Abstract
Background
This study reports the findings of the first large-scale Phase III investigator-driven clinical trial to slow the rate of cognitive decline in Alzheimer disease with a dihydropyridine (DHP) calcium channel blocker, nilvadipine. Nilvadipine, licensed to treat hypertension, reduces amyloid production, increases regional cerebral blood flow, and has demonstrated anti-inflammatory and anti-tau activity in preclinical studies, properties that could have disease-modifying effects for Alzheimer disease. We aimed to determine if nilvadipine was effective in slowing cognitive decline in subjects with mild to moderate Alzheimer disease.
Methods and findings
NILVAD was an 18-month, randomised, placebo-controlled, double-blind trial that randomised participants between 15 May 2013 and 13 April 2015. The study was conducted at 23 academic centres in nine European countries. Of 577 participants screened, 511 were eligible and were randomised (258 to placebo, 253 to nilvadipine). Participants took a trial treatment capsule once a day after breakfast for 78 weeks. Participants were aged >50 years, meeting National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer’s disease Criteria (NINCDS-ADRDA) for diagnosis of probable Alzheimer disease, with a Standardised Mini-Mental State Examination (SMMSE) score of ≥12 and <27. Participants were randomly assigned to 8 mg sustained-release nilvadipine or matched placebo. The a priori defined primary outcome was progression on the Alzheimer's Disease Assessment Scale Cognitive Subscale-12 (ADAS-Cog 12) in the modified intention-to-treat (mITT) population (n = 498), with the Clinical Dementia Rating Scale sum of boxes (CDR-sb) as a gated co-primary outcome, eligible to be promoted to primary end point conditional on a significant effect on the ADAS-Cog 12. The analysis set had a mean age of 73 years and was 62% female. Baseline demographic and Alzheimer disease–specific characteristics were similar between treatment groups, with reported mean of 1.7 years since diagnosis and mean SMMSE of 20.4. The prespecified primary analyses failed to show any treatment benefit for nilvadipine on the co-primary outcome (p = 0.465). Decline from baseline in ADAS-Cog 12 on placebo was 0.79 (95% CI, −0.07–1.64) at 13 weeks, 6.41 (5.33–7.49) at 52 weeks, and 9.63 (8.33–10.93) at 78 weeks and on nilvadipine was 0.88 (0.02–1.74) at 13 weeks, 5.75 (4.66–6.85) at 52 weeks, and 9.41 (8.09–10.73) at 78 weeks. Exploratory analyses of the planned secondary outcomes showed no substantial effects, including on the CDR-sb or the Disability Assessment for Dementia. Nilvadipine appeared to be safe and well tolerated. Mortality was similar between groups (3 on nilvadipine, 4 on placebo); higher counts of adverse events (AEs) on nilvadipine (1,129 versus 1,030), and serious adverse events (SAEs; 146 versus 101), were observed. There were 14 withdrawals because of AEs. Major limitations of this study were that subjects had established dementia and the likelihood that non-Alzheimer subjects were included because of the lack of biomarker confirmation of the presence of brain amyloid.
Conclusions
The results do not suggest benefit of nilvadipine as a treatment in a population spanning mild to moderate Alzheimer disease.
Trial registration
Clinicaltrials.gov NCT02017340, EudraCT number 2012-002764-27.
In a randomised controlled trial, Brian Lawlor and colleagues investigate whether the blood pressure medication nivaldipine can slow the progression of Alzheimer disease.
Author summary
Why was this study done?
There are few licensed drug treatments for Alzheimer disease and none are effective in slowing the rate of disease progression.
Nilvadipine is a licensed blood pressure medication and has been shown to lower brain amyloid and improve memory function in animal models of Alzheimer disease.
If nilvadipine were shown to be effective in slowing the rate of progression of Alzheimer disease, because it is already licensed and available to treat high blood pressure, it would be possible to introduce the drug for use in Alzheimer disease relatively quickly.
What did the researchers do and find?
We carried out an investigator-led clinical trial funded by the European Union across 23 academic university sites and involving 511 patients with mild- and moderate-stage Alzheimer disease, as diagnosed by a clinician.
We tested whether a single dose of nilvadipine, compared with placebo, was safe and slowed the progression of Alzheimer disease over a period of 18 months.
We found that nilvadipine appeared safe and was well tolerated but did not slow decline in cognition or function in this group of mild- and moderate-stage Alzheimer disease patients.
What do these findings mean?
Nilvadipine does not appear to be effective as a treatment for people with mild- or moderate-stage Alzheimer disease.
We cannot rule out that this medication may help at an earlier stage of the disease process, before the person experiences loss of function.
Introduction
Observational studies have suggested a benefit of certain blood pressure medications on reducing the risk of developing dementia [1]. Particular antihypertensive agents have also been shown to decrease Alzheimer disease pathology in the brains of people with hypertension, independently of blood pressure control, suggesting a direct effect of these medications against the biological processes underpinning Alzheimer disease [2,3]. One antihypertensive, for which there is clinical and scientific rationale for disease-modifying efficacy in Alzheimer disease, is nilvadipine. Nilvadipine is a dihydropyridine (DHP) calcium channel blocker and is licensed in a number of countries to treat patients with hypertension. Nilvadipine is reported to have a number of neuroprotective mechanisms of action other than direct calcium channel blockade and maintenance of intracellular calcium homeostasis, including lowering Amyloid beta 40 and 42 amino acid peptides (Aβ40 and Aβ42) production in vitro and in vivo in transgenic mouse models of Alzheimer disease, and enhancing Aβ clearance across the blood–brain barrier in in vivo mouse models [4,5]. However, many other DHPs do not share these properties and some may actually increase Aβ40 and Aβ42 production in vitro [4], demonstrating that amyloid lowering is not a class effect of DHPs. In addition to effects on Aβ production and clearance, nilvadipine specifically has also shown efficacy against a broad range of other putative Alzheimer disease pathological mechanisms, including tau-phosphorylation, reduced cerebral blood flow, and neuroinflammation [6–9].
In clinical studies, nilvadipine stabilised cognitive decline and reduced conversion to Alzheimer disease in a small study of patients with hypertension and mild cognitive impairment [10]. Another 6-week open label study demonstrated that nilvadipine was safe and well tolerated in patients with Alzheimer disease and did not reduce blood pressure in nonhypertensive patients with Alzheimer disease, but appropriately lowered blood pressure in hypertensive cases [11].
These studies are complemented by a number of epidemiological and interventional studies involving different calcium channel blockers that have reported on the potential benefit of this drug class in the prevention of Alzheimer disease. In the treatment of Systolic Hypertension in Europe (Syst-Eur) trial, which involved over 2,400 older participants with systolic hypertension treated with the DHP calcium channel blocker, nitrendipine, there was a reported 55% reduction in the incidence of Alzheimer disease [12,13]. The Baltimore Longitudinal Study of Aging found a nonsignificant apparent benefit towards reduced relative risk of Alzheimer disease in patients treated with DHP calcium channel blockers, with no lowered risk observed in the non-DHP calcium channel blocker treatment group [14].
To our knowledge, there has been no definitive intervention study with a calcium channel blocker to test for an effect on slowing the rate of cognitive decline in patients with Alzheimer disease.
Given the previous preclinical and clinical data suggesting the potential efficacy for nilvadipine and related compounds against Alzheimer disease, the objective of this 78-week randomised, placebo-controlled study was to determine whether treatment with nilvadipine sustained-release 8 mg, once a day, was effective and safe in slowing the rate of cognitive decline in patients with mild to moderate Alzheimer disease.
Methods
Study design
This 18-month Phase III, randomised, placebo-controlled, double-blind, parallel-group study was carried out at 23 academic centres in nine European countries: Ireland (two sites), United Kingdom (one site), Italy (four sites), the Netherlands (three sites), France (seven sites), Greece (three sites), Sweden (one site), Germany (one site), and Hungary (one site) (S1 Table). The trial project office was based at St. James’s Hospital, Dublin, Ireland, which was also the sponsor. The trial coordinating institution was Trinity College, University of Dublin, and the trial was funded by the European Commission, under a Framework 7 Programme Health Theme collaborative project grant. The trial database, randomisation, and allocation system were maintained by the Clinical Trials Unit at King’s College London, and the statistical analysis was conducted at the University College Dublin Centre for Support and Training in Analysis and Research (UCD CSTAR). As part of the overall governance of the trial, there was a Scientific Advisory Board, an independent Ethics Advisory Board, and an independent Data Safety Monitoring Board. Approval of the study protocol and all related documents was obtained from the appropriate National Competent Authorities, Independent Ethics Committees, and Institutional Review Boards for all study sites. Additional information is provided below and in supplementary files S1 Text (study design and treatment), S2 Text (detailed statistical methods), and S3 Text (trial-associated boards).
Participants
A detailed list of inclusion and exclusion criteria is provided in the published protocol [15]. Briefly, participants were aged >50 years, meeting National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer’s disease Criteria (NINCDS-ADRDA) for diagnosis of probable Alzheimer disease [16], with a Standardised Mini-Mental State Examination (SMMSE) [17] score of ≥12 and <27, and having a caregiver available to complete relevant assessment instruments. If on a cholinesterase inhibitor or memantine, the dose had to be stable for >12 weeks. People with dementia because of other causes or with known sensitivity to calcium channel blockers were excluded.
All participants provided written informed consent before enrolling in the study. The consent form was amended as required in each country to comply with local ethics requirements. All caregivers also provided consent for involvement.
Randomisation and masking
Participants were randomly assigned to nilvadipine sustained-release 8 mg or placebo using block randomisation with randomly varying block sizes, stratified by site, using an online system integrated with stock control across sites. Participants, caregivers, and assessors were blinded to treatment assignment.
Procedures
Participants took a trial treatment capsule once a day after breakfast for 78 weeks and returned their used treatment boxes at subsequent dispensing visits, when the number of returned capsules was recorded. Participants were assessed at 6, 13, 26, 39, 52, 65, and 78 weeks after commencing treatment. Participants were followed up 4 weeks after the final, week 78 visit.
Outcomes
The co-primary outcome measures were the change from baseline in the 12-item Alzheimer Disease Assessment Scale–Cognitive Subscale (ADAS-Cog 12) [18] and the Clinical Dementia Rating scale sum of boxes (CDR-sb) [19]. The key secondary outcome measure was the Disability Assessment for Dementia (DAD) [20], as maintenance of functional abilities is considered a crucial benefit of any potential treatment. Data on all primary and secondary outcome measures were collected at baseline and at 13, 52, and 78 weeks. Safety was assessed through the collection of data on adverse events (AEs), blood pressure, and laboratory tests.
Statistical analysis
The sample size of 250 patients in each group was calculated to allow detection of a 50% reduction in cognitive decline in the nilvadipine group over the 78 weeks of follow-up [15]. This resulted in 90% power to detect a 3.5-point group difference in the decline in ADAS-Cog 12 (SD = 10), and 81% power to also detect a significant effect on the CDR-sb as a gated co-primary end point. The sample size calculation included allowance for 30% loss to follow-up.
The primary and secondary efficacy analyses were conducted in a modified intention-to-treat (mITT) population, including all participants randomised who had both a baseline assessment and at least one later assessment. The safety set included all patients who took at least one dose of the trial treatment.
A secondary per-protocol analysis was carried out using only those patients compliant with medication (defined as taking >80% of doses) and with all assessments on schedule.
The primary and secondary end point analyses consisted of linear mixed-effects models, with country as a random effect and correlated residuals over time. Findings hinged on a p-value less than 0.05 for a (Visit × Arm) interaction test using change scores from baseline and adjusting for the baseline score. We adopted a gated approach to control the false positive rate over multiple end points. The ordered outcomes were as follows: change from baseline of ADAS-Cog 12 (analysed in discrete time); followed by the change in CDR-sb; then, in order, ADAS-Cog 12 and CDR-sb were to be tested for a linear improvement over continuous time. The key secondary outcome of DAD was next on the list, followed by the other secondary outcomes. In the case of a nonsignificant result, any further analyses are purely exploratory, with no further tests of a null hypothesis. Full technical details and description of the gated approach and statistical models are given in the S1 Text file.
Responder analyses were conducted on a dichotomised change score from baseline to week 78 using logistic regression, with no imputation for missing values. Preplanned subgroup analyses included examination of a difference in nilvadipine effect size between mild and moderate Alzheimer disease (≥20 versus <20 on baseline SMMSE, respectively), between males and females, and between Apolipoprotein E gene (APOE) ε4 allele carriers and noncarriers. The latter analysis was limited to the patient subgroup that participated in the blood biomarker study [21]. Subgroup differences in efficacy were examined by a three-way interaction of the subgroup with visit and treatment arms.
Baseline and safety end points were tested by standard tests for proportions (Pearson chi-squared test) or rates (Poisson count model), with no corrections applied for multiple testing.
An independent Data Safety Monitoring Board, blind to group assignment, reviewed safety data throughout the trial.
This trial adhered to the Declaration of Helsinki and International Conference on Harmonisation Good Clinical Practice (ICH GCP) guidelines and was conducted in compliance with the protocol, data protection regulations, and all other regulatory requirements, as appropriate.
Results
Participants
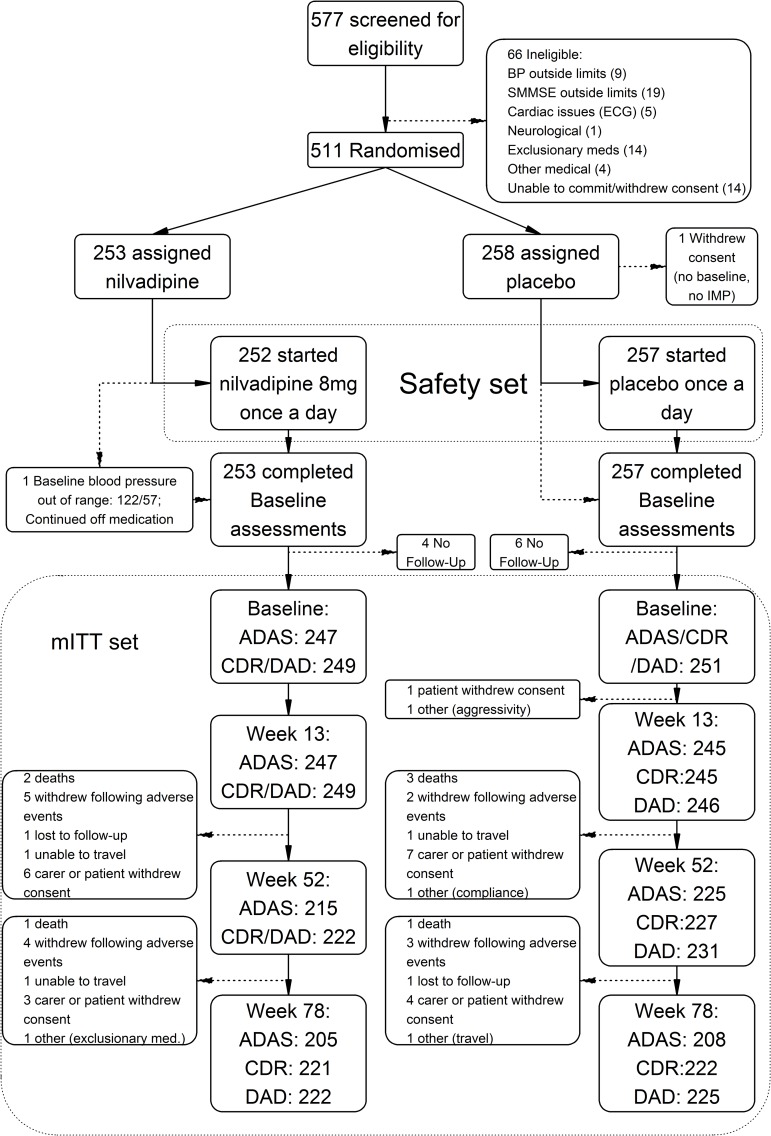
Between 15 May 2013 and 13 April 2015, 511 eligible participants were randomised; the last outcomes visit was in November 2016. Of the 511 randomised, 498 had at least one post-baseline ADAS-Cog 12 assessment and comprised the mITT population (Fig 1), with 247 on nilvadipine and 251 on placebo. The proportion of ADAS-Cog 12 assessments completed was high, allowing us to exceed our sample size target (see Fig 1). Trial medication was interrupted by 103 patients during the course of the study (55 nilvadipine, 48 placebo), of whom 4 resumed medication; mean treatment compliance was 88% (capsules taken over days in study), and 80.4% of patients were compliant with assigned medication at a threshold of 80% of capsules taken, balanced between arms.
Fig 1. Flowchart of the NILVAD study according to the CONSORT guideline.
ADAS, Alzheimer’s Disease Assessment Scale-Cognitive Subscale 12 item; BP, blood pressure; CDR, Clinical Dementia Rating Scale sum of boxes; DAD, Disability Assessment for Dementia; ECG, electrocardiogram; IMP, investigational medicinal product; NILVAD, Nilvadipine in Alzheimer disease; SMMSE, Standardised Mini-Mental State Examination.
Baseline demographic and Alzheimer disease–specific characteristics were similar between treatment groups (Table 1, Table 2). There were no significant differences at baseline or end of trial in the prescribing of Alzheimer disease medications (acetylcholinesterase inhibitors and/or memantine) or non-Alzheimer disease concomitant medications (Table 1). Vascular risk factors, notably hypertension, hypercholesterolemia, and kidney disease, were also similar, with the exception of diabetes, which was more common in the nilvadipine group (Table 1). Comorbid medical conditions at baseline were substantially more prevalent in the nilvadipine group than in the placebo group, and predominantly in the endocrine class, which included diabetes (Table 1). APOE genotype was available from 161 participants in the nilvadipine group and 167 in the placebo group.
Table 1. Characteristics of the modified intention-to-treat sample.
| Characteristics | Nilvadipine (N = 247) |
Placebo (N = 251) |
|
|---|---|---|---|
| Demographics and anthropometrics | |||
| Sex N (%) | Female | 161 (65%) | 147 (59%) |
| Baseline age (years) | mean (SD) | 73.1 (8.66) | 72.8 (7.84) |
| Ethnicity N (%) | White | 241 (98%) | 244 (97%) |
| Asian | 1 (0.4%) | 2 (0.8%) | |
| Black | 1 (0.4%) | 4 (1.6%) | |
| Other | 4 (1.6%) | 1 (0.4%) | |
| Baseline BMI | mean (SD) | 25.3 (3.92) | 25.8 (4.45) |
| Baseline blood pressure | mean (SD) SBP/DBP | 138 (14) / 77 (9) | 137 (14) / 77 (9) |
| Week 13 blood pressure | mean (SD) SBP/DBP | 131 (15) / 73 (9) | 137 (16) / 76 (9) |
| Week 52 blood pressure | mean (SD) SBP/DBP | 131 (15) / 74 (9) | 135 (14) / 76 (9) |
| Week 78 blood pressure | mean (SD) SBP/DBP | 132 (16) / 74 (10) | 135 (16) / 75 (9) |
| Baseline vascular risk factors | |||
| Diabetes | 28 (12%) | 12 (5%) | |
| Kidney disease | 3 (1%) | 5 (2%) | |
| Hypercholesterolemia | 82 (34%) | 83 (33%) | |
| Hypertension | 82 (34%) | 87 (35%) | |
| Baseline blood pressure (median SBP / DBP) | 140 / 77 | 138 / 77 | |
| Week 78 blood pressure (median SBP / DBP) | 130 / 74 | 135 / 74 | |
| Baseline medical status | |||
| Patients on AD concomitant medications | 173 (69%) on 1; 65 (26%) on 2+ | 170 (66%) on 1; 75 (29%) on 2+ | |
| Patients on non-AD concomitant medications | 221 (88%) | 219 (85%) | |
| Comorbid medical conditions (per patient) | mean (SD) | 2.84 (2.09) | 2.43 (1.87) |
Data are mean (standard deviation), median (IQR: first and third quartiles), n (%), or n/N (%).
Abbreviations: AD, Alzheimer disease; BMI, body mass index; DBP, diastolic blood pressure; IQR, interquartile range; SBP, systolic blood pressure.
Table 2. Baseline Alzheimer disease characteristics.
| Characteristics | Sample statistics | Nilvadipine (N = 247) |
Placebo (N = 251) |
|---|---|---|---|
| Years since diagnosis | mean (SD) | 1.73 (1.66) | 1.70 (1.78) |
| Years since symptom onset | mean (SD) | 4.31 (2.56) | 4.28 (2.72) |
| APOE ε4 carrier | n/N (%) | 94/161 (58%) | 100/167 (60%) |
| SMMSE | mean (SD) | 20.3 (3.76) | 20.5 (3.89) |
| SMMSE < 20 | N (%) | 93 (38%) | 94 (37%) |
| Baseline ADAS-Cog 12 | mean (SD) | 34.4 (10.5) | 34.5 (10.8) |
| Baseline CDR-sb (N = 249 + 251) | mean (SD) | 5.34 (2.76) | 5.17 (2.73) |
| Baseline DAD (N = 249 + 251) | mean (SD) | 29.7 (8.0) | 30.4 (8.1) |
Data are mean (standard deviation), n (%), or n/N (%).
Abbreviations: ε4, epsilon 4 allele; ADAS-Cog 12, Alzheimer Disease Assessment Scale–Cognitive Subscale (12 item); APOE, Apolipoprotein E gene; CDR-sb, Clinical Dementia Rating sum of boxes; DAD, Disability Assessment for Dementia; SMMSE, Standardised Mini-Mental State Examination.
Efficacy end points
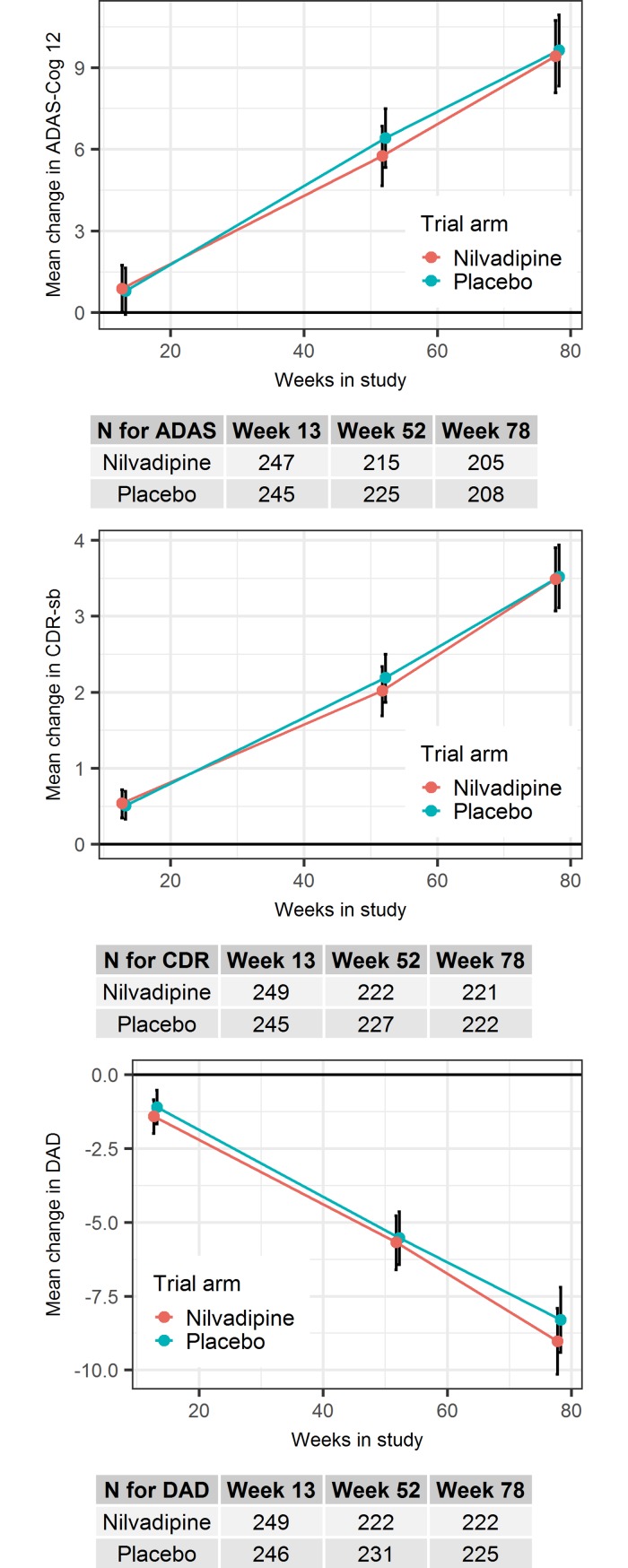
No treatment effect was observed at a statistically significant level for the first primary outcome analysis (p = 0.465). The nilvadipine difference from placebo, in change from baseline in the ADAS-Cog 12 score, was −0.22 (95% CI, −2.01–1.57) (Table 3). Similarly, nilvadipine did not show any clinically meaningful effects on CDR-sb and DAD (Table 3, Fig 2).
Table 3. Efficacy analyses for primary outcomes (ADAS-Cog 12 and CDR-sb), and the key secondary outcome (DAD).
| Outcomes | Week 0 | Week 13 | Week 52 | Week 78 | p-value* | ||
|---|---|---|---|---|---|---|---|
| ADAS-Cog 12Ɨ | Nilvadipine | Mean ± SD | 34.4 ± 10.5 | 35.2 ± 11.3 | 39.4 ± 13.1 | 41.9 ± 14.6 | |
| Δ (95% CI) | 0.88 (0.02–1.74) | 5.75 (4.66–6.85) | 9.41 (8.09–10.73) | ||||
| Placebo | Mean ± SD | 34.5 ± 10.8 | 35.3 ± 11.6 | 40.3 ± 13.9 | 41.9 ± 14.5 | ||
| Δ (95% CI) | 0.79 (−0.07–1.64) | 6.41 (5.33–7.49) | 9.63 (8.33–10.93) | ||||
| Group difference | 0.09 (−0.96–1.15) | −0.65 (−2.10–0.80) | −0.22 (−2.01–1.57) | 0.465 | |||
| CDR-sbǂ | Nilvadipine | Mean ± SD | 5.34 ± 2.76 | 5.87 ± 2.96 | 7.29 ± 3.69 | 8.72 ± 4.60 | |
| Δ (95% CI) | 0.54 (0.35–0.72) | 2.02 (1.69–2.34) | 3.49 (3.07–3.90) | ||||
| Placebo | Mean ± SD | 5.17 ± 2.73 | 5.71 ± 3.17 | 7.22 ± 4.02 | 8.38 ± 4.45 | ||
| Δ (95% CI) | 0.51 (0.33–0.70) | 2.19 (1.87–2.50) | 3.52 (3.11–3.94) | ||||
| Group difference | 0.02 (−0.25–0.29) | −0.17 (−0.62–0.28) | −0.04 (−0.62–0.55) | N/A | |||
| DAD§ | Nilvadipine | Mean ± SD | 29.7 ± 8.0 | 28.4 ± 8.2 | 24.3 ± 10.2 | 21.1 ± 11.5 | |
| Δ (95% CI) | −1.42 (−1.99–−0.85) | −5.68 (−6.60–−4.77) | −9.02 (−10.14–−7.91) | ||||
| Placebo | Mean ± SD | 30.4 ± 8.1 | 29.2 ± 8.6 | 25.1 ± 10.4 | 22.8 ± 11.3 | ||
| Δ (95% CI) | −1.10 (−1.67–−0.52) | −5.53 (−6.43–−4.64) | −8.30 (−9.40–−7.20) | ||||
| Group difference | −0.32 (−1.13–0.49) | −0.15 (−1.43–1.13) | −0.73 (−2.29–0.84) | N/A¶ | |||
| ADAS-Cog 12 | Placebo time trend (per week) | 0.120 (0.109–0.132) | N/A¶ | ||||
| Nilvadipine change in trend | −0.002 (−0.019–0.014) | ||||||
| CDR-sb | Placebo time trend (per week) | 0.043 (0.040–0.047) | N/A¶ | ||||
| Nilvadipine change in trend | 0.0005 (−0.005–0.005) | ||||||
Δ Change from baseline.
*From F test for Visit × Arm interaction term. All models were controlled for baseline measurement and included country as a random effect and unstructured correlation between time points.
ƗScores on the ADAS-Cog 12 range from 0 to 80, with higher scores indicating greater cognitive impairment [18].
ǂScores on the CDR-sb range from 0 to 18, with higher scores indicating worse functioning [19].
§Scores on the DAD range from 0 to 100, with higher scores indicating less impairment [20].
¶ After the nonsignificant outcome on the primary outcome ADAS-Cog 12, all other primary and secondary gated outcomes are not judged for significance, as defined by the preplanned analysis.
Abbreviations: ADAS-Cog 12, Alzheimer's Disease Assessment Scale Cognitive-12; CDR-sb, Clinical Dementia Rating Scale sum of boxes; DAD, Disability Assessment for Dementia.
Fig 2. Estimated marginal means and 95% confidence intervals of the per-visit change from baseline in cognition and functional performance, as measured by the primary and key secondary outcomes, respectively.
ADAS/ADAS-Cog 12, Alzheimer's Disease Assessment Scale Cognitive-12; CDR/CDR-sb, Clinical Dementia Rating Scale sum of boxes; DAD, Disability Assessment for Dementia.
Per-protocol analyses showed identical patterns to the primary analysis. The prespecified responder analysis showed no effects of nilvadipine on the proportion of patients maintaining cognition or function as measured by the ADAS-Cog 12: odds ratio 1.09 (95% CI, 0.65–1.84), the CDR-sb: odds ratio 1.74 (95% CI, 0.99–3.06), or the DAD: odds ratio 0.90 (95% CI, 0.54–1.51).
The predefined subgroup analyses were inspected to identify group differences (S2 Table, S3 Table, S4 Table); we note that no hypothesis tests were performed for these exploratory analyses. Comparing those with mild to those with moderate Alzheimer disease, there was less decline in the mild group on nilvadipine compared to placebo. However, a greater decline was seen in the moderate group treated with nilvadipine. For gender, males showed less decline than females on nilvadipine compared to placebo. Furthermore, APOE ε4 allele carriers showed less decline than noncarriers on nilvadipine (S2 Table, S3 Table, S4 Table).
Safety
Participants who received at least one dose of the study drug comprised the safety population (n = 509). Despite a higher total number of AEs or serious adverse events (SAEs) in the nilvadipine group (Table 4) the number of patients with at least one AE or SAE were substantially similar. The median change in systolic blood pressure from baseline to week 78 was −5 mmHg and the number of falls, complaints of dizziness, or syncope were very similar between groups (Table 4). The number of deaths was 10 (7participants died during the study duration and a further 3 during the longer-term follow-up of SAEs). No deaths were judged by the investigators to be related to treatment. Emergent clinically significant blood test results on nilvadipine and placebo from baseline to week 78 were too rare to draw conclusions but were not elevated in the nilvadipine group. Between-group differences were observed on aggregated significant and nonclinically significant abnormal blood markers; these reflected more elevated results on placebo at trial end for creatinine (9%–13%) and calcium (7%–11%), or fewer elevated results on nilvadipine for mean corpuscular volume (MCV) results (10%–7%) (S5 Table). A comparison of the Medical Dictionary for Regulatory Activities (MedDRA)-coded AEs (S6 Table) showed small differences (<6%) between groups for the following events: fall (worse on placebo), cough, cellulitis, peripheral edema, insomnia, and hypotension.
Table 4. Safety end points.
| Characteristics | Nilvadipine (N = 252) |
Placebo (N = 257) |
|---|---|---|
| Adverse Events | ||
| Total logged | 1,129 | 1,030 |
| Possibly, Probably, or Definitely related to IMP | 223 | 178 |
| Patients | 206 (82%) | 201 (78%) |
| Patients with Possibly, Probably, or Definitely related | 142 (56%) | 145 (56%) |
| Patients with Dizziness | 30 (12%) | 29 (11%) |
| Patients with Fall | 40 (16%) | 38 (15%) |
| Patients with Fracture | 16 (6%) | 9 (4%) |
| Patients with Peripheral edema | 15 (6%) | 3 (1%) |
| Patients with Syncope | 12 (5%) | 10 (4%) |
| Serious Adverse Events | ||
| Total logged | 146 | 101 |
| Possibly, Probably, or Definitely related to IMP | 17 | 19 |
| Patients | 50 (20%) | 42 (16%) |
| Patients with Possibly, Probably, or Definitely related | 7 (3%) | 9 (4%) |
| MortalityƗ | 3 (1.2%) | 4 (1.6%) |
ƗThree further individuals died at or after week 82 of the study (all in the placebo group).
Note that patients are counted if they had one or more events of the type listed.
Abbreviations: CDR, CDR-sb (Clinical Dementia Rating sum of boxes); DAD, Disability Assessment for Dementia; DBP, diastolic blood pressure; IMP, investigational medicinal product; mITT, modified intention-to-treat; SBP, systolic blood pressure; SMMSE, Standardised Mini-Mental State Examination.
Discussion
To our knowledge, this is the first definitive intervention study of nilvadipine, a DHP calcium channel blocker with demonstrated Aβ-lowering properties in animal studies, for the treatment of Alzheimer disease. The results of this study indicated no benefit of nilvadipine as a treatment in a population spanning mild to moderate Alzheimer disease. There were no obvious methodological limitations that could have contributed to these negative findings for the primary and secondary outcomes in the overall treatment population. Recruitment was to target, the dropout and missing data rates were low. The rate of decline in the placebo group on the ADAS-Cog 12 was consistent with previous Phase III clinical trials involving mild to moderate Alzheimer disease participants. Treatment and placebo arms were well balanced, although there were more patients with abnormal glucose levels and with diabetes in the nilvadipine group at baseline. The higher frequency of diabetes in the nilvadipine group is unlikely to have had a bearing on the overall negative finding, as the effect of diabetes on cognitive decline in established Alzheimer disease is unclear [22]. Furthermore, data from a sub-study confirm that there was no significant imbalance between the nilvadipine and the placebo groups in terms of antihypertensive use (J. Claassen & M.G.M. Olde Rikkert, personal communication, see S4 Text).
The overall safety and AE profile for nilvadipine was favourable in this older population. There was no significant difference in the number of deaths, AEs, or SAEs that could be attributed to treatment. Blood pressure effects were modest, with only a median 5 mmHg drop in systolic blood pressure from baseline to week 78 in the nilvadipine treated group.
The findings from the predefined subgroup analyses suggest differential effects of nilvadipine in those at a milder disease stage, in APOE ε4 allele carriers, and in males. However, no significance tests were conducted on these subgroups, and these findings will require further investigation to determine if there are specific subgroups within the overall population that respond either positively or negatively to nilvadipine treatment. For instance, consistent with other anti-amyloid treatment trials suggesting that milder patients may respond better [23], in these exploratory analyses, those with an SMMSE >20 appeared to decline at a slower rate than those with an SMMSE <20. However, greater decline on the ADAS-Cog 12 in moderate-stage patients on nilvadipine treatment should also be noted. Similarly, the gender and APOE ε4 allele carrier results warrant further exploration, although the number of patients participating in the APOE study (64%) was fewer than the overall treatment population. Further exploratory analyses, making use of the sub-study data, will look for correlation between biomarkers (in both blood and cerebrospinal fluid [CSF]), cerebral blood flow, and other brain imaging data to better understand whether specific mechanisms, e.g., via a blood pressure–lowering pathway or changes in Aβ or tau correlate with cognitive change.
The strengths of this investigator-driven clinical trial include the successful recruitment and retention of participants and the conduct of the study to a high standard. There are, however, a number of issues related to the study design that could be considered for future trials of this nature that are suggested by our main findings. Firstly, a single-dose strategy was used, and it is possible that an insufficient dose was given to effect a treatment response. The side effect profile for nilvadipine in this older, mild to moderate Alzheimer disease population was favourable and the effect on blood pressure quite modest, so it would probably have been safe to give a higher dose. While we predicted that any effect of nilvadipine on cognition would be via an anti-amyloid rather than a blood pressure–lowering pathway, it is possible that a lack of benefit in the overall population may have been contributed to by the modest blood pressure–lowering effect of nilvadipine in this study. Secondly, the lack of biomarker confirmation of the diagnosis of Alzheimer disease, which could mean that up to 20% of patients included in the trial may not have had significant amyloid pathology [24], could be taken into account in the design of future trials of this nature. A third issue to consider is the timing of the intervention in the course of Alzheimer disease. Many anti-amyloid treatments have failed in populations with established mild to moderate Alzheimer disease, and it is a commonly held belief that it may be too late to treat established dementia with amyloid-lowering drugs when there is already associated significant neuronal damage [25]. Similarly, if cerebral hypoperfusion triggers or accelerates the deposition of amyloid pathology, intervention with a drug that can improve cerebral blood flow should occur at the earliest possible stage if it is to be effective as a disease-modifying agent. The latter two limitations reflect the rapidly evolving evidence over recent years since this study was designed, highlighting the ability and necessity of more detailed phenotyping and a focus on earlier-stage intervention. Treatment at the prodromal stage of the Alzheimer disease process might therefore be a more successful point at which to intervene with nilvadipine.
Conclusions
This study of Nilvadipine at a dose of 8 mg found no overall effect on slowing the rate of cognitive decline in a population spanning mild to moderate Alzheimer disease.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
ADAS-Cog 12, Alzheimer's Disease Assessment Scale Cognitive-12.
(DOCX)
ε4, epsilon 4 allele; ADAS-Cog 12, Alzheimer's Disease Assessment Scale Cognitive-12; APOE, Apolipoprotein E gene.
(DOCX)
ADAS-Cog 12, Alzheimer's Disease Assessment Scale Cognitive-12.
(DOCX)
(DOCX)
MedDRA, Medical Dictionary for Regulatory Activities.
(XLSX)
(DOCX)
Acknowledgments
NILVAD Study Group
Brian Lawlor, Mercer’s Institute for Research on Ageing, St. James’s Hospital and Department of Medical Gerontology, Trinity College, Dublin, Ireland; Ricardo Segurado, CSTAR and School of Public Health, Physiotherapy and Sport Science, University College Dublin (UCD), Dublin, Ireland; Sean Kennelly, Department of Age Related Healthcare, Tallaght Hospital, Dublin 24 and Department of Medical Gerontology, Trinity College Dublin; Marcel G. M. Olde Rikkert, Department of Geriatric Medicine, Radboudumc Alzheimer Center, Donders Institute of Medical Neurosciences, Radboudumc, Nijmegen, the Netherlands; Robert Howard, Division of Psychiatry, University College London and King’s College London; Florence Pasquier, CHU Lille, Univ. Lille, DISTALZ Laboratory of Excellence, F-59000 Lille, France; Anne Börjesson-Hanson, Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, Sahlgrenska Academy, University of Gothenburg; Magda Tsolaki, Papanikolaou General Hospital of Thessaloniki, Greece; Ugo Lucca, Laboratory of Geriatric Neuropsychiatry, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy; D. William Molloy, University College Cork Centre for Gerontology and Rehabilitation, Cork, Ireland; Robert Coen, Mercer's Institute for Research on Ageing, St. James's Hospital, Dublin, Ireland; Matthias W. Riepe, Department of Geriatrics and Old Age Psychiatry, Psychiatry II, Ulm University at BKH Günzburg, Germany; János Kálmán, Department of Psychiatry, University of Szeged, Hungary; Rose Anne Kenny, Department of Medical Gerontology, Trinity College Dublin (TCD), Dublin, Ireland; Fiona Cregg, Department of Medical Gerontology, Trinity College Dublin (TCD), Dublin, Ireland; Sarah O'Dwyer, Mercer's Institute for Research on Ageing, St. James's Hospital, Dublin, Ireland; Cathal Walsh, Health Research Institute and MACSI, Department of Mathematics and Statistics, University of Limerick, Ireland; Jessica Adams, Department of Old Age Psychiatry, King's College London; Rita Banzi, Laboratory of Geriatric Neuropsychiatry, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy; Laetitia Breuilh, CHU Lille, Univ. Lille, DISTALZ Laboratory of Excellence, F-59000 Lille, France; Leslie Daly, CSTAR and School of Public Health, Physiotherapy and Sport Science, University College Dublin (UCD), Dublin, Ireland; Suzanne Hendrix, Pentara Corporation, 2180 Claybourne Avenue, Salt Lake City, Utah; Paul Aisen, Department of Neurology, University of Southern California; Siobhan Gaynor, Molecular Medicine Ireland (MMI), Dublin, Ireland; Ali Sheikhi, Health Research Institute and MACSI, Department of Mathematics and Statistics, University of Limerick, Ireland; Diana G. Taekema, Department of Geriatric Medicine, Rijnstate Hospital, Arnhem, the Netherlands; Frans R. Verhey, Department of Psychiatry and Neuropsychology, School of Mental Health and Neuroscience, Alzheimer Center Limburg, Maastricht University, Maastricht, the Netherlands; Raffaello Nemni, IRCCS Don Gnocchi Foundation-University of Milan, Italy; Flavio Nobili, Dept. of Neuroscience (DINOGMI), University of Genoa, and IRCCS AOU Polyclinic, Hospital San Martino, Genoa, Italy; Massimo Franceschi, Neurology Department, Multimedica, Castellanza, Italy; Giovanni Frisoni, Centro San Giovanni di Dio—IRCCS Fatebenefratelli, Brescia, Italy; Orazio Zanetti, Centro San Giovanni di Dio—IRCCS Fatebenefratelli, Brescia, Italy; Anastasia Konsta, Aristotle University of Thessaloniki (AUTH), First Psychiatric Department, Papageorgiou General Hospital, Greece; Orologas Anastasios, Ahepa University General Hospital of Thessaloniki, Greece; Styliani Nenopoulou, Papanikolaou General Hospital of Thessaloniki, Greece; Fani Tsolaki-Tagaraki, Papanikolaou General Hospital of Thessaloniki, Greece; Magdolna Pakaski, Department of Psychiatry, University of Szeged, Hungary; Olivier Dereeper, Centre Hospitalier de Calais, France; Vincent de la Sayette, Centre Hospitalier Universitaire de Caen, France; Olivier Sénéchal, Centre Hospitalier de Lens, France; Isabelle Lavenu, Centre Hospitalier de Béthune, France; Agnès Devendeville, Centre Hospitalier Universitaire d'Amiens, France; Gauthier Calais, Groupement des Hôpitaux de l'Institut Catholique de Lille (GHICL), France; Fiona Crawford, Archer Pharmaceuticals, Sarasota, Florida, and Roskamp Institute, Sarasota, Florida; Michael Mullan, Archer Pharmaceuticals, Sarasota, Florida, and Roskamp Institute, Sarasota, Florida, Pauline Aalten, PhD, Department of Psychiatry and Neuropsychology, School of Mental Health and Neurosciences, Alzheimer Center Limburg, Maastricht University, Maastricht, the Netherlands; Maria A. Berglund, RN, Sahlgrenska University Hospital, Gothenburg, Sweden; Jurgen A. Claassen MD, PhD, Department of Geriatric Medicine, Radboudumc Alzheimer Center, Donders Institute of Medical Neurosciences, Radboudumc, Nijmegen, the Netherlands; Rianne A. De Heus, MSc, Department of Geriatric Medicine, Radboudumc Alzheimer Center, Donders Institute of Medical Neurosciences, Radboudumc, Nijmegen, the Netherlands; Daan L. K. De Jong, MSc, Department of Geriatric Medicine, Radboudumc Alzheimer Center, Donders Institute of Medical Neurosciences, Radboudumc, Nijmegen, the Netherlands; Olivier Godefroy, MD, PhD, Centre Hospitalier Universitaire d'Amiens, France; Siobhan Hutchinson, MD, St. James's Hospital, Dublin, Ireland; Aikaterini Ioannou, MD, 1st Department of Neurology, Ahepa University General Hospital, Aristotle University of Thessaloniki, Greece; Michael Jonsson, MD, PhD, Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, Sahlgrenska Academy, University of Gothenburg, Sweden; Annette Kent, PhD, Trinity College Dublin (TCD), Dublin, Ireland; Jürgen Kern MD, PhD, Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, Sahlgrenska Academy, University of Gothenburg, Sweden; Petros Nemtsas MD, PhD, 1st Department of Neurology, Ahepa University General Hospital, Aristotle University of Thessaloniki, Greece; Minoa-Kalliopi Panidou, BSc, MA, 1st Department of Neurology, Ahepa University General Hospital, Aristotle University of Thessaloniki, Greece; Laila Abdullah, PhD, Roskamp Institute, Sarasota, Florida; Daniel Paris, PhD, Roskamp Institute, Sarasota, Florida; Angelina M. Santoso, MD, MSc, Department of Geriatric Medicine, Radboudumc Alzheimer Center, Donders Institute of Medical Neurosciences, Radboudumc, Nijmegen, the Netherlands; Gerrita J. van Spijker, MSc, Department of Geriatric Medicine, Radboudumc Alzheimer Center, Donders Institute of Medical Neurosciences, Radboudumc, Nijmegen, the Netherlands; Martha Spiliotou MD, PhD, 1st Department of Neurology, Ahepa University General Hospital, Aristotle University of Thessaloniki, Greece; Georgia Thomoglou, BSc, 1st Department of Neurology, Ahepa University General Hospital, Aristotle University of Thessaloniki, Greece; and Anders Wallin, MD, Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, Sahlgrenska Academy, University of Gothenburg, Sweden.
Additional acknowledgments
We gratefully acknowledge the support of the following individuals to the conduct of the NILVAD trial at the study sites: William G. Aalst, MSc, Department of Geriatric Medicine, Radboudumc Alzheimer Center, Donders Institute of Medical Neurosciences, Radboudumc, Nijmegen, the Netherlands; Catherine Adnet, MD, Meotis Regional Network & Centre Hospitalier Universitaire Lille, France; Ghania Ait-Ghezala, PhD, Roskamp Institute, Sarasota, Florida; Margherita Alberoni, MD, IRCCS Don Gnocchi Foundation, Italy; Monica Almici, MS, Centro San Giovanni di Dio—IRCCS Fatebenefratelli, Brescia, Italy; Lauren Armstrong, Department of Psychology, King’s College London, UK; Barna Babik, MD, PhD, University of Szeged, Department of Anaesthesiology and Intensive Therapy, Hungary; Kaj Blennow, MD, PhD, Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, Sahlgrenska Academy, University of Gothenburg, Neurochemistry Laboratory, Sahlgrenska University Hospital/Mölndal, 431 80 Mölndal, Sweden; Stéphanie Bombois, MD, PhD, Inserm 1171, Centre Hospitalier Universitaire Lille, France; Gábor Borbás, BSc, University of Szeged, Department of Psychiatry, Hungary; Justine Boutantin, PsyD, Centre Hospitalier Universitaire Lille, France; Eva Bringman, RN, Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, Sahlgrenska Academy, University of Gothenburg, Sweden; Elena Calabrese, MD, IRCCS Don Gnocchi Foundation, Italy; Mareeta Calnan, RN, University College Cork Centre for Gerontology and Rehabilitation, Cork, Ireland; Fiona Campbell, MB, Mercer's Institute for Research on Ageing, St. James's Hospital, Dublin, Ireland; Pascaline Cassagnaud, MD, Inserm 1171, Centre Hospitalier Universitaire Lille, France; Lisa Crosby, RGN, Mercer's Institute for Research on Ageing, St. James's Hospital, Dublin, Ireland; Danira Damiani, MS, Laboratory of Geriatric Neuropsychiatry, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy; Alessandra D'Amico, MS, IRCCS Don Gnocchi Foundation, Italy; Silvia De Battistii, MSc, Neurology Department, Multimedica, Castellanza, Italy; Xavier Delbeuck, PsyD, PhD, Centre Hospitalier Universitaire Lille, France; Vincent Deramecourt, MD, PhD, Univ. Lille, Inserm 1171, Centre Hospitalier Universitaire Lille, France; Rosa Di Costanzo, MS, Laboratory of Geriatric Neuropsychiatry, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy; Noel Ellison, PhD, Pentara Corporation, Salt Lake City, Utah; Elisabetta Farina, MD, IRCCS Don Gnocchi Foundation, Italy; Michela Ferrara, MD, Dept. of Neuroscience (DINOGMI), University of Genoa, and IRCCS AOU San Martino-IST, Genoa, Italy; Samantha Galluzzi, MD, Centro San Giovanni di Dio—IRCCS Fatebenefratelli, Brescia, Italy; Sara Gipponi, MS, Centro San Giovanni di Dio—IRCCS Fatebenefratelli, Brescia, Italy; Nicola Girtler, PsyD, Dept. of Neuroscience (DINOGMI), University of Genoa, and IRCCS AOU San Martino-IST, Genoa, Italy; Maria Grazia Buratti, MS, Laboratory of Geriatric Neuropsychiatry, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy; Hannah Grocott, Department of Old Age Psychiatry, King's College London, UK;
Danique Hellebrekers, MSc, Department of Psychiatry and Neuropsychology, School of Mental Health and Neurosciences, Alzheimer Center Limburg, Maastricht University, Maastricht, the Netherlands; Ing-Marie Isgaard, R.N., Memory Clinic Department of Neuropsychiatry, Sahlgrenska University Hospital, Wallinsgatan 6, SE-431 41 Mölndal, Sweden; Robin Jacoby, D.M., F.R.C.P., F.R.C.Psych., The University of Oxford Department of Psychiatry, The Warneford Hospital, Oxford, UK; Anikó Jász, BA, University of Szeged, Department of Psychiatry, Hungary; Sára Kálmán, MD, PhD, University of Szeged, Department of Psychiatry, Hungary; Joanna Kelly, Clinical Trials Unit, King's College London, Institute of Psychiatry, London, UK; Sinead Larkin, Trinity College Dublin (TCD), Dublin, Ireland; Susan Lennon, Molecular Medicine Ireland (MMI), Dublin, Ireland; Alexandra Leroy, PsyD, Centre Hospitalier Universitaire Lille, France; Marie-Anne Mackowiak, MD, Inserm 1171, Centre Hospitalier Universitaire Lille, France; Ulla-Britt Mattsson, MD, Sahlgrenska University Hospital, Sweden; Kevin McCarroll, MD, Mercer's Institute for Research on Ageing, St. James's Hospital, Dublin, Ireland; Hannah McCarthy, PhD, Trinity College Dublin (TCD), Dublin, Ireland; Olga V. Meulenbroek, PhD, Department of Geriatric Medicine, Radboudumc Alzheimer Center, Donders Institute of Medical Neurosciences, Radboudumc, Nijmegen, the Netherlands; Caroline Murphy, MSc, Clinical Trials Unit, King's College London, Institute of Psychiatry, London, UK; Vonnie Nally, M.Sc., Trinity College Dublin (TCD), Dublin, Ireland; Maurice O'Connell, The Alzheimer Society of Ireland, Temple Road, Blackrock, Ireland; Maura Parapini, MS, Centro San Giovanni di Dio—IRCCS Fatebenefratelli, Brescia; Oriana Pelati, MSc, Neurology Department, Multimedica, Castellanza; Marianne Pollet, PsyD, Centre Hospitalier Universitaire Lille, France; Astrid Quist, MSc, Department of Psychiatry and Neuropsychology, School of Mental Health and Neurosciences, Alzheimer Center Limburg, Maastricht University, Maastricht, the Netherlands; Jennifer Rogers, MSc, Trinity College Dublin (TCD), Dublin, Ireland; Adeline Rollin, MD, Inserm 1171, Centre Hospitalier Universitaire Lille, France; Johan Sandelin, MD, Department of Neuropsychiatry, Sahlgrenska University Hospital, Wallinsgatan 6, SE-431 41 Mölndal, Sweden; Susanna Scioli, MS, IRCCS Don Gnocchi Foundation, Italy; Elena Seletti, MS, Laboratory of Geriatric Neuropsychiatry, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy; Anne-Cécile Troussiere, MD, Centre Hospitalier Universitaire Lille, France; Anne van der Vorst, MSc, Department of Psychiatry and Neuropsychology, School of Mental Health and Neurosciences, Alzheimer Center Limburg, Maastricht University, Maastricht, the Netherlands; Olivier Vercruysse, MD, Centre Hospitalier Universitaire Lille, France; Frances Verholen, MSc, Department of Geriatric Medicine, Rijnstate Hospital Arnhem, the Netherlands; Laura E. Versteeg, MSc, Department of Geriatric Medicine, Radboudumc Alzheimer Center, Donders Institute of Medical Neurosciences, Radboudumc, Nijmegen, the Netherlands; and Marta Zuffi, MD, Neurology Department, Multimedica, Castellanza, Italy.
The authors also wish to thank all other study partners: Alzheimer Europe and Newsweaver, which helped with dissemination and promotion of the trial, and GABO:mi Gesellschaft für Ablauforganisation: milliarium mbH & Co. KG, which was the project management company for the majority of the trial. The authors especially wish to thank all of the patients and caregivers who so generously gave of their time. We also acknowledge the time and effort contributed by Muireann O'Briain; Oliver Gupta; the members of the Ethics Advisory Board (Ursula Collins, Mary Donnelly, Tony O’Brien, and Shaun O’Keefe); the members of Scientific Advisory Board (Paul Aisen, Suzanne Hendrix, Robin Jacoby, and Maurice O’Connell); and the members of the Data Safety Monitoring Board (Bernadette McGuinness, John Newell, Martin O’Donnell, and Peter Passmore).
Abbreviations
- Aβ40
Amyloid beta 40 amino acid peptide
- Aβ42
Amyloid beta 42 amino acid peptide
- ADAS-Cog 12
Alzheimer's Disease Assessment Scale Cognitive Subscale-12
- AE
adverse event
- APOE
Apolipoprotein E gene
- CDR-sb
Clinical Dementia Rating Scale sum of boxes
- CSF
cerebrospinal fluid
- DAD
Disability Assessment for Dementia
- DBP
diastolic blood pressure
- DHP
dihydropyridine
- ECG
electrocardiogram
- ICH GCP
International Conference on Harmonisation Good Clinical Practice
- IMP
investigational medicinal product
- IQR
interquartile range
- MCV
mean corpuscular volume
- MedDRA
Medical Dictionary for Regulatory Activities
- mITT
modified intention-to-treat
- NINCDS-ADRDA
National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer’s disease Criteria
- SAE
serious adverse event; SBP, systolic blood pressure
- SMMSE
Standardised Mini-Mental State Examination
- Syst-Eur
Systolic Hypertension in Europe
- UCD CSTAR
University College Dublin Centre for Support and Training in Analysis and Research
Data Availability
In accordance with Irish and European data protection law, the terms under which ethical approval for the trial were granted, and the consortium agreement entered into by the NILVAD centres, we are unable to make public any patients’ personal data, even deidentified. Researchers interested in access to the trial data may contact the Trinity College Dublin officer for Data and Material Transfer Agreements, Emily Vereker at Trinity Research and Innovation, O’Reilly Institute, Trinity College, Dublin 2, Ireland (https://www.tcd.ie/innovation/exchange/technologies/) (verekee@tcd.ie), to apply for access.
Funding Statement
This work was funded by the European Commission (https://ec.europa.eu/research/fp7/index_en.cfm) under grant agreement number 279093 to principal investigator BL. Additional funding not specifically for this trial was received from UCLH NIHR Biomedical Research Centre, UK (RH), and Hauts-de-Freance Region, France (FP). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Rouch L, Cestac P, Hanon O, Cool C, Helmer C, Bouhanick B, et al. Antihypertensive drugs, prevention of cognitive decline and dementia: a systematic review of observational studies, randomized controlled trials and meta-analyses, with discussion of potential mechanisms. CNS Drugs. 2015. Feb; 29(2):113–130. 10.1007/s40263-015-0230-6 [DOI] [PubMed] [Google Scholar]
- 2.Hoffman LB, Schmeidler J, Lesser GT, Beeri MS, Purohit DP, Grossman HT, et al. Less Alzheimer disease neuropathology in medicated hypertensive than nonhypertensive persons. Neurology. 2009. May 19; 72(20):1720–1726 10.1212/01.wnl.0000345881.82856.d5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hajjar I, Brown L, Mack WJ, Chui H. Impact of Angiotensin receptor blockers on Alzheimer disease neuropathology in a large brain autopsy series. Arch Neurol. 2012. December; 69(12):1632–1638. 10.1001/archneurol.2012.1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paris D, Bachmeier C, Patel N, Quadros A, Volmar CH, Laporte V, et al. Selective Antihypertensive Dihydropyridines Lower Aβ Accumulation by Targeting both the Production and the Clearance of Aβ across the Blood-Brain Barrier. Mol Med. 2011; 17:149–162. 10.2119/molmed.2010.00180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachmeier C, Beaulieu-Abdelahad D, Mullan M, Paris D. Selective dihydropyridine compounds facilitate the clearance of β-amyloid across the blood-brain barrier. Eur J Pharmacol. 2011; 659:124–129. 10.1016/j.ejphar.2011.03.048 [DOI] [PubMed] [Google Scholar]
- 6.Iwasaki Y, Asai M, Yoshida M, Nigawara T, Kambayashi M, Oiso Y, et al. Nilvadipine inhibits nuclear factor-kappa B-dependent transcription in hepatic cells. Clin Chim Acta. 2004; 350:151–157. 10.1016/j.cccn.2004.07.012 [DOI] [PubMed] [Google Scholar]
- 7.Kagawa H, Nomura S, Ozaki Y, Nagahama M, Fukuhara S. Effects of nilvadipine on cytokine levels and soluble factors in collagen disease complicated with essential hypertension. Clin Exp Hypertens. 1999; 21:1177–1188. [DOI] [PubMed] [Google Scholar]
- 8.Paris D, Ait-Ghezala G, Bachmeier C, Laco G, Beaulieu-Abdelahad D, Lin Y, et al. The spleen tyrosine kinase (Syk) regulates Alzheimer amyloid-β production and Tau hyperphosphorylation. J Biol Chem. 2014; 289:33927–33944. 10.1074/jbc.M114.608091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paris D, Quadros A, Humphrey J, Patel N, Crescentini R, Crawford F, et al. Nilvadipine antagonizes both Abeta vasoactivity in isolated arteries, and the reduced cerebral blood flow in APPsw transgenic mice. Brain Res. 2004; 999:53–61. [DOI] [PubMed] [Google Scholar]
- 10.Hanyu H, Hirao K, Shimizu S, Sato T, Kiuchi A, Iwamoto T. Nilvadipine prevents cognitive decline in patients with mild cognitive impairment. Int J Geriatr Psychiatry. 2007; 22:1264–1266. 10.1002/gps.1851 [DOI] [PubMed] [Google Scholar]
- 11.Kennelly SP, Abdullah L, Paris D, Parish J, Mathura V, Mullan M, et al. Demonstration of safety in Alzheimer's patients for intervention with an anti-hypertensive drug Nilvadipine: results from a 6-week open label study. Int J Geriatr Psychiatry. 2011; 10:1038–1045. [DOI] [PubMed] [Google Scholar]
- 12.Forette F, Seux ML, Staessen JA, Thijs L, Birkenhäger WH, Babarskiene MR, et al. Prevention of dementia in randomised double-blind placebo-controlled systolic hypertension in Europe (Syst-Eur) trial. Lancet. 1998; 352:1347–1351. [DOI] [PubMed] [Google Scholar]
- 13.Forette F, Seux ML, Staessen JA, Thijs l, Babarskiene MR, Babeanu S, et al. Systolic Hypertension in Europe Investigators. The prevention of dementia with antihypertensive treatment: new evidence from the Systolic Hypertension in Europe (Syst-Eur) study. Arch Intern Med. 2002; 162:2046–2052. [DOI] [PubMed] [Google Scholar]
- 14.Yasar S, Corratta M, Brookmeyer R, Kawas C. Calcium channel blockers and risk of AD: The Baltimore Longitudinal Study of Aging. Neurobiol Aging. 2005; 26:157–163. 10.1016/j.neurobiolaging.2004.03.009 [DOI] [PubMed] [Google Scholar]
- 15.Lawlor B, Kennelly S, O'Dwyer S, Cregg F, Walsh C, Coen R, et al. NILVAD protocol: a European multicentre double-blind placebo-controlled trial of nilvadipine in mild-to-moderate Alzheimer's disease. BMJ Open. 2014. October 9 10.1136/bmjopen-2014-006364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984; 34:939–944. [DOI] [PubMed] [Google Scholar]
- 17.Molloy W, Alemayehu E, Roberts RS. Reliability of a standardised Mini-Mental State Examination compared with traditional Mini-Mental State examination. Am J Psychiatry. 1991; 148:102–105. 10.1176/ajp.148.1.102 [DOI] [PubMed] [Google Scholar]
- 18.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984; 141:1356–1364. 10.1176/ajp.141.11.1356 [DOI] [PubMed] [Google Scholar]
- 19.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993; 43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 20.Gauthier S, Gelinas I, Gauthier L. Functional disability in Alzheimer's disease. Int Psychogeriatr. 1997; 9(Suppl 1):163–165. [DOI] [PubMed] [Google Scholar]
- 21.Meulenbroek O, O'Dwyer S, de Jong D, van Spijker G, Kennelly S, Cregg F, et al. European multicentre double-blind placebo-controlled trial of Nilvadipine in mild-to-moderate Alzheimer's disease-the substudy protocols: NILVAD frailty; NILVAD blood and genetic biomarkers; NILVAD cerebrospinal fluid biomarkers; NILVAD cerebral blood flow. BMJ Open. 2016. July 19 10.1136/bmjopen-2016-011584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Cesari M, Liu F, Dong B, Vellas B. Effects of Diabetes Mellitus on Cognitive Decline in Patients with Alzheimer Disease: A Systematic Review. Can J Diabetes. 2017; 41:114–119. 10.1016/j.jcjd.2016.07.003 [DOI] [PubMed] [Google Scholar]
- 23.Rinne JO, Brooks DJ, Rossor MN, Fox RC, Bullock R, Klunk WE, et al. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer's disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 2010; 9:363–372. 10.1016/S1474-4422(10)70043-0 [DOI] [PubMed] [Google Scholar]
- 24.Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. N Engl J Med. 2014; 370:322–333. 10.1056/NEJMoa1304839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sperling RA, Jack CR Jr, Aisen PS. Testing the right target and right drug at the right stage. Sci Transl Med 2011. November 30 10.1126/scitranslmed.3002609 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
ADAS-Cog 12, Alzheimer's Disease Assessment Scale Cognitive-12.
(DOCX)
ε4, epsilon 4 allele; ADAS-Cog 12, Alzheimer's Disease Assessment Scale Cognitive-12; APOE, Apolipoprotein E gene.
(DOCX)
ADAS-Cog 12, Alzheimer's Disease Assessment Scale Cognitive-12.
(DOCX)
(DOCX)
MedDRA, Medical Dictionary for Regulatory Activities.
(XLSX)
(DOCX)
Data Availability Statement
In accordance with Irish and European data protection law, the terms under which ethical approval for the trial were granted, and the consortium agreement entered into by the NILVAD centres, we are unable to make public any patients’ personal data, even deidentified. Researchers interested in access to the trial data may contact the Trinity College Dublin officer for Data and Material Transfer Agreements, Emily Vereker at Trinity Research and Innovation, O’Reilly Institute, Trinity College, Dublin 2, Ireland (https://www.tcd.ie/innovation/exchange/technologies/) (verekee@tcd.ie), to apply for access.