Abstract
The role of Y chromosome in prostate cancer progression and incidence is not well known. Among the 46 chromosomes, Y chromosome determines the male gender. The Y chromosome is smaller than the X chromosome and contains only 458 genes compared to over 2000 genes found in the X chromosome. The Y chromosome is prone to high mutation rates, created exclusively in sperm cells due to the highly oxidative environment of the testis. Y chromosome harbors epigenetic information, which affects the expression of genes associated with the incidence and progression of prostate cancer. In this review, we focus on Y chromosome related genetic abnormalities, likely to be involved in the development and progression of prostate cancer.
Keywords: Y chromosome, Prostate cancer, DYZ1, Cancer Genetics
2. INTRODUCTION
Prostate cancer is the leading non-cutaneous cancer among men (1). About 1 in 7 men is diagnosed with prostate cancer at some point in his lifetime (2). Prostate cancer cells like other cancer cells migrate and metastasize. The small walnut shaped prostate gland produces seminal fluid required to nourish and transport sperm (3). Prostate cancer grows slowly and initially remains confined to the gland, however, in time will, like other tumors, metastasize (4). Metastasis usually occurs in the lymph nodes, bones, liver, lungs and brain (5). Prostate cancers are mostly adenocarcinomas (6). Measuring the levels of prostate specific antigen (PSA) in men’s blood is the standard screening practice for prostate cancer patients. An elevated level of PSA predicts the patient’s risk of developing prostate cancer in future. Clinical presentations may include urinary difficulty, blood in the semen, bone pain, erectile dysfunction and discomfort in the pelvic region (7). A complete pathophysiology of prostate cancer is unclear (8). A Gleason score determines prostate cancer severity and is a system of grading prostate cancer tissue based on microscopic appearance (9). Gleason scores range from 2 to 10 and indicate how likely a tumor will spread. A low Gleason score means the cancer tissue is similar to normal prostate tissue and the tumor is less likely to spread; a high Gleason score indicates the cancer tissue is very different from normal prostate and is more likely to spread. Several factors contribute to prostate cancer, one major risk factor is increased age until 70 years and then declines thereafter (10). Another risk factor is gonorrhea; however, the reason for this association has not been established (11). Race and family history are also established risk factors, with men of African American ancestry having higher documented prostate cancer rates than Caucasian men (12). There are several risk factors associated with prostate cancer progression and incidence such as smoking (13), diet (14), family history (12) and environmental exposure (15).
Reports suggest prostate cancer develops in prostate cells that have abnormal repetitive mutations (16–19), understanding how these mutations are initiated will enable exploration of the factors determining mutation and its evolution. After spermatogenesis, sperm are stored in an oxidative environment in the testis contributes to mutational events. Chromosomes including Y chromosome goes through multiple cell divisions. Each cellular division provides an opportunity for mutation. Researchers reported a provisional association between Y chromosome modification and prostate cancer (20–24). Evidence found using computerized based method to gather population-based genetic information to establish the connection between Y chromosome gene alterations and prostate cancer after conducting analysis (23). Deep understanding of Y chromosome s and its genes may provide a link to prostate cancer and using different approaches to modulate the altered gene expression may have potential cure to prostate cancer. However, research regarding Y chromosome mutations is limited because of its size, high levels of repetitive genetic information and low levels of recombination (25). In reviewing the Y chromosome we hope to explore the potential role of the Y chromosome and associated genes in prostate cancer development.
3. PROSTATE CANCER AND THE Y CHROMOSOME
3.1. The Y chromosome and genetic epidemiology
Epidemiologic data illustrates men have higher rates of cancer than females (26). In prostate cancer progression genetics (27), age (28), ethnicity (12), dietary factors (14), and environmental factors (15) all play a role. In terms of the Y chromosome and prostate cancer, there are several distinct disparities between certain groups such as African American men and their susceptibility to the disease (29). Interestingly, there has been a recent increase in the incidence of prostate cancer rates in Japanese men (30). These increased rates can be accounted for several external factors such as the change to a western diet, pollution and screening for disease. These factors may be related to environmental and genetic influences in Japanese immigrants in Hawaii (30). Globally, there has been a change to a more westernized diet that could be having detrimental effects on these men’s susceptibility to prostate cancer (31, 32). Moreover, in European ancestry population, 3,995 prostate cancer cases and 3,815 control cases were genotyped for Y chromosome binary markers (25). The Y chromosome based genetic information in different ethnic groups represented the incidence of prostate cancer in African American men is twice that of Caucasian men and 10 times higher in Japanese men (25). Similarly, other reports suggest higher incidence of prostate cancer in African American men (33). It was determined that risk variants of rs114798100 and rs111906923 - a new association signal are found exclusively in US men with African ancestry (33). These variants found close to long noncoding RNAs (lncRNAs) which are associated with prostate cancer. These lncRNAs include PRNCR1, PCAT1 and PCAT2 (33). Therefore, this may be the apparent disparities between men of African ancestry in terms of prostate cancer (33).
Furthermore, there is a connection between the rare haplogroup, E1b1b1c, which is associated with Ashkenazi Jewish ancestry and stage I prostate cancer (25). Additionally in a cohort study report, four different ethnic groups were selected from Hawaii and California (34). In one Japanese men study group, discovered to have a significant predisposition to prostate cancer, with high rates in younger individuals (35). These racial differences are shown Table 1. Based on the accumulated literature this is apparent the genetic variance in Y chromosome s play significant role in prostate cancer.
Table 1.
Racial differences are shown with genetic variance in patients with prostate cancer
| Population | Genetic Variance | Relation to Prostate Cancer | References |
|---|---|---|---|
| White | n/a | (34) | |
| African American | E3a | lineage associated with increased risk | (34) |
| Japanese | O3 | lineage associated with increased risk | (34) |
| Latino | n/a | (34) | |
| Ashkenazi Jewish | E,E1b1b, E1b1b1a1, E1b1b1b1 | haplogroup protective effect against prostate cancer | (25) |
3.2. Genetic mutations
Prostate cancer patient’s genome wide next generation sequencing analysis detected variants in chromosomal abnormalities (36). The genomic instability of 3.4. kb DYZ1 was observed in individuals with prostate cancer, cases of repeated abortion and males who were exposed to natural background radiation (37). The variation of DYZ1 in these males correlated with genetic constraints/anomalies. The mechanisms of genomic instability of DYZ1 is not well explored; however, it is understood that it plays a vital role in the structural integrity of the Y chromosome maybe by absorbing mutations, and can be used as a marker for Y chromosome integrity. There were well defined deletions observed in three different regions (265, 773 and 275 bp) of DYZ1 when compared with DNA from normal males. Moreover, the copy number of DYZI was inconsistent and fluctuated below and above the normal range associated with an abnormal genotype (38). Additional reports explore the fate of DYZ1 in monozygotic male twins. It was determined that DYZ1 varied in both sequence polymorphisms and copy number between the twins. The sequence variation observed in germline and blood DNA of the same individual. Therefore, genetic changes in DYZ1 can be used as a marker (37).
3.2.1. Loss of Y chromosome
Age related loss of the Y chromosome is a well-known phenomenon in normal hematopoietic cells. Swedish men blood samples (approximately 6000) and medical records examined using single-nucleotide polymorphism (SNP) array analysis in order to quantify the loss of the Y chromosome (LOY) in blood cells for potential causes of LOY (39). The association between LOY rate and other variables includes age, education level, exercise, smoking and cholesterol levels. The same research group also hypothesized that LOY potentially gives cells a proliferative advantage presumably through elimination of tumor suppressor genes on the Y chromosome (40). Recent report suggest that LOY in cancer patients is more significant predictor than age, however age does not contribute to even increased number of subjects with detectable LOY in cancer patients cohort (26). The higher rates of LOY is associated with death at a younger age and more susceptibility to cancer (41). LOY may contribute to compromising the cancer fighting abilities of immune cells (26). However, another study suggested that, who smoke occasionally have less LOY rates than chain smokers (35). Complete loss of the Y chromosome was reported in seminal vesicles of 28 prostate cancer patients and 11 bladder cancer patients (42). However, loss of the Y chromosome has been reported in only 12 prostate cancer tissue microarray containing samples of 3,261 patients treated with radical prostatectomy, no significant associations found between LOY and patient age, tumor stage and risk of PSA recurrence (41). Y chromosome loss was significantly higher than expected percent seen in lymphocytes, which may be indicative of aging rather than alteration in the prostate due to carcinogenesis. The loss of the Y chromosome in epithelial cells is a predictive biomarker for prostate cancer in men (41). Available literature suggest that loss of Y chromosome and its genes are closely associated with immunosurveillance modification and various cancers.
3.2.2. Y chromosome loci
The Y chromosome has specific loci genetically linked to familial prostate cancer inheritance (22). On the Y chromosome, 51 sequence tagged sites were screened on coding region of SRY gene related with male-specific region and sequence and copy number variations in DYZ1 gene in LNCaP and DU145 prostate cancer cells (43). Though both of these cell lines were derived from different origins; LNCaP cells (androgen dependent metastasize to lymph nodes) and DU145 (androgen independent, from brain metastasis) prostate cancer cells. In another study, Malaysian men with prostate cancer reported to have four Y-linked short tandem repeats (STRs) were; DYS388, DYS435, DYS437 and DYS439 on the DYS loci (31). STRs, referred to as microsatellites or simple sequence repeats (SSRs; are stretches of DNA) that contain core repeated sequences between two and seven nucleotides in length (20). Men who have allele 12 of DYS388, allele 14 of DYS439, or haplotype CAAA are more likely to develop prostate cancer; and that those belonging to lineages with allele 10 of DYS388 or haplotype AABC were more resistant to develop prostate cancer (33). In another study, 23 prostate cancer susceptibility loci were identified (44). Table 2 summarizes Y chromosome related loci. These loci were able to explain approximately 30 percent of the familial risk for the disease (44). Another specific region of the Y chromosome that contains risk variants for prostate cancer is the 8q24 region. This observation was made after using fine mapping identified a rare variation of the risk region of 8q24 when prostate cancer patients were compared to healthy control subjects (45).
Table 2.
Specific loci associated with prostate cancer found on the Y chromosome
| Loci | High incidence of prostate cancer | References |
|---|---|---|
| rs114798100 | High incidence of prostate cancer | (45) |
| rs111906923 | High incidence of prostate cancer | (45) |
| allele 12 of DYS388 | High incidence of prostate cancer | (45) |
| allele 14 of DYS439 | Risk variant for prostate cancer in Europeans | (22) |
| rs1218582, rs119022336, rs4245739, rs3771570, rs761694, rs1894292, rs6869841, rs3096702, rs2273669, rs1933488, rs12155172, rs11135910, rs3850699, rs11568818, rs1270884, rs8008270, rs7141529, rs6844232, rs11650494, rs7241993, rs2427345, rs6062509, rs2405942 | Low incidence of prostate cancer | (45) |
| allele 10 of DYS388 | High incidence of prostate cancer in African Americans | (22) |
| 8q24 region | High incidence of prostate cancer in African Americans | (22), (63) |
| PRNCR1 | High incidence of prostate cancer in African Americans | (45, 64) |
| PCAT1 | High incidence of prostate cancer in African Americans | (45, 65) |
| PCAT2 | (45, 65) |
3.2.3. Y chromosome splice variants
Splice variation or alternative splicing is a process resulting in multiple transcripts generated from a single gene (46). The effect of splice variation of Y chromosome genes has not been well-characterized (47). However, Lystine (K)-specific demethlase 5D (KDM5D), is one gene found on the Y chromosome, located in the AZFb region that encodes for a JmjC-domain-containing protein. The KDM5D gene is capable of demethylating di and tri-methyl H3K4, a known Y chromosome suppressor. Two novel splice variants of KDM5D were detected with lengths of 2650bp and 2400bp corresponding to proteins in DU-145 human prostate cancer cell line (24). Silencing these variants increased the growth of prostate cancer cells and reduced cell-mediated death. These variants reduced cell-mediated death. Playing a role in RNA processing, protein synthesis, apoptosis, cell cycle and cell growth, variants of KDM5D are promising targets for tumor specific chemotherapeutics (24).
3.2.4. Y chromosomes and related genes
Reports suggest that certain genes present on the Y chromosome have a connection to the incidence of prostate cancer (44). Prostate cancer known to have complex polygenetic properties. Experimentally 15 genes on Y chromosome were observed link to prostate cancer (48). In these genes; SRY, XKRY2, AMELY, UTY, DDX3Y and EIF1AY were observed replaced by BPY2 and RPS4Y1 in cancer network. BPY2 found on Y chromosome, interacts with ubiquitin protein ligase E3A, which may be involved in male germ cell development and infertility. RPS4Y1 encodes for the protein Y isoform 1, which is related the function of ribosomes; organelles involved in protein translation. The roles of following genes are summarized in Table 3 and represented increased expression in prostate cancer. Next, 19 genes on Y chromosome are linked to prostate cancer; however, 12 genes have already been identified as having a putative role in prostate cancer. These genes were identified using an independent co-expression network approach to reconstruct normal and cancerous stages (49). The genes, which represented low expression on the Y chromosomes are summarized in Table 4. The most prominent genes in prostate cancer are BPY2, UTY, SRY and EIF1AY (48).
Table 3.
Genes associated with high expression in prostate cancer found on the Y chromosome
| Genes | Full Name | Role | References |
|---|---|---|---|
| BPY2 | Basic Charge, Y-Linked, 2 | Cell growth | (48, 62) |
| RPS4Y1 | Ribosomal Protein S4, Y-Linked | Cell growth | (48,62,66) |
| NLGN4Y | Neuroligin 4 Y linked | Cell growth | (48, 67) |
| VCY1B | Variable Charge, Y-Linked 1B | Cell growth | (48) |
| RBMY1E | RNA Binding Motif Protein, Y-Linked, Family 1, Member E | Cell growth | (48) |
| ZFY | Zinc Finger Protein, | ||
| Y-Linked | Cell growth | (48, 62) | |
| TMSB4Y | Thymosin Beta 4, Y-Linked | Cell growth | (48, 67, 68) |
| XKRY2 | XK Related, Y-Linked 2 | Cell growth | (48, 69) |
| GPI | Glucose-6-Phosphate Isomerase | Tumor growth | (49, 70) |
| SMAD3 | SMAD Family Member 3 | Prostate cancer cell growth | (49, 70) |
| HMGB2 | High Mobility Group Box 2 | Cell growth castration-resistant in prostate cancer | (49) |
| SF1 | Steroidogenic factor 1 | Promotes aggressive growth of castration-resistant prostate cancer cells by stimulating steroid synthesis and cell proliferation. | (49,71–73), |
| IL10RB | Interleukin 10 Receptor Subunit Beta | Benign prostate hyperplasia | (49, 74) |
| RPS4Y1 | Ribosomal Protein S4, Y-Linked | Protein synthesis | (48,62,66,75) |
| FGFR1 | Fibroblast Growth Factor Receptor 1 | Tissue development | (49, 76) |
| MYB | Transcriptional activator Myb | Proliferation and differentiation of hematopoietic progenitor cells | (49, 77) |
| KLK3 | Kallikrein-3 | Biomarker for prostate cancer | (49, 78, 79) |
| HEXA | Hexosaminidase Subunit Alpha | Protein synthesis | (49, 80) |
| OLFM1 | Olfactomedin 1 | Nerve tissue | (48) |
| CYL1B | Chromodomain Y-Linked 1B | Gene repression | (48) |
| SFN | Stratifin | Cell growth | (49, 81, 82) |
| CD44 | CD44 Blood molecule (Indian Blood Group) | Cell migration | (49, 83) |
| Slug | Snail family transcriptional repressor 2 | Antiapoptosis Activity | (50, 84) |
| UBE3A | Ubiquitin Protein. Ligase E3A | Targeting for cell degradation | (49, 85) |
| TSPY | Testis Specific Protein, Y linked | Prostate cancer cell progression | (59) (60) (55) |
| H3K4 | Histone H3 Lysine 4 | Cell Growth | (51, 86) |
| CYORF15B | Chromosome Y Open Reading Frame 15B | Cell Growth | (48) |
| PRY2 | PTPN13-Like, Y-Linked 2 | Cell Growth | (48) |
| DAZ4 | Deleted In Azoospermia 4 | Cell growth | (48) |
| PRKY | Protein Kinase, Y-Linked, Pseudogene | Cell Growth | (48) |
| PCDH11Y | Protocadherin 11 Y-Linked | Cell Growth | (48) |
Table 4.
Genes associated with high expression in prostate cancer found on the Y chromosome
| Genes | Full Name | Role | References |
|---|---|---|---|
| RB1 | RB Transcriptional Corepressor 1 | Negative regulator of the cell cycle | (49, 87, 88) |
| FAS | Fas Cell Surface Death Receptor | Apoptosis | (49) |
| SRY | Sex-Determining Region Y | Prostate cancer cell growth | (48, 89, 90) |
| XKRY2 | XK Region, Y linked | Prostate cancer cell growth | (48, 89, 90) |
| AMELY | Amelogenin, Y linked | Prostate cancer cell growth | (48, 62) |
| UTY | Ubiquitously Transcribed Tetratricopeptide Repeat Containing, Y linked | Prostate cancer cell growth | (48, 62, 67, 68) |
| DDX3Y | DEAD-Box Helicase 3, Y-Linked | Prostate cancer cell growth | (48) |
| EIF1AY | Eukaryotic Translation Initiation Factor 1A, Y-Linked | Prostate cancer cell growth | (48, 62, 68) |
| USP9Y | Ubiquitin Specific Peptidase 9, Y-Linked | Cell growth, prevent protein degradation | (48, 67) |
3.2.5. Y Chromosome and tumor suppressor genes
Prognosis of prostate cancer requires sequence of events leading to the development of tumor and metastasis. To deal with such successive events we need to have good modulators of prognostic markers and tumor suppressor genes. KDM5D and MSY, represses gene expression associated with cell invasion (50) (Table 5). The male specific protein is believed to be involved in inter- and intra-sexual communication (51), and found to specifically repress invasion-associated genes MMP1, MMP2, MMP3, MMP7 when demethylated (50). These genes are involved in extracellular matrix degradation and occurs under normal physiological conditions such as embryonic development, reproduction, and tissue remodeling (52). However, matrix metalloproteinases play major role in arthritis as well as in cancer metastasis. Suppression of these genes may control metastasis (53). The suppressor KDM5D was significantly down regulated in metastatic prostate tissues compared to normal prostate tissues (51). Therefore, we emphasize Y chromosome gene involvement in suppressing prostate cancer. Low levels of this gene were associated with slow disease progression. Moreover, in metastatic prostate cancer these genes are frequently deleted (50). These findings highlight the role Y chromosome mediated transcriptional regulation plays in the prevention of prostate cancer metastasis (50).
Table 5.
Genes associated with prostate cancer tumor suppression
3.2.6. Alleles
Allele is a variant form of a gene and micro-allele is a short tandem repeat with a fractional value. Micro-alleles sometimes referred as microvariants as fractional markers or partial repeats. Micro variant alleles DYS458 sequence of DYZI gene are over expressed in individuals with prostate cancer. Moreover report suggested that allele 12 of DYS393 and allele 19 of DYS458 might have a protective effect in patients. However, patients carrying allele 13 of DYS393 appeared to have an increased risk to prostate cancer (38). A recent report found a significant amount of prostatic cancerous tumors had recurrent, non-coding sequences of genes (38). The analyzed genome sequences from non-indolent prostate tumors represented recurrent molecular aberrations and novel prognostic translocations, inversions and epigenetic events (54). Further in-depth knowledge of short tandem repeat allele may add more information to genetic profiling of prostate cancer by overcoming from resistance or susceptibility.
4. EXPERIMENTAL MODELS:
There are limited animal models available to determine the Y-linked nature of prostate cancer. Various transgenic mice models recapitulate different aspects of prostate cancer development and metastasis. The TSPY (testis specific protein, is Y-linked), is a proto-oncogene involved in the onset and progression of several human cancers such as skin, liver and prostate (55). Moreover, TSPY’s binding partner EEF1A is elevated in clinical cases of prostate cancer compared to latent prostate cancer or non-cancerous cases. These findings suggest the expression of TSPY is associated with the growth and progression of prostate cancer. Y chromosome of rat harbors a single functional copy of TSPY gene, and this is only expressed in elongating spermatids while the human TSPY is primarily expressed in spermatogonia and spermatocytes (56, 57). Later Schubert et al. generated a transgenic mouse line (58), which was harboring 50 copies of human TSPY gene on Y chromosome of the mouse, known as TgTSPY9. More detail about TgTSPY9 transgene, it was 8.2.-kb transgene contains 2.9.5-kb the promoter region, 2.8.-kb structural gene and 2.4.5-kb 3’ flanking sequence of the human TSPY gene. Like human TSPY gene, it was expressed at early stages of spermatogenesis in spermatogonia and spermatocytes (58). There are potential limitations with available mouse models of prostate cancer in mimicking the ectopic expression of TSPY, when Y-located TSPY transgene (TgTSPY9) was introduced to LADY mouse model of prostate cancer. TgTSPY9 transgene was expressed in FoxA1-negative hypercellular stroma of LADY mice prostate, conversely in human clinical prostate cancer specimens TSPY is expressed in FoxA1-positive epithelial cells (59).
Another approach utilizes mice by transplanting non myelo-ablative MHC-matched; single Y chromosome-encoded, or multiple minor histocompatibility antigen-mismatched hematopoietic cells (52). Transplanting allogeneic hematopoietic stem cells synergized with vaccination to cure prostate cancer (60). However, this approach is not well understood enough to be used as a method to treat solid tumors (60).
One report, which evaluated the role of genes on Y chromosome in human prostate cancer using athymic nude mice (61). Histidinol gene tagged Y chromosome was transferred into parental PC-3 cells lacking Y chromosome. TSA–FISH was able to detect Histidino gene on the Y chromosome. Tumorigenicity of these PC-3 hybrids were evaluated in vivo and in vitro. PC-3 hybrid injected mice showed tumor growth in only one mouse; however, tumors grew in all mice injected with parental PC-3 cells. Results showed the addition of Y chromosome prevented tumor formation in athymic nude mice, and blocked tumorigenesis in-vitro (61).
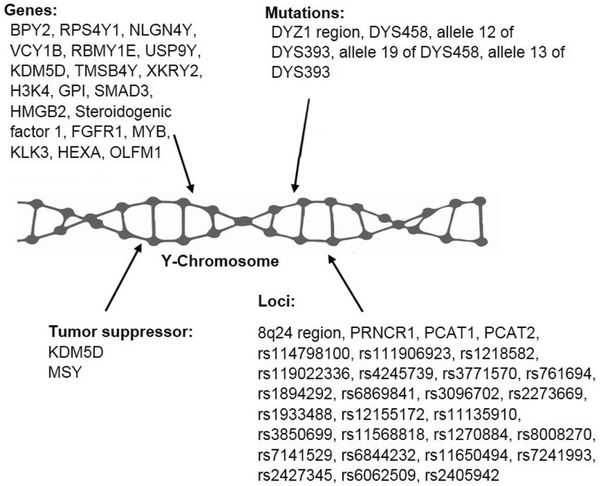
5. SUMMARY AND CONCLUSIONS
Available studies suggest the involvement of the Y chromosome in contributing to the development of prostate cancer (Figure 1). Prostate cancer risk and susceptibility are associated with several epidemiological factors. Mutations on DYZI region of the Y chromosome can act as a marker for the disease (37, 41). Specific alleles found on the DYZ1 region were associated with an increased risk to prostate cancer while other alleles shown protective effects. Another abnormality seen in prostate cancer was LOY (39). LOY correlates with incidence of prostate cancer. The Y chromosome encodes for numerous genes associated with prostate cancer such as BPY2 and RPS4Y1 (48). These genes are upregulated during prostate cancer, associated with cell growth, and may contribute to tumor progression and metastasis. Other genes on the Y chromosome, specifically CDY1B, RB1, SFN and TNFRSF25 are downregulated in prostate cancer (48). These genes specifically reduce cell growth and promote apoptosis so the low expression may suppress prostate cancer progression. Silencing of KDM5D splice variants increased prostate cancer cell growth and reduced apoptosis in DU-145 cells (24). Furthermore, tumor suppressor effects on the Y chromosome revealed that genes; KDM5D and MSY act as tumor suppressors and are down regulated in metastatic prostate (50, 62).
Figure 1.
Y chromosome related genes, loci, mutations and involved tumor suppressor genes.
Based on published research articles, Y chromosome mutations may play a significant role in prostate cancer progression. Once we have in-depth knowledge about the pathophysiology of the mutations, then we may have novel solutions to prevent or reverse these genetic alterations.
6. ACKNOWLEDGEMENTS
This study was supported by NIH grants R21 CA190921 funded to SS.
7. REFERENCES
- 1.Benafif S and Eeles R: Genetic predisposition to prostate cancer. Br Med Bull, 120(1), 75–89 (2016) 10.1093/bmb/ldw039 PMid: [DOI] [PubMed] [Google Scholar]
- 2.Machnes Z, Avtalion R, Shirak A, Trombka D, Wides R, Fellous M and Don J: Male-specific protein (MSP): a new gene linked to sexual behavior and aggressiveness of tilapia males. Horm Behav, 54(3), 442–9 (2008) 10.1016/j.yhbeh.2008.03.014 PMid: [DOI] [PubMed] [Google Scholar]
- 3.Thalmann GN, Anezinis PE, Chang SM, Zhau HE, Kim EE, Hopwood VL, Pathak S, von Eschenbach AC and Chung LW: Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res, 54(10), 2577–81 (1994) [PubMed] [Google Scholar]
- 4.Logothetis CJ and Lin SH: Osteoblasts in prostate cancer metastasis to bone. Nat Rev Cancer, 5(1), 21–8 (2005) 10.1038/nrc1528 PMid: [DOI] [PubMed] [Google Scholar]
- 5.Jin JK, Dayyani F and Gallick GE: Steps in prostate cancer progression that lead to bone metastasis. Int J Cancer, 128(11), 2545–61 (2011) 10.1002/ijc.26024 PMid: PMCid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boxer RJ: Adenocarcinoma of the prostate gland. Urol Surv, 27(3), 75–94 (1977) [PubMed] [Google Scholar]
- 7.Kayser M, Roewer L, Hedman M, Henke L, Henke J, Brauer S, Kruger C, Krawczak M, Nagy M, Dobosz T, Szibor R, de Knijff P, Stoneking M and Sajantila A: Characteristics and frequency of germline mutations at microsatellite loci from the human Y chromosome, as revealed by direct observation in father/son pairs. Am J Hum Genet, 66(5), 1580–8 (2000) 10.1086/302905 PMid: PMCid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siegel RL, Miller KD and Jemal A: Cancer statistics, 2016. CA Cancer J Clin, 66(1), 7–30 (2016) 10.3322/caac.21332 PMid: [DOI] [PubMed] [Google Scholar]
- 9.Gordetsky J and Epstein J: Grading of prostatic adenocarcinoma: current state and prognostic implications. Diagn Pathol, 11, 25 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tosoian JJ, Alam R, Gergis C, Narang A, Radwan N, Robertson S, McNutt T, Ross AE, Song DY, DeWeese TL, Tran PT and Walsh PC: Unscreened older men diagnosed with prostate cancer are at increased risk of aggressive disease. Prostate Cancer Prostatic Dis (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lian WQ, Luo F, Song XL, Lu YJ and Zhao SC: Gonorrhea and Prostate Cancer Incidence: An Updated Meta-Analysis of 21 Epidemiologic Studies. Med Sci Monit, 21, 1902–10 (2015) 10.12659/MSM.893579 PMid: PMCid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vertosick EA, Poon BY and Vickers AJ: Relative value of race, family history and prostate specific antigen as indications for early initiation of prostate cancer screening. J Urol, 192(3), 724–8 (2014) 10.1016/j.juro.2014.03.032 PMid: PMCid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson KM, Markt SC, Fang F, Nordenvall C, Rider JR, Ye W, Adami HO, Stattin P, Nyren O and Mucci LA: Snus use, smoking and survival among prostate cancer patients. Int J Cancer, 139(12), 2753–2759 (2016) 10.1002/ijc.30411 PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hackshaw-McGeagh LE, Perry RE, Leach VA, Qandil S, Jeffreys M, Martin RM and Lane JA: A systematic review of dietary, nutritional, and physical activity interventions for the prevention of prostate cancer progression and mortality. Cancer Causes Control, 26(11), 1521–50 (2015) 10.1007/s10552-015-0659-4 PMid: PMCid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva JF, Mattos IE, Luz LL, Carmo CN and Aydos RD: Exposure to pesticides and prostate cancer: systematic review of the literature. Rev Environ Health, 31(3), 311–27 (2016) 10.1515/reveh-2016-0001 PMid: [DOI] [PubMed] [Google Scholar]
- 16.Heaphy CM, Gaonkar G, Peskoe SB, Joshu CE, De Marzo AM, Lucia MS, Goodman PJ, Lippman SM, Thompson IM Jr., Platz EA and Meeker AK: Prostate stromal cell telomere shortening is associated with risk of prostate cancer in the placebo arm of the Prostate Cancer Prevention Trial. Prostate, 75(11), 1160–6 (2015) 10.1002/pros.22997 PMid: PMCid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song JZ, Stirzaker C, Harrison J, Melki JR and Clark SJ: Hypermethylation trigger of the glutathione-S-transferase gene (GSTP1) in prostate cancer cells. Oncogene, 21(7), 1048–61 (2002) 10.1038/sj.onc.1205153 PMid: [DOI] [PubMed] [Google Scholar]
- 18.Tilley WD, Buchanan G, Hickey TE and BenTel JM: Mutations in the androgen receptor gene are associated with progression of human prostate cancer to androgen independence. Clin Cancer Res, 2(2), 277–85 (1996) [PubMed] [Google Scholar]
- 19.MacGrogan D, Levy A, Bostwick D, Wagner M, Wells D and Bookstein R: Loss of chromosome arm 8p loci in prostate cancer: mapping by quantitative allelic imbalance. Genes Chromosomes Cancer, 10(3), 151–9 (1994) 10.1002/gcc.2870100302 PMid: [DOI] [PubMed] [Google Scholar]
- 20.Van Den Berg C, Guan XY, Von Hoff D, Jenkins R, Bittner C Griffin, Kallioniemi O, Visakorpi, McGill, Herath J and et al. : DNA sequence amplification in human prostate cancer identified by chromosome microdissection: potential prognostic implications. Clin Cancer Res, 1(1), 11–8 (1995) [PubMed] [Google Scholar]
- 21.Cannon-Albright LA, Farnham JM, Bailey M, Albright FS, Teerlink CC, Agarwal N, Stephenson RA and Thomas A: Identification of specific Y chromosomes associated with increased prostate cancer risk. Prostate, 74(9), 991–8 (2014) 10.1002/pros.22821 PMid: PMCid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khosravi P, Gazestani VH, Asgari Y, Law B, Sadeghi M and Goliaei B: Network-based approach reveals Y chromosome influences prostate cancer susceptibility. Comput Biol Med, 54, 24–31 (2014) 10.1016/j.compbiomed.2014.08.020 PMid: [DOI] [PubMed] [Google Scholar]
- 23.Yao L, Ren S, Zhang M, Du F, Zhu Y, Yu H, Zhang C, Li X, Yang C, Liu H, Wang D, Meng H, Chang S, Han X, Sun Y and Sun Y: Identification of specific DNA methylation sites on the Y chromosome as biomarker in prostate cancer. Oncotarget, 6(38), 40611–21 (2015) 10.18632/oncotarget.6141 PMid: PMCid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jangravi Z, Tabar MS, Mirzaei M, Parsamatin P, Vakilian H, Alikhani M, Shabani M, Haynes PA, Goodchild AK, Gourabi H, Baharvand H and Salekdeh GH: Two Splice Variants of Y Chromosome-Located Lysine-Specific Demethylase 5D Have Distinct Function in Prostate Cancer Cell Line (DU-145). J Proteome Res, 14(9), 3492–502 (2015) 10.1021/acs.jproteome.5b00333 PMid: [DOI] [PubMed] [Google Scholar]
- 25.Wang Z, Parikh H, Jia J, Myers T, Yeager M, Jacobs KB, Hutchinson A, Burdett L, Ghosh A, Thun MJ, Gapstur SM, Ryan Diver W, Virtamo J, Albanes D, Cancel-Tassin G, Valeri A, Cussenot O, Offit K, Giovannucci E, Ma J, Stampfer MJ, Michael Gaziano J, Hunter DJ, Dutra-Clarke A, Kirchhoff T, Alavanja M, Freeman LB, Koutros S, Hoover R, Berndt SI, Hayes RB, Agalliu I, Burk RD, Wacholder S, Thomas G and Amundadottir L: Y chromosome haplogroups and prostate cancer in populations of European and Ashkenazi Jewish ancestry. Hum Genet, 131(7), 1173–85 (2012) 10.1007/s00439-012-1139-5 PMid: PMCid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noveski P, Madjunkova S, Sukarova Stefanovska E, Matevska Geshkovska N, Kuzmanovska M, Dimovski A and Plaseska-Karanfilska D: Loss of Y Chromosome in Peripheral Blood of Colorectal and Prostate Cancer Patients. PLoS One, 11(1), e0146264 (2016) 10.1371/journal.pone.0146264 PMid: PMCid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eeles R, Goh C, Castro E, Bancroft E, Guy M, Al Olama AA, Easton D and Kote-Jarai Z: The genetic epidemiology of prostate cancer and its clinical implications. Nat Rev Urol, 11(1), 18–31 (2014) 10.1038/nrurol.2013.266 PMid: [DOI] [PubMed] [Google Scholar]
- 28.Hussein S, Satturwar S and Van der Kwast T: Young-age prostate cancer. J Clin Pathol, 68(7), 511–5 (2015) 10.1136/jclinpath-2015-202993 PMid: [DOI] [PubMed] [Google Scholar]
- 29.Ewis AA, Lee J, Naroda T, Sano T, Kagawa S, Iwamoto T, Shinka T, Shinohara Y, Ishikawa M, Baba Y and Nakahori Y: Prostate cancer incidence varies among males from different Y chromosome lineages. Prostate Cancer Prostatic Dis, 9(3), 303–9 (2006) 10.1038/sj.pcan.4500876 PMid: [DOI] [PubMed] [Google Scholar]
- 30.Severson RK, Nomura AM, Grove JS and Stemmermann GN: A prospective study of demographics, diet, and prostate cancer among men of Japanese ancestry in Hawaii. Cancer Res, 49(7), 1857–60 (1989) [PubMed] [Google Scholar]
- 31.Bashir MN: Epidemiology of Prostate Cancer. Asian Pac J Cancer Prev, 16(13), 5137–41 (2015) 10.7314/APJCP.2015.16.13.5137 PMid: [DOI] [PubMed] [Google Scholar]
- 32.Ito K: Prostate cancer in Asian men. Nat Rev Urol, 11(4), 197–212 (2014) 10.1038/nrurol.2014.42 PMid: [DOI] [PubMed] [Google Scholar]
- 33.Nargesi MM, Ismail P, Razack AH, Pasalar P, Nazemi A, Oshkoor SA and Amini P: Linkage between prostate cancer occurrence and Y-chromosomal DYS loci in Malaysian subjects. Asian Pac J Cancer Prev, 12(5), 1265–8 (2011) [PubMed] [Google Scholar]
- 34.Paracchini S, Pearce CL, Kolonel LN, Altshuler D, Henderson BE and Tyler-Smith C: A Y chromosomal influence on prostate cancer risk: the multi-ethnic cohort study. J Med Genet, 40(11), 815–9 (2003) 10.1136/jmg.40.11.815 PMid: PMCid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, Antipin Y, Mitsiades N, Landers T, Dolgalev I, Major JE, Wilson M, Socci ND, Lash AE, Heguy A, Eastham JA, Scher HI, Reuter VE, Scardino PT, Sander C, Sawyers CL and Gerald WL: Integrative genomic profiling of human prostate cancer. Cancer Cell, 18(1), 11–22 (2010) 10.1016/j.ccr.2010.05.026 PMid: PMCid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee Y, Kim C, Park Y, Pyun JA and Kwack K: Next generation sequencing identifies abnormal Y chromosome and candidate causal variants in premature ovarian failure patients. Genomics, 108(5–6), 209–215 (2016) [DOI] [PubMed] [Google Scholar]
- 37.Yadav SK, Kumari A, Javed S and Ali S: DYZ1 arrays show sequence variation between the monozygotic males. BMC Genet, 15, 19 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pathak D, Premi S, Srivastava J, Chandy SP and Ali S: Genomic instability of the DYZ1 repeat in patients with Y chromosome anomalies and males exposed to natural background radiation. DNA Res, 13(3), 103–9 (2006) 10.1093/dnares/dsl002 PMid: [DOI] [PubMed] [Google Scholar]
- 39.Lindstrom S, Adami HO, Adolfsson J and Wiklund F: Y chromosome haplotypes and prostate cancer in Sweden. Clin Cancer Res, 14(20), 6712–6 (2008) 10.1158/1078-0432.CCR-08-0658 PMid: [DOI] [PubMed] [Google Scholar]
- 40.Dumanski JP, Rasi C, Lonn M, Davies H, Ingelsson M, Giedraitis V, Lannfelt L, Magnusson PK, Lindgren CM, Morris AP, Cesarini D, Johannesson M, Tiensuu Janson E, Lind L, Pedersen NL, Ingelsson E and Forsberg LA: Mutagenesis. Smoking is associated with mosaic loss of chromosome Y. Science, 347(6217), 81–3 (2015) 10.1126/science.1262092 PMid: PMCid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stahl PR, Kilgue A, Tennstedt P, Minner S, Krohn A, Simon R, Krause GV, Izbicki J, Graefen M, Sauter G, Schlomm T and Wilczak W: Y chromosome losses are exceedingly rare in prostate cancer and unrelated to patient age. Prostate, 72(8), 898–903 (2012) 10.1002/pros.21492 PMid: [DOI] [PubMed] [Google Scholar]
- 42.Nadal M, Pera G, Pujadas J, Abril J, Gonzalez L, Aguilo F, Condom E, Gomez-Zaera M and Nunes V: Aneuploidy of chromosome Y in prostate tumors and seminal vesicles: a possible sign of aging rather than an indicator of carcinogenesis? Mol Carcinog, 46(7), 543–52 (2007) [DOI] [PubMed] [Google Scholar]
- 43.Yadav SK, Kumari A and Ali S: Fate of the human Y chromosome linked genes and loci in prostate cancer cell lines DU145 and LNCaP. BMC Genomics, 14, 323 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eeles RA, Olama AA, Benlloch S, Saunders EJ, Leongamornlert DA, Tymrakiewicz M, Ghoussaini M, Luccarini C, Dennis J, Jugurnauth-Little S, Dadaev T, Neal DE, Hamdy FC, Donovan JL, Muir K, Giles GG, Severi G, Wiklund F, Gronberg H, Haiman CA, Schumacher F, Henderson BE, Le Marchand L, Lindstrom S, Kraft P, Hunter DJ, Gapstur S, Chanock SJ, Berndt SI, Albanes D, Andriole G, Schleutker J, Weischer M, Canzian F, Riboli E, Key TJ, Travis RC, Campa D, Ingles SA, John EM, Hayes RB, Pharoah PD, Pashayan N, Khaw KT, Stanford JL, Ostrander EA, Signorello LB, Thibodeau SN, Schaid D, Maier C, Vogel W, Kibel AS, Cybulski C, Lubinski J, Cannon-Albright L, Brenner H, Park JY, Kaneva R, Batra J, Spurdle AB, Clements JA, Teixeira MR, Dicks E, Lee A, Dunning AM, Baynes C, Conroy D, Maranian MJ, Ahmed S, Govindasami K, Guy M, Wilkinson RA, Sawyer EJ, Morgan A, Dearnaley DP, Horwich A, Huddart RA, Khoo VS, Parker CC, Van As NJ, Woodhouse CJ, Thompson A, Dudderidge T, Ogden C, Cooper CS, Lophatananon A, Cox A, Southey MC, Hopper JL, English DR, Aly M, Adolfsson J, Xu J, Zheng SL, Yeager M, Kaaks R, Diver WR, Gaudet MM, Stern MC, Corral R, Joshi AD, Shahabi A, Wahlfors T, Tammela TL, Auvinen A, Virtamo J, Klarskov P, Nordestgaard BG, Roder MA, Nielsen SF, Bojesen SE, Siddiq A, Fitzgerald LM, Kolb S, Kwon EM, Karyadi DM, Blot WJ, Zheng W, Cai Q, McDonnell SK, Rinckleb AE, Drake B, Colditz G, Wokolorczyk D, Stephenson RA, Teerlink C, Muller H, Rothenbacher D, Sellers TA, Lin HY, Slavov C, Mitev V, Lose F, Srinivasan S, Maia S, Paulo P, Lange E, Cooney KA, Antoniou AC, Vincent D, Bacot F, Tessier DC, Kote-Jarai Z and Easton DF: Identification of 23 new prostate cancer susceptibility loci using the iCOGS custom genotyping array. Nat Genet, 45(4), 385–91, 391e1–2 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han Y, Rand KA, Hazelett DJ, Ingles SA, Kittles RA, Strom SS, Rybicki BA, Nemesure B, Isaacs WB, Stanford JL, Zheng W, Schumacher FR, Berndt SI, Wang Z, Xu J, Rohland N, Reich D, Tandon A, Pasaniuc B, Allen A, Quinque D, Mallick S, Notani D, Rosenfeld MG, Jayani RS, Kolb S, Gapstur SM, Stevens VL, Pettaway CA, Yeboah ED, Tettey Y, Biritwum RB, Adjei AA, Tay E, Truelove A, Niwa S, Chokkalingam AP, John EM, Murphy AB, Signorello LB, Carpten J, Leske MC, Wu SY, Hennis AJ, Neslund-Dudas C, Hsing AW, Chu L, Goodman PJ, Klein EA, Zheng SL, Witte JS, Casey G, Lubwama A, Pooler LC, Sheng X, Coetzee GA, Cook MB, Chanock SJ, Stram DO, Watya S, Blot WJ, Conti DV, Henderson BE and Haiman CA: Prostate Cancer Susceptibility in Men of African Ancestry at 8q24. J Natl Cancer Inst, 108(7) (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Chan SC, Brand LJ, Hwang TH, Silverstein KA and Dehm SM: Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res, 73(2), 483–9 (2013) 10.1158/0008-5472.CAN-12-3630 PMid: PMCid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jangravi Z, Alikhani M, Arefnezhad B, Sharifi Tabar M, Taleahmad S, Karamzadeh R, Jadaliha M, Mousavi SA, Ahmadi Rastegar D, Parsamatin P, Vakilian H, Mirshahvaladi S, Sabbaghian M, Mohseni Meybodi A, Mirzaei M, Shahhoseini M, Ebrahimi M, Piryaei A, Moosavi-Movahedi AA, Haynes PA, Goodchild AK, Nasr-Esfahani MH, Jabbari E, Baharvand H, Sedighi Gilani MA, Gourabi H and Salekdeh GH: A fresh look at the male-specific region of the human Y chromosome. J Proteome Res, 12(1), 6–22 (2013) 10.1021/pr300864k PMid: [DOI] [PubMed] [Google Scholar]
- 48.Khosravi P, Zahiri J, Gazestani VH, Mirkhalaf S, Akbarzadeh M, Sadeghi and M B. Goliaei: Analysis of candidate genes has proposed the role of y chromosome in human prostate cancer. Iran J Cancer Prev, 7(4), 204–11 (2014) [PMC free article] [PubMed] [Google Scholar]
- 49.Prensner JR, Sahu A, Iyer MK, Malik R, Chandler B, Asangani IA, Poliakov A, Vergara IA, Alshalalfa M, Jenkins RB, Davicioni E, Feng FY and Chinnaiyan AM: The IncRNAs PCGEM1 and PRNCR1 are not implicated in castration resistant prostate cancer. Oncotarget, 5(6), 1434–8 (2014) 10.18632/oncotarget.1846 PMid: PMCid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carvalho R, Pinheiro MF and Medeiros R: Localization of candidate genes in a region of high frequency of microvariant alleles for prostate cancer susceptibility: the chromosome region Yp11.2. genetic variation. DNA Cell Biol, 29(1), 3–7 (2010) 10.1089/dna.2009.0905 PMid: [DOI] [PubMed] [Google Scholar]
- 51.Li N, Dhar SS, Chen TY, Kan PY, Wei Y, Kim JH, Chan CH, Lin HK, Hung MC and Lee MG: JARID1D Is a Suppressor and Prognostic Marker of Prostate Cancer Invasion and Metastasis. Cancer Res, 76(4), 831–43 (2016) 10.1158/0008-5472.CAN-15-0906 PMid: PMCid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kido T and Lau YF: The human Y-encoded testis-specific protein interacts functionally with eukaryotic translation elongation factor eEF1A, a putative oncoprotein. Int J Cancer, 123(7), 1573–85 (2008) 10.1002/ijc.23697 PMid: [DOI] [PubMed] [Google Scholar]
- 53.Harlap S, Paltiel O, Friedlander Y, Calderon-Margalit R, Deutsch L, Kleinhaus KR, Manor O, Neugut AI, Opler M, Perrin MC, Terry MB, Tiram E and Yanetz R: Prostate cancer in fathers with fewer male offspring: the Jerusalem Perinatal Study cohort. J Natl Cancer Inst, 99(1), 77–81 (2007) 10.1093/jnci/djk007 PMid: [DOI] [PubMed] [Google Scholar]
- 54.Fraser M, Sabelnykova VY, Yamaguchi TN, Heisler LE, Livingstone J, Huang V, Shiah YJ, Yousif F, Lin X, Masella AP, Fox NS, Xie M, Prokopec SD, Berlin A, Lalonde E, Ahmed M, Trudel D, Luo X, Beck TA, Meng A, Zhang J, D’Costa A, Denroche RE, Kong H, Espiritu SM, Chua ML, Wong A, Chong T, Sam M, Johns J, Timms L, Buchner NB, Orain M, Picard V, Hovington H, Murison A, Kron K, Harding NJ, P’ng C, Houlahan KE, Chu KC, Lo B, Nguyen F, Li CH, Sun RX, de Borja R, Cooper CI, Hopkins JF, Govind SK, Fung C, Waggott D, Green J, Haider S, Chan-Seng-Yue MA, Jung E, Wang Z, Bergeron A, Pra AD, Lacombe L, Collins CC, Sahinalp C, Lupien M, Fleshner NE, He HH, Fradet Y, Tetu B, van der Kwast T, McPherson JD, Bristow RG and Boutros PC: Genomic hallmarks of localized, non-indolent prostate cancer. Nature, 541(7637), 359–364 (2017) 10.1038/nature20788 PMid: [DOI] [PubMed] [Google Scholar]
- 55.Vijayakumar S, Garcia D, Hensel CH, Banerjee M, Bracht T, Xiang R, Kagan J and Naylor SL: The human Y chromosome suppresses the tumorigenicity of PC-3, a human prostate cancer cell line, in athymic nude mice. Genes Chromosomes Cancer, 44(4), 365–72 (2005) 10.1002/gcc.20250 PMid: [DOI] [PubMed] [Google Scholar]
- 56.Mazeyrat S and Mitchell MJ: Rodent Y chromosome TSPY gene is functional in rat and non-functional in mouse. Hum Mol Genet, 7(3), 557–62 (1998) 10.1093/hmg/7.3.557 PMid: [DOI] [PubMed] [Google Scholar]
- 57.Kido T and Lau YF: The rat Tspy is preferentially expressed in elongated spermatids and interacts with the core histones. Biochem Biophys Res Commun, 350(1), 56–67 (2006) 10.1016/j.bbrc.2006.08.191 PMid: PMCid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schubert S, Skawran B, Dechend F, Nayernia K, Meinhardt A, Nanda I, Schmid M, Engel W and Schmidtke J: Generation and characterization of a transgenic mouse with a functional human TSPY. Biol Reprod, 69(3), 968–75 (2003) 10.1095/biolreprod.103.016501 PMid: [DOI] [PubMed] [Google Scholar]
- 59.Kido T, Schubert S, Hatakeyama S, Ohyama C, Schmidtke J and Lau YF: Expression of a Y-located human protooncogene TSPY in a transgenic mouse model of prostate cancer. Cell Biosci, 4(1), 9 (2014) 10.1186/2045-3701-4-9 PMid: PMCid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hess Michelini R, Manzo T, Sturmheit T, Basso V, Rocchi M, Freschi M, Listopad J, Blankenstein T, Bellone M and Mondino A: Vaccine-instructed intratumoral IFN-gamma enables regression of autochthonous mouse prostate cancer in allogeneic T-cell transplantation. Cancer Res, 73(15), 4641–52 (2013) 10.1158/0008-5472.CAN-12-3464 PMid: [DOI] [PubMed] [Google Scholar]
- 61.Gupta S, Hastak K, Ahmad N, Lewin JS and Mukhtar H: Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Natl Acad Sci U S A, 98(18), 10350–5 (2001) 10.1073/pnas.171326098 PMid: PMCid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lau YF and Zhang J: Expression analysis of thirty one Y chromosome genes in human prostate cancer. Mol Carcinog, 27(4), 308–21 (2000) [DOI] [PubMed] [Google Scholar]
- 63.Dimitrakopoulou VI, Travis RC, Shui IM, Mondul A, Albanes D, Virtamo J, Agudo A, Boeing H, Bueno-de-Mesquita HB, Gunter MJ, Johansson M, Khaw KT, Overvad K, Palli D, Trichopoulou A, Giovannucci E, Hunter DJ, Lindstrom S, Willett W, Gaziano JM, Stampfer M, Berg C, Berndt SI, Black A, Hoover RN, Kraft P, Key TJ and Tsilidis KK: Interactions Between Genome-Wide Significant Genetic Variants and Circulating Concentrations of 25-Hydroxyvitamin D in Relation to Prostate Cancer Risk in the National Cancer Institute BPC3. Am J Epidemiol, 185(6), 452–464 (2017) 10.1093/aje/kww143 PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Teerlink CC, Leongamornlert D, Dadaev T, Thomas A, Farnham J, Stephenson RA, Riska S, McDonnell SK, Schaid DJ, Catalona WJ, Zheng SL, Cooney KA, Ray AM, Zuhlke KA, Lange EM, Giles GG, Southey MC, Fitzgerald LM, Rinckleb A, Luedeke M, Maier C, Stanford JL, Ostrander EA, Kaikkonen EM, Sipeky C, Tammela T, Schleutker J, Wiley KE, Isaacs SD, Walsh PC, Isaacs WB, Xu J, Cancel-Tassin G, Cussenot O, Mandal D, Laurie C, Laurie C, Thibodeau SN, Eeles RA, Kote-Jarai Z and Cannon-Albright L: Genome-wide association of familial prostate cancer cases identifies evidence for a rare segregating haplotype at 8q24.2.1. Hum Genet, 135(8), 923–38 (2016) 10.1007/s00439-016-1690-6 PMid: PMCid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wen J, Xu J, Sun Q, Xing C and Yin W: Upregulation of long non coding RNA PCAT-1 contributes to cell proliferation, migration and apoptosis in hepatocellular carcinoma. Mol Med Rep, 13(5), 4481–6 (2016) 10.3892/mmr.2016.5075 PMid: [DOI] [PubMed] [Google Scholar]
- 66.Chida J, Araki H and Maeda Y: Specific growth suppression of human cancer cells by targeted delivery of Dictyostelium mitochondrial ribosomal protein S4. Cancer Cell Int, 14, 56 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seim I, Jeffery PL, Thomas PB, Nelson CC and Chopin LK: Whole-Genome Sequence of the Metastatic PC3 and LNCaP Human Prostate Cancer Cell Lines. G3 (Bethesda) (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dasari VK, Goharderakhshan RZ, Perinchery G, Li LC, Tanaka Y, Alonzo J and Dahiya R: Expression analysis of Y chromosome genes in human prostate cancer. J Urol, 165(4), 1335–41 (2001) DOI: 10.1016/S0022-5347(01)69895-1 DOI: 10.1016/S0022-5347(01)69895-1 DOI: 10.1097/00005392-200104000-00080 PMid: [DOI] [PubMed] [Google Scholar]
- 69.Cao Y, Cao M, Chen Y, Yu W, Fan Y, Liu Q, Gao G, Zhao Z, Wang X and Jin J: The combination of prostate imaging reporting and data system version 2 (PIRADS v2) and periprostatic fat thickness on multi-parametric MRI to predict the presence of prostate cancer. Oncotarget (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang J, Mondul AM, Weinstein SJ, Karoly ED, Sampson JN and Albanes D: Prospective serum metabolomic profile of prostate cancer by size and extent of primary tumor. Oncotarget (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ho Y and Dehm SM: Androgen Receptor Rearrangement and Splicing Variants in Resistance to Endocrine Therapies in Prostate Cancer. Endocrinology (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Munkley J, Livermore K, Rajan P and Elliott DJ: RNA splicing and splicing regulator changes in prostate cancer pathology. Hum Genet (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee AR, Li Y, Xie N, Gleave ME, Cox ME, Collins CC and Dong X: Alternative RNA splicing of the MEAF6 gene facilitates neuroendocrine prostate cancer progression. Oncotarget, 8(17), 27966–27975 (2017) 10.18632/oncotarget.15854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Winchester DA, Till C, Goodman PJ, Tangen CM, Santella RM, Johnson-Pais TL, Leach RJ, Xu J, Zheng SL, Thompson IM, Lucia MS, Lippman SM, Parnes HL, Isaacs WB, De Marzo AM, Drake CG and Platz EA: Association between variants in genes involved in the immune response and prostate cancer risk in men randomized to the finasteride arm in the Prostate Cancer Prevention Trial. Prostate, 77(8), 908–919 (2017) 10.1002/pros.23346 PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Beaver LM, Kuintzle R, Buchanan A, Wiley MW, Glasser ST, Wong CP, Johnson GS, Chang JH, Lohr CV, Williams DE, Dashwood RH, Hendrix DA and Ho E: Long noncoding RNAs and sulforaphane: a target for chemoprevention and suppression of prostate cancer. J Nutr Biochem, 42, 72–83 (2017) 10.1016/j.jnutbio.2017.01.001 PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Feng S, Shao L, Castro P, Coleman I, Nelson PS, Smith PD, Davies BR and Ittmann M: Combination treatment of prostate cancer with FGF receptor and AKT kinase inhibitors. Oncotarget, 8(4), 6179–6192 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bishop JA, Yonescu R, Epstein JI and Westra WH: A subset of prostatic basal cell carcinomas harbor the MYB rearrangement of adenoid cystic carcinoma. Hum Pathol, 46(8), 1204–8 (2015) 10.1016/j.humpath.2015.05.002 PMid: [DOI] [PubMed] [Google Scholar]
- 78.Wilson S, Fan L, Sahgal N, Qi J and Filipp FV: The histone demethylase KDM3A regulates the transcriptional program of the androgen receptor in prostate cancer cells. Oncotarget, 8(18), 30328–30343 (2017) 10.18632/oncotarget.15681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barfeld SJ, Urbanucci A, Itkonen HM, Fazli L, Hicks JL, Thiede B, Rennie PS, Yegnasubramanian S, DeMarzo AM and Mills IG: c-Myc Antagonises the Transcriptional Activity of the Androgen Receptor in Prostate Cancer Affecting Key Gene Networks. EBioMedicine, 18, 83–93 (2017) 10.1016/j.ebiom.2017.04.006 PMid: PMCid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Costanzi E, Urbanelli L, Bellezza I, Magini A, Emiliani C and Minelli A: Hypermethylation contributes to down-regulation of lysosomal beta-hexosaminidase alpha subunit in prostate cancer cells. Biochimie, 101, 75–82 (2014) 10.1016/j.biochi.2013.12.016 PMid: [DOI] [PubMed] [Google Scholar]
- 81.Yano S, Matsuyama H, Hirata H, Inoue R, Matsumoto H, Ohmi C, Miura K, Shirai M, Iizuka N and Naito K: Identification of genes linked to gefitinib treatment in prostate cancer cell lines with or without resistance to androgen: a clue to application of gefitinib to hormone-resistant prostate cancer. Oncol Rep, 15(6), 1453–60 (2006) 10.3892/or.15.6.1453 [DOI] [PubMed] [Google Scholar]
- 82.Cheng L, Pan CX, Zhang JT, Zhang S, Kinch MS, Li L, Baldridge LA, Wade C, Hu Z, Koch MO, Ulbright TM and Eble JN: Loss of 14–3-3sigma in prostate cancer and its precursors. Clin Cancer Res, 10(9), 3064–8 (2004) 10.1158/1078-0432.CCR-03-0652 PMid: [DOI] [PubMed] [Google Scholar]
- 83.Ceder JA, Aalders TW and Schalken JA: Label retention and stem cell marker expression in the developing and adult prostate identifies basal and luminal epithelial stem cell subpopulations. Stem Cell Res Ther, 8(1), 95 (2017) 10.1186/s13287-017-0544-z PMid: PMCid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hao M, Li Y, Wang J, Qin J, Wang Y, Ding Y, Jiang M, Sun X, Zu L, Chang K, Lin G, Du J, Korinek V, Ye DW and Wang J: HIC1 loss promotes prostate cancer metastasis by triggering epithelial-mesenchymal transition. J Pathol (2017) [DOI] [PubMed] [Google Scholar]
- 85.Srinivasan S and Nawaz Z: E3 ubiquitin protein ligase, E6-associated protein (E6-AP) regulates PI3K-Akt signaling and prostate cell growth. Biochim Biophys Acta, 1809(2), 119–27 (2011) 10.1016/j.bbagrm.2010.08.011 PMid: PMCid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Han M, Xu W, Cheng P, Jin H and Wang X: Histone demethylase lysine demethylase 5B in development and cancer. Oncotarget, 8(5), 8980–8991 (2017) 10.18632/oncotarget.13858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nguyen HM, Vessella RL, Morrissey C, Brown LG, Coleman IM, Higano CS, Mostaghel EA, Zhang X, True LD, Lam HM, Roudier M, Lange PH, Nelson PS and Corey E: LuCaP Prostate Cancer Patient-Derived Xenografts Reflect the Molecular Heterogeneity of Advanced Disease an--d Serve as Models for Evaluating Cancer Therapeutics. Prostate, 77(6), 654–671 (2017) 10.1002/pros.23313 PMid: PMCid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stone L: Prostate cancer: Hand in hand - Rb1 and Trp53 cooperate to suppress resistance. Nat Rev Urol, 14(3), 131 (2017) 10.1038/nrurol.2017.13 PMid: [DOI] [PubMed] [Google Scholar]
- 89.Moreno CS: The Sex-determining region Y-box 4 and homeobox C6 transcriptional networks in prostate cancer progression: crosstalk with the Wnt, Notch, and PI3K pathways. Am J Pathol, 176(2), 518–27 (2010) 10.2353/ajpath.2010.090657 PMid: PMCid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu P, Ramachandran S, Ali Seyed M, Scharer CD, Laycock N, Dalton WB, Williams H, Karanam S, Datta MW, Jaye DL and Moreno CS: Sex-determining region Y box 4 is a transforming oncogene in human prostate cancer cells. Cancer Res, 66(8), 4011–9 (2006) 10.1158/0008-5472.CAN-05-3055 PMid: [DOI] [PubMed] [Google Scholar]
- 91.Komura K, Jeong SH, Hinohara K, Qu F, Wang X, Hiraki M, Azuma H, Lee GS, Kantoff PW and Sweeney CJ: Resistance to docetaxel in prostate cancer is associated with androgen receptor activation and loss of KDM5D expression. Proc Natl Acad Sci U S A, 113(22), 6259–64 (2016) 10.1073/pnas.1600420113 PMid: PMCid: [DOI] [PMC free article] [PubMed] [Google Scholar]