Abstract
Background
Many studies have indicated the detrimental effect of ambient ozone to respiratory health in different countries. The levels of ozone in Hanoi, Vietnam are frequently above the WHO guideline but very few studies on the effects of ambient ozone on human health have been conducted in this location. This study aimed to examine the effects of ozone on hospital admission for respiratory diseases in Hanoi, by diseases, ages and seasons.
Methods
Hospital admissions, air pollutants and meteorological data were collected from January 2010 to June 2014. We used generalized linear models and distributed lag linear model to assess the association. In addition to full year analysis, we conducted restricted analysis of the data for two summer (from June-August) and winter (from December-February) seasons and grouped hospital admissions by diseases and ages (all ages, children 0 to 5 years and elderly >65 years). The delayed effect of ozone was assessed using lags of up to 5 days.
Results
Ozone has a stronger effect on the risk of hospital admission for respiratory diseases and wheeze-associated disorders in the winter. For respiratory diseases, children were affected by ozone more than other age groups in both winter and summer. Each increase of 10 μg/m3 of ozone is associated with an increase of 6.2% risk of admission for respiratory disease among children in the winter and 1.2% in the summer. For wheeze-associated disorders, the elderly group seemed to be more affected by ozone in full year and winter but no significant association was found between ozone and admission for wheeze-associated diseases in any age group.
Conclusions
Ozone is a risk factor for respiratory admission, especially amongst children under 5 years old in Hanoi, and ozone has a stronger effect in the winter than in the summer in this city.
Introduction
Long-term monitoring of air quality indicates that air pollution levels are rising in many cities throughout the world [1]. Among the criteria air pollutants, ground-level ozone (O3) commonly exceeds the recommended upper limit in many parts of the world, and is generally increasing in East Asia [2–4].
The strong oxidant, O3, has been reported to associate with adverse effects on human health including respiratory, cardiovascular diseases or even premature death [5, 6]. Acute exposure to air with high levels of O3 can trigger chest pain, cough and wheeze, throat irritation, and airway inflammation. Longer term exposure can reduce lung function and lung growth, lead to the development of asthma, and induce exacerbations of bronchitis, chronic obstructive pulmonary disease (COPD) and asthma [6]. Increased ground-level O3 has been associated with asthma and respiratory-related exacerbations [5, 7–11]. However, not all studies report these associations [12–14]. Clarifying associations between O3 and respiratory ill health, especially in ethnically and geographically diverse populations, is important as wheeze-associated disorders and asthma are common causes of hospitalization in children [11].
Young children, especially children younger than 5 years old, are more sensitive to air pollutants than adults; for a variety of reasons, including; their smaller airways; higher breathing rate and minute ventilation relative to body size; immature and developing lungs and immune systems; and they spend more time outdoors exposed to ambient air. [9, 15]. The elderly (> 65 years old) are also most susceptible to air pollution, with an increased need for clinic visits for respiratory illnesses [16–18].
Most of the studies in the literature come from North America, Europe or China [19] although the burden of air pollution is heavy in the developing countries of South and South East Asia [20]. Vietnam is one of the countries where rapid economic expansion and urbanization has been associated with serious air pollution [21]. The levels of air pollutants in Hanoi are frequently above the WHO guidelines for both particulate and gaseous pollutants including O3 [21]. According to the data from air quality monitoring stations, the highest level of ozone recorded in Hanoi was 455 μg/m3 (1-hour mean) and 346 μg/m3 (8-hour mean) which was 3.5 times higher than the WHO suggested level for O3 (100 μg/m3 8-hour mean) [22]. Major contributors to the high level of ozone and other air pollutants in Hanoi are the increasing number of road vehicles, crop stubble burning in suburban areas, and the effects of fossil fuel power plants in the neighbouring provinces.
Daily mean O3 values vary across seasons, increasing from spring to reach peak values during summer, with a gradual decline into winter troughs [6, 23]. Some studies have reported a stronger association between ambient O3 and adverse health outcomes in summer while others found a more pronounced association during the cold season [19, 24–28].
While some studies have examined the effects of ambient air pollution on various health outcomes in Vietnam, [29–31], only one has evaluated the effect of O3 on respiratory disease [32]. The present study aims to examine the association between ambient O3 and respiratory-related hospitalisations, especially among children and the elderly, in Hanoi in two different seasons, winter and summer.
Materials and methods
Research location
The study was conducted in Hanoi, which has a population of about 7 million with a density of more than 2000 people/ km2. Children aged < 5 years old and the elderly aged >65 years old accounted for 9% and 8%, respectively, of the total population of the city. The annual population growth rate was 2% [33].
During 2013, Hanoi’s economy grew by 8%, significantly higher than the national average of 5.3% [34, 35]. Hanoi has seen a large increase in the number of road vehicles, second only to Ho Chi Minh City in Vietnam. During the ten years from 2003 to 2013, the number of cars and motorbikes in Hanoi increased from 1 million to 5 million vehicles [34, 36].
Data collection
Hospital admissions
Data on daily hospital admissions from January 2010 to June 2014 were collected from three hospitals in Hanoi, the Vietnam National Hospital of Paediatrics (Paediatrics) and two multi-department hospitals, Bach Mai (BM) and Duc Giang (DG) hospitals. Data extracted included age, address, date of admission and the International Classification Diseases 10th revision (ICD10) code. Patients who came from locations other than Hanoi were excluded. We grouped hospitalizations as: all-cause respiratory diseases (ICD10 code: J00-J99) and wheeze-associated disorders (ICD10 code: J21, J22, J45, J46). We examined the effects of O3 on all ages group including patients at any age and 2 specific age groups including children and the elderly. Children included all patients aged from 28 days to less than 5 years old. Neonatal admissions (aged 0–27 days) were excluded as they are likely to be affected by perinatal conditions [37]. The elderly included all patients aged > 65 years old.
Ozone and meteorological data
Ozone and meteorological data (hourly values) were obtained from the Centre for Environmental Monitoring Portal (Vietnam Environment Administration) from January 2010 to June 2014. The data were recorded from a national automatic air quality monitoring station in Hanoi. Daily average temperature (°C), relative humidity (%) and daily average concentration of O3 (μg/m3) were calculated from collected hourly values. A 75% completeness criterion was applied to calculate daily aggregate data, meaning that if less than 18 hours of temperature, relative humidity and O3 concentration data were available in a day then the daily average concentration for the day was assigned as ‘missing’ data. All missing values were replaced by the value generated by the mean- before-after method for 1-day gap and the multiple imputation method with linear regression for continuous variables for longer gaps [38, 39].
Data analysis
We performed a time series regression analysis to examine the relationship between daily concentrations of O3 and hospital admissions due to respiratory diseases and wheeze-associated disorders. We used generalized linear models and distributed lag linear model with the family of quasi-Poisson distribution to assess the effect of daily O3 on respiratory admissions while adjusting for the effect of temperature and humidity [40–42].
Our model included two cross-basis matrices. The first cross-basis for O3 comprised a linear function for the space of the predictor. The delayed effect of O3 was assessed using lags of up to 5 days. The second cross-basis was for daily temperatures, applying a quadratic B-spline function with 3 strata intervals at lags of 0–1, 2–5, 6–10 days. The maximum lag for temperature was set to 10 days [41–43].
A smooth function of time with 7 degree of freedom (df) per year was included in the regression models to correct for seasonality and long-time trend [41]. Time variable is a series of equally-spaced point taken each day in time order during the period of study. In order to adjust for the effects of relative humidity, we used a natural spline function of daily average humidity with 3 df. In addition, we also added the day of the week (DOW) into the model to adjust for the potential DOW’s effect on hospital admissions (Eq 1)
To estimate the effect of ozone on hospital admission in hot and cold weather separately, we also restricted analysis of the data to two specific seasons: summer (from June-August) and winter (from December–February) in addition to the full year analysis. We used natural splines for day of the year with 4 df and time with 3 df in the regression models to describe the seasonal effect within each year and the long-time trend, respectively (Eq 2) [41].
The final models are shown below:
| (Eq 1) |
| (Eq 2) |
Where Yt is the observed daily count of hospital admissions (respiratory and wheeze-associated disorders) on day t; α is the intercept; cbOz is the cross-basic for O3; Ozt is daily average concentration of O3 on day t; cbT is the cross-basic for temperature; Tt is the daily average temperature on day t; ns is a natural cubic spline function; doy is day of the year; time is variable running from 1 to 1642; RH is the daily average humidity; DOW is the categorical day of the week with a reference day of Sunday.
Autocorrelation in the residuals was checked for and pre-removed before analysing in the time series models. A sensitivity analysis using DLNM with a threshold (mean concentration of O3 in winter and summer) was run to check if there is any significantly different outcomes from our non-threshold approach. Results of sensitivity analysis are presented as supplementary material (S1–S3 Tables).
All statistical analyses were performed using R software version 2.3.2 (http://www.r-project.org), using the “dlnm”, “mvmeta”, “splines”, “tsModel” and “lubridate” packages. The results are presented as the Risk Ratio (RR) and its 95% confidence interval (CI) for daily respiratory hospital admissions, per 10 μg/m3 increase in O3 at single lag days and cumulative lag of 5 days, by using STATA 12 (Stat Corporation, College Station, Texas, USA).
The ethical clearance of this study was approved by the Vietnam National Hospital of Paediatrics, Biomedical Research Ethics Committee (NHP–RICH– 15–014) and UQ School of Medicine Low Risk Ethical Review Committee (#2016-SOMILRE-0155). This ethical clearance allows us to use the hospital admission data (includes age, province level address, date of admission and the ICD10 code), which have been de-identified, in our research. As the data are anonymous, we are not required to obtain informed written consent from patients (or their parents/guardians in the case of minors).
Results
A total of 92,183 hospital admissions for respiratory diseases were recorded during the study period in the three participating hospitals in Hanoi (Table 1), with 78% for children aged <5 years old and 10% for the elderly aged >65 years old. Wheeze-associated disorders accounted for 10,031 admissions of all ages combined (about 11% of the all cause respiratory diseases). Admissions among children and the elderly made up 69% and 7%, respectively.
Table 1. Descriptive statistics of hospitalisation, air pollution and meteorological variables.
| Percentile | Minimum | Maximum | Mean (SD) | |||
|---|---|---|---|---|---|---|
| 25th | 50th | 75th | ||||
| Daily hospital admissions | ||||||
| All ages | ||||||
| All causes of respiratory diseases (92,183 admissions) | ||||||
| 43 | 55 | 67 | 13 | 121 | 56 (18) | |
| Wheeze-associated disorders (10,031 admissions) | ||||||
| 4 | 6 | 8 | 0 | 23 | 6 (3) | |
| Children (<5 years old) | ||||||
| All causes of respiratory diseases (66,685 admissions) | ||||||
| 31 | 39 | 49 | 10 | 92 | 41 (14) | |
| Wheeze-associated disorders (6,902 admissions) | ||||||
| 2 | 4 | 6 | 0 | 19 | 4(3) | |
| The elderly (>65 years old) | ||||||
| All causes of respiratory diseases (9,544 admissions) | ||||||
| 3 | 5 | 8 | 0 | 19 | 6 (3) | |
| Wheeze-associated disorders (698 admissions) | ||||||
| 0 | 0 | 1 | 0 | 3 | 0.4 (0.7) | |
| Air pollutants (μg/m3) and meteorological variables | ||||||
| Full year average | ||||||
| 24-hour mean O3 (μg/m3) | 28.9 | 41.8 | 57.8 | 0.1 | 196.6 | 46.9 (28.6) |
| 1-hour mean O3 (μg/m3) | 15.4 | 31 | 63.8 | 0.0 | 455 | 50.2 (53.9) |
| Temperature (°C) | 20.1 | 25.7 | 29.1 | 9.6 | 35.9 | 24.5 (5.8) |
| Relative humidity (%) | 71.5 | 78.3 | 85.3 | 39.8 | 99.4 | 78.2 (10.7) |
| Winter average | ||||||
| 24-hour mean O3 (μg/m3) | 30.9 | 40.3 | 51.4 | 4.1 | 115.4 | 42.4 (17.5) |
| Temperature (°C) | 14.2 | 17 | 20 | 9.6 | 26.8 | 17.4 (3.7) |
| Summer average | ||||||
| 24-hour mean O3 (μg/m3) | 25.5 | 41 | 69.4 | 7.3 | 196.6 | 52 (37.6) |
| Temperature (°C) | 28.7 | 30.1 | 31.5 | 18.5 | 35.9 | 30.1 (2.2) |
SD: Standard Deviation
The average daily mean level of O3 was 46.9 μg/m3 over the entire study period, 42.4 μg/m3 in winter, and 52 μg/m3 in summer (Table 1). There were 193 days (46%) in the winter where the daily mean of O3 was above 42.4 μg/m3 and 190 days (45.2%) in the summer where it was above 52 μg/m3. The maximum level for 24-hour mean O3 was 196.6 μg/m3 and for 1-hour mean O3 was recorded at 455 μg/m3 which was more than twice of the Vietnam national air quality standard level (200 μg/m3). During the study period, the O3 levels in Hanoi exceeded the Vietnam air quality guideline for O3 1-hour mean (200 μg/m3) in nearly one thousand occasions (on more than 70 days) and exceeded the 1-hour mean value suggested by WHO (100 μg/m3) in more than 3700 occasions (on more than 350 days).
The average daily mean temperature in Hanoi during the study period was 24.5° C with a minimum of 9.6° C and a maximum of 35.9° C. In the winter, the average temperature was 17.4° C, and 30.1° C in summer. The average daily mean relative humidity was 78.2%, with a minimum of 39.8% and maximum of 99.4% (Table 1).
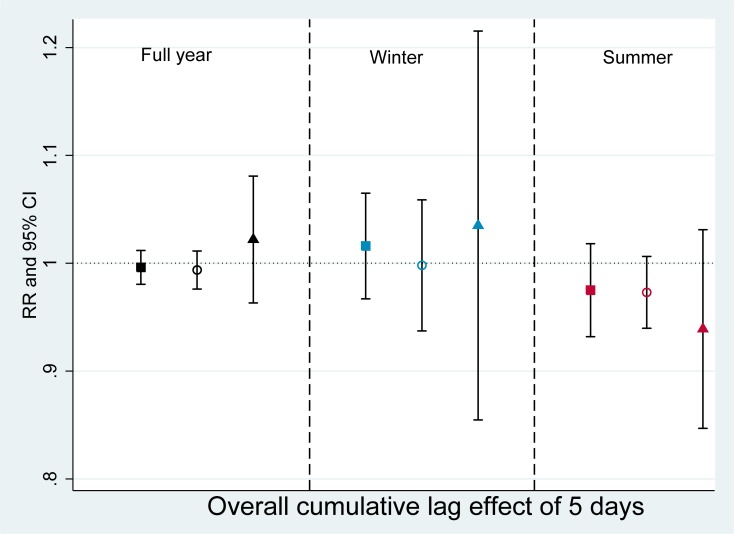
Fig 1 presents the overall effects of O3 on hospital admission for respiratory diseases. The overall cumulative lag effect of 5 days exposure to O3 on hospital admission was statistically significant in each age groups. An increase of 10 μg/m3 in O3 level was associated with an increase of 0.7% (95% CI 0.1%– 1.3%) risk of admissions for respiratory diseases among both all ages and children group and of 2.1% (95% CI 0.5%-3.7%) among elderly group. When the data were restricted to specific seasons, in the winter, RR for respiratory admission was highest among children aged < 5 year old as compared to all ages and the elderly. For instance, the RR for each 10 μg/m3 increase of O3 was highest among children group (RR = 1.062, 95%CI 1.037–1.088) in the winter and lower in all ages (RR = 1.056, 95%CI 1.034–1.079) and the elderly group (RR 1.024, 95%CI 0.977–1.073). A similar pattern was also found in the summer for each age group but the effect of O3 was clearly weaker than in the winter, especially among children and all ages group which were (RR 1.012, 95%CI 1.003–1.021) and (RR 1.012, 95% CI 1.004–1.020), respectively. For elderly group, we did not observe statistically significant effect of O3 in specific season (Fig 1, S1 Table).
Fig 1. Association between O3 and hospital admissions for respiratory diseases (Overall cumulative lag effect of 5 days).
RR: risk ratio, CI: confidence interval. Black square: full year, all ages; Blue square: winter, all ages; Red square: summer, all ages. Black hollow circle: full year, <5 years old; Blue hollow circle: winter, <5 years old; Red, hollow circle: summer, <5 years old. Black triangle: full year, >65 years old, Blue triangle: winter, >65 years old; Red triangle: summer, >65 years old.
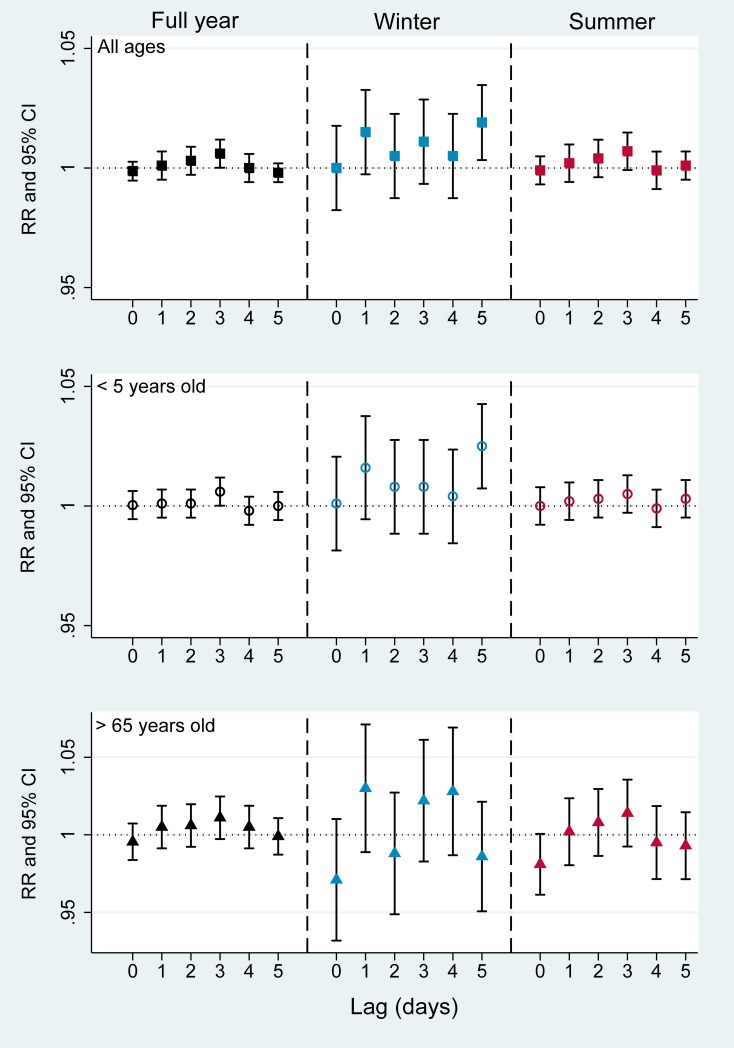
Fig 2 presents single day lag effects of O3 on hospital admission for respiratory diseases by seasons and age groups. In the full year model, O3 tended to increase its effect after exposure at lag 0 to the strongest effect at lag 3 and decreased after that. At lag 3, the RR for each 10 μg/m3 increased of O3 was 1.006 (95%CI 1.001–1.011), 1.006 (95%CI 1.001–1.012) and 1.011 (95%CI 0.998–1.024) for all ages, children and the elderly group, respectively. A similar pattern with equivalent RRs was found with the summer models for each age groups but the O3 effects in the summer failed to reach statistical significance. In the winter, the effects of O3 significantly stronger than those in the summer or full year. Statistically significant associations were observed at a lag of 5 days among all ages (RR 1.019, 95% CI 1.003–1.036) and children aged <5 years old (RR 1.025, 95% CI 1.007–1.044). Ozone had a greater effect on children group compared to others (Fig 2, S1 Table).
Fig 2. Associations between O3 and hospital admissions for respiratory diseases by seasons and age groups (Single day lag effect).
RR: risk ratio, CI: confidence interval. Black square: full year, all ages; Blue square: winter, all ages; Red square: summer, all ages. Black hollow circle: full year, <5 years old; Blue hollow circle: winter, <5 years old; Red, hollow circle: summer, <5 years old. Black triangle: full year, >65 years old, Blue triangle: winter, >65 years old; Red triangle: summer, >65 years old.
Figs 3 and 4 show that O3 had some positive but non-statistically significant effects on admission for wheeze-associated disorders. The RR for overall cumulative association after 5 days exposure were estimated highest among elderly group in the full year model (RR = 1.021, 95%CI 0.966–1.082) and the winter model (RR = 1.035, 95% CI 0.880–1.216) but lowest in the summer model (RR = 0.939, 95%CI 0.856–1.031) (Fig 3, S2 Table).
Fig 3. Association between O3 and hospital admissions for wheeze-associated disorder (Overall cumulative lag effect of 5 days).
RR: risk ratio, CI: confidence interval. Black square: full year, all ages; Blue square: winter, all ages; Red square: summer, all ages. Black hollow circle: full year, <5 years old; Blue hollow circle: winter, <5 years old; Red, hollow circle: summer, <5 years old. Black triangle: full year, >65 years old, Blue triangle: winter, >65 years old; Red triangle: summer, >65 years old.
Fig 4. Associations between O3 and hospital admissions for wheeze-associated disorder by seasons and age groups (Single day lag effect).
RR: risk ratio, CI: confidence interval. Black square: full year, all ages; Blue square: winter, all ages; Red square: summer, all ages. Black hollow circle: full year, <5 years old; Blue hollow circle: winter, <5 years old; Red, hollow circle: summer, <5 years old. Black triangle: full year, >65 years old, Blue triangle: winter, >65 years old; Red triangle: summer, >65 years old.
Regards to delayed effects of O3, the highest effects were observed in the winter and higher in elderly group. For instance, the greatest effect was estimated at lags 4 in all ages (RR = 1.018, 95% CI 0.979–1.059), lag 3 in children (RR = 1.023, 95% CI 0.975–1.074) and lag 3 among elderly (RR = 1.059, 95% CI 0.925–1.213). Ozone in summer seems to have lowest effect on wheeze-associated disorders but we observed a very high positive effect of O3 on elderly group at lag 1 (RR = 1.064, 95% CI 0.983–1.150). However, no statistically significant effects were found for O3 on the admissions for wheeze-associated disorders in any age group or season (Fig 4, S2 Table).
Discussion
This present study is amongst the first to examine the short-term effect of air pollution on the risk of hospital admission in Hanoi, Vietnam with specific focus on the relationship between ground-level ozone and daily hospital admission for respiratory diseases and wheeze-associated disorders.
Although there is abundance of peer-reviewed published literature supporting an association between ambient ozone concentrations and adverse health effects so far, the most recent Integrated Science Assessment (ISA) document of the US EPA on ozone also showed that the evidence of ozone effect is mixed [6]. The ISA also overlooked the Zemp et al. (1999) study [13] from a large cohort study in Europe whose main conclusion was no association between ozone and respiratory symptoms. Additionally, a review paper on air pollution and health [44] has pointed out that there were “large inter individual differences in responsiveness to inhaled ozone”, which was reflected in the ISA as well. Therefore, our study with the aim of evaluating and quantifying the impact of ambient ozone level on the local population with different socio-economic conditions than the US is well justified.
The data from the present study show that there was a positive effect of ozone on the risk of hospital admissions for respiratory diseases, especially amongst young children. Ozone showed significant cumulative effects on hospitalization of children and all ages in both winter and summer. These results are in agreement with some previous studies in other parts of the world which reported significant increases in respiratory admission due to increases in the level of O3 [45, 46]. In the present study, O3 increased the risk of respiratory-related hospitalisations, with the maximum effect at 5-day lag between exposure and effect, especially in winter. While this was significant for all ages and for children <5 years old, the all age-effects are likely to be primarily due to effects on children, as evidenced by the high risk ratio for children and the lack of effect on the older adults.
Previous studies also observed significant effects of ozone on total respiratory disease among patients of all ages [47–50] and among children or the elderly [10, 51, 52] at some specific lags. For example, in one study conducted in Canada, the authors found respiratory admissions were associated with elevated ozone levels at 2, 3, 4, and 5 days prior to admission with the strongest association observed at a lag of 4 days, and with the odds ratio for children aged <3 years old of 1.22 (95% CI: 1.15–1.30) and for the elderly of 1.13 (1.09–1.18), based on an increment in ozone level of 19 μg/m3 [10]. Similarly, Vahedian et al. (2017) reported in their study that O3 showed a negative association with respiratory hospital admissions among all ages at lag 0 but a positive association at lag 1 day with a corresponding RRs (95% CI) of 1.010 (1.002–1.020) per 10 μg/m3 increase [50]. However, in another study, Fusco et al (2001) found O3 was associated with admissions only among children (0-14yo) (lag 1, 5.5% increase per IQR, 23.9 μg/m3) for total respiratory admissions while no association was observed for all ages.
Not all studies in the literature find positive associations between O3 and hospital admissions for wheeze-associated disorders [32, 51, 53, 54]. In a study conducted in Italy, Fusco et al. (2001) found no significant effect of ozone on admission for asthma among all ages (RR: 1.038, 95% CI: 0.997–1.011) and a negative effect for children aged <14 years old (RR: 0.984, 95% CI: 0.887–0.909). Another study in Malaysia did not observe significant associations between ozone and admissions for acute bronchitis (RR = 1.002, 95% CI 1.000–1.004), acute bronchiolitis (RR = 0.990, 95% CI 0.978–1.002) or asthma (RR = 1.000, 95% CI 0.999–1.000) among children < 5 years old [54]. In our previous study in Ho Chi Minh city, Vietnam, we did not observe any significant association between O3 and respiratory hospitalizations for any age groups (<5 years old, all ages and >65 years old) [31], not did a recent Vietnamese study with hospital admission for bronchitis and asthma (RR = 1.003, 95% CI 0.903–1.114) among children aged 1–5 years old [32].
Few studies have specifically accounted for seasonal differences in O3. Positive associations with respiratory admissions have been reported in summer only [45, 47], the warm season [48], or in the winter only [55]. In the present study, we observed positive associations with a 5-day cumulative lag in both winter and summer months but single day lag effects were only seen in winter. The reasons for these inconsistent findings is not clear but may be contributed to by: the absolute level of ambient ozone, differing patient characteristics (such as age, sex, occupation or poverty), the amount of outdoor activity undertaken and/or any adaptive behaviours (such as the use of open windows or using air conditioning), which can differ by location [17, 56]. The average mean and maximum concentration of ozone in Hanoi is generally higher than in Ho Chi Minh city [31]. In Vietnam, people often avoid going out when it is hot and sunny, when the photochemical formation of O3 from NO2 is maximal. Therefore, despite the higher ozone concentration during summer in Hanoi the adverse effects on health were less than those seen in winter. Another possible reason for inconsistent reports of seasonal effects may be insufficient adjustment for temperature [57]. Previous studies did not adequately control for temperature using the same day lags as for O3 [19]. In addition, since ozone has been receiving increasing attention over the past 30 years, associations between ozone and hospitalizations may have changed over this time frame due to changes in socioeconomic factors which vary by region [56]. An increase in the use of air conditioning, which is related to socioeconomic status, can modify exposure to ozone [56, 58].
Limitations
We acknowledge that there were some limitations to this study. First, admission data were obtained from 3 hospitals which are not at the same level. Paediatrics and BM are national hospitals with 1300 and 1400 beds, respectively, while DG is a regional hospital, with 510 beds. However, Paediatrics is the tertiary level hospital for children while BM and DG are the general hospital which receive patients of all ages. Due to their heterogeneous characteristics, we could not check their effects and assume there is no hospital effects in our study. Second, the ambient monitoring data we used were collected from the only functioning monitoring station in Hanoi, which may not be representative of the whole city and it is possible that the effects of ozone might be under or overestimated. In addition, using imputation methods to replace missing ozone data may have introduced bias to the results of our analyses although the imputation amount is minimal. Third, data of other pollutants (particulate matters, nitrogen dioxide, sulphur dioxide, carbon monoxide) and other environmental variables (rainfall, wind speed) were not controlled in the models to see if they may attenuate the ozone effect as they were not available for the entire study period. Next, no data on individual exposure to ambient ozone or information on adaptive measures (e.g. air- conditioning usage, outdoor activity) were available in Vietnam which might be factors that we could not adjust in our models. Finally, the short time frame of this study might hinder the possibility of analysing the effects of all confounding factors to the impact of ozone on respiratory admissions.
Conclusions
The findings of this study demonstrated that ozone was associated with an increased risk of respiratory-related admissions, especially for children under the age of 5 years. The effect was stronger in the winter than in the summer in each age group. In the winter, for each increase of 10 μg/m3 of O3, the risk of admissions for respiratory diseases after 5 days of exposure increased 6.2% (95% CI 3.7%– 8.8%) among children, 5.6% (95% CI 3.4%-7.9%) for all ages. No significant association between O3 and hospital admission for wheeze-associated disorders was found for any age. These findings suggested that O3 is a risk factor for respiratory admission among population, especially among children aged < 5 years old and the ozone effect was different between winter and summer season. Further studies on the effects of other air pollutants and their interaction on paediatric admission in Hanoi should be considered.
Supporting information
RR: risk ratio, CI: confidence interval.
(DOCX)
RR: risk ratio, CI: confidence interval.
(DOCX)
RR: risk ratio, CI: confidence interval.
(DOCX)
Acknowledgments
The authors would like to thank the staff of Vietnam National Hospital of Paediatrics, Bach Mai and Duc Giang Hospital, Centre for Environmental Monitoring, especially Dr Lien Nguyen, Dr Hong Minh for providing the admission data.
Data Availability
The use of our hospital admission data are restricted by the ethical approval, and can only be made accessible with the permission of the relevant hospitals. Data requests may be sent to The Biomedical Research Ethics Committees of National Hospital of Paediatrics (http://www.nhp.org.vn/), Bach Mai Hospital (http://bachmai.gov.vn/english.html), and Duc Giang Hospital (http://benhvienducgiang.com/). The authors of this paper can also help facilitate the communication if other researchers are interested in requesting the data.
Funding Statement
LL is funded by an Australia Award Scholarship. DP is funded by a Griffith Postdoctoral Research Fellowship. PS is funded by a National Health and Medical Research Council Senior Principal Research Fellowship. PT is funded by a Queensland University of Technology Vice-Chancellor's Research Fellowship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
References
- 1.WHO. Air pollution levels rising in many of the world’s poorest cities. 2016. doi: http://www.who.int/mediacentre/news/releases/2016/air-pollution-rising/en/.
- 2.Guerreiro CBB, Foltescu V, de Leeuw F. Air quality status and trends in Europe. Atmospheric Environment. 2014;98(Supplement C):376–84. doi: 10.1016/j.atmosenv.2014.09.017. [DOI] [Google Scholar]
- 3.CAI-Asia Center. Air Quality in Asia: Status and Trends. 2010;(2010 Edition).
- 4.Cooper OR, Parrish D, Ziemke J, Balashov N, Cupeiro M, Galbally I, et al. Global distribution and trends of tropospheric ozone: An observation-based review. Elem Sci Anth. 2014;2. [Google Scholar]
- 5.Ghaffari HR, Aval HE, Alahabadi A, Mokammel A, Khamirchi R, Yousefzadeh S, et al. Asthma disease as cause of admission to hospitals due to exposure to ambient oxidants in Mashhad, Iran. Environmental Science and Pollution Research. 2017;24(35):27402–8. 10.1007/s11356-017-0226-5 [DOI] [PubMed] [Google Scholar]
- 6.U.S.EPA. Intergrated Science Assesment (ISA) of Ozone and Related Photochemical Oxidants (Final Report, Feb 2013). US Emvironmental Protection Agency, Washington, DC, EPA/600/R-10/076F. 2013.
- 7.Paulu C, Smith AE. Tracking Associations Between Ambient Ozone and Asthma-Related Emergency Department Visits Using Case-Crossover Analysis. Journal of Public Health Management and Practice. 2008;14(6):581–91. 10.1097/01.PHH.0000338371.53242.0e. 00124784-200811000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Kim B-J, Kwon J-W, Seo J-H, Kim H-B, Lee S-Y, Park K-S, et al. Association of ozone exposure with asthma, allergic rhinitis, and allergic sensitization. Annals of Allergy, Asthma & Immunology. 2011;107(3):214–9. e1. [DOI] [PubMed] [Google Scholar]
- 9.Lin S, Liu X, Le LH, Hwang S- A. Chronic exposure to ambient ozone and asthma hospital admissions among children. Environmental Health Perspectives. 2008;116(12):1725 10.1289/ehp.11184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Q, Chen Y, Shi Y, Burnett RT, McGrail KM, Krewski D. Association between ozone and respiratory admissions among children and the elderly in Vancouver, Canada. Inhalation toxicology. 2003;15(13):1297–308. 10.1080/08958370390241768 [DOI] [PubMed] [Google Scholar]
- 11.Pershagen G, Rylander E, Norberg S, Eriksson M, Nordvall SL. Air Pollution Involving Nitrogen Dioxide Exposure and Wheezing Bronchitis in Children. International Journal of Epidemiology. 1995;24(6):1147–53. 10.1093/ije/24.6.1147 [DOI] [PubMed] [Google Scholar]
- 12.Karr C, Lumley T, Schreuder A, Davis R, Larson T, Ritz B, et al. Effects of subchronic and chronic exposure to ambient air pollutants on infant bronchiolitis. American journal of epidemiology. 2006;165(5):553–60. 10.1093/aje/kwk032 [DOI] [PubMed] [Google Scholar]
- 13.Zemp E, Elsasser S, Schindler C, Kunzli N, Perruchoud AP, Domenighetti G, et al. Long-term ambient air pollution and respiratory symptoms in adults (SAPALDIA study). American journal of respiratory and critical care medicine. 1999;159(4):1257–66. [DOI] [PubMed] [Google Scholar]
- 14.Glad JA, Brink LL, Talbott EO, Lee PC, Xu X, Saul M, et al. The relationship of ambient ozone and PM2. 5 levels and asthma emergency department visits: possible influence of gender and ethnicity. Archives of environmental & occupational health. 2012;67(2):103–8. [DOI] [PubMed] [Google Scholar]
- 15.Health CoE. Ambient air pollution: health hazards to children. Pediatrics. 2004;114(6):1699–707. 10.1542/peds.2004-2166 [DOI] [PubMed] [Google Scholar]
- 16.Hwang J-S, Chan C-C. Effects of air pollution on daily clinic visits for lower respiratory tract illness. American journal of epidemiology. 2002;155(1):1–10. [DOI] [PubMed] [Google Scholar]
- 17.Bell ML, Zanobetti A, Dominici F. Who is More Affected by Ozone Pollution? A Systematic Review and Meta-Analysis. American Journal of Epidemiology. 2014;180(1):15–28. 10.1093/aje/kwu115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandström T, Frew A, Svartengren M, Viegi G. The need for a focus on air pollution research in the elderly. European Respiratory Journal. 2003;21(40 suppl):92s–5s. [DOI] [PubMed] [Google Scholar]
- 19.Qin L, Gu J, Liang S, Fang F, Bai W, Liu X, et al. Seasonal association between ambient ozone and mortality in Zhengzhou, China. International Journal of Biometeorology. 2017;61(6):1003–10. 10.1007/s00484-016-1279-8 [DOI] [PubMed] [Google Scholar]
- 20.WHO. Ambient air pollution: A global assessment of exposure and burden of disease. 2016. doi: http://www.who.int/phe/publications/air-pollution-global-assessment/en/.
- 21.MONRE. National State of Environment Report 2013 on Air quality. Vietnam Environment Administration, 2014.
- 22.WHO. WHO Air quality guildelines for particulate matter, ozone, nitrogen dioxide and sulfur dioxide—Global update 2005. 2005. doi: http://www.who.int/airpollution/guidelines/en/.
- 23.Roberts–Semple D, Song F, Gao Y. Seasonal characteristics of ambient nitrogen oxides and ground–level ozone in metropolitan northeastern New Jersey. Atmospheric Pollution Research. 2012;3(2):247–57. doi: 10.5094/APR.2012.027. [DOI] [Google Scholar]
- 24.Yang C, Yang H, Guo S, Wang Z, Xu X, Duan X, et al. Alternative ozone metrics and daily mortality in Suzhou: the China Air Pollution and Health Effects Study (CAPES). Science of the Total Environment. 2012;426:83–9. 10.1016/j.scitotenv.2012.03.036 [DOI] [PubMed] [Google Scholar]
- 25.Tao Y, Huang W, Huang X, Zhong L, Lu S-E, Li Y, et al. Estimated acute effects of ambient ozone and nitrogen dioxide on mortality in the Pearl River Delta of southern China. Environmental health perspectives. 2012;120(3):393 10.1289/ehp.1103715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Almeida SP, Casimiro E, Calheiros J. Short-term association between exposure to ozone and mortality in Oporto, Portugal. Environmental research. 2011;111(3):406–10. 10.1016/j.envres.2011.01.024 [DOI] [PubMed] [Google Scholar]
- 27.Peng RD, Samoli E, Pham L, Dominici F, Touloumi G, Ramsay T, et al. Acute effects of ambient ozone on mortality in Europe and North America: results from the APHENA study. Air Quality, Atmosphere & Health. 2013;6(2):445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gryparis A, Forsberg B, Katsouyanni K, Analitis A, Touloumi G, Schwartz J, et al. Acute effects of ozone on mortality from the “air pollution and health: a European approach” project. American journal of respiratory and critical care medicine. 2004;170(10):1080–7. 10.1164/rccm.200403-333OC [DOI] [PubMed] [Google Scholar]
- 29.Mehta S, Ngo LH, Van Dzung D, Cohen A, Thach TQ, Dan VX, et al. Air pollution and admissions for acute lower respiratory infections in young children of Ho Chi Minh City. Air Quality, Atmosphere and Health. 2013;6(1):167–79. 10.1007/s11869-011-0158-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luong LM, Phung D, Sly PD, Morawska L, Thai PK. The association between particulate air pollution and respiratory admissions among young children in Hanoi, Vietnam. Science of the Total Environment. 2017;578:249–55. 10.1016/j.scitotenv.2016.08.012 [DOI] [PubMed] [Google Scholar]
- 31.Phung D, Hien TT, Linh HN, Luong LMT, Morawska L, Chu C, et al. Air pollution and risk of respiratory and cardiovascular hospitalizations in the most populous city in Vietnam. Science of The Total Environment. 2016;557–558:322–30. doi: 10.1016/j.scitotenv.2016.03.070. 10.1016/j.scitotenv.2016.03.070 [DOI] [PubMed] [Google Scholar]
- 32.Nhung NTT, Schindler C, Dien TM, Probst-Hensch N, Perez L, Künzli N. Acute effects of ambient air pollution on lower respiratory infections in Hanoi children: An eight-year time series study. Environment International. 2018;110:139–48. doi: 10.1016/j.envint.2017.10.024. 10.1016/j.envint.2017.10.024 [DOI] [PubMed] [Google Scholar]
- 33.Hanoi DS. Hanoi Department of Statistics 2014. Available from: thongkehanoi.gov.vn/.
- 34.Hanoi DPI. Hanoi Department of Planning and Investment,. 2013. doi: http://www.hapi.gov.vn/english.aspx.
- 35.The World Bank. An Update on Vietnam's Recent Economic Development July 2013. 2013.
- 36.Vietnam M. Ministry of Transport of Vietnam. 2013. doi: http://mt.gov.vn/en/Pages/default.aspx.
- 37.Mehta S, Ngo LH, Cohen A, Thach T, Dan VX, Tuan ND. Air pollution and admissions for acute lower respiratory infections in young children of Ho Chi Minh City. Air Quality, Atmosphere & Health. 2013;6(1):167–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norazian MN, Shukri YA, Azam RN, Al Bakri AMM. Estimation of missing values in air pollution data using single imputation techniques. ScienceAsia. 2008;34(3):341–5. 10.2306/scienceasia1513-1874.2008.34.341 [DOI] [Google Scholar]
- 39.Marchenko Y, editor Multiple-imputation analysis using Stata’s mi command. Stata Conference, Boston, MA; 2010.
- 40.Schwartz J. The distributed lag between air pollution and daily deaths. Epidemiology. 2000;11(3):320–6. [DOI] [PubMed] [Google Scholar]
- 41.Gasparrini A. Distributed lag linear and non-linear models for time series data. Document Is Available at R Project: Https://cran R-Project org/web/packages/dlnm/(Accessed: 4 May 2015) http://143107.2013;212.
- 42.Gasparrini A. Distributed lag linear and non-linear models in R: the package dlnm. Journal of statistical software. 2011;43(8):1 [PMC free article] [PubMed] [Google Scholar]
- 43.Bae S, Lim Y-H, Kashima S, Yorifuji T, Honda Y, Kim H, et al. Non-linear concentration-response relationships between ambient ozone and daily mortality. PloS one. 2015;10(6):e0129423 10.1371/journal.pone.0129423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brunekreef B, Holgate ST. Air pollution and health. The Lancet. 2002;360(9341):1233–42. 10.1016/S0140-6736(02)11274-8 [DOI] [PubMed] [Google Scholar]
- 45.Burnett RT, Smith-Doiron M, Stieb D, Raizenne ME, Brook JR, Dales RE, et al. Association between ozone and hospitalization for acute respiratory diseases in children less than 2 years of age. American journal of epidemiology. 2001;153(5):444–52. [DOI] [PubMed] [Google Scholar]
- 46.Farhat S, Paulo R, Shimoda T, Conceição G, Lin C, Braga A, et al. Effect of air pollution on pediatric respiratory emergency room visits and hospital admissions. Brazilian Journal of Medical and Biological Research. 2005;38(2):227–35. [DOI] [PubMed] [Google Scholar]
- 47.Burnett RT, Brook JR, Yung WT, Dales RE, Krewski D. Association between Ozone and Hospitalization for Respiratory Diseases in 16 Canadian Cities. Environmental Research. 1997;72(1):24–31. doi: 10.1006/enrs.1996.3685. 10.1006/enrs.1996.3685 [DOI] [PubMed] [Google Scholar]
- 48.De Leon AP, Anderson HR, Bland JM, Strachan DP, Bower J. Effects of air pollution on daily hospital admissions for respiratory disease in London between 1987–88 and 1991–92. Journal of Epidemiology & Community Health. 1996;50(Suppl 1):s63–s70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petroeschevsky A, Simpson RW, Thalib L, Rutherford S. Associations between outdoor air pollution and hospital admissions in Brisbane, Australia. Archives of Environmental Health: An International Journal. 2001;56(1):37–52. [DOI] [PubMed] [Google Scholar]
- 50.Vahedian M, Khanjani N, Mirzaee M, Koolivand A. Associations of short-term exposure to air pollution with respiratory hospital admissions in Arak, Iran. Journal of Environmental Health Science and Engineering. 2017;15(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fusco D, Forastiere F, Michelozzi P, Spadea T, Ostro B, Arca M, et al. Air pollution and hospital admissions for respiratory conditions in Rome, Italy. European respiratory journal. 2001;17(6):1143–50. [DOI] [PubMed] [Google Scholar]
- 52.Gouveia N, Fletcher T. Respiratory diseases in children and outdoor air pollution in Sao Paulo, Brazil: a time series analysis. Occupational and environmental medicine. 2000;57(7):477–83. 10.1136/oem.57.7.477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwartz J. Air pollution and hospital admissions for the elderly in Detroit, Michigan. American journal of respiratory and critical care medicine. 1994;150(3):648–55. 10.1164/ajrccm.150.3.8087333 [DOI] [PubMed] [Google Scholar]
- 54.Abdul Rahman S, Ismail SNS, Sahani M, Ramli M, Latif M. A case crossover analysis of primary air pollutants association on acute respiratory infection (ARI) among children in urban region of Klang valley, Malaysia. Annals of Tropical Medicine and Public Health. 2017;10(1):44–55. 10.4103/atmph.atmph_75_17 [DOI] [Google Scholar]
- 55.Wong TW, Lau TS, Yu TS, Neller A, Wong SL, Tam W, et al. Air pollution and hospital admissions for respiratory and cardiovascular diseases in Hong Kong. Occupational and Environmental Medicine. 1999;56(10):679–83. 10.1136/oem.56.10.679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ji M, Cohan DS, Bell ML. Meta-analysis of the association between short-term exposure to ambient ozone andrespiratory hospital admissions. Environmental Research Letters. 2011;6(2):024006 10.1088/1748-9326/6/2/024006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen R, Cai J, Meng X, Kim H, Honda Y, Guo YL, et al. Ozone and daily mortality rate in 21 cities of East Asia: how does season modify the association? American journal of epidemiology. 2014;180(7):729–36. 10.1093/aje/kwu183 [DOI] [PubMed] [Google Scholar]
- 58.Medina-Ramon M, Schwartz J. Who is more vulnerable to die from ozone air pollution? Epidemiology. 2008;19(5):672–9. 10.1097/EDE.0b013e3181773476 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RR: risk ratio, CI: confidence interval.
(DOCX)
RR: risk ratio, CI: confidence interval.
(DOCX)
RR: risk ratio, CI: confidence interval.
(DOCX)
Data Availability Statement
The use of our hospital admission data are restricted by the ethical approval, and can only be made accessible with the permission of the relevant hospitals. Data requests may be sent to The Biomedical Research Ethics Committees of National Hospital of Paediatrics (http://www.nhp.org.vn/), Bach Mai Hospital (http://bachmai.gov.vn/english.html), and Duc Giang Hospital (http://benhvienducgiang.com/). The authors of this paper can also help facilitate the communication if other researchers are interested in requesting the data.