Abstract
The endoplasmic reticulum (ER) is the main site of protein synthesis, folding, and secretion to other organelles. The capacity of the ER to process proteins is limited, and excessive accumulation of unfolded and misfolded proteins can induce ER stress, which is associated with plant diseases. Here, a transgenic Arabidopsis system was established to express anti-cancer monoclonal antibodies (mAbs) that recognize the tumor-associated antigen GA733-2. Monoclonal antibody (mAb) CO17-1A recognize a tumor-associated epitope expressed on the colorectal cancer cell surface. The ER retention Lys-Asp-Glu-Leu (KDEL) motif sequence was added to the C-terminus of the heavy chain to retain anti-colorectal cancer mAbs in the ER, consequently boosting mAb production. Agrobacterium-mediated floral dip transformation was used to generate T1 transformants, and homozygous T4 seeds obtained from transgenic Arabidopsis plants expressing anti-colorectal cancer mAbs were used to confirm the physiological effects of KDEL tagging. Germination rates were not significantly different between both plants expressing mAb CO without KDEL mAb CO (CO plant) and mAb CO with KDEL mAb COK (COK plant). However, COK plants primary root lengths were shorter than those of CO plants and non-transgenic Arabidopsis plants in in vitro media. Most ER stress-related genes, with the exception of bZIP28 and IRE1a, were upregulated in COK plants compared to CO plants. Western blot and SDS-PAGE analyses showed that COK plants exhibited up to five times higher expression and mAb amounts than plants. Enhanced expression in mAb COK plants was confirmed by immunohistochemical analyses. mAb COK was distributed across most of the area of leaf tissues, whereas mAb CO was mainly distributed in extracellular areas. Surface plasmon resonance analyses revealed that mAb CO and mAb COK possessed equivalent or slightly better binding activities to antigen EpCAM compared to a commercially available parental antibody. N-glycosylation analysis showed that mAb CO had plant specific residues whereas mAb COK mainly showed an oligo-mannose N-glycan structure without the plant specific glycan residues. In this study, the reduction of plant growth and biomass induced by ER retention signal peptide might be only in in vitro conditions, and thus should be carefully considered for the initial screening for transgenic lines on culture media. Taken together, nevertheless the fusion of ER retention signal peptide is an effective approach for enhancing the yields of recombinant proteins in vivo.
Introduction
Colorectal cancer (CRC) is the second most frequently diagnosed cancer in women and the third most commonly diagnosed cancer in men worldwide [1]. Highly valuable recombinant therapeutic antibodies for the diagnosis and treatment of cancer have been produced in transgenic plant systems [2, 3]. In fact, to express anti-CRC mAbs, Arabidopsis was selected as a production platform because of its short life cycle and high total soluble protein content [4, 5]; however, therapeutic glycoproteins produced in plant cells had plant-specific N-glycans. The fusion of the endoplasmic reticulum (ER) retention KDEL (Lys-Asp-Glu-Leu) motif is frequently used to retain and accumulate recombinant therapeutic proteins in the ER, enhancing their stability and production yields in plants. KDEL is added to the C-terminus of the heavy chain (HC) to induce high mannose glycan structures without plant-specific glycan residues [α(1,3)-fucose and β(1,2)-xylose], which can trigger a host immune response [6, 7].
The accumulation of recombinant anti-colorectal cancer mAbs in the ER requires a large ER-mediated protein quality control (ERQC) capacity, which results in ER stress [8–10]. ER stress activates three main unfolded protein response (UPR) signaling pathways [11, 12], namely ER-membrane-associated activating transcription factor 6 (ATF6), inositol-requiring enzyme 1 (IRE1), and protein kinase RNA-like ER kinase (PERK) [13, 14]. In this study, three basic-leucine zipper transcription factor family proteins (bZIP17, bZIP28, and bZIP60), two binding immunoglobulin proteins (BiP1 and BiP3), two plant-specific NAC transcription factors (NAC103 and NAC089), regulator of ER stress-induced programmed cell death (BAX inhibitor 1), B-cell lymphoma 2 (Bcl-2)-associated athanogene 7 (BAG7), and ER oxidoreductin 1 (ERo1) were analyzed in terms of ER stress regulation [15–17].
We obtained homozygous seeds from transgenic Arabidopsis thaliana plants expressing anti-CRC mAbPs (mAb CO and mAb COK) to recognize CRC-associated antigen GA733 (EpCAM), which is highly expressed in CRC cells [18, 19]. To confirm the effects of ER retention motif tagging on transgenic Arabidopsis, the KDEL motif was fused to the C-terminus of the original HC. Germination rates, primary root lengths, regulation of ER stress-related genes, transcriptional and translational levels, subcellular distribution of mAbs in fresh leaves, purification amount per unit fresh leaf, and antigen-binding functions were compared between the non KDEL-tagged group (CO plant) and the KDEL-tagged group (COK plant).
Materials and methods
Vector construction and Arabidopsis transformation
Plant expression vectors pBI CO17-1A (pBI CO) and pBI CO17-1AK (pBI COK), carrying mAb CO and mAb CO tagged with the KDEL ER retention motif (mAb COK), were transformed into Agrobacterium tumefaciens strain GV3101::pMP90 via electroporation. Wild-type Arabidopsis plants were transformed using the floral dip method [20] (Fig 1). Approximately one month prior to transformation, non-transgenic (NT) Arabidopsis seeds were sown with approximately 4–5 plants per pot in eight pots. The plants were grown under standard conditions (16 h light/8 h dark) in a growth chamber. To induce the proliferation of multiple secondary bolts, the first bolts of Arabidopsis were trimmed. Two days before plant transformation, A. tumefaciens strain GV3101::pMP90, carrying mAb CO or mAb COK expression cassettes, was cultured at 28–30°C in LB (Luria-Bertani) with kanamycin. Agrobacteria were centrifuged (4,000 rpm for 10 min) and pellets were resuspended with infiltration media until the OD values were adjusted to 0.8–1.0. Before transformation, Silwet L-77 was added to the infiltration media at a concentration of 0.02%. The pots were inverted into the infiltration solution for 5 min. After dipping transformation, pots were covered with a black plastic bag to ensure highly humid and dark conditions, and the trays were placed in a growth chamber. The following day, the plastic bags were removed, and the plants were maintained under standard conditions in a growth chamber until seeds were well-ripened. Transformed seedlings were selected on agar plates containing Murashige and Skoog medium (adjusted pH 5.7) (10 g·L-1 of sucrose, 8 g·L-1 of plant agar, and 4.3 g·L-1 of MS B5 vitamin; Duchefa Biochemie, Haarlem, Netherlands), containing 50 mg·L-1 kanamycin and 25 mg·L-1 cefotaxime. All transformed seedlings were grown in a growth chamber at 22°C under a 16 h light/8 h dark cycle. After T1 seeds were obtained, kanamycin selection was repeated over generations until homozygous lines were obtained. Homozygous T4 seeds were used in this experiment.
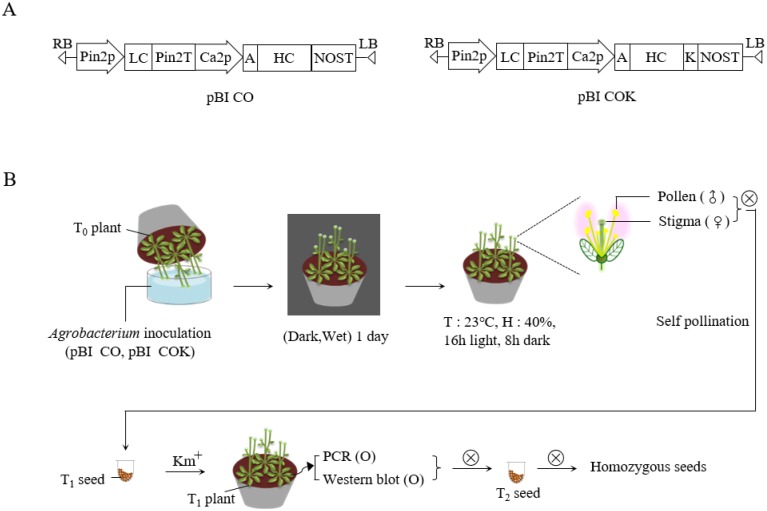
Fig 1. Schematic diagram of the floral dip transformation process.
Agrobacterium tumefaciens strain GV3101::pMP90, carrying plant binary vectors pBI CO and pBI COK, were used for floral dip transformation. (A) Schematic diagram of mAb CO and mAb COK gene expression cassettes in plant expression vector pBI121, which was used for Agrobacterium-mediated transformation. Pin2p, promoter of the Pin2 gene from potato, and Ca2p, the cauliflower mosaic virus 35S promoter, control the light and heavy chains (LC and HC), respectively. KDEL is the 3′ endoplasmic reticulum (ER) retention motif. An alfalfa mosaic virus untranslated leader sequence of RNA4; Pin2T, terminator of the Pin2 gene from potato; NOST, terminator of nopaline synthase (NOS) gene. (B) After floral dip transformation, plants were placed in a box, watered, and maintained under a dark and humid atmosphere for one day. Plants were cultured for a few weeks until mature seeds were obtained. Seeds were germinated on kanamycin-containing medium to select transformants. Kanamycin selection was repeated over generations until homozygous lines were identified.
PCR analysis
Rosette leaves (approximately 100 mg) from four-week-old NT and transgenic plants expressing mAb CO were used for polymerase chain reaction (PCR) analysis. A DNA extraction kit (RBC Bioscience, Seoul, Korea) was used to extract genomic DNA from plant leaves, following the manufacturer’s recommended protocol. PCR was performed to confirm the presence HC (1,416 bp) and LC (717 bp) genes associated with mAb CO, and transformants were selected from T1 plants. Primers were designed as follows: HC forward primer, 5′-GCGAATTCATGGAATGGAGCAGAGTCTT TATC-3′; HC reverse primer, 5′-GATTAATCGATTTTACCCGGAGTCCG-3′; LC forward primer, 5′-GCCTCGAGATGGGCATCAAGATGGAATCACAG-3′; LC reverse primer, 5′-GAGGTACCCTAACACTCATTCCTGTTGAAGCTC-3′. Leaves from NT plants were used as a negative control, and the pBI CO gene was used as a positive control. PCR analysis was replicated more than three times.
Western blot analysis
The rosette leaves of T1 plants, with confirmed foreign gene insertion via PCR, were subjected to western blot analyses to confirm target protein expression. Fifty milligram of fresh rosette leaves was frozen in liquid nitrogen and immediately crushed. To extract total soluble protein, leaf samples were mixed with 100 μL of sample buffer (1 M Tris-HCl, 50% glycerol, 10% SDS, 5% 2-mercaptoethanol, and 0.1% bromophenol blue), boiled for 10 min, and cooled on ice. Total soluble proteins were separated by 12.5% SDS-PAGE and transferred to a nitrocellulose membrane (Millipore, Billerica, MA). Membranes were incubated with 5% skimmed milk (Sigma, St. Louis, MO) for 16 h at 4°C. The nitrocellulose blots were incubated with goat anti-murine IgG Fcγ and anti-murine IgG F(ab)′2, which recognize HC and LC of mAb CO, respectively. After washing three times for 10 min at room temperature, proteins were detected with the SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, Rockford, IL), and X-ray film (Fuji, Tokyo, Japan) was used for visualization. Rosette leaves of NT Arabidopsis plants were used as a negative control.
Plant growth conditions and morphological assessments
After three continuous screenings on kanamycin media, homozygous T4 seeds were obtained. Homozygous T4 seeds of transgenic Arabidopsis plants expressing mAb CO were used as the material for this experiment. Two-hundred surface-sterilized seeds of each plant expressing mAb CO (CO) and mAb COK (COK), including NT plants, were sown on agar plates containing Murashige and Skoog medium (adjusted pH 5.7; 10 g·L-1 of sucrose, 8 g·L-1 of plant agar, and 4.3 g·L-1 of MS B5 vitamin (Duchefa Biochemie, Haarlem, Netherlands), containing 50 mg·L-1 kanamycin and 25 mg·L-1 cefotaxime. The seedling plates were vernalized at 4°C for two days under dark conditions to improve the rate and synchrony of germination, and were then placed vertically in a growth chamber at 23°C under 16 h light/8 h dark conditions. The germination rates were calculated as follows: (number of germinated seeds/total seeds) × 100. Germination was checked four days after transfer to the growth chamber, and primary root lengths were checked at four, six, eight, 10, and 12 days. Thirty-two shoots of each plant (CO, COK, and NT) were transferred to pots for further study. Rosette leaf lengths were measured from the petiole to the leaf blade using a ruler (in cm) over a period of four weeks at one-week intervals. After 10 weeks of soil culture, the roots of 96 plants were rinsed with tap water for 1 min, and the main root lengths were measured using a ruler. Photographs were taken with a camera (Digital Gross System) (Humintec, Suwon, Korea), and at least two independent experiments were conducted for all data analyses.
RNA extraction and reverse transcription (RT) PCR analyses
Total RNA was extracted from NT plants and transgenic Arabidopsis plants expressing mAb CO and mAb COK that were grown under standard conditions in a growth chamber. Fresh rosettes leaves were sampled and homogenized after freezing in liquid nitrogen. RNA was extracted using TRIzol, as previously described [21, 22]. Extracted RNA was stored in a deep freezer (-70°C) for further study. Genomic DNA was removed, and cDNA was synthesized using a Quantitect reverse transcription kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. Each cDNA sample was used as a template for RT-PCR analyses. RT-PCR was performed using the Maxime PCR Premix Kit (Intron Biotechnology, Seoul, Korea). Transcription of HC and LC genes was confirmed with primers as described above. Actin 8 was used as a housekeeping control gene, and the primer sets were used to amplify the gene: actin 8 forward primer, 5′-CAACTATGTTCTCAGGTATTGCAGA-3′; actin 8 reverse primer: 5′-GTCATGGAAACGATGTCTCTTTAGT-3′. NT plants were used as a negative control. RT-PCR analyses were performed in duplicate, and the reverse transcription PCR product was run on an agarose gel for UV detection.
RNA isolation and quantitative real time PCR (qRT-PCR) analysis
Arabidopsis plants were cultivated (vertical growth) in MS (Murashige and Skoog) media for 14 days after seeding, and were then harvested to dissect root tissues. Total RNA was extracted from Arabidopsis roots using an RNeasy Mini Kit (Qiagen, Valencia, CA), and the extracted RNA samples were the processed with an RNase-free DNase kit (Qiagen, Valencia, CA), according to the supplied protocol. cDNA was synthesized from 2 μg of total RNA using the Superscript III First Strand Synthesis kit (Invitrogen, Carlsbad, CA). Regarding real-time qPCR, each primer was designed to amplify a 200–300 bp region of the corresponding transcript, and the control transcript for qRT-PCR was UBI10. Real-time PCR analyses were performed on a C1000 Thermal Cycler (Bio-Rad, Hercules, CA). Primers were tested on NT plants via quantitative PCR using the Bio-Rad CFX96 real time system, and primer quality was determined based on melting curve analyses and amplification efficiency. Gene expression was measured in three technical replicates for each sample. cDNA was generated via reverse transcription of 2 μg total RNA as described above. Primers were designed by AtRTPrimer [23], and the primer sequences used for qRT-PCR are provided in Table 1.
Table 1. Primers specific for ER stress-related genes (bZIP17, bZIP28, bZIP60, BiP1, BiP3, IRE1a, NAC103, NAC089, BAG7, BAX inhibitor 1, and ERO1) in Arabidopsis thaliana [23].
| No. | Gene name | Primer name | Sequence information |
|---|---|---|---|
| 1 | bZIP17 | AT2G40950-2704F | TGACGTGGGAAGGACAGAGGAAT |
| AT2G40950-2705R | CTGTGGTGTTCTTACCGTGTTCAGC | ||
| 2 | bZIP28 | AT3g10800-2702F | ATTAGCTGTTCCGGGGGTAAGAGG |
| AT3g10800-2703R | TTGCCGTGGGTAGTGACATTTTGG | ||
| 3 | bZIP60 | AT1G42990-2706F | CGGGAAGGAGAATTCGGATTTGG |
| AT1G42990-2707R | GCAACGAAGCACTCAAGCATACG | ||
| 4 | BIP1 | AT5G28540-2708F | CGGAGTGTTTGAGGTTCTCTCCACA |
| AT5G28540-2709R | CTCACATTCCCTTCGGAGCTTACC | ||
| 5 | BIP3 | AT1G09080-2722F | CCCAACGAAGAAGTCTCAGGTGTTC |
| AT1G09080-2723R | TCACCTGCAGGATCCCATTTGC | ||
| 6 | IRE1a | AT2G17520-2720F | AGGAGGGAAATGGGATGAAAAGCTA |
| AT2G17520-2721R | CAATTTCGGAAACCGTACAGCAAAG | ||
| 7 | NAC103 | AT5G64060-2716F | TGGTGGCGAGTTAAATTCGATGC |
| AT5G64060-2717R | TGGTCATTGCTCAGGTCAAACATTC | ||
| 8 | NAC089 | AT5G22290-2714F | TGTGCGCGTGGGAAAAAGTATCC |
| AT5G22290-2715R | AACCCGGCAAACAACCATAGCAT | ||
| 9 | BAG7 | AT5G62390-2710F | CTGATTGCTCGTGATGGAGAGGAG |
| AT5G62390-2711R | TGCGGGTCTACCACATCTAACATTG | ||
| 10 | BAX INHIBITOR 1 | AT5G47120-2724F | GGCTCCAGTTTGCCTCTTCAATCTT |
| AT5G47120-2725R | ATGTCACCGAGGTGTGCCTTTTCTA | ||
| 11 | ERO1 | AT1G72280-2718F | CTTTGGGGGAAACTTCAGGTTCAGG |
| AT1G72280-2719R | CCGTTGCAGTTGCAGTGTTTGG | ||
| 12 | UBI10 | Ubiquitin10-2712F | GGCCTTGTATAATCCCTGATGAATAA |
| Ubiquitin10-2713R | AAAGAGATAACAGGAACGGAAACATA |
Immunohistochemical analysis
The intracellular distributions of mAb CO and mAb COK in the rosette leaf tissues of transgenic Arabidopsis were assessed using immunohistochemical analyses. Rosette leaf tissues, freshly harvested from 3–4-week-old Arabidopsis plants, were fixed in 10% formalin overnight and processed for paraffin embedding. Paraffin sections were cut to 4 μm thickness by a microtome. The sections were deparaffinized and rehydrated through xylene and serially diluted ethanol. Slides were then treated with 3% H2O2 for 10 min to block endogenous peroxidase activity. After washing twice with 1× PBS, tissue sections were treated with antigen retrieval solution (Dako, Glostrup, Denmark) and kept at 120°C for 5 min in a pressure cooker. Tissue sections were then cooled to room temperature (23°C). After treatment with a protein block serum-free reagent for 30 min, the Dako REAL™ EnVision™ Detection System (Dako, Glostrup, Denmark) was applied according to manufacturer’s instructions, and samples were then counterstained with Mayer’s hematoxylin (Muto Pure Chemicals CO., Tokyo, Japan). Slides were mounted with cover glass using Permount solution and visualized with a microscope (×400 magnification, BX53F (Olympus, Tokyo, Japan)).
Purification of anti-cancer mAb
To purify anti-CRC mAbs (CO and COK) from plants, 200 g of each transgenic Arabidopsis leaf was homogenized in a 1.2 L extraction buffer (37.5 mM Tris-HCl pH 7.5, 50 mM NaCl, 15 mM EDTA, 75 mM sodium citrate, and 0.2% sodium thiosulfate) using a HR2094 grinder (Philips, Seoul, Korea). After centrifugation at 8,800 × g for 30 min at 4°C, the supernatant was filtered through Miracloth (Biosciences, La Jolla, CA), and the pH was adjusted to 5.1 with acetic acid. The solution was further centrifuged at 10,200 × g for 30 min. The supernatant was filtered through Miracloth and adjusted to 7.0 with the addition of 3M Tris-HCl, and ammonium sulfate was added up to an 8% concentration. After centrifugation at 8,800 × g for 30 min at 4°C, ammonium sulfate was added to the supernatant to reach a 24% concentration, and samples were incubated at 4°C overnight. The final solution was centrifuged at 4°C for 30 min, and the pellet was resuspended in one-twelfth of the starting volume of extraction buffer. The obtained solution was centrifuged at 10,200 × g for 30 min at 4°C. Plant-derived mAbs (mAbP CO and mAbP COK) were purified using protein A Sepharose 4 Fast Flow (GE Healthcare, Piscataway, NJ), according to the manufacturer’s recommendations. Both mAbP CO and mAbP COK proteins were dialyzed with 1× PBS (pH 7.4). The protein concentration was determined via Nanodrop analysis (Biotek, Highland, VT), and the purified protein was visualized using SDS-PAGE. Purified mAb proteins were stored at -70°C for further study.
Surface plasmon resonance analysis
To compare the binding affinities of anti-CRC mAbsP to the epithelial cell adhesion molecule (EpCAM). Surface plasmon resonance [24] analyses were performed using a ProteOn XPR36 instrument (Bio-Rad, Hercules, CA). The EpCAM antigen (R&D systems, Minneapolis, MN) was immobilized onto the surface of a GLC sensor chip and stabilized with PBS-T buffer (PBS buffer containing 0.05% v/v Tween-20). After stabilization, anti-EpCAM mAb (600 nM), mAbP CO (600 nM), and mAbP COK (600 nM) were applied to the sensor chip with a flow rate of 80 μl·min-1 at 25°C. The surface of the GLC chip was regenerated using phosphoric acid. Data analyses were performed with ProteOn Manager 2.1 software, and data were corrected by subtraction of the zero antibody concentration column as well as interspot correction.
MALDI-TOF mass spectrometry analysis of mAbP CO17-1A
Purified mAb CO17 and mAbP COK were treated with pepsin to digest the protein into glycopeptides, and digested peptides were passed through a C18 sep-pak cartridge (Waters, Lexington, MA). The samples were washed with 5% acetic acid to remove contaminants such as salts and free glycans. The fraction was eluted in series solutions with 20, 40, and 100% iso-propanol, and the eluted fractions were dried using a speed vaccum dryer. To release N-glycans, PNGase A (Roche, Basel, Switzerland) was added to each sample and incubated overnight at 37°C. The released N-glycans were purified using a graphitized carbon resin (Alltech, Lexington, MA). Purified glycans were resolubilized in HPLC water, spotted onto a stainless steel test plate, and treated by 0.8 μL of 50 mg·mL-1 2,5-dihydroxybenzoic acid in 50% acetonitrile. The spot was rapidly dried under vacuum for more homogenous crystallization. Analysis was carried out by MALDI-TOF MS using a Bruker ultrafleXtreme™ system.
Results
Generation of T1 Arabidopsis plants expressing anti-CRC mAb CO and mAb COK
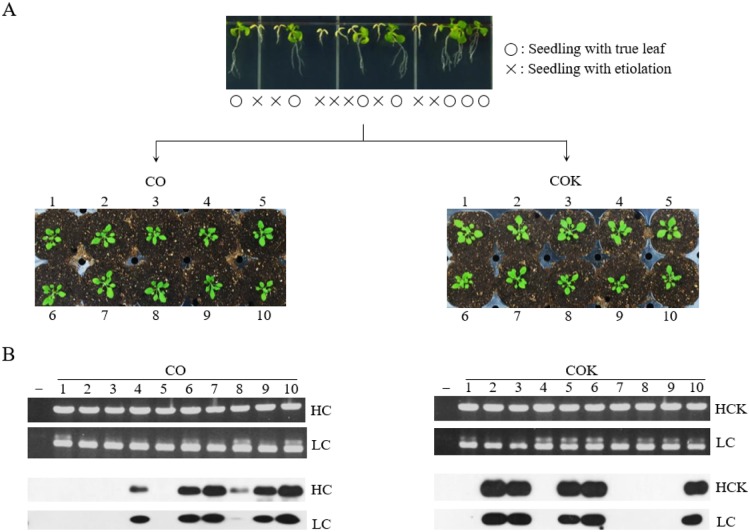
Plant expression vectors pBI CO and pBI COK were transferred into Arabidopsis plants via Agrobacterium-mediated dipping transformation (Fig 1). A total of 2,000 T1 seeds were obtained after floral dip transformation, and seeds were then plated on MS medium containing kanamycin prior to transformant selection. Most of the sown seeds failed to develop true leaves, and were etiolated with light yellow-colored shoots (Fig 2A; top). Approximately 40 seedlings with true leaves that survived in the kanamycin medium, for each transgenic plant expressing mAb CO and mAb COK (CO and COK, respectively), were transplanted to a growth chambers under standard conditions (Fig 2A; bottom). The rosette leaves of 40 plants were used to confirm transgene insertion. PCR analysis of HC (1,416 bp) and LC (717 bp) gene products were observed in all Arabidopsis plants (Fig 2B; top). No PCR bands were observed in NT Arabidopsis (Fig 2B; top). After confirmation of transgene insertion, protein expression of HC and LC proteins in leaves was determined by western blot analysis (Fig 2B; bottom), and HC and LC protein bands were detected at approximately 50 and 25 kDa, respectively. Among the randomly selected 10 plants, six CO and five COK plants exhibited HC and LC protein bands, respectively (Fig 2B; bottom). Kanamycin selection was repeated across generations to confirm homozygous T4 seeds for plant growth observations and the stable mass production of transgenic Arabidopsis plants expressing mAb CO or mAb COK (data not shown). Three lines of CO plants and two lines of COK plants were obtained using repeated antibiotic selections (S1 Fig).
Fig 2. Screening T1 transformants expressing mAb CO and mAb COK using antibiotic selection, PCR, and western blot analyses.
Selection of transformants after sowing T1 seeds on kanamycin media (top). o: seedling with true leaf; ×: seedling with etiolation. Surviving shoots were transplanted to soil pots and placed in a growth chamber with 16 h of light and 8 h of darkness at 23°C (middle). Ten rosette leaves (mAb CO and mAb COK) were sampled from each T1 plant to confirm the existence of HC and LC gene insertions and protein expression levels (bottom). Goat anti-murine IgG Fcγ and goat anti-murine IgG F(ab)′2 were used to detect HC and LC, respectively.
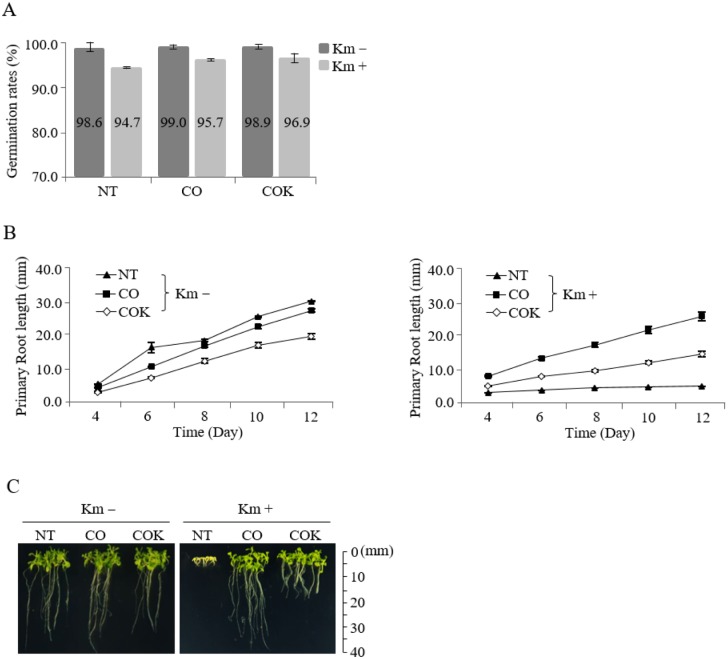
Effects of ER retention motif KDEL on germination rates and primary root growth of NT, CO, and COK seedlings in germination media with and without kanamycin
A total of 200 surface-sterilized seeds from each experimental group (NT, CO, and COK) were sown on MS agar plates containing kanamycin, and the same sets were sown on non-kanamycin media. In general, almost all seeds evenly started to germinate after two or three days of in vitro culture regardless of kanamycin presence. The germination rates of NT, CO plant, and COK plant in the non-kanamycin media were 98.6, 99.0, and 98.9%, respectively. The germination rates of NT, CO, and COK in the kanamycin-containing media were 94.7, 95.7, and 96.9%, respectively (Fig 3A). Twelve days after germination, the average primary root lengths of NT, CO, and COK plants specimens were 30.6, 28.0, and 20.7 mm, respectively, in media without kanamycin (Fig 3B; left). In kanamycin-containing media, the average primary root lengths of CO and COK plants were 23.2 and 12.2 mm, respectively, 12 days after germination (Fig 3B; right). In general, the primary root length of COK plants was shorter than that of plants in both the NT and CO groups (Fig 3B and 3C). All NT seeds were etiolated in MS media containing kanamycin within 4–5 days. The root lengths of plants in the CO group were approximately twice those of plants in the COK group (Fig 3C and S1 Fig). The primary root growth rate of COK plant was lower than that of CO plant in media, regardless of the presence of kanamycin.
Fig 3. Effects of KDEL tagging on the plant growth of transgenic Arabidopsis plants expressing mAb CO and mAb COK under in vitro conditions with and without kanamycin.
(A) Germination rates (number of germinated seeds/total seeds × 100) were calculated four days after the seeding of homozygous seeds on non-kanamycin or kanamycin-containing media. The values on the graphs represent the mean value of each experimental group. (B) Average primary root lengths of CO and COK plants in vitro with kanamycin (left) and without kanamycin (right) at four, six, eight, 10, and 12 days under the same conditions described above. (C) Photographs were taken 12 days after seeding on each MS media. Scales are displayed at 10 mm intervals. Standard deviation is indicated with error bars.
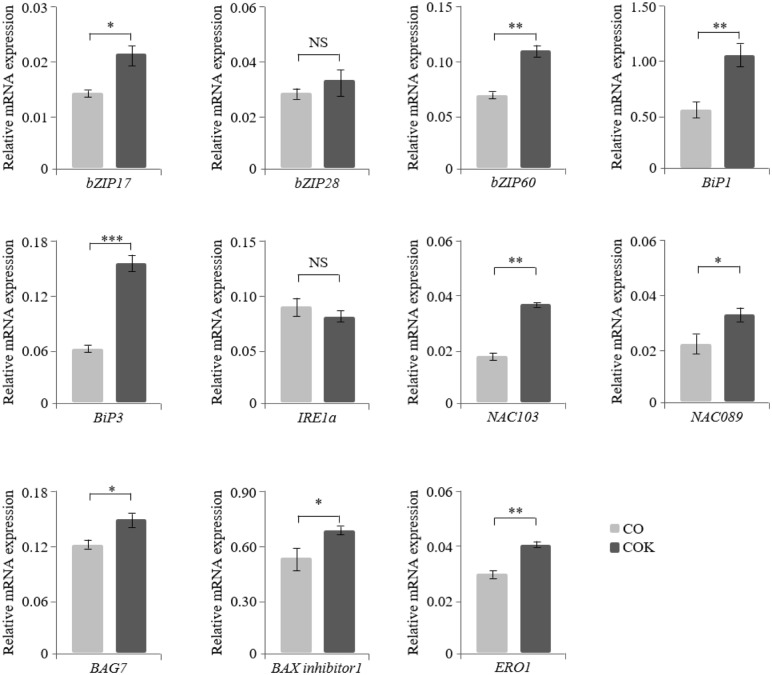
mRNA expression of ER stress-related genes in transgenic Arabidopsis plants expressing mAb CO and mAb COK
The mRNA expression levels of 11 ER stress-related genes (bZIP17, bZIP28, bZIP60, BiP1, BiP3, IRE1a, NAC103, NAC089, BAG7, BAX inhibitor 1, and ERO1) were quantified using qRT-PCR analyses to investigate the effects of ER retention motif tagging on transgenic Arabidopsis plants expressing mAb CO and mAb COK (Fig 4). Expression of ER stress-related genes (bZIP17, bZIP60, BiP1, BiP3, NAC103, NAC089, BAG7, BAX inhibitor 1, and ERO1) were significantly upregulated in COK plant lines compared to CO plant lines, with the exception of bZIP28 and IRE1a genes. Among the tested genes, the mRNA levels of BiP3 in COK plant lines were almost three times higher compared to CO plant lines.
Fig 4. Regulation of mRNA expression of ER stress-related genes (bZIP17, bZIP28, bZIP60, BiP1, BiP3, IRE1a, NAC103, NAC089, BAG7, BAX inhibitor 1, and ERO1) in transgenic Arabidopsis plants expressing mAb CO and mAb COK measured with qRT-PCR analysis.
RNA was isolated from the roots of plants from each experimental group (mAb CO and mAb COK) and grown on kanamycin-containing media for 12 days. The ubiquitin 10 (UBI 10) gene was used as a control gene for normalization. Error bars represent the mean ± standard deviation (SD) of three technical replicates with two biological replicates. Statistically significant differences are indicated by asterisks (NS: not significant; *: p < 0.05; **: p < 0.01; and ***: p < 0.001). The X-axis represents ER stress-related genes, and the relative mRNA expression levels of each transformant are expressed on the Y-axis.
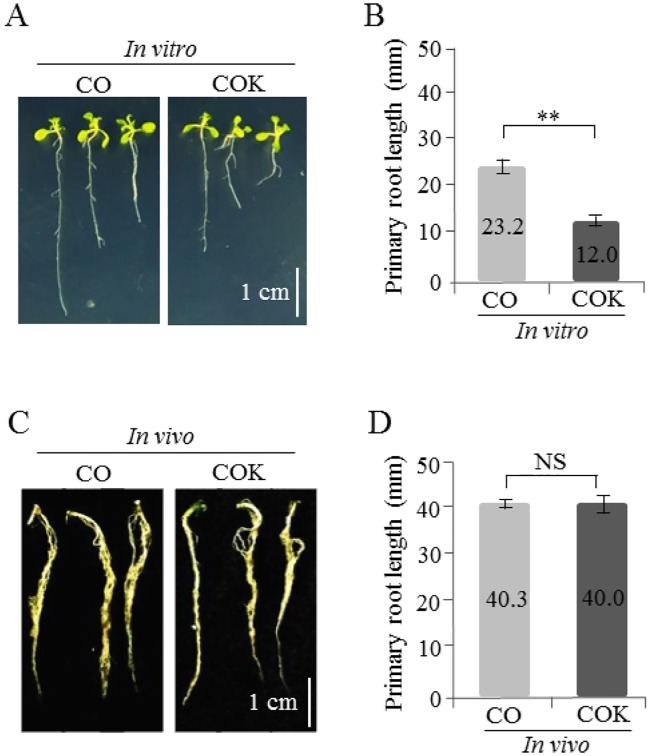
Comparisons of root lengths of CO and COK plants grown under in vitro and in vivo conditions
A total of 100 vernalized and sterilized seeds for each experimental group (CO and COK plants) were sown on agar plates containing kanamycin. Two-weeks after seeding on an agar plate containing kanamycin, the average root lengths of CO and COK plants were 23.2 and 12.0 mm, respectively, and the difference between the two was significant (Fig 5A and 5B). Following an in vitro antibiotic susceptibility test, true leaf-generated shoots [CO (36 shoots), and COK (32 shoots)] were transplanted to a soil pot for further study. Ten-weeks after transplanting to a pot, the average root lengths of CO and COK plants were 40.3 and 40.0 mm, respectively (Fig 5C and 5D). Under in vitro conditions, the root length of plants in the CO plant group was twice that of plants in the COK plant group. However, under in vivo conditions, there was no significant difference between the root length of plants in the CO and COK groups.
Fig 5. Comparison of primary root growth of transgenic Arabidopsis plants expressing mAb CO (CO) and mAb COK (COK) under in vitro and in vivo conditions.
(A) In vitro germinated seedlings examined two weeks after sowing on MS media. The scale bar represents 1 cm in each photograph. (B) Comparison of average primary root lengths of transgenic Arabidopsis plants expressing CO and COK grown under in vitro conditions. (C) Roots of in vivo CO and COK plant seedlings 10 weeks after transplanting from in vitro growth to a soil pot. (D) Comparison of average root lengths of transgenic Arabidopsis plants expressing CO and COK grown in a chamber for 10 weeks. Error bars represent the mean ± SD. Statistically significant differences are indicated by asterisks (**: p < 0.01; NS: not significant).
Growth trends of rosette leaves of transgenic Arabidopsis plants expressing mAb CO and mAb COK under in vivo conditions
Among the total seeds sown on kanamycin-containing MS medium, true leaf-generated shoots [NT (16 shoots), CO (32 shoots), and COK (30 shoots)] were transplanted to a soil pot for further study. One-week after transplantation to a pot, the average rosette leaf lengths (measured from the petiole to the blade) of NT, CO, and COK specimens were 0.81, 0.85, and 0.78 cm, respectively (Figure B in S2 Fig). Two-weeks after transplantation to a pot, the average rosette leaf lengths of NT, CO, and COK specimens were 1.90, 1.94, and 1.88 cm, respectively (Figure B in S2 Fig). Three-weeks after transplantation to a pot, the average rosette leaf lengths of NT, CO, and COK were 2.80, 2.70, and 2.65 cm, respectively (Figure B in S2 Fig). Four-weeks after transplantation to a pot, the average rosette leaf lengths of NT, CO, and COK were 3.44, 3.32, and 3.22 cm, respectively (Figure B in S2 Fig). Under in vivo conditions, the rosette leaf lengths of NT, CO, and COK were not significantly different.
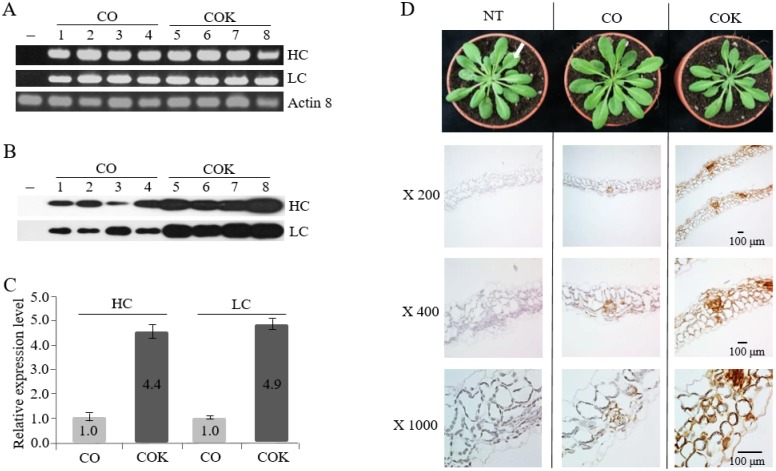
Effects of KDEL on mAbs CO and COK protein expression and localization in rosette leaf tissues
To investigate the effects of ER retention motif tagging on transcription and translation levels of mAbs CO and COK, RT-PCR and western blot analyses were conducted, respectively (Fig 6A and 6B). Rosette leaves of the T4 generation of CO (30 plants, five lines), COK (25 plants, four lines), and NT (eight plants) plants were used to extract mRNA and proteins, respectively. The HC and LC transcription levels did not differ significantly between CO and COK lines. However, HC expression levels in COK plants were 4.4-fold higher than those of CO plants, and LC expression levels in COK plants were 4.9-fold higher than those of CO plants (Fig 6C). In addition, COK plants exhibited more stable expression patterns than CO plants. These results indicated that ER retention motif tagging to HC affects protein expression levels rather than transcription levels. Immunohistochemical analyses using a Dako REAL™ EnVision™ Detection System and Mayer’s hematoxylin were conducted to confirm the localization of mAb CO and mAb COK in rosette leaves of Arabidopsis plants (NT, CO, and COK) (Fig 6D). Brown color staining was observed in leaf tissues of both CO and COK plants, whereas brown color stained cells were not detected in the leaf tissues of NT plants (Fig 6D). The brown color density of COK plants was stronger than that of CO plants (Fig 5D; ×200, ×400, and ×1000). In COK plants, strong brown color staining was localized in the intracellular space including xylem and phloem areas, whereas the staining was weak in CO plant leaf tissues (Fig 6D; ×1000).
Fig 6. Effects of the ER retention motif on the expression and distribution of mAbs in transgenic Arabidopsis plants expressing mAb CO and mAb COK.
(A) RT-PCR and (B) western blot analyses were performed to confirm HC and LC expression based on RNA and protein expression levels. Lane 1: non-transgenic (NT); lanes 2–5: transgenic plants expressing mAb CO; and lanes 6–9: transgenic plants expressing mAb COK. (C) Relative expression levels of HC and LC determined by immunoblot analyses. Values represent the mean for each experimental group, and SD is indicated by error bars. (D) Intracellular distribution of mAb CO and mAb COK in the rosette leaf tissues of transgenic Arabidopsis plants. Freshly harvested rosette leaf tissues (indicated by a white arrow) were fixed in 10% formalin overnight and processed for paraffin embedding. A Dako REAL EnVision Detection System was used, according to the manufacturer’s instructions, and samples were then counterstained with Mayer’s hematoxylin. Lane 1: non-transgenic (NT) plant; lane 2: mAb CO; and lane 3: mAb COK. Slides were observed under a microscope (magnification: ×200, ×400, and ×1000; BX53F; Olympus, Tokyo, Japan)]. Scale bar represents 100 μm.
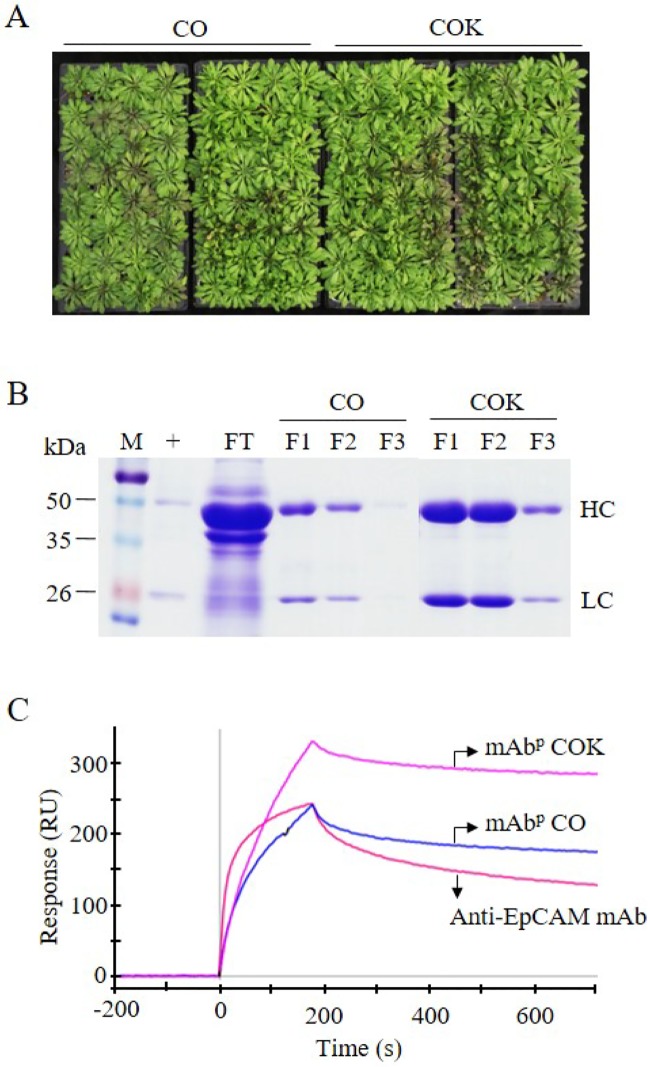
Mass production and purification of anti-CRC mAbs from Arabidopsis plants using protein A affinity chromatography
Amplified seeds of transgenic CO and COK plants were sown in soil and cultivated in a greenhouse facility (Fig 7A). A total of 40 g of transgenic Arabidopsis leaves were harvested in each tray, and 200 g of plant biomass (each CO and COK plant) were used to purify anti-CRC mAbs (CO and COK). Ammonium sulfate-mediated total soluble protein precipitation and protein A affinity chromatography were used to purify anti-CRC mAbs from plant leaf biomass, and the two processes efficiently isolated the majority of total soluble proteins and separated specific mAbs CO and COK from plant biomass extracts, respectively. SDS-PAGE analyses were used to identify HC (50 kDa) and LC (25 kDa) of anti-CRC mAbPs from elution fractions (Fig 7B). Both mAb CO and mAb COK were mainly eluted in fraction samples (Fig 7B; F1 and F2). Each eluted sample (F1–F3) was quantified, and the total amounts of mAbs obtained were 410 (CO) and 1,950 μg (COK). The amount of mAb obtained from COK plants was five times higher than that obtained from CO plants.
Fig 7. Purification and functions of mAb CO and mAb COK expressed in Arabidopsis plants.
(A) Representative images depicting the mass production of mAb CO and mAb COK in transgenic Arabidopsis plants. Seeds were sown in a greenhouse and conditioned at 24°C, 30% humidity, 16 h light, and 8 h dark. Photographs were taken seven weeks after sowing homozygous seeds. (B) SDS-PAGE analysis of eluted F1–F3 fractions of purified anti-cancer mAbs obtained from Arabidopsis plants. Lane 1: protein marker; lane 2: anti-EpCAM mAb; lane 3: flow-through; lanes 4–6: mAb CO; and lanes 7–9: mAb COK. Samples (20 μL) were loaded into each well. (C) Surface plasmon resonance analysis was used to confirm the binding activity of mAb CO and mAb COK to an epithelial cell adhesion molecule (EpCAM-Fc). To confirm antigen-antibody binding activity of both mAb CO and mAb COK purified from transgenic Arabidopsis plants, the surface of a GLC sensorchip was immobilized with EpCAM fused to human IgG Fc fragment (EpCAM-Fc) molecules. Anti-EpCAM mAb (300 nM), mAb CO (300 nM), and mAb COK (300 nM) were applied to the sensor chip with a flow rate of 80 μL·min-1 at 25°C, and the response curves shown in the figure were consequently obtained.
Binding activity of anti-CRC mAbs to antigen EpCAM proteins
Purified samples (mAbP CO and mAbP COK) were dialyzed with 1× PBS and normalized to adjust the concentration (25 μg·mL-1) for SPR analyses (Fig 7C), and 250-μL samples (anti-EpCAM mAb, mAbP CO, and mAbP COK) were used for each SPR analysis. Both mAb types (mAbP CO and mAbP COK) had higher and similar binding affinities to EpCAM compared to mammalian-derived anti-EpCAM mAb. Results of SPR analyses showed that mAbP COK had the highest equilibrium concentration to the immobilized chip coated with EpCAM-Fc after 200 seconds (Fig 7C). Anti-EpCAM mAb and mAbP CO had lower equilibrium concentrations compared to mAbP COK, and dissociation rates were almost equal between CO and COK. However, anti-EpCAM mAb dissociated faster than both mAbP CO and mAbP COK (Fig 7C).
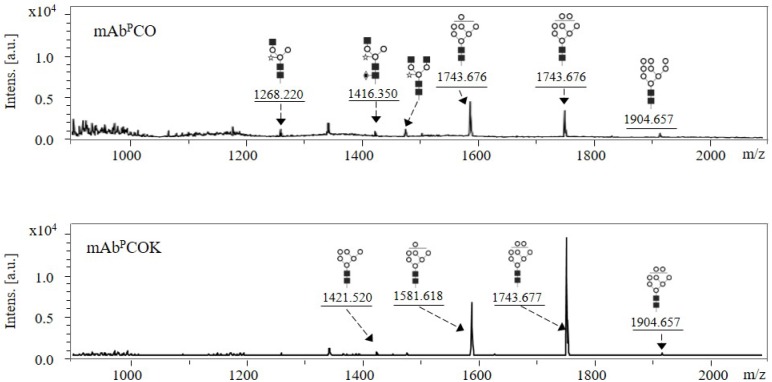
N-glycosylation profiles of anti-colorectal cancer mAbs purified from transgenic Arabidopsis
N-glycan profiles of mAbP CO and mAbP COK purified from transgenic Arabidopsis were confirmed using MALDI-TOF mass spectrometry analysis (Fig 8). In mAbP CO, Man7-8GlcNAc2 was mainly detected whereas Man3GlcNAc3Xyl, Man3GlcNAc3XylFuc, Man3GlcNAc4Xyl, and Man9GlcNAc2 were observed with quite small peaks (Fig 8; upper panel). In mAbP COK, the two main peaks were identified as Man7GlcNAc2 and Man8GlcNAc2, and the two small peaks were identified as Man6GlcNAc2 and Man9GlcNAc2 (Fig 8; bottom panel).
Fig 8. N-glycan profiles of mAbPCO and mAbPCOK purified from transgenic Arabidopsis biomass using MALDI-TOF mass spectrometry.
The symbols of the glycan structures are as follows: mannose, white circle; N-acetylglucosamine, black square; α(1,3)-fucose, diamond with a dot inside; β(1,2)-xylose, white star.
Discussion
This study demonstrated the effects of ER retention motif KDEL on plant growth phenotypes and anti-CRC mAb expression in transgenic Arabidopsis plants. The effects of KDEL tagging were previously discussed in transgenic tobacco plants, and it resulted in the retention of KDEL-tagged recombinant proteins in the ER, leading to a high mannose glycan protein structure [25, 26]. In the current study, A. thaliana, for molecular biofarming, was used to express mAb CO and mAb COK.
T1 seeds were obtained using a traditional floral dip Arabidopsis transformation protocol, and positive transformants were screened by PCR and western blot analyses, consequently leading to the identification of stable homozygous transgenic lines. Sterilized seeds of transgenic Arabidopsis plants, expressing mAb CO and mAb COK, and NT plant seeds were vertically sown on kanamycin or non-kanamycin media to confirm the effects of ER retention motif tagging on germination rates and the primary root growth of each group. mAb CO or mAb COK transgene insertions in Arabidopsis plants had little influence on germination rates, and this result was similar to that of the NT group. However, the main root lengths of plants expressing mAb COK were approximately two times shorter than those of plants expressing mAb CO. We hypothesized that the accumulation of anti-CRC mAbs in the ER might induce ER stress-related genes in Arabidopsis plants, consequently affecting the primary root phenotype of transgenic Arabidopsis plants [27, 28]. Previous studies demonstrated that the accumulation of recombinant proteins in the ER might induce ER stress, which activates UPR to reduce ER stress and maintain homeostasis [8, 9, 28]. Among the three major UPR branches [12], ATF6 and IRE1 pathway-related genes were analyzed in the present study. The main transcription factors related to the UPR pathway (bZIP17, bZIP60, BiP1, BiP3, NAC103, NAC089, BAG7, BAX inhibitor 1, and ERO1) [27] were upregulated in the roots of plants expressing mAb COK compared with those of plants expressing mAb CO.
In vitro seedlings that survived antibiotic selection were transferred to soil pots in a growth chamber to confirm plant growth, transcription levels, and translation levels of mAbs in each experimental group. Unlike in vitro conditions, plant phenotypes such as rosette leaf and primary root length of mAb COK plants were similar to those of the mAb CO and NT groups under in vivo conditions. In addition, genes related to ER stress and UPR did not differ significantly between both CO and COK plants grown under in vivo conditions (data not shown). It is speculated that conditions such as light, fertilizer, and soil (non-kanamycin added) led to reduce stress compared to in vitro conditions.
Although C-terminal KDEL tagging induced ER stress and resulted in slightly altered plant growth phenotypes, the ER retention motif boosted the amount of translated mAb COK per unit to nearly four times higher in fresh leaves, and it exhibited a more stable expression pattern compared to mAb CO [29, 30]. The transcript levels of mAb HC and LC genes were nearly equal between CO and COK plants, and these results suggested that ER retention motifs efficiently accumulated recombinant anti-CRC mAbs in plant tissues.
The protein expression-boosting effects of KDEL tagging were confirmed by immunohistochemical analyses using fresh rosette leaf tissues obtained from transgenic Arabidopsis plants expressing mAb CO and mAb COK. Our study showed that ER retention motif KDEL led to high accumulation of mAb COK without any damage to plant cells, consequently inducing high protein production levels per unit leaf biomass in vivo conditions. Thus, it is important to consider careful selection and screening of healthy transgenic lines with high transgene expression both in vitro and in vivo conditions. In this study, the mAb-expressing cells seemed to be limited to those near the veins or vascular tissues. These results are expected since among plant cells, vain and vascular tissues are mainly composed of actively dividing and growing cells.
To obtain anti-CRC mAbs, approximately 2,000 seeds from each group (mAb CO and mAb COK) were sown in a greenhouse facility, and mature plants were harvested for purification. No difference was observed in the phenotypes of transgenic plants, and this was consistent with growth chamber culture results. Both mAb CO and mAb COK expressed from transgenic Arabidopsis plants were efficiently purified with ammonium sulfate-mediated precipitation and protein A affinity column processes, without any deviation from the original protocol [31]. SDS-PAGE results showed that overall amounts of mAbPs per unit mass were greatly increased up to five times in COK plants compared to CO plants.
The binding affinities of both mAbP CO and mAbP COK to target antigen EpCAM molecules were higher or similar to mammalian-derived anti-EpCAM mAb counterparts, respectively. Moreover, these results were previously discussed in transgenic tobacco system [18]. Taken together, our findings suggest that Arabidopsis plants are a promising platform for the generation of anti-CRC mAbs with biological activities that are consistent with the anti-EpCAM mAb, a counterpart antibody. The introduction of the ER retention motif enabled the bulk production of mAbs in plants with enhanced ER accumulation, without the generation of significant in vivo stress regardless of in vitro ER stress. Therefore, an ER retention strategy is recommended to induce high accumulation of recombinant antibodies in a plant expression system.
Supporting information
Kanamycin selection was repeated over generations to obtain homozygous seeds for further study. Photographs were taken 14 days after seed germination using a camera (Digital Gross System) (Humintec, Suwon, Korea). CO: seedling expressing mAbP CO; COK: seedling expressing mAbP COK; Km+: kanamycin containing media.
(TIF)
(A) Seeds (NT, mAb CO, and mAb COK) were sown into pots containing soil in a growth chamber. Plants were photographed one month after transfer to pots. NT: non-transgenic plants; CO: transgenic plants expressing mAb CO; COK: transgenic plants expressing mAb COK. (B) Rosette leaf lengths were measured from the petiole to the blade using a ruler at 1-week intervals after transplantation from in vitro conditions. Scale bar represents 1 cm in each photograph.
(TIF)
Acknowledgments
This research was supported by a grant (Code# PJ0134372018) from the Korean Rural Development Administration and the National Research Foundation of Korea Grant that was funded by the Korean Government (MEST) (NRF-2017R1A2A2A0569788).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by a grant (Code#PJ0134372018) from the Korean Rural Development Administration and National Research Foundation of Korea Grant funded by the Korean Government (MEST) (NRF-2017R1A2A2A0569788).
References
- 1.Tariq K, Ghias K. Colorectal cancer carcinogenesis: a review of mechanisms. Cancer biology & medicine. 2016;13(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ko K. Expression of recombinant vaccines and antibodies in plants. Monoclonal antibodies in immunodiagnosis and immunotherapy. 2014;33(3):192–8. 10.1089/mab.2014.0049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moussavou G, Ko K, Lee J-H, Choo Y-K. Production of monoclonal antibodies in plants for cancer immunotherapy. BioMed research international. 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koornneef M, Meinke D. The development of Arabidopsis as a model plant. The Plant Journal. 2010;61(6):909–21. 10.1111/j.1365-313X.2009.04086.x [DOI] [PubMed] [Google Scholar]
- 5.Song I, Kim MK, Jamal A, Hwang K-A, Ko K. Comparison of total soluble protein in various horticultural crops and evaluation of its quantification methods. Horticulture, Environment, and Biotechnology. 2015;56(1):123–9. [Google Scholar]
- 6.Ko K, Ahn M-H, Song M, Choo Y-K, Kim HS, Ko K, et al. Glyco-engineering of biotherapeutic proteins in plants. Molecules & Cells (Springer Science & Business Media BV). 2008;25(4). [PubMed] [Google Scholar]
- 7.Kim D-S, Lee S-H, Ko K. Expression and function of plant-derived recombinant multiple monoclonal antibodies for the recognition of human colorectal cancer cells. Plant Biotechnology Reports. 2015;9(6):361–8. [Google Scholar]
- 8.Yamamoto K, Hamada H, Shinkai H, Kohno Y, Koseki H, Aoe T. The KDEL receptor modulates the endoplasmic reticulum stress response through mitogen-activated protein kinase signaling cascades. Journal of Biological Chemistry. 2003;278(36):34525–32. 10.1074/jbc.M304188200 [DOI] [PubMed] [Google Scholar]
- 9.Liu L, Cui F, Li Q, Yin B, Zhang H, Lin B, et al. The endoplasmic reticulum-associated degradation is necessary for plant salt tolerance. Cell research. 2011;21(6):957 10.1038/cr.2010.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schäfer P, Eichmann R. The endoplasmic reticulum in plant immunity and cell death. Frontiers in plant science. 2012;3:200 10.3389/fpls.2012.00200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J, Ozcan U. Unfolded protein response signaling and metabolic diseases. Journal of Biological Chemistry. 2014;289(3):1203–11. 10.1074/jbc.R113.534743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanderson TH, Gallaway M, Kumar R. Unfolding the unfolded protein response: unique insights into brain ischemia. International journal of molecular sciences. 2015;16(4):7133–42. 10.3390/ijms16047133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, Wang A. Virus-induced ER stress and the unfolded protein response. Frontiers in plant science. 2012;3:293 10.3389/fpls.2012.00293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roy G, Zhang S, Li L, Higham E, Wu H, Marelli M, et al. Development of a fluorescent reporter system for monitoring ER stress in Chinese hamster ovary cells and its application for therapeutic protein production. PloS one. 2017;12(8):e0183694 10.1371/journal.pone.0183694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tajima H, Iwata Y, Iwano M, Takayama S, Koizumi N. Identification of an Arabidopsis transmembrane bZIP transcription factor involved in the endoplasmic reticulum stress response. Biochemical and biophysical research communications. 2008;374(2):242–7. 10.1016/j.bbrc.2008.07.021 [DOI] [PubMed] [Google Scholar]
- 16.Liu J-X, Howell SH. Endoplasmic reticulum protein quality control and its relationship to environmental stress responses in plants. The Plant Cell. 2010;22(9):2930–42. 10.1105/tpc.110.078154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Z-T, Wang M-J, Sun L, Lu S-J, Bi D-L, Sun L, et al. The membrane-associated transcription factor NAC089 controls ER-stress-induced programmed cell death in plants. PLoS genetics. 2014;10(3):e1004243 10.1371/journal.pgen.1004243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim D-S, Song I, Kim J, Kim D-S, Ko K. Plant recycling for molecular biofarming to produce recombinant anti-cancer mAb. Frontiers in plant science. 2016;7:1037 10.3389/fpls.2016.01037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han S, Zong S, Shi Q, Li H, Liu S, Yang W, et al. Is Ep-CAM Expression a Diagnostic and Prognostic Biomarker for Colorectal Cancer? A Systematic Meta-Analysis. EBioMedicine. 2017;20:61–9. 10.1016/j.ebiom.2017.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clough SJ, Bent AF. Floral dip: a simplified method forAgrobacterium-mediated transformation ofArabidopsis thaliana. The plant journal. 1998;16(6):735–43. [DOI] [PubMed] [Google Scholar]
- 21.Chomczynski P, Mackey K. Short technical reports. Modification of the TRI reagent procedure for isolation of RNA from polysaccharide-and proteoglycan-rich sources. Biotechniques. 1995;19(6):942–5. [PubMed] [Google Scholar]
- 22.Kang YJ, Kim D-S, Myung S-C, Ko K. Expression of a Human Prostatic Acid Phosphatase (PAP)-IgM Fc Fusion Protein in Plants Using In vitro Tissue Subculture. Frontiers in plant science. 2017;8:274 10.3389/fpls.2017.00274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han S, Kim D. AtRTPrimer: database for Arabidopsis genome-wide homogeneous and specific RT-PCR primer-pairs. BMC bioinformatics. 2006;7(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zalevsky J, Chamberlain AK, Horton HM, Karki S, Leung IW, Sproule TJ, et al. Enhanced antibody half-life improves in vivo activity. Nature biotechnology. 2010;28(2):157 10.1038/nbt.1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.So Y, Lee K-J, Kim D-S, Lee J-H, Oh D-B, Hwang K-A, et al. Glycomodification and characterization of anti-colorectal cancer immunotherapeutic monoclonal antibodies in transgenic tobacco. Plant Cell, Tissue and Organ Culture (PCTOC). 2013;113(1):41–9. [Google Scholar]
- 26.Lu Z, Lee K-J, Shao Y, Lee J-H, So Y, Choo Y-K, et al. Expression of GA733-Fc fusion protein as a vaccine candidate for colorectal cancer in transgenic plants. BioMed Research International. 2012;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozgur R, Turkan I, Uzilday B, Sekmen AH. Endoplasmic reticulum stress triggers ROS signalling, changes the redox state, and regulates the antioxidant defence of Arabidopsis thaliana. Journal of experimental botany. 2014;65(5):1377–90. 10.1093/jxb/eru034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng Y, Srivastava R, Howell SH. Endoplasmic reticulum (ER) stress response and its physiological roles in plants. International journal of molecular sciences. 2013;14(4):8188–212. 10.3390/ijms14048188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okamoto T, Shimada T, Hara-Nishimura I, Nishimura M, Minamikawa T. C-terminal KDEL sequence of a KDEL-tailed cysteine proteinase (sulfhydryl-endopeptidase) is involved in formation of KDEL vesicle and in efficient vacuolar transport of sulfhydryl-endopeptidase. Plant Physiology. 2003;132(4):1892–900. 10.1104/pp.103.021147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee J-H, Park D-Y, Lee K-J, Kim Y-K, So Y-K, Ryu J-S, et al. Intracellular reprogramming of expression, glycosylation, and function of a plant-derived antiviral therapeutic monoclonal antibody. PloS one. 2013;8(8):e68772 10.1371/journal.pone.0068772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park S-R, Lim C-Y, Kim D-S, Ko K. Optimization of ammonium sulfate concentration for purification of colorectal cancer vaccine candidate recombinant protein GA733-FcK isolated from plants. Frontiers in plant science. 2015;6:1040 10.3389/fpls.2015.01040 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Kanamycin selection was repeated over generations to obtain homozygous seeds for further study. Photographs were taken 14 days after seed germination using a camera (Digital Gross System) (Humintec, Suwon, Korea). CO: seedling expressing mAbP CO; COK: seedling expressing mAbP COK; Km+: kanamycin containing media.
(TIF)
(A) Seeds (NT, mAb CO, and mAb COK) were sown into pots containing soil in a growth chamber. Plants were photographed one month after transfer to pots. NT: non-transgenic plants; CO: transgenic plants expressing mAb CO; COK: transgenic plants expressing mAb COK. (B) Rosette leaf lengths were measured from the petiole to the blade using a ruler at 1-week intervals after transplantation from in vitro conditions. Scale bar represents 1 cm in each photograph.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.