Abstract
Background:
Data on new-onset seizures after treatment of aneurysmal subarachnoid hemorrhage (aSAH) patients are limited and variable. We examined the association between new-onset seizures after aSAH and aneurysm treatment modality, as well their relationship with initial clinical severity of aSAH and outcomes.
Methods:
This is a retrospective cohort study of all aSAH patients admitted to our institution over a 6-year period. ‘Seizures’ were defined as any observed clinical seizure, or electrographic seizure on continuous electric encephalography (cEEG) recordings, as determined by the reviewing neurophysiologist. Subgroup analyses were performed in low-grade (Hunt Hess 1–3) and high-grade (Hunt Hess 4–5) patients. Outcomes measures were Glasgow Coma Score (GCS) at ICU discharge and modified Rankin Scale (mRS) at outpatient follow-up.
Results:
There were 282 patients with aSAH; 203 (72.0%) suffered low-grade and 79 (28%) high-grade aSAH. Patients were treated with endovascular coiling (N=194, 68.8%) or surgical clipping (N=66, 23.4%). Eighteen (6.4%) patients had seizures, of whom 10 (5.5%) had aneurysm coiling and 7 (10.6%) underwent clipping (p=0.15). In low-grade patients, seizures occurred less frequently (p=0.016) and were more common after surgical clipping (p=0.0089). Seizures correlated with lower GCS upon ICU discharge (p<0.001), in clipped (p=0.011) and coiled (p<0.001) patients, and in low-grade aSAH (p<0.001). Seizures correlated with higher mRS on follow-up (p<0.001), in clipped (p=0.032) and coiled (p=0.004) patients, and in low-grade aSAH (p=0.003).
Conclusions:
New onset seizures after aSAH occurred infrequently, and their incidence after aneurysm clipping versus coiling was not significantly different. However, in low-grade patients, new seizures were more frequently associated with clipping than coiling. Additionally, non-convulsive seizures did not occur in low-grade patients treated with coiling. These findings may explain, in part, previous work suggesting better outcomes in coiled patients, and encourage physicians to have a lower threshold for cEEG utilization in low-grade patients suspected to have acute seizures after surgical clipping.
Keywords: Seizures, aneurysmal subarachnoid hemorrhage, clipping, coiling
Introduction
Non-traumatic subarachnoid hemorrhage (SAH) occurs in roughly 30,000 people per year in the USA. Approximately 85% of these are secondary to ruptured intracranial aneurysms at an annual incidence of roughly 9 per 100,000 people (1), and account for 3–5% of strokes annually. While mortality secondary to aneurysmal subarachnoid hemorrhage (aSAH) has decreased from 30% to 20% over the last 3 decades (2), it remains a significant cause of long-term disability, healthcare spending and lost productivity (3). Survivors suffer cognitive, behavioral and social impairments and perform poorly on neuropsychological testing (4, 5).
Seizures and epilepsy are a well-known consequence of aSAH and have been associated with poor functional outcomes (6). However, reports on the incidence of acute seizures after aSAH are highly variable, ranging from 6% to 26% (7–12), and limited data exists on the occurrence of acute seizures in patients treated with endovascular coiling versus surgical clipping of aneurysm after aSAH. Importantly, studies showed high variability in incidence of seizures ranging from 0.7% to 10.7% for endovascular coiling and from 4.95 to 21.2% for surgical clipping. The large disparity in reported seizure incidence is based on variability in the methodology of seizure reporting, i.e. reported seizures, witnessed clinical seizures, electrographic seizures captured on continuous video electroencephalography (cEEG) and billing code searches.
In this study, our aim was three-fold: one, to examine the incidence of all new acute onset seizures in patients with aSAH admitted to our institution; two, to compare seizure incidence by clinical grade with modality of aneurysm treatment, viz. endovascular coiling and surgical clipping; and three, to study the relationship between seizures and SAH outcomes. Of note, ‘seizures’ were defined as any clinical seizure noted in a physician daily progress report or an electrographic seizure documented in a physician’s official report on cEEG. Whether a patient was subsequently diagnosed with epilepsy is not examined.
Methods
Subjects
We reviewed data on all adult (18 years of age or older) patients with SAH admitted to the Neurosciences Intensive Care Unit (Neuro-ICU) of Weill Cornell Medical Center/New York Presbyterian Hospital between January 1, 2010 and October 31, 2015. Patients were included only if they had a confirmed aneurysmal source of SAH and were treated with either endovascular coiling or surgical clipping. Patients with untreated aneurysmal SAH, spontaneous non-aneurysmal SAH, and SAH secondary to trauma, arteriovenous malformation rupture, vasculitis, or any other structural lesion or unknown cause were excluded from the study.
Clinical Management
All patients were admitted to the Neurosciences intensive care unit (neuro-ICU). Patients underwent CTA on admission to identify aneurysm(s), location(s) and morphology. Aneurysms were either surgically clipped or endovascularly coiled based on their location, size, morphology and consensus between neurosurgeon and endovascular surgeon and discussion with the patient and/or the surrogate decision maker. Patients remained in the ICU for at least 14 days for monitoring and were treated according to standard clinical treatment protocols based on American Heart Association/American Stroke Association (AHA/ASA) guidelines (8). This included treatment with nimodipine, and treatment for delayed cerebral ischemia (DCI) with hypertensive hyperdynamic therapy as indicated. Intra-arterial calcium channel antagonists, angioplasty and stenting were used to treat refractory vasospasm. Hydrocephalus was treated with ventriculostomy, if needed. All SAH patients with unexplained altered or fluctuating mental status (defined as GCS≤13) or suspected seizures were monitored with cEEG for 24–48 hours regardless of hemorrhagic grade. Prior to definitive aneurysm treatment, seizure prophylaxis with levetiracetam or phenytoin was routinely used and continued at the discretion of the treating physician.
cEEG Recordings
cEEG was recorded digitally using 21 electrodes placed per the international 10–20 system. This was performed using an XLTEK 32 channel computerized video EEG system (Natus Medical Incorporated, Pleasanton, CA) with digital analysis of EEG for spike detections, topographic and occurrence analysis, computerized seizure detection algorithms, compressed spectral array for quantification of EEG frequency content and a patient event marker. Electrodes over surgical sites may have been omitted on a case-by-case basis. Digital video was included with all records and available to screen for clinical correlations for detected seizures. Recordings were interpreted by a board-certified electroencephalographer and were reviewed periodically. EEG reports were written once daily, and the clinical team was updated over the course of the day when seizures were detected. The presence of any seizure recorded on cEEG was recorded for our analysis, including electroclinical seizures, non-convulsive electrographic seizures, convulsive and non-convulsive status epilepticus.
Clinical Variables
All medical records were reviewed retrospectively using two-person review. Demographic data and Hunt Hess scale (HH) upon admission were collected for all patients. We defined low-grade SAH as HH score 1–3 and high-grade SAH as HH score 4–5. Daily clinical notes were also reviewed for reported seizure events from periods when cEEG recording was not being performed, and these are recorded in this study as clinical seizures. Outcome measures included Glasgow Coma Scale score (GCS) on discharge from neuro-ICU, in-hospital mortality, and modified Rankin Scale (mRS) computed at a single time point using a two-person review based upon outpatient clinical notes at 6-month follow-up.
Statistical Analysis
Characteristics of study patients are described using the mean (SD) for continuous variables and frequencies (percentages) for categorical variables. Chi-Square statistics were computed for all categorical variables (Fisher’s test was used when appropriate). Continuous variables were analyzed using the Wilcoxon Rank Sum test. Significance was defined as a p-value<0.05. Only two-sided probabilities were utilized for assessing significance in both Fisher’s and Wilcoxon Rank Sum tests.
Results
Patient Characteristics
A total of 312 patients were admitted with a diagnosis of SAH to the neuro-ICU at our institution between January 1, 2010 and October 31, 2015 (Figure 1). Thirty patients with non-aneurysmal SAH were excluded. The mean age of patients was 52.7 (SD: 14.7) years, and 183 (64.9%) were female. Within our aSAH population, 194 (68.8%) patients were treated by endovascular coiling and 66 (23.4%) by surgical clipping; 22 patients were neither coiled nor clipped (19 untreated, 1 wrapped, 1 bypassed, and 1 sacrificed parent vessel) due to aneurysm morphology, comorbid condition and or family preference for no aggressive intervention. These patients were included in the overall analysis, but not in the group comparisons.
Fig 1.
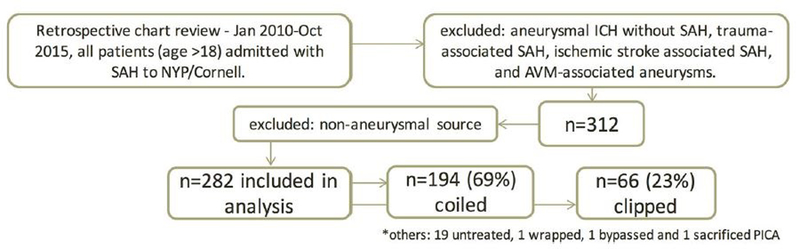
Flow diagram showing the selection of patients for study
Seizure Occurrence
cEEG monitoring was performed on 115 (40%) patients for suspicion of seizures. Twenty four (8.5%) patients had seizures, 18 detected during cEEG monitoring and 6 based on clinical reports. In those patients found to have seizure on cEEG, 11 (3.9%) had non-convulsive seizures, 7 (2.4%) of whom had non-convulsive status epilepticus. In addition 4 (1.4%) patients monitored with cEEG suffered convulsive status epilepticus. There were 6 patients who had clinically reported seizures at presentation, none of whom had further clinical seizures reported or seizures recorded on cEEG in the neuro-ICU. These 6 patients were not included in the sub-group analyses of seizures by treatment modality as these seizures occurred prior to treatment. Of the remaining 18 patients, 10 had undergone endovascular coiling, 7 had surgical clipping, and 1 patient was not treated. Of note, this single patient was not included in sub-group analyses.
Seizures by severity
Based on Hunt Hess classification, 203 (72.0%) patients suffered low grade and 79 (28.0%) suffered high grade aSAH (Table 1). Seizures occurred less frequently in low grade than in high grade patients (p = 0.016). Only 1.4% of low-grade aSAH patients had seizures and seizure occurrence was significantly higher (p = 0.009) in patients treated with surgical clipping compared to those treated with endovascular coiling (Table 2). There was not a significant difference in seizure occurrence overall or in high-grade aSAH patients beween the two treatment strategies. There was no statistical association between seizure occurrence and modified Fisher score in either clinical grade comparison or aneurysm treatment group.
Table 1.
Demographics and clinical characteristics of study population
| Overall | Clipped | Coiled | |
|---|---|---|---|
| N=282 | N=66 | N=194 | |
| Age1 | 52.7 (14.7) |
46 (12.8) |
55 (14.0) |
| N (%) | N (%) | N (%) | |
| Female | 183 (64.9) | 40 (60.6) | 131 (67.5) |
|
Hunt Hess Grade |
|||
| 1 | 36 (12.8) | 11 (16.7) | 23 (11.9) |
| 2 | 99 (35.1) | 31 (47.0) | 66 (34.0) |
| 3 | 68 (24.1) | 16 (24.2) | 50 (25.8) |
| 4 | 57 (20.2) | 7 (10.6) | 46 (23.7) |
| 5 | 22 (7.8) | 1 (1.5) | 9 (4.6) |
| mFS | |||
| 0 | 2 (0.7) | 0 (0.0) | 0 (0.0) |
| 1 | 22 (7.8) | 9 (13.6) | 12 (6.2) |
| 2 | 32 (11.4) | 9 (13.6) | 21 (10.8) |
| 3 | 106 (37.6) | 29 (43.9) | 72 (37.1) |
| 4 |
119 (42.2) |
18 (27.3) |
87 (44.8) |
| Treatment Modality | 282 | 66 (23.4) | 194 (68.8) |
|
Risk Factors |
|||
| Vasospasm | 167 (59.2) | 47 (71.2) | 113 (58.2) |
| Ventriculitis | 8 (2.8) | 1 (1.5) | 7 (3.6) |
| Infarction | 94 (33.3) | 26 (39.4) | 57 (29.4) |
| cEEG Utilization | 167 (40.8) | 31 (47.0) | 76 (39.2) |
[Mean (SD)]
Table 2.
Seizure occurrence by Hunt Hess grade and modality of aneurysm treatment.
| Overall N(%) |
Clipped N(%) |
Coiled N(%) |
||
|---|---|---|---|---|
| Overall | 18 (6.4) | 7 (10.6) | 10 (5.5) | p=0.150 |
| HH1–3 | 8 (3.9) | 6 (10.3) | 2 (1.4) | p=0.009 |
| HH4–5 | 10 (12.7)¶ | 1 (12.5) | 8 (14.6) | p=1.000 |
(comparison between HH 1–3 and 4–5 in overall group, p=0.016)
Seizures by secondary insults
The incidence of vasospasm, infarction on imaging, and ventriculitis were also examined for association with seizure occurrence. Infarction on imaging was noted in 33% of all patients, 24% of low-grade patients and 57% of high-grade patients; 29% of patients who underwent coiling and 39% of those undergoing clipping had infarcts. There was a significant association of seizure occurrence with the presence of infarction on imaging in the clipping group (p = 0.013), the coiling group (p = 0.008), and in low-grade aSAH overall (p < 0.001). Seizures occurred more frequently in patients with infarcts in clipping group (33%) compared to coiling group (12%), however this did not reach significance (p = 0.056). No other signs of secondary injury were found to be associated with seizure incidence. (Table 3)
Table 3.
Seizure occurrence and secondary brain injury by modality of aneurysm treatment
| Seizure incidence |
Clipping N (%) |
Coiling N (%) |
|---|---|---|
|
with vasospasm |
6 (12.8) | 6 (5.3) |
|
without vasospasm |
1 (5.3) | 4 (4.9) |
|
with infarct |
6 (23.1)* | 7 (12.3)* |
|
without infarct |
1 (2.5) | 3 (2.2) |
|
with ventriculitis |
0 (0.0) | 0 (0.0) |
|
without ventriculitis |
7 (10.8) | 10 (5.3) |
(p<0.05)
Seizures and Outcome
Mean GCS on discharge from ICU was significantly lower in patients with seizures overall (p < 0.001), in the clipping group (p = 0.0108), in the coiling group (p < 0.001) and in the low-grade aSAH group (p < 0.001). Follow-up visits were available for 244 (86%) patients formRS abstraction at an average of 4.1 months after SAH; the remaining 38 (14%) patients died prior to discharge from the ICU. mRS was 0–3 for 198 (70%) patients and 4–6 for 84 (29%) patients. Seizures were associated with significantly worse mRS on follow-up overall (p < 0.001), in the clipping group (p = 0.032), in the coiled group (p = 0.004) and in the low-grade aSAH group (p = 0.003). Additionally, the presence of clinical or non-convulsive status epilepticus was associated with worse mRS on follow-up (p = 0.04). (Table 4)
Table 4.
Outcome analysis by Hunt Hess grade and modality of aneurysm treatment
| All |
Clipping |
Coiling |
|
|---|---|---|---|
| GCS on discharge | Mean (SD) |
Mean (SD) |
Mean (SD) |
| Seizure | 8.11 (4.96) | 9.86 (5.30) | 7.0 (4.88) |
| No seizure | 12.76 (4.12) | 13.61 (3.22) | 13.27 (3.50) |
| Wilcoxon Probability |
p<0.001 |
p=0.011 |
p<0.001 |
| Hunt Hess grade | |||
| HH1–3 | HH4–5 | ||
| GCS on discharge | Mean (SD) | Mean (SD) | |
| Seizure | 8.38 (5.88) | 7.90 (4.41) | |
| No seizure | 14.02 (2.80) | 9.19 (5.08) | |
| Wilcoxon Probability |
p<0.001 |
p=0.401 |
|
| mRS on Follow-up | |||
| 0–3 | 4–6 | ||
| Overall | N (%) | N (%) | |
| Seizure | 6 (33.3) | 12 (66.7) | |
| No seizure | 192 (72.7) | 72 (27.3) | p<0.001 |
| Clipped | |||
| Seizure | 3 (42.9) | 4 (57.1) | |
| No seizure | 49 (83.0) | 10 (17.0) | p=0.032 |
| Coiled | |||
| Seizure | 3 (30.0) | 7 (70.0) | |
| No seizure | 139 (75.5) | 45 (24.5) | p=0.004 |
| HH 1–3 | 172 (84.7) | 31 (15.3) | |
| HH 4–5 | 26 (32.9) | 53 (67.1) | p<0.001 |
| HH 1–3 w seizure | 3 (37.5) | 5 (62.5) | |
| HH 1–3 w/o seizure | 169 (86.7) |
26 (13.3) |
p=0.003 |
| HH 4–5 w seizure | 3 (30.0) | 7 (70.0) | |
| HH 4–5 w/o seizure | 23 (33.3) | 46 (66.7) | p=1.000 |
| Seizure w/o SE | 5 (50.0) | 5 (50.0) | |
| SE/NCSE | 0 (0.0) | 7 (100.0) | p=0.044 |
Discussion
The results of our study did not demonstrate a significant difference in acute new onset seizures after aneurysm clipping versus coiling. However, new onset seizures occurred more commonly in low-grade aSAH patients after surgical clipping than after endovascular coiling and were associated with greater morbidity. Seizure incidence was similar in higher-grade patients regardless of how aneurysm was secured, and higher in patients with evident infarction on imaging. These findings are from a single center and therefore not confounded by other treatment variables. Our data provide evidence that should be considered in addition to existing factors in deciding modality of aneurysm treatment, especially in low-grade SAH patients, in an effort to reduce subsequent morbidity.
Current data on acute new-onset seizure incidence after aSAH based on treatment modalities is limited, while data on long-term epilepsy exists. As surgical practice continues to change and more patients are treated with endovascular coiling over surgical clipping, it is increasingly important to understand the acute risks that may be associated with different treatment strategies.
In this single center retrospective study, we found several important associations between incidence of seizures and treatment modality of aneurysm in relation to SAH severity and outcomes. In-hospital seizures were associated with unfavorable outcomes at ICU discharge and were associated with worse functional outcome at follow-up in the entire study group, in both treatment groups individually and in low-grade aSAH patients. These findings are consistent with a recently published retrospective cohort analysis using the National Inpatient Sample database (13). An important novel finding of our study is that low-grade aSAH patients treated with surgical clipping suffered a greater incidence of acute new onset in-hospital seizures.
It is unclear how treatment modality of aSAH may affect the incidence of seizures in relation to functional outcomes. The primary mechanisms of aSAH, including delayed cerebral ischemia, re-bleeding, local and systemic electrolyte abnormalities, hydrocephalus, and the irritative effects of blood all increase the risk of seizures. In addition, some studies have shown that delayed cerebral ischemia is more likely to occur after surgical clipping than endovascular coiling (14). The incidence of seizures following a craniotomy in general has been reported between 8 and 18% but the potential risk factors such as surgical contusion, post-operative bleeding, and infection have not been individually quantified (15–19). One possible mechanism to explain increased seizures in clipped versus coiled patients was demonstrated by Wostrack et al, who found an increased incidence of hippocampal damage in those treated with open surgery, although this study examined neuropsychiatric changes rather than seizures (20). Conversely, it was also proposed that seizures themselves may contribute to secondary injuries as they may be linked to increased metabolic demand, increased heart rate, blood pressure, and cerebral perfusion pressure, and can in turn cause rises in intracranial pressure and delayed rise in regional cerebral blood flow. As Claassen et al demonstrated, if the increased metabolic demand caused by the seizures cannot be matched by compensatory mechanisms, it may lead to secondary neuronal injury. This in turn could explain worse outcomes in patients with seizures (21).
In contrast to our study, which focuses specifically on the question of seizures during the initial hospitalization, most studies have focused on long-term epilepsy in patients treated with surgical clipping. Claassen et al found a 7% incidence of post-SAH epilepsy after 1 year (6), and Olafsson et al found an 18, 23, and 25% risk of developing epilepsy at 1, 2, and 5 years, respectively (22). In both studies, epilepsy was associated with worse outcomes and persistent neurologic deficits. The first large study to focus on aSAH patients treated by endovascular coiling by Byrne et al, found that 11% of patients had ictal seizures, none had clinical post-procedural seizures while in hospital, and 1.7% self-reported new onset seizures more than 30 days after treatment (7). The study does not provide detail into how seizure occurrence was determined, and there is no discussion of the use of EEG in their patient population during hospitalization. In a secondary analysis from the International Subarachnoid Aneurysm Trial (ISAT) multi-center study that involved 2143 patients with aSAH, the frequency of seizures after surgical clipping and coil embolization was found to be 10.9% after randomization with a significant difference based on treatment modality: 8.3% had seizures after coil embolization and 13.6% after surgical clipping, and 6.4% of coiled and 9.6% of clipped patients had had a seizure at 5 years after discharge from the hospital (13).
Two additional studies focused on seizure or epilepsy occurrence in the setting of aSAH. Hoh et al used the Nationwide Inpatient Sample Database to examine the incidence of seizures or epilepsy after treatment for both ruptured and unruptured aneurysms (23). This study found no difference in seizure occurrence in the ruptured aneurysm group (10.7% for clipped and 11.1% for coiled), and found seizure or epilepsy to be associated with longer hospitalizations and higher cost. Finally, Huttunen et al examined outcomes in 876 patients from the Kuopio University Hospital in Finland (24). In this population, 3% and 5% of patients treated with endovascular coiling developed epilepsy at 1 and 5 years, respectively, compared to 10% and 15% of those treated with surgical clipping. Importantly, when examined with a multi-variate analysis, treatment modality was no longer significant, but Hunt-Hess grade III-V, presence of ICH >15 cm3 on admission, and presence of acute seizures were.
In addition, our data also has implications on cEEG utilization in low-grade aSAH patients. In patients undergoing coiling, the incidence of seizures is extremely low and non-clinical seizures were not seen in our dataset. Therefore, the utilization of cEEG could be restricted in such patients. In this specific population, clinical seizures could be treated when seen, without necessitating cEEG monitoring, which increases cost and length of stay without clear-cut diagnostic or therapeutic yield. However, in surgically treated low-grade patients, physicians should have a lower threshold for cEEG monitoring, as this group had a higher seizure incidence. There is a paucity of data to provide high level recommendations regarding the use of cEEG after aSAH. AHA/ASA guidelines do not provide specific recommendations for the use of cEEG monitoring in aSAH patients based on clinical severity (8), while the 2011 Neurocritical Care Society (NCS) guidelines makes a strong recommendation - with low-quality evidence - to consider cEEG monitoring in patients with high-grade SAH who “fail to improve or who have neurological deterioration of undetermined etiology” (25).
Our study has some limitations. It is a retrospective single center study. We may have underestimated the impact of treatment modality on seizure occurrence due to the low incidence of seizures overall, the low number of patients in the surgical clipping group and the propensity to treat high-grade patients with endovascular coiling. These factors may cause the study to have insufficient power. The indications for cEEG monitoring, beyond patients with GCS ≤ 13, were dependent on the attending physician and may have included a clinical bias. Additionally, given that cEEGs were interpreted by several different neurophysiologists, variation in applying objective criteria for electrographic seizure may have influenced the results.
In conclusion, our study provides data that new acute onset seizures in low-grade aSAH patients were more frequently associated with surgical clipping than endovascular coiling of aneurysms, whereas in high grade patients, seizure incidence was not significantly different between the two treatments. While overall seizure occurrence was approximately twice as likely in patients treated with surgical clipping compared to endovascular coiling, this did not reach significance in our study. In addition, our findings demonstrate that non-convulsive seizures rarely occur in low-grade aSAH patients who have undergone coiling, and confirm existing data that both convulsive and non-convulsive status epilepticus are associated with poor functional outcome. Our results also help to further clarify that cEEG monitoring for seizure may not be necessary in low-grade aSAH patients who have undergone aneurysm coiling and could be more important in those who have undergone surgical clipping, highlighting the need for better strategies in appropriate utilization of cEEG monitoring in these patients stratified by SAH severity and treatment modality.
Acknowledgements
Dr. Linda Gerber and Ms. Xian Wu were supported in part by funds from the Clinical and Translational Science Center (CTSC) and National Center for Advancing Translational Sciences (NCAS) Grant #2UL1-TR000457-06. Dr. Peter B. Forgacs was supported in part by funds from the NIH NINDS K23 NS096222; Leon Levy Neuroscience Fellowship Award; NIH UL1 TR000043 NCATS Rockefeller CTSA Program and The Stavros Niarchos Foundation. Dr. Santosh B. Murthy was supported in part by research fellowships from the American Academy of Neurology, American Brain Foundation, and the Leon Levy Foundation.
Footnotes
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings of this paper.
Parts of these results were presented in poster form at the annual meeting of the American Academy of Neurology (AAN), Vancouver, Canada, April 18, 2016.
References
- 1.Ziemba-Davis M, Bohnstedt BN, Payner TD, Leipzig TJ, Palmer E, and Cohen-Gadol AA. Incidence, Epidemiology, and Treatment of Aneurysmal Subarachnoid Hemorrhage in 12 Midwest Communities. J Stroke Cerebrovasc Dis 2014; 23 (5): 1073–1082. [DOI] [PubMed] [Google Scholar]
- 2.Rincon F, Rossenwasser RH, and Dumont A. The Epidemiology of Admissions of Nontraumatic Subarachnoid Hemorrhage in the United States. Neurosurgery 2013; 73: 217–223. [DOI] [PubMed] [Google Scholar]
- 3.Ridwan S, Urbach H, Greschus S, von Hagen J, Esche J, and Boström A. Health Care Costs of Spontaneous Aneurysmal Subarachnoid Hemorrhage for Rehabilitation, Home Care, and In-Hospital Treatment for the First Year. World Neurosurgery 2017; 97: 495–500. [DOI] [PubMed] [Google Scholar]
- 4.Huttter BO, Gilsbach JM, and Kreitschmann I. Quality of life and cognitive deficits after subarachnoid hemorrhage. Br J Neurosurg 1995; 9:465–75. [DOI] [PubMed] [Google Scholar]
- 5.Brand C, Alber B, Fladung A-K, Knauer K, Konig R, Oechsner A, et al. Cognitive performance following spontaneous subarachnoid hemorrhage versus other forms of intracranial hemorrhage. Br J Neurosurg 2014; 28:68–80. [DOI] [PubMed] [Google Scholar]
- 6.Claassen J, Peery S, Kreiter KT, Hirsch LJ, Du EY, Connolly ES, et al. Predictors and clinical impact of epilepsy after subarachnoid hemorrhage. Neurology 2003; 60: 208–214. [DOI] [PubMed] [Google Scholar]
- 7.Byrne JV, Boardman P, Ioannidis I, Adcock J, and Traill Z. Seizures after Aneurysmal Subarachnoid Hemorrhage Treated with Coil Embolization. Neurosurgery 2003; 52: 545–552. [DOI] [PubMed] [Google Scholar]
- 8.Connolly ES, Rabinstsein AA, Carhuaporna JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the Management of Aneurysmal Subarachnoid Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2012; 43: 1711–1737. [DOI] [PubMed] [Google Scholar]
- 9.Claassen J, Hirsch LJ, Frontera JA, Fernandez A, Schmidt M, Kapinos G, et al. Prognostic Significance of Continuous EEG Monitoring in Patients With Poor-Grade Subarachnoid Hemorrhage. Neurocrit Care 2006; 04: 103–112. [DOI] [PubMed] [Google Scholar]
- 10.Dennis LJ, Claasen J, Hirsch LJ, Emerson RG, Connolly ES, and Mayer SA. Nonconvulsive Status Epilepticus after Subarachnoid Hemorrhage. Neurosurgery 2002; 51: 1136–1144. [DOI] [PubMed] [Google Scholar]
- 11.Little AS, Kerrigan JF, McDougall CG, Zabramski JM, Albuquerque FC, Nakaji P, et al. Nonconvulsive status epilepticus in patients suffering spontaneous subarachnoid hemorrhage. J Neurosurg 2007; 106: 805–811. [DOI] [PubMed] [Google Scholar]
- 12.Molyneux A, Kerr R, Yu L, Clarke M, Sneade M, Yarnold JA, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping vs endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005; 366: 809–817. [DOI] [PubMed] [Google Scholar]
- 13.Rush B, Wiskar K, Fruhstorfer C, and Hertz P. Association between seizures and mortality in patients with aneurysmal subarachnoid hemorrhage: A nationwide retrospective cohort analysis. Seizure 2016; 41: 66–69. [DOI] [PubMed] [Google Scholar]
- 14.Dorhout Mees SM, Kerr RS, Rinkel GJE, Algra A, and Molyneux AJ. Occurrence and impact of delayed cerebral ischemia after coiling and after clipping in the International Subarachnoid Aneursym Trial (ISAT). J Neurol 2012; 259: 679–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.North JB, Penhall RK, Hanieh A, Frewin DB, and WB Taylor. Phenytoin and postoperative epilepsy: A double-blind study. J Neurosurg 1983; 58 (5): 672–677. [DOI] [PubMed] [Google Scholar]
- 16.Shaw MD and Foy PM. Epilepsy after craniotomy and the place of prophylactic anticonvulsant drugs: discussion paper. J R Soc Med 1991; 84 (4): 221–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franceschetti S, Binelli S, Casazza M, Lodrini S, Panzica F, Pluchino F, et al. Influence of surgery and antiepileptic drugs on seizures symptomatic of cerebral tumours. Acta Neurochir (Wien) 1990; 103 (1–2): 47–51. [DOI] [PubMed] [Google Scholar]
- 18.De Santis A, Villani R, Sinisi M, Stocchetti N, and Perucca E. Add-on phenytoin fails to prevent early seizures after surgery for supratentorial brain tumors: a randomized controlled study. Epilepsia 2002; 43 (2): 175–82 [DOI] [PubMed] [Google Scholar]
- 19.Thorpe ML, Cordato DJ, Morgan MK, and Herkes GK. Postoperative seizure outcome in a series of 114 patients with supratentorial arteriovenous malformations. J Clin Neurosci 2000; 7 (2): 107–111. [DOI] [PubMed] [Google Scholar]
- 20.Wostrack M, Friedrich B, Hammer K, Harmening K, Stankewitz A, Ringel F, et al. J Neurol 2014; 261: 2128–2135. [DOI] [PubMed] [Google Scholar]
- 21.Claassen J, Perotte A, Albers D, Kleinberg S, Schmidt JM, Tu B, et al. Nonconvulsive seizures after subarachnoid hemorrhage: multimodal detection and outcomes. Ann Neurol 2013; 74 (1): 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olafsson E, Gudmundsson G, and Hauser WA. Risk of Epilepsy in Long-Term Survivors of Surgery for Aneurysmal Subarachnoid Hemorrhage: A Population-Based Study in Iceland. Epilepsia 2000; 41 (9): 1201–1205. [DOI] [PubMed] [Google Scholar]
- 23.Hoh BL, Nathoo S, Chi Y, Mocco J, and Barker FG II. Incidence of Seizures or Epilepsy After Clipping or Coiling of Ruptured and Unruptured Cerebral Aneurysms in the Nationwide Inpatient Sample Database: 2002–2007. Neurosurgery 2011; 69: 644–650. [DOI] [PubMed] [Google Scholar]
- 24.Huttunen J, Kurki MI, von und zu Fraunberg M, Koivisto T, Ronkainen A, Rinne J, et al. Epilepsy after aneurysmal subarachnoid hemorrhage: A population-based, long-term follow-up study. Neurology 2015; 84: 2229–2237. [DOI] [PubMed] [Google Scholar]
- 25.Diringer MN, Bleck TP, Hemphill C III, Menon D, Shutter L, Vespa P, et al. ; Neurocritical Care Society. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society’s Multidisciplinary Consensus Conference. Neurocrit Care 2011; 15 (2): 211–240. [DOI] [PubMed] [Google Scholar]


