. Biol. Chem. 2012;287:16454–16466. doi: 10.1074/jbc.M112.362137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y., Slee R.B., Fukai N., Rawadi G., Roman-Roman S., Reginato A.M., Wang H., Cundy T., Glorieux F.H., Lev D., Zacharin M., Oexle K., Marcelino J., Suwairi W., Heeger S., Sabatakos G., Apte S., Adkins W.N., Allgrove J., Arslan-Kirchner M., Batch J.A., Beighton P., Black G.C.M., Boles R.G., Boon L.M., Borrone C., Brunner H.G., Carle G.F., Dallapiccola B., De Paepe A., Floege B., Halfhide M.L., Hall B., Hennekam R.C., Hirose T., Jans A., Jüppner H., Kim C.A., Keppler-Noreuil K., Kohlschuetter A., Lacombe D., Lambert M., Lemyre E., Letteboer T., Peltonen L., Ramesar R.S., Romanengo M., Somer H., Steichen-Gersdorf E., Steinmann B., Sullivan B., Superti-Furga A., Swoboda W., van den Boogaard M.-J., Van Hul W., Vikkula M., Votruba M., Zabel B., Garcia T., Baron R., Olsen B.R., Warman M.L. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- Holroyd C., Cooper C., Dennison E. Epidemiology of osteoporosis. Best Pract. Res. Clin. Endocrinol. Metab. 2008;22:671–685. doi: 10.1016/j.beem.2008.06.001. (Osteoporosis) [DOI] [PubMed] [Google Scholar]
- Hurley M., Tetradis S., Huang Y.F., Hock J., Kream B.E., Raisz L.G., S. M.G. Parathyroid hormone regulates the expression of fibroblast growth factor-2 mRNA and fibroblast growth factor receptor mRNA in osteoblastic cells. J. Bone Miner. Res. 1999;14:776–783. doi: 10.1359/jbmr.1999.14.5.776. [DOI] [PubMed] [Google Scholar]
- Hurley M., Marie P., Florkiewics R.Z. Fibroblast growth factor and fibroblast growth factor receptor families. In: Bilezikian J.P., Raisz L.G., Rodan G., editors. Principles of Bone Biology. Academic Press; San Diego: 2002. pp. 627–645. [Google Scholar]
- Hurley M., Yao W., Ne L. Changes in serum fibroblast growth factor 2 in patients with glucocorticoid-induced osteoporosis treated with human parathyroid hormone (1–34) Osteoporos. Int. 2005;16:2080–2084. doi: 10.1007/s00198-005-1998-x. [DOI] [PubMed] [Google Scholar]
- Hurley M.M., Okada Y., Xiao L., Tanaka Y., Ito M., Okimoto N., Nakamura T., Rosen C.J., Doetschman T., Coffin J.D. Impaired bone anabolic response to parathyroid hormone in Fgf2−/− and Fgf2+/− mice. Biochem. Biophys. Res. Commun. 2006;341:989–994. doi: 10.1016/j.bbrc.2006.01.044. [DOI] [PubMed] [Google Scholar]
- Hurley M.M., Gronowicz G., Zhu L., Kuhn L.T., Rodner C., Xiao L. Age-related changes in FGF-2, fibroblast growth factor receptors and β-catenin expression in human mesenchyme-derived progenitor cells. J. Cell. Biochem. 2016;117(3):721–729. doi: 10.1002/jcb.25357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaniec U.T., Wronski T.J., Liu J., Rivera M.F., Arzaga R.R., Hansen G., Brommage R. PTH stimulates bone formation in mice deficient in Lrp5. J. Bone Miner. Res. 2007;22:394–402. doi: 10.1359/jbmr.061118. [DOI] [PubMed] [Google Scholar]
- Jilka R.L. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40:1434–1446. doi: 10.1016/j.bone.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya N., Ye L., Kobayashi T., Mochida Y., Yamauchi M., Kronenberg H.M., Feng J.Q., Mishina Y. BMP signaling negatively regulates bone mass through sclerostin by inhibiting the canonical Wnt pathway. Development. 2008;135:3801–3811. doi: 10.1242/dev.025825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi H., Oka H., Jingushi S., Izumi T., Fukunaga M., Sato K., Matsushita T., N. K., T. Group A local application of recombinant human fibroblast growth factor 2 for tibial shaft fractures: a randomized, placebo-controlled trial. J. Bone Miner. Res. 2010;25:2459–2467. doi: 10.1002/jbmr.146. [DOI] [PubMed] [Google Scholar]
- Kawano Y., Kypta R. Secreted antagonists of the Wnt signalling pathway. J. Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- Keller H., Kneissel M. SOST is a target gene for PTH in bone. Bone. 2005;37:148–158. doi: 10.1016/j.bone.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Kitamura M., Akamatsu M., Machigashira M., Hara Y., Sakagami R., Hirofuji T., Hamachi T., Maeda K., Yokota M., Kido J., Nagata T., Kurihara H., Takashiba S., Sibutani T., Fukuda M., Noguchi T., Yamazaki K., Yoshie H., Ioroi K., Arai T., Nakagawa T., Ito K., Oda S., Izumi Y., Ogata Y., Yamada S., Shimauchi H., Kunimatsu K., Kawanami M., Fujii T., Furuichi Y., Furuuchi T., Sasano T., Imai E., Omae M., Watanuki M., Murakami S. FGF-2 stimulates periodontal regeneration. J. Dent. Res. 2011;90:35–40. doi: 10.1177/0022034510384616. [DOI] [PubMed] [Google Scholar]
- Korinek V., Barker N., Morin P.J., van Wichen D., de Weger R., Kinzler K.W., Vogelstein B., Clevers H. Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Kulkarni N.H., Halladay D.L., Miles R.R., Gilbert L.M., Frolik C.A., Galvin R.J.S., Martin T.J., Gillespie M.T., Onyia J.E. Effects of parathyroid hormone on Wnt signaling pathway in bone. J. Cell. Biochem. 2005;95:1178–1190. doi: 10.1002/jcb.20506. [DOI] [PubMed] [Google Scholar]
- Li X., Liu P., Liu W., Maye P., Zhang J., Zhang Y., Hurley M., Guo C., Boskey A., Sun L., Harris S.E., Rowe D.W., Ke H.Z., Wu D. Dkk2 has a role in terminal osteoblast differentiation and mineralized matrix formation. Nat. Genet. 2005;37:945–952. doi: 10.1038/ng1614. [DOI] [PubMed] [Google Scholar]
- Li X., Liu H., Qin L., Tamasi J., Bergenstock M., Shapses S., Feyen J.H.M., Notterman D.A., Partridge N.C. Determination of dual effects of parathyroid hormone on skeletal gene expression in vivo by microarray and network analysis. J. Biol. Chem. 2007;282:33086–33097. doi: 10.1074/jbc.M705194200. [DOI] [PubMed] [Google Scholar]
- Mani A., Radhakrishnan J., Wang H., Mani A., Mani M.-A., Nelson-Williams C., Carew K.S., Mane S., Najmabadi H., Wu D., Lifton R.P. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007;315:1278–1282. doi: 10.1126/science.1136370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min J.-K., Park H., Choi H.-J., Kim Y., Pyun B.-J., Agrawal V., Song B.-W., Jeon J., Maeng Y.-S., Rho S.-S., Shim S., Chai J.-H., Koo B.-K., Hong H.J., Yun C.-O., Choi C., Kim Y.-M., Hwang K.-C., Kwon Y.-G. The WNT antagonist Dickkopf2 promotes angiogenesis in rodent and human endothelial cells. J. Clin. Invest. 2011;121:1882–1893. doi: 10.1172/JCI42556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero A., Okada Y., Tomita M., Ito M., Tsurukami H., Nakamura T., Doetschman T., Coffin J.D., Hurley M.M. Disruption of the fibroblast growth factor-2 gene results in decreased bone mass and bone formation. J. Clin. Invest. 2000;105:1085–1093. doi: 10.1172/JCI8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOF . National Osteoporosis Foundation; 2013. Fast Facts on Osteoporosis.http://www.nof.org/node/40 [Google Scholar]
- Okazaki M., Ferrandon S., Vilardaga J.P., Bouxsein M.L., Potts J.T., Jr., Gardella T.J. Prolonged signaling at the parathyroid hormone receptor by peptide ligands targeted to a specific receptor conformation. Proc. Natl. Acad. Sci. U. S. A. 2008;(43):16525–16530. doi: 10.1073/pnas.0808750105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyia J.E., H. L., Gelbert L., Wei T., Huang S., Chen P., Dow E.R., Maran A., Zhang M., Lotinun S., Lin X., Halladay D.L., Miles R.R., Kulkarni N.H., Ambrose E.M., Ma Y.L., Frolik C.A., Sato M., Bryant H.U., Turner R.T. Molecular profile of catabolic versus anabolic treatment regimens of parathyroid hormone (PTH) in rat bone: an analysis by DNA microarray. J. Cell. Biochem. 2005;95:403–418. doi: 10.1002/jcb.20438. [DOI] [PubMed] [Google Scholar]
- Ornitz D.M., Marie P.J. Fibroblast growth factor signaling in skeletal development and disease. Genes Dev. 2015;29(14):1463–1486. doi: 10.1101/gad.266551.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29 doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piters E., Boudin E., Van Hul W. Wnt signaling: a win for bone. Arch. Biochem. Biophys. 2008;473:112–116. doi: 10.1016/j.abb.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Prisby R., Guignandon A., Vanden-Bossche A., Mac-Way F., Linossier M.-T., Thomas M., Laroche N., Malaval L., Langer M., Peter Z.-A., Peyrin F., Vico L., Lafage-Proust M.-H. Intermittent PTH(1–84) is osteoanabolic but not osteoangiogenic and relocates bone marrow blood vessels closer to bone-forming sites. J. Bone Miner. Res. 2011;26:2583–2596. doi: 10.1002/jbmr.459. [DOI] [PubMed] [Google Scholar]
- Qin L., Qiu P., Wang L., Li X., Swarthout J.T., Soteropoulos P., Tolias P., Partridge N.C. Gene expression profiles and transcription factors involved in parathyroid hormone signaling in osteoblasts revealed by microarray and bioinformatics. J. Biol. Chem. 2003;278:19723–19731. doi: 10.1074/jbc.M212226200. [DOI] [PubMed] [Google Scholar]
- Sabbieti M.G., A. D., Xiao L., Marchetti L., Coffin J.D., Doetschman T., Hurley M.M. Endogenous FGF-2 is critically important in PTH anabolic effects on bone. J. Cell. Physiol. 2009;219:143–151. doi: 10.1002/jcp.21661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidak Z., Le Henaff C., Azzi S., Marty C., Marie P.J. Low-dose PTH increases osteoblast activity via decreased Mef2c/Sost in senescent osteopenic mice. J. Endocrinol. 2014;223:25–33. doi: 10.1530/JOE-14-0249. [DOI] [PubMed] [Google Scholar]
- Sawakami K., Robling A.G., Ai M., Pitner N.D., Liu D., Warden S.J., Li J., Maye P., Rowe D.W., Duncan R.L., Warman M.L., Turner C.H. The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J. Biol. Chem. 2006;281:23698–23711. doi: 10.1074/jbc.M601000200. [DOI] [PubMed] [Google Scholar]
- Stevens J.R., Miranda-Carboni G.A., Singer M.A., Brugger S.M., Lyons K.M., Lane T.F. Wnt10b deficiency results in age-dependent loss of bone mass and progressive reduction of mesenchymal progenitor cells. J. Bone Miner. Res. 2010;25:2138–2147. doi: 10.1002/jbmr.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai K., Zeng X., Liu C., Zhang X., Harada Y., Chang Z., He X. A mechanism for Wnt coreceptor activation. Mol. Cell. 2004;13:149–156. doi: 10.1016/s1097-2765(03)00484-2. [DOI] [PubMed] [Google Scholar]
- Tobimatsu T., Kaji H., Sowa H., Naito J., Canaff L., Hendy G.N., Sugimoto T., Chihara K. Parathyroid hormone increases β-catenin levels through Smad3 in mouse osteoblastic cells. Endocrinology. 2006;147:2583–2590. doi: 10.1210/en.2005-1627. [DOI] [PubMed] [Google Scholar]
- Towler D.A. Skeletal anabolism, PTH, and the bone–vascular axis. J. Bone Miner. Res. 2011;26:2579–2582. doi: 10.1002/jbmr.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wesenbeeck L., C. E., Gram J., Beals R.K., Bénichou O., Scopelliti D., Key L., Renton T., Bartels C., Gong Y., Warman M.L., De Vernejoul M.C., Bollerslev J., Van Hul W. Six novel missense mutations in the LDL receptor-related protein 5 (LRP5) gene in different conditions with an increased bone density. Am. J. Hum. Genet. 2003;72:763–771. doi: 10.1086/368277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan M., Yang C., Li J., Wu X., Yuan H., Ma H., He X., Nie S., Chang C., Cao X. Parathyroid hormone signaling through low-density lipoprotein-related protein 6. Genes Dev. 2008;22:2968–2979. doi: 10.1101/gad.1702708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L.P., Liu P., Li X.F., Doetschman T., Coffin J.D., Drissi H., Hurley M.M. Exported 18-kDa isoform of fibroblast growth factor-2 is a critical determinant of bone mass in mice. J. Biol. Chem. 2009;284:3170–3182. doi: 10.1074/jbc.M804900200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L., E. A., Sobue T., Kronenberg M.S., Coffin J.D., Doetschman T., Hurley M.M. Disruption of the Fgf2 gene activates the adipogenic and suppresses the osteogenic program in mesenchymal marrow stromal stem cells. Bone. 2010;47:360–370. doi: 10.1016/j.bone.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M., S. R., Paul R.J., Lorenz J.N., Hoying J.B., Haudenschild C.C., Yin M., Coffin J.D., Kong L., Kranias E.G., Luo W., Boivin G.P., Duffy J.J., Pawlowski S.A., Doetschman T. Fibroblast growth factor 2 control of vascular tone. Nat. Med. 1998;4:201–207. doi: 10.1038/nm0298-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.