Abstract
Central nervous tumors are the leading cause of death from cancer in the pediatric population. Advances in care for pediatric neuro-oncology patients have led to improved survival rates. As survivorship increases, care of the sequelae of the tumor and its treatment become more important for long-term quality of life.
A significant portion of the brain is involved in vision. Pediatric brain tumors can distort, damage, and destroy portions of the brain involved in both the afferent and efferent vision pathways. This interruption of normal visual pathways can lead to permanent vision loss or other morbidities such as strabismus and nystagmus. This article reviews the presenting symptoms and signs of brain tumors in children and adolescents, as well as the effects of the tumor and its treatment on the afferent and efferent visual pathways. Strategies for monitoring during treatment, and management of sequelae are reviewed. Through systematic evaluation and monitoring of pediatric neuro-oncology patients, those at risk for vision loss or tumor progression can be identified.
Introduction
Despite advances in detection, treatment, and long-term care for survivors, cancer is the second most common cause of death in the United States.1 Among children and adolescents, cancer is the most common cause of non-accidental death, with central nervous systems being the second most common site of involvement.1 Brain and central nervous system tumors are the most common solid tumor among children 0–14 years of age.2,3 The incidence of malignant central nervous system tumors in children and adolescents (aged birth to 19 years) has increased over the period of time between 2000 and 2008.4 It is estimated that for the year 2018 4,610 children and young adults will have been diagnosed with a new brain or central nervous system neoplasm.3 However survival rates have been improving, likely due to a combination of new treatment modalities, improved surveillance methods for recurrence, improved surgical techniques and radiation therapies, as well as risk-adapted chemotherapeutic regimens.5,6 As children are more often transferring care to long-term survivorship clinics outcome measures are also moving toward quality of life measures, and beyond survival alone.7,8
Treatment decisions for pediatric central nervous system tumor patients are often made by a multi-disciplinary team, which may include the neuro-oncologist, neuro-radiologist, neuro-ophthalmologist, neurosurgeon, and pediatric neurologist. Accurate data on visual function in a young, sick child may be difficult to obtain, but obtaining the highest quality data is important as treatment decisions may be made based on the observations of the ophthalmologist.
Involvement of the ophthalmologist in care of the pediatric central nervous system tumor patient should be standard of care. Early assessment of the visual system in these children may not be performed for many reasons, including lack of awareness of providers of the potential involvement of the visual system despite lack of direct involvement of the tumor in the primary visual pathways. Other limitations to obtaining a timely ophthalmologic examination include the difficulty in examining an acutely ill child, priority given to treatment of the primary lesion rather than sequelae of the lesion, inability of a young child to clearly communicate symptoms of vision loss or abnormal visual symptoms, and lack of access to an ophthalmologist or neuro-ophthalmologist. Brain tumors can alter the normal anatomy of structures involved in both the afferent and efferent visual systems, leading to dysfunction of these systems, vision loss, and ocular motility disturbances. The signs, symptoms, and examination techniques to evaluate these alterations will be discussed.
Demographics
The incidence of childhood cancers has been gradually increasing
About 6% of all central nervous system tumors reported between 2010 and 2014 occurred in children and young adults (ages 0–19).2 Tumors of the brain parenchyma (temporal lobe, frontal lobe, parietal lobe, and occipital lobe) were most common (16.7%) followed by neoplasms of the pituitary and craniopharyngeal duct (15.6%), tumors of the cerebellum (13%), brain stem (11%), cranial nerves (7.1%), and ventricle (5.7%).2 Younger children were more likely to have cerebellar tumors, while adolescents were more likely to have involvement of the pituitary gland and craniopharyngeal duct.2 Children 14 years of age and younger were most likely to have pilocytic astrocytomas (18.2%), malignant gliomas (13.9%), and embryonal tumors (13.5%). Adolescents most commonly had pituitary and craniopharyngeal duct tumors (32.1%).2
Visual System in Children
The visual system of a child is still developing, and this puts children with central nervous system tumors at particular risk of vision loss from failure to develop normally. The continued development of the visual system must be taken into account when assessing vision in the ophthalmic examination, as normal visual acuities vary by age. If evaluated with visual evoked potentials, the Snellen visual acuity equivalent of a newborn is approximately 20/200, and approached 20/40 by 6 months to one year of age.9 Visual fields rapidly mature after birth, and continue to mature throughout childhood. The fovea also matures after birth, until approximately 15–45 months of age.9 The occipital cortex continues to develop and retains plasticity through approximately 8 years of age. Visual input from each eye is essential for monocular and binocular visual development, and interruption of the visual input from one eye from occlusion, strabismus, or refractive error can lead to amblyopia, which if left untreated can lead to permanent vision loss. Children with central nervous system tumors are at risk for having strabismus or ptosis, as discussed below, and therefore may be at higher risk for the development of amblyopia in addition to the risk of visual pathway damage from their underlying tumor. Additionally post-treatment cataracts can lead to deprivation amblyopia.
Signs and symptoms of brain tumors in children
Symptoms of brain tumors in children may be entirely non-specific. For example, very young children may present with signs of irritability, lethargy, anorexia, and psychomotor regression.10 However a combination of signs and symptoms may lead to high suspicion of an intracranial lesion.
Effects on the afferent visual pathways include visual field defects from parenchymal brain lesions, involvement of the lateral geniculate ganglion, optic tracts, optic chiasm, or optic nerves themselves. Compressive lesions including and anterior to the lateral geniculate ganglion result in optic atrophy. Papilledema (optic nerve head swelling from elevated intracranial pressure) can develop in cases of intracranial tumors as a result of mass effect with increased total brain volume, brain edema, invasion or obstruction of the venous sinus system, meningeal involvement by the tumor, or due to obstructive hydrocephalus. Early papilledema can produce peripheral visual field defects while preserving central vision. Chronic papilledema can lead to permanent, severe vision loss, including loss of peripheral vision and central vision. Optic neuropathy and optic atrophy from chronic papilledema can be from progressive loss of axonal nerve fibers, central retinal artery occlusion, or central retinal vein occlusion. Retinopathy and maculopathy can occur in cases of subretinal macular edema, choroidal folds, retinal nerve fiber layer hemorrhages, infarcts, and exudates.11–13 A meta-analysis of cohort studies evaluated the presenting signs and symptoms of children with central nervous system tumors and found that, in addition to symptoms of increased intracranial pressure (headache, nausea, and vomiting), papilledema was present in 13% of patients evaluated, strabismus was present in 7%, cranial nerve palsies were present in 7%, and 6% had abnormal eye movements.14 Location of the tumor can affect which symptoms and signs are present, though papilledema and elevated intracranial pressure can be present with any tumor location.14
Alswaina et al. highlighted the important role of the ophthalmologist in making the diagnosis of a brain tumor in children, with 46% of these patients presenting with optic nerve atrophy, 46% with vision loss, 24% with papilledema, 24% with nystagmus, 19% with sixth nerve palsies, and 12% with a third nerve palsy.15 Many of these children had multiple ocular findings. Presentation frequently included optic nerve edema or pallor with decreased vision or strabismus.
A retrospective chart review of patients presenting to the emergency room who were diagnosed with brain tumors evaluated their presenting signs and symptoms.16 Headache was the most frequent symptom (67%), with 20% of patients presenting with vision changes.16 Signs on examination included 23% of patients with cranial nerve deficits, 6% with ptosis, and 1% with anisocoria. Significantly, 13% of patients presented with papilledema, however only 57% of patients were examined for the presence of papilledema.16 This emphasizes the importance of performing funduscopic examination on all patients who present with headache and signs of increased intracranial pressure to the emergency room.
Visual Outcomes of Children with Brain Tumors
Central nervous system neoplasms can affect both afferent and efferent visual systems, and treatment can affect the ocular structures as well. Adult survivors of pediatric brain tumors were found to have a relative risk of cataracts of 11.9 compared to their non-affected siblings, a relative risk of legal blindness in one or both eyes of 14.8, and a relative risk of diplopia of 8.8.17
An analysis of ten children presenting to a neuro-ophthalmic clinic who underwent surgical intervention for an intracranial neoplasm found that of the eight children who had preoperative ophthalmological examinations, all but one had significant reductions in vision prior to their surgeries.18 Postoperatively all ten children had optic atrophy and vision loss, and eight had strabismus.18 Four of those patients experienced vision loss in the perioperative period.18 Craniopharyngiomas commonly cause vision loss as a consequence of the close proximity of the remnants of Rathke’s pouch, from which the tumor is thought to arise, to the optic chiasm, optic nerves, and optic tracts. As a result craniopharyngiomas can cause optic neuropathies and chiasmal syndromes. [Figure 1] Additionally the foramen of Munro can become obstructed, leading to papilledema and hydrocephalus. In a review of 59 patients with craniopharyngioma 10% were found to meet the criteria for legal blindness, and 58% had some degree of visual impairment, highlighting the severe nature of vision loss secondary to craniopharygiomas.19 Risk factors for vision loss included younger age at diagnosis, presence of optic nerve edema at presentation, and tumor recurrence.19 A retrospective chart review of 20 children with pineal tumors found a 5% rate of optic atrophy, and 5% rate of the development of a homonymous hemianopia.20 Another retrospective study of 29 children presenting with pineal tumors found similar results, with 4% having a visual field defect, and no patients with decreased vision at final follow-up.21 Papilledema was present in 69% of patients.21 Peeler et al. found that patients who had posterior fossa tumors (medulloblastoma, juvenile pilocytic astrocytoma, and ependymoma, most frequently) had rates of fair to poor visual acuities (defined as 20/40 or worse) following treatment of their underlying lesion of 17.2%.22 [Figure 2] Kedar et al. evaluated the causes and locations of lesions leading to homonymous hemianopias in 86 children, and found that tumors caused 27% of all cases of pediatric homonymous hemianopias in comparison to 10% in adults.23 This rate is similar to the 39% of pediatric homonymous hemianopias caused by neoplasms that Liu et al. found in their series of 36 children.24 Importantly Harbert et al. found that 15.2% of children previously diagnosed with a primary brain tumor had unrecognized visual fields defects that were later (up to 13 years later) discovered on systematic neuro-ophthalmic examination.25 The most common tumor type causing an unrecognized visual field defect was a juvenile pilocytic astrocytoma, and the most frequent locations were temporal lobe lesions and hemispheric lesions.25 As many of these children become adolescents and adults, their visual field defects may affect their ability to drive, and the discovery of an unrecognized visual field defect may come as a shock to these children and their parents. This makes the systematic ophthalmologic evaluation of children with primary brain tumors even more important.
Figure 1:
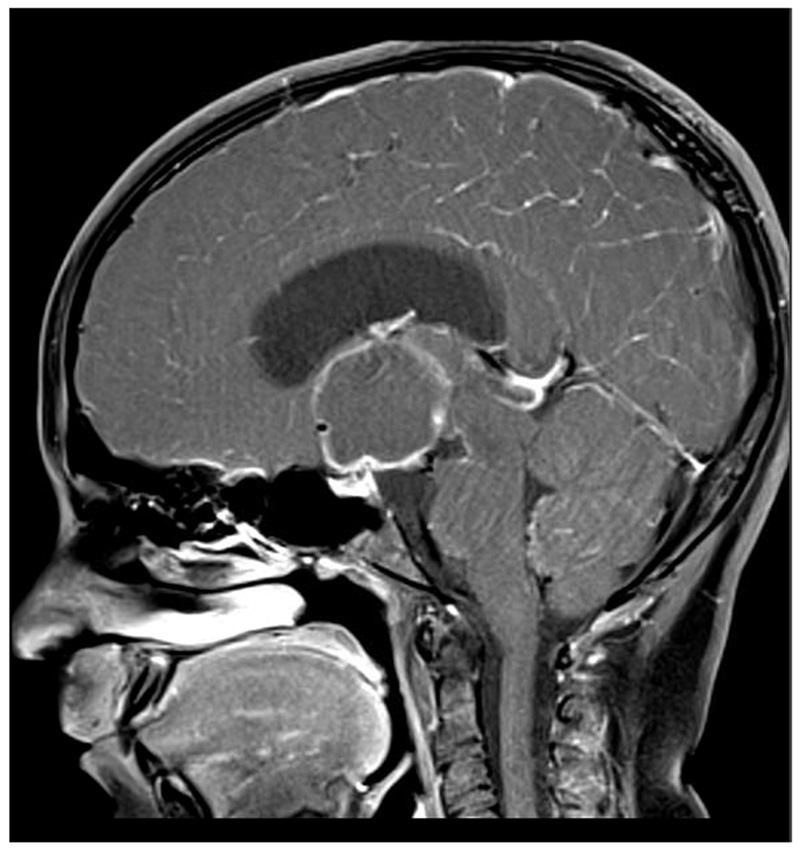
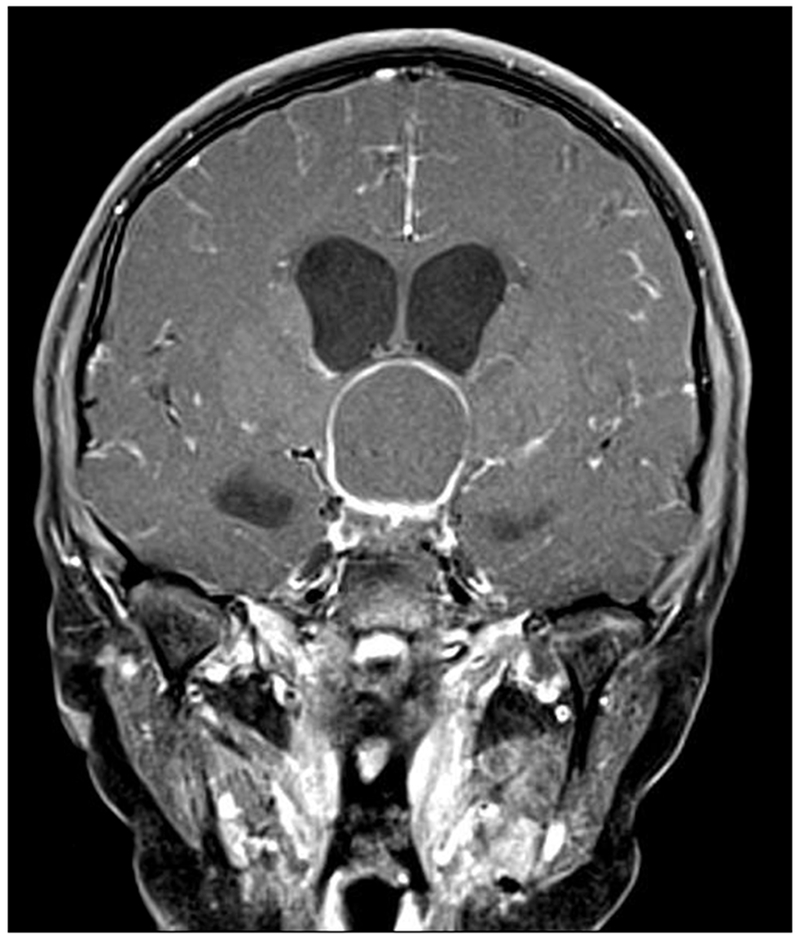
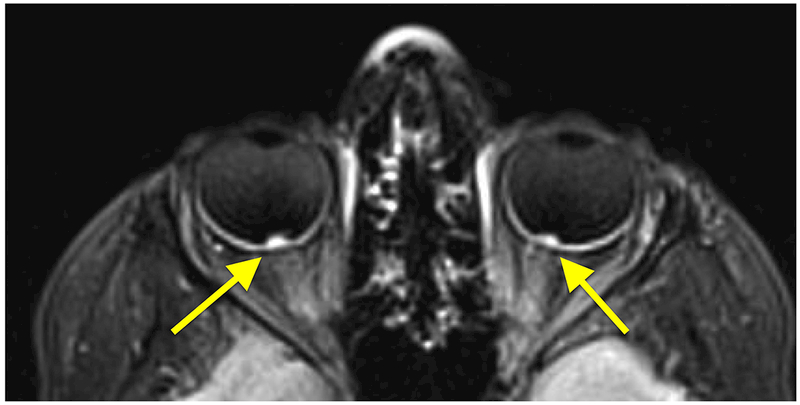
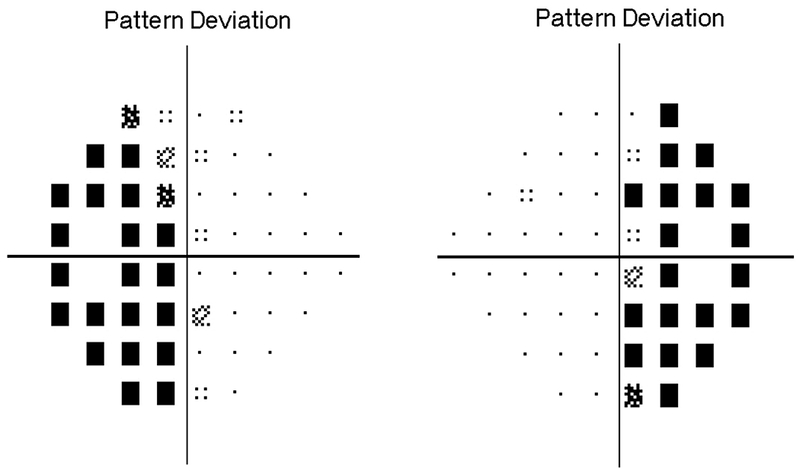
A 10-year-old girl presented with headaches, diplopia, nausea, and vomiting. Sagittal T1-weighted post-gadolinium with fat suppression magnetic resonance image (A) and coronal T1-weighted post-gadolinium with fat suppression image (B) revealed a large solid and cystic suprasellar mass resulting in obstructive hydrocephalus and compression of the optic chiasm. Ophthalmologic examination revealed bilateral sixth nerve palsies and moderate papilledema, which can be seen on the axial FLAIR post-contrast image (C, arrows). The patient underwent resection, and pathology returned consistent with craniopharyngioma. Compression of the optic chiasm led to a bitemporal hemianopia (D). Papilledema and compression of the optic chiasm led to eventual optic nerve pallor with peripapillary changes (E) after resolution of the disc edema.
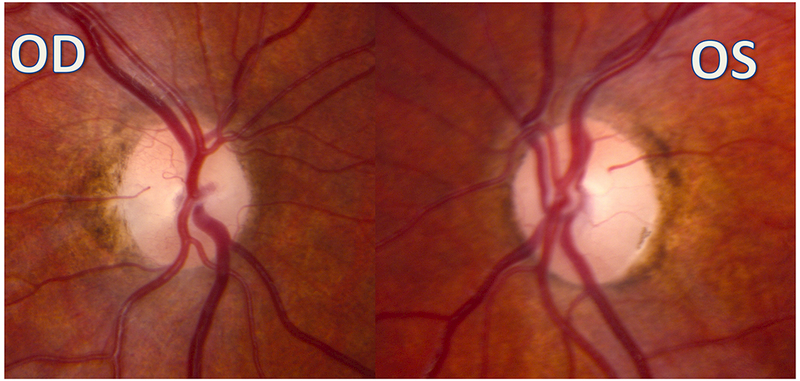
Figure 2:
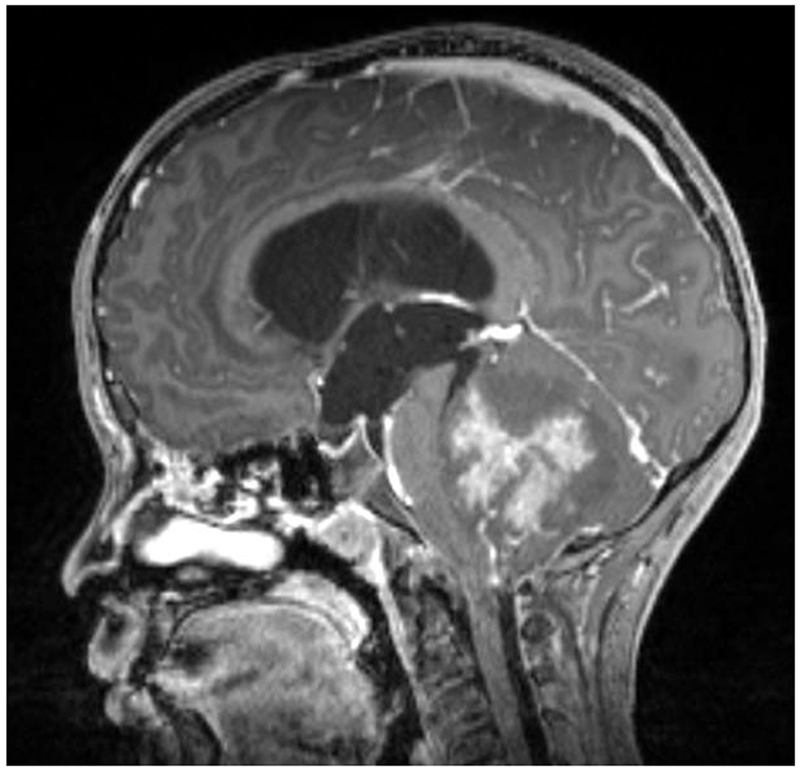
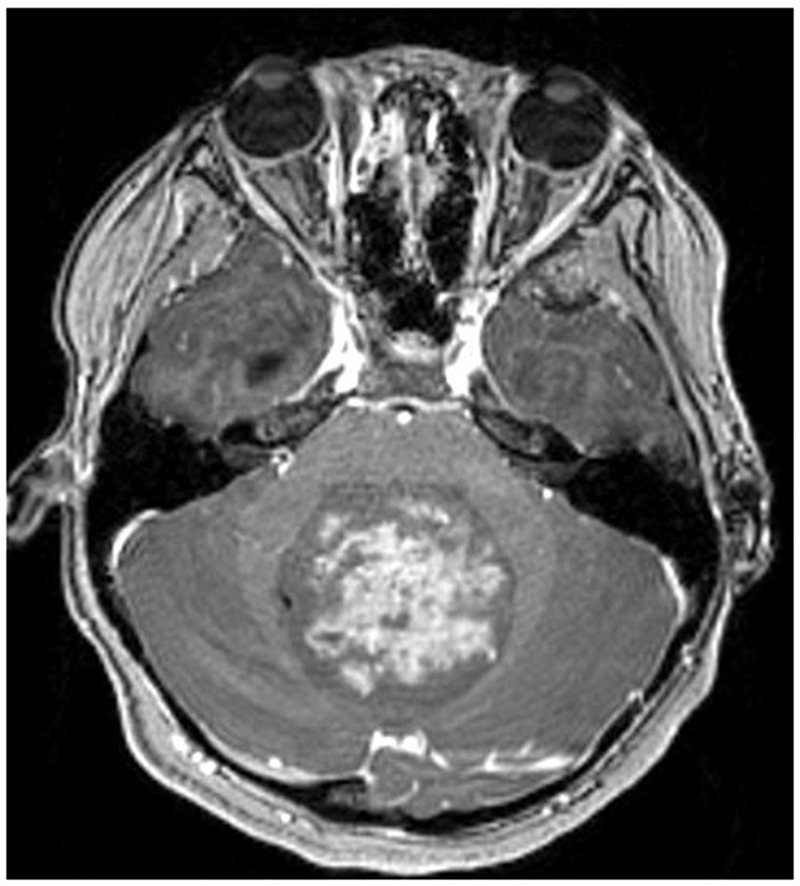
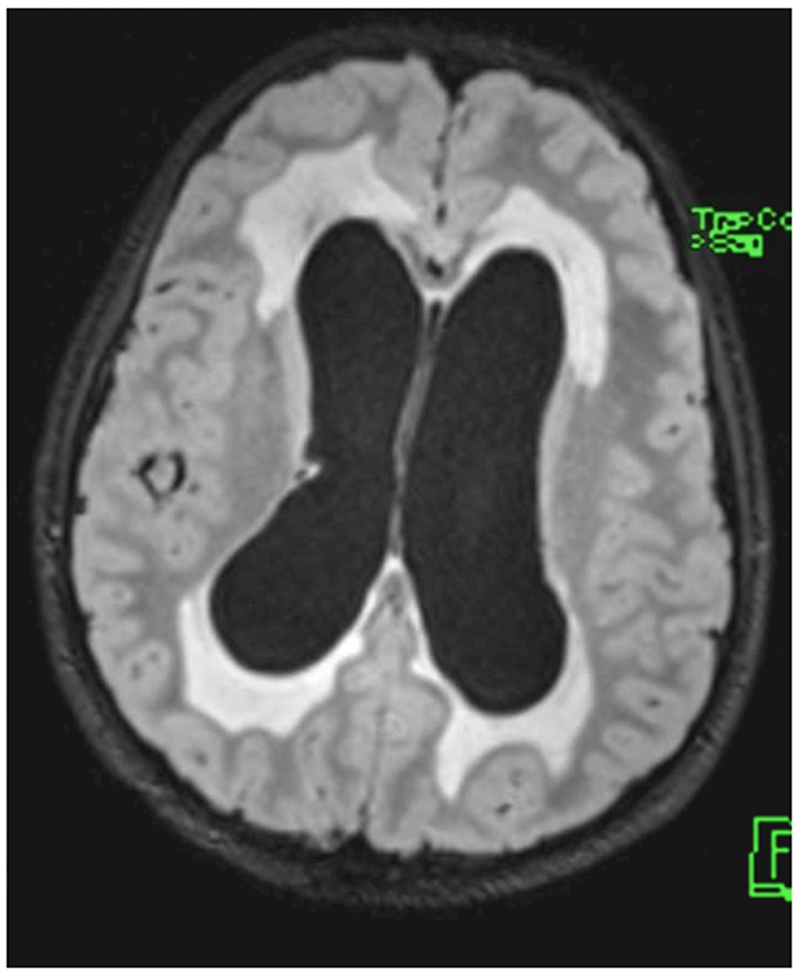
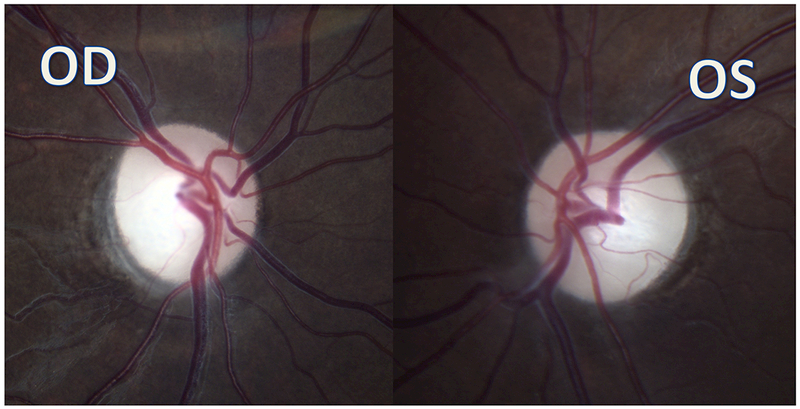
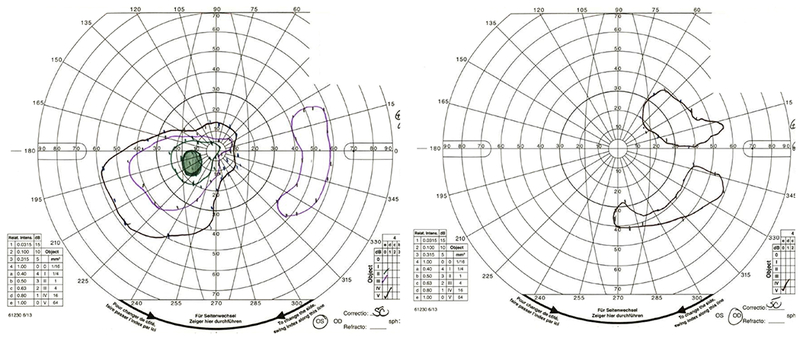
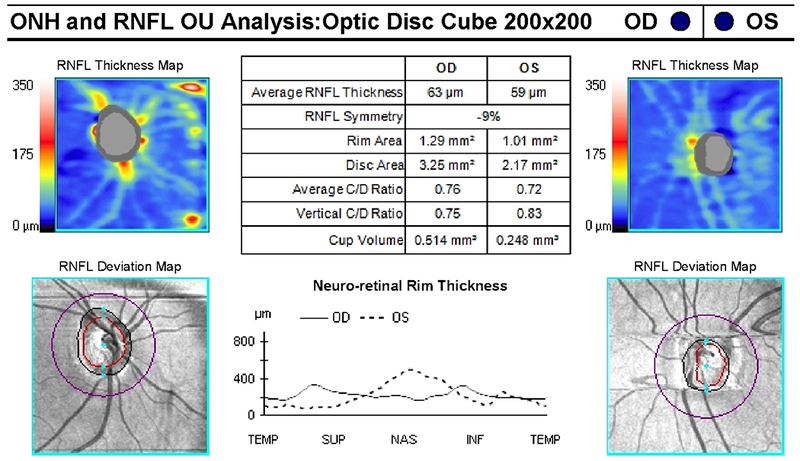
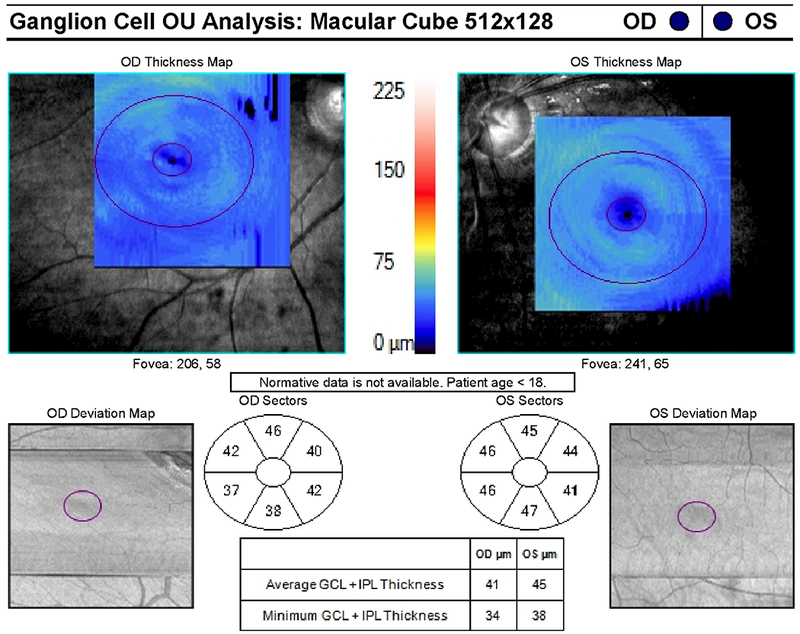
A 9-year-old boy presented with headaches, nausea, vomiting, and imbalance. Sagittal T1-weighted post-gadolinium magnetic resonance image (A) and axial T1-weighted post-gadolinium image (B) demonstrated a large posterior fossa mass with obstructive hydrocephalus and transependymal cerebrospinal fluid flow on axial FLAIR post-gadolinium image (C). Resection of the mass was performed and pathology returned consistent with medulloblastoma. Outpatient neuro-ophthalmologic examination several months after treatment demonstrated visual acuities of hand motions OD and 20/125 OS, with severe bilateral optic nerve pallor (D), and diffuse visual field depression OD worse than OS with a retained central and paracentral island OS (E). Optical coherence tomography (OCT) revealed diffuse retinal nerve fiber layer loss OU (F), as well as diffuse ganglion cell layer complex loss (G). He also had see-saw nystagmus and a right hypertropia thought to be due to a skew deviation.
Although patients with pineal tumors can develop afferent visual system dysfunction, more commonly these patients develop disorders of ocular motility, including some element of Parinaud syndrome (upgaze palsy, convergence-retraction nystagmus, light-near dissociation of pupils, and lid retraction) in all patients, horizontal deviations in 25%, and skew deviation in 15%.20 Hankinson et al. found a 62% prevalence of Parinaud syndrome at final follow-up in their study of 29 pineal tumor patients.21 Similarly, while patients with posterior fossa tumors can develop decreased vision, likely from optic atrophy from chronic papilledema and hydrocephalus, these patient are at higher risk of efferent visual pathway disorders.26 Patients who underwent treatment of posterior fossa lesions developed nystagmus (23%), strabismus (42%), and a significant number underwent strabismus surgery to improve ocular alignment (17%).22 Shalev et al. found in a cohort of 23 children with brain tumors who had strabismus that 60% were restored to orthotropia after either treatment of the tumor itself, or strabismus procedures (9/23).27 The authors suggest that restoration of binocular vision is possible with surgical intervention. Treatment plans should be tailored to the individual patient, as some patients may be at high risk for short-term clinical deterioration from their underlying central nervous system tumor.
Treatment of the tumor itself can lead to dysfuction of the visual system. Direct surgical trauma to the visual apparatus is possible, as is creating a visual field defect due to surgical approach to the tumor. Perioperative vision loss is also possible due to a sudden decrease in intracranial pressure, or interruption of the blood supply of the optic nerves. Radiotherapy can damage the visual pathways, either from late necrosis, or radiation-induced optic neuropathies, usually 5 months to 7 years after treatment.28–30 Lower rates of radiation optic neuropathies are found when reducing the treatment dose to <50Gy total dose, and <8Gy for a single dose.31 Reports of chemotherapy leading to optic neuropathy, optic neuritis, papilledema, maculopathy, cataracts, keratitis, dry eye, retinal detachment, myasthenia gravis, inflammatory orbitopathy, retinopathy, cranial nerve palsies, and uveitis have been described.32-40
Quality of Life for Pediatric Brain Tumor Survivors
Vision loss, strabismus, and diplopia can be devastating and lead to long-term effects on quality of life for a child, and the child’s family. Survivors of pediatric brain tumors have lower health-related quality of life scores compared with other cancer survivors and healthy patients.41,42 For example, vision loss can lead to decreased quality of life due to poor self-perception, school difficulties, driving ineligibility, and decreased access to gainful employment.43 A study evaluating the long term effects of brain tumors on children and health related quality of life found that only 57% of survivors had a drivers license and were eligible to drive.44 Decreased visual acuities are associated with decreased total quality of life scores, and children with visual impairment have 35.6% lower quality of life scores in comparison to age-matched controls.43 Survivors of pediatric astroglial tumors were 4.7 times more likely to unmarried, 3.1 times more likely to live with a caregiver, and 2.2 times more likely to be unemployed if they were bilaterally blind in comparison to survivors without visual impairment.45 Visual pathway disorders are associated with lower quality of life scores than abnormalities of the eye itself.46 Jariyakasol et al. reviewed studies evaluating quality of life in children with vision loss from brain tumors, and found that most health-related quality of life evaluations demonstrated lower scores for patients who reported vision abnormalities in comparison to controls and patients without vision abnormalities.7 Vision-related quality of life evaluations have thus far only been used in the pediatric population in a study of children with optic pathway gliomas.47 Quality of life evaluations will likely become more important in evaluating children with brain tumors as more of these children survive their primary disease process and enter adolescence and adulthood.
Examination of Children with Central Nervous System Neoplasms
Maximizing the information regarding visual pathway status in the presence of a central nervous system neoplasm is essential. Visual acuities are considered to be the most important measurable ophthalmic endpoint of pediatric brain tumors.48,49 Vision in infants can be assessed by reaction to light (blinking, withdrawal, pupil constriction). The ability to fixate may develop around 4 to 6 weeks of age, and following objects begins around 2 to 4 months of age. The central, steady, maintained method can be used for cooperative infants. Teller preferential looking cards can be used for cooperative infants for a quantitative measure of vision in preverbal children, and are the preferred method for quantitative assessment of vision in this age group.49 Older children can be evaluated using HOTV or Lea symbol matching, followed by reading age-appropriate eye charts. Color vision can be assessed using pseudoisochromatic plates or a red cap for color desaturation. Pupil assessment is essential for detecting relative afferent pupillary defects. The extraocular motility examination should include ductions and versions, as well as alignment testing, again dependent on age. Slit-lamp examination, funduscopy, and a basic cranial nerve examination should be performed. Visual field testing can be more difficult in very young children, but attempts should be made with confrontation with interesting objects in the quadrants of the peripheral field of vision while maintaining fixation on a central target for the youngest children, with saccadic movements toward the introduced peripheral object counted as positive responses. Older children can perform more traditional finger counting as a basic test of visual fields. More cooperative children can perform kinetic perimetry, and eventually automated perimetry. Optic nerve photographs can be useful for serial monitoring of optic nerve atrophy or edema. Optical coherence tomography (OCT) is a useful adjunctive test for monitoring children with central nervous system neoplasms. Bialer et al. described a correlation of visual acuity and visual field defects with retinal nerve fiber layer thickness in children with craniopharyngioma.50 Hand-held OCT has been used to distinguish between optic pathway glioma patients with vision loss, and those without vision loss, and can be performed on children who are sedated for their MRIs.51 Ganglion cell layer complex analysis of macular OCT scans can demonstrate thinning that suggests a compressive optic neuropathy, correlates with visual fields, and can potentially be useful as an adjunctive test for evaluating and monitoring brain tumor patients.52
Conclusions
Survival rates for pediatric central nervous system tumors continue to improve with time. However, pediatric central nervous system tumors can lead to devastating long-term consequences for patients due to vision loss, ocular motor abnormalities, and decreased quality of life. Awareness of the potential for ophthalmic monitoring for progression and the opportunity to intervene to prevent vision loss from tumor involvement is important for the ophthalmologist and the multi-disciplinary team caring for the patient. Involvement of the ophthalmologist in systematic treatment monitoring and decision-making is essential for optimal outcomes. Through earlier interventions the ophthalmologist may be able to improve long-term vision outcomes and quality of life.
Acknowledgments
Support: Supported in part by an unrestricted departmental grant (Department of Ophthalmology) from Research to Prevent Blindness, Inc., New York, and by NIH/NEI core grant P30-EY06360 (Department of Ophthalmology). This work was not industry supported.
Footnotes
Disclosures:
The authors report no conflicts of interest.
References:
- 1).Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7–10. [DOI] [PubMed] [Google Scholar]
- 2).Ostrom QT, Gittleman H, Liao P, et al. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol 2017;19(Suppl 5):v1–v88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Ostrom QT, de Blank PM, Kruchko C, et al. Alex’s Lemonade Stand Foundation infant and childhood primary brain and central nervous tumors diagnosed in the United States in 2007–2011. Neuro Oncol 2015; 16(Suppl 10):x1–x36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Gittleman HR, Ostrom QT, Rouse CD, et al. Trends in central nervous system tumor incidence relative to other common cancers in adults, adolescents, and children in the United States, 2000 to 2010. Cancer 2015;121:102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Pollack IF. Multidisciplinary management of childhood brain tumors: a review of outcomes, recent advances, and challenges. J Neurosurg Pediatrics 2011;8:135–148. [DOI] [PubMed] [Google Scholar]
- 6).Pollack IF, Jakacki RI. Childhood brain tumors: epidemiology, current management and future directions. Nat Rev Neurol 2011;7:495–506. [DOI] [PubMed] [Google Scholar]
- 7).Jariyakosol S, Peragallo JH. The effects of primary brain tumors on vision and quality of life in pediatric patients. Semin Neurol 2015;35:587–598. [DOI] [PubMed] [Google Scholar]
- 8).Pollack IF. Diagnosis and treatment of childhood brain tumors: Current perspectives. J Child Neurol 2009;24:1464–1465. [DOI] [PubMed] [Google Scholar]
- 9).Møller HU, Larsen DA. Milestones and normative data In Lambert SR, Lyons CJ, eds. Taylor and Hoyt’s Pediatric Ophthalmology and Strabismus, ed 5 Edinburgh, Scotland: Elsevier, 2017: 40–49. [Google Scholar]
- 10).Goldman RD, Cheng S, Cochrane DD. Improving diagnosis of pediatric central nervous system tumours: aiming for early detection. CMAJ 2017;189:E459–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Schirmer CM, Hedges TR III. Mechanisms of visual loss in papilledema. Neurosurg Focus 2007;23:E5. [DOI] [PubMed] [Google Scholar]
- 12).Green GJ, Lessell S, Loewenstein JI. Ischemic optic neuropathy in chronic papilledema. Arch Ophthalmol 1980;98:502–504. [DOI] [PubMed] [Google Scholar]
- 13).Baker RS, Buncic JR. Sudden visual loss in pseudotumor cerebri due to central retinal artery occlusion. Arch Neurol 1984;41:1274–1276. [DOI] [PubMed] [Google Scholar]
- 14).Wilne S, Collier J, Kennedy C, et al. Presentation of childhood CNS tumours: a systematic review. Lancet Oncol 2007;8:685–695. [DOI] [PubMed] [Google Scholar]
- 15).Alswaina N, Elkhamary SM, Shammari MA, et al. Ophthalmic features of outpatient children diagnosed with intracranial space-occupying lesions by ophthalmologists. Middle East Afr J Ophthalmol 2015;22:327–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Lanphear J, Sarnaik S. Presenting symptoms of pediatric brain tumors diagnosed in the emergency department. Pediatr Emer Care 2014;30:77–80. [DOI] [PubMed] [Google Scholar]
- 17).Packer RJ, Gurney JG, Punkyo JA, et al. Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: Childhood cancer survivor study. J Clin Oncol 2003;21:3255–3261. [DOI] [PubMed] [Google Scholar]
- 18).Goldenberg-Cohen N, Ehrenberg M, Toledano H, et al. Preoperative visual loss is the main cause of irreversible poor vision in children with a brain tumor. Front Neurol 2011;2:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Wan MJ, Zapotocky M, Bouffet E, et al. Long-term visual outcomes of craniopharyngioma in children. J Neurooncol 2018;137:645–651. [DOI] [PubMed] [Google Scholar]
- 20).Hart MG, Sarkies NJ, Santarius T, et al. Ophthalmological outcome after resection of tumors based on the pineal gland. J Neurosurg 2013;119:420–426. [DOI] [PubMed] [Google Scholar]
- 21).Hankinson EB, Lyons CJ, Hukin J, et al. Ophthalmological outcomes of patients treated for pineal region tumors. J Neurosurg Pediatr; 2016;17:558–563. [DOI] [PubMed] [Google Scholar]
- 22).Peeler CE, Edmond JC, Hollander J, et al. Visual and ocular motor outcomes in children with posterior fossa tumors. J AAPOS 2017;21:375–379. [DOI] [PubMed] [Google Scholar]
- 23).Kedar S, Zhang X, Lynn MJ, et al. Pediatric homonymous hemianopia. J AAPOS 2006;10:249–252. [DOI] [PubMed] [Google Scholar]
- 24).Liu GT, Galetta SL. Homonymous hemifield loss in childhood. Neurology 1997;49:1748–1749. [DOI] [PubMed] [Google Scholar]
- 25).Harbert MJ, Yeh-Nayre LA, O’Halloran HS, et al. Unrecognized visual field deficits in children with primary central nervous system brain tumors. J Neurooncol 2012;107:545–549. [DOI] [PubMed] [Google Scholar]
- 26).Peeler CE. A review of visual and oculomotor outcomes in children with posterior fossa tumors. Semin Pediatr Neurol 2017;24:100–103. [DOI] [PubMed] [Google Scholar]
- 27).Shalev B, Repka MX. Restoration of fusion in children with intracranial tumors and incomitant strabismus. Ophthalmology 2000;107:1880–1883. [DOI] [PubMed] [Google Scholar]
- 28).Lessell S Friendly fire: neurogenic visual loss from radiation therapy. J Neuroophthalmol 2004;24:243–250. [DOI] [PubMed] [Google Scholar]
- 29).Donahue B Short- and long-term complications of radiation theraphy for pediatric brain tumors. Pediatr Neurosurg 1992;18:207–217, [DOI] [PubMed] [Google Scholar]
- 30).al-Mefty O, Kersh JE, Routh A, et al. The long-term side effects of radiation therapy for benign brain tumors in adults. J Neurosurg 1990;73:502–512. [DOI] [PubMed] [Google Scholar]
- 31).Mayo C, Martel MK, Marks LB, et al. Radiation dose-volume effects of optic nerves and chiasm. Int J Radiat Oncol Biol Phys 2010;76(Suppl 3):S28–S35. [DOI] [PubMed] [Google Scholar]
- 32).Schmid KE, Kornek GV, Scheithauer W, et al. Update on ocular complications of systemic cancer chemotherapy. Surv Ophthalmol 2006;51:19–40. [DOI] [PubMed] [Google Scholar]
- 33).al-Tweigeri T, Nabholtz JM, Mackey JR. Ocular toxicity and cancer chemotherapy. A review. Cancer 1996;78:1359–1373. [DOI] [PubMed] [Google Scholar]
- 34).Wantanabe W, Kuwabara R, Nakahara T, et al. Severe ocular and orbital toxicity after intracarotid injection of carboplatin for recurrent glioblastomas. Graefes Arch Clin Exp Ophthalmol 2002;240:1033–1035. [DOI] [PubMed] [Google Scholar]
- 35).Urba S, Forasteire AA. Retrobulbar neuritis in a patient treated with intaarterial cisplatin for head and neck cancer. Cancer 1988;62:2094–2097. [DOI] [PubMed] [Google Scholar]
- 36).Shurin SB, Rekate HL, Annable W. Optic atrophy induced by vincristine. Pediatrics 1982;70:288–291. [PubMed] [Google Scholar]
- 37).Dalvin LA, Shields CL, Orloff M, et al. Checkpoint inhibitor immune therapy: Systemic indications and ophthalmic side effects. Retina 2018;38:1063–1078. [DOI] [PubMed] [Google Scholar]
- 38).Bhatti MT, Salama AKS. Neuro-ophthalmic side effects of molecularly targeted cancer drugs. Eye 2018;32:287–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Stjepanovic N, Velazquez-Martin JP, Bedard PL. Ocular toxicities of MEK inhibitors and other targeted therapies. Ann Oncol 2016;27:998–1005 [DOI] [PubMed] [Google Scholar]
- 40).Kheir WJ, Sniegowski MC, El-Sawy T, et al. Ophthalmic complications of targeted cancer therapy and recently recognized ophthalmic complications of traditional therapy. Surv Ophthalmol 2014;59:493–502. [DOI] [PubMed] [Google Scholar]
- 41).Macartney G, Harrison MB, VanDenKerkhof E, et al. Quality of life and symptoms in pediatric brain tumor survivors: A systematic review. J Pediatr Oncol Nurs 2014;31:65–77. [DOI] [PubMed] [Google Scholar]
- 42).Gupta P, Jalali R. Long-term survivors of childhood brain tumors: Impact on general health and quality of life. Curr Neurol Neurosci Rep 2017;17:99. [DOI] [PubMed] [Google Scholar]
- 43).Chadha RK, Subramanian A. The effect of visual impairment on quality of life in children aged 3–16 years. Br J Ophthamol 2011;95:642–645. [DOI] [PubMed] [Google Scholar]
- 44).Bhat SR, Goodwin TL, Burwinkle TM. Profile of daily life in children with brain tumors: An assessment of health-related quality of life. J Clin Oncol 2005;23:5493–5500. [DOI] [PubMed] [Google Scholar]
- 45).de Blank PM, Fisher MJ, Lu L, et al. Impact of vision loss among survivors of childhood central nervous system astroglial tumors. Cancer 2016;122:730–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Boulton M, Haines L, Smyth D, et al. Health-related quality of life of children with vision impairment or blindness. Dev Med Child Neurol 2006;48:656–661. [DOI] [PubMed] [Google Scholar]
- 47).Avery RA, Hardy KK. Vision specific quality of life in children with optic pathway gliomas. J Neurooncol 2014;116:341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48).Avery RA, Fisher MJ, Liu GT. Optic pathway gliomas. J Neuroophthalmol 2011;31:269–278. [DOI] [PubMed] [Google Scholar]
- 49).Fisher MJ, Avery RA, Allen JC, et al. REiNS International Collaboration. Functional outcome measures for NF1-associated optic pathway glioma clinical trials. Neurology 2013;81(Suppl 1):S15–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50).Bialer OY, Goldenberg-Cohen N, Toledano H, et al. Retinal RNFL thinning on OCT correlates with visual field loss in pediatric craniopharyngioma. Can J Ophthalmol 2013;48:494–499. [DOI] [PubMed] [Google Scholar]
- 51).Avery RA, Hwang EI, Ishikawa H, et al. Handheld optical coherence tomography during sedation in young children with optic pathway gliomas. JAMA Ophthalmol 2014;132:265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52).Vuong LN, Hedges TR. Ganglion cell layer complex measurements in compressive optic neuropathy. Curr Opin Ophthalmol 2017;28:573–578. [DOI] [PubMed] [Google Scholar]


