Abstract
Assessment of naming in children has been hampered by the use of tests that were developed, either to assess naming in adults, or to assess related verbal functions in children. We developed comparable visual naming (VNT) and auditory description naming tests (ANT) specifically for children. We collected normative data, not only for accuracy, typically the sole performance measure, but also for response time (RT) and reliance on phonemic cuing. The normative sample consisted of 200 typically developing children, ages 6–15, with 40 children per 2-year age group (6–7, 8–9, 10–11, 12–13, and 14–15). Children were tested individually by a trained examiner. Based on item analysis, naming tests were finalized at 36 items for ages 8–15, and 28 items for ages 6–7. Age-stratified normative data are provided for accuracy, mean RT, Tip-of-the-tongues (i.e., delayed but accurate responses plus items named following phonemic cueing) and a summary score, which incorporates all performance measures. Internal and test-retest reliability coefficients for both tests were reasonable. Accuracy scores were high across age groups, indicating that item names were within the mental lexicon of most typically developing children. By contrast, time and cue based scores improved with age, reflecting greater efficiency in word retrieval with development. These complimentary auditory and visual naming tests for children address a longstanding clinical need, improving upon the current standard with respect to the sensitivity of performance measures and the addition of an auditory verbal component to the assessment of naming in children.
Keywords: child language, naming assessment, word production, auditory naming, visual naming
INTRODUCTION
Neuropsychological tests aim to determine the level of functioning of a particular skill, thereby enabling inference regarding the functional integrity of the brain area(s) that mediate the function of interest. When tests are developed prospectively, steps can be taken to minimize potential confounds, normative data can be collected from an appropriate sample, and the instrument can be refined to obtain a sound assessment of the targeted function. However, test development is time and labor intensive, and typically costly; consequently, it is not uncommon for existing tests to be used for purposes and populations that diverge from original intentions. Although, on the surface, this might appear to be an efficient and cost effective strategy, this type of repurposing can potentially compromise the assessment, increasing the likelihood of misleading results and erroneous interpretations.
The assessment of naming in children exemplifies the substitution of related tests in the absence of measures developed specifically for this purpose. Naming, i.e., the capacity to retrieve from the mental lexicon and produce the precise name of an item on demand, encompasses the integration of perceptual, semantic and phonological processes (Caramazza, 1997). Importantly, although naming is dependent on vocabulary knowledge, naming refers to the targeted retrieval and production of a particular word from an established mental lexicon. Therefore, in assessing naming, it is critical that the influence of vocabulary knowledge is minimized. Specifically, a naming test should assess retrieval of item names that are that are highly likely to be part of the examinee’s mental vocabulary.
While seemingly effortless under normal circumstances, naming can become disrupted in a number of developmental and neurological conditions; thus, naming assessment is an integral component of neuropsychological evaluation. Although developed to assess adults, the Boston Naming Test (BNT), consisting of 60 line drawn objects, (Kaplan, Goodglass, & Weintraub, 1983) is by far, the most frequently used measure of object naming in children (Camara, Nathan, & Puente, 2000); (Rabin, Barr, & Burton, 2005). In fact, results of a recent international survey of tests used by neuropsychologists in pediatric epilepsy practice found the BNT to be the most commonly used naming test in both English-speaking (77.9%) and non-English-Speaking (37.5%) countries (Berl, Smith, Bulteau, ILAE, & Commissions of Pediatrics, in press). Literature review of studies on naming in children reveals less frequent use of the Expressive Vocabulary Test (Williams, 1997), Expressive One Word Vocabulary Test (Martin & Brownell, 2011) and Naming Vocabulary subtest of the Differential Abilities Scale-II (Elliot, 2006); however, consistent with their names, these tests assess expressive vocabulary, and therefore, are not true measures of naming. The Wechsler Preschool and Primary Scale of Intelligence-IV(Wechsler, 2012) includes a picture naming subtest; however, the age range is limited (2.5 – 7.7 years). Rapid automatized naming tests (Fastenau et al., 2004) which require rapid naming of a series of illustrations depicting objects, actions, or concepts, have also been used to assess naming in children, yet, these tests are considered primarily, measures of visual processing speed and working memory (Denckla & Cutting, 1999).
The BNT has had profound influence on the neuropsychology of naming in adults. Clinically, the BNT has been a cornerstone in the assessment of productive language in adult based neurological disorders including stroke, epilepsy, dementia, and virtually all types of aphasic syndromes. Similarly, the BNT has been the most widely used measure of object naming across decades of neuroscientific investigations of brain and language. Inarguably, the BNT has been instrumental in illuminating the central role of naming in the assessment and understanding of brain-language relations.
However, with several decades of use, it has become evident that BNT performance in adults is influenced significantly by education level and vocabulary knowledge (Baron, 2004; Mitrushina, Boone, Razani, & D’Elia, 2005; Randolph, Lansing, Ivnik, Munro Cullum, & Hermann, 1999; Roberts & Hamsher, 1984). Therefore, it is not surprising that BNT performance in children has been shown to relate more to word knowledge than to naming ability (Guilford & Nawojczyk, 1988; Halperin, Healey, Zeitchik, Ludman, & Weinstein, 1989; Kirk, 1992). Kirk (Kirk, 1992) found that among children ages 5–13, items 28–50 of the 60 items were not reliably part of children’s working vocabulary, depending on age, and that items 51–60 could not be considered part of the lexicon at any age. Other investigators have reported similar findings, attributing such results to the advanced vocabulary level of BNT items, and more recently, the datedness of many of the stimuli as well (e.g. yoke, trellis, abacus, and palette) (Martielli & Blackburn, 2016a; Schmitter-Edgecombe, Vesneski, & Jones, 2000). Additionally, there are some concerns regarding cultural sensitivity of test items (e.g., noose).
In light of these concerns, attempts have been made to render the BNT more usable for children, for example, by reordering the stimuli (Kirk, 1992), providing caveats when interpreting performance (Martielli & Blackburn, 2016b) or collecting more extensive or updated normative data sets (Cohen, Town, & Buff, 1988; Yeates, 1994). However, these modifications in practice cannot change the intrinsic level or quality of the test stimuli.
Rather than modify an adult naming test comprised of stimuli that already exhibit datedness and cohort effects with young people, we opted to develop a new naming instrument that would be specifically designed for children. This approach also provided an opportunity to incorporate additional improvements, based on advances in the neuropsychology and cognitive neuroscience of naming:
Assessment of both visual object naming and auditory description naming. Word retrieval difficulty can occur, not only when naming visible objects, but also during every day, auditory-based discourse. Previous work with adults, together with preliminary findings in children with epilepsy, has shown that auditory description naming is particularly sensitive to left temporal lobe abnormalities (Bell, Seidenberg, Hermann, & Douville, 2003; Hamberger & Seidel, 2003). Moreover, cortical stimulation studies have revealed a neuroanatomical distinction between dominant, temporal lobe areas that support auditory naming and visual object naming (Hamberger, Goodman, Perrine, & Tamny, 2001). Similar to measures available for adults, we aimed to develop complimentary visual (VNT) and auditory naming tests (ANT) comprised of age-appropriate items for children.
Normative data that incorporate response time. Conventional naming tests such as the BNT use accuracy (number correct) as the sole performance measure, with each item allotted 20 seconds for a response. Without formalized scoring for delayed but accurate responses, examinees receive the same one-point credit for items named immediately, or, delayed up to 20 seconds, thereby failing to capture this classic manifestation of word finding difficulty. When word retrieval exceeds 1.5 seconds, automatic processes have ceased and deliberate search strategies are typically initiated (Goodglass, Theurkauf, & Wingfield, 1984). The children’s ANT and VNT incorporate response time into performance evaluation.
Normative data for phonemic cueing. Word retrieval failure followed by success elicited by phonemic cueing (e.g., “ha” for “hammer”), represents another demonstration of word finding difficulty, indicating that the item-name is within the individual’s mental lexicon, yet is temporarily inaccessible. The BNT includes the provision of phonemic cues in its administration; however, interpretation is limited in the absence of normative data. The children’s ANT and VNT capture the reliance on cueing within the naming performance measures, and provide age-stratified normative data for cueing to aid interpretation.
Quality and longevity of naming stimuli. The test stimuli in conventional naming tests consist of line-drawn objects, which, in current times, appear dated in quality and style (Gollan, Weissberger, Runnqvist, Montoya, & Cera, 2012; Kaplan et al., 1983). Additionally, the use of line drawings potentially confounds the assessment of naming with visual perceptual demands. The current, children’s VNT is comprised of color photographs, consistent with experience in contemporary society, where high quality, digitized images are commonplace and readily accessible. Further, in the interest of longevity of the tests, we avoided items that would likely become dated within a relatively short time interval (e.g., technical items such as phones or computers).
Comparability of ANT and VNT. Prior behavioral and cortical mapping work with adults suggests maximal benefit when auditory and visual naming tasks are used together. As word frequency is related to both vocabulary knowledge and efficiency in word retrieval (Graves, Boettcher, Peacock, & Ryder, 1980; Ryder & Slater, 1988), the children’s ANT and VNT target words are matched for word frequency, by both mean values and distribution of word frequency between sets (detailed in Methods section).
METHODS
Participants
Participants were 200 typically developing children, ages 6–15, with 40 children per 2-year age group (6–7, 8–9, 10–11, 12–13, and 14–15). Children were recruited via advertising at Columbia University Medical Center, word of mouth, posted notices in local community centers, and select internet websites (Craig’s List, www.researchmatch.org, www.volunteermatch.org). All participants were required to be native English speakers, or to have learned English by age 5 and to be educated in English. A telephone pre-screening queried parents of prospective child participants about their child’s neurological, psychiatric and academic history. Individuals with a history of learning disabilities, language problems, head injury, stroke, or other neurological disorders were excluded. This study was approved by the Institutional Review Board at Columbia University Medical Center.
Stimuli
For the VNT, 63 pictured objects were selected from bigstockphoto.com. Pictured objects were required to be “isolated” on a white background for visual uniformity across items and to eliminate contextual cues (see examples in Figure 1).
Figure 1 Title:

Test item examples, Caption. Select test items from the Children’s Auditory and Visual Naming Tests
For the ANT, item descriptions were required to be presentable within 4 seconds at a natural speaking rate, with low likelihood that target words could be named before the final word of the description (e.g., “an object used for weighing”). Forty –five descriptions were generated by the authors (MH, WTS) and 18 items were taken from the adult ANT (Hamberger & Seidel, 2003)
For the initial pilot testing, 63 ANT and 63 VNT items were administered to 14 children ages 6–13 years. Twenty- two ANT and 20 VNT items were eliminated due to items eliciting errors (including alternate responses) or delayed responses (≥ 2 seconds) in ≥50% of the group (e.g., ANT: flag, shampoo; VNT: grasshopper, celery). Two additional ANT items were eliminated due to target words being provided before the final word of the description (witch, chalk), resulting in 41 ANT and 43 VNT items. Interim analyses were conducted on the 41 ANT and 43 VNT stimuli following data collection with 140 typically developing children, ages 6–15. Based on poor accuracy (including alternate responses) and delayed responses, similar to that described above, 5 ANT and 5 VNT items were eliminated. In addition, 2 VNT items were eliminated due to perfect, rapid scores obtained across participants (balloon, frog), as these items provided no variance. This resulted in 36 stimuli each for the ANT and VNT that best met the following criteria: 1) correct response from a minimum of 90% of subjects, and 2) median RT < 2 sec. Word frequencies, based on spoken English, were obtained from http://subtlexus.lexique.org/. A T test comparing mean word frequency for target words between tasks (ANT: 23.2, SD=27.4; VNT: 16.7, SD=25.2) indicated no significant difference (P = 0.30). Furthermore, there was no significant difference in the distributions of word frequency between tasks, as assessed by the Kolmogorov Smirnov test (P = 0.19). Auditory description stimuli and the item names for visual stimuli are listed in Appendix A.
Procedure
All participants were administered the two naming tasks, the two-subtest Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999) and the Word Reading subtest of the Wechsler Individual Achievement Test (WIAT-II or WIAT III; (Wechsler, 2009). These scores and demographic information were obtained to characterize the normative sample (Table 2).
Table 2.
Normative group characteristics
| Mean age(sd) |
m/f | FSIQ | Vocabulary T score |
Word Reading Standard Score |
Matrix Reasoning Tscore |
Mother’s Education (years) |
|
|---|---|---|---|---|---|---|---|
| Age 6–7 | 7.0 (0.6) |
20/20 | 105.9 (11.5) 84–129 |
54.4 (7.3) 33–70 |
107.0 (16.6 14–148 |
52.5 (9.4) 33–70 |
15.4 (2.2) 12–20 |
| Age 8–9 | 9.0 (0.6) |
22/18 | 104.4 (13.6) 77–125 |
53.6 (10.0) 23–73 |
107.7 (13.6) 78–136 |
51.1 (11.3) 24–73 |
15.3 (2.4) 12–20 |
| Age 10–11 | 10.9 (0.6) |
20/20 | 101.4 (11.9) 76–121 |
52.4 (7.7) 38–66 |
107.8 (13.6) 72–128 |
49.0 (9.0) 29–63 |
15.1 (2.6) 11–20 |
| Age 12–13 | 13.0 (0.5) |
21/19 | 104.8 (10.4) 85–128 |
54.3 (6.9) 40–67 |
109.3 (9.5) 84–127 |
51.5 (8.1) 38–68 |
16.4 (2.4) 12–20 |
| Age 14–15 | 14.8 (0.6) |
22/18 | 104.1 (15.1) 72–138 |
55.8 (10.0) 32–76 |
107.3 (9.9) 79–122 |
48.3 (10.0) 20–65 |
15.3 (2.6) 12–20 |
Order of naming tasks was counterbalanced across subjects. Standardized instructions were read aloud by a trained examiner. For the ANT, timing, via stopwatch, began when the examiner completed the final word of the description and terminated with the subject’s correct response. For the VNT, timing began with picture presentation and terminated with the subject’s correct response. Participants were permitted a maximum of 20 seconds to provide a correct response. On trials in which the child provided an incorrect response(s) before the time limit, the examiner queried, “What else?” If the child failed to provide the correct response within the allotted 20 seconds, these trials were coded as “incorrect,” and the examiner provided a phonemic cue. Subjects were given 5 additional seconds to provide the correct response following this cue before the next trial was initiated. The provision of a correct response was noted on the record form.
A small subset of participants (n=30) was tested approximately one month after the initial testing for assessment of test –retest reliability.
Performance measures
Performance measures consisted of those used in the development of the original adult tests plus additional measures derived from clinical experience with those tests. Performance measures from the original test include: 1) Accuracy scores, i.e., “Number Correct,” calculated by summing the number of correct responses within 20 seconds of stimulus presentation; 2) mean response time (RT), and 3) tip-of-the tongue (“TOT”) responses, defined as the sum of items named accurately in 2–20 seconds (“delayed responses”), plus items not named by 20 seconds, yet named accurately following a phonemic cue. Both delayed and cued responses represent instances in which the word is clearly within the individual’s mental lexicon, yet, additional time, or a phonemic cue was necessary to retrieve the word.
Based on over a decade of use in both clinical and research settings, we have found utility in additional performance scores. Just as the number of delayed responses is informative, so is the counterpart to this measure, i.e., items named in less than 2 seconds, as this score represents the absence of word finding difficulty. Moreover, we reasoned there would likely be utility in an overall summary score that captured the components of performance represented by the subscores. Accordingly, we developed a score that utilized best performance as its base (i.e., number of items named in less than 2 seconds), with a penalty for TOTs (i.e., delayed yet accurate and cued responses). Given the use of a manual stopwatch, we use 2 seconds (rather than 1.5 (Goodglass et al., 1984)) as a demarcation of automatic verses conscious processing to allow for human error and variability. Performance measures are defined in Table 1 below.
Table 1.
Naming scores
| Performance measure | Definition | Rationale |
|---|---|---|
| Number Correct | Number of items named within 20 seconds | The purpose of this measure is primarily to confirm that the test assesses naming rather than vocabulary. |
| Mean RT | Mean RT across items named within 20 seconds | RT is highly sensitive measure, yet, might be impractical in the clinical setting. (For adults, we have used RT mainly for research) |
| Less than 2 seconds “<2 sec” |
Number of items named in less than 2 seconds following picture presentation (VNT) or following last word of description (ANT) | Best performance; responses represent rapid, automatic word retrieval |
| TOT | Sum of delayed yet accurate, (2–20 seconds) and cued responses | Represents inefficiency in word retrieval from the mental lexicon |
| Summary score | Number of items named < 2 sec ) - TOT score | Best performance, subtracting delayed and cued responses (TOT) |
Statistical Analyses
Item analysis.
Internal consistency for the ANT and VNT was assessed using split-half (odd/even) Cronbach’s alpha correlations with Spearman Brown corrections for the reduced number of items per set (Nunnally, 1978) using two measures that incorporate time based performance. Accuracy alone (within 20 seconds) was not used due to the severely restricted range, and although RT was considered, the absence of RT for items not named in 20 seconds reduced the data set list-wise. Therefore, we used TOT scores for each item as described above and coded responses from 1–4, defined as follows: 1 = correct target word provided in less than 2 seconds, 2 = correct target word provided in 2–20 seconds, 3= correct target word provided following phonemic cue, and 4= no correct response provided. This coded measure essentially captures every aspect of performance.
Sample characteristics and naming performance.
Demographic data and performance measures are presented as means (+/−SD) by age group. Multivariate ANOVAs assessed potential demographic and performance differences among age groups, and T tests were used to assess potential gender differences within age groups. Scheffe’s post hoc tests were used to assess group differences following significant Age Group effects indicated by ANOVA. Test-retest reliability was assessed via Spearman’s rho correlations.
RESULTS
Table 2 demonstrates that group mean scores on standardized measures fall in the average range in all groups. Accordingly, multivariate ANOVA revealed no significant differences among age groups in IQ [F(4/195)= 0.68, P = 0.60] Vocabulary T scores [F(4/195) = 0.86, P = 0.48], Matrix Reasoning T scores [F(4/195)= 0.68, P = 0.60] or WIAT-3 standard scores [F(4/185) =0.17, P = 0.95]. Additionally, there were no significant differences between boys and girls in IQ, Vocabulary or WIAT Word Reading scores, across or within in any of the age groups (all P > .15). Level of mother’s education was available for 180 of the 200 participants, and was also comparable among age groups [F(4/175) =1.67, P = 0.15].
Preliminary analyses: Accuracy
As detailed above, we anticipated high Number Correct scores (within 20 seconds), reflecting that test item names are established within the mental lexicon of most healthy children. Accordingly, accuracy scores approached ceiling level and were comparable across age groups, with the exception of the youngest age group.
Results of one-way ANOVA followed by post hoc testing revealed a significant effect of Age Group for ANT accuracy scores [F(4/166) =15.7, P < .001]. Post hoc tests revealed significantly lower accuracy scores for age 6–7 compared to that of all other age groups, with no significant differences among the other four age groups. VNT accuracy showed a similar pattern: [F(4/166) =6.2, P < .001] although post hoc tests indicated the difference between age 6–7 and age 8–9 was borderline significant (P = .05).
Closer examination of the 6–7 year group revealed that >25% of the group failed to name eight of the ANT items. These eight ANT items, along with eight VNT items with the lowest accuracy scores were removed from the test for age 6–7, resulting in 28 items for both the ANT and VNT for this group. For analysis comparing performance among age groups, performance scores from age 6–7 group were transformed to their equivalent 36-item score (e.g., Number correct score of 26/28 items transformed to 33.4).
Internal consistency
Internal consistency was assessed using performance data from ages 8–15. Using TOT scores, Cronbach’s Alpha with Spearman Brown correction was 0.78 for the VNT and 0.74 for the ANT. For coded (1–4) responses, these values were 0.80 for the VNT and 0.87 for the ANT, overall, reflecting a reasonable level of internal consistency.
Naming performance.
Results of RMANOVA showed a significant effect of Age Group for both ANT group [F(4/195) =14.9, P < .001] and VNT group [F(4/166) =3.8, P < .005]. However, inspection of the data (Figure 2, top panel) suggests that these statistical findings are not clinically meaningful, as group means were close to ceiling in all groups. Results of RMANOVA for all the other performance measures, which incorporate response time, also revealed an effect of Age Group (ANT: RT [F(4/195) =24.1 P < .001; TOT: [F(4/195) =30.1 P < .001; Summary Score: [F(4/195) =33.5 P < .001; VNT: RT [F(4/195) =15.1 P < .001; TOT [F(4/195) =16,5 P < .001; Summary score [F(4/195) =16.2 P < .001]. In contrast to accuracy scores, however, inspection of these scores by Age group (see Table 3 and Figure 2, bottom panel), suggests a developmental progression in the efficiency of word retrieval with increasing age, potentially plateauing at approximately age 12. This finding is also reflected in the results of post hoc analyses (include below Table 3).
Figure 2 Title:
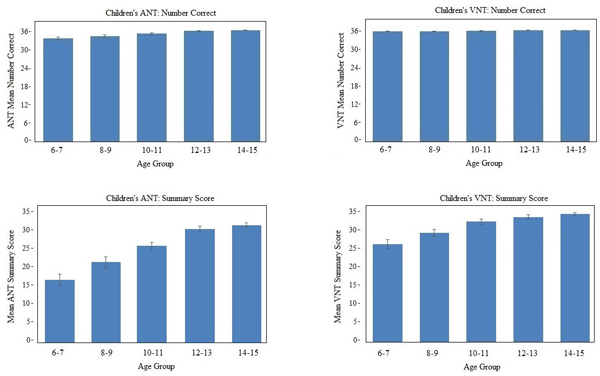
Auditory and Visual Naming Performance by Age Group, Caption. Upper left: Auditory Naming Number Correct; Upper right Visual Naming Number Correct; Lower left: Auditory Naming Summary Scores; Lower right: Visual Naming Summary Scores. Note: Performance scores from the Age 6–7 group were transformed to their equivalent 36-item score for ease of comparison with other groups.
Table 3.
Results of ANOVA and post hoc analyses
| AN Number Correct |
AN RT | AN TOT | AN Summary Score |
VN Number Correct |
VN RT | VN TOT | VN Summary Score |
|
|---|---|---|---|---|---|---|---|---|
| Age 6–7 | ||||||||
| mean | 25.9 | 1.97 f | 7.0 a | 13.2 a | 27.7 | 1.40 f | 3.8 f | 20.4 f |
| SD | (1.9) | (0.6) | (3.1) | (6.7) | (0.6) | (0.5) | (2.9) | (5.8) |
| range | 22–28 | 0.93–3.69 | 1–15 | −3–26 | 26–28 | 0.73–3.09 | 0–11 | 6–28 |
| Age 8–9 | ||||||||
| mean | 33.9 | 1.72 f | 6.9 a | 21.4 a | 35.7 | 1.21g | 3.3 g | 29.4 g |
| SD | (2.3) | (0.7) | (4.2) | (8.7) | (0.7) | (0.4) | (3.0) | (6.1) |
| range | 27–36 | 0.68–3.21 | 0–17 | 2–36 | 33–36 | 0.63–2.12 | 0–11 | 14–36 |
| Age 10–11 | ||||||||
| mean | 34.7 | 1.35 e | 4.8 a | 25.9 a | 35.8 | 1.08 b | 1.9 b | 32.1 b |
| SD | (1.9) | (0.5) | (3.3) | (6.5) | (0.5) | (0.3) | (2.3) | (4.7) |
| range | 29–36 | 0.55–2.56 | 0–11 | 10–36 | 34–36 | 0.49–2.39 | 0–9 | 17–36 |
| Age 12–13 | ||||||||
| mean | 35.7 | 1.12 c | 2.7 d | 30.6 d | 36.0 | 0.93 c | 1.3 c | 33.5 c |
| SD | (0.7) | (0.3) | (2.3) | (4.6) | (0.0) | (0.3) | (1.7) | (3.5) |
| range | 33–36 | 0.60–2.44 | 0–8 | 19–36 | 36–36 | 0.52–1.74 | 0–9 | 18–36 |
| Age 12–13 | ||||||||
| mean | 35.8 | 1.03 d | 2.2 d | 31.6 d | 36.0 | .87 c | 0.8 c | 34.3 c |
| SD | (0.6) | (0.4) | (2.1) | (4.1) | (0.2) | (0.2) | (1.2) | (2.5) |
| mean | 34–36 | 0.53–2.27 | 0–9 | 17–36 | 35–36 | 0.41–1.35 | 0–4 | 27–36 |
Superscript denotes significant difference from listed age groups at p<.05
≠ all other groups;
≠ Age 6–7;
≠ Age 6–7, Age 8–9;
≠ Age 6–7, Age 8–9, Age 10–11;
≠ Age 6–7, Age 8–9, Age 14–15;
≠ Age 10–11, Age 12–13, Age 14–15;
≠ Age 12–13, Age 14–15
Notes: 1) For Age 6–7, actual values are shown, however, transformed scores were used in analyses comparing Age groups; 2) Normative data for additional performance scores (<2 sec, > 2 sec, phonemic cue) are available in Appendix Table 1.
Comparison of ANT and VNT performance within each Age group revealed stronger VNT performance (Number Correct, RT, TOT, Summary Score, all P < .05).
There were no significant differences in IQ or in any naming performance measures between girls and boys in the total sample or within individual age groups (all P > .10). Therefore, normative data are presented for girls and boys combined.
Test retest reliability
Thirty children (15 girls, 15 boys), with relatively equal representation across the age groups (Age 6–7, n=6; Age 8–9, n=7; Age 10–11, n=7; Age 12–13, n= 6; Age 14–15, n= 6), were retested approximately one month after their initial testing (mean: 37 days, SD=10). There were no significant differences in IQ between subjects who did (mean 105.0, SD=10.7) and did not return (mean 103.0, SD=12.9; P =.67). Spearman rank correlations for Summary scores, which we consider the most comprehensive clinical measure and less problematic than accuracy with regard to restricted range given the normative sample, were .84 for Auditory Naming and .81 for Visual Naming.
Relations between naming performance and other measures
IQ and reading performance were obtained, primarily, to characterize the normative sample; however, we examined relations between these measures and naming performance. With Bonferroni’s correction applied for multiple correlations, only correlations between IQ and ANT (r = 0.28) and VNT (r =0 .25) accuracy scores reached significance (both P <.001). Correlations between WIAT word reading and several ANT, yet no VNT scores, were significant: ANT accuracy (r=0.25), TOT (r = −0.27) Summary Score (r = 0.25; all P < .001).
DISCUSSION
Addressing a longstanding need in neuropsychological assessment of children, this normative study developed complementary auditory and visual naming tests for children, ages 6 through 15 years. Test development was guided by a number of recent developments in the neuropsychology and cognitive neuroscience of language. The current naming measures add an auditory verbal modality to the assessment of naming in children, utilize items that are highly likely to be within the lexicon of most typically developing children, and additionally provide age-stratified normative data for performance measures that capture the time and cue related features of word finding difficulty that reflect efficiency in word retrieval.
Child specific naming assessment
A key motivating factor for developing naming tests for children was to reduce the influence of vocabulary knowledge on the assessment of naming. In developing the tasks, we selected target words that, based on word frequency, would likely be known to school aged children, and used interim analyses to eliminate items that were not readily named by a sizeable proportion of the normative sample. Importantly, our normative sample appears to be reasonable representation of typically developing children, as reflected by general intelligence scores solidly in the average range for each two-year age group. Normative performance data confirmed that the vast majority of children named most, if not all items on both tasks within the allotted 20 seconds (Table 3), underscoring that, overall, target words on these naming tests have been assimilated into the mental lexicon of most typically developing children. The current naming results stand in clear contrast to children’s’ BNT performance, in which the average score (i.e., number correct ) is approximately 47/60 (78%) in school aged children (Guimaraes et al., 2007; Hermann et al., 2008). With the issue of vocabulary knowledge minimized, the current tasks can be used to truly assess naming, i.e., retrieval of known words from the mental lexicon.
Performance measures
We have emphasized that untimed accuracy essentially confirms that the items named are in the mental lexicon of the individual, whereas, response time and reliance on cueing reflect the efficiency by which the names are retrieved—i.e., the function naming measures are intended to assess. To capture the timing component of word retrieval, we created several performance measures to be calculated separately for the ANT and VNT, including mean RT, sum of items named in < 2 seconds, sum of items named in 2–20 sec, as well as summary scores that incorporate these more basic scores. Although mean response time could be considered the purest time-based measure, mean RT carries some undesirable features. Statistically, mean RT could potentially be skewed by a few outliers, rendering the value misleading, and in the clinical setting, it can be a burden to calculate. What might be most meaningful is whether a word is retrieved automatically (< 2 sec), or with conscious effort (≥2 sec), and logistically, summing each of these scores is a straightforward and rapid calculation.
In adults with unilateral temporal lobe epilepsy, we have found the TOT score, which combines number items named in ≥2 seconds and number of items named following a phonemic cue, to be particularly sensitive to left (dominant) temporal lobe dysfunction(Hamberger & Seidel, 2003). In children with unilateral epilepsy, preliminary findings suggest that the number of delayed responses, i.e., ≥2 sec, is particularly sensitive to left hemisphere dysfunction, with minimal additional contribution from number item items named from phonemic cueing (Hamberger, Smith, MacAllister, Williams, & Seidel, 2015). As it is as yet unknown which scores will be most sensitive in which clinical populations in children, we provide age stratified normative data for multiple scores in simple and combined forms (Table 3 and Appendix, Table 1).
In retrospect, one regret with the adult measures has been the absence of a global performance score that would encompass the individual sub-scores. As such, we created a Summary Score for the children’s ANT and VNT which uses best performance as its base (number of items <2 sec) with a penalty for delayed responses and reliance on phonemic cueing (TOT). Additionally, given preliminary evidence of particular sensitivity of time-based scores in children, we provide normative data for an additional variation of the summary score that uses only time-related subscores (items <2 – items ≥ 2).
Psychometric considerations
Assessment of reliability, which could potentially present a challenge for a measure of an intact function in a normative sample, was conducted using time based performance scores that capture the inter-individual and developmental differences in efficiency of word retrieval in a healthy sample. Internal consistency values, calculated using split-half correlations, were well within an acceptable range of .74 to .87. Test–retest correlations ranged from .73 to .84, overall, reflecting a reasonable level of reliability for clinical application (Nunnally & Bernstein, 1994).
Age related naming in children
Based on accuracy scores, we found that several of the item names were too advanced for a sizable proportion of the 6–7 year old group, whereas 8–9 year olds earned accuracy scores similar to those of older children in the normative sample. The expansion in vocabulary from ages 6–7 to 8–9 years has been shown to be related to the beginning of formal schooling and the development of reading skills (Chen & Truscott, 2010; Verhoeven, van Leeuwe, & Vermeer, 2011). To minimize the influence of vocabulary, the tests for 6–7 year-olds were shortened to include only items that procured high accuracy scores, similar to that in the older groups (indicated via “stop here” on record form). Accordingly, with the 28-item scores transformed to their 36-item equivalents, accuracy scores were comparable across all groups.
Whereas Figure 2 (top) suggests that untimed word retrieval plateaus close to ceiling level around 10–12 years of age, Figure 2 (bottom) reflects greater efficiency in word retrieval with increasing age (Table 3). Certainly, improved efficiency and more rapid response times are not unique to naming; response time improves with age in childhood for virtually all motor and cognitive functions (Hale, 1990; Ridderinkhof & van der Molen, 1997). The time-based performance data bring to light and provide normative for this aspect of naming ability.
Visual and Auditory Naming: modality related considerations
Both the auditory and visual naming stimuli evoke semantic, lexical and phonological processes that culminate in the retrieval of a specific word. Additionally, as with the adult naming tasks, we aimed for the tasks to be commensurate with each other by controlling for word frequency of the target words. Nevertheless, alongside these similarities, the tasks also carry inherently different processing demands. Whereas pictured objects in the VNT are perceived instantaneously, the ANT requires auditory verbal comprehension and serial processing. Importantly, these different task requirements simulate the different contexts in which word retrieval occurs in day to day living. At times, we name objects in the visual environment (e.g., “Please pass the salt.”), whereas, at other times we produce words in response to another speaker’s auditory verbal message (e.g., “This food is bland, what does it need?” “Salt”).
In light of its verbal processing demands, it could be argued that the ANT is more “difficult” than the VNT. Consistent with this, as we found in adults (Hamberger & Seidel, 2003) and now replicated in children, VNT RTs are more rapid and VNT performance scores overall, are stronger than ANT scores. However, we would contend that the task differences are not simply reducible to level of difficulty; rather, auditory naming and visual naming recruit task-specific cognitive mechanisms supported by distinct neural substrates.
Cortical mapping studies of adolescents and adults with epilepsy have shown that stimulation of anterior temporal cortex tends to disrupt auditory naming, but not visual naming, whereas stimulation in the posterior temporal-parietal region tends to disrupt both visual naming and auditory naming, or at some posterior sites, visual naming only (Hamberger et al., 2001; Hamberger, McClelland, Williams, Goodman, & McKhann, 2007). Further, anatomical distinctions between auditory and visual naming are not limited to clinical samples; results from functional neuroimaging of healthy young adults, likewise, have shown both overlapping and task specific areas involved in auditory versus visual naming (Hamberger, Habeck, Pantazatos, Williams, & Hirsch, 2014; Tomaszewki-Farias, Harrington, Broomand, & Seyal, 2005). Perhaps most notable in this regard and consistent with stimulation findings, patients with posterior temporal lesions or epileptogenic regions perform more poorly on visual naming compared to auditory naming – underscoring neurocognitive specificity rather than task difficulty -- with the reverse task related asymmetry (auditory naming poorer than visual naming) found for patients with lesions or seizure onset in anterior temporal areas (Hamberger & Seidel, 2009). Taken together, results from behavioral, cortical stimulation and neuroimaging studies suggest that auditory and visual naming tasks probe different aspects of word retrieval that draw on a combination of overlapping as well as distinct neural substrates and cognitive mechanisms.
Relations between naming and other domains
The relatively low but significant correlations between IQ and naming accuracy scores are consistent with correlations between IQ and neuropsychological test performances previously reported for both adults (Diaz-Asper, Schretlen, & Pearlson, 2004) and children (Foley, Garcia, Shaw, & Golden, 2009), reflecting an expected, relative consistency between general intellectual function and performance in particular cognitive domains. Correlations between WIAT word reading and several ANT, yet no VNT scores, reached significance. We speculate that this might be due to the greater demands in accessing meaning to process the descriptions in the auditory naming task. Thus, correlations between auditory naming and reading performance might reflect common mechanisms related to efficiency in accessing the semantic store.
Limitations
Our decision to include 40 children per age group was driven by available resources, and with regard to consistency with the pediatric neuropsychological literature. Certainly, larger sample sizes would have been desirable, enabling more detailed analyses, and potentially, greater accuracy in diagnosing deficits. Additionally, although maternal education level was fairly consistent across age groups of the normative sample, the level of education could be considered relatively high. Most mothers indicated having completed high school and some college. Thus, the relatively high range of maternal education levels should be taken into consideration in using the normative data.
Closing comments
The auditory and visual naming tasks presented here were developed specifically for children, with target item names shown to be within the mental lexicon of most typically developing 6–15 year olds. Age stratified normative data are provided for multiple performance measures that incorporate time and cue related features of word finding difficulty, thereby reflecting efficiency in word retrieval. As such, the examiner or investigator can select those which address their particular needs. The tests are relatively easy to administer, and scoring requires only simple summations or straightforward calculations.
The ANT and VNT can be used separately or together, depending on the questions at hand. The tests improve upon the current standard with respect to the sensitivity of performance measures and the addition of an auditory verbal component to the assessment of naming. It is our hope and expectation that utilization of these measures in both clinical and research settings will improve clinical assessment, facilitate advancements in clinical research on naming in children, and deepen our understanding of neurodevelopmental aspects of word retrieval.
Acknowledgements:
We thank Alicia Williams for assistance with data collection, data management and graphics, Kaitlin Walsh and Leslie Church for assistance with data collection, and the children and parents who generously contributed their time and effort toward this project.
Funding: This work was supported by the National Institutes of Health/the National Institute of Neurological Disorders and Stroke (MH) grant NIH R01 NS35140. Ironshore Pharmaceuticals Inc. did not fund or contribute to this research.
APPENDIX
Table A1.
Naming Scores by Age Group
| ANCorr | ANLess2 | ANRT (sec) |
ANTOT | ANGR2 | ANCue | ANSumm Score |
AN SS2 |
VNCorr | VNLess2 | VNRT (sec) |
VNTOT | VNGR2 | VNCue | VNSumm Score |
VN SS2 |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age 6–7 | 25.9 (1.9) |
20.2 (3.6) |
1.97 (0.6) |
7.0 (3.1) |
5.7 (2.5) |
1.4 (1.3) |
13.2 (6.7) |
14.5 (5.9) |
27.7 (0.6) |
24.2 (3.0) |
1.40 (0.5) |
3.8 (2.9) |
3.6 (2.7) |
0.2 (0.5) |
20.4 (5.8) |
20.6 (5.7) |
| Age 8–9 | 33.9 (2.3) |
28.3 (5.1) |
1.72 (0.7) |
6.9 (4.2) |
5.6 (3.5) |
1.3 (1.3) |
21.4 (8.7) |
22.7 (7.9) |
35.7 (0.7) |
32.7 (3.1) |
1.21 (0.4) |
3.3 (3.0) |
3.0 (2.8) |
0.3 (0.6) |
29.4 (6.1) |
29.7 (5.9) |
| Age 10–11 | 34.7 (1.9) |
30.7 (4.0) |
1.35 (0.5) |
4.8 (3.3) |
4.1 (2.6) |
0.7 (1.0) |
25.9 (6.5) |
26.6 (5.8) |
35.8 (0.5) |
34.0 (2.4) |
1.08 (0.3) |
1.9 (2.3) |
1.8 (2.1) |
0.1 (0.3) |
32.1 (4.7) |
32.2 (4.5) |
| Age 12–13 | 35.7 (0.7) |
33.2 (2.4) |
1.12 (0.3) |
2.7 (2.3) |
2.4 (2.2) |
0.3 (0.5) |
30.6 (4.6) |
30.8 (4.4) |
36.0 (0.0) |
34.7 (1.7) |
0.93 (0.3) |
1.3 (1.7) |
1.3 (1.7) |
0.0 (0.0) |
33.5 (3.5) |
33.5 (3.5) |
| Age 14–15 | 35.8 (0.6) |
33.8 (2.3) |
1.03 (0.4) |
2.2 (2.1) |
2.0 (1.9) |
0.2 (0.4) |
31.6 (4.1) |
31.8 (3.9) |
36.0 (0.2) |
35.1 (1.3) |
.87 (0.2) |
0.8 (1.2) |
0.8 (1.2) |
0.0 (0.0) |
34.3 (2.5) |
34.3 (2.5) |
Mean (SD); note: Age 6–7: 28-item test, 8–15: 36-item test
AN Auditory Naming
VN Visual Naming
Corr Mean Number Correct
RT Mean response time
TOT Mean TOT (number of items >2 seconds or >20 correct with cue)
Gr2 Mean number of items >2 sec but < 20 sec (correct in 2–20 seconds)
Cue Mean number of items >20 sec but correct with cue
Less2 Mean number of items correct in < 2 seconds
SummScore Summary Score: Mean number of items <2 sec – TOT score (range: −36 – 36)
SS2 Summary Score-2: Number of items <2 sec – number of items >2 (i.e., correct in 2–20 seconds; range: −36– 36)
APPENDIX A2.
| AUDITORY NAMING TEST ITEMS | VISUAL NAMING TEST ITEMS | |
|---|---|---|
| Description |
Target word |
Item |
| 1. The planet we live on | EARTH | 1. POPCORN |
| 2. Where you go to borrow books | LIBRARY | 2. SHARK |
| 3. A long, yellow fruit with a thick peel | BANANA | 3. GUITAR |
| 4. The room of a house where people cook | KITCHEN | 4. TIGER |
| 5. The part of the body used for smelling | NOSE | 5. LADDER |
| 6. What you use to dry off your body | TOWEL | 6. STRAWBERRY |
| 7. The red sauce you put on a hamburger | KETCHUP | 7. ZEBRA |
| 8. The big grey animal with a long trunk | ELEPHANT | 8. BROCCOLI |
| 9. What a king wears on his head | CROWN | 9. MICROPHONE |
| 10. An instrument you beat with sticks | DRUM | 10. HAMBURGER |
| 11. The prickly plant that grows in the desert | CACTUS | 11. DRESS |
| 12. What swimmers wear to protect their eyes | GOGGLES | 12. SKUNK |
| 13. A pet that purrs | CAT | 13. TABLE |
| 14. The part of the shirt that covers your arms | SLEEVES | 14. DUCK |
| 15. What you look in to see yourself | MIRROR | 15. PUMPKIN |
| 16. The person who flies a plane | PILOT | 16. TROPHY |
| 17. A place with sand along a shore | BEACH | 17. SNOWMAN |
| 18. Something used to pound a nail | HAMMER | 18. HORSE |
| 19. What you wear to help you see | GLASSES | 19. ZIPPER |
| 20. A person who goes into space | ASTRONAUT | 20. STARFISH |
| 21. Where birds lay their eggs | NEST | 21. ORANGE |
| 22. What falls from trees in autumn | LEAVES | 22. WHEEL |
| 23. A kind of boat that travels underwater | SUBMARINE | 23. KANGAROO |
| 24. Something chewy you blow bubbles with | GUM | 24. GLOVE |
| 25. What you use to slice food | KNIFE | 25. SQUIRREL |
| 26. A small flying but that leaves an itchy bite | MOSQUITO | 26. BELT |
| 27. The imaginary animal that breathes fire | DRAGON | 27. PENGUIN |
| 28. What you use to wipe your face at a meal | NAPKIN* | 28. UMBRELLA* |
| 29. The white stuff used to write on a blackboard | CHALK | 29. LIZARD |
| 30. The meal you eat in the middle of the day | LUNCH | 30. NECKLACE |
| 31. What smoke comes out of on a roof | CHIMNEY | 31. RULER |
| 32. The list of foods served at a restaurant | MENU | 32. CHAIN |
| 33. The vegetable that grows on a cob | CORN | 33. POTATO |
| 34. What you use to measure temperature | THERMOMETER | 34. NAIL |
| 35. The part of the tree that grows underground | ROOT | 35. GIRAFFE |
| 36. The hard outside edges of bread | CRUST | 36. RAKE |
Last item for ages 6 to 7
Footnotes
Disclosure: The authors have no conflicts of interest to disclose.
References
- Baron IS (2004). Neuropsychological evaluation of the child. New York, NY: Oxford University Press. [Google Scholar]
- Bell B, Seidenberg M, Hermann B, & Douville K (2003). Visual and auditory naming in patients with left or bilateral temporal lobe epilepsy. Epilepsy Research, 55, 29–37. [DOI] [PubMed] [Google Scholar]
- Berl MM, Smith ML, Bulteau C, ILAE, Task Force for Pediatric Epilepsy Surgery for the, & Commissions of Pediatrics, Surgical Therapies. (in press). ILAE survey of neuropsychology practice in pediatric epilepsy surgery evaluation. Epileptic Disorders, in press. [DOI] [PubMed] [Google Scholar]
- Camara WJ, Nathan JS, & Puente AE (2000). Psychological test usage: Implications in professional psychology. Professional Psychology: Research and Practice, 31(2), 141–154. doi: doi: 10.1037/0735-7028.31.2.141 [DOI] [Google Scholar]
- Caramazza A (1997). How many levels of processing are there in lexical access? Cognitive Neuropsychology, 14, 177–208. [Google Scholar]
- Chen C, & Truscott J (2010). The Effects of Repetition and L1 Lexicalization on Incidental Vocabulary Acquisition. Applied Linguistics, 31(5), 693–713. doi: 10.1093/applin/amq031 [DOI] [Google Scholar]
- Cohen MJ, Town P, & Buff A (1988). Neurodevelopmental differences in confrontational naming in children. Developmental Neuropsychology, 4(1), 75–81. doi: 10.1080/87565648809540392 [DOI] [Google Scholar]
- Denckla MB, & Cutting LE (1999). History and significance of rapid automatized naming. Journal of Dyslexia, 49(1), 29–42. [Google Scholar]
- Diaz-Asper CM, Schretlen DJ, & Pearlson GD (2004). How well does IQ predict neuropsychological test performance in normal adults? Journal of the International Neuropsychological Society, 10(1), 82–90. doi: 10.1017/S1355617704101100 [DOI] [PubMed] [Google Scholar]
- Elliot CD (2006). Differential Ability Scales-Second Edition. Toronto: Pearson Canada Assessment, Inc. . [Google Scholar]
- Fastenau PS, Shen J, Dunn DW, Perkins SM, Hermann BP, Austin JK, . . . Austin, Joan K. (2004). Neuropsychological predictors of academic underachievement in pediatric epilepsy: moderating roles of demographic, seizure, and psychosocial variables. Epilepsia, 45(10), 1261–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley J, Garcia J, Shaw L, & Golden C (2009). IQ predicts neuropsychological performance in children. International Journal of Neuroscience, 119(10), 1830–1847. [DOI] [PubMed] [Google Scholar]
- Gollan Tamar H., Weissberger Gali H., Runnqvist Elin, Montoya Rosa I., & Cera Cynthia M. (2012). Self-ratings of spoken language dominance: A Multilingual Naming Test (MINT) and preliminary norms for young and aging Spanish-English bilinguals. Bilingualism: Language and Cognition, 15(3), 594–615. doi: 10.1017/S1366728911000332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodglass H, Theurkauf JC, & Wingfield A (1984). Naming latencies as evidence for two modes of lexical retrieval. Applied Psycholinguistics, 5, 135–146. [Google Scholar]
- Graves MF, Boettcher JA, Peacock JL, & Ryder RJ (1980). Word frequency as a predictor of students’ reading vocabularies. Journal of Reading Behavior, 12(2), 117–127. [Google Scholar]
- Guilford AM, & Nawojczyk DC (1988). Standardization of the Boston Naming Test at the kindergarten and elementary school levels. Language, Speech, and Hearing Services in Schools, 19(4), 395–400. [Google Scholar]
- Guimaraes CA, Li LM, Rzezak P, Fuentes D, Franzon RC, Augusta Montenegro M, . . . Guerreiro MM (2007). Temporal lobe epilepsy in childhood: comprehensive neuropsychological assessment. Journal of Child Neurology, 22(7), 836–840. [DOI] [PubMed] [Google Scholar]
- Hale S (1990). A global developmental trend in cognitive processing speed. Child Development, 61(3), 653–663. [PubMed] [Google Scholar]
- Halperin JM, Healey JM, Zeitchik E, Ludman WL, & Weinstein L (1989). Developmental aspects of linguistic and mnestic abilities in normal children. Journal of Clinical & Experimental Neuropsychology: Official Journal of the International Neuropsychological Society, 11(4), 518–528. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, Habeck CG, Pantazatos SP, Williams AC, & Hirsch J (2014). Shared space, separate processes: Neural activation patterns for auditory description and visual object naming in healthy adults. Human Brain Mapping, 35(6), 2507–2520. doi: 10.1002/hbm.22345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberger MJ, Goodman RR, Perrine K, & Tamny T (2001). Anatomical dissociation of auditory and visual naming in the lateral temporal cortex. Neurology, 56, 56–61. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, McClelland S, Williams AC, Goodman RR, & McKhann GM (2007). Distribution of auditory and visual naming sites in nonlesional temporal lobe epilepsy patients and patients with space-occupying temporal lobe lesions. Epilepsia, 48(3), 531–538. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, & Seidel WT (2003). Auditory and visual naming tests: Normative and patient data for accuracy, response time and tip-of-the-tongue. Journal of the International Neuropsychological Society, 9, 479–489. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, & Seidel WT (2009). Localization of cortical dysfunction based on auditory and visual naming performance. Journal of the International Neuropsychological Society(15), 529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberger MJ, Smith ML, MacAllister WS, Williams AC, & Seidel WT (2015). Auditory and visual naming in children with lateralized epilepsy Paper presented at the American Epilepsy Society Annual Meeting, Philadelphia. [Google Scholar]
- Hermann BP, Jones JE, Sheth R, Koehn M, Becker T, Fine J, . . . Seidenberg M (2008). Growing up with epilepsy: a two-year investigation of cognitive development in children with new onset epilepsy. Epilepsia, 49(11), 1847–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan EF, Goodglass H, & Weintraub S (1983). The Boston Naming Test, 2nd Edition. Philadelphia: Lea & Febiger. [Google Scholar]
- Kirk Ursula. (1992). Confrontation naming in normally developing children: Word-retrieval or word knowledge? Clinical Neuropsychologist, 6(2), 156–170. doi: 10.1080/13854049208401852 [DOI] [PubMed] [Google Scholar]
- Martielli TM, & Blackburn LB (2016. a). When a funnel becomes a martini glass: Adolescent performance on the Boston Naming Test. Child Neuropsychology, 22(4), 381–393. doi: 10.1080/09297049.2015.1014899 [DOI] [PubMed] [Google Scholar]
- Martielli TM, & Blackburn LB (2016. b). When a funnel becomes a martini glass: Adolescent performance on the Boston Naming Test. Child Neuropsychology, 22(4), 381–393. doi: 10.1080/09297049.2015.1014899 [DOI] [PubMed] [Google Scholar]
- Martin NA, & Brownell R (2011). Expressive one-word picture vocabulary test: Academic Therapy Publications. [Google Scholar]
- Mitrushina M, Boone KB, Razani J, & D’Elia LF (2005). The Boston Naming Test In Mitrushina M, Boone KB, Razani J & D’Elia LF (Eds.), Handbook of normative data for neuropsychological assessment (2 ed., pp. 173–199). New York, NY: Oxford University Press. [Google Scholar]
- Nunnally JC (1978). Psychometric theory (2 ed.). New York: McGraw-Hill. [Google Scholar]
- Nunnally JC, & Bernstein I (1994). Psychometric theory (3 ed.). New York: McGraw-Hill. [Google Scholar]
- Rabin LA, Barr WB, & Burton LA (2005). Assessment practices of clinical neuropsychologists in the United States and Canada: a survey of INS, NAN, and APA Division 40 members. Archives of Clinical Neuropsychology, 20(1), 33–65. doi: 10.1016/j.acn.2004.02.005 [DOI] [PubMed] [Google Scholar]
- Randolph C, Lansing AE, Ivnik RJ, Munro Cullum C, & Hermann BP (1999). Determinants of Confrontation Naming Performance. Archives of Clinical Neuropsychology, 14(6), 489–496. [PubMed] [Google Scholar]
- Ridderinkhof KR, & van der Molen MW (1997). Mental resources, processing speed, and inhibitory control: a developmental perspective. Biological Psychology, 45(1–3), 241–261. [DOI] [PubMed] [Google Scholar]
- Roberts RJ, & Hamsher KD (1984). Effects of minority status on facial recognition and naming performance. Journal of Clinical Psychology, 40(2), 539–545. [DOI] [PubMed] [Google Scholar]
- Ryder RJ, & Slater WH (1988). The Relationship Between Word Frequency and Word Knowledge. The Journal of Educational Research 81(5), 213–317. doi: 10.1080/00220671.1988.10885840 [DOI] [Google Scholar]
- Schmitter-Edgecombe M, Vesneski M, & Jones DWR (2000). Aging and word-finding: A comparison of spontaneous and constrained naming tests. Archives of Clinical Neuropsychology, 15(6), 479–493. [PubMed] [Google Scholar]
- Tomaszewki-Farias S, Harrington G, Broomand C, & Seyal M (2005). Differences in functional MR imaging activation patterns associated with confrontation naming and responsive naming. American Journal of Neuroradiology, 26(10), 2492–2499. [PMC free article] [PubMed] [Google Scholar]
- Verhoeven L, van Leeuwe J, & Vermeer A (2011). Vocabulary growth and reading development across the elementary school years. Scientific Studies of Reading, 15(1), 8–25. doi: 10.1080/10888438.2011.536125 [DOI] [Google Scholar]
- Wechsler D (1999). Wechsler Abbreviated Scale of Intelligence. San Antonio: Psychological Corporation. [Google Scholar]
- Wechsler D (2009). Wechsler Individual Achievement Test®-Third Edition (WIAT®-III). San Antonio. [Google Scholar]
- Wechsler D (2012). Wechsler Preschool and Primary Scale of Intelligence™ - Fourth Edition. New York: Pearson, Inc. [Google Scholar]
- Williams KT (1997). Expressive Vocabulary Test. Circle Pines: American Guidance Service. [Google Scholar]
- Yeates KO (1994). Comparison of developmental norms for the Boston Naming Test. Clinical Neuropsychologist, 8(1), 91–98. [Google Scholar]


