Abstract
Objective
To evaluate antigen-specific immune responses for leprosy diagnosis in a hyperendemic area in China.
Methods
Eighty-three leprosy patients and 161 non-leprosy controls were enrolled from Hani-yi Autonomous Prefecture of Honghe, Yunnan Province, China. Leprosy patients were divided into multibacillary (MB, n = 38), paucibacillary (PB, n = 23), and post-multi-drug therapy (MDT, n = 22) groups. Controls were divided into the following groups: healthy household contacts (HHC, n = 119), tuberculosis (TB, n = 11), and endemic controls (EC, n = 31). The NDO-LID Rapid Test, M. leprae antigen-specific ELISA and antigen-specific IFN-γ secretion in a whole blood assay (WBA) were used to evaluate these subjects.
Results
The NDO-LID Rapid Test achieved higher positive response rates in MB than in PB patients[94.7%(36/38) vs 65.2%(15/23)], and these rates were higher than those observed by ELISA using anti-LID-1[92.1%(35/38) vs 52.2%(12/23)], anti-NDO-LID[92.1%(35/38) vs 47.8% (11/23)], and anti-ND-O-BSA[89.5%(34/38) vs 60.9%(14/23)]. However, the NDO-LID Rapid Test also showed a higher positive response rate in the EC group (33.3%,10/31), which was higher than the rates observed for anti-NDO-LID (12.9%,4/31) and anti-ND-O-BSA (16.1%,5/31). M. leprae antigen-specific ELISA demonstrated relatively high specificity (86.84–97.37%) but low sensitivity (15.97–72.73%) in discriminating between leprosy patients and non-leprosy controls by ROC curve analysis. In contrast, M. leprae antigen-specific IFN-γ secretion detection achieved higher positive response rates in PB than in MB patients (positive ratio of MB vs PB: 40% vs 56% for LID-1, 28.6% vs 47.8% for ML89, 31.4% vs 60.7% for ML2044, and 31.4 vs 47.8% for ML2028) and could distinguish MB from EC when stimulated with ML89(AUC = 0.6664) and PB fromTB when stimulated with ML2044 and ML2028(AUC = 0.7549 and 0.7372, respectively).
Conclusion
The NDO-LID Rapid Test and M. leprae antigen-specific ELISA are useful tools to assist in the diagnosis of leprosy patients, especially MB patients, although the former had higher sensitivity but lower specificity than the latter. M. leprae antigen-specific IFN-γ release assessed by WBA has diagnostic value for distinguishing PB from TB but not for distinguishing PB from HHC or EC. Screening novel M. leprae-specific antigens, combining different M. leprae antigens and a multi-cytokine analyte model may be needed for more effective diagnosis of leprosy.
Author summary
Although the implementation of World Health Organization (WHO) multidrug therapy (MDT) treatment has drastically reduced the number of registered leprosy cases, new case detection rates have stabilized over the last decade, and leprosy remains an important health problem in many regions. Antigen-specific immune diagnostic tools are helpful for leprosy diagnosis but require broad evaluation in different populations from areas with hyperendemic leprosy. The NDO-LID Rapid Test, M. leprae antigen-specific ELISA and antigen-specific secretion of IFN-γ in a whole blood assay (WBA) can be used to diagnose multibacillary (MB) and paucibacillary (PB) leprosy patients. The authors found that in Honghe Autonomous Prefecture,Yunnan Province, China, the NDO-LID Rapid Test and M. leprae antigen-specific ELISA have the potential to be used as tools to assist in the diagnosis of patients with MB leprosy. The NDO-LID Rapid Test has higher sensitivity but lower specificity than the M. leprae antigen-specific ELISA. M. leprae antigen-specific IFN-γ secretion in WBA exhibited diagnostic value for distinguishing PB from TB but not for distinguishing PB from HHC or EC. This study provides an evaluation of antigen-specific immune responses for leprosy diagnosis in a hyperendemic area in China.
Introduction
Leprosy, a chronic disease caused by Mycobacteriumleprae (M. leprae) infection, has a wide range of clinical outcomes correlated with the host's immune response to the bacilli[1,2].
Current leprosy control strategies rely on diagnosing the disease as early as possible, followed by prompt treatment with multi-drug therapy (MDT)[1]. The implementation of World Health Organization (WHO) MDT for widespread, worldwide treatment has drastically reduced registered leprosy cases from the approximately 12 million reported in 1985 to fewer than 250,000 reported in 2006[3]. Currently, leprosy is mainly diagnosed by clinicians using defined criteria, slit-skin smears and biopsies[4]. However, as the prevalence of the disease decreases, clinical expertise is diminishing, leading to extended delays between the onset of clinical signs and the diagnosis and consequent sustained transmission of M. leprae[5].
Leprosy patients are predominantly diagnosed by the appearance of disease signs, but they can also be characterized by the physical and histological attributes of skin or nerve lesions or by their immune response to crude or recombinant M. leprae antigens[6, 7, 8, 9]. It has been demonstrated that the immune response to crude or recombinant M. leprae antigens is helpful for detecting multibacillary (MB) leprosy patients by their antibody response[6], for the diagnosis of paucibacillary (PB) patients by antigen-specific CMI[7], and for monitoring the effectiveness of MDT in MB and PB leprosy patients by the antibody response and antigen-specific CMI, respectively[8]. The M. leprae antigens used for ELISA in this study were Leprosy IDRI diagnostic-1 (LID-1), a fusion protein developed by fusing the ML0405 and ML2331 genes[9, 10];NDO-LID, a conjugate of LID-1 with natural octyl disaccharide (NDO)[11];and ND-O-BSA, a synthetic PGL-I derivative. The NDO-LID Rapid Test in lateral flow-based format has been developed using NDO-LID. The single tetravalent 89-kDa fusion protein(ML89), designated LEP-F1, consists of the ML2028, ML2055 and ML2380 antigens[12]. A list of accession numbers/ID numbers for genes and proteins included in the NCBI search and mentioned in the text is shown inTable 1.
Table 1. List of accession numbers/ID numbers for genes and proteins included in the NCBI search and mentioned in the text.
| Name | Gene ID | Description | Location | Aliases |
|---|---|---|---|---|
| ML0405 | ID: 909138 | hypothetical protein [Mycobacterium leprae TN] | NC_002677.1 (503217..504401) | ML0405 |
| ML2331 | ID: 908688 | hypothetical protein [Mycobacterium leprae TN] | NC_002677.1 (2761703..2762473) | ML2331 |
| ML2044 | ID: 909000 | hypothetical protein [Mycobacterium leprae TN] | NC_002677.1 (2434368..2434589, complement) | ML2044 |
| ML2028 | ID: 909036 | fbpB diacylglycerol acyltransferase/mycolyltransferase [Mycobacterium leprae TN] | NC_002677.1 (2418620..2419603) | fbpB |
The purpose of this study was to evaluate the diagnostic value of three antigen-specific immune diagnostic tests, namely, the NDO-LID Rapid Test(antibody response), an antigen-specific enzyme linked immunosorbent assay (ELISA)(anti-LID-1, anti-NDO-LID, and anti-ND-O-BSA)(antibody response), and antigen-specific IFN-γ secretion in a whole blood assay (WBA) (stimulated by LID-1, ML89, ML2044 and ML2028)(antigen-specific CMI) for diagnosing leprosy in a hyperendemic area in China.
Methods
Ethics statement
This study was approved by the Medical Ethics Committee of Beijing Friendship Hospital, Capital Medical University, Beijing, P.R. China. Written informed consent was obtained from all adult participants, and all parents or guardians of child participants provided informed consent on their behalf. All of the procedures in the study involving human participants were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Study area and subjects
Eighty-three leprosy patients, who were referred to the Honghe Prefecture Skin Disease Prevention and Cure Institute in Honghe Autonomous Prefecture, Yunnan Province, were included in the study. Leprosy diagnosis was established based on clinical signs and symptoms, skin smears, skin biopsy, and neuro-physiologic examinations. The leprosy patients were classified into five groups based on the Ridley and Jopling[13] classification: tuberculoid (TT), borderline-tuberculoid (BT), borderline-borderline (BB), borderline-lepromatous (BL), and lepromatous (LL) groups. For data analysis in this study, leprosy patients were also classified into three groups: PB and MB, according to the WHO operational classification[14] during MDT, or post-MDT. One hundred and sixty-one controls from the same endemic region were included as non-leprosy controls. The controls were further classified into three groups: healthy household contacts (HHC), tuberculosis (TB), and endemic controls (EC).
NDO-LID Rapid Test
Antigen-specific antibody detection by NDO-LID was performed as previously described[15]. Serum antibodies were measured by the NDO-LID rapid diagnostic test (RDT; procured from Orange Life, Rio de Janeiro, Brazil). Briefly, NDO-LID RDT was performed by first adding undiluted serum (10 μl) into the sample well within the test cassette, followed by the addition of running buffer (100 μl). Samples migrated through the cassette such that interactions with the test and/or control lines were revealed as red colored lines within the reading window. Tests were valid if the control line was observed. A positive result was defined by the presence of the test line. Visual results were interpreted after 20 minutes by two independent readers and scored subjectively as (±/+/++/+ + +), with faint (±) or no test line considered a negative result.
Anti-LID-1, anti-NDO-LID and anti-ND-O-BSA by ELISA
ELISA microplate wells were coated overnight with the M. leprae-specific antigens LID-1 (1 μg/ml), NDO-LID (200 ng/ml) or synthetic PGL-I (200 ng/ml ND-O-BSA) in 0.1 M carbonate/bicarbonate coating buffer, pH 9.6 (50 μl). After 1 h in blocking buffer (1% bovine serum albumin in phosphate-buffered saline, pH 7.2, with 0.05% tween and 1% BSA/PBS/T), sera were diluted in blocking solution, tested at a 1:200 dilution (100 μl), and subsequently incubated for 2 h at room temperature (RT). Then, the wells were washed with PBS with 0.05% tween 20 (PBS/T, wash buffer) six times. Secondary peroxidase-conjugated anti-human IgM (anti-PGL-I), anti-human IgG (anti-LID-1) (1:20,000, Abcam, Cambridge, UK), or a combination of anti-human IgM and IgG antibodies (anti-NDO-LID) was added for another 2-h incubation period. Following this incubation, the wells were washed with PBS/T six times, followed by the addition of 100 μl of substrate (3,3’,5,5’-tetramethylbenzidine; TMB). After 15-minute incubation at RT, 50 μl of stop solution (H2SO4, 1 M) was added. Optical density (OD) values were determined with an ELISA plate reader (Asys Expert Plus-Microplate Reader UK) at 450 nm. The cut off for ELISA positivity was calculated from an OD value of 0.2, as described previously[15].
IFN-γ release by M. leprae-specific antigen Stimulated WBA
WBA was performed as previously described. Briefly, undiluted, heparinized venous whole blood (Greiner) was collected. Whole blood was plated into 24-well plates (450 μl/well; Sigma, St. Louis, MO) within 2 h of collection and incubated with stimulants for 24 h at 37°C and 5% CO2. Each assay included stimulation with individual M. leprae recombinant proteins, including LID-1, ML89, ML2044, and ML2028 (provided by Dr. M.S. Duthie, Infectious Disease Research Institute (IDRI), Seattle, USA), at 100 μg/ml in PBS for experimental evaluations or 750 μg/ml PHA (Sigma) as a control treatment. Approximately 150 μl of plasma was collected and stored at -20°C until IFN-γ assessment. IFN-γ concentration was determined by ELISA according to the manufacturer’s instructions (U-CyTech Biosciences Human IFN-γ ELISA kit, CT201A, The Netherlands, CM). The IFN-γ ELISA employed had a detection limit of 2 pg/ml, and a threshold for positive responses was arbitrarily selected at 50pg/ml according to a previous study[15].
Statistical analysis
Statistical analysis was performed primarily with GraphPad Prism software version 5.0 (GraphPad Software Inc., San Diego, CA, USA). The nonparametric Mann-Whitney U test was used to analyze differences between two groups. The Kruskal-Wallis test was used to analyze differences among three or more groups. Probability (p) values less than 0.05 were considered significant. The diagnostic utility of individual M. leprae antigen-specific responses for leprosy disease, including sensitivity, specificity, Youden’s index, and area under the receiver operator characteristic curve (AUC), were ascertained by receiver operator characteristics (ROC) curve analysis. The concordance between results was determined by kappa values (κ),and p values were calculated (Statistical Package for the Social Sciences (SPSS) version 16.0).
Results
Study area
The study was undertaken mainly in counties in Honghe Autonomous Prefecture, Yunnan (YN) Province, southwest China. Other cases were enrolled from the nearby autonomous prefectures of Chuxiong, Zhaotong and Kunming (provincial capital city) in YN. Honghe Autonomous Prefecture hadan estimated population of 4,470,000 in 2015 and is considered highly endemic for leprosy in China (annual new case detection rate of 1.13/100,000 from 2000–2007). According to data from the Honghe Prefecture Skin Disease Prevention and Cure Institute, 190 new cases were reported from 2010 to 2014[16].
Basic characteristics of leprosy patients and controls
Eighty-three leprosy cases[MB, n = 38; PB, n = 23; and MDT, n = 22] and 161 controls [HHC, n = 119; TB, n = 11; and EC, n = 31] from the same endemic region were included. The basic information for each study group is summarized in Table 2.
Table 2. Clinical characteristics of the leprosy patients enrolled in this study.
| Leprosy | Leprosy classification (n, %) | n | Gender ratio | Mean age | Bacterial index (BI) | ||
| WHO* | RJ** | (n, %) | (M/F) | Year (range) | Skin-slit smear | Pathology | |
| MB | LL | 4 | 3/1 | 40.0 (24–59) | 1–3.5+ | 5.5–6+ | |
| BL | 32 | 20/12 | 42.3 (21–91) | 1–5+ | 2.2–5+ | ||
| BB | 2 | 2/0 | 43.0 (34–52) | 4+ | 2.6+ | ||
| PB | BT | 14 | 7/7 | 46.0 (17–84) | 0–1.2+ | 0–3.5+ | |
| TT | 9 | 7/2 | 44.9 (29–62) | 0 | 0 | ||
| Post-MDT | LL | 3 | 3/0 | 60.3 (54–68) | - | - | |
| BL | 8 | 5/3 | 65.8 (48–80) | - | - | ||
| BB | 0 | - | - | - | - | ||
| BT | 8 | 6/2 | 62.9 (52–80) | - | - | ||
| TT | 3 | 3/0 | 62.3 (42–78) | - | - | ||
| Controls | HHC | 119 | 57/62 | 33.7 (2–87) | - | - | |
| TB - |
11 | 8/3 | 44.5 (28–77) | - | - | ||
| EC | 31 | 18/13 | 39.2 (32–48) | - | - | ||
n: number of patients, with percentages in parentheses.
*WHO: Operational classification proposed by the World Health Organization.
** RJ: Ridley-Jopling classification.
Comparison of NDO-LID Rapid Test, M. leprae antigen-specific ELISA and IFN-γ in WBA by positive responses
Serum samples were evaluated by the NDO-LID Rapid Test, M. leprae antigen-specific ELISA, and M. leprae antigen-specific secretion of IFN-γ in WBA based on the positive response rate (Table 3).
Table 3. Comparison of NDO-LID Rapid Test, M. leprae antigen-specific ELISA and WBA positive response rates.
| Leprosy classification | Rapid Test | ELISA (OD) | IFN-γ Secretion by WBA (pg/ml) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NDO-LID | LID-1 | NDO-LID | ND-O-BSA | LID-1 | ML89 | ML2044 | ML2028 | |||
| Cut offα | - | 0.2 | 0.2 | 0.2 | Cut offβ | 50 | 50 | 50 | 50 | |
| Total (n) | n (%) | n (%) | n (%) | n (%) | Total (n) | n (%) | n (%) | n (%) | n (%) | |
| MB | 38 | 36 (94.7%) | 35(92.1%) | 35(92.1%) | 34(89.5%) | 35 | 14/35 (40%) | 10/35 (28.6%) | 11/35 (31.4%) | 11/35 (31.4%) |
| PB | 23 | 15 (65.2%) | 12(52.2%) | 11(47.8%) | 14(60.9%) | 23 | 13 (56.5%) | 11 (47.8%) | 14 (60.7%) | 11 (47.8%) |
| Post-MDT | 22 | 7 (31.8%) | 9(45.0%) | 9(45.0%) | 11(55.0%) | 20 | 12/20 (54.5%) | 14/20 (63.6%) | 7/20 (31.8%) | 9/20 (40.9%) |
| HHC | 119 | 34 (28.6%) | 53(44.5%) | 31(26.0%) | 63(52.9%) | 116 | 56/116 (48.3%) | 51/116 (44.0%) | 49/115 (42.6%) | 40/116 (34.5%) |
| TB | 11 | 1 (9.1%) | 2(18.2%) | 0 | 2(18.2%) | 11 | 0 | 0 | 0 | 0 |
| EC | 31 | 10 (33.3%) | 4(12.9%) | 5(16.1%) | 12(38.7%) | 31 | 16 (51.6%) | 15 (48.4%) | 13 (41.9%) | 8 (25.8%) |
α: Cut off value of M. leprae-specific antigen ELISA was defined as 0.2 OD value.
β: Cut off value of IFN-γ was defined as 50 pg/ml.
For the NDO-LID Rapid Test, the positive response rates were higher in the MB than in the PB group[MB vs PB: 94.7% (36/38) vs 65.2% (15/23)]. For M. leprae antigen-specific ELISA, a trend similar to that observed for the NDO-LID Rapid Test was noted: the positive response rates were also higher in the MB than in the PB group[MB vs PB: 92.1% (35/38) vs 52.2% (12/23) against LID-1, 92.1%(35/38) vs 47.8%(11/23) against NDO-LID, and 89.5% (34/38) vs 60.9% (14/23) against ND-O-BSA]. Both methods also demonstrated higher response rates in the MB group than in the post-MDT, HHC, EC, and TB groups.
For WBA, however, the positive response rates were higher in the PB group than in the MB group[MB:PB: 40%(14/35) vs 56.5% (13/23)for LID-1, 28.6%(10/35) vs 47.8%(11/23) for ML89, 31.4%(11/35) vs 60.7%(14/23) for ML2044, and 31.4%(11/35) vs 47.8%(11/23) for ML2028]. WBA also showed higher response rates in the PB group than in the post-MDT, HHC, EC, and TB groups, except for the ML89 antigen in the post-MDT and EC groups.
When the same samples were evaluated using the NDO-LID Rapid Test, confirmation was achieved in 94.7%(36/38) of MB patients, and a high degree of agreement was observed between LID-1(92.1%), NDO-LID (92.1%), and ND-O-BSA (89.5%) ELISA. For PB patients, the NDO-LID Rapid Test reached 65.2% confirmation, which was slightly higher than the results obtained for LID-1(52.2%), NDO-LID(47.8%), and ND-O-BSA (60.9%) ELISA (Table 2). However, the NDO-LID Rapid Test showed positive responses in 33.3% (10/31) of the EC group, which was similar to the rate for ND-O-BSA(38.7%, 12/31) but higher than those for LID-1(12.9%, 4/31) and NDO-LID(16.1%, 5/31) (Table 3).This finding indicates that the NDO-LID Rapid Test is more sensitive than M. leprae-specific antigen ELISA (anti-LID-1 and anti-NDO-LID) for detecting leprosy patients, especially MB patients, but has reduced specificity.
Comparing the consistency of NDO-LID Rapid Test, M. leprae antigen-specific ELISA and WBA by kappa test
A kappa test analyzes for the agreement of results collected from various test formats. When a kappa test was performed between the NDO-LID Rapid Test and M. leprae antigen-specific ELISA and between the NDO-LID Rapid Test and WBA, good agreement was only observed between the NDO-LID Rapid Testand M. leprae antigen-specific ELISA (anti-LID-1, anti-NDO-LID, and anti-ND-O-BSA), with indexes of 0.868, 0.868 and 0.842, respectively (p values of 0.000, 0.000, and 0.000, respectively) for the MB group(Table 4). This finding indicates that the two tests showed high consistency for the diagnosis of MB leprosy patients.
Table 4. Comparison of NDO-LID Rapid Test, M. leprae antigen-specific ELISA and WBA by kappa test.
| NDO-LID Rapid Test vs | ELISA | Kappa test | WBA | Kappa test | ||
| Kappa | p value | Kappa | p value | |||
| MB | LID-1 | 0.8680 | 0.0000 | LID-1 | 0.2860 | 0.0070 |
| NDO-LID | 0.8680 | 0.0000 | ML89 | 0.2390 | 0.0070 | |
| ND-O-BSA | 0.8420 | 0.0000 | ML2044 | 0.2680 | 0.0040 | |
| ML2028 | 0.2680 | 0.0040 | ||||
| PB | ELISA | Kappa test | WBA | Kappa test | ||
| Kappa | p value | Kappa | p value | |||
| LID-1 | 0.1740 | 0.2340 | LID-1 | 0.2170 | 0.1390 | |
| NDO-LID | 0.1300 | 0.3690 | ML89 | 0.1300 | 0.3690 | |
| ND-O-BSA | 0.2610 | 0.0770 | ML2044 | 0.2610 | 0.0770 | |
| ML2028 | 0.1300 | 0.3690 | ||||
| Post-MDT | ELISA | Kappa test | WBA | Kappa test | ||
| Kappa | p value | Kappa | p value | |||
| LID-1 | -0.2730 | 0.0690 | LID-1 | 0.8100 | 0.5800 | |
| NDO-LID | -0.2730 | 0.0690 | ML89 | 0.1800 | 0.8990 | |
| ND-O-BSA | -0.1820 | 0.2200 | ML2044 | -0.3300 | 0.0320 | |
| ML2028 | -0.2300 | 0.1290 | ||||
Discriminating between leprosy patients and controls with M. leprae antigen-specific ELISA
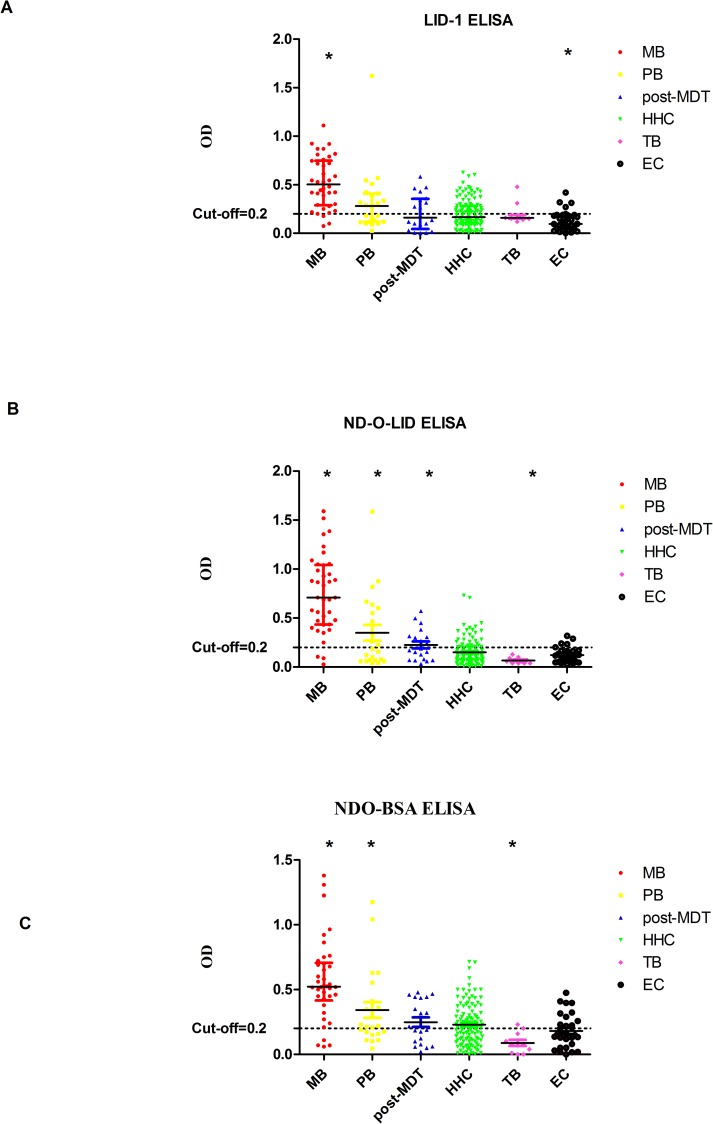
For all three M. leprae antigens (LID-1, NDO-LID and ND-O-BSA ELISA), the OD values showed significant differences for MB vs the PB, post-MDT, HHC, TB or EC groups, and PB vs EC(Fig 1). Of note, NDO-LID was better than the other two antigens (LID-1 and ND-O-BSA) at discriminating PB leprosy patients from non-leprosy controls (Table 5). In addition, we evaluated the diagnostic ability of M. leprae antigen-specific ELISA using ROC curve analysis, AUC, sensitivity and specificity (Table 6) and demonstrated that this method had a relatively high specificity but low sensitivity.
Fig 1. Scatter-dot plots of OD detected in M. leprae proteins by ELISA.
Differences between analyte levels were evaluated by the Mann Whitney U test for non-parametric data analysis. Representative plots show the analyte OD in the LID-1, NDO-LID, and ND-O-BSA ELISA for participants with leprosy (MB, PB and post-MDT) and without leprosy disease controls(HHC, TB and EC). (A)* = p<0.05 for LID-1(MB vs PB, post-MDT, HHC, TB and EC; EC vs PB, HHC and TB);(B) * = p<0.05 for NDO-LID (MB vs PB, post-MDT, HHC, TB and EC; PB vs HHC, TB and EC; post-MDT vs HHC, TB and EC; TB vs HHC and EC); (C)* = p<0.05 for ND-O-BSA (MB vs PB, post-MDT, HHC, TB and EC; PB vs TB and EC; TB vs post-MDT, HHC and EC). Bars in the scatter-dot plots represent the median plus interquartile range of analyte concentrations. M. leprae Ag: LID-1, ML89, ML2044, ML2028; IFN-γ: Interferon gamma.
Table 5. P values of M. leprae antigen-specific ELISA between the leprosy group and control groupsfrom the Kruskal-Wallis test(among three groups) and the Mann-Whitney U test(between two groups).
| M. leprae antigens | Leprosy classification | Total | PB | post-MDT | HHC | TB | EC |
|---|---|---|---|---|---|---|---|
| MB | <0.0001 | 0.0007 | <0.0001 | <0.0001 | 0.0001 | <0.0001 | |
| PB | 0.2280 | 0.1041 | 0.6321 | 0.002 | |||
| post-MDT | 0.8336 | 0.5773 | 0.1800 | ||||
| HHC | 0.6818 | 0.005 | |||||
| TB | 0.0478 | ||||||
| NDO-LID | MB | <0.0001 | 0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| PB | 0.6523 | 0.0378 | 0.0037 | 0.0373 | |||
| post-MDT | 0.0331 | 0.0024 | 0.0191 | ||||
| HHC | 0.0091 | 0.4935 | |||||
| TB | 0.0265 | ||||||
| ND-O-BSA | MB | <0.0001 | 0.0022 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| PB | 0.4216 | 0.1408 | 0.0005 | 0.0209 | |||
| post-MDT | 0.6462 | 0.0111 | 0.1619 | ||||
| HHC | 0.0018 | 0.1489 | |||||
| TB | 0.0319 |
Table 6. ROC of the diagnostic potential of OD detected in M. leprae antigen-specific ELISA for leprosy diagnosis.
| M. leprae Antigens | Subgroups of Cases | p value | AUC | Sensitivity (%) | Specificity (%) | Youden's Index | |
|---|---|---|---|---|---|---|---|
| LID-1 | MB vs | PB | 0.0007 | 0.7603 | 39.13% | 94.74% | 0.3387 |
| post MDT | <0.0001 | 0.823 | 35.00% | 97.37% | 0.3237 | ||
| HHC | <0.0001 | 0.8505 | 50.42% | 94.74% | 0.4516 | ||
| TB | 0.0001 | 0.8804 | 54.55% | 94.74% | 0.4929 | ||
| EC | <0.0001 | 0.9334 | 51.61% | 97.37% | 0.4898 | ||
| PB vs | EC | 0.0020 | 0.7482 | 41.94% | 95.65% | 0.3759 | |
| HHC vs | EC | 0.0050 | 0.6641 | 6.46% | 99.16% | 0.0562 | |
| TB vs | EC | 0.0468 | 0.7038 | 58.06% | 90.91% | 0.4897 | |
| NDO-LID | MB vs | PB | 0.0001 | 0.7929 | 34.78% | 97.37% | 0.3215 |
| post MDT | <0.0001 | 0.8895 | 30.00% | 97.37% | 0.2737 | ||
| HHC | <0.0001 | 0.9285 | 38.66% | 97.37% | 0.3603 | ||
| TB | <0.0001 | 0.9653 | 72.73% | 97.37% | 0.7010 | ||
| EC | <0.0001 | 0.9423 | 38.71% | 97.37% | 0.3608 | ||
| PB vs | HHC | 0.0377 | 0.6372 | 13.45% | 91.30% | 0.0475 | |
| TB | 0.0037 | 0.8123 | 36.36% | 95.65% | 0.3201 | ||
| EC | 0.0366 | 0.6676 | 29.03% | 91.30% | 0.2033 | ||
| post-MDT vs | HHC | 0.0329 | 0.6494 | 20.17% | 95.00% | 0.1517 | |
| TB | 0.0023 | 0.8364 | 36.36% | 95.00% | 0.3136 | ||
| EC | 0.0186 | 0.6968 | 29.03% | 95.00% | 0.2403 | ||
| HHC vs | TB | 0.0091 | 0.7383 | 36.36% | 86.55% | 0.2291 | |
| TB vs | EC | 0.0257 | 0.7287 | 58.06% | 90.91% | 0.4897 | |
| ND-O-BSA | MB vs | PB | 0.0022 | 0.7357 | 56.52% | 86.84% | 0.4336 |
| post MDT | <0.0001 | 0.8434 | 20.00% | 97.37% | 0.1737 | ||
| HHC | <0.0001 | 0.8430 | 15.97% | 97.37% | 0.1334 | ||
| TB | <0.0001 | 0.9414 | 36.36% | 97.37% | 0.3373 | ||
| EC | <0.0001 | 0.8862 | 22.58% | 97.37% | 0.1995 | ||
| PB vs | TB | 0.0005 | 0.8755 | 63.64% | 95.65% | 0.5929 | |
| EC | 0.0205 | 0.6858 | 25.81% | 95.65% | 0.2146 | ||
| post-MDT vs | TB | 0.0105 | 0.7818 | 36.36% | 95.00% | 0.3136 | |
| HHC vs | TB | 0.0017 | 0.7861 | 18.18% | 99.16% | 0.1734 | |
| TB vs | EC | 0.0308 | 0.7214 | 38.71% | 90.91% | 0.2962 | |
All of the analytes that showed significant differences (p<0.05) between leprosy and non-leprosy controls according to receiver operating characteristic (ROC) analysis are shown. Sensitivity and specificity were selected based on Youden’s index. AUC = Area under the receiver operator characteristic curve.
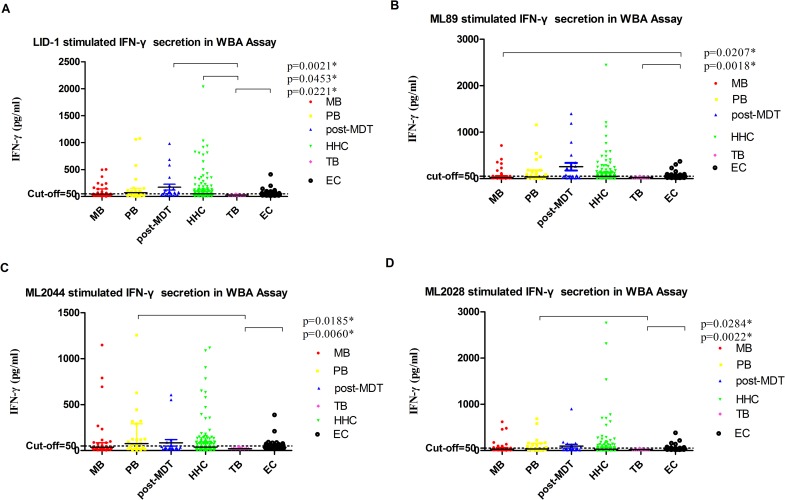
Evaluation of IFN-γ as a potential diagnostic host biomarker for leprosy
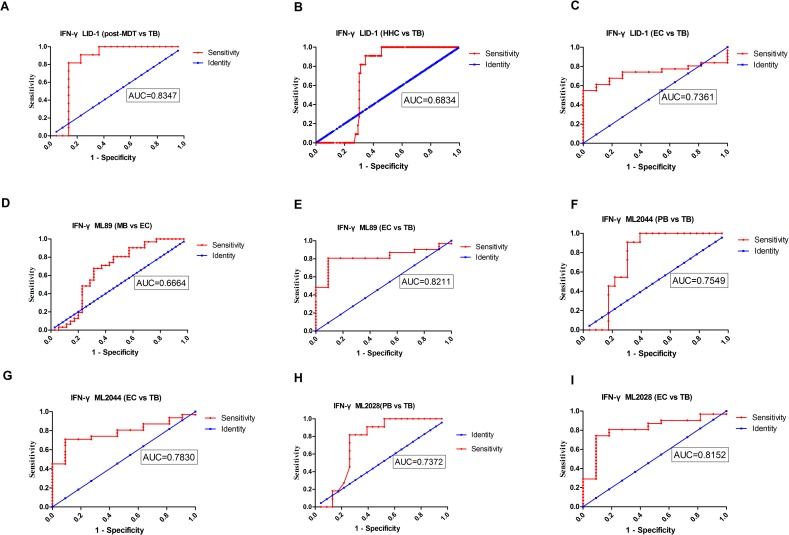
We compared the analyte levels detected in M. leprae antigen-stimulated WBA supernatants in leprosy patients with the levels obtained from the non-leprosy control groups using the mean and standard deviation(SD)(Fig 2) and the median and range(Table 7). As described previously, newly diagnosed PB patients produce more IFN-γ than MB patients. We also evaluated the diagnostic potential of IFN-γ by ROC curve analysis and AUC. IFN-γ levels were significantly different in (1) MB vs EC when stimulated with ML89(AUC = 0.6664); (2) PB vs TB when stimulated with ML2044 and ML2028(AUC = 0.7549 and 0.7372, respectively); (3) post-MDT vs TB when stimulated with LID-1(AUC = 0.8347); (4) HHC vs TB when stimulated with LID-1(AUC = 0.6834); and (5) EC vs TB when stimulated with LID-1, ML89, ML2044 and ML2028(AUC = 0.8211, 0.8152, 0.7830, and 0.7361, respectively)(Fig 3, Table 8).
Fig 2. Scatter-dot plots of host IFN-γ detected in antigen-specific overnight WBA supernatants.
Differences in analyte levels were evaluated by the Mann Whitney U test for non-parametric data analysis. Representative plots show the levels of analytes in the overnight whole blood culture supernatants of participants with leprosy (MB, PB and post-MDT) and without leprosy controls(HHC, TB and EC). Bars in the scatter-dot plots represent the median plus interquartile range of the analyte concentration. M.leprae Ag: LID-1(A), ML89(B), ML2044(C), ML2028(D); IFN-γ: Interferon gamma.
Table 7. Median and range values of IFN-γ as a potential host biomarker detected in overnight culture supernatant by WBA for the leprosy group and control groups.
| Marker/M.leprae Antigens | MB | PB | post-MDT | HHC | EC | TB | |
|---|---|---|---|---|---|---|---|
| IFN-γ/LID-1 | Median | 44.56 | 68.76 | 73.51 | 49.04 | 25.9 | 53.46 |
| Minimum | 0.93 | 1.78 | 14.27 | 1.12 | 22.18 | 14.41 | |
| Maximum | 502.7 | 1079 | 981.3 | 2036 | 46.17 | 411.9 | |
| IFN-γ/ML89 | Median | 23.7 | 36.72 | 60.95 | 48.36 | 22.18 | 49.33 |
| Minimum | 0.75 | 1.29 | 10.76 | 0.89 | 17.31 | 14.38 | |
| Maximum | 710.4 | 1155 | 1397 | 2441 | 51.63 | 371.4 | |
| IFN-γ/ML2044 | Median | 37.33 | 76.14 | 29.57 | 38.26 | 20.38 | 35.3 |
| Minimum | 0.78 | 1.78 | 10.45 | 0.89 | 16.45 | 16.05 | |
| Maximum | 1150 | 1257 | 606.9 | 1118 | 42.44 | 388 | |
| IFN-γ/ML2028 | Median | 29.73 | 33.19 | 25.75 | 26.83 | 18.61 | 32.5 |
| Minimum | 0.75 | 1.29 | 10.45 | 0.85 | 14.35 | 14.15 | |
| Maximum | 620.1 | 685.1 | 900.2 | 2758 | 47.79 | 380.6 |
Median levels of analytes (pg/ml) and ranges (minimum and maximum) showing accuracies at discriminating between leprosy and controls in overnight culture supernatants for all of the study participants.
Fig 3. ROC curves of host marker IFN-γ detected in stimulated overnight WBA supernatants.
Representative ROC curves showing the accuracy of IFN-γ as a marker discriminating between participants with leprosy (MB, PB and post-MDT) and controls without leprosy disease (HHC, TB and EC). All of the markers had AUC≥0.70 except IFN-γ LID-1 (HHC vs TB), and IFN-γ ML89 (MB vs EC). M. leprae Ag: (A) post-MDT, (B) HHC, and (C) EC vs TB stimulated by LID-1;(D) MB and (E) TB vs EC stimulated by ML89;(F) PB and (G) EC vs TB stimulated by ML2044;(H) PB and (I) EC vs TB stimulated by ML2028. IFN-γ: Interferon gamma.
Table 8. ROC of the diagnostic potential of IFN-γ detected in overnight culture supernatant for leprosy.
| Subgroups of Cases | IFN-γ/M.leprae Antigens | p value | AUC | Sensitivity (%) | Specificity (%) | Youden's Index |
|---|---|---|---|---|---|---|
| MB vs EC | IFN-γ/ML89 | 0.0204 | 0.6664 | 67.74% | 68.57% | 0.3631 |
| PB vs TB | IFN-γ/ML2044 | 0.0176 | 0.7549 | 90.91% | 69.57% | 0.6048 |
| IFN-γ/ML2028 | 0.0272 | 0.7372 | 81.82% | 73.91% | 0.5573 | |
| post-MDT vs TB | IFN-γ/LID-1 | 0.0020 | 0.8347 | 81.82% | 86.36% | 0.6818 |
| HHC vs TB | IFN-γ/LID-1 | 0.0449 | 0.6834 | 81.82% | 68.97% | 0.5079 |
| EC vs TB | IFN-γ/ML89 | 0.0017 | 0.8211 | 80.65% | 90.91% | 0.7156 |
| IFN-γ/ML2028 | 0.0021 | 0.8152 | 74.19% | 90.91% | 0.651 | |
| IFN-γ/ML2044 | 0.0058 | 0.783 | 70.97% | 90.91% | 0.6188 | |
| IFN-γ/LID-1 | 0.0213 | 0.7361 | 61.29% | 90.91% | 0.522 |
All of the analytes that showed significant differences (p<0.05) between leprosy and uninfected controls according to ROC analysis are shown. Sensitivity and specificity were selected based on Youden’s index. AUC = Area under the receiver operator characteristics curve.
Discussion
Widespread application of MDT therapy has led to major advances in leprosy control, with sharp declines in prevalence rates in the vast majority of countries over the last 20 years[15,17]. However, the disease remains a public health concern in many regions. In 2010, China reported 1324 new cases of leprosy to the WHO[18]. The majority of cases in China came from the ethnically diverse, mountainous, and underdeveloped southwest provinces of Yunnan, Guizhou, and Sichuan[19,20]. Honghe Autonomous Prefecture inYunan is considered a highly endemic area for leprosy in China. We enrolled 83 leprosy patients and 161 controls from this endemic region in this study to evaluate the ability of several diagnostic tests to correctly diagnose different categories of leprosy patients. We found that the NDO-LID Rapid Test and M. leprae antigen-specific ELISA was useful to diagnose leprosy patients in hyperendemic areas of leprosy disease, especially MB patients. The former method provides a point-of-care measurement of antibodies and had higher sensitivity but lower specificity than the latter. M. leprae antigen-specific IFN-γ secretion in WBA has diagnostic value for distinguishing PB from TB but not for distinguishing PB and HHC or EC.
M. leprae-specific antigen tests have been developed as useful tools to diagnose leprosy. LID-1, NDO-LID, and ND-O-BSA(also named PGL-I), as representative M. leprae-specific antigens, have been widely evaluated as leprosy diagnostics in the hyper-endemic regions of Brazil[20–23], Colombia, the Philippines[24], and China[15] and have been demonstrated to be excellent tools for detecting MB leprosy patients in a simple and highly quantitative manner[24], predicting patients susceptible to developing leprosy type 2 reactions (T2R)[23], and distinguishing leprosy from other confounding dermatoses[18].
The NDO-LID Rapid Testwas compared with M. leprae antigen-specific ELISA and demonstrated a high degree of sensitivity but significant differences in specificity for leprosy diagnosis[25]. Therefore, this test is an effective tool for screening and identifying individuals at high risk who might benefit from regular monitoring[26]. Our previous study showed that confirmation was achieved in 95% of MB leprosy patients with the NDO-LID Rapid Test, and a high degree of agreement was observed with LID-1, NDO-LID, and ND-O-BSA ELISA[15]. In addition, 63.6% of PB leprosy patients were confirmed, and the NDO-LID Rapid Test had a higher detection rate in PB leprosy patients than LID-1, ND-O-BSA, and NDO-LID ELISA[15]. In this study, we enlarged the sample size and obtained results similar to those of previous studies. These data indicate an improved capacity of the NDO-LID Rapid Test over M. leprae ELISA for detecting the disease. However, the test also suffers from higher positive responses in the EC group than did NDO-LID and LID-1 ELISA. This implies that the NDO-LID Rapid Test was more sensitive but less specific than M. leprae antigen-specific ELISA(anti-LID-1 and anti-NDO-LID) for discriminating the leprosy patient group fromthen on-leprosy EC group. Despite the relatively low specificity, the NDO-LID Rapid Test, as a low-tech, robust assay, can still be applied in resource-poor settings to measure the immune response to M. leprae infection and can be used as a tool for leprosy screening in combination with good specificity confirmation tests, which will lead to timely treatment and reduced transmission[27].
We also evaluated the capacity of M. leprae antigen-specific ELISA to discriminate between the leprosy and control groups. All three M. leprae antigens (LID-1, NDO-LID and ND-O-BSA) were able to discriminate the MB group from all other leprosy and non-leprosy groups and the PB leprosy group from the non-leprosy EC groups, whereas only NDO-LID was able to discriminate the PB leprosy group from the non-leprosy HHC group. This indicates that all three M. leprae antigens have potential and specific value for research and medical applications. As described before, the M. leprae antigen-specific ELISA had lower sensitivity but better specificity than the NDO-LID Rapid Test. ELISA detection of specific antibodies may be preferred for confirming diagnoses, differentiating leprosy from other dermatological conditions, and performing follow-up studies for leprosy HHC and indeterminate leprosy, which are very early signs of the disease that are often missed by family members and medical personnel in the endemic area[27].
Cytokines such as IFN-γ have recently been studied as diagnostic host biomarkers for leprosy. M. leprae-specific antigens, such as M. Leprae crude antigens (M. leprae cell sonicate, MLCS), M. leprae recombinant protein (rML)(LID-1), M. leprae diffusion proteins[46f(ML0405+ML0568) and 73f(ML2028+ML2346+ML2044)] and combinations of rML (46f+LID-1, ML0276+LID-1, ML2055+ML1632+ML2044, ML0276+46f, and ML2055+LID-1) were used in these studies[20,28–31]. IFN-γ and CXCL10 were evaluated as potential diagnostic host markers for PB leprosy patients in the hyper-endemic regions of Brazil[20,28–31] and China[15]. Newly diagnosed PB patients produced more IFN-γ than MB patients[28–31], and IFN-γ was helpful in the differential diagnosis of leprosy from other confounding dermatoses[2]. CXCL10 discriminated PB from EC only in ML0276+LID-1 WBA; however, CXCL10 could not discriminate active disease (PB) from HHC individuals[28]. In this study, we also demonstrated that for new cases, PB patients produced more IFN-γ than MB patients; however, IFN-γ did not discriminate active disease (PB) from HHC or EC individuals.
In this study, we investigated the accuracy of IFN-γ as a host marker detected in supernatants after stimulation of whole blood with M. leprae-specific antigens (LID-1, ML89, ML2044, and ML2028) in an overnight culture assay and compared the IFN-γ marker levels in leprosy and non-leprosy control groups. Although IFN-γ can be useful as a host biomarker that contributes to a diagnostic signature of MB vs EC and that distinguishes PB vs TB groups, there was no evidence that IFN-γ was able to discriminate between the PB and HHC or EC groups. To screen novel M. leprae-specific antigens, combining different M. leprae antigens and facilitating a multi-cytokine analyte model may achieve improved diagnostic potential.
This study is limited by the small sample size, especially in the PB group. Antigen-specific immune responses have had limited diagnostic ability for leprosy disease and until recently have only been used for seroepidemiological investigation in hyperendemic areas of leprosy disease or in patients clinically suspected of having leprosy disease. However, the results should be interpreted with caution. Only a very limited number of M. leprae-specific antigens(LID-1, ML89, ML2044, and ML2028) and only one potential diagnostic host biomarker (IFN-γ) were tested for leprosy diagnosis in this study. Future studies should focus on additional M. leprae-specific antigens as well as additional host biomarkers.
In conclusion, the NDO-LID Rapid Test and M. leprae antigen-specific ELISA were helpful for diagnosing leprosy in hyperendemic areas of leprosy disease, especially for MB patients. The former had higher sensitivity but lower specificity than the latter. Although IFN-γ has been widely studied as a potential biomarker for PB leprosy patients, more research is needed to identify feasible M. leprae-specific antigens and other appropriate host biomarkers to improve its diagnostic value in PB patients in future studies.
Acknowledgments
We thank Prof. P.J. Brennan for providing the M. leprae antigen ND-O-BSA and Dr. M.S. Duthie for providing the M. leprae recombinant antigens LID-1, NDO-LID, ML89, ML2044, and ML2028 and the NDO-LID Rapid Test kit.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Duthie MS, Sampaio LH, Oliveira RM, Raman VS, O'Donnell J, et al. (2013) Development and pre-clinical assessment of 73 kD chimeric fusion protein as a defined sub-unit vaccine for leprosy. Vaccine 21;31(5):813–819. 10.1016/j.vaccine.2012.11.073 [DOI] [PubMed] [Google Scholar]
- 2.Freitas AA, Hungria EM, Costa MB, Sousa AL, Castilho ML, et al. (2016). Application of Mycobacterium Leprae-specific cellular and serological tests for the differential diagnosis of leprosy from confounding dermatoses. Diagn Microbiol Infect Dis 86(2):163–168. 10.1016/j.diagmicrobio.2016.07.024 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO) (2007) Global leprosy situation, 2007. Wkly Epidemiol Rec 82:225–232. [PubMed] [Google Scholar]
- 4.World Health Organisation.(2012) WHO Expert Committee on Leprosy, 8th Report. World Health Organization; 2012, Geneva. [PubMed] [Google Scholar]
- 5.Roset Bahmanyar E, Smith WC, Brennan P, Cummings R, Duthie M, et al. (2016) Leprosy Diagnostic Test Development As a Prerequisite Towards Elimination: Requirements from the User's Perspective. PLoS Negl Trop Dis 11;10(2):e0004331 10.1371/journal.pntd.0004331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duthie MS, Goto W, Ireton GC, Reece ST, Sampaio LH, et al. (2008) Antigen-specific T-cell responses of leprosy patients. Clin Vaccine Immunol.15(11):1659–1665. 10.1128/CVI.00234-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duthie MS, Raychaudhuri R, Tutterrow YL, Misquith A, Bowman J, et al. (2014) A rapid ELISA for the diagnosis of MB leprosy based on complementary detection of antibodies against a novel protein-glycolipid conjugate. Diagn Microbiol Infect Dis 79(2):233–239. 10.1016/j.diagmicrobio.2014.02.006 [DOI] [PubMed] [Google Scholar]
- 8.Freitas AA, Oliveira RM, Hungria EM, Cardoso LP, Sousa AL,et al. (2015) Alterations to antigen-specific immune responses before and after multidrug therapy of leprosy. Diagn Microbiol Infect Dis 83(2):154–161. 10.1016/j.diagmicrobio.2015.06.021 [DOI] [PubMed] [Google Scholar]
- 9.Rada E, Duthie MS, Reed SG, Aranzazu N, Convit J.(2012) Serologic follow-up of IgG responses against recombinant mycobacterial proteins ML0405, ML2331 and LID-1 in a leprosy hyperendemic area in Venezuela. Mem Inst Oswaldo Cruz 107 Suppl 1:90–4 [DOI] [PubMed] [Google Scholar]
- 10.Qiong-Hua P, Zhong-Yi Z, Jun Y, Yan W, Lian-Chao Y, et al. (2013) Early Revelation of Leprosy in China by Sequential Antibody Analyses with LID-1 and PGL-I. J Trop Med 352689 10.1155/2013/352689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duthie Malcolm S.,Orcullo Florenda M.,Abbelana Junie, Maghanoy Armi, Balagon Marivic F.(2016) Comparative evaluation of antibody detection tests to facilitate the diagnosis of multibacillary leprosy. Appl Microbiol Biotechnol 100:3267o3275 10.1007/s00253-016-7328-8 [DOI] [PubMed] [Google Scholar]
- 12.Duthie MS, Pena MT, Ebenezer GJ, Gillis TP, Sharma R, et al. (2018) LepVax, a defined subunit vaccine that provides effective pre-exposure and post-exposure prophylaxis of M. leprae infection. NPJ Vaccines 28;3:12 10.1038/s41541-018-0050-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ridley D. S. & Jopling W. H.(1966) Classification of leprosy according to immunity. A five-group system. Int. J. Lepr. Other Mycobact. Dis 34, 255–273. [PubMed] [Google Scholar]
- 14.WHO. (1998) WHO expert commitee on leprosy: seventh report (World Health Organization, 1998). [Google Scholar]
- 15.Wen Y, You YG, Yuan LC, Yuan YH, Zhang Y,et al. (2014). Evaluation of Novel tools to facilitate the detection and characterization of leprosy patients in China. Biomed Res Int 371828 10.1155/2014/371828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu jie, Li juan. (2016) Analysis of leprosy epidemic situation in Honghe prefecture 1930 to 2014. Chinese Community Doctors.32(2):179–180. [Google Scholar]
- 17.Muñoz M, Beltrán-Alzate JC, Duthie MS, Serrano-Coll H, Cardona-Castro N.(2018).Comparison of Enzyme-Linked Immunosorbent Assay Using Either Natural Octyl Disaccharide-Leprosy IDRI Diagnostic or Phenolic Glycolipid-I Antigens for the Detection of Leprosy Patients in Colombia. Am J Trop Med Hyg 98(1):274–277. 10.4269/ajtmh.17-0500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO, 2018 Jan 1.oi: 1situation,”(2012) The Weekly Epidemiological Record, vol. 87, pp. 317328.
- 19.Shen J., Zhang G., Chen X., Zhou M., Yu M., et al. (2008) Along-term evolution on the epidemiological characteristics of leprosy, towards the goal of its elimination in 1949–2007 in China, Zhonghua Liu Xing Bing Xue Za Zhi 29(11):1095sy,. [PubMed] [Google Scholar]
- 20.Yu M., Yan L., Shen J., Sun Y., and Zhang G.(2010)“Epidemiological analysis on leprosy in China, 2009,”Zhonghua Liu Xing Bing XueZaZhi 31(10): 1155–1157. [PubMed] [Google Scholar]
- 21.Freitas AA, Hungria EM, Costa MB, Sousa AL, Castilho ML, et al. (2016) Application of Mycobacterium Leprae-specific cellular and serological tests for the differential diagnosis of leprosy from confounding dermatoses. Diagn Microbiol Infect Dis 86(2):163–168. 10.1016/j.diagmicrobio.2016.07.024 [DOI] [PubMed] [Google Scholar]
- 22.Amorim FM, Nobre ML, Ferreira LC, Nascimento LS, Miranda AM,et al. (2016) Identifying Leprosy and Those at Risk of Developing Leprosy by Detection of Antibodies agaist LID-1 and LID_NDO. PLoS Negl Trop Dis 22;10(9):e0004934 10.1371/journal.pntd.0004934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizoguti Dde F, Hungria EM, Freitas AA, Oliveira RM, Cardoso LP, et al. (2015) Multibacillary leprosy patients with high and persistent serum antibodies to leprosy IDRI diagnostic-1/LID-1: higher susceptibility to develop type 2 reactions. a) Mem Inst Oswaldo Cruz. 110(7):914–920. 10.1590/0074-02760150198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duthie MS, Raychaudhuri R, Tutterrow YL, Misquith A, Bowman J, et al. (2014) A rapid ELISA for the diagnosis of MB leprosy based on complementary detection of antibodies agaist a novel protein-glycolipid conjugate.Diagn Microbiol Infect Dis 79(2):233–239. 10.1016/j.diagmicrobio.2014.02.006 [DOI] [PubMed] [Google Scholar]
- 25.Duthie MS, Orcullo FM, Abbelana J, Maghanoy A, Balagon MF.(2016) Comparative evaluation of antibody detection tests to facilitate the diagnosis of multi bacillary leprosy. Appl Microbiol Biotechnol. 100(7):3267–3275. 10.1007/s00253-016-7328-8 [DOI] [PubMed] [Google Scholar]
- 26.Frade MA, de Paula NA, Gomes CM, Vernal S, Bernardes Filho F, et al. (2017). Unexpectedly high leprosy seroprevalence detected using a random surveillance strategy in midwestern Brazil: A comparison of ELISA and a rapid diagnostic test. PLoS Negl Trop Dis 11(2):e0005375 10.1371/journal.pntd.0005375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wen Y, Xing Y, Yuan LC, Liu J, Zhang Y, et al. (2013) Whole-blood nested-PCR amplification of M. leprae-specific DNA for early diagnosis of leprosy. Am J Trop Med Hyg 88(5):918–22. 10.4269/ajtmh.11-0253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hungria EM, Freitas AA, Pontes MA, Gonçalves HS, Sousa AL, et al. (2017). Antigen-specific secretion of IFN-γ and CXCL10 in whole blood assay detects Mycobacterium leprae infection but does not discriminate asymptomatic infection from symptomatic leprosy. Diagn Microbiol Infect Dis 87(4):328–334. 10.1016/j.diagmicrobio.2017.01.002 [DOI] [PubMed] [Google Scholar]
- 29.Freitas AA, Oliveira RM, Hungria EM, Cardoso LP, Sousa AL, et al. (2015). Alterations to antigen-specific immune responses before and after multidrug therapy of leprosy. therapy of leprosy. Diagn Microbiol Infect Dis. 83(2):154–161. 10.1016/j.diagmicrobio.2015.06.021 [DOI] [PubMed] [Google Scholar]
- 30.Oliveira RM, Hungria EM, de Araújo Freitas A, de Sousa AL, Costa MB, et al. (2014) Synergistic antigen combinations for the development of interferon gamma release assays for paucibacillary leprosy. Eur J Clin Microbiol Infect Dis 33(8):1415–1424. 10.1007/s10096-014-2077-z [DOI] [PubMed] [Google Scholar]
- 31.Duthie MS, Sampaio LH, Oliveira RM, Raman VS, O'Donnell J, et al. (2013) Development and pre-clinical assessment of a 73 kD chimeric fusion protein as defined sub-unit vaccine for leprosy. Vaccine. 21;31(5):813–819. 10.1016/j.vaccine.2012.11.073 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.