Abstract
Background.
Limited data exist regarding population effectiveness of human papillomavirus (HPV) catch-up vaccination, defined in the United States as first vaccination at ages 13–26 years. We evaluated the risk of cervical intraepithelial neoplasia 2, 3, adenocarcinoma in situ or cancer (CIN2+ and CIN3+) by prior HPV vaccination status, age at first dose and number of doses among women participating in a screening program within a large integrated healthcare system.
Methods.
Case-control study of 4,357 incident CIN2+ cases and 5:1 age-matched, incidence-density selected controls (N=21,773). Cases and controls were aged 26 years or younger when the HPV quadrivalent vaccine became available in 2006. Rate ratios (RR) from conditional logistic regression were estimated by age at first dose (≤17, 18–20, ≥21 years), and number of doses (1, 2, ≥3 doses) compared with no prior vaccination, with adjustment for smoking, hormonal contraceptive prescription, race/ethnicity, sexually transmitted infections, immunosuppression, parity and number of outpatient visits.
Findings.
In the study population, the youngest age at first vaccination was 14 years. One or more HPV vaccine doses conferred protection against CIN2+ (RR 0.82; 95% confidence interval 0.73–0.93) and CIN3+ (RR 0.77; 0.64–0.94). We observed the strongest protection for CIN2+ among women with ≥3 vaccine doses aged 14–17 years at first dose (RR 0.52; 0.36–0.74) and among women with ≥3 vaccine doses aged 18–20 years at first dose (RR 0.65; 0.49–0.88). No significant protection was observed for women aged 21 years or older at first dose (RR 0.94; 95% CI 0.81–1.09). Inferences were similar for CIN3+, but with stronger RRs for women with ≥3 vaccine doses aged 14–17 years at first dose (RR 0.27; 0.13–0.56) and among women with ≥3 vaccine doses aged 18–20 years at first dose (RR 0.59; 0.36–0.97).
Interpretation.
Catch-up quadrivalent HPV vaccination with 3 doses was effective against CIN2+ and CIN3+ in girls and women aged 14–20 years at first vaccine dose, but not for women aged 21 years and older at first dose.
INTRODUCTION
Human papillomavirus (HPV) vaccination in the United States is currently recommended for girls ages 11–12 years with catch-up vaccination for girls and women ages 13–26 years. While originally approved as a 3-dose series, recommendations from the Centers for Disease Control and Prevention in 2016 allow for a 2-dose series for girls who initiate the vaccine series ages 9–14 years.1 The potential protection afforded by the available HPV vaccines against cervical neoplasia is substantial, but the actual vaccine impact has yet to be fully observed due to the fact that girls vaccinated in early adolescence are only recently old enough to initiate cervical cancer screening. Vaccine effectiveness can be evaluated, however, among girls and women who initiated the vaccine series during catch-up years.
Several studies have reported evidence of vaccination population effectiveness, including decreases in prevalence of abnormal cervical cytology or HPV vaccine-type specific infections .2–9 Recent data from a randomized clinical trial of the quadrivalent HPV vaccine in India suggested that catch-up vaccination with fewer doses was both immunogenic and protective against HPV.10 Limited evidence exists for vaccine impact on high-grade precancerous lesions, including cervical intraepithelial neoplasia grades 2, 2/3, 3, adenocarcinoma in situ or cancer (CIN2+). The evidence mainly consists of studies showing ecologic declines over time in CIN2+ incidence11–18, with most showing no change for older women.11,13,14,16,17 Randomized clinical trials have demonstrated that the quadrivalent HPV vaccine decreases risk of CIN2+ in women who initiate at ages 15–2619 years but another trial among women initiating at 24–45 years of age did not demonstrate vaccine effectiveness for CIN2+.20 Studies in Australia and Scotland21–24 which have achieved high vaccination coverage in school aged programs of 70–90% have demonstrated vaccine effectiveness for CIN2+, although few23,24 included women who initiated at ≥17 years of age. Here we estimated the effectiveness of catch-up quadrivalent HPV vaccination to prevent CIN2+ and CIN3+ by age at first dose and by number of doses.
METHODS
The study was conducted among women enrolled in Kaiser Permanente Northern California (KPNC), a large integrated health care delivery system providing comprehensive care for over 3.9 million members in the greater San Francisco Bay Area, representing 28% of insured Californians in same region.25 The study population was a subset of participants in a previously described26 nested case-control study which included cases defined as women with a new diagnosis of CIN2+ or CIN3+, and incidence density selected controls consisting of women without CIN2+ or CIN3+ at the time each case occurred.
The source population for the parent case-control study included >2 million women who had cytology between January 1995 and June 2014. To focus on women targeted for screening, we excluded girls aged <18 years, women aged >70 years and all women with prior hysterectomy. For each case, we randomly selected 5 controls that met these same eligibility criteria, matched by age (within one year), time since first cytology in the health system (within one year), and years of continuous prior health plan membership (within one year). The 5:1 sampling scheme provided adequate power for rare exposures as described previously.26 The index date for cases was the diagnosis date, and controls were assigned the same index date as the case to which they were matched. Cases and controls were also required to have a cytology test performed within 12 months prior to their assigned index date. The current study was limited to women eligible for the HPV vaccine since its availability in 2006 and who were old enough to participate in the cervical cancer screening program, corresponding to women ages 18–26 years at some point between 2006 and 2014. The final study population included 4,357 cases and 21,773 controls. Although a cohort study design was a viable alternative approach, we used the nested case-control design to enhance computational efficiency, since a cohort study would have involved a multivariable analysis of >2,000,000 women and relies on covariate adjustment, instead of precise matching of risk factors between cases and controls.
The primary data source, including vaccination data, was the electronic medical record. Histopathology results of cervical biopsies were ascertained by Systematized Nomenclature of Medicine topology and morphology codes. Text-based natural language processing of the corresponding pathology reports was used to more accurately assign the diagnosis (e.g., CIN2, CIN3). Since 2006, KPNC offered the quadrivalent HPV vaccine; the nonavalent HPV vaccine was introduced in August 2015, after the end of the study period. Other clinical risk factors ascertained included recent (within 18 months) history of smoking and high parity (defined as 3 or more live births). We also identified factors potentially associated with increased screening frequency, including number of recent outpatient visits and race/ethnicity. Finally, factors that were both clinical risk factors and associated with screening frequency included recently documented sexually transmitted infections (herpes, gonorrhea, syphilis and chlamydia), recent prescription of hormonal contraceptives, and immunosuppression (HIV-infected, prior solid organ transplantation and recent prescription of immunosuppressive medication), defined in detail previously.26
HPV vaccination history prior to index date was obtained for all women. We only ascertained vaccine doses received at least six months prior to index, since more proximal vaccine doses are not likely to have an impact on disease risk. The following comparisons by vaccination status were made: 1) prior HPV vaccination (i.e., ≥1 HPV vaccine doses) versus no prior HPV vaccination; 2) age at first dose (i.e., 14–17 years, 18–20 years, and ≥21 years) versus no prior HPV vaccination; 3) number of doses received (i.e., 1, 2, and ≥3 doses) versus no prior HPV vaccination; and 4) combined age at first dose and number of doses received, including 6 categories for 3 age strata (i.e., 14–17 years, 18–20 years and ≥21 years) and 2 dose strata (i.e., ≥3 and <3 doses), each compared with women with no prior HPV vaccination. Conditional logistic regression was used to estimate odds ratios, which represent unbiased estimates of rate ratios (RR) in a nested case-control study with incidence density sampling.27 Adjusted models included covariates representing recent (i.e., within 18 months) smoking (yes or no); recent hormonal contraceptives (yes or no); race and ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, other, unknown); recent sexually transmitted infections (yes or no); three or more live births (yes or no); prior outpatient visits (continuous); and immunosuppression status (yes or no). For the model including the combined variable of age at first dose and number of doses, a custom contrast tested the interaction of whether the effects of age at first dose (14–17, 18–20, and ≥21, each compared with no prior HPV vaccination) significantly differed by number of doses (<3 vs. ≥3). Sensitivity analyses were performed, including: (1) limiting to cases and controls with continuous membership since 2006 to minimize misclassification of HPV vaccine history; (2) excluding controls with abnormal cytology from recent cytology to minimize misclassification of outcome status (i.e., misclassification may have occurred if controls with abnormal cytology had not yet been followed up with colposcopy to identify CIN2+); and (3) replacing all “recent” versions of covariates with “ever” versions (i.e., any time in past as a proxy for lifetime exposure) to minimize potential for residual confounding.
Analyses were conducted using the LOGISTIC procedure in SAS, Version 9.3 (Cary, North Carolina). The institutional review board at Kaiser Permanente Northern California approved this study with a waiver of written informed consent.
Role of the Funding Source
The study sponsor had no role in study design, data collection, analysis, interpretation of data, or manuscript development. The corresponding author had full access to all study data and had final responsibility for the decision to submit for publication.
RESULTS
The study population included 4,357 CIN2+ cases and 21,773 controls. Among women in the case group, 4,348/4,357 cases had five matched women in the control group, 8 women in the case group had four matched women in the control group, and 1 woman in the case group had one matched woman in the control group. Some women in the control group (n=1,599) matched to more than one woman in the case group, and other women in the control group (n=211) became cases at a later date. Cases and controls were similar with respect to matching parameters of age, index year and mean years prior health plan membership (Table 1). Compared with controls, cases were more likely to be non-Hispanic White, had a higher mean number of outpatient visits per year, higher recent smoking history), higher recent hormonal contraceptive use, more recent sexually transmitted infections; fewer cases than controls had 3 or more live births and a history of immunosuppression. Of 4,357 CIN2+ cases, 874 (20%) were CIN2, 1,634 (38%) were CIN2/3, 1,744 (40%) were CIN3 and 23 (<1%) were cancer (9 adenocarcinoma, 13 squamous cell carcinoma and 1 other cancer).
Table 1.
Baseline characteristics, cervical intraepithelial neoplasia (CIN2+) cases and matched controls, Kaiser Permanente, 2006–2014
| Characteristic | Cases | Controls | P-value1 |
|---|---|---|---|
| n=4,357 | n=21,773 | ||
| Mean age at index (SD), years | 26.3 (4) | 26.3 (4) | matching |
| Mean years prior membership (SD) | 7.4 (5) | 7.5 (5) | matching |
| Index year, n (%) | matching | ||
| 2006–2008 | 1,189 (27) | 5,945 (27) | |
| 2009–2011 | 1,420 (33) | 7,099 (33) | |
| 2012–2014 | 1,748 (40) | 8,729 (40) | |
| Mean outpatient visits per year (SD) | 7.5 (6) | 6.9 (5) | <0.001 |
| Race/ethnicity, n (%) | <0.001 | ||
| Non-Hispanic White | 2,155 (49) | 9,611 (44) | |
| Non-Hispanic Black | 459 (11) | 2,107 (10) | |
| Hispanic | 946 (22) | 5,035 (23) | |
| Other | 660 (15) | 4,153 (19) | |
| Unknown | 137 (3) | 867 (4) | |
| Smoking | |||
| Recent2, n (%) | 1,044 (24) | 3,725 (17) | <0.001 |
| Ever, n (%) | 1,730 (40) | 7,051 (32) | <0.001 |
| Hormonal contraceptive use | |||
| Recent2, n (%) | 2,880 (66) | 12,696 (58) | <0.001 |
| Ever, n (%) | 3,730 (86) | 17,969 (83) | <0.001 |
| Sexually transmitted infection3 | |||
| Recent3, n (%) | 314 (7) | 953 (4) | <0.001 |
| Ever, n (%) | 996 (23) | 3272 (15) | <0.001 |
| 3 or more live births, n (%) | 212 (5) | 1,207 (6) | 0.065 |
| Immunosuppressed4, n (%) | 506 (12) | 2,672 (12) | 0.013 |
SD, standard deviation
P-value based on bivariate conditional logistic regression models; not computed for matching variables.
Within 18 months prior to index
Herpes, gonorrhea, syphilis, chlamydia
HIV-infected, solid organ transplant, or immunosuppressive therapy in prior 18 months
HPV vaccine effectiveness against CIN2+
Table 2 displays vaccine history percentages for CIN2+ cases and controls and unadjusted RRs. Among 4,357 cases and 21,773 controls, 429 (10%) and 2,408 (11%) women, respectively, had any prior HPV vaccination (RR 0.86; 95% CI 0.76–0.96). Age at first dose at 14–17 years (RR 0.62; 95% CI 0.46– 0.83) and 18–20 years (RR 0.76; 95% CI 0.61–0.94) conferred protection against CIN2+ compared with women with no prior vaccination, while age at first dose at ≥21 years of age was not protective (RR 0.98; 95% CI 0.84–1.13). Receipt of ≥3 HPV vaccine doses compared with no prior vaccination was associated with CIN2+ protection (RR 0.78; 95% CI 0.66–0.91), while 2 doses (RR 1.02; 95% CI 0.82–1.28) and 1 dose (RR 0.89; 95% CI 0.73–1.09) were not. For analyses that considered the combined association of age at first dose and number of doses, CIN2+ protection was only observed for women with ≥3 HPV vaccine doses with first dose at age 14–17 (RR 0.52; 95% CI 0.36–0.74), and for women with ≥3 HPV vaccine doses and ages 18–20 years at first dose (RR 0.68; 95% CI 0.50–0.91) compared with women with no prior vaccination.
Table 2:
Human papillomavirus vaccine history and unadjusted rate ratios among cervical intraepithelial neoplasia grade 2 or worse (CIN2+) cases and matched controls, Kaiser Permanente 1996–2014
|
Cases n=4,357 |
Controls n=21,773 |
||||
|---|---|---|---|---|---|
| N | (%) | N | (%) | RR (95% CI)1 | |
| HPV vaccine history | |||||
| Prior vaccination | 429 | (10) | 2,408 | (11) | 0.86 (0.76–0.96) |
| No prior vaccination | 3,928 | (90) | 19,365 | (89) | 1 (reference) |
| HPV vaccine history, age at first dose | |||||
| Prior vaccination, 14–17 years | 77 | (2) | 516 | (2) | 0.62 (0.46–0.83) |
| Prior vaccination, 18–20 years | 113 | (3) | 686 | (3) | 0.76 (0.61–0.94) |
| Prior vaccination, ≥21 years | 239 | (5) | 1,206 | (6) | 0.98 (0.84–1.13) |
| No prior vaccination | 3,928 | (90) | 19,365 | (89) | 1 (reference) |
| HPV vaccine history, # doses | |||||
| Prior vaccination, ≥3 doses2 | 214 | (5) | 1,313 | (6) | 0.78 (0.66–0.91) |
| Prior vaccination, 2 doses | 97 | (2) | 457 | (2) | 1.02 (0.82–1.28) |
| Prior vaccination, 1 dose | 118 | (3) | 638 | (3) | 0.89 (0.73–1.09) |
| No prior vaccination | 3,928 | (90) | 19,365 | (89) | 1 (reference) |
| HPV vaccine history, age at first dose, # doses | |||||
| Prior vaccination, 14–17 years, ≥3 doses | 42 | (1) | 333 | (2) | 0.52 (0.36–0.74) |
| Prior vaccination, 18–20 years, ≥3 doses | 56 | (1) | 379 | (2) | 0.68 (0.50–0.91) |
| Prior vaccination, ≥21 years, ≥3 doses | 116 | (3) | 601 | (3) | 0.95 (0.78–1.17) |
| Prior vaccination, 14–17 years, <3 doses | 35 | (1) | 183 | (1) | 0.80 (0.54–1.19) |
| Prior vaccination, 18–20 years, <3 doses | 57 | (1) | 307 | (1) | 0.86 (0.64–1.15) |
| Prior vaccination, ≥21 years, <3 doses | 123 | (3) | 605 | (3) | 1.00 (0.82–1.22) |
| No prior vaccination | 3,928 | (90) | 19,365 | (89) | 1 (reference) |
Based on bivariate conditional logistic regression models
Only 4 cases and 16 controls had four or more vaccine doses
Adjusted RRs for CIN2+ are presented in Figure 1 with similar inferences as for unadjusted results. Women with ≥1 HPV vaccine doses were at an overall decreased risk for CIN2+ compared with women with no prior vaccination (RR 0.82; 95% CI 0.73–0.93). A significant reduced CIN2+ risk was also observed for women with first HPV vaccine dose at ages 14–17 years (RR 0.61; 95% CI 0.46–0.81) and 18–20 years (RR 0.72; 95% CI 0.58–0.90), but not at ≥21 years (RR 0.94; 95% CI 0.81–1.09) compared with women with no prior vaccination. Regarding number of doses, a significant reduced CIN2+ risk was observed for women with ≥3 HPV vaccine doses (RR 0.76; 95% CI 0.64–0.89), but not 2 (RR 0.98; 95% CI 0.78–1.24) or 1 dose (RR 0.84; 95% CI 0.68–1.03) compared with women with no prior vaccination. Finally, in adjusted models that considered the combined association of age at first dose and number of doses, we only observed protection against CIN2+ for women with ≥3 HPV vaccine doses and age 14–17 years at first dose (RR 0.52; 95% CI 0.36–0.74), and for women with ≥3 HPV vaccine doses and age 18–20 years at first dose (RR 0.65; 95% CI 0.49–0.88), compared with no prior vaccination. No statistically significant protection against CIN2+ was observed for women with <3 vaccine doses, although point estimates were protective for those aged 14–17 years (RR 0.77; 95% CI 0.52–1.15) and 18– 20 years (RR 0.80; 95% CI 0.60–1.08) at first dose. Finally, while the associations of age at first dose with CIN2+ appeared to be stronger for women with ≥3 HPV vaccine doses, the test for interaction between age at first dose and number of doses among vaccinated women was not statistically significant (P=0.41).
Figure 1: Adjusted rate ratios and 95% confidence intervals (CI) for cervical intraepithelial neoplasia grade 2 or worse (CIN2+) by human papillomavirus (HPV) vaccination history.
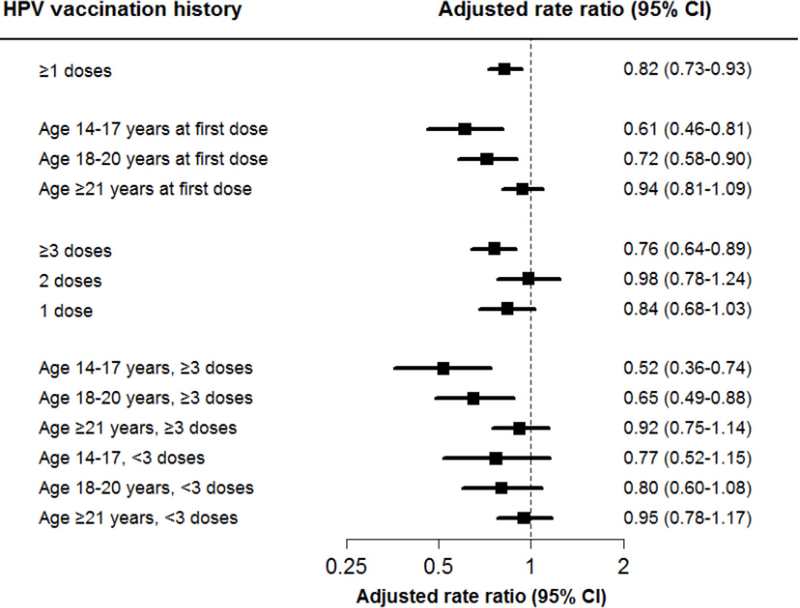
Variables are: ≥1 HPV vaccine doses versus no prior HPV vaccination; age at first dose (i.e., 14–17 years, 18–20 years, and ≥21 years) versus no prior HPV vaccination; number of doses received (i.e., 1, 2, and ≥3 doses) versus no prior HPV vaccine; and combined age at first dose and number of doses received, including 6 categories for 3 age strata (i.e., 14–17 years, 18–20 years and ≥21 years) and 2 dose strata (i.e., ≥3 and <3 doses), each compared with women with no prior HPV vaccination. Rate ratios obtained from conditional logistic regression models adjusted for smoking, hormonal contraceptives, race/ethnicity, recent sexually transmitted infections, parity, and prior outpatient visits, and immunosuppression status.
HPV vaccine effectiveness against CIN3+
Table 3 displays vaccine history percentages for CIN3+ cases and controls and unadjusted RRs. Among 1,849 cases and 9,242 controls, 154 (8%) and 893 (10%) women, respectively, had prior HPV vaccination (RR 0.82; 95% CI 0.68–1.00). Age at first dose 14–17 years (RR 0.45; 95% CI 0.27–0.76) conferred protection against CIN3+ compared with women with no prior vaccination, while protection was not observed for women with first dose at ages 18–20 years (RR 0.84; RR 0.59–1.21) or ≥21 years (RR 0.92; 95% CI 0.73–1.17) at first dose compared with women with no prior vaccination. Receipt of ≥3 HPV vaccine doses compared with women with no prior vaccination was associated with CIN3+ protection (RR 0.68; 95% CI 0.52–0.90), while 2 doses (RR 1.02; 95% CI 0.71, 1.48) and 1 dose (RR 0.94; RR 0.68, 1.30) were not. For analyses that considered the combined association of age at first dose and number of doses, CIN3+ protection was only observed for women with ≥3 HPV vaccine doses and first dose at ages 14–17 years (0.29; 95% CI 0.14–0.60). Of the 23 cancers observed, only 3 had prior HPV vaccination, all of whom had ≥3 doses, and all were aged ≥21 years at first dose.
Table 3:
Human papillomavirus vaccine history and unadjusted rate ratios among cervical intraepithelial neoplasia grade 3 or worse (CIN3+) cases and matched controls, Kaiser Permanente 1996–2014
|
Cases n=1,849 |
Controls n=9,242 |
||||
|---|---|---|---|---|---|
| N | (%) | N | (%) | RR (95% CI)1 | |
| HPV vaccine history | |||||
| Prior vaccination | 154 | (8) | 893 | (10) | 0.82 (0.68–1.00) |
| No prior vaccination | 1,695 | (92) | 8,349 | (90) | 1 (reference) |
| HPV vaccine history, age at first dose | |||||
| Prior vaccination, 14–17 years | 22 | (1) | 188 | (2) | 0.45 (0.27–0.76) |
| Prior vaccination, 18–20 years | 56 | (3) | 312 | (3) | 0.84 (0.59–1.21) |
| Prior vaccination, ≥21 years | 76 | (4) | 393 | (4) | 0.92 (0.73–1.17) |
| No prior vaccination | 1,695 | (92) | 8,349 | (90) | 1 (reference) |
| HPV vaccine history, # doses | |||||
| Prior vaccination, ≥3 doses2 | 71 | (4) | 486 | (5)) | 0.68 (0.52–0.90) |
| Prior vaccination, 2 doses | 36 | (2) | 168 | (2) | 1.02 (0.71–1.48) |
| Prior vaccination, 1 dose | 47 | (3) | 239 | (3) | 0.94 (0.68–1.30) |
| No prior vaccination | 1,695 | (92) | 8,349 | (90) | 1 (reference) |
| HPV vaccine history, age at first dose, # doses | |||||
| Prior vaccination, 14–17 years, ≥3 doses | 10 | (1) | 126 | (1) | 0.29 (0.14–0.60) |
| Prior vaccination, 18–20 years, ≥3 doses | 20 | (1) | 132 | (1) | 0.67 (0.41–1.10) |
| Prior vaccination, ≥21 years, ≥3 doses | 41 | (2) | 228 | (2) | 0.88 (0.62–1.25) |
| Prior vaccination, 14–17 years, <3 doses | 12 | (1) | 62 | (1) | 0.77 (0.40–1.49) |
| Prior vaccination, 18–20 years, <3 doses | 22 | (1) | 93 | (1) | 1.08 (0.66–1.75) |
| Prior vaccination, ≥21 years, <3 doses | 49 | (3) | 252 | (3) | 0.95 (0.70–1.30) |
| No prior vaccination | 1,695 | (92) | 8,349 | (90) | 1 (reference) |
Based on bivariate conditional logistic regression models
No cases and 4 controls had four or more vaccine doses
Adjusted RRs for CIN3+ are presented in Figure 2 with similar inferences as for unadjusted results. Women with ≥1 HPV vaccine doses were at an overall decreased risk for CIN3+ compared with women with no prior vaccination (RR 0.77; 95% CI 0.64–0.94). Protection against CIN3+ was also observed for women with first HPV vaccine dose at ages 14–17 years (RR 0.44; 95% CI 0.26–0.74), but not for women aged 18–20 years (RR 0.75; 95% CI 0.52–1.08) or women aged ≥21 years (RR 0.88; 95% CI 0.69–1.12) compared with no prior vaccination. Regarding number of doses, significant protection against CIN3+ was observed for women with ≥3 HPV vaccine doses (RR 0.64; 95% CI 0.48–0.84), but not 2 (RR 0.97; 95% CI 0.67–1.41) or 1 dose (RR 0.90; 95% CI 0.65–1.24) compared with women with no prior vaccination. Finally, in adjusted models that considered the combined association of age at first dose and number of doses, we observed protection against CIN3+ for women with ≥3 HPV vaccine doses and ages 14–17 years at first dose (RR 0.27; 95% CI 0.13–0.56), and in contrast to unadjusted results, for women with ≥3 HPV vaccine doses and age 18–20 years at first dose (RR 0.59; 95% CI 0.36–0.97), compared with women with no prior vaccination. No statistically significant protection against CIN3+ was observed within any of the age strata for women with <3 vaccine doses, although point estimates were protective for those aged 14–17 years (RR 0.79; 95% CI 0.40–1.55) at first dose. Finally, while the associations of age at first dose with CIN3+ appeared to be stronger for women with ≥3 HPV vaccine doses, the test for interaction between age at first dose and number of doses among vaccinated women was not statistically significant (P=0.13).
Figure 2: Adjusted rate ratios and 95% confidence intervals (CI) for cervical intraepithelial neoplasia grade 3 or worse (CIN3+) by human papillomavirus (HPV) vaccination history.
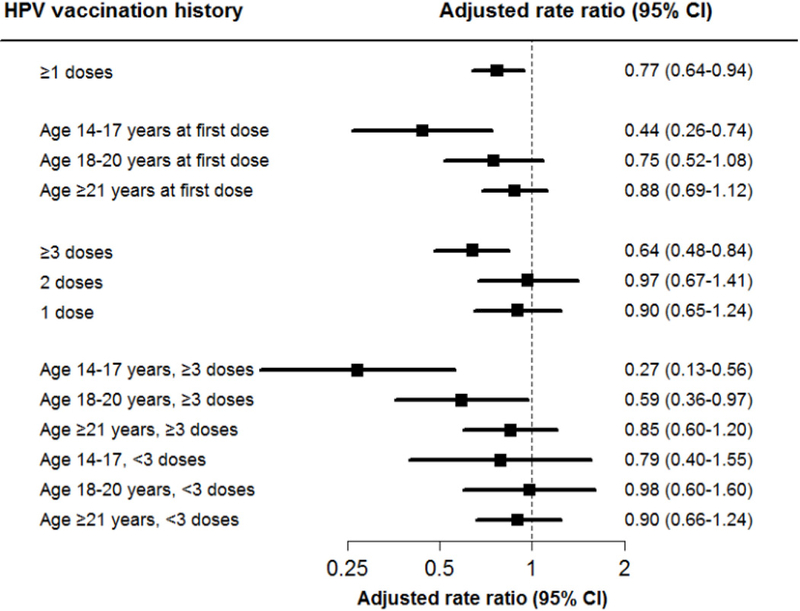
Variables are: ≥1 HPV vaccine doses versus no prior HPV vaccination; age at first dose (i.e., 14–17 years, 18–20 years, and ≥21 years) versus no prior HPV vaccination; number of doses received (i.e., 1, 2, and ≥3 doses) versus no prior HPV vaccine; and combined age at first dose and number of doses received, including 6 categories for 3 age strata (i.e., 14–17 years, 18–20 years and ≥21 years) and 2 dose strata (i.e., ≥3 and <3 doses), each compared with women with no prior HPV vaccination. Rate ratios obtained from conditional logistic regression models adjusted for smoking, hormonal contraceptives, race/ethnicity, recent sexually transmitted infections, parity, and prior outpatient visits, and immunosuppression status.
Sensitivity analyses
Sensitivity analyses limiting to cases and controls with continuous health plan membership since 2006, did not change inferences for association with ≥1 HPV vaccine doses, although the association strengthened for CIN2+ (RR 0.69; 95% CI 0.59–0.81) and CIN3+ (RR 0.71; 95% CI 0.55–0.91). Other sensitivity analyses had negligible impacts on findings (data not shown).
DISCUSSION
In this large population-based study in an integrated healthcare system, catch-up HPV vaccination with 3 doses was effective against CIN2+ and CIN3+ in women aged less than 21 years at first dose. We found no significant effectiveness, however, among women who initiated vaccination at ages 21–26 years, or for women who received less than the full 3-dose series. These results support current guidelines recommending the full 3-dose series for girls and women who begin vaccination after age 14 years; the finding of the limited effectiveness of catch-up vaccination for women ages 21–26 years should be confirmed in other settings.
Our observed 18% reduction in CIN2+ with ≥1 doses of the vaccine is similar to findings from randomized clinical trials of the quadrivalent vaccine19 showing a 19% reduction in the intention-to-treat analysis for CIN2+ among girls/women aged 15–26 who received ≥1 vaccine doses. A trial of women aged 24–45 randomized to the quadrivalent vaccine versus placebo reported 89% efficacy against the combined outcome of persistent infection, CIN and external genital lesions related to HPV vaccine types; 20 although not powered for CIN2+, the trial also noted no decrease in CIN2+.
Few epidemiologic studies have evaluated the population effectiveness of HPV vaccination for CIN2+.21–24,28 A large study in Scotland22 evaluated the effectiveness of the bivalent HPV vaccine and noted a 50% reduction in CIN2 and 55% reduction in CIN3 for girls ages 13–17 years vaccinated with 3 doses compared with no vaccination; these effects attenuated with increasing age. An Australian study evaluated vaccine effectiveness in girls ages 12–17 (mean age 16 years) and noted a statistically significant 28% reduction in CIN2+ for ≥1 vaccine doses, similar in magnitude to our results, with reduced protection for girls vaccinated at older ages.21 They only observed protection for girls who were fully vaccinated. A follow-up Australian case-control study23 of girls and women ages 11–27 years at first dose who were just entering the cervical cancer screening program demonstrated 46% effectiveness for CIN2+ among those receiving 3 doses and 21% effectiveness for those receiving 2 doses. They also reported 26% effectiveness for ≥1 vaccine doses compared with those receiving no vaccine, but no evidence of any vaccine effectiveness for women ages 23–27. Another Australian study among girls and women ages 11–27 years compared vaccine effectiveness by age and number of doses for women before and after cervical cancer screening initiation.24 They reported 29% effectiveness against CIN2+ for girls/women fully vaccinated prior to screening initiation and 13% effectiveness for girls/women fully vaccinated after screening initiation, but no protection with <3 doses. Effectiveness was reduced, but remained significant for women aged 20–23 years of age at vaccination, and for women aged 24–26 but only for women vaccinated after screening initiation.
Some study limitations should be acknowledged. First, clinically ascertained study measurements such as smoking may have been subject to misclassification. Other measurements based on pharmacy or laboratory data (i.e., hormonal contraceptive use, STIs) were more accurately ascertained; sensitivity analyses that replaced recent versions of covariates with ever versions (with potentially more complete data) had no effect on results. Second, our sensitivity analysis limited to women with continuous membership since the introduction of the vaccine demonstrated moderately stronger results suggesting there may have been some misclassification of vaccination status. Third, it is possible that residual confounding related to screening vigilance may have influenced our results. Cases and controls, however, were carefully matched to reflect similar engagement in the health plan and all subjects. Fourth, while the case-control design offered advantages with respect to analytical efficiency and careful adjustment for confounders, the design precluded the calculation of absolute rates. Fifth, the increased HPV type vaccine coverage of the recently introduced nonavalent HPV vaccine is anticipated to prevent more CIN2+ compared with the quadrivalent HPV vaccine, thus requiring future investigation. Sixth, despite the large sample size, the low uptake of catch-up vaccination resulted in limited statistical power and wide confidence intervals for some comparisons (e.g., <3 doses). Seventh, given the observational design, it remains likely that vaccinated and unvaccinated women differed in ways that we could not fully measure. Finally, results may have limited generalizability given the setting of a single healthcare setting. However, results are highly generalizable to other integrated healthcare settings and insured women in the San Francisco Bay Area, given the current membership of more than 2 million members, representing a quarter of all insured women in the region.25
The study has several key strengths. First, it is among the first to evaluate vaccine effectiveness in a large sample in the United States where vaccine uptake has been low compared with other countries. It is one of few studies to evaluate effectiveness in women vaccinated after age 17 and among the largest; compared with a study in Australia,23 our study had four times as many cases overall (n=4,357 vs. 1,062) enhancing our ability to detect small differences between cases and controls. The randomized trial20 that evaluated vaccine efficacy in older women identified only 62 and 51 CIN2+ cases in the vaccinated and placebo arms, respectively. Our study also adjusted for clinical risk factors such as smoking, hormonal contraceptive use and sexually transmitted infections, which was not done in previous studies.21–24 An additional strength was the comprehensive available data allowing for complete ascertainment of clinical data and the ability to precisely match controls on factors associated with engagement with the healthcare system.
In summary, our findings support current US guidelines recommending 3 HPV vaccine doses for girls and women initiating vaccination between the ages of 14 and 20 years. Consistent with some20,23, but not all studies24, our findings do not support catch-up vaccination of women ages 21–26 years. This finding conflicts with recent calls to extend HPV vaccination to older ages,29 thus results should be confirmed in other settings, and with increasing use of the nonavalent HPV vaccine.
Research in context.
Evidence before this study
Human papillomavirus (HPV) vaccination in the United States is currently recommended for girls ages 11–12 years with catch-up vaccination for girls and women ages 13–26 years who have not started the vaccine series. We searched PubMed, without language restrictions, to identify studies which compared cervical intraepithelial neoplasia 2, 3, adenocarcinoma in situ or cancer (CIN2+ and CIN3+) in HPV-vaccinated and unvaccinated women. The search was limited to articles published before March 21, 2018. Search terms used included combinations of: “papillomavirus vaccine”, “papillomavirus vaccination”, “HPV vaccine”, “HPV vaccination”, “effectiveness”, “papillomavirus infection”, “cervical intraepithelial neoplasia”, “cervical dysplasia”, “cervical neoplasm”, “HPV related diseases”, “histology”, “biopsy”, and “colposcopy”. We found few epidemiologic studies which have evaluated the effectiveness of HPV vaccination to reduce the risk of CIN2+, especially among girls and women vaccinated at older ages.
Added value of this study
In a large sample of women with uniform access to comprehensive care and engaged in a robust cervical cancer screening program, we found that catch-up HPV vaccination with 3 doses was effective against CIN2+ and CIN3+ in women between the ages of 14 and 20 years at first dose. We found no significant effectiveness, however, among women who initiated at ages 21–26 years, or for women who received less than the full 3-dose series.
Implications of all the available evidence
These results support current guidelines recommending the full 3-dose series for girls and women who start the series after their 15th birthday. Additional research is needed to confirm our findings regarding the limited effectiveness of catch-up vaccination for women ages 21–26 years.
Acknowledgments
Funding. US-based National Cancer Institute
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
All authors report no financial or personal relationships with other people or organizations that could inappropriately influence this work.
REFERENCES
- 1.Meites E, Kempe A, Markowitz LE. Use of a 2-Dose Schedule for Human Papillomavirus Vaccination - Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2016; 65(49): 1405–8. [DOI] [PubMed] [Google Scholar]
- 2.Drolet M, Bénard É, Boily M-C, et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. The Lancet Infectious Diseases 2015; 15(5): 565–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heard I, Tondeur L, Arowas L, Demazoin M, Falguieres M, Parent Du Chatelet I. Effectiveness of Human Papillomavirus Vaccination on Prevalence of Vaccine Genotypes in Young Sexually Active Women in France. The Journal of infectious diseases 2017; 215(5): 757–63. [DOI] [PubMed] [Google Scholar]
- 4.Hofstetter AM, Ompad DC, Stockwell MS, Rosenthal SL, Soren K. Human Papillomavirus Vaccination and Cervical Cytology Outcomes Among Urban Low-Income Minority Females. JAMA Pediatr 2016; 170(5): 445–52. [DOI] [PubMed] [Google Scholar]
- 5.Mahmud SM, Kliewer EV, Lambert P, Bozat-Emre S, Demers AA. Effectiveness of the quadrivalent human papillomavirus vaccine against cervical dysplasia in Manitoba, Canada. J Clin Oncol 2014; 32(5): 438–43. [DOI] [PubMed] [Google Scholar]
- 6.Merckx M, Broeck DV, Benoy I, Depuydt C, Weyers S, Arbyn M. Early effects of human papillomavirus vaccination in Belgium. European Journal of Cancer Prevention 2015; 24(4): 340–2. [DOI] [PubMed] [Google Scholar]
- 7.Mollers M, King AJ, Knol MJ, et al. Effectiveness of human papillomavirus vaccine against incident and persistent infections among young girls: Results from a longitudinal Dutch cohort study. Vaccine 2015; 33(23): 2678–83. [DOI] [PubMed] [Google Scholar]
- 8.Powell SE, Hariri S, Steinau M, et al. Impact of human papillomavirus (HPV) vaccination on HPV 16/18-related prevalence in precancerous cervical lesions. Vaccine 2012; 31(1): 109–13. [DOI] [PubMed] [Google Scholar]
- 9.Kim J, Bell C, Sun M, et al. Effect of human papillomavirus vaccination on cervical cancer screening in Alberta. CMAJ 2016; 188(12): E281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sankaranarayanan R, Prabhu PR, Pawlita M, et al. Immunogenicity and HPV infection after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre prospective cohort study. Lancet Oncol 2016; 17(1): 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baldur-Felskov B, Dehlendorff C, Junge J, Munk C, Kjaer SK. Incidence of cervical lesions in Danish women before and after implementation of a national HPV vaccination program. Cancer Causes Control 2014; 25(7): 915–22. [DOI] [PubMed] [Google Scholar]
- 12.Benard VB, Castle PE, Jenison SA, et al. Population-Based Incidence Rates of Cervical Intraepithelial Neoplasia in the Human Papillomavirus Vaccine Era. JAMA Oncol 2016; 3(6): 833–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brotherton JML, Fridman M, May CL, Chappell G, Saville AM, Gertig DM. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. The Lancet 2011; 377(9783): 2085–92. [DOI] [PubMed] [Google Scholar]
- 14.Flagg EW, Torrone EA, Weinstock H. Ecological Association of Human Papillomavirus Vaccination with Cervical Dysplasia Prevalence in the United States, 2007–2014. Am J Public Health 2016; 106(12): 2211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hariri S, Johnson ML, Bennett NM, et al. Population-based trends in high-grade cervical lesions in the early human papillomavirus vaccine era in the United States. Cancer 2015; 121(16): 2775–81. [DOI] [PubMed] [Google Scholar]
- 16.Niccolai LM, Julian PJ, Meek JI, McBride V, Hadler JL, Sosa LE. Declining rates of high-grade cervical lesions in young women in Connecticut, 2008–2011. Cancer epidemiology, biomarkers & prevention 2013; 22(8): 1446–50. [DOI] [PubMed] [Google Scholar]
- 17.Niccolai LM, Meek JI, Brackney M, Hadler JL, Sosa LE, Weinberger DM. Declines in Human Papillomavirus (HPV)–Associated High-Grade Cervical Lesions After Introduction of HPV Vaccines in Connecticut, United States, 2008–2015. Clinical Infectious Diseases 2017. [DOI] [PubMed]
- 18.Hariri S, Bennett NM, Niccolai LM, et al. Reduction in HPV 16/18-associated high grade cervical lesions following HPV vaccine introduction in the United States - 2008–2012. Vaccine 2015; 33(13): 1608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munoz N, Kjaer SK, Sigurdsson K, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst 2010; 102(5): 325– 39. [DOI] [PubMed] [Google Scholar]
- 20.Castellsague X, Munoz N, Pitisuttithum P, et al. End-of-study safety, immunogenicity, and efficacy of quadrivalent HPV (types 6, 11, 16, 18) recombinant vaccine in adult women 24–45 years of age. Br J Cancer 2011; 105(1): 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gertig DM, Brotherton JM, Budd A, Drennan K, Chappell G, Saville AM. Impact of a population-based HPV vaccination program on cervical abnormalities: a data linkage study. BMC medicine 2013; 11(227): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollock KG, Kavanagh K, Potts A, et al. Reduction of low- and high-grade cervical abnormalities associated with high uptake of the HPV bivalent vaccine in Scotland. British journal of cancer 2014; 111(9): 1824–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crowe E, Pandeya N, Brotherton JM, et al. Effectiveness of quadrivalent human papillomavirus vaccine for the prevention of cervical abnormalities: case-control study nested within a population based screening programme in Australia. BMJ 2014; 348: g1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brotherton JML, Malloy M, Budd AC, Saville M, Drennan KT, Gertig DM. Effectiveness of less than three doses of quadrivalent human papillomavirus vaccine against cervical intraepithelial neoplasia when administered using a standard dose spacing schedule: Observational cohort of young women in Australia. Papillomavirus Research 2015; 1: 59–73. [Google Scholar]
- 25.Gordon NP. Similarity of the Adult Kaiser Permanente Membership in Northern California to the Insured and General Population in Northern California: Statistics from the 2011 California Health Interview Survey 2015. https://divisionofresearch.kaiserpermanente.org/projects/memberhealthsurvey/SiteCollectionDocuments/chis_non_kp_2011.pdf (accessed March 22, 2018). [Google Scholar]
- 26.Silverberg MJ, Leyden WA, Chi A, et al. Human Immunodeficiency Virus (HIV)- and Non-HIV-Associated Immunosuppression and Risk of Cervical Neoplasia. Obstet Gynecol 2018; 131(1): 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langholz B Case-control studies = odds ratios: blame the retrospective model. Epidemiology 2010; 21(1): 10–2. [DOI] [PubMed] [Google Scholar]
- 28.Ozawa N, Ito K, Tase T, Shibuya D, Metoki H, Yaegashi N. Lower Incidence of Cervical Intraepithelial Neoplasia among Young Women with Human Papillomavirus Vaccination in Miyagi, Japan. Tohoku J Exp Med 2017; 243(4): 329–34. [DOI] [PubMed] [Google Scholar]
- 29.Bosch FX, Robles C, Diaz M, et al. HPV-FASTER: broadening the scope for prevention of HPV-related cancer. Nat Rev Clin Oncol 2016; 13(2): 119–32. [DOI] [PubMed] [Google Scholar]


