High-density lipoprotein (HDL) is dysfunctional in chronic kidney disease (CKD) (1), but the clinical relevance of this is unknown. Cholesterol efflux capacity (CEC), a key HDL function, and total HDL particle number (HDL-P) each inversely associates with cardiovascular (CV) events, independent of HDL-C and of each other (2,3). Although some reports suggested no such associations for CEC among those with advanced CKD (4), none have analyzed HDL-P among those with CKD, and none have assessed these relationships in lower risk populations. Therefore, we investigated whether impaired renal function modifies the association of CEC and HDL-P with incident CV events in a low-risk cohort.
Participants enrolled in the Dallas Heart Study, a population-based cohort aged 30–65 years, (3) without prevalent CVD were included in the analysis (n = 2,805). As previously published, CKD was defined as impaired renal function at a single time point based on an estimated glomerular filtration rate (eGFR, calculated by the 4-variable Modification of Diet in Renal Disease formula) <60 mL/min per 1.73 m2 OR gender-based urinary albumin-to-creatinine ratio (ACR) ≥17 mg/g in men or ≥25 mg/g in women as defined by NHANES (5). The MDRD formula has been used in all DHS studies based on the alkaline picrate method of creatinine determination in the DHS. HDL-P concentration was determined by NMR (Liposcience, now LabCorp). CEC was determined by quantifying the capacity of a participant’s plasma depleted of apolipoprotein B to accept BODIPY-cholesterol from J774 macrophages. Cox proportional hazards models were used to assess the associations of continuous CEC and HDL-P with outcomes over 11.3 years of follow-up. Pre-specified outcomes were ASCVD (first nonfatal MI, nonfatal stroke, or death from CVD), total CVD (ASCVD + percutaneous coronary intervention or coronary-artery bypass grafting, and hospitalizations for atrial fibrillation, heart failure, or peripheral arterial disease), CV death and non-CV death. Interactions of CEC and HDL-P with CKD were tested in models adjusted for age, sex, race, diabetes, hypertension, smoking, total cholesterol, HDL-C, log eGFR, body mass index, hs-CRP, and ACR. Two-sided P-values were used.
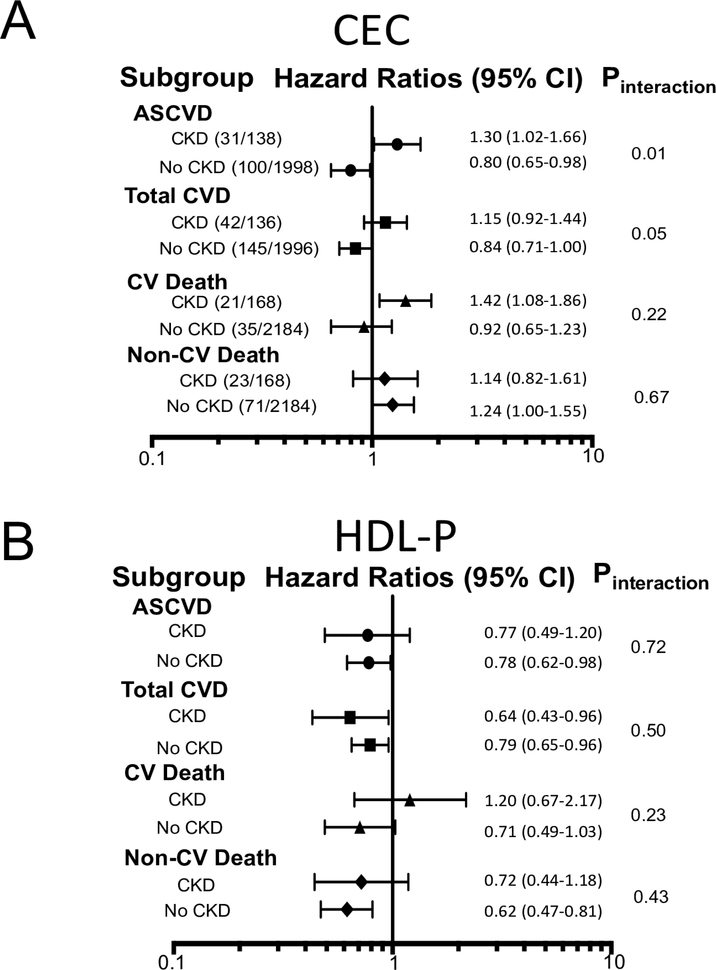
Of the 2,805 participants included, 7.5% (210) had baseline CKD, of which 54% (113) met the definition with elevated ACR alone. Those with prevalent CKD had increased HTN (61% vs. 29%), diabetes (35% vs. 9%), and statin use (7% vs. 4%) when compared to those without CKD. The median HDL-C (46 vs. 48 mg/dL), LDL-C (104 vs. 104 mg/dL), CEC (1.0 vs. 1.0 normalized ratio), and HDL-P (33 vs. 33 nmol/L) and smoking (31% vs. 28%) were not significantly different between groups. Those with CKD had higher incident ASCVD (22% vs. 5%, n= 131, p<0.0001) and total CVD (31% vs. 7%, n= 187, p<0.0001). In multivariate Cox models, there was a significant interaction between CEC and CKD on associations with ASCVD (p=0.01) and total CVD (p=0.05). Specifically, in those without CKD, CEC was inversely associated with incident ASCVD and total CVD (Figure 1A). However, in those with CKD, CEC was directly associated with ASCVD and CV death and was not associated with total CVD (Figure 1A). HDL-P inversely associated with each of the CV events without effect modification by CKD status (Figure 1B). In sensitivity analyses, use of the CKD-EPI formula (5) or analyses by eGFR or ACR alone did not affect these findings.
Figure 1: Associations of CEC (A) and HDL-P (B) with events by CKD status.
Adjusted hazard ratios for 1 standard deviation. Event proportions are in parentheses.
In a large low-risk cohort free of known CVD, higher CEC may actually connote paradoxically increased CV risk in those with CKD. These findings provide new insights into how HDL-related markers perform in the setting of CKD. Specifically, future investigations regarding CEC should account for CKD status. Interestingly, the ability of HDL-P to predict CVD risk is preserved in CKD patients. Further studies are warranted to determine the clinical utility of HDL function and HDL particles as risk predictors and as therapeutic targets across the spectrum of kidney disease.
Acknowledgments
Financial Support: The Dallas Heart Study is supported by grants from the Donald W. Reynolds Foundation and the National Center for Advancing Translational Sciences of the NIH (UL1TR001105). Anand Rohatgi is supported by the National Heart, Lung, and Blood Institute of the NIH under Award Number R01HL136724, K08HL118131, and R21HL137450, and by the American Heart Association under Award Number 15CVGPSD27030013. NMR measurements were provided by LipoScience (now 8 LabCorp, Burlington, North Carolina).
Abbreviations
- CKD
Chronic Kidney disease
- CEC
Cholesterol efflux capacity
- HDL-P
High density lipoprotein particle
- CVD
Cardiovascular disease
- ACR
Albumin to creatinine ratio
- BMI
Body mass index
Footnotes
Disclosures: Chindhy: None; Joshi: None; Khera: None; Ayers: None; Hedayati: None; Rohatgi: Merck, research grant, significant Merck, consultant, modest CSL Limited, consultant, modest HDL Diagnostics, Advisory Board, modest Cleveland HeartLabs, consultant, modest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson JL, Gautier T, Nijstad N et al. High density lipoprotein (HDL) particles from end-stage renal disease patients are defective in promoting reverse cholesterol transport. Sci Rep 2017;7:41481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mackey RH, Greenland P, Goff DC, Lloyd-Jones D, Sibley CT, Mora S. High-density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: MESA (multi-ethnic study of atherosclerosis). J Am Coll Cardiol 2012;60:508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rohatgi A, Khera A, Berry JD et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med 2014;371:2383–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer L, Kern S, Rogacev KS et al. HDL Cholesterol Efflux Capacity and Cardiovascular Events in Patients With Chronic Kidney Disease. J Am Coll Cardiol 2017;69:246–247. [DOI] [PubMed] [Google Scholar]
- 5.Gregg LP, Adams-Huet B, Li X et al. Effect Modification of Chronic Kidney Disease on the Association of Circulating and Imaging Cardiac Biomarkers With Outcomes. J Am Heart Assoc 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]