Abstract
Stage I small-cell lung cancer is increasing in incidence and there are limited clinical data upon which to make treatment recommendations for this population. In this study we compared outcomes for patients receiving surgery, stereotactic body radiation therapy (SBRT), and conventional radiation therapy. Patients who underwent surgery had the best survival outcomes. For those who did not have surgery, SBRT resulted in better outcomes that standard radiotherapy.
Introduction:
The diagnosis of stage I small-cell lung cancer (SCLC) is increasing in incidence with the advent of low-dose screening computed tomography. Surgery is considered the standard of care but there are very few data to guide clinical decision-making. The purpose of this study was to compare outcomes for patients receiving definitive surgery, stereotactic body radiation therapy (SBRT), or external beam radiation therapy (EBRT) for stage I SCLC.
Patients and Methods:
Patients with a primary diagnosis of stage I SCLC were identified in the National Cancer Database. Patients were defined as having a first course of treatment of either surgery, EBRT, or SBRT. Overall survival (OS) was determined using the Kaplane—Meier method and Cox proportional hazards regression methods were used to estimate risk of overall mortality.
Results:
A total of 2678 patients were included in the analysis. The 2- and 3-year OS for the whole cohort was 62% and 50%. Comparing treatment strategies in a multivariate model, surgical resection showed improved OS over EBRT (P < .001) and SBRT (P < .001), however, the OS benefit over SBRT did not persist for patients who underwent limited resection. When excluding patients who underwent surgery, SBRT showed improved OS compared with EBRT (P ¼ .04). Additional use of chemotherapy with any treatment modality resulted in improved OS (P < .001).
Conclusion:
In this hospital-based registry study, definitive surgical resection and use of chemotherapy resulted in improved survival for patients with early stage SCLC. For patients who are not candidates for surgery, SBRT may offer a survival benefit compared with standard EBRT.
Keywords: Radiation, SBRT, SCLC, Stage I, Surgery
Introduction
Small-cell lung cancer (SCLC) represents approximately 15% of new diagnoses of thoracic malignancies.1 In general, definitive concurrent chemoradiotherapy remains the standard management strategy for patients with limited stage disease, with historical studies showing a survival advantage of radiotherapy over surgical resection.2 The optimal management of patients with stage I (T1-2a N0 M0) SCLC, however, remains less clear. For these patients, surgical resection with lobectomy with mediastinal lymph node dissection is the primary recommendation of the National Comprehensive Cancer Network guidelines.3 Because of the relative rarity of early stage SCLC, there are few published studies to help guide decision-making for these patients. A recently published study using the National Cancer Database (NCDB) described that the patterns of surgical management of patients with stage I and II SCLC showed marked variability in the use of surgery across the United States, with more frequent use of lobectomy and improved survival at higher-volume centers.4 In a separate population-based study comparing outcomes on the basis of primary modality of therapy, it was noted that there was improved survival among patients who underwent surgery and radiation or surgery alone compared with patients who received nonsurgical therapy. Despite this apparent improvement in survival, it was noted that fewer than one-third of all patients with stage I SCLC underwent surgery.5
For patients who are not candidates for surgical resection, definitive radiotherapy remains the standard, although the optimal dose and fractionation have not been established for this small subset of patients. Historically, radiotherapy has been delivered using either once-daily or twice-daily fractionation to a dose of 45 to 70 Gy. However, a recently published study by Verma and colleagues showed an increasing trend over the past decade toward the use of stereotactic body radiation therapy (SBRT) with a median survival of 23 months in patients with early stage SCLC.6 To date, there have been no studies comparing the outcomes of patients managed with surgery, conventional radiation therapy, or SBRT. The purpose of this study, therefore, was to compare treatment strategies for stage I SCLC using the NCDB.
Patients and Methods
After institutional approval, a retrospective study was performed using a participant user file obtained from the NCDB. The NCDB is a clinical oncology database compiling registry data from >1500 Commission on Cancer-accredited hospitals.7 Briefly, patients with no history of previous malignancy diagnosed between 2004 and 2013 with stage I (T1-T2a N0 M0) SCLC (codes 8041-8045) were included in the initial cohort. Patients were clinically staged unless surgery was performed, in which case pathologic stage was used. From this group, patients were further classified according to initial treatment approach as having had either definitive surgery, external beam radiation therapy (EBRT), or SBRT; patients who did not meet any of these criteria were excluded (Figure 1). Definitive surgery was defined as including limited resection (eg, wedge resection, lobectomy, extended lobectomy, or pneumonectomy [codes 20-70]). For the purpose of a secondary analysis, surgical patients were further classified according to definitive (codes 30-70, including lobectomy or pneumonectomy) or limited resection (codes 21-25, including wedge resection and segmental resection). EBRT was defined as 45 to 74 Gy directed at the thorax and delivered in 15 to 40 fractions to account for variance in fractionation pattern (once-daily vs. twice-daily). SBRT was defined as 40 to 60 Gy directed at the thorax and delivered in <5 fractions. Receipt and timing of chemotherapy was recorded and included in the analysis.
Figure 1.
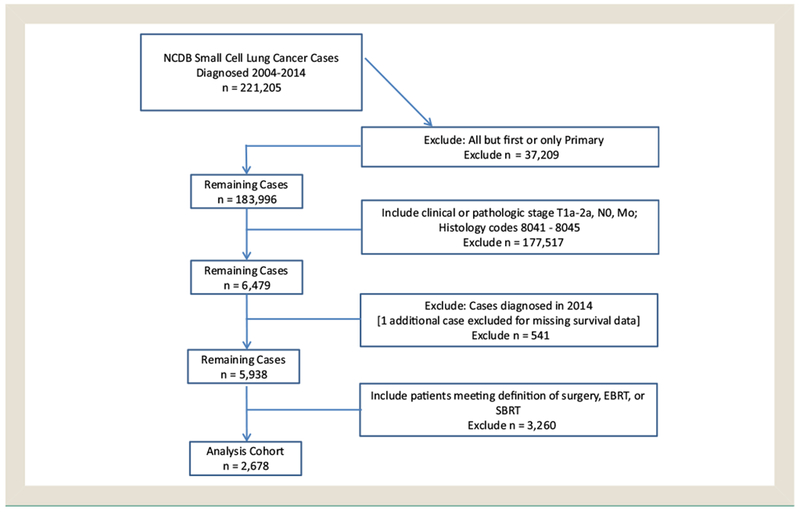
Consolidated Standards of Reporting Trials (CONSORT) Diagram Showing Selection Criteria for the Study Population
Abbreviation: NCDB = National Cancer Database.
Kaplan—Meier analysis was used to generate survival plots and estimate 2- and 3-year overall survival (OS) rates and survival was compared using the log rank test. Cox proportional hazards regression methods were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). Risk of death was estimated using a multivariate hazard model adjusting for age at diagnosis, sex, race, facility type, Charlson—Deyo score, T stage, as well as treatment modality. Analyses were conducted using SAS software version 9.4 (SAS Institute Inc, Cary, NC).
Results
Results of the initial query of the NCDB for patients with a diagnosis of SCLC returned 221,205 cases over the specified time period. From this initial cohort, 5938 patients with stage I SCLC were identified (Table 1). The median age of all patients was 69 years. Most patients were female (56%), Caucasian (90%), and treated in a community setting (65%). For the survival analysis, the patient cohort was further restricted according to the specified definitions of surgery, SBRT, and EBRT to generate a final cohort of 2678 (Table 2). The median 2-year and 3-year OS rates were 62% and 50% with a median follow-up period of 45.6 months. Classifying patients according to initial management approach, 943 (35%) underwent surgery, 1595 (60%) received definitive EBRT, and 140 (5%) underwent SBRT. Of the patients who underwent surgery, 286 (30%) underwent a limited resection versus 657 (70%) who underwent a definitive resection. The most commonly performed procedures were lobectomy (n = 617 [66%]) and wedge resection (n = 235 [26%]); 9 patients (<1%) underwent pneumonectomy. For patients receiving EBRT, there was a wide variation in fractionation schemes, with the most common being 60 Gy in 30 fractions (n = 224 [14%]), followed by 45 Gy in 30 fractions twice daily (n = 173 [11%]), and 59.4 Gy in 33 fractions (n = 106 [7%]). For patients receiving SBRT, the most common fractionation schemes were 50 Gy in 5 fractions (n = 35 [25%]), followed by 48 Gy in 4 fractions (n = 28 [20%]), 60 Gy in 3 fractions (n = 21 [15%]), and 54 Gy in 3 fractions (n = 18 [13%]). Pathologic nodal assessment was performed in 782 patients (83%) of surgical cases compared with 96 (6%) in the EBRT and 8 (6%) SBRT cases.
Table 1.
Patient and Treatment Characteristics for All Patients With Stage I SCLC (n = 5938)
| Characteristic | Value |
|---|---|
| Sex | |
| Male | 2615 (44.0) |
| Female | 3323 (56.0) |
| Mean Age, Years | 69 |
| Race | |
| Caucasian | 5327 (89.7) |
| African American | 458 (7.7) |
| Other or not identified | 153 (2.6) |
| Charlson—Deyo Comorbidity Score | |
| 0 | 3315 (55.8) |
| 1 | 1796 (30.2) |
| 2+ | 827 (13.9) |
| Histologic Subtype | |
| Small cell carcinoma NOS | 5418 (91.2) |
| Oat cell | 104 (1.8) |
| Small-cell carcinoma, fusiform cell | 11(0.2) |
| Small-cell carcinoma, intermediate cell | 47 (0.8) |
| Combined small-cell carcinoma | 358 (6.0) |
| T Stage | |
| 1 | 3237 (54.5) |
| 2 | 2701 (45.5) |
| Receipt of Chemotherapy | |
| Yes | 4219 (71.1) |
| No | 1591 (26.8) |
| Unknown | 128 (2.1) |
| Chemotherapy | |
| Concurrent | 432 (81.1) |
| Sequential | 55 (10.3) |
| None | 46 (8.6) |
| Facility Type | |
| Community cancer program | 883 (14.9) |
| Comprehensive community cancer program | 2978 (50.2) |
| Academic/research program | 1486 (25.0) |
| Other/not recorded | 591 (9.9) |
Data are presented as n (%) except where otherwise noted.
Abbreviation: NOS = not otherwise specified.
Table 2.
Patient and Treatment Characteristics for Patients Undergoing Surgery, EBRT or SBRT for Stage I SCLC
| Characteristic | All Patients (n = 2678) | Surgery (n = 943) | EBRT (n = 1595) | SBRT (n = 140) |
|---|---|---|---|---|
| Mean Age, Years | 68 | 68 | 68 | 73 |
| Sex | ||||
| Male | 1157 (43.2) | 393 (41.7) | 699 (43.8) | 65 (46.4) |
| Female | 1521 (56.8) | 550 (58.3) | 896 (56.2) | 75 (53.6) |
| Race | ||||
| Caucasian | 2413 (90.1) | 864 (91.6) | 1424 (89.3) | 125 (89.3) |
| African American | 195 (7.3) | 57 (6.0) | 128 (8.0) | 10(7.1) |
| Other or not identified | 70 (2.6) | 22 (2.3) | 43 (2.7) | 5 (3.6) |
| Charlson—Deyo Score | ||||
| 0 | 1452 (54.2) | 407 (43.2) | 978 (61.3) | 67 (47.9) |
| 1 | 855 (31.9) | 381 (40.4) | 426 (26.7) | 48 (34.3) |
| 2+ | 371 (13.9) | 155 (16.4) | 191 (12.0) | 25 (17.9) |
| T Stage | ||||
| 1 | 1602 (59.8) | 736 (78.0) | 752 (47.1) | 114 (81.4) |
| 2 | 1076 (40.2) | 207 (22.0) | 843 (52.9) | 26 (18.6) |
| Facility Type | ||||
| Community cancer program | 359 (13.4) | 93 (9.9) | 260 (16.3) | 6 (4.3) |
| Comprehensive community cancer program | 1300 (48.5) | 432 (45.8) | 814 (51.0) | 54 (38.6) |
| Academic/research program | 743 (27.7) | 324 (34.4) | 366 (22.9) | 53 (37.9) |
| Other/not recorded | 276 (10.3) | 94 (9.9) | 155 (9.7) | 27 (19.3) |
| Chemotherapy | ||||
| Yes | 2064 (77.1) | 505 (53.6) | 1488 (93.3) | 71 (50.7) |
| No | 569 (21.2) | 401 (42.5) | 101 (6.3) | 67 (47.9) |
| Unknown | 45 (1.7) | 37 (3.9) | 6 (0.4) | 2(1.4) |
| Chemotherapy Sequencing | ||||
| Neoadjuvant | 991 (37.0) | 12 (1.3) | 951 (59.6) | 28 (20.0) |
| Concurrent | 478 (17.8) | NA | 469 (29.4) | 9 (6.4) |
| Adjuvant | 478 (17.8) | 436 (46.2) | 12 (0.8) | 30 (21.4) |
| Other/timing not reported | 117 (4.4) | 57 (6.0) | 56 (3.5) | 4 (2.9) |
Data are presented as n (%) except where otherwise noted.
Abbreviation: SCLC = small-cell lung cancer.
In survival analysis, factors that were significantly associated with improved OS were female sex, Charlson—Deyo score of 0, T stage 1, and receipt of chemotherapy (Table 3). When comparing treatment modalities, EBRT (HR, 1.99 [95% CI, 1.74-2.28], P < .001) and SBRT (HR, 1.67 [95% CI, 1.32-2.10], P < .001) were associated with inferior survival compared with surgery (Figure 2). The 2- and 3-year OS for patients receiving surgery, EBRT, and SBRT were 72% and 62%, 56% and 44%, and 56% and 40%, respectively. Stratifying patients according to surgical extent, lobectomy was associated with improved survival compared with limited resection (HR, 0.64 [95% CI, 0.53-0.78], P < .001). Limited resection resulted in improved OS compared with patients who received EBRT (HR, 1.46 [95% CI, 1.22-1.76], P < .001) but not SBRT (HR, 1.24 [95% CI, 0.95-1.61], P = .11; Table 4; Figure 3). When comparing radiotherapeutic management approaches alone, SBRT was associated with improved survival compared with EBRT (HR, 1.30 [95% CI, 1.02-1.66], P = .037) in multivariable analysis.
Table 3.
Multivariable Survival Analysis for Patients Receiving Surgery, EBRT, or SBRT for Stage I SCLC
| Characteristic | Overall Survival, % | Adjusted Hazard Ratios | |||
|---|---|---|---|---|---|
| 2-Year | 3-Year | Hazard Ratio | 95% CI | P | |
| Mean Age | 1.02 | 1.01-1.03 | <.001 | ||
| Sex | |||||
| Male | 58 | 46 | 1.00 | ||
| Female | 64 | 53 | 0.84 | 0.76-0.92 | <.001 |
| Race | |||||
| Caucasian | 62 | 49 | 1.00 | ||
| African American | 65 | 57 | 0.89 | 0.73-1.08 | .23 |
| Other or not identified | 54 | 50 | 0.96 | 0.71-1.30 | .81 |
| Charlson—Deyo Score | |||||
| 0 | 62 | 50 | 1.00 | ||
| 1 | 63 | 51 | 1.12 | 1.00-1.25 | .05 |
| 2+ | 55 | 44 | 1.36 | 1.18-1.57 | <.001 |
| T Stage | |||||
| 1 | 65 | 53 | 1.00 | ||
| 2 | 57 | 45 | 1.14 | 1.02-1.26 | .02 |
| Facility Type | |||||
| Academic/research program | 64 | 52 | 1.00 | ||
| Community program | 61 | 50 | 1.03 | 0.92-1.16 | .30 |
| Chemotherapy | |||||
| Yes | 62 | 50 | 0.66 | 0.57-0.76 | <.001 |
| No | 58 | 47 | 1.00 | ||
| Unknown | 68 | 61 | 0.71 | 0.46-1.09 | .113 |
| Treatment Modality | |||||
| Surgery | 72 | 62 | 1.00 | ||
| EBRT | 56 | 44 | 1.99 | 1.74-2.28 | <.001 |
| SBRT | 56 | 40 | 1.67 | 1.32-2.10 | <.001 |
Abbreviations: EBRT = external beam radiation therapy; SBRT = stereotactic body radiation therapy; SCLC = small-cell lung cancer.
Figure 2.
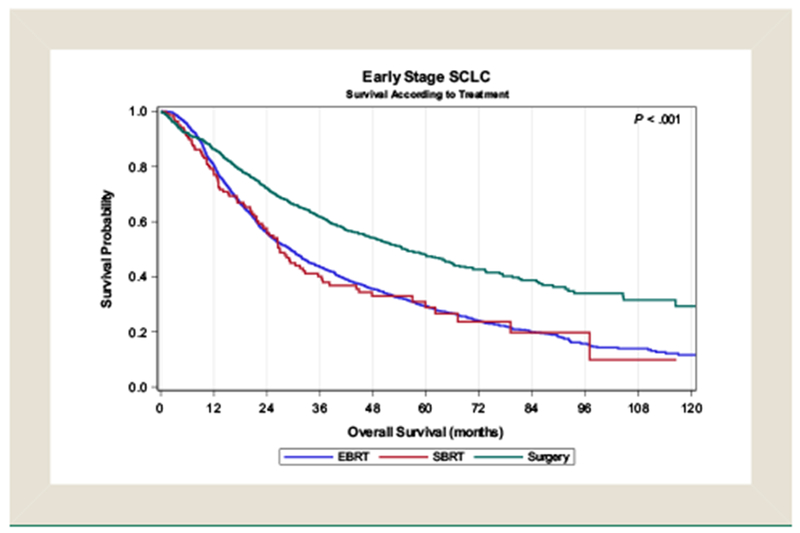
Kaplane—Meier Survival Analysis of Patients With Stage I Small-Cell Lung Cancer Undergoing Surgery, Conventionally-Fractionated External Beam Radiotherapy (EBRT) or Stereotactic Body Radiation Therapy (SBRT)
Abbreviation: SCLC = small-cell lung cancer.
Table 4.
Subgroup Survival Analysis Including Extent of Surgical Resection
| Treatment Modality | Adjusted Hazard Ratios | ||
|---|---|---|---|
| Hazard Ratio | 95% CI | P | |
| Limited Resection | 1.0 | ||
| Lobectomy | 0.64 | 0.53-0.78 | <.001 |
| EBRT | 1.46 | 1.22-1.76 | <.001 |
| SBRT | 1.24 | 0.95-1.61 | .11 |
Abbreviations: EBRT = external beam radiation therapy; SBRT = stereotactic body radiation therapy.
Figure 3.
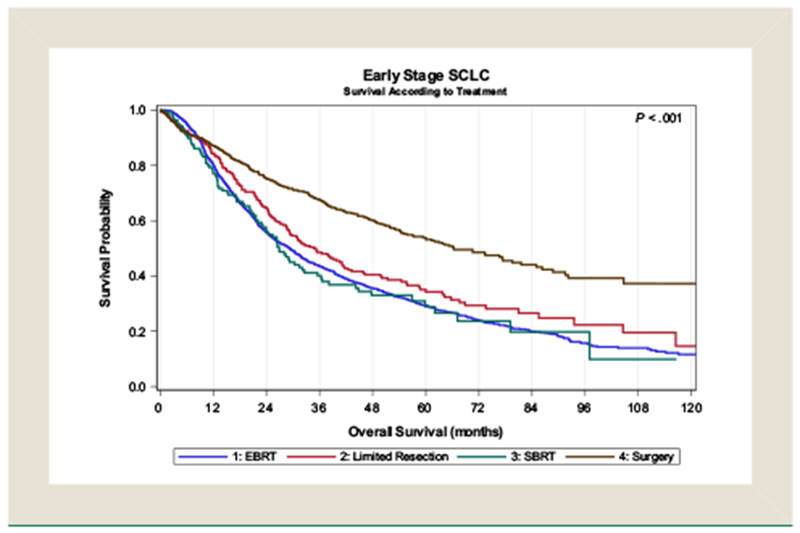
Kaplane—Meier Survival Analysis of Patients With Stage I Small-Cell Lung Cancer Undergoing Surgery, Limited Resection, Conventionally-Fractionated External Beam Radiotherapy (EBRT) or Stereotactic Body Radiation Therapy (SBRT)
Abbreviation: SCLC = small-cell lung cancer.
Discussion
Until the advent of lung cancer screening with low-dose computed tomography (CT), the incidence of stage I SCLC was exceedingly low. In the National Lung Screening Trial, it was noted that approximately 1% of the detected lung cancers in the CT arm were stage I small cell.8 As the incidence of stage I SCLC increases in tandem with screening CT, it becomes more important to establish evidence-based recommendations for the management of these patients. In operable patients, surgical resection is typically the recommendation for early stage small-cell cancer, although the evidence supporting this approach over nonsurgical treatment is limited mainly to population-based studies and metaanalyses. In a population-based study in Britain, it was reported that patients who underwent surgical resection of SCLC had markedly improved survival compared with nonsurgical patients.9 Similarly, a registry study from Norway reported improved outcomes of patients who underwent surgery compared with those who received concurrent chemoradiotherapy, albeit with small numbers of surgical patients.10 Conversely, in a Cochrane metaanalysis, it was shown that lobectomy resulted in superior outcomes over wedge resection for stage I to IIIA SCLC, however, there was no difference noted between surgical and nonsurgical approaches.11 Despite the presumed improved outcomes from surgery compared with nonsurgical management, a recent population-based study in the United States reported that fewer than one-third of patients with stage I SCLC underwent surgery during the study period.5 In patients who are medically fit, adjuvant chemotherapy is typically recommended after surgical resection. A recent population-based study reported by Yang et al who investigated the effect of adjuvant therapy after surgical resection of early-stage SCLC and showed a survival benefit to the additional use of chemotherapy after surgery, with or without prophylactic cranial irradiation (PCI).12
For patients with stage I SCLC who are medically inoperable or otherwise elect against surgery, there are very few data to direct therapeutic management, including radiotherapy dose, fractionation, and technique. For patients with limited stage disease, treatment with either once-daily or twice-daily concurrent chemoradiotherapy has been established as the standard of care.13,14 Initial results of the Concurrent Once-Daily versus Twice-Daily Radiotherapy (CONVERT) trial, in which patients received either twice-daily radiation therapy to 45 Gy or once-daily to 66 Gy showed favorable survival outcomes compared with historical rates but no significant difference between the 2 arms.15 For patients with stage I SCLC, however, treatment with conventionally-fractionated chemoradiotherapy might be unnecessarily toxic and might not be as effective as stereotactic ablation. For patients with stage I non-SCLC, it has been shown that SBRT results in excellent local control and survival outcomes compared with historical controls.16,17 Indeed, even when comparing SBRT with surgical resection in medically operable patients, a combined analysis of 2 prospective, randomized studies showed equivalence, if not superiority, of SBRT over surgery.18 Because of the tendency for systemic spread, however, simple extrapolation of these data to the small cell population is not prudent and there are decidedly fewer data to support its routine use. The largest clinical study published on the use of SBRT in SCLC is a multi-institutional retrospective series in which 74 patients were treated for stage I disease.6 In this study, 1- and 3-year local control rates were 97.4% and 96.1%, respectively, with 1- and 3-year OS rates of 69.6% and 34%. Despite very high rates of local control, distant relapse was common with a disease-free survival of 53.2% at 3 years. It was noted in this study that the additional use of chemotherapy improved survival in multivariable analysis. In a similar retrospective series from Japan, 64 patients with stage I SCLC treated with SBRT showed disease-specific, progression-free and OS rates of 79.1%, 49.3%, and 76.3% at 2 years.19 This study also showed a survival benefit to the additional use of chemotherapy in univariable as well as multivariable analysis.
In the present study, we report the finding that surgical resection results in improved survival outcomes compared with conventionally-fractionated radiation therapy or SBRT. When analyzing the surgical subset according to type of surgery, however, it was noted that the survival outcomes for patients who received limited surgery, such as wedge or segmental resection, have inferior outcomes compared with those who have more extensive resection, most of which were lobectomy. Interestingly, there was no survival advantage of limited resection compared with SBRT, although limited resection did result in superior outcomes compared with conventionally-fractionated radiation. These data support the notion that lobectomy should be the treatment of choice for patients with stage I SCLC who are medically operable, but that SBRT might be equivalent to limited resection. As reported in other analyses, administration of chemotherapy was noted to improve survival regardless of treatment modality and thus should be incorporated into the multimodal treatment of patients who are candidates for systemic therapy.
We also report the novel finding that SBRT has improved survival outcomes compared with conventionally-fractionated EBRT in multivariable analysis. We believe that these data support the continued exploration of SBRT as a viable treatment alternative to conventionally-fractionated radiation therapy for these patients, which is of particular interest because of the possibility of not only improving survival outcomes, but also potentially reducing the side effects associated with standard chemoradiotherapy.15,20 As with patients who underwent surgery, approximately half of the patients who underwent SBRT received chemotherapy compared with 93% of those who received EBRT, representing a possible avenue for further improvement in outcomes because of the marked improvement on OS that is evident with the use of chemotherapy.
As with any population-based analysis, our study has a number of potential weaknesses. The data are retrospectively reported and subject to selection bias, as well as potential errors in data entry. Most patients in the surgical group had pathologic nodal assessment whereas only 6% underwent pathologic staging in the EBRT and SBRT groups. This is a source of potential bias in that the clinically staged patients likely had a higher incidence of nodal disease than detected. The potential discrepancy in true staging could have partially accounted for the decrease in OS rates observed in the non-surgical cohort. Treatment-specific details such as chemotherapy type and dose are unavailable and thus could not be included in the multivariable model, which could potentially confound the results. Because of the widely differing treatment paradigms that were compared in this analysis, we could also not examine the effect of chemotherapy timing on outcomes. Furthermore, because we selected for first course of treatment, we are unable to report the effect of subsequent PCI for patients who received any form of radiotherapy as their definitive treatment. A similar analysis focusing only on surgical patients, however, showed no significant benefit to the additional use of PCI in these patients.12 Finally, there are no toxicity outcomes reported in the NCDB and thus we cannot take this into consideration when interpreting these findings. Because of the relative rarity of stage I SCLC, however, there are unlikely to be any prospective, randomized trials to guide clinical decision-making and this type of analysis likely represents the highest level of evidence that can be attained and thus is of significant value and potentially practice changing.
Conclusion
In this population-based analysis, we found that surgery is superior to nonsurgical management of stage I SCLC. Patients who undergo limited resection, however, have inferior outcomes compared with lobectomy and there was no statistical difference between limited resection and SBRT. Regardless of treatment modality, administration of chemotherapy results in improved outcomes and should be recommended for all patients who are candidates for systemic therapy. For patients who are not candidates for surgery, SBRT might have superior outcomes compared with conventionally-fractionated radiation therapy and should be considered as an alternative to EBRT. Because of the multitude of treatment options that can be reasonably considered for patients with stage I SCLC, treatment in a true multidisciplinary setting is imperative for the appropriate management of these patients.
Clinical Practice Points.
There are few published studies specifically addressing the management of stage I SCLC.
Because surgery is the primary consideration for patients with early stage SCLC, there are very few studies describing outcomes for those who are not surgical candidates and who are undergoing primary radiotherapy for stage I SCLC.
Although it has been established that SBRT is the preferred approach compared with conventionally fractionated radiation therapy in medically inoperable patients with NSCLC, this technique has largely gone unused in the context of early stage SCLC.
Stereotactic body radiation therapy has been reported to be well tolerated and effective in patients with early stage SCLC, but there are no studies comparing outcomes for patients who received conventionally-fractionated radiation therapy versus SBRT
This study describes outcomes for a large cohort of patients with stage I SCLC according to treatment type and controlling for patient-specific factors.
Our study confirms the benefit of surgical resection for patients who are operable, but also provides insight into the appropriate radiotherapeutic approach for patients who are not surgical candidates.
Specifically, we find that SBRT might have outcomes superior to conventionally-fractionated radiation therapy.
In addition, we report that patients who undergo limited resection (eg, wedge resection) might have inferior outcomes compared with more definitive resection (eg, lobectomy) and that SBRT might result in similar outcomes for these patients.
Finally, we confirm the benefit of chemotherapy in all patients, irrespective of primary treatment.
Taken together in context with the rest of the published literature, surgical resection should remain the standard of care for patients with stage I SCLC.
For patients who are not candidates for lobectomy, SBRT should be considered as a viable alternative to limited resection.
For inoperable patients, SBRT may be superior to conventionally-fractionated radiation therapy.
Regardless of primary modality, chemotherapy should be offered to all patients who are candidates for systemic therapy.
Footnotes
Disclosure
The authors have stated that they have no conflicts of interest.
Presented, in part, at the 2017 American Society of Clinical Oncology Annual Meeting, Chicago, Illinois.
References
- 1.American Cancer Society. Cancer Facts and Figures, 2017. Available at: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html.
- 2.Fox W, Scadding JG. Medical Research Council comparative trial of surgery and radiotherapy for primary treatment of small-celled or oat-celled carcinoma of bronchus. Ten-year follow-up. Lancet 1973; 2:63–5. [DOI] [PubMed] [Google Scholar]
- 3.NCCN Guidelines - Small-cell lung Cancer (ed 22018), 2018. Available at: https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf.
- 4.Wakeam E, Byrne JP, Darling GE, et al. Surgical treatment for early small-cell lung cancer: variability in practice and impact on survival. Ann Thorac Surg 2017; 104: 1872–80. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed Z, Kujtan L, Kennedy KF, et al. Disparities in the management of patients with stage I small-cell lung carcinoma (SCLC): a Surveillance, Epidemiology, and End Results (SEER) Analysis. Clin Lung Cancer 2017; 18: e315–25. [DOI] [PubMed] [Google Scholar]
- 6.Stahl JM, Corso CD, Verma V, et al. Trends in stereotactic body radiation therapy for stage I small-cell lung cancer. Lung Cancer 2017; 103:11–6. [DOI] [PubMed] [Google Scholar]
- 7.American College of Surgeons and the American Cancer Society. National Cancer Database, 2017. Available at: https://www.facs.org/quality-programs/cancer/ncdb.
- 8.National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luchtenborg M, Riaz SP, Lim E, et al. Survival of patients with small-cell lung cancer undergoing lung resection in England, 1998–2009. Thorax 2014; 69:269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rostad H, Naalsund A, Jacobsen R, et al. Small-cell lung cancer in Norway. Should more patients have been offered surgical therapy? Eur J Cardiothorac Surg 2004; 26:782–6. [DOI] [PubMed] [Google Scholar]
- 11.Manser R, Wright G, Hart D, et al. Surgery for early stage non—small-cell lung cancer. Cochrane Database Syst Rev 2005:CD004699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang CF, Chan DY, Speicher PJ, et al. Role of adjuvant therapy in a population based cohort of patients with early-stage small-cell lung cancer. J Clin Oncol 2016; 34:1057–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watkins JM, Fortney JA, Wahlquist AE, et al. Once-daily radiotherapy to > or ¼ 59.4 Gy versus twice-daily radiotherapy to > or ¼ 45.0 Gy with concurrent chemotherapy for limited-stage small-cell lung cancer: a comparative analysis of toxicities and outcomes. Jpn J Radiol 2010; 28:340–8. [DOI] [PubMed] [Google Scholar]
- 14.Turrisi AT 3rd, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 1999; 340:265–71. [DOI] [PubMed] [Google Scholar]
- 15.Faivre-Finn C, Snee M, Ashcroft L, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol 2017; 18:1116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non—small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys 2009; 75:677–82. [DOI] [PubMed] [Google Scholar]
- 17.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010; 303:1070–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non—small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015; 16:630–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onishi H, Araki T. Stereotactic body radiation therapy for stage I non—small-cell lung cancer: a historical overview of clinical studies. Jpn J Clin Oncol 2013; 43: 345–50. [DOI] [PubMed] [Google Scholar]
- 20.Bastos BR, Hatoum GF, Walker GR, et al. Efficacy and toxicity of chemoradiotherapy with carboplatin and irinotecan followed by consolidation docetaxel for unresectable stage III non—small-cell lung cancer. J Thorac Oncol 2010; 5:533–9. [DOI] [PubMed] [Google Scholar]


