Abstract
Several diseases share misfolding of different peptides and proteins as a key feature for their development. This is the case of important neurodegenerative diseases such as Alzheimer’s and Parkinson’s diseases and type II diabetes mellitus. Even more, metal ions such as copper and zinc might play an important role upon interaction with amyloidogenic peptides and proteins, which could impact their aggregation and toxicity abilities. In this review, the different coordination modes proposed for copper and zinc with amyloid-β, α-synuclein and IAPP will be reviewed as well as their impact on the aggregation, and ROS production in the case of copper. In addition, a special focus will be given to the mutations that affect metal binding and lead to familial cases of the diseases. Different modifications of the peptides that have been observed in vivo and could be relevant for the coordination of metal ions are also described.
Keywords: Amyloid-β, α-synuclein, amylin, familial mutations, copper, zinc
1. Introduction
In the last decades, the discovery of a common characteristic to different diseases is leading the research on their development. Neurodegenerative diseases such as Alzheimer’s (AD), Parkinson’s (PD), Huntington’s and prions diseases share with Type II diabetes mellitus (T2D) the misfolding of specific proteins or peptides, which causes the deposition of amyloid fibrils and plaques in different tissues [1]. Furthermore, metal ions dyshomeostasis has been linked to AD, PD and T2D and could be a key factor in their development as they can greatly impact the aggregation and the redox activity of the implicated peptides and proteins [2–4]. Several studies have found a relative high concentration of metal ions (Zn, Cu and Fe) in aggregates such as senile plaques (AD) formed by the amyloid-β peptide [5–9], and Lewy’s bodies (PD) formed by α-synuclein protein [10]; and a more than probable correlation between amyloid deposits formation of islet amyloid polypeptide (IAPP, amylin), Zn deficiency and T2D [11]. Nevertheless, there is still no clear evidence of the in vivo metal-binding to Aβ, αSyn and IAPP upstream of the aggregates. One parameter to take into account is the relatively low binding constant for these peptides and protein, which makes metal-binding a low probability scenario in the cytosol [12]. However, interaction between Cu and Zn and intrinsically disordered peptides/proteins (IDPs) could be plausible in the extracellular space. In the case of AD, a “labile copper pool” was proposed [13]. Similarly, spots where high concentration of metal ions, especially loosely bound metals, would be present such as in β-cells in the pancreas could be key for the interaction of IDPs and these metal ions. Moreover, some deregulation of metal ions might need to occur, which would increase the available metal ions concentration, and hence permits metal binding to Aβ, αSyn and IAPP.
In this review, we will focus on Cu and Zn, due to their relatively high concentration in the synaptic cleft in the brain and β-cells of the pancreas. The coordination chemistry of Zn(II) and Cu(II/I) are studied since more than two decades, at least for amyloid-β. Thus, we do not go into past controversies, which mainly concern the native structure of amyloid-β. We just report on the most accepted structures (and refer to past review) and concentrate on the most recent advancements often obtained on mutations or modified forms of the peptides/proteins. A general perspective of the metal-induced aggregation and ROS production will be covered in sections 3 and 4, aiming to compare the last data for the three peptides and protein. Surely this work will complete the reviews aimed to cover the interaction of metal ions and amyloidogenic peptides individually. Moreover, we aim at outlining their coordination not only to amyloid-β, α-synuclein and amylin, the main disordered peptides and proteins of the mentioned diseases; but also, to the mutated peptides which cause familial pathologies, and the murine peptides, which show different aggregating propensity features (Table 1). Studying how these mutations impact the coordination of metals ions, and consequently their aggregation and ROS production could be an important step to help elucidate this very interesting chemistry.
Table 1.
Amino acid sequences of hAβ1-42 peptide and its FAD mutants, mAβ1-42, hIAPP* and its mutants, mIAPP* peptides, and α- and β- synucleins proteins†. Metal-binding residues are marked in orange; point mutations in blue. The main sequence involved in aggregation is underlined. In gray: *C-terminal amidation of IAPP [14] and † N-terminal acetylation of αSyn and βSyn [15,16] occur in vivo.
| hAβ1-42 | DAEFRHDSGY10EVHHQKLVFF20AEDVGSNKGA30IIGLMVGGVV40IA |
| hAβ1-42 (A2T) |
DTEFRHDSGY10EVHHQKLVFF20AEDVGSNKGA30IIGLMVGGVV40IA |
| hAβ1-42 (A2V) |
DVEFRHDSGY10EVHHQKLVFF20AEDVGSNKGA30IIGLMVGGVV40IA |
| hAβ1-42 (H6R) |
DAEFRRDSGY10EVHHQKLVFF20AEDVGSNKGA30IIGLMVGGVV40IA |
| hAβ1-42 (D7N) |
DAEFRHNSGY10EVHHQKLVFF20AEDVGSNKGA30IIGLMVGGVV40IA |
| hAβ1-42 (D7H) |
DAEFRHHSGY10EVHHQKLVFF20AEDVGSNKGA30IIGLMVGGVV40IA |
| mAβ1-42 | DAEFGHDSGF10EVRHQKLVFF20AEDVGSNKGA30IIGLMVGGVV40IA |
| hIAPP | 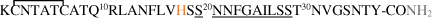 |
| mIAPP | 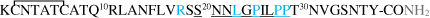 |
| hIAPP (S20G) |
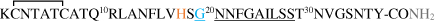 |
| αSyn1-60 | Ac_MDVFMKGLSKAKEGVVAAAEKTKQG25VAEAAGKTKEGVLYVGSKTKEGVVH50GVATVAEKTK |
| αSyn61-95 | EQVTNVGGQVVTGVT75AVAQKTVEGQGSIAAATGFV |
| αSyn96-140 | KKDQL100GKNEEGAPQEGILEDMPVDPDNEAY125EMPSEEGYQDYEPEA |
| βSyn1-60 | Ac_MDVFMKGLSMAKEGVVAAAEKTKQG25VTEAAEKTKEGVLYVGSKTREGVVQ50GVASVAEKTK |
2. Coordination of Cu and Zn to amyloid-β, α-synuclein and amylin and their mutants
The coordination of metal ions to the different amyloidogenic peptides and proteins has been thoroughly studied. There is still debate regarding some coordination modes. Nonetheless, in the next paragraphs the different coordination modes, including the most accepted and the new ones, proposed for Aβ, αSyn, hIAPP and their mutated and murine homologues will be outlined (table 1). In order to give a more global understanding of Cu and Zn binding to these peptides and protein, their association constants have been gathered in table 2 at the end of this section.
Table 2. Binding constants (Ka, M-1) for the highest affinity binding sites of the different peptides and proteins revised in this review.
| peptide/protein | Cu(II) | Cu(I) | Zn(II) |
|---|---|---|---|
| hAβ16 | 109 – 1010 [55,117–119] | 107 – 1010 [57,118] | 105 [120,121] |
| hAβ16 (A2T) | ≈ hAβ16 WT [53] | ||
| hAβ16 (A2V) | |||
| hAβ16 (H6R) | 108 [55,119] | 2.4·103 [58] † | |
| hAβ16 (D7N) | 109 [55,119] | 0.88·105 [121] | |
| hAβ (D7H) | > Aβ40 WT [122] | 4.1·104 [59] ‡ | |
| mAβ16 | 3·109 [119] | 2·106 [57] | 1.53·104 [62] |
| hIAPP | 105 – 106 [99,123] | 106 [109] | |
| hIAPP (S20G) | |||
| mIAPP | 9·104 [123] | 9·103 [116] | |
| αSyn | 107 [65,78] | 105 – 106 [77,78] | < 103 [83] |
| Ac-αSyn | ca. 104 [72] * | 104 – 105 [79] | |
| βSyn | 5·106 [67] | ||
| αSyn (H50Q) | 105 [124] | ||
| Ac-αSyn (H50Q) | too low [73] | ||
This value is taken from the one for the low-binding affinity site found at His50, given in ref. [65].
Value calculated for the dimer formation Zn-(AβH6R)2 (M-2).
Value calculated for dimer formation of Zn2-(Aβ1-10D7H)2 (M-3).
2.1. Coordination to Aβ, its FAD mutants and murine Aβ
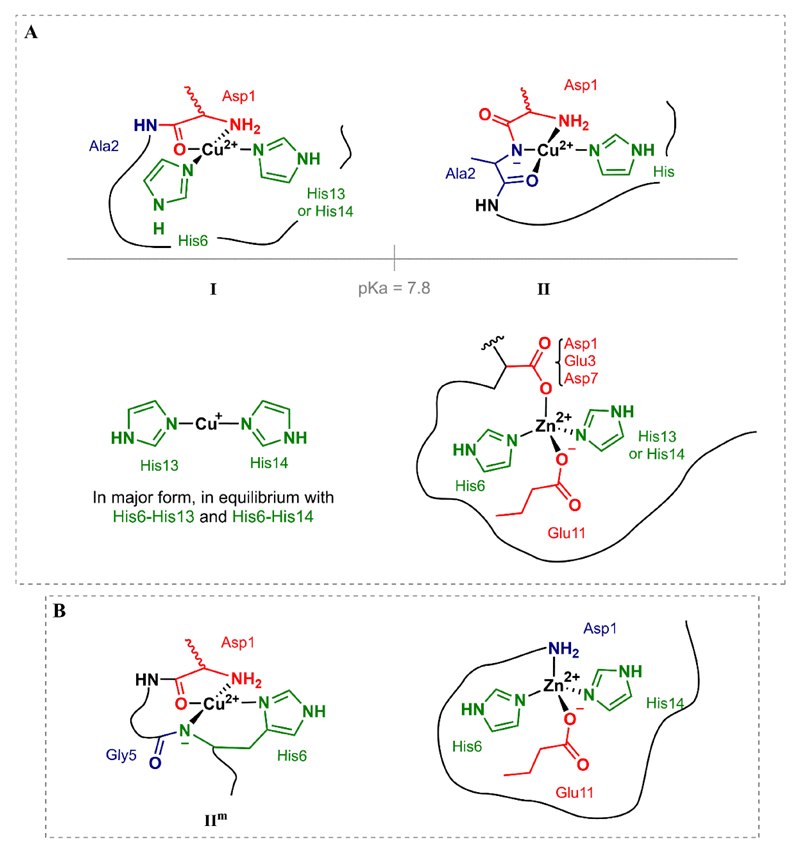
The high affinity metal binding site of amyloid-β (Aβ) peptide is found at residues 1-16, which is proposed as an appropriate model of its coordination and redox properties. The most accepted metal binding structures involving the native peptide will be described below. Meanwhile, the structures concerning mutated peptides will be discussed more profoundly. At physiological pH, two different binding modes can be found for Cu(II), known as component I (favored at lower pH) and II (at higher pH) [17–21]. They both present a distorted square-planar geometry and the coordination through the terminal amine of the Asp residue (Fig. 1.A). In Component I Cu(II) is also bound through the carbonyl from the Asp1-Ala2 amide bond, and the imidazole nitrogen atom from two histidine residues, His6 and His13 or His14 in equilibrium. In component II, the nitrogen atom from the Asp1-Ala2 amide bond is deprotonated and binds to the Cu(II), together with the CO from the Ala2-Glu3 peptide bond and one histidine residue with no preference. A different structure has been proposed for the component II, involving the oxygen from the carbonyl group of the Ala2-Glu3 bond and the three Nim from His6, His13 and His14 [22]. Some authors have also proposed a carboxylate function being involved in the apical position [23,24]. A second site has been described for the Aβ1-28 peptide but its low affinity would mean a lower biological relevance [25]. Cu(I) is bound in a linear fashion by the Nim of His6, His13 and His14 in an equilibrium, in which His13 and His14 seem to be the preferred ligands [26,27] (Fig.1.A.). The amino acids involved in the coordination of Zn(II) are also found in the Aβ1-16 sequence. First studies reported the involvement of the three histidine residues in the coordination site [28–34], being the fourth and, in some cases, fifth ligands the NH2- terminal and the Glu11 residue among others. A more recent work proposes a different coordination sphere after the study by 1H-NMR and X-Ray absorption of Zn-Aβ complexes with Aβ16 and a wide series of modified peptides [35]. The Zn(II) ion would have a tetrahedral binding to two histidine residues (His6 and His13 or His14), and two carboxylate residues (Glu11 and Asp1, Glu3, Asp7, with a preference for Asp1) (Fig. 1.A). Some authors have stated the Cu(II) coordination sphere to be independent to the oligomerization state [36,37], but a polymorphism has also been found in Cu(II)-Aβ oligomers [38,39]. In the case of Zn(II), the monomeric complex would be lost upon aggregation [40], leading to a polymorphism, of intra- and inter- molecular binding which would trigger the fast Zn-induced aggregation [41–44]. The metal-cross linking has also been observed for Cu(II) in a minor relevance [39,41,45]. Recently, the effect of N-terminal truncation of Aβ on Cu(II) binding ability, ROS production and Cu-mediated Aβ aggregation has been reviewed [46]. Such Aβ modification will thus not be addressed in the present review. Another point which could be of great interest is the mutual interference of Cu and Zn on their coordination to Aβ and the metal-induced aggregation and ROS production, which has been recently revised as well [47].
Fig. 1.
Main coordination modes of (A) hAβwt (for Cu(II), Cu(I) and Zn(II)) and (B) mAβ for Cu(II) at physiological pH and for Zn(II).
Familial Alzheimer’s disease (FAD) comprises the subtypes of this dementia characterized by mutations on proteins linked to Aβ: the presenilin-1 and -2 and APP. FAD mutations are generally associated with an early onset of the illness. Mutations on the APP can affect either cleavage sites, or fragments apart from it [48]. In this review, we focus on those found in the N-terminal part of the Aβ peptide, which can have an impact on the coordination of metal ions. Most of these mutations lead to either an increase of the fibrillation of Aβ or an alteration the Aβ40/Aβ42 ratio [49]. Understanding the mechanism of the enhanced toxicity could be key to better disentangle the molecular mechanisms of AD even if the number of FAD cases is not significantly high. The coordination to some of these mutants has been recently reviewed [24,49]. The mutation of Ala2 is an interesting one: A2V is highly pathogenic [50], while A2T has shown a protective effect [51]. EPR studies show a similar Cu(II) coordination for the WT and the A2 mutants, but changes on the pKa values of the transition between component I and component II, which could be key for a pathological role of Cu-Aβ binding [52,53]. In fact, with a pKa for A2V of 8.4 (7.4 for A2T, 7.8 for WT), component I is stabilized at physiological pH. Furthermore, this mutant shows a faster inter-peptide Cu(II) exchange linked to a higher pathogenic character of component I [53]. Similar features were observed for the Tottori mutation D7N, involved in early onset AD, although the shift of pKa value between components I and II is less accused [54,55]. His residues are key for the coordination of Cu, and greater differences would be expected when the mutations affect this amino acid. This could be the case of the English (H6R) and Taiwan (D7H) mutations, with -1 or +1 His residues respectively. In the case of the mutation H6R, only His13 and His14 can coordinate Cu(II), and its impact can be observed mainly in component I, which lowers the transition pKa from 7.8 (Aβwt) to 7.2 [54]. However, no big differences have been found in either Cu(II) coordination or pKa in the case of the additional His-mutation D7H [56]. Regarding Cu(I) binding, a similar coordination sphere with 2 Nim implicated would be expected, as in the case of Aβwt [56,57]. For the case of Zn(II) binding, mainly the mutation of His6, and in some extent D7N, would have an impact on the coordination and affinity [35]. The punctual mutation H6R seems to favor the formation of dimers, with residues His13, His14 and Glu11 being implicated in the coordination of Zn(II) [58]. Induction of homodimers is also found for the D7H mutation upon Zn-binding [59].
Murine Aβ (mAβ) differs from hAβ in three-point mutations: R5G, Y10F and H13R. At least two different coordination modes have been found: Im and IIm, with a pKa much lower than for hAβ, i.e. 6.2 [60]. Some studies on the coordination have shown that the N-terminal amine is also implicated in the coordination of Cu(II). In the predominant mode at physiological pH, IIm, the main difference between hAβ and mAβ is the intervention of the deprotonated N- from the amide bond between Gly5 and His6 (murine) instead of Asp1-Ala2 (human) [60] (Fig. 1.B, left). In the case of Zn(II) binding, the implication of the two available histidine ligands (His6 and His14) has been corroborated, as well as the implication of the NH2-terminal of Asp1 and the COO- group of Glu11 [61] (Fig. 1.B, right). Another study has remarked the strong propensity of Zn(II) to induce dimerization mAβ, by binding to the His6 and His14 of two different peptide chains [62].
2.2. Coordination of Cu(II), Cu(I) and Zn(II) to αSyn, its H50Q mutated form and βSyn
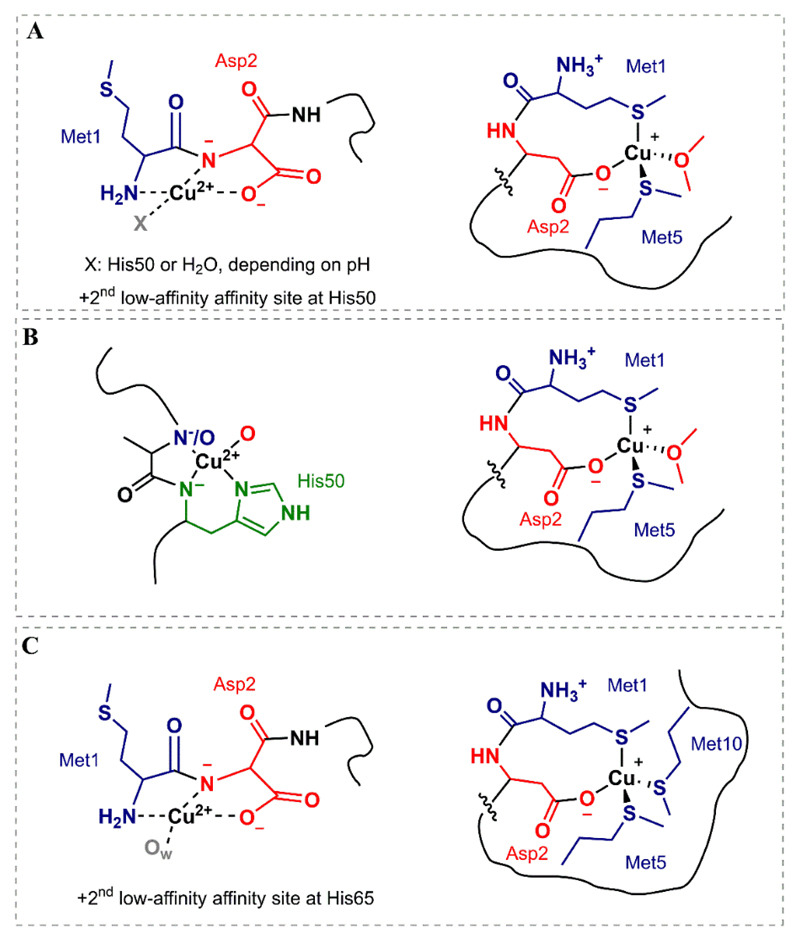
α-Synuclein is a protein with 140 amino acid residues and three main domains: (i) the N-terminal (1-60); (ii) the non-amyloid component (NAC) (61-95) where fibrillation is initiated; (iii) C-terminal region (96-140) rich in Pro, Asp and Glu residues. The high affinity binding site (table 2) has been found within the first 9 amino acids (MDVFMKGLS), where Cu(II) forms either a 2N2O or a 3N1O complex binding to the terminal amine, even the co-presence of both forms has been proposed [63]. Several amino acids have been identified as binding residues. One of the possibilities would be the deprotonated nitrogen of the Met1-Asp2 amide bond and the carboxylate of the Asp2 side chain, with either a molecule of H2O or the imidazole side chain of His50 [64–70] (Fig. 2.A, left). Other possibilities have related the Nim and the deprotonated N-amide from His50, an unidentified oxygen atom, and a H2O molecule, with the presence of the N-terminal as an axial ligand [71]. Most coordination studies have been carried out for the αSyn, although it has been found to be in the acetylated form (Ac-αSyn) in Lewy bodies in vivo [15]. In this case, coordination through the N-terminal domain is lost, but binding to the lower affinity binding site (His50) is maintained (Fig. 2.B, left) and Asp121 are maintained, although the binding affinity (table 2) lowers with respect to the one of unmodified α-synuclein [72,73]. The coordination of Cu(II) ions to the N-terminal motif and His50 might promote the formation of oligomers by favoring intra-molecule interaction [74]. Cu(I), being a softer cation than Cu(II), shows preference for the S atoms of the Met1 and Met5 residues in a tetrahedral structure together with the carboxyl group of Asp2 and a water molecule [75–78] (Fig. 2.A, right). This coordination sphere is maintained in the Ac-αSyn protein, as well as the affinity [79] (Fig. 2.B, right). As it should be relevant for the ROS production of Cu-αSyn complexes (section 4.2.3), the only common coordination groups for both Cu(II) and Cu(I) ions are the carboxylate of Asp2 [80], and in some extent the His50 imidazole ring [76]. In contrast to the wide number of studies regarding Zn-Aβ complexes and its role on amyloid-β aggregation, the coordination and influence of zinc ions on αSyn is still unclear [81,82], as maybe the low affinity of αSyn for Zn(II) (table 2) may lead to a low relevancy of the metal ion in the development of PD [83,84]. Two amino acids residues might be involved in the binding: Asp121 and His50 [83].
Fig. 2.
Cu(II) and Cu(I) coordination to (A) α-synuclein, (B) Ac-α-synuclein and (C) β-synuclein.
Six single amino acid pathological mutations of αSyn have been linked to familial PD: A30P, E46K, H50Q, G51D, A53T and A53E [85,86]. Nevertheless, the most significant one from a bioinorganic point of view would be H50Q, as it implies the metal-coordinating residue His [87,88]. Proukakis et al. [87] confirmed the Cu(II)-binding ability of the His50-mutated αSyn; but their Cu(II)-complexes showed a different EPR signature to that of the Cu(II)-αSynWT, consistent with a change of an equatorial oxygen by nitrogen. Further studies have confirmed the similar binding capacity of the mutant, with the only loss of the second binding site at His50 [63,89]. Nevertheless, Mason et al. [73] have remarked the importance of the N-acetylation in Cu(II) binding: not only for the WT αSyn, but also for its H50Q. In this study, the presence of both N-acetylation and H50Q mutation prevents Cu(II) coordination.
It could also be interesting to review the coordination ability of β-synuclein: (i) this conformation represents between 75 and 80% of the whole synuclein content in the brain [90]; (ii) it shares sequence with α-synuclein, with the exception of six point mutations between residues; (iii) it seems less prone to aggregation [91], and has shown to inhibit the aggregation of αSyn [92]. The coordination of Cu(II) does not differ between αSyn and βSyn, apart from the shift for the low-affinity binding site from His50 to His65 [67]. Nevertheless, the point mutation K10M induces slight changes on the Cu(I) coordination environment [75].
2.3. Cu(II) and Zn(II) coordination to Amylin
Type 2 diabetes (T2D) has also been linked to the formation of amyloid aggregates in the pancreas. The fibrils are formed by amylin (hIAPP), a peptide stored in granules of β-cells, altogether with insulin and a considerable concentration of zinc (in the millimolar range) [93,94]. The ProIAPP undergoes proteolysis and post-translational modifications, which produce a C-terminal amidation, by the peptidylamidating monooxygenase complex from a Gly residue. Formation of a disulfide bond between Cys2 and Cys7 leads to “mature” amylin. Both C-term amidation and the disulfide bond are believed to have a biological role [14] (table 1). Amylin is co-secreted with insulin upon high glucose concentrations. Three fragments can be differentiated in the sequence: the N-terminal, containing residues 1-19, is related to the binding to insulin and membranes [95]; the amyloidogenic control peptide sequence (20-29) is the fragment most prone to aggregation, while residues 30-37 and 8-20 would also contribute to self-association processes [96]. The metal ion binding properties of amylin arise mainly from the His18 residue and can be modulated by other amino acids or mutations in the sequence, as will be further described.
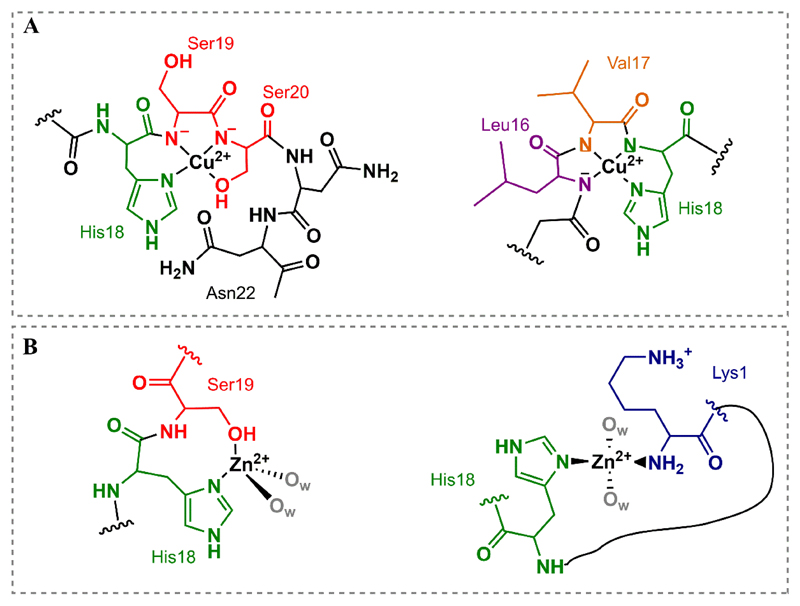
There is a controversy on the coordination mode of Cu(II) to monomers of human IAPP. The two common conclusions that can be extrapolated from the different studies are: the formation of a 1:1 complex and the anchoring role played by His18 [97–102]. The main differences arise from the amides adjacent to the His residue, which determine a coordination towards the N-terminal or the C- terminal region, using the model peptides hIAPP(14-22) and hIAPP(15-22) (Fig. 3.A). Quintanar and co-workers [99,102] have proposed a 3N1O equatorial binding mode at pH 7.5 for Ac-hIAPP(15-22)-NH2 supported by the use of different fragments of the peptide and spectroscopic and computational results (Fig. 3.A, left). Apart from the N1 of His18 being involved, the two following nitrogen amides (Ser19 and Ser20) would deprotonate and contribute to the coordination of Cu(II). The oxygen atom is provided by Ser20, either from the carbonyl group or mainly from the alcohol side chain. Moreover, Asn22 might contribute as an apical ligand. In the case of Magrì et al. [101] two species are found at physiological pH for the peptide Ac-PEG-hIAPP(14-22)-NH2: one with two deprotonated amides and another with 3 deprotonated amides, forcing the coordination toward the N-terminal region (Fig. 3.A, right). Both groups have used mutations and truncations of the peptide to more carefully study the Cu(II) coordination to hIAPP, but both are lacking control experiments by blocking the N- or C- terminal respectively. Moreover, the use of mutated peptides gives useful but not complete information on the case of very dynamic peptides. Using more advanced EPR methods or specific site labeling would help on the elucidation of the structure for Cu(II)-hIAPP complexes. In addition, Rowińska-Żyrek [100] has researched the coordination of Cu(II) and Zn(II) to the monomeric form of the membrane disrupting fragment of the peptide, which compromises residues 1-19. With a 4N (1Nim and 3NN-), besides His18 being crucial, the coordination mode is forced towards the N-terminal as only one amide is available after His18. Two very recent articles have also described the importance of the N-terminal amine in the coordination of Cu(II) at physiological pH, as described for Aβ and non-acetylated αSyn [103,104]. Cu(II) binding to the full-length peptide had not been studied before, and therefore the coordination to the N-terminal amine was not considered. These are relevant results, but it should be noted that, further and more specialized research should be done to specifically address the controversies. As in the case of Aβ and αSyn, which have been studied for longer time, many structures are proposed at the beginning. Converging to a widely-accepted coordination mode takes time and effort from different groups. As for example, whether the NH2- and the Nim constitute two different binding sites with different affinities, and how they would be modified by pH, ratio, etc. conditions must still be carefully researched. We encourage to further study the coordination chemistry of Cu(II)-hIAPP complexes.
Fig. 3.
(A) Representation of the two Cu(II) coordination models proposed for hIAPP with His18 as anchoring ligand, towards the C-terminal (left) and N-terminal (right). (B) Two different propositions for Zn(II) binding to monomeric hIAPP.
Determining the role of zinc in the coordination and aggregation of amylin could be crucial in the development of T2D. Zn(II) ions are stored in the granules of β-cells in the pancreatic islet, and are secreted into the blood stream altogether with insulin [105]. In addition, zinc concentration in the pancreas is one of the highest in the body, and many patients share a zinc deficiency [3,106]. Therefore, many are the groups who have studied the effect of Zn(II) in the aggregation of IAPP. Up to now, the role of His18 on the coordination of zinc is quite undeniable, as in the case of Cu(II). An oxygen atom of the contiguous residue Ser19 could also bind zinc [107] (Fig. 3.B, left). For the membrane disrupting fragment, hIAPP(1-19), the N-terminal amine, together with the Nim of His18 and 2 molecules of water coordinate zinc at physiological pH [100] (Fig. 3.B, right). As zinc contributes to the formation of hexamers of insulin [108], some authors have also stated the possibility of zinc bridging several molecules of amylin, which could have an impact on the aggregation [109–111].
While hIAPP amyloid fibrils have been found in vivo in diabetic patients, mIAPP is thought to be not amyloidogenic, as there is no evidence of amyloid deposit formation in rats. The two significative differences between the two forms of peptide are: the mutation of His18 by an arginine residue, and the presence of three Pro at positions 25, 28, 29. One of the hypothesis which could explain the lack of amyloidogenity is the increase water solubility upon the proline substitution [112,113]. Besides, the role of His18 in the binding of metal ions should not be dismissed. Cu(II) coordination to different fragments of mIAPP has been studied by spectroscopic [114] and spectrometric methods [115], as well as using the hIAPP(H18A) mutation [99]. A 1:1 complex would be formed at physiological pH, but with a lower affinity than hIAPP. Lacking His18, deprotonated amides and Arg, Ser or Asp side chains would contribute to the coordination of Cu(II). Mainly, the oxygen from the hydroxyl group of Ser19. A very recently study on the full-length mIAPP reveals an important role for the N-terminal amine on the coordination of Cu(II) [104]. The authors proposed a different metal-loading for mIAPP and hIAPP as one of the possible origins in the different toxicity. In the case of Zn(II) a dynamic binding could be stabilized between different conformations [116], with an important contribution of Asn14 [115].
As it has been seen in the previous section 2, mutations on the peptide and proteins sequences can have direct effects on the coordination of Cu and Zn, but mainly there are indirect consequences to their binding modes. For example, as seen for A2V and A2T Aβ mutants, for which their coordination modes are similar to the WT, but there is an impact on the pKa transition between component I and component II. Something similar has been described for the mutant Aβ (H6R) or (Ac-)αSyn (H50Q). This could be further reflected on their aggregative properties or the redox capability of Cu-Aβ complexes, for example. For this it is important, not only to check the coordination mode but also the implications on other parameters such as pKa, binding affinities, net charge, morphology of the complex, etc.
3. Impact of Cu and Zn on the aggregation of Aβ, αSyn and hIAPP
3.1. Common ground
Metal ions such as Cu and Zn can impact the aggregation of intrinsically disordered peptides and proteins, either by changing the kinetics of the process or the morphologies of the aggregates formed (Fig. 4). The influence of the metal ions in the aggregation of IDPs’ has been thoroughly reviewed in the last years (especially for Aβ) [1,3,12,85,125–129], including an article in this special issue [130].
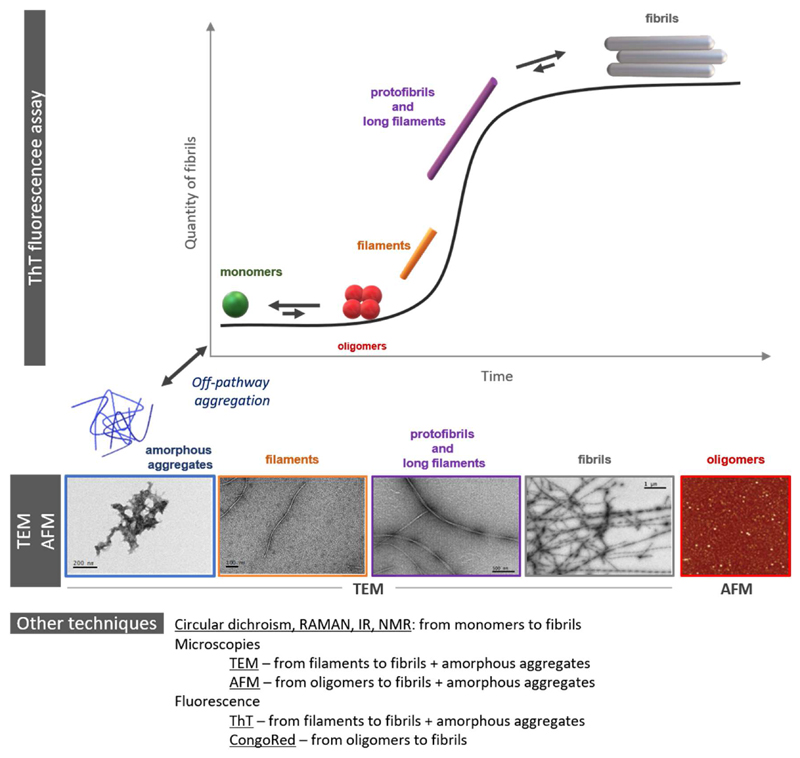
Fig. 4.
Schematic representation of some of the morphologies found in the aggregation pathway and off-pathway (amorphous aggregates) of amyloid peptides followed by ThT fluorescence assay and their TEM and AFM images. The most common techniques used to study the aggregation are listed.
Literature in the field of aggregation of amyloidogenic peptides and proteins is very extensive and it is common to find different tendencies in aggregation. A typical issue encountered by researchers working on amyloid aggregation is the irreproducibility of their experiments. Indeed, the aggregation of the peptides/proteins is highly dependent on many different factors such as concentration, starting state of the peptide/protein batch (i.e. fully monomeric vs trace of oligomers’ seeds), pH, temperature, nature of container (i.e. glass vs plastic), etc. Even a subtle variation on an unknown critical parameter will dramatically modify the outcome of the aggregation assay.
Another difficulty of amyloid aggregation field is the nature of the aggregates themselves: from amorphous to organized morphologies. If organized, it could be as oligomers (i.e. an assembly made of a limited number of peptides/proteins) or fibrils (i.e. an assembly made of a large number of peptides/proteins). Recent structures have been obtained using full-peptide or fragments (as well as chemically modified fragments) and X-ray crystallography, solid-state NMR and cryo-electronic microscopy [131–135]. These visualizations are possible organized states (i.e. oligomers/fibril) of amyloidogenic peptides/protein when they aggregate.
The techniques used to investigate aggregation are another pitfall. Indeed, depending of the assay chosen, it will favor a specific type of aggregate. For instance, the Thioflavin T (ThT) assay is sensitive for fibrils whereas oligomers will be observed using specific antibodies or FRET-pair fluorophores [136,137]. Amyloidogenic peptides/proteins (i.e. Aβ, αSyn, IAPP) share the ability to aggregate via a similar autocatalytic pathway where the starting point is monomers and the end point is fibrils. Transient species include oligomers, protofibrils and protofilaments. Oligomers are considered as the most toxic ones. The off-pathway aggregation leads to amorphous aggregates. Figure 4 illustrates these aggregation pathways as well as the most common techniques used to study them. On the top of this intricate mechanism, metal ions can shift equilibrium toward various directions. Due to this intrinsic complexity, experimental conditions and techniques used, the interpretation of the impact of metal ions on amyloid aggregation may vary a lot. Nevertheless, some common trends can be found in presence of metal ions. Especially relevant is the case of zinc, for which even small sub-stoichiometric quantities have a big influence on the aggregation of the Aβ peptide [43,44,138–142]. Intra and inter-molecular Zn binding promotes a fast aggregation of amorphous aggregates. Zn-induced aggregation even predominates in the presence of Cu [138], for which a protecting role has been assigned for zinc [143]. The influence of Cu(II) on the Aβ aggregation seems to be mainly dependent on its concentration [144], and leads to the production of highly cytotoxic oligomers [139,145,146]. There is also in vitro evidence of the impact of metal ions on the fibrillation of αSyn [67,74,81,82,147,148], and hIAPP [94,98,99,107,109,110,149–153], with notable controversy as well on the pro- or anti-aggregatory roles of Cu and Zn, as well be further outlined.
3.2. Effect of mutations on metal-induced aggregation of Aβ, αSyn and hIAPP
3.2.1. Amyloid-β peptide
Whereas H6R and D7N familial mutation do not affect the Aβ production via secretases pathway from APP [154], D7H mutation increase the total amount of Aβ and the Aβ42/Aβ40 ratio [122]. Aggregates promoted by these mutant peptides are described as oligomers. Consistent with the nature of aggregates, viability assays of these mutated Aβs showed higher level of toxicity [122,155]. It is likely that the H6R mutation will influence aggregating properties since this mutation has been show to impact Cu(II) binding. In contrast, it is possible that Cu(II)-mediated aggregation with D7N-Aβ will not significantly change from WT as its Cu(II) coordination remains unchanged and so it does not have an important impact on the aggregation [54].
Cu(II)-mediated aggregation of D7H-Aβ also seem to differ from WT (likely due its extra histidine ligand) [122]. Ying and co-workers [55] propose an important effect of the Cu-Aβ interaction kinetics on the roles of metal-Aβ interaction linked to aggregation, since formation of a copper-bridge complex is faster for H6R and D7N modified peptides than for the WT. Likewise, D7H-Aβ has shown a high propensity to aggregate in presence of Zn(II) ions. A recent study has proposed an important role for the extra His by the formation of a homodimer by two Zn(II) ions [59]. The English mutation, H6R, also seems to favor the Zn-induced dimer formation, as seen in section 2.1 [58].
Other FAD N-term mutations are also very interesting. Whereas A2T is protective against AD [51], A2V cause an early onset of disease at least on homozygous carriers [50], as it has been said in section 2.1. Since the difference between a threonine and a valine is subtle, various studies were conducted to disentangle molecular basis of the remarkable difference in term of AD [50,51][156–162]. Whereas A2T mutation induces a decrease of the overall amount of Aβ (Aβ40/Aβ42) via β-secretase pathway, A2V increases it [50,51,158,162]. The A2 mutation also affects the Aβ aggregation propensity [50,157,158,161,162]. Indeed, Molecular Dynamics (MD) simulation predicts that monomeric Aβ state differ between WT, A2T and A2V [159], as well as the oligomers formed and analyzed by Ion Mobility-Mass Spectrometry [160], in contrast to D7N [163]. Another intriguing point is that A2V mutation is causative of AD, only on homozygous carriers [50], suggesting a mixture of WT and A2V Aβ peptides might be less toxic via different aggregation properties. These hypotheses have been tested and confirmed that a mixture of A2V and WT is less toxic than individual peptides [50], and oligomer formation and aggregation properties are altered in presence of A2 mutants [157,158,160].
Furthermore, in the study of Somavarapu et al. [53], reviewed in section 2.1, Cu(II) shows a more pronounced propensity to extend the lag phase, especially for the pathogenic A2V. A fast, low intensity ThT response has been observed at the first hours of the in vitro aggregation experiment. They hypothesize that a faster interpeptide exchange due to the stabilization of component I (see section 2.1) could be the underlying cause of the formation of amorphous aggregates in the early hours of aggregation. Although A2 mutations are really interesting due to their fickle effects on the disease, only too few studies have been investigated the impact of metal ions on these mutations.
As the coordination of Cu(II) between the human and murine Aβ peptides differs, aggregation is also influenced by alteration of Cu(II) binding mode. As for the coordination, R5G is the key mutation impacting the reduction of Cu(II)-induced aggregation [164].
3.2.2. α-Synuclein
As with Aβ, metal ions modulate aggregation of αSyn. In presence of Cu(II) or Zn(II) and cross-linking reagents, multimeric species were observed on SDS-PAGE. Other metal ions in the same conditions were not effective to do so, suggesting these two metal ions would be able to promote αSyn self-assembly [165]. The effect of Cu(II) on aggregation kinetics has been investigated using ThT fluorescence [124,148,166,167], and signals broadening NMR spectroscopy [78]. Cu(II) promotes aggregation by accelerating it. In contrast, Anandhan et al. [168] based on PAGE experiments suggested Cu(II) changes the folded state of αSyn rather than increase its aggregation. Cu(II) also affect αSyn aggregation morphology as well as its pathway. Indeed, highlighted by time-dependent electronic microscopy and statistical analysis, Zhang et al. [166] showed that Cu(II) enhances the formation of small annular species likely oligomers. Moreover, predominance of these species occurs at an early stage of aggregation (before the appearance of fibrils) supporting their oligomeric or protofibrils nature [166,167]. Similar annular morphology enhanced in presence of Cu(II) has been observed on former independent studies [169,170].
Although less studied and maybe less potent than Cu(II), Zn(II) seems also able to impact αSyn aggregation. Zn(II) dramatically increases intermolecular interaction illustrated with αSyn bound to surface and on the tip of AFM [171]. It can induce multimeric species observed in SDS-PAGE when co-incubated with cross-linking reagents [165]. However, other study suggested Zn(II) has a minimal effect on the aggregation kinetic followed by ThT [81]. Disparity between studies may be explained by difference in experiments conditions as aggregation is highly dependent of concentration, pH, metal/protein ratio, techniques used, as described in section 3.1. Taking together, these various results suggest that metal ions, especially Cu(II), can modulate aggregation propensity of αSyn via different means. For instance, Cu(II) seems capable of accelerating aggregation as well as drifting its pathway towards oligomers.
It is worth noting that genetic mutations that exacerbate PD also impact αSyn aggregation [89,172], and some of them are related to metal binding site. In the case of the pathological H50Q mutant, Cu(II) has been described to enhance the aggregation of the protein [63,73,124], and change from fibrillar morphology to amorphous aggregates. The exact role of Cu(II) in the increasing of aggregation remains unclear, as H50Q mutation can enhance aggregation by itself [89]. Acetylation of the N-term of αSyn and βSyn has shown to result in α-helical forms in vitro [92,173]. Cu(I) stabilizes the formation of long and structure α-helical segments [79], while for Cu(II), more equivalents are needed to see an effect on the aggregation of Ac-αSyn compared to the non-acetylated form [72].
3.2.3. Amylin
hIAPP is a highly amyloidogenic peptide. Nevertheless, its aggregation can be modulated by different factors as pH and β-cell components [174]. In vitro experiments have shown that a lower pH slows the fibrillation of IAPP, via protonation of His18 [175]. Considering this residue as key for the coordination of copper and zinc, it would be interesting to elucidate the role of metal ions in the modulation of hIAPP and mIAPP aggregation. A consensus is given for the inhibitory effect on fibril formation upon zinc binding [3,11]. The origin of the amyloid formation inhibition could be electrostatic, due to repulsion of adjacent β-sheets in presence of zinc [107], although this result could be concentration-dependent [109,110]. Another hypothesis is the ability of Zn(II) to stabilize prefibrillar aggregates during lag phase, preventing the equilibrium towards mature fibrils [150]. A similar effect has been found for Cu(II): the formation of several conformers, such as small globular aggregates and oligomers, produces an off-pathway that could increase the energetic barrier to form amyloid fibrils, explaining the inhibition of fibril formation [98,99,102,152]. These Cu-induced conformers could be more toxic than fibrils and zinc-induced aggregates [152]. What is clear is that in vitro results are not able yet to elucidate the role of metal-binding in the toxicity of amyloid deposits in T2D.
Genetic factors such as the mutation of key residues in the sequence, can lead to drastic changes in the coordination of metal ions, and the impact these have on the aggregation and ROS production. Mutation of Ser20 by a Gly residue is linked to the early onset of T2D in Asiatic populations. The S20G mutation accelerates the aggregation of the peptide, compared to the wild-type amylin. One possible explanation is that as Gly is less bulky than Ser, the effect can be due to a decrease of sterically impediments [176–178]. Moreover, as Ser20 would be implicated in the coordination of Cu(II), its substitution reduces the ability to bind copper, and its inhibitory effect could be affected [102].
The impact that metal ions could have on the aggregation of mIAPP has not been thoroughly studied. Using the fragment hIAPP(15-22)H18A, which contains the key mutation of His18, showed that Cu(II) was not able to induce such an inhibitory effect on the aggregation of this fragment [99]. In the case of zinc, it has been proposed that the aggregation of mIAPP would be accelerated by increasing the pH and the concentration of zinc, although it could have, as in the case of the hIAPP, a ratio-dependent impact [116].
3.3. Conclusion
The impact of Zn and Cu on the aggregation of amyloidogenic peptides and proteins is undeniable. Furthermore, metal ions could be key also in presence of mutations and post-translational modifications. Since, even in some cases the coordination is not greatly modified, the impact of metal ions is visible on most of the cases. Mutations can also help to understand the link between ROS and aggregation and will be further reviewed in the next section.
4. Production of reactive oxygen species catalyzed by Cu-peptides
4.1. Introduction and biological relevance
Cu-ions can be very efficient catalysts for the reduction of dioxygen resulting in the generation of reactive oxygen species (ROS, like HO•, H2O2 and O2•-) [179–183]. An overproduction of ROS induces oxidative stress. Oxidative stress has been observed in several amyloidogenic diseases, including AD, PD and diabetes [184–187]. However, it is not known to what extent or if at all the complexes of Cu with amyloidogenic peptides and proteins (Aβ, αSyn and IAPP) contribute directly to the observed oxidative stress.
Although for AD and PD is clear that 1) Cu can bind to Aβ/αSyn [188–190], 2) oxidative stress occurs [181] and 3) Cu metabolism is altered [191–193], the proof that the Cu bound to Aβ or αSyn contributes directly to the oxidative stress is missing. The same holds also for in vivo studies in AD model organisms, i.e. an altered metabolism on amyloidogenic peptides, ROS production and Cu is reported [191,192], but how they are connected is not very well described.
In cellulo studies with Cu and amyloidogenic peptides were also conflicting: some showed that Cu increases the toxicity of Aβ or αSyn, others showed the contrary. Moreover, one of the inherent problem is that Cu can be a catalytic center and a structural element. As a structural element, Cu-binding to the amyloidogenic peptides changes the structure and hence the aggregation behavior. As a catalytic center it can trigger reactions, in particular ROS production. This means that changes in toxicity upon Cu-binding to Aβ/αSyn or amylin could be direct due ROS production of Cu bound to the peptides or indirect via the change in aggregation states. Analyzing the toxicity of mutants can suffer from the same problem, to decipher if the mutations impact Cu-binding only, aggregation or both.
4.2. ROS Chemistry of Cu complexes with Aβ, αSyn and IAPP in the test tube
During the last two decades, understanding of the chemistry of ROS production by these Cu-peptides in the test tube has significantly advanced, especially for Cu-Aβ, but also for Cu-αSyn, since the first studies reported for Cu complexes with APP [194], Aβ [195,196] and αSyn [197]. However, less is known for amylin compared to the other ones. Here we report mainly on the mechanistic studies performed in vitro. These studies generally showed that the Cu complexes with Aβ and αSyn are active in the production of ROS in the presence of dioxygen and the physiological relevant reducing agent ascorbate (Fig. 5). Cu-Aβ and Cu–αSyn were more active than several tested Cu-binding proteins and peptides [198–200]. They were less active than Cu in buffer [180], but this seems less relevant as non-biomolecule bound Cu is quasi inexistent in vivo.
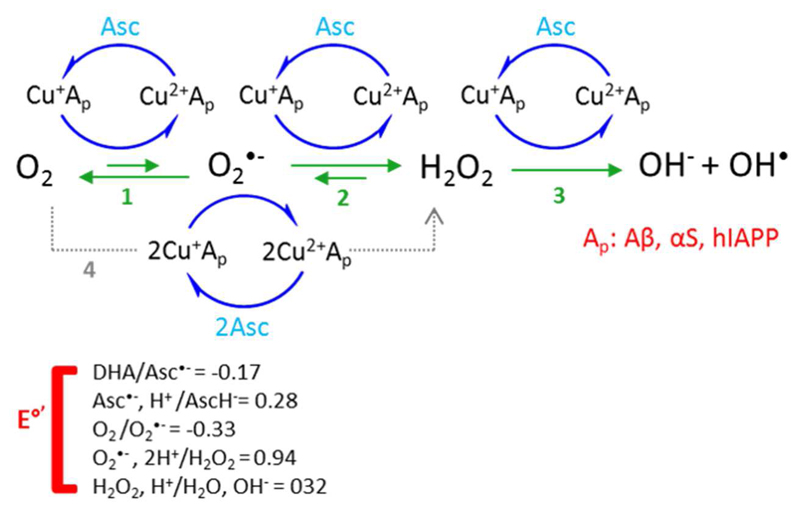
Fig. 5.
Schematic mechanism of the ROS production by Cu-amyloidogenic peptides/proteins complexes in the presence of ascorbate (Asc) and dioxygen. Amyloidogenic peptides and proteins considered in this work are Aβ, αSyn and IAPP.
For Cu-Aβ it has been shown that the production of H2O2 goes via two consecutive one electron reductions (reaction 1 and 2) and not via a simultaneously two electrons reduction (reaction 4) [201]. For αSyn and IAPP this is not known. The abbreviation DHA in the figure stands for dihydro ascorbic acid. The redox potential values were reported by Halliwell and Gutteridge [202].
These studies aimed mostly at answering the following questions: (i) how active the Cu complexes (Aβ, αSyn and IAPP) are in ROS production? (ii) What is the impact of mutations, specifically disease related mutations and the impact of the aggregation state? (iii) What is the molecular mechanism? (iv) What is the impact of oxidative damages to peptide/protein itself on coordination, aggregation and ROS production?
4.2.1. Comparison of activity in ROS production
It has been proposed that Cu bound to the flexible peptides and proteins Aβ, αSyn and IAPP, contributes to oxidative stress observed in AD, PD and T2D by catalyzing the production of ROS. There is a discussion in the literature if Aβ and αSyn quench Cu-based ROS production or promotes it. It is clear that Aβ and αSyn quench ROS production compared to Cu in the buffer. However, in biological environment Cu is always bound to biomolecules, mostly proteins. Thus, the real question is if Cu-complexes of amyloidogenic proteins produce more ROS than Cu-biomolecules present under healthy conditions. Comparison made with several Cu-proteins and peptides suggested indeed, that Cu-Aβ and –αSyn are more efficient in ROS production [200].
The activity of Cu-Aβ and Cu-αSyn has been compared in presence of ascorbate, monitoring the production of H2O2 and HO•, at a ratio of 1.2 or 2 peptide to Cu [198,199]. Both report a faster ROS production by Cu-αSyn and Cu-βsyn compared to Cu-Aβ. However, in such type of experiments it is important to make sure that there is no or neglectable contribution of non-peptide bound Cu, because as stated above, free Cu is very efficient in catalyzing ROS production.
There is no report on a direct comparison between IAPP with Cu-αSyn or Cu-Aβ. Nonetheless, ROS production by Cu-IAPP was observed in the presence and absence of a reducing agent [123,203,204].
4.2.2. Impact of mutations on ROS production
The impact of mutation or derivatization has been studied, including mutations connected to familial AD [56]. Single mutations have a relatively modest impact and no clear-cut correlation between ROS production and familial mutations was observed. The murine Aβ1-16 showed a slight decrease in the ROS production rate [56]. However, these studies have been done on the soluble Cu-binding domain, and hence does not involve the possible change in ROS production via a change in aggregation state.
To our knowledge, no study evaluating the impact of familial mutations on the rate of ROS production by Cu-αSyn is reported. As proposed by Valensin and co-workers, the effect on the ROS production upon acetylation of the terminal amine should be checked, as it influences the Cu(II) coordination but not that of Cu(I) [80] (and ref. therein).
For IAPP the only comparison known was with the rat sequence, which lacks the amino acid His18, hence the main Cu(II)-binding site is missing. Moreover, the comparison was limited to the H2O2 detection in the absence of any reducing agent and thus not under catalytic conditions [203]. This might explain the surprising effect when Cu-rat-IAPP did not show H2O2 production in contrast to human-IAPP. Considering the absence of a main Cu(II/I) binding site (His), one would expect more Cu in the buffer, that is very competent in ROS production.
4.2.3. Impact of aggregation state on ROS production
For αSyn and Aβ, the production of ROS seemed to be lower in the fibrillary or extensive aggregated forms [198]. If this is due to a different coordination or a lower accessibility (or both) is not clear. An important question, is the capacity of oligomeric forms to catalyze ROS. As these are supposed to be the most toxic species, a higher competence for ROS activity could contribute to explain their higher toxicity whenever in presence of metal ions as it has been proposed for αSyn [205,206]. Here the results are conflicting, as some publications report a higher activity for oligomers [200] and some others not [198]. However, there are different types of oligomers with different structures, which could explain the different reactivity observed. More work, particularly on the characterization of the oligomeric species present, would be necessary to clarify this point.
4.2.4. Mechanism of ROS production
The mechanism has been mainly studied on Cu-Aβ1-16, containing the Cu-binding domain. This peptide is non-aggregating and hence a fairly complete model for the monomeric Aβ, but a more limited model for the aggregated Cu-Aβ.
Cu-Aβ catalyzes the stepwise one electron reduction of dioxygen to HO• i.e. dioxygen is bound to Cu(I)-Aβ, reduced and superoxide is released [201]. This is likely the rate limiting step, as this is thermodynamically an uphill reaction [207]. Then another Cu(I)-Aβ can reduce superoxide to H2O2 etc. No indication for a two electrons reduction going directly from dioxygen to H2O2 was observed (Fig. 5) [201].
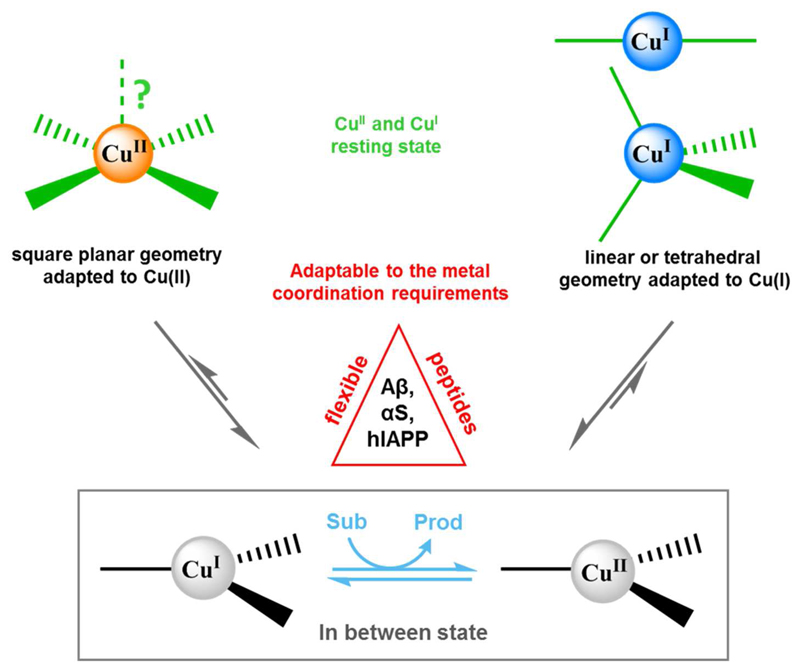
So far, the most developed model is based on the so-called resting states and in-between state [23]. The resting states are the most populated coordination of Cu(II) and Cu(I). As described above, their coordination sphere is very different, which implies a large reorganization from the resting state of Cu(II) to the resting state of Cu(I). This reorganization includes also at least one proton transfer at the N-terminal amine (protonated in Cu(I), deprotonated in Cu(II)). Such a large reorganization is sluggish and hence not efficient in redox cycling between Cu(II) and Cu(I), which is the underlying base of ROS production. Indeed, this view has been supported by electrochemical studies. These studies suggested that the redox cycling passes via an in-between state, which is very competent in Cu redox cycling and supposed not to involve a proton transfer on the peptide (but on the substrate depending on the reaction (Fig. 5)) [208].
Based on these electrochemical studies in the absence of a substrate, an in-between state has also been suggested for the chemical redox reactions, by dioxygen and ascorbate (Fig. 5). Recently, the analysis of a multitude of Aβ derivatives resulted in the proposition of an in-between state and the ligands involved (Fig. 6) [56].
Fig. 6.
Schematic representation of the different coordination geometries around the Cu center in the equilibrium between the “resting state” (for Cu(II) on the left and Cu(I) on the right) and the “in between state”. While for Cu(II) a square planar geometry is favored, Cu(I) prefers a diagonal or tetrahedral geometry. In the “in between state”, where the electron transfer between the Cu and the substrate occurs, we can imagine a highly similar coordination sphere for Cu(II) and Cu(I) in which the substrate is involved. In this way a low re-organization energy would be needed. Aβ, αSyn and IAPP are flexible peptides thus able to adapt to the Cu coordination requirements.
No mechanistic studies of this type were reported for Cu-αSyn or Cu-IAPP. However, some comments and suggestions can be given. Cu-αSyn has also two very different “resting” states for Cu(II) and Cu(I) (see above). Thus, by analogy with Aβ, an “in-between state” may also be anticipated, since αSyn is also a very flexible protein and such an in-between state would be dynamically achievable. Moreover, analyzing the damage of HO• attack in Cu-αSyn, which is assumed to mainly react with the most nearby residues (i.e. ligands) showed that Met1 is much more efficiently oxidized than Met5 [209,210]. This indicates that the ROS producing state is not the Cu(I) resting state (see Fig. 2). Thus, the in-between state in Cu-αSyn likely involves the ligation of S of Met1, and maybe Asp2 (as this residue is bound in either resting state).
In the case of IAPP, the resting state of Cu(I) is not known. But because IAPP has only one His and no Cys or Met, one can assume that Cu(I) is very loosely bound, and once Cu(II) is reduced to Cu(I), the latter will decoordinate rapidly. The Cu(II) resting state involves two amidate ligations, that stabilize Cu(II) against reduction. Thus, in ROS measurements, more care must be taken for IAPP, to exclude contribution of unbound Cu.
4.2.5. Redox potentials
Measuring of reduction and oxidation potentials of Cu-Aβ and Cu-αSyn (but not IAPP) complexes by cyclic voltammetry has been reported in the literature (Table 3). Such studies are important to understand the reaction mechanism; however, one must keep in mind that in these electrochemical studies no chemical substrate is present, and hence the mechanism could be different. The electrochemical study on Cu-Aβ in the absence of substrates revealing a low populated in-between state was the base of the proposition that also in the mechanism of ROS production an in-between state is present as discussed in section 4.2.4 (although of different structure than the one in of electrochemistry) [201,208].
Table 3. Compilation of electrochemical studies of Cu-Aβ and Cu-αSyn complexes.
| Peptide Complex | Redox Potential (V) [NHE]† | Experimental Conditions | Ref. | |||
|---|---|---|---|---|---|---|
| Electrolyte Solution | Electrode | Scan Rate (mV/s) | Cu:peptide ratio‡ | |||
| Cu-Aβ1-42 | E° ~ 0.500–0.550 [0.699 – 0.749] (quasi-reversible) |
PB pH 7.3 | Ag/AgCl (1M KCl) (indium/tinoxide working electrode) |
- | 1:6 (17 μM) |
[212] |
| Cu-Aβ1-16 | Epa = 0.340 Epc = 0.650 |
Tris/HCl pH 7.4, 100 mM NaCl |
NHE | - | 1:1 (1 mM) |
[200] |
| Cu-Aβ1-28 | Epa = 0.330 Epc = 0.630 |
Tris/HCl pH 7.4, 100 mM NaCl |
NHE | - | 1:1 (1 mM) |
[200] |
| Cu-Aβ1-16 | Epa = 0.780 [0.979] Epc = 0.085 [0.284] |
PB 5 mM pH 7.4, 0.1M Na2SO4 10% DMSO |
Ag/AgCl | 20 | 1:1 (50 μM) |
[213] |
| Cu-Aβ1-42 | Epa = 0.600 [0.799] Epc ~ 0.020 [0.219] |
PB 5 mM, pH 7.4, 0.1M Na2SO4 10% DMSO |
Ag/AgCl | 20 | 1:1 (50 μM) |
[213] |
| Cu-Aβ1-40 | E° = 0.100 [0.299] | KCl 0.2 M, pH 6.9 |
Ag/AgCl | 50 | 1:1 | [214] |
| Cu-Aβ1-16 | 0.300 vs (NHE) (In-between state) |
PIPES buffer 25 mM, pH 6.7, 0.2 M KCl |
SCE | 20, 50, 100 | 1:5 (0.2 mM) |
[208] |
| Cu-Aβ1-16 | Epc = -0.076 [0.123] Epa =-0.140 [0.059] E°= 0.032 [0.231] (pH 6.5, mainly Component I) E° = -0.376 [-0.177] (pH 8.5, mainly Component II) |
PB 10 mM, 50 mM Na2SO4 |
Ag/AgCl | 5 | 1:4 (100 μM) |
[215] |
| Cu-Ac-Aβ1-16 | E°=0.060 [0.259] (pH 7.4, mainly Component I) E° = -0.353 [-0.154] (pH 9.5, Component II) |
PB 10 mM, 50 mM Na2SO4 |
Ag/AgCl | 5 | 1:4 (100 μM) |
[215] |
| Cu-Aβ1-16 | Epa - Epc = 0.450 [0.649] | KNO3 96 mM HNO3 4 mM pH 7.4 |
Ag/AgCl | 20, 100 | 0.9:1 (0.45 mM) |
[216] |
| Cu-Aβ4-16 | Epa = 0.830 [1.029] | KNO3 96 mM HNO3 4 mM pH 7.4 |
Ag/AgCl | 20, 100 | 0.9:1 (0.45 mM) |
[216] |
| Cu-αSyn1-140 | 0.018 [0.217] (quasi-reversible) |
PB 5 mM pH 7.4, 0.1 M Na2SO4 |
Ag/ AgCl | 5 | 1:2 (50 μM) |
[217] |
| Cu-αSyn1-19 | 0.053 [0.252] (quasi-reversible) |
PB 5 mM pH 7.4, 0.1 M Na2SO4 |
Ag/ AgCl | 5 | 1:2 (50 μM) |
[217] |
| Cu-αSyn1-6 | Epc (1) ≈ −0.365 [-0.166] Epc(2) = −0.461 [-0.262] Epa (1) ≈ −0.101 [0.098] Epa (2) = −0.024 [0.175] |
PB 10 mM pH 7.4, 50 mM Na2SO4 |
Ag/ AgCl | 5 | 1:1 (300 μM) |
[209] |
All the redox potential values were obtained using a glassy carbon working electrode unless otherwise stated.
Conversion value for the Ag/AgCl electrode to NHE obtained from [218].
Cu concentration is indicated in parenthesis.
These studies were done under different experimental conditions and therefore the redox potential values differ from one to another when comparing the same peptide fragments. Parameters that can have an influence in the redox potential values include pH, Cu-Peptide ratio, and scan rate. It is important to add enough peptide to avoid contribution of free Cu (or Cu bound elsewhere). A titration of Aβ to Cu at 0.2 mM showed that up to 5 equivalents were needed to suppress completely the contribution of Cu outside the main binding site at pH 6.7 [208]. Such experiment must be performed for each peptide and condition, since peptide, buffer, pH and concentration can impact the over-stoichiometry needed. Several studies use just 1:1 ratios, likely too low to suppress to measure purely the Cu bound to the main site (Table 3).
pH can influence the reduction and oxidation potential as well. For instance, the reduction of Cu(II)-Aβ in the resting state is more difficult in the component II compared to I, as the amide bond stabilizes Cu(II) (Table 3, ref. [211]). In line with this, the reduction potential is also lower for Cu-αSyn compared to component I on Aβ, as it involves an amide bond. Thus, one could predict that the reduction potential is even lower for Cu-IAPP, as Cu is bound to two amides (Fig. 3A).
This consideration is based on the reduction of the resting state. The cyclic voltammograms of all measurements show non-reversible behavior, in line with the different coordination site of the resting state of Cu(I) and Cu(II). However, the deepest analysis has been performed for Cu-Aβ and showed that redox properties are more complicated. Balland et al. proposed a new mechanism, called preorganization electron transfer (POET) [208].
The POET consists of a low populated state (with similar coordination sphere for Cu(I) and (II)), that undergoes very efficient redox cycling due to very small reorganization energy. For Cu-Aβ a redox potential of 0.300 V (vs NHE) was found. Considering the flexibility of αSyn and IAPP, as well as the coordination difference between Cu(II) and Cu(I), a POET mechanism can be proposed for these complexes. It is therefore important, that a deep electrochemical analysis is made by using different scan rates, concentrations, buffers, ratios etc. to reveal these properties, as it was done for Cu-Aβ [208].
4.2.6. Oxidative damage of peptide and impact ROS production
The produced reactive oxygen species, and in particular HO•, can attack and modify the peptide itself. In the case of Aβ it was shown that the HO• radical attack mainly the N-terminal amine and the different His residues. The derivatization of these ligands reduces their Cu-coordination and therefore, the coordination sphere is modified. This leads to an increase in ROS production, as kind of a vicious circle [219]. ROS production measurements for Cu-αSyn showed an increase in the rate with time, indicating that Cu-coordination changes would be due to oxidative damage of the peptide [198]. If this higher active state of Cu is still bound to oxidized αSyn or if the higher activity is just due to the release of Cu from αSyn, and hence higher active “free” Cu, is unknown.
For Cu-Aβ it has been shown that the main peptide degradation occurs on the N-terminal Asp and the His. This leads to changes in the Cu(I) and Cu(II) coordination (resting state) and might also affect the in-between state explaining then the higher ROS production rate.
For the case of αSyn the main oxidation targets are Met1 and Met5 to form the respective sulfoxides, with the possibility to further oxidize them to the sulfone or to decompose. Decarboxylation of Asp2 and cleavage of the Met1-Asp2 bond, as well as other peptide bonds further away from the Cu-biding sites, were also observed. The oxidation of Met sulfur will highly impact the binding of Cu(I), but not much Cu(II) [210,220].
An important feature is also the fact that peptide oxidative damage can not only change Cu-coordination and ROS production, but also the aggregation behavior. Lee and coworkers [197] have also shown that ROS production by Cu-αSyn yields protein oligomers, ending up in aggregation. Also in the case of Aβ, oxidative damage by Cu catalyzed ROS production can impact aggregation behavior and structure of aggregates [128,221]. For instance, when Tyr cross-linking was observed, stabilization of oligomers and fibrils can occur [222,223].
4.3. Conclusion
Cu-Aβ and Cu-αSyn are quite competent in catalyzing the production of ROS in the presence of dioxygen and ascorbate, at least more as the tested Cu-peptides and proteins. The efficiency of IAPP is not clear.
All three peptides are flexible and have very different coordination spheres between the two Cu redox states (called resting states). Supported by electrochemistry, a large reorganization is needed to pass from Cu(I) to Cu(II) and vice versa.
Based on this, a similar mechanism can be proposed for Cu-αSyn (and maybe IAPP), that includes an in-between state. This in-between state is different from the resting state, which has a lower populated state but is dynamically accessible. This state is responsible for the ROS production. The efficiency of the ROS production depends on the population of the in-between state in its reactivity. The reactivity is depending on the redox potential (of the in-between state) and substrate binding/accessibility. The generated ROS can damage biomolecules like DNA, lipids, etc. but also the peptide itself. This will lead to a change in the Cu-coordination and for Cu-Aβ and Cu-αSyn to an increase in ROS production. Such oxidations can also affect the aggregation behavior, another important factor to consider.
Although misregulation of amyloidogenic peptides, copper and ROS are well documented in AD and PD (but not in diabetes), the importance of the direct ROS production by Cu-Aβ/αSyn in cellulo and in vivo is not clear and remains an important open question.
5. General conclusions
In the present review, we have described the coordination modes of Cu and Zn ions to three peptides and protein of biological interest: Aβ, αSyn and amylin and to their familial and murine mutants. General coordination trends are shared by these peptides: (i) when available and close to other binding amino-acid residues, the N-terminal amine is the preferred anchoring sites for Cu(II), else His residues serve as the anchoring residues. As N ligands, His and deprotonated amide groups from the peptide bond complete the Cu(II) environment while as O ligands, carboxylate and carbonyl groups are endogenous ligands, and water exogenous ligand. (ii) His and to a lesser extent the N-terminal amine are the two preferred N-donor ligand of Zn(II), while carboxylate and water are the O-donor ligands. (iii) Cu(I) prefers Met, His and water. (iv) In addition, each metal ion adopts its favored geometry indicating that the nature of the metal binding site in these disordered peptides is not only metal-dependent but metal-driven too. This has two main consequences. First, regarding the modulation of the aggregation propensity of the amyloidogenic peptides, each metal ion does influence the process differently than the other ones. Secondly, regarding the production of ROS, the postulated mechanism relies on a complex redox process and relies on the intervention of an “in-between” state where the geometry around the Cu center is intermediate between those of Cu(I) and Cu(II).
As another very general feature, Cu and Zn coordination to familial and murine mutants are modified for the three kinds of peptides and protein, in line with mutations involving binding amino-acids residues. Mutations are also associated with different aggregation and ROS production ability, in link with different metal ions – peptides interactions. As mutations are either associated with early-onset of the disease (familial ones) or absence of disease (murine ones), this underlines the connection between metal ions and amyloid diseases.
From current literature, it seems that the link between metal ions and amyloid diseases would be particularly relevant in the case of metal-induced aggregation, especially in the case of Aβ. Hence, further studies are needed to validate a similar connection mediated by ROS production. Future works will also address the connection between metal ions and amyloid diseases, via aggregation and/or ROS production, for the other two diseases (PD and T2D) of interest in the present review as well as for any amyloid-related pathologies.
Acknowledgements
The ERC aLzINK grant (ERC-StG-638712) is acknowledged for financial support (granted to C.H.). We acknowledge financial support from the University of Strasbourg Institute for Advanced Study (USIAS; to P.G., C.H. and P.F.), University of Strasbourg (IDEX program, PhD grant to A.S.) and the Frontier Research in Chemistry Foundation of Strasbourg (Installation grant to P.F.)
Abbreviations
- AD
Alzheimer's disease
- AFM
Atomic Force Microscopy
- APP
Amyloid Precursor Protein
- Aβ
Amyloid-β peptide
- DHA
Dihydro Ascorbic acid
- EPR
Electron Paramagnetic Resonance
- FAD
Familial Alzheimer's disease
- hAβ
hIAPP, human peptides
- IAPP
islet amyloid polypeptide
- mAβ
mIAPP, murine peptides
- IDPs
intrinsically disordered peptides/proteins
- MD
Molecular Dynamics
- NAC
Non-amyloid Component
- Nim
imidazole nitrogen
- NMR
Nuclear Magnetic Resonance
- N-term
N-terminal
- PB
phosphate buffer
- PD
Parkinson's disease
- rAβ, rIAPP
rat peptides
- SDS-PAGE
Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis
- T2D
Type II diabetes mellitus
- ThT
Thioflavin T
- WT
Wild Type (no mutated peptide)
- αSyn
alpha-synuclein
- βSyn
beta-synuclein
Footnotes
Conflicts of interest: none.
References
- [1].Ke PC, Sani M-A, Ding F, Kakinen A, Javed I, Separovic F, Davis TP, Mezzenga R. Implications of peptide assemblies in amyloid diseases. Chem Soc Rev. 2017 doi: 10.1039/C7CS00372B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Barnham KJ, Bush AI. Metals in Alzheimer’s and Parkinson’s Diseases. Curr Opin Chem Biol. 2008;12:222–228. doi: 10.1016/j.cbpa.2008.02.019. [DOI] [PubMed] [Google Scholar]
- [3].DeToma AS, Salamekh S, Ramamoorthy A, Lim MH. Misfolded proteins in Alzheimer’s disease and type II diabetes. Chem Soc Rev. 2012;41:608–621. doi: 10.1039/C1CS15112F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kepp KP. Bioinorganic Chemistry of Alzheimer’s Disease. Chem Rev. 2012;112:5193–5239. doi: 10.1021/cr300009x. [DOI] [PubMed] [Google Scholar]
- [5].Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, Markesbery WR. Copper, iron and zinc in Alzheimer’s disease senile plaques. J Neurol Sci. 1998;158:47–52. doi: 10.1016/S0022-510X(98)00092-6. [DOI] [PubMed] [Google Scholar]
- [6].Suh SW, Jensen KB, Jensen MS, Silva DS, Kesslak PJ, Danscher G, Frederickson CJ. Histochemically-reactive zinc in amyloid plaques, angiopathy, and degenerating neurons of Alzheimer’s diseased brains. Brain Res. 2000;852:274–278. doi: 10.1016/S0006-8993(99)02096-X. [DOI] [PubMed] [Google Scholar]
- [7].Dong J, Atwood CS, Anderson VE, Siedlak SL, Smith MA, Perry G, Carey PR. Metal Binding and Oxidation of Amyloid-β within Isolated Senile Plaque Cores: Raman Microscopic Evidence. Biochemistry. 2003;42:2768–2773. doi: 10.1021/bi0272151. [DOI] [PubMed] [Google Scholar]
- [8].Miller LM, Wang Q, Telivala TP, Smith RJ, Lanzirotti A, Miklossy J. Synchrotron-based infrared and X-ray imaging shows focalized accumulation of Cu and Zn co-localized with β-amyloid deposits in Alzheimer’s disease. J Struct Biol. 2006;155:30–37. doi: 10.1016/j.jsb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- [9].Leskovjan AC, Lanzirotti A, Miller LM. Amyloid plaques in PSAPP mice bind less metal than plaques in human Alzheimer’s disease. Neuroimage. 2009;47:1215–1220. doi: 10.1016/j.neuroimage.2009.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Davies KM, Bohic S, Carmona A, Ortega R, Cottam V, Hare DJ, Finberg JPM, Reyes S, Halliday GM, Mercer JFB, Double KL. Copper pathology in vulnerable brain regions in Parkinson’s disease. Neurobiol Aging. 2014;35:858–866. doi: 10.1016/j.neurobiolaging.2013.09.034. [DOI] [PubMed] [Google Scholar]
- [11].Tomasello MF, Sinopoli A, Pappalardo G. On the Environmental Factors Affecting the Structural and Cytotoxic Properties of IAPP Peptides. J Diabetes Res. 2015;2015 doi: 10.1155/2015/918573. 918573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Faller P, Hureau C, La Penna G. Metal Ions and Intrinsically Disordered Proteins and Peptides: From Cu/Zn Amyloid-β to General Principles. Acc Chem Res. 2014;47:2252–2259. doi: 10.1021/ar400293h. [DOI] [PubMed] [Google Scholar]
- [13].James SA, Volitakis I, Adlard PA, Duce JA, Masters CL, Cherny RA, Bush AI. Elevated labile Cu is associated with oxidative pathology in Alzheimer disease. Free Radic Biol Med. 2012;52:298–302. doi: 10.1016/j.freeradbiomed.2011.10.446. [DOI] [PubMed] [Google Scholar]
- [14].Roberts AN, Leighton B, Todd JA, Cockburn D, Schofield PN, Sutton R, Holt S, Boyd Y, Day AJ, Foot EA, Willis AC, et al. Molecular and functional characterization of amylin, a peptide associated with type 2 diabetes mellitus. Proc Natl Acad Sci USA. 1989;86:9662–9666. doi: 10.1073/pnas.86.24.9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Öhrfelt A, Zetterberg H, Andersson K, Persson R, Secic D, Brinkmalm G, Wallin A, Mulugeta E, Francis PT, Vanmechelen E, Aarsland D, et al. Identification of Novel α-Synuclein Isoforms in Human Brain Tissue by using an Online NanoLC-ESI-FTICR-MS Method. Neurochem Res. 2011;36:2029–2042. doi: 10.1007/s11064-011-0527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Polevoda B, Sherman F. N-terminal Acetyltransferases and Sequence Requirements for N-terminal Acetylation of Eukaryotic Proteins. J Mol Biol. 2003;325:595–622. doi: 10.1016/S0022-2836(02)01269-X. [DOI] [PubMed] [Google Scholar]
- [17].Kowalik-Jankowska T, Ruta M, Wiśniewska K, Łankiewicz L. Coordination abilities of the 1-16 and 1-28 fragments of β-amyloid peptide towards copper(II) ions: A combined potentiometric and spectroscopic study. J Inorg Biochem. 2003;95:270–282. doi: 10.1016/S0162-0134(03)00128-4. [DOI] [PubMed] [Google Scholar]
- [18].Syme CD, Nadal RC, Rigby SEJ, Viles JH. Copper binding to the amyloid-β (aβ) peptide associated with Alzheimer’s disease: Folding, coordination geometry, pH dependence, stoichiometry, and affinity of Aβ-(1-28): Insights from a range of complementary spectroscopic techniques. J Biol Chem. 2004;279:18169–18177. doi: 10.1074/jbc.M313572200. [DOI] [PubMed] [Google Scholar]
- [19].Karr JW, Kaupp LJ, Szalai VA. Amyloid-β binds Cu2+ in a mononuclear metal ion binding site. J Am Chem Soc. 2004;126:13534–13538. doi: 10.1021/ja0488028. [DOI] [PubMed] [Google Scholar]
- [20].Drew SC, Noble CJ, Masters CL, Hanson GR, Barnham KJ. Pleomorphic Copper Coordination by Alzheimer ’ s Disease Amyloid- Peptide. J Am Chem Soc. 2009;131:1195–1207. doi: 10.1021/ja808073b. [DOI] [PubMed] [Google Scholar]
- [21].Dorlet P, Gambarelli S, Faller P, Hureau C. Pulse EPR spectroscopy reveals the coordination sphere of copper(II) Ions in the 1-16 amyloid-β peptide: A key role of the first two N-terminus residues. Angew Chemie Int Ed. 2009;48:9273–9276. doi: 10.1002/anie.200904567. [DOI] [PubMed] [Google Scholar]
- [22].Drew SC, Masters CL, Barnham KJ. Alanine-2 carbonyl is an oxygen ligand in Cu2+ coordination of Alzheimer’s disease amyloid-β peptide - Relevance to N-terminally truncated forms. J Am Chem Soc. 2009;131:8760–8761. doi: 10.1021/ja903669a. [DOI] [PubMed] [Google Scholar]
- [23].Hureau C. Coordination of redox active metal ions to the amyloid precursor protein and to amyloid-β peptides involved in Alzheimer disease. Part 1: An overview. Coord Chem Rev. 2012;256:2164–2174. doi: 10.1016/j.ccr.2012.03.037. [DOI] [Google Scholar]
- [24].Hureau C, Dorlet P. Coordination of redox active metal ions to the amyloid precursor protein and to amyloid-β peptides involved in Alzheimer disease. Part 2: Dependence of Cu(II) binding sites with Aβ sequences. Coord Chem Rev. 2012;256:2175–2187. doi: 10.1016/j.ccr.2012.03.034. [DOI] [Google Scholar]
- [25].Guilloreau L, Damian L, Coppel Y, Mazarguil H, Winterhalter M, Faller P. Structural and thermodynamical properties of CuII amyloid-β16/28 complexes associated with Alzheimer’s disease. J Biol Inorg Chem. 2006;11:1024–1038. doi: 10.1007/s00775-006-0154-1. [DOI] [PubMed] [Google Scholar]
- [26].Shearer J, Szalai VA. The Amyloid-β Peptide of Alzheimer’s Disease Binds Cu I in a Linear Bis-His Coordination Environment: Insight into a Possible Neuroprotective Mechanism for the Amyloid-β Peptide. J Am Chem Soc. 2008;130:17826–17835. doi: 10.1021/ja805940m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hureau C, Balland V, Coppel Y, Solari P-L, Fonda E, Faller P. Importance of dynamical processes in the coordination chemistry and redox conversion of copper amyloid-β complexes. J Biol Inorg Chem. 2009;14:995–1000. doi: 10.1007/s00775-009-0570-0. [DOI] [PubMed] [Google Scholar]
- [28].Mekmouche Y, Coppel Y, Hochgräfe K, Guilloreau L, Talmard C, Mazarguil H, Faller P. Characterization of the ZnII Binding to the Peptide Amyloid-β1-16 linked to Alzheimer’s Disease. ChemBioChem. 2005;6:1663–1671. doi: 10.1002/cbic.200500057. [DOI] [PubMed] [Google Scholar]
- [29].Syme CD, Viles JH. Solution 1H NMR investigation of Zn2+ and Cd2+ binding to amyloid-beta peptide (Aβ) of Alzheimer’s disease. Biochim Biophys Acta - Proteins Proteomics. 2006;1764:246–256. doi: 10.1016/j.bbapap.2005.09.012. [DOI] [PubMed] [Google Scholar]
- [30].Zirah S, Kozin SA, Mazur AK, Blond A, Cheminant M, Segalas-Milazzo I, Debey P, Rebuffat S. Structural Changes of Region 1-16 of the Alzheimer Disease Amyloid beta-Peptide upon Zinc Binding and in Vitro Aging. J Biol Chem. 2006;281:2151–2161. doi: 10.1074/jbc.M504454200. [DOI] [PubMed] [Google Scholar]
- [31].Danielsson J, Pierattelli R, Banci L, Gräslund A. High-resolution NMR studies of the zinc-binding site of the Alzheimer’s amyloid β-peptide. FEBS J. 2007;274:46–59. doi: 10.1111/j.1742-4658.2006.05563.x. [DOI] [PubMed] [Google Scholar]
- [32].Gaggelli E, Janicka-Klos A, Jankowska E, Kozłowski H, Migliorini C, Molteni E, Valensin D, Valensin G, Wieczerzak E. NMR Studies of the Zn 2+ Interactions with Rat and Human β-Amyloid (1–28) Peptides in Water-Micelle Environment. J Phys Chem B. 2008;112:100–109. doi: 10.1021/jp075168m. [DOI] [PubMed] [Google Scholar]
- [33].Damante CA, Ősz K, Nagy Z, Pappalardo G, Grasso G, Impellizzeri G, Rizzarelli E, Sóvágó I. Metal Loading Capacity of Aβ N-Terminus: a Combined Potentiometric and Spectroscopic Study of Zinc(II) Complexes with Aβ(1–16), Its Short or Mutated Peptide Fragments and Its Polyethylene Glycol–ylated Analogue. Inorg Chem. 2009;48:10405–10415. doi: 10.1021/ic9012334. [DOI] [PubMed] [Google Scholar]
- [34].Tsvetkov PO, Kulikova AA, Golovin AV, Tkachev YV, Archakov AI, Kozin SA, Makarov AA. Minimal Zn2+ Binding Site of Amyloid-β. Biophys J. 2010;99:L84–L86. doi: 10.1016/j.bpj.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Alies B, Conte-Daban A, Sayen S, Collin F, Kieffer I, Guillon E, Faller P, Hureau C. Zinc(II) Binding Site to the Amyloid-β Peptide: Insights from Spectroscopic Studies with a Wide Series of Modified Peptides. Inorg Chem. 2016;55:10499–10509. doi: 10.1021/acs.inorgchem.6b01733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Karr JW, Szalai VA. Cu(II) Binding to Monomeric, Oligomeric, and Fibrillar Forms of the Alzheimer’s Disease Amyloid-β Peptide. Biochemistry. 2008;47:5006–5016. doi: 10.1021/bi702423h. [DOI] [PubMed] [Google Scholar]
- [37].Sarell CJ, Syme CD, Rigby SEJ, Viles JH. Copper(II) Binding to Amyloid-β Fibrils of Alzheimer’s Disease Reveals a Picomolar Affinity: Stoichiometry and Coordination Geometry Are Independent of Aβ Oligomeric Form. Biochemistry. 2009;48:4388–4402. doi: 10.1021/bi900254n. [DOI] [PubMed] [Google Scholar]
- [38].Parthasarathy S, Long F, Miller Y, Xiao Y, McElheny D, Thurber K, Ma B, Nussinov R, Ishii Y. Molecular-Level Examination of Cu 2+ Binding Structure for Amyloid Fibrils of 40-Residue Alzheimer’s β by Solid-State NMR Spectroscopy. J Am Chem Soc. 2011;133:3390–3400. doi: 10.1021/ja1072178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gunderson WA, Hernández-Guzmán J, Karr JW, Sun L, Szalai VA, Warncke K. Local Structure and Global Patterning of Cu 2+ Binding in Fibrillar Amyloid-β [Aβ(1–40)] Protein. J Am Chem Soc. 2012;134:18330–18337. doi: 10.1021/ja306946q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Tõugu V, Palumaa P. Coordination of zinc ions to the key proteins of neurodegenerative diseases: Aβ, APP, α-synuclein and PrP. Coord Chem Rev. 2012;256:2219–2224. doi: 10.1016/j.ccr.2011.12.008. [DOI] [Google Scholar]
- [41].Miura T, Suzuki K, Kohata N, Takeuchi H. Metal Binding Modes of Alzheimer’s Amyloid β-Peptide in Insoluble Aggregates and Soluble Complexes. Biochemistry. 2000;39:7024–7031. doi: 10.1021/bi0002479. [DOI] [PubMed] [Google Scholar]
- [42].Minicozzi V, Stellato F, Comai M, Dalla Serra M, Potrich C, Meyer-Klaucke W, Morante S. Identifying the minimal copper- and zinc-binding site sequence in amyloid-beta peptides. J Biol Chem. 2008;283:10784–10792. doi: 10.1074/jbc.M707109200. [DOI] [PubMed] [Google Scholar]
- [43].Miller Y, Ma B, Nussinov R. Zinc ions promote Alzheimer A aggregation via population shift of polymorphic states. Proc Natl Acad Sci USA. 2010;107:9490–9495. doi: 10.1073/pnas.0913114107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Pan L, Patterson JC. Molecular Dynamics Study of Zn(Aβ) and Zn(Aβ)2. PLoS ONE. 2013;8:e70681. doi: 10.1371/journal.pone.0070681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Han D, Wang H, Yang P. Molecular modeling of zinc and copper binding with Alzheimer’s amyloid β-peptide. BioMetals. 2008;21:189–196. doi: 10.1007/s10534-007-9107-6. [DOI] [PubMed] [Google Scholar]
- [46].Borghesani V, Alies B, Hureau C. Cu(II) binding to various forms of amyloid-β peptides. Are they friends or foes? Eur J Inorg Chem. 2017 doi: 10.1002/ejic.201700776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Atrián-Blasco E, Conte-Daban A, Hureau C. Mutual interference of Cu and Zn ions in Alzheimer’s disease: perspectives at the molecular level. Dalton Trans. 2017;46:12750–12759. doi: 10.1039/C7DT01344B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Weggen S, Beher D. Molecular consequences of amyloid precursor protein and presenilin mutations causing autosomal-dominant Alzheimer’s disease. Alzheimers Res Ther. 2012;4:9. doi: 10.1186/alzrt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kepp KP. Alzheimer’s disease: How metal ions define β-amyloid function. Coord Chem Rev. 2017 doi: 10.1016/j.ccr.2017.05.007. [DOI] [Google Scholar]
- [50].Di Fede G, Catania M, Morbin M, Rossi G, Suardi S, Mazzoleni G, Merlin M, Giovagnoli AR, Prioni S, Erbetta A, Falcone C, et al. A Recessive Mutation in the APP Gene with Dominant-Negative Effect on Amyloidogenesis. Science. 2009;323:1473–1477. doi: 10.1126/science.1168979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, Stefansson H, Sulem P, Gudbjartsson D, Maloney J, Hoyte K, et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature. 2012;488:96–99. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- [52].Drew SC, Masters CL, Barnham KJ. Alzheimer’s Aβ peptides with diseaseassociated N-Terminal modifications: Influence of isomerisation, truncation and mutation on Cu2+ coordination. PLoS ONE. 2010;5:e15875. doi: 10.1371/journal.pone.0015875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Somavarapu AK, Shen F, Teilum K, Zhang J, Mossin S, Thulstrup PW, Bjerrum MJ, Tiwari MK, Szunyogh D, Søtofte PM, Kepp KP, et al. The Pathogenic A2V Mutant Exhibits Distinct Aggregation Kinetics, Metal Site Structure, and Metal Exchange of the Cu 2+ -Aβ Complex. Chem Eur J. 2017;23:13591–13595. doi: 10.1002/chem.201703440. [DOI] [PubMed] [Google Scholar]
- [54].Alies B, Eury H, Bijani C, Rechignat L, Faller P, Hureau C. pH-Dependent Cu(II) Coordination to Amyloid-β Peptide: Impact of Sequence Alterations, Including the H6R and D7N Familial Mutations. Inorg Chem. 2011;50:11192–11201. doi: 10.1021/ic201739n. [DOI] [PubMed] [Google Scholar]
- [55].Girvan P, Miyake T, Teng X, Branch T, Ying L. Kinetics of the Interactions between Copper and Amyloid-β with FAD Mutations and Phosphorylation at the N terminus. ChemBioChem. 2016;17:1732–1737. doi: 10.1002/cbic.201600255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Cheignon C, Jones M, Atrián-Blasco E, Kieffer I, Faller P, Collin F, Hureau C. Identification of key structural features of the elusive Cu–Aβ complex that generates ROS in Alzheimer’s disease. Chem Sci. 2017;8:5107–5118. doi: 10.1039/C7SC00809K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Alies B, Badei B, Faller P, Hureau C. Reevaluation of Copper(I) Affinity for Amyloid-β Peptides by Competition with Ferrozine-An Unusual Copper(I) Indicator. Chem Eur J. 2012;18:1161–1167. doi: 10.1002/chem.201102746. [DOI] [PubMed] [Google Scholar]
- [58].Kozin SA, Kulikova AA, Istrate AN, Tsvetkov PO, Zhokhov SS, Mezentsev YV, Kechko OI, Ivanov AS, Polshakov VI, Makarov AA. The English (H6R) familial Alzheimer’s disease mutation facilitates zinc-induced dimerization of the amyloid-β metal-binding domain. Metallomics. 2015;7:422–425. doi: 10.1039/C4MT00259H. [DOI] [PubMed] [Google Scholar]
- [59].Polshakov VI, Mantsyzov AB, Kozin SA, Adzhubei AA, Zhokhov SS, van Beek W, Kulikova AA, Indeykina MI, Mitkevich VA, Makarov AA. A Binuclear Zinc Interaction Fold Discovered in the Homodimer of Alzheimer’s Amyloid-β Fragment with Taiwanese Mutation D7H. Angew Chemie Int Ed. 2017;56:11734–11739. doi: 10.1002/anie.201704615. [DOI] [PubMed] [Google Scholar]
- [60].Eury H, Bijani C, Faller P, Hureau C. Copper(II) Coordination to Amyloid β: Murine versus Human Peptide. Angew Chemie Int Ed. 2011;50:901–905. doi: 10.1002/anie.201005838. [DOI] [PubMed] [Google Scholar]
- [61].Gaggelli E, Janicka-Klos A, Jankowska E, Kozlowski H, Migliorini C, Molteni E, Valensin D, Valensin G, Wieczerzak E. NMR studies of the Zn2+ interactions with rat and human β-amyloid (1-28) peptides in water-micelle environment. J Phys Chem B. 2008;112:100–109. doi: 10.1021/jp075168m. [DOI] [PubMed] [Google Scholar]
- [62].Istrate AN, Tsvetkov PO, Mantsyzov AB, Kulikova AA, Kozin SA, Makarov AA, Polshakov VI. NMR solution structure of rat Aβ(1-16): Toward understanding the mechanism of rats’ resistance to Alzheimer’s disease. Biophys J. 2012;102:136–143. doi: 10.1016/j.bpj.2011.11.4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Villar-Piqué A, Lopes da Fonseca T, Sant’Anna R, Szegö ÉM, Fonseca-Ornelas L, Pinho R, Carija A, Gerhardt E, Masaracchia C, Abad Gonzalez E, Rossetti G, et al. Environmental and genetic factors support the dissociation between α-synuclein aggregation and toxicity. Proc Natl Acad Sci USA. 2016;113:E6506–E6515. doi: 10.1073/pnas.1606791113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Dudzik CG, Walter ED, Millhauser GL. Coordination Features and Affinity of the Cu 2+ Site in the α-Synuclein Protein of Parkinson’s Disease. Biochemistry. 2011;50:1771–1777. doi: 10.1021/bi101912q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Binolfi A, Rodriguez EE, Valensin D, D’Amelio N, Ippoliti E, Obal G, Duran R, Magistrato A, Pritsch O, Zweckstetter M, Valensin G, et al. Bioinorganic Chemistry of Parkinson’s Disease: Structural Determinants for the Copper-Mediated Amyloid Formation of Alpha-Synuclein. Inorg Chem. 2010;49:10668–10679. doi: 10.1021/ic1016752. [DOI] [PubMed] [Google Scholar]
- [66].Drew SC, Ling Leong S, Pham CLL, Tew DJ, Masters CL, Miles LA, Cappai R, Barnham KJ. Cu 2+ Binding Modes of Recombinant α-Synuclein − Insights from EPR Spectroscopy. J Am Chem Soc. 2008;130:7766–7773. doi: 10.1021/ja800708x. [DOI] [PubMed] [Google Scholar]
- [67].Binolfi A, Lamberto GR, Duran R, Quintanar L, Bertoncini CW, Souza JM, Cerveñansky C, Zweckstetter M, Griesinger C, Fernández CO. Site-Specific Interactions of Cu(II) with α and β-Synuclein: Bridging the Molecular Gap between Metal Binding and Aggregation. J Am Chem Soc. 2008;130:11801–11812. doi: 10.1021/ja803494v. [DOI] [PubMed] [Google Scholar]
- [68].Kowalik-Jankowska T, Rajewska A, Jankowska E, Grzonka Z. Copper(II) binding by fragments of a-synuclein containingM1-D2- and -H50-residues; a combined potentiometric and spectroscopic study. Dalton Trans. 2006;42:5068–5076. doi: 10.1039/B610619F. [DOI] [PubMed] [Google Scholar]
- [69].Rasia RM, Bertoncini CW, Marsh D, Hoyer W, Cherny D, Zweckstetter M, Griesinger C, Jovin TM, Fernández CO. Structural characterization of copper(II) binding to α-synuclein: Insights into the bioinorganic chemistry of Parkinson’s disease. Proc Natl Acad Sci USA. 2005;102:4294–4299. doi: 10.1073/pnas.0407881102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].De Ricco R, Valensin D, Dell’Acqua S, Casella L, Dorlet P, Faller P, Hureau C. Remote His50 Acts as a Coordination Switch in the High-Affinity N-Terminal Centered Copper(II) Site of α-Synuclein. Inorg Chem. 2015;54:4744–4751. doi: 10.1021/acs.inorgchem.5b00120. [DOI] [PubMed] [Google Scholar]
- [71].Bortolus M, Bisaglia M, Zoleo A, Fittipaldi M, Benfatto M, Bubacco L, Maniero AL. Structural Characterization of a High Affinity Mononuclear Site in the Copper(II)-α-Synuclein Complex. J Am Chem Soc. 2010;132:18057–18066. doi: 10.1021/ja103338n. [DOI] [PubMed] [Google Scholar]
- [72].Moriarty GM, Minetti CASA, Remeta DP, Baum J. A Revised Picture of the Cu(II)−α-Synuclein Complex: The Role of N-Terminal Acetylation. Biochemistry. 2014;53:2815–2817. doi: 10.1021/bi5003025. [DOI] [PubMed] [Google Scholar]
- [73].Mason RJ, Paskins AR, Dalton CF, Smith DP. Copper Binding and Subsequent Aggregation of α-Synuclein Are Modulated by N-Terminal Acetylation and Ablated by the H50Q Missense Mutation. Biochemistry. 2016;55:4737–4741. doi: 10.1021/acs.biochem.6b00708. [DOI] [PubMed] [Google Scholar]
- [74].Drew SC. The N Terminus of α-Synuclein Forms Cu II -Bridged Oligomers. Chem Eur J. 2015;21:7111–7118. doi: 10.1002/chem.201500236. [DOI] [PubMed] [Google Scholar]
- [75].De Ricco R, Valensin D, Dell’Acqua S, Casella L, Gaggelli E, Valensin G, Bubacco L, Mangani S. Differences in the Binding of Copper(I) to α- and β- Synuclein. Inorg Chem. 2015;54:265–272. doi: 10.1021/ic502407w. [DOI] [PubMed] [Google Scholar]
- [76].Miotto MC, Binolfi A, Zweckstetter M, Griesinger C, Fernández CO. Bioinorganic chemistry of synucleinopathies: Deciphering the binding features of Met motifs and His-50 in AS-Cu(I) interactions. J Inorg Biochem. 2014;141:208–211. doi: 10.1016/j.jinorgbio.2014.08.012. [DOI] [PubMed] [Google Scholar]
- [77].Camponeschi F, Valensin D, Tessari I, Bubacco L, Dell’Acqua S, Casella L, Monzani E, Gaggelli E, Valensin G. Copper(I)-α-Synuclein Interaction: Structural Description of Two Independent and Competing Metal Binding Sites. Inorg Chem. 2013;52:1358–1367. doi: 10.1021/ic302050m. [DOI] [PubMed] [Google Scholar]
- [78].Binolfi A, Valiente-Gabioud AA, Duran R, Zweckstetter M, Griesinger C, Fernández CO. Exploring the Structural Details of Cu(I) Binding to α-Synuclein by NMR Spectroscopy. J Am Chem Soc. 2011;133:194–196. doi: 10.1021/ja107842f. [DOI] [PubMed] [Google Scholar]
- [79].Miotto MC, Valiente-Gabioud AA, Rossetti G, Zweckstetter M, Carloni P, Selenko P, Griesinger C, Binolfi A, Fernández CO. Copper Binding to the N-Terminally Acetylated, Naturally Occurring Form of Alpha-Synuclein Induces Local Helical Folding. J Am Chem Soc. 2015;137:6444–6447. doi: 10.1021/jacs.5b01911. [DOI] [PubMed] [Google Scholar]
- [80].Valensin D, Dell’Acqua S, Kozłowski H, Casella L. Coordination and redox properties of copper interaction with α-synuclein. J Inorg Biochem. 2016;163:292–300. doi: 10.1016/j.jinorgbio.2016.04.012. [DOI] [PubMed] [Google Scholar]
- [81].Uversky VN, Li J, Fink AL. Metal-triggered structural transformations, aggregation, and fibrillation of human alpha-synuclein. A possible molecular link between Parkinson’s disease and heavy metal exposure. J Biol Chem. 2001;276:44284–44296. doi: 10.1074/jbc.M105343200. [DOI] [PubMed] [Google Scholar]
- [82].Yamin G, Glaser CB, Uversky VN, Fink AL. Certain metals trigger fibrillation of methionine-oxidized α-synuclein. J Biol Chem. 2003;278:27630–27635. doi: 10.1074/jbc.M303302200. [DOI] [PubMed] [Google Scholar]
- [83].Valiente-Gabioud AA, Torres-Monserrat V, Molina-Rubino L, Binolfi A, Griesinger C, Fernández CO. Structural basis behind the interaction of Zn2+ with the protein α-synuclein and the Aβ peptide: A comparative analysis. J Inorg Biochem. 2012;117:334–341. doi: 10.1016/j.jinorgbio.2012.06.011. [DOI] [PubMed] [Google Scholar]
- [84].Meloni G, Vašák M. Redox activity of α-synuclein-Cu is silenced by Zn7-metallothionein-3. Free Radic Biol Med. 2011;50:1471–1479. doi: 10.1016/j.freeradbiomed.2011.02.003. [DOI] [PubMed] [Google Scholar]
- [85].McDowall JS, Brown DR. Alpha-synuclein: relating metals to structure, function and inhibition. Metallomics. 2016;8:385–397. doi: 10.1039/C6MT00026F. [DOI] [PubMed] [Google Scholar]
- [86].Flagmeier P, Meisl G, Vendruscolo M, Knowles TPJ, Dobson CM, Buell AK, Galvagnion C. Mutations associated with familial Parkinson’s disease alter the initiation and amplification steps of α-synuclein aggregation. Proc Natl Acad Sci USA. 2016;113:10328–10333. doi: 10.1073/pnas.1604645113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Proukakis C, Dudzik CG, Brier T, MacKay DS, Cooper JM, Millhauser GL, Houlden H, Schapira AH. A novel α-synuclein missense mutation in Parkinson disease. Neurology. 2013;80:1062–1064. doi: 10.1212/WNL.0b013e31828727ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Villar-Piqué A, Rossetti G, Ventura S, Carloni P, Fernández CO, Outeiro TF. Copper(II) and the pathological H50Q α-synuclein mutant: Environment meets genetics. Commun Integr Biol. 2017;10:e1270484. doi: 10.1080/19420889.2016.1270484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Khalaf O, Fauvet B, Oueslati A, Dikiy I, Mahul-Mellier AL, Ruggeri FS, Mbefo MK, Vercruysse F, Dietler G, Lee S-J, Eliezer D, et al. The H50Q mutation enhances α-synuclein aggregation, secretion, and toxicity. J Biol Chem. 2014;289:21856–21876. doi: 10.1074/jbc.M114.553297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Rockenstein E, Hansen LA, Mallory M, Trojanowski JQ, Galasko D, Masliah E. Altered expression of the synuclein family mRNA in Lewy body and Alzheimer’s disease. Brain Res. 2001;914:48–56. doi: 10.1016/S0006-8993(01)02772-X. [DOI] [PubMed] [Google Scholar]
- [91].Uversky VN, Li J, Souillac P, Millett IS, Doniach S, Jakes R, Goedert M, Fink AL. Biophysical Properties of the Synucleins and Their Propensities to Fibrillate. J Biol Chem. 2002;277:11970–11978. doi: 10.1074/jbc.M109541200. [DOI] [PubMed] [Google Scholar]
- [92].Janowska MK, Wu K-P, Baum J. Unveiling transient protein-protein interactions that modulate inhibition of alpha-synuclein aggregation by beta-synuclein, a pre-synaptic protein that co-localizes with alpha-synuclein. Sci Rep. 2015;5 doi: 10.1038/srep15164. 15164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Foster MC, Leapman RD, Li MX, Atwater I. Elemental composition of secretory granules in pancreatic islets of Langerhans. Biophys J. 1993;64:525–532. doi: 10.1016/S0006-3495(93)81397-3. S0006-3495(93)81397-3 [pii]\r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Ge X, Kakinen A, Gurzov EN, Yang W, Pang L, Pilkington EH, Govindan-Nedumpully P, Chen P, Separovic F, Davis TP, Ke PC, et al. Zinc-coordination and C-peptide complexation: a potential mechanism for the endogenous inhibition of IAPP aggregation. Chem Commun. 2017;53:9394–9397. doi: 10.1039/C7CC04291D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Nanga RPR, Brender JR, Xu J, Veglia G, Ramamoorthy A. Structures of Rat and Human Islet Amyloid Polypeptide IAPP1– 19 in Micelles by NMR Spectroscopy. Biochemistry. 2008;47:12689–12697. doi: 10.1021/bi8014357. Structures. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Jaikaran ETAS, Clark A. Islet amyloid and type 2 diabetes: From molecular misfolding to islet pathophysiology. Biochim Biophys Acta - Mol Basis Dis. 2001;1537:179–203. doi: 10.1016/S0925-4439(01)00078-3. [DOI] [PubMed] [Google Scholar]
- [97].Yu Y-P, Lei P, Hu J, Wu W-H, Zhao Y-F, Li Y-M. Copper-induced cytotoxicity: reactive oxygen species or islet amyloid polypeptide oligomer formation. Chem Commun. 2010;46:6909–6911. doi: 10.1039/c0cc02141e. [DOI] [PubMed] [Google Scholar]
- [98].Sinopoli A, Magrì A, Milardi D, Pappalardo M, Pucci P, Flagiello A, Titman JJ, Nicoletti VG, Caruso G, Pappalardo G, Grasso G. The role of copper(II) in the aggregation of human amylin. Metallomics. 2014;6:1841–1852. doi: 10.1039/C4MT00130C. [DOI] [PubMed] [Google Scholar]
- [99].Rivillas-Acevedo L, Sánchez-López C, Amero C, Quintanar L. Structural Basis for the Inhibition of Truncated Islet Amyloid Polypeptide Aggregation by Cu(II): Insights into the Bioinorganic Chemistry of Type II Diabetes. Inorg Chem. 2015;54:3788–3796. doi: 10.1021/ic502945k. [DOI] [PubMed] [Google Scholar]
- [100].Rowińska-Żyrek M. Coordination of Zn 2+ and Cu 2+ to the membrane disrupting fragment of amylin. Dalton Trans. 2016;45:8099–8106. doi: 10.1039/C6DT00628K. [DOI] [PubMed] [Google Scholar]
- [101].Magrì A, La Mendola D, Nicoletti VG, Pappalardo G, Rizzarelli E. New Insight in Copper-Ion Binding to Human Islet Amyloid: The Contribution of Metal-Complex Speciation To Reveal the Polypeptide Toxicity. Chem Eur J. 2016;22:13287–13300. doi: 10.1002/chem.201602816. [DOI] [PubMed] [Google Scholar]
- [102].Sánchez-López C, Cortés-Mejía R, Miotto MC, Binolfi A, Fernández CO, del Campo JM, Quintanar L. Copper Coordination Features of Human Islet Amyloid Polypeptide: The Type 2 Diabetes Peptide. Inorg Chem. 2016;55:10727–10740. doi: 10.1021/acs.inorgchem.6b01963. [DOI] [PubMed] [Google Scholar]
- [103].Seal M, Dey SG. Active-Site Environment of Copper-Bound Human Amylin Relevant to Type 2 Diabetes. Inorg Chem. 2017 doi: 10.1021/acs.inorgchem.7b02266. acs.inorgchem.7b02266. [DOI] [PubMed] [Google Scholar]
- [104].Magrì A, Pietropaolo A, Tabbì G, LaMendola D, Rizzarelli E. From Peptide Fragments to Whole Protein: Copper(II) Load and Coordination Features of IAPP. Chem Eur J. 2017;23:17898–17902. doi: 10.1002/chem.201704910. [DOI] [PubMed] [Google Scholar]
- [105].Li D, Chen S, Bellomo EA, Tarasov AI, Kaut C, Rutter GA, Li W. Imaging dynamic insulin release using a fluorescent zinc indicator for monitoring induced exocytotic release (ZIMIR) Proc Natl Acad Sci USA. 2011;108:21063–21068. doi: 10.1073/pnas.1109773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Taylor CG. Zinc, the pancreas, and diabetes: Insights from rodent studies and future directions. BioMetals. 2005;18:305–312. doi: 10.1007/s10534-005-3686-x. [DOI] [PubMed] [Google Scholar]
- [107].Brender JR, Hartman K, Nanga RPR, Popovych N, de la Salud Bea R, Vivekanandan S, Marsh ENG, Ramamoorthy A. Role of zinc in human islet amyloid polypeptide aggregation. J Am Chem Soc. 2010;132:8973–8983. doi: 10.1021/ja1007867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Dunn MF. Zinc-ligand interactions modulate assembly and stability of the insulin hexamer - A review. BioMetals. 2005;18:295–303. doi: 10.1007/s10534-005-3685-y. [DOI] [PubMed] [Google Scholar]
- [109].Salamekh S, Brender JR, Hyung S-J, Nanga RPR, Vivekanandan S, Ruotolo BT, Ramamoorthy A. A two-site mechanism for the inhibition of IAPP amyloidogenesis by zinc. J Mol Biol. 2011;410:294–306. doi: 10.1016/j.jmb.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Nedumpully-Govindan P, Yang Y, Andorfer R, Cao W, Ding F. Promotion or Inhibition of Islet Amyloid Polypeptide Aggregation by Zinc Coordination Depends on Its Relative Concentration. Biochemistry. 2015;54:7335–7344. doi: 10.1021/acs.biochem.5b00891. [DOI] [PubMed] [Google Scholar]
- [111].Wineman-Fisher V, Miller Y. Insight into a New Binding Site of Zinc Ions in Fibrillar Amylin. ACS Chem Neurosci. 2017 doi: 10.1021/acschemneuro.7b00221. acschemneuro.7b00221. [DOI] [PubMed] [Google Scholar]
- [112].Betsholtz C, Christmansson L, Engström U, Rorsman F, Svensson V, Johnson KH, Westermark P. Sequence divergence in a specific region of islet amyloid polypeptide (IAPP) explains differences in islet amyloid formation between species. FEBS Lett. 1989;251:261–264. doi: 10.1016/0014-5793(89)81467-X. [DOI] [PubMed] [Google Scholar]
- [113].Westermark P, Engström U, Johnson KH, Westermark GT, Betsholtz C. Islet amyloid polypeptide: pinpointing amino acid residues linked to amyloid fibril formation. Proc Natl Acad Sci USA. 1990;87:5036–5040. doi: 10.1073/pnas.87.13.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Kállay C, Dávid A, Timári S, Nagy EM, Sanna D, Garribba E, Micera G, De Bona P, Pappalardo G, Rizzarelli E, Sóvágó I. Copper(II) complexes of rat amylin fragments. Dalton Trans. 2011;40:9711–9721. doi: 10.1039/c1dt10835b. [DOI] [PubMed] [Google Scholar]
- [115].Bellia F, Grasso G. The role of copper (II) and zinc (II) in the degradation of human and murine IAPP by insulin-degrading enzyme. J Mass Spectrom. 2014;49:274–279. doi: 10.1002/jms.3338. [DOI] [PubMed] [Google Scholar]
- [116].Erthal LCS, Marques AF, Almeida FCL, Melo GLM, Carvalho CM, Palmieri LC, Cabral KMS, Fontes GN, Lima LMTR. Regulation of the assembly and amyloid aggregation of murine amylin by zinc. Biophys Chem. 2016;218:58–70. doi: 10.1016/j.bpc.2016.09.008. [DOI] [PubMed] [Google Scholar]
- [117].Alies B, Renaglia E, Rózga M, Bal W, Faller P, Hureau C. Cu(II) Affinity for the Alzheimer’s Peptide: Tyrosine Fluorescence Studies Revisited. Anal Chem. 2013;85:1501–1508. doi: 10.1021/ac302629u. [DOI] [PubMed] [Google Scholar]
- [118].Young TR, Kirchner A, Wedd AG, Xiao Z. An integrated study of the affinities of the Aβ16 peptide for Cu(I) and Cu(II): Implications for the catalytic production of reactive oxygen species. Metallomics. 2014;6:505–517. doi: 10.1039/c4mt00001c. [DOI] [PubMed] [Google Scholar]
- [119].Conte-Daban A, Borghesani V, Sayen S, Guillon E, Journaux Y, Gontard G, Lisnard L, Hureau C. Link between Affinity and Cu(II) Binding Sites to Amyloid-β Peptides Evaluated by a New Water-Soluble UV–Visible Ratiometric Dye with a Moderate Cu(II) Affinity. Anal Chem. 2017;89:2155–2162. doi: 10.1021/acs.analchem.6b04979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Talmard C, Bouzan A, Faller P. Zinc binding to amyloid-β: Isothermal titration calorimetry and Zn competition experiments with Zn sensors. Biochemistry. 2007;46:13658–13666. doi: 10.1021/bi701355j. [DOI] [PubMed] [Google Scholar]
- [121].Noël S, Bustos Rodriguez S, Sayen S, Guillon E, Faller P, Hureau C. Use of a new water-soluble Zn sensor to determine Zn affinity for the amyloid-β peptide and relevant mutants. Metallomics. 2014;6:1220–1222. doi: 10.1039/c4mt00016a. [DOI] [PubMed] [Google Scholar]
- [122].Chen W-T, Hong C-J, Lin Y-T, Chang W-H, Huang H-T, Liao J-Y, Chang Y-J, Hsieh Y-F, Cheng C-Y, Liu H-C, Chen Y-R, et al. Amyloid-beta (Aβ) D7H mutation increases oligomeric Aβ42 and alters properties of Aβ-zinc/copper assemblies. PLoS ONE. 2012;7:e35807. doi: 10.1371/journal.pone.0035807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Lee EC, Ha E, Singh S, Legesse L, Ahmad S, Karnaukhova E, Donaldson RP, Jeremic AM. Copper(II)–human amylin complex protects pancreatic cells from amylin toxicity. Phys Chem Chem Phys. 2013;15:12558. doi: 10.1039/c3cp44542a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Ranjan P, Ghosh D, Yarramala DS, Das S, Maji SK, Kumar A. Differential copper binding to alpha-synuclein and its disease-associated mutants affect the aggregation and amyloid formation. Biochim Biophys Acta - Gen Subj. 2017;1861:365–374. doi: 10.1016/j.bbagen.2016.11.043. [DOI] [PubMed] [Google Scholar]
- [125].Faller P, Hureau C, Berthoumieu O. Role of Metal Ions in the Self-assembly of the Alzheimer’s Amyloid-β Peptide. Inorg Chem. 2013;52:12193–12206. doi: 10.1021/ic4003059. [DOI] [PubMed] [Google Scholar]
- [126].Leal SS, Botelho HM, Gomes CM. Metal ions as modulators of protein conformation and misfolding in neurodegeneration. Coord Chem Rev. 2012;256:2253–2270. doi: 10.1016/j.ccr.2012.04.004. [DOI] [Google Scholar]
- [127].Viles JH. Metal ions and amyloid fiber formation in neurodegenerative diseases. Copper, zinc and iron in Alzheimer’s, Parkinson’s and prion diseases. Coord Chem Rev. 2012;256:2271–2284. doi: 10.1016/j.ccr.2012.05.003. [DOI] [Google Scholar]
- [128].Gamez P, Caballero AB. Copper in Alzheimer’s disease: Implications in amyloid aggregation and neurotoxicity. AIP Adv. 2015;5:92503. doi: 10.1063/1.4921314. [DOI] [Google Scholar]
- [129].Stewart KL, Radford SE. Amyloid plaques beyond Aβ: a survey of the diverse modulators of amyloid aggregation. Biophys Rev. 2017;9:405–419. doi: 10.1007/s12551-017-0271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Quintanar L. Copper and protein aggregation: from amyloids in diabetes to non-amyloids in cataracts disease. Coord Chem Rev. 2017 this issue. [Google Scholar]
- [131].Tuttle MD, Comellas G, Nieuwkoop AJ, Covell DJ, Berthold DA, Kloepper KD, Courtney JM, Kim JK, Barclay AM, Kendall A, Wan W, et al. Solid-state NMR structure of a pathogenic fibril of full-length human α-synuclein. Nat Struct Mol Biol. 2016;23:409–415. doi: 10.1038/nsmb.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Kreutzer AG, Spencer RK, McKnelly KJ, Yoo S, Hamza IL, Salveson PJ, Nowick JS. A Hexamer of a Peptide Derived from Aβ16-36. Biochemistry. 2017;56:6061–6071. doi: 10.1021/acs.biochem.7b00831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Kreutzer AG, Hamza IL, Spencer RK, Nowick JS. X-ray Crystallographic Structures of a Trimer, Dodecamer, and Annular Pore Formed by an Aβ17-36β-Hairpin. J Am Chem Soc. 2016;138:4634–4642. doi: 10.1021/jacs.6b01332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Soriaga AB, Sangwan S, MacDonald R, Sawaya MR, Eisenberg D. Crystal structures of IAPP amyloidogenic segments reveal a novel packing motif of out-of-register beta sheets. J Phys Chem B. 2016;120:5810–5816. doi: 10.1021/acs.jpcb.5b09981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Gremer L, Schölzel D, Schenk C, Reinartz E, Labahn J, Ravelli RBG, Tusche M, Lopez-Iglesias C, Hoyer W, Heise H, Willbold D, et al. Fibril structure of amyloid-ß(1-42) by cryoelectron microscopy. Science. 2017;358:116–119. doi: 10.1126/science.aao2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Klaver AC, Patrias LM, Finke JM, Loeffler DA. Specificity and sensitivity of the Abeta oligomer ELISA. J Neurosci Methods. 2011;195:249–254. doi: 10.1016/j.jneumeth.2010.12.001. [DOI] [PubMed] [Google Scholar]
- [137].Alies B, Eury H, Essassi EM, Pratviel G, Hureau C, Faller P. Concept for Simultaneous and Specific in Situ Monitoring of Amyloid Oligomers and Fibrils via Förster Resonance Energy Transfer. Anal Chem. 2014;86:11877–11882. doi: 10.1021/ac503509g. [DOI] [PubMed] [Google Scholar]
- [138].Matheou CJ, Younan ND, Viles JH. The Rapid Exchange of Zinc2 + Enables Trace Levels to Profoundly Influence Amyloid-β Misfolding and Dominates Assembly Outcomes in Cu2 +/Zn2 + Mixtures. J Mol Biol. 2016;428:2832–2846. doi: 10.1016/j.jmb.2016.05.017. [DOI] [PubMed] [Google Scholar]
- [139].Sharma AK, Pavlova ST, Kim J, Kim J, Mirica LM. The effect of Cu2+ and Zn2+ on the Aβ42 peptide aggregation and cellular toxicity. Metallomics. 2013;5:1529–1536. doi: 10.1039/c3mt00161j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Giannozzi P, Jansen K, La Penna G, Minicozzi V, Morante S, Rossi GC, Stellato F. Zn induced structural aggregation patterns of β-amyloid peptides by first-principle simulations and XAS measurements. Metallomics. 2012;4:156–165. doi: 10.1039/C2MT00148A. [DOI] [PubMed] [Google Scholar]
- [141].Alies B, Solari P-L, Hureau C, Faller P. Dynamics of Zn II Binding as a Key Feature in the Formation of Amyloid Fibrils by Aβ11-28. Inorg Chem. 2012;51:701–708. doi: 10.1021/ic202247m. [DOI] [PubMed] [Google Scholar]
- [142].Bush AI, Pettingell WH, Multhaup G, Paradis MD, Vonsattel J-P, Gusella JF, Beyreuther K, Masters CL, Tanzi RE. Rapid Induction of Alzheimer ABeta Amyloid Formation By Zinc. Science. 1994;265:1464–1467. doi: 10.1126/science.8073293. [DOI] [PubMed] [Google Scholar]
- [143].Garai K, Sahoo B, Kaushalya SK, Desai R, Maiti S. Zinc Lowers Amyloid-β Toxicity by Selectively Precipitating Aggregation Intermediates. Biochemistry. 2007;46:10655–10663. doi: 10.1021/bi700798b. [DOI] [PubMed] [Google Scholar]
- [144].Jun S, Saxena S. The aggregated state of amyloid-β peptide in vitro depends on Cu 2+ ion concentration. Angew Chemie Int Ed. 2007;46:3959–3961. doi: 10.1002/anie.200700318. [DOI] [PubMed] [Google Scholar]
- [145].Matheou CJ, Younan ND, Viles JH. Cu 2+ accentuates distinct misfolding of Aβ (1–40) and Aβ (1–42) peptides, and potentiates membrane disruption. Biochem J. 2015;466:233–242. doi: 10.1042/BJ20141168. [DOI] [PubMed] [Google Scholar]
- [146].Pedersen JT, Teilum K, Heegaard NHH, Østergaard J, Adolph H-W, Hemmingsen L. Rapid formation of a preoligomeric peptide-metal-peptide complex following copper(II) binding to amyloid β peptides. Angew Chemie Int Ed. 2011;50:2532–2535. doi: 10.1002/anie.201006335. [DOI] [PubMed] [Google Scholar]
- [147].Carboni E, Lingor P. Insights on the interaction of alpha-synuclein and metals in the pathophysiology of Parkinson’s disease. Metallomics. 2015;7:395–404. doi: 10.1039/C4MT00339J. [DOI] [PubMed] [Google Scholar]
- [148].Binolfi A, Rasia RM, Bertoncini CW, Ceolin M, Zweckstetter M, Griesinger C, Jovin TM, Fernández CO. Interaction of α-Synuclein with Divalent Metal Ions Reveals Key Differences: A Link between Structure, Binding Specificity and Fibrillation Enhancement. J Am Chem Soc. 2006;128:9893–9901. doi: 10.1021/ja0618649. [DOI] [PubMed] [Google Scholar]
- [149].Ward B, Walker K, Exley C. Copper(II) inhibits the formation of amylin amyloid in vitro. J Inorg Biochem. 2008;102:371–375. doi: 10.1016/j.jinorgbio.2007.09.010. [DOI] [PubMed] [Google Scholar]
- [150].Brender JR, Krishnamoorthy J, Messina GML, Deb A, Vivekanandan S, La Rosa C, Penner-Hahn JE, Ramamoorthy A. Zinc stabilization of prefibrillar oligomers of human islet amyloid polypeptide. Chem Commun. 2013;49:3339–3341. doi: 10.1039/c3cc40383a. [DOI] [PubMed] [Google Scholar]
- [151].Ma L, Li X, Wang Y, Zheng W, Chen T. Cu(II) inhibits hIAPP fibrillation and promotes hIAPP-induced beta cell apoptosis through induction of ROS-mediated mitochondrial dysfunction. J Inorg Biochem. 2014;140:143–152. doi: 10.1016/j.jinorgbio.2014.07.002. [DOI] [PubMed] [Google Scholar]
- [152].Lee SJC, Choi TS, Lee JW, Lee HJ, Mun D-G, Akashi S, Lee S-W, Lim MH, Kim HI. Structure and assembly mechanisms of toxic human islet amyloid polypeptide oligomers associated with copper. Chem Sci. 2016;7:5398–5406. doi: 10.1039/C6SC00153J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Wineman-Fisher V, Miller Y. Effect of Zn 2+ ions on the assembly of amylin oligomers: insight into the molecular mechanisms. Phys Chem Chem Phys. 2016;18:21590–21599. doi: 10.1039/C6CP04105A. [DOI] [PubMed] [Google Scholar]
- [154].Hori Y, Hashimoto T, Wakutani Y, Urakami K, Nakashima K, Condron MM, Tsubuki S, Saido TC, Teplow DB, Iwatsubo T. The Tottori (D7N) and English (H6R) Familial Alzheimer Disease Mutations Accelerate Aβ Fibril Formation without Increasing Protofibril Formation. J Biol Chem. 2007;282:4916–4923. doi: 10.1074/jbc.M608220200. [DOI] [PubMed] [Google Scholar]
- [155].Ono K, Condron MM, Teplow DB. Effects of the English (H6R) and Tottori (D7N) Familial Alzheimer Disease Mutations on Amyloid β-Protein Assembly and Toxicity. J Biol Chem. 2010;285:23186–23197. doi: 10.1074/jbc.M109.086496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Diomede L, Di Fede G, Romeo M, Bagnati R, Ghidoni R, Fiordaliso F, Salio M, Rossi A, Catania M, Paterlini A, Benussi L, et al. Expression of A2V-mutated Aβ in Caenorhabditis elegans results in oligomer formation and toxicity. Neurobiol Dis. 2014;62:521–532. doi: 10.1016/j.nbd.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Messa M, Colombo L, del Favero E, Cantù L, Stoilova T, Cagnotto A, Rossi A, Morbin M, Di Fede G, Tagliavini F, Salmona M. The Peculiar Role of the A2V Mutation in Amyloid-β (Aβ) 1–42 Molecular Assembly. J Biol Chem. 2014;289:24143–24152. doi: 10.1074/jbc.M114.576256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Benilova I, Gallardo R, Ungureanu A-A, Castillo Cano V, Snellinx A, Ramakers M, Bartic C, Rousseau F, Schymkowitz J, De Strooper B. The Alzheimer disease protective mutation A2T modulates kinetic and thermodynamic properties of amyloid-β (Aβ) aggregation. J Biol Chem. 2014;289:30977–30989. doi: 10.1074/jbc.M114.599027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Das P, Murray B, Belfort G. Alzheimer’s Protective A2T Mutation Changes the Conformational Landscape of the Aβ1–42 Monomer Differently Than Does the A2V Mutation. Biophys J. 2015;108:738–747. doi: 10.1016/j.bpj.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Zheng X, Liu D, Roychaudhuri R, Teplow DB, Bowers MT. Amyloid β-Protein Assembly: Differential Effects of the Protective A2T Mutation and Recessive A2V Familial Alzheimer’s Disease Mutation. ACS Chem Neurosci. 2015;6:1732–1740. doi: 10.1021/acschemneuro.5b00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Murray B, Sorci M, Rosenthal J, Lippens J, Isaacson D, Das P, Fabris D, Li S, Belfort G. A2T and A2V Aβ peptides exhibit different aggregation kinetics, primary nucleation, morphology, structure, and LTP inhibition. Proteins Struct Funct Bioinforma. 2016;84:488–500. doi: 10.1002/prot.24995. [DOI] [PubMed] [Google Scholar]
- [162].Colombo L, Gamba A, Cantù L, Salmona M, Tagliavini F, Rondelli V, Del Favero E, Brocca P. Pathogenic Aβ A2V versus protective Aβ A2T mutation: Early stage aggregation and membrane interaction. Biophys Chem. 2017;229:11–18. doi: 10.1016/j.bpc.2017.05.001. [DOI] [PubMed] [Google Scholar]
- [163].Gessel MM, Bernstein S, Kemper M, Teplow DB, Bowers MT. Familial Alzheimer’s Disease Mutations Differentially Alter Amyloid β-Protein Oligomerization. ACS Chem Neurosci. 2012;3:909–918. doi: 10.1021/cn300050d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Hong L, Carducci TM, Bush WD, Dudzik CG, Millhauser GL, Simon JD. Quantification of the Binding Properties of Cu2+ to the Amyloid Beta Peptide: Coordination Spheres for Human and Rat Peptides and Implication on Cu2+-Induced Aggregation. J Phys Chem B. 2010;114:11261–11271. doi: 10.1021/jp103272v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Paik SR, Shin H-J, Lee J-H, Chang C-S, Kim J. Copper(II)-induced self-oligomerization of alpha-synuclein. Biochem J. 1999;340:821–828. doi: 10.1042/0264-6021:3400821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Zhang H, Griggs A, Rochet J-C, Stanciu LA. In vitro study of α-synuclein protofibrils by Cryo-EM suggests a Cu2+-dependent aggregation pathway. Biophys J. 2013;104:2706–2713. doi: 10.1016/j.bpj.2013.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Zhang H, Rochet J-C, Stanciu LA. Cu(II) promotes amyloid pore formation. Biochem Biophys Res Commun. 2015;464:342–347. doi: 10.1016/j.bbrc.2015.06.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Anandhan A, Rodriguez-Rocha H, Bohovych I, Griggs AM, Zavala-Flores L, Reyes-Reyes EM, Seravalli J, Stanciu LA, Lee J, Rochet J-C, Khalimonchuk O, et al. Overexpression of alpha-synuclein at non-toxic levels increases dopaminergic cell death induced by copper exposure via modulation of protein degradation pathways. Neurobiol Dis. 2015;81:76–92. doi: 10.1016/j.nbd.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Lowe R, Pountney DL, Jensen PH, Gai WP, Voelcker NH. Calcium(II) selectively induces α-synuclein annular oligomers via interaction with the C-terminal domain. Protein Sci. 2004;13:3245–3252. doi: 10.1110/ps.04879704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Bharathi, Indi SS, Rao KSJ. Copper- and iron-induced differential fibril formation in α-synuclein: TEM study. Neurosci Lett. 2007;424:78–82. doi: 10.1016/j.neulet.2007.06.052. [DOI] [PubMed] [Google Scholar]
- [171].Yu J, Warnke J, Lyubchenko YL. Nanoprobing of α-synuclein misfolding and aggregation with atomic force microscopy. Nanomedicine Nanotechnology Biol Med. 2011;7:146–152. doi: 10.1016/j.nano.2010.08.001. [DOI] [PubMed] [Google Scholar]
- [172].Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant α-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4:1318–1320. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- [173].Trexler AJ, Rhoades E. N-terminal acetylation is critical for forming α-helical oligomer of α-synuclein. Protein Sci. 2012;21:601–605. doi: 10.1002/pro.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Westermark P, Li Z-C, Westermark GT, Leckström A, Steiner DF. Effects of beta cell granule components on human islet amyloid polypeptide fibril formation. FEBS Lett. 1996;379:203–206. doi: 10.1016/0014-5793(95)01512-4. [DOI] [PubMed] [Google Scholar]
- [175].Abedini A, Raleigh DP. The Role of His-18 in Amyloid Formation by Human Islet Amyloid Polypeptide. Biochemistry. 2005;44:16284–16291. doi: 10.1021/bi051432v. [DOI] [PubMed] [Google Scholar]
- [176].Sakagashira S, Hiddinga HJ, Tateishi K, Sanke T, Hanabusa T, Nanjo K, Eberhardt NL. S20G mutant amylin exhibits increased in vitro amyloidogenicity and increased intracellular cytotoxicity compared to wild-type amylin. Am J Pathol. 2000;157:2101–2109. doi: 10.1016/S0002-9440(10)64848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Xu W, Jiang P, Mu Y. Conformation preorganization: Effects of S20G mutation on the structure of human islet amyloid polypeptide segment. J Phys Chem B. 2009;113:7308–7314. doi: 10.1021/jp8106827. [DOI] [PubMed] [Google Scholar]
- [178].Cao P, Tu L-H, Abedini A, Levsh O, Akter R, Patsalo V, Schmidt AM, Raleigh DP. Sensitivity of amyloid formation by human islet amyloid polypeptide to mutations at residue 20. J Mol Biol. 2012;421:282–295. doi: 10.1016/j.jmb.2011.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Chen P, Miah MR, Aschner M. Metals and Neurodegeneration. F1000Research. 2016;5:366. doi: 10.12688/f1000research.7431.1. [version 1; referees: 3 approved] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Cheignon C, Collin F, Faller P, Hureau C. Is ascorbate Dr Jekyll or Mr Hyde in the Cu(Aβ) mediated oxidative stress linked to Alzheimer’s disease? Dalton Trans. 2016;45:12627–12631. doi: 10.1039/C6DT01979J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- [182].Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/1781154a0. [DOI] [PubMed] [Google Scholar]
- [183].Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Rochette L, Zeller M, Cottin Y, Vergely C. Diabetes, oxidative stress and therapeutic strategies. Biochim Biophys Acta - Gen Subj. 2014;1840:2709–2729. doi: 10.1016/j.bbagen.2014.05.017. [DOI] [PubMed] [Google Scholar]
- [185].Jomova K, Vondrakova D, Lawson M, Valko M. Metals, oxidative stress and neurodegenerative disorders. Mol Cell Biochem. 2010;345:91–104. doi: 10.1007/s11010-010-0563-x. [DOI] [PubMed] [Google Scholar]
- [186].Ahmad W, Ijaz B, Shabbiri K, Ahmed F, Rehman S. Oxidative toxicity in diabetes and Alzheimer’s disease: mechanisms behind ROS/ RNS generation. J Biomed Sci. 2017;24:76. doi: 10.1186/s12929-017-0379-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Cheignon C, Tomas M, Bonnefont-Rousselot D, Faller P, Hureau C, Collin F. Oxidative stress and the amyloid beta peptide in Alzheimer’s Disease. Redox Biol. 2018;14:450–464. doi: 10.1016/j.redox.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Rózga M, Kłoniecki M, Dadlez M, Bal W. A Direct Determination of the Dissociation Constant for the Cu(II) Complex of Amyloid β 1–40 Peptide. Chem Res Toxicol. 2010;23:336–340. doi: 10.1021/tx900344n. [DOI] [PubMed] [Google Scholar]
- [189].Faller P, Hureau C. Bioinorganic chemistry of copper and zinc ions coordinated to amyloid-β peptide. Dalton Trans. 2009;1080:1094. doi: 10.1039/B813398K. [DOI] [PubMed] [Google Scholar]
- [190].Binolfi A, Quintanar L, Bertoncini CW, Griesinger C, Fernández CO. Bioinorganic chemistry of copper coordination to alpha-synuclein: Relevance to Parkinson’s disease. Coord Chem Rev. 2012;256:2188–2201. doi: 10.1016/j.ccr.2012.05.004. [DOI] [Google Scholar]
- [191].Pall HS, Blake DR, Williams AC, Lunec J. Evidence of Enhanced Lipid Peroxidation in the Cerebrospinal Fluid of Patients Taking Phenothiazines. Lancet. 1987;330:596–599. doi: 10.1016/s0140-6736(87)92987-4. [DOI] [PubMed] [Google Scholar]
- [192].Xu J, Church SJ, Patassini S, Begley P, Waldvogel HJ, Curtis MA, Faull RLM, Unwin RD, Cooper GJS. Evidence for widespread, severe brain copper deficiency in Alzheimer’s dementia. Metallomics. 2017;9:1106–1119. doi: 10.1039/C7MT00074J. [DOI] [PubMed] [Google Scholar]
- [193].Faller P. Copper in Alzheimer disease: Too much, too little, or misplaced? Free Radic Biol Med. 2012;52:747–748. doi: 10.1016/j.freeradbiomed.2011.11.005. [DOI] [PubMed] [Google Scholar]
- [194].Multhaup G, Schlicksupp A, Hesse L, Beher D, Ruppert T, Masters CL, Beyreuther K. The amyloid precursor protein of Alzheimer’s disease in the reduction of copper (II) to copper (I) Science. 1996;271:1406–1409. doi: 10.1126/science.271.5254.1406. [DOI] [PubMed] [Google Scholar]
- [195].Bondy SC, Guo-Ross SX, Truong AT. Promotion of transition metal-induced reactive oxygen species formation by β-amyloid. Brain Res. 1998;799:91–96. doi: 10.1016/S0006-8993(98)00461-2. [DOI] [PubMed] [Google Scholar]
- [196].Huang X, Atwood CS, Hartshorn MA, Multhaup G, Goldstein LE, Scarpa RC, Cuajungco MP, Gray DN, Lim J, Moir RD, Tanzi RE, et al. The Aβ Peptide of Alzheimer’s Disease Directly Produces Hydrogen Peroxide through Metal Ion Reduction. Biochemistry. 1999;38:7609–7616. doi: 10.1021/bi990438f. [DOI] [PubMed] [Google Scholar]
- [197].Paik SR, Shin H-J, Lee J-H. Metal-Catalyzed Oxidation of α-Synuclein in the Presence of Copper(II) and Hydrogen Peroxide. Arch Biochem Biophys. 2000;378:269–277. doi: 10.1006/abbi.2000.1822. [DOI] [PubMed] [Google Scholar]
- [198].Pedersen JT, Chen SW, Borg CB, Ness S, Bahl JM, Heegaard NHH, Dobson CM, Hemmingsen L, Cremades N, Teilum K. Amyloid-β and α- Synuclein Decrease the Level of Metal-Catalyzed Reactive Oxygen Species by Radical Scavenging and Redox Silencing. J Am Chem Soc. 2016;138:3966–3969. doi: 10.1021/jacs.5b13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].De Ricco R, Valensin D, Dell’Acqua S, Casella L, Hureau C, Faller P. Copper(I/II), α/β-Synuclein and Amyloid-β: Menage à Trois? ChemBioChem. 2015;16:2319–2328. doi: 10.1002/cbic.201500425. [DOI] [PubMed] [Google Scholar]
- [200].Guilloreau L, Combalbert S, Sournia-Saquet A, Mazarguil H, Faller P. Redox chemistry of copper-amyloid-β: The generation of hydroxyl radical in the presence of ascorbate is linked to redox-potentials and aggregation state. ChemBioChem. 2007;8:1317–1325. doi: 10.1002/cbic.200700111. [DOI] [PubMed] [Google Scholar]
- [201].Reybier K, Ayala S, Alies B, Rodrigues JV, Bustos Rodriguez S, La Penna G, Collin F, Gomes CM, Hureau C, Faller P. Free Superoxide is an Intermediate in the Production of H 2 O 2 by Copper(I)-Aβ Peptide and O 2. Angew Chemie Int Ed. 2016;55:1085–1089. doi: 10.1002/anie.201508597. [DOI] [PubMed] [Google Scholar]
- [202].Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Third edit. Oxford University Press; 1999. [Google Scholar]
- [203].Masad A, Hayes L, Tabner BJ, Turnbull S, Cooper LJ, Fullwood NJ, German MJ, Kametani F, El-Agnaf OMA, Allsop D. Copper-mediated formation of hydrogen peroxide from the amylin peptide: A novel mechanism for degeneration of islet cells in type-2 diabetes mellitus? FEBS Lett. 2007;581:3489–3493. doi: 10.1016/j.febslet.2007.06.061. [DOI] [PubMed] [Google Scholar]
- [204].Masad A, Tabner BJ, Mayes J, Allsop D. The amylin peptide implicated in type 2 diabetes stimulates copper-mediated carbonyl group and ascorbate radical formation. Free Radic Biol Med. 2011;51:869–875. doi: 10.1016/j.freeradbiomed.2011.05.033. [DOI] [PubMed] [Google Scholar]
- [205].Deas E, Cremades N, Angelova PR, Ludtmann MHR, Yao Z, Chen S, Horrocks MH, Banushi B, Little D, Devine MJ, Gissen P, et al. Alpha-Synuclein Oligomers Interact with Metal Ions to Induce Oxidative Stress and Neuronal Death in Parkinson’s Disease. Antioxid Redox Signal. 2016;24:376–391. doi: 10.1089/ars.2015.6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Wright JA, Wang X, Brown DR. Unique copper-induced oligomers mediate alpha-synuclein toxicity. FASEB J. 2009;23:2384–2393. doi: 10.1096/fj.09-130039. [DOI] [PubMed] [Google Scholar]
- [207].Wood PM. The potential diagram for oxygen at pH 7. Biochem J. 1988;253:287–289. doi: 10.1042/bj2530287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Balland V, Hureau C, Savéant J-M. Electrochemical and homogeneous electron transfers to the Alzheimer amyloid-ß copper complex follow a preorganization mechanism. Proc Natl Acad Sci USA. 2010;107:17113–17118. doi: 10.1073/pnas.1011315107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Rodríguez EE, Arcos-López T, Trujano-Ortiz LG, Fernández CO, González FJ, Vela A, Quintanar L. Role of N-terminal methionine residues in the redox activity of copper bound to alpha-synuclein. J Biol Inorg Chem. 2016;21:691–702. doi: 10.1007/s00775-016-1376-5. [DOI] [PubMed] [Google Scholar]
- [210].Miotto MC, Rodriguez EE, Valiente-Gabioud AA, Torres-Monserrat V, Binolfi A, Quintanar L, Zweckstetter M, Griesinger C, Fernández CO. Site-Specific Copper-Catalyzed Oxidation of α-Synuclein: Tightening the Link between Metal Binding and Protein Oxidative Damage in Parkinson’s Disease. Inorg Chem. 2014;53:4350–4358. doi: 10.1021/ic4031377. [DOI] [PubMed] [Google Scholar]
- [211].Trujano-Ortiz LG, González FJ, Quintanar L. Redox Cycling of Copper–Amyloid β 1–16 Peptide Complexes Is Highly Dependent on the Coordination Mode. Inorg Chem. 2015;54:4–6. doi: 10.1021/ic501941a. [DOI] [PubMed] [Google Scholar]
- [212].Huang X, Cuajungco MP, Atwood CS, Hartshorn MA, Tyndall JDA, Hanson GR, Stokes KC, Leopold M, Multhaup G, Goldstein LE, Scarpa RC, et al. Cu(II) Potentiation of Alzheimer Abeta Neurotoxicity: correlation with cell-free hydrogen peroxide production and metal reduction. J Biol Chem. 1999;274:37111–37116. doi: 10.1074/jbc.274.52.37111. [DOI] [PubMed] [Google Scholar]
- [213].Jiang D, Men L, Wang J, Zhang Y, Chickenyen S, Wang Y, Zhou F. Redox Reactions of Copper Complexes Formed with Different β-Amyloid Peptides and Their Neuropathalogical Relevance. Biochemistry. 2007;46:9270–9282. doi: 10.1021/bi700508n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Brzyska M, Trzesniewska K, Wieckowska A, Szczepankiewicz A, Elbaum D. Electrochemical and Conformational Consequences of Copper (Cu I and Cu II) Binding to β-Amyloid(1-40) ChemBioChem. 2009;10:1045–1055. doi: 10.1002/cbic.200800732. [DOI] [PubMed] [Google Scholar]
- [215].Trujano-Ortiz LG, González FJ, Quintanar L. Redox cycling of copper-amyloid β 1-16 peptide complexes is highly dependent on the coordination mode. Inorg Chem. 2015;54:4–6. doi: 10.1021/ic501941a. [DOI] [PubMed] [Google Scholar]
- [216].Wiloch MZ, Wawrzyniak UE, Ufnalska I, Bonna A, Bal W, Drew SC, Wróblewski W. Tuning the Redox Properties of Copper(II) Complexes with Amyloid-β Peptides. J Electrochem Soc. 2016;163:G196–G199. doi: 10.1149/2.0641613jes. [DOI] [Google Scholar]
- [217].Wang C, Liu L, Zhang L, Peng Y, Zhou F. Redox reactions of the α-synuclein-Cu(2+) complex and their effects on neuronal cell viability. Biochemistry. 2010;49:8134–8142. doi: 10.1021/bi1010909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Bard AJ, Faulkner LR. Electrochemical methods: fundamentals and applications. Wiley; 2001. [Google Scholar]
- [219].Cheignon C, Faller P, Testemale D, Hureau C, Collin F. Metal-catalyzed oxidation of Aβ and the resulting reorganization of Cu binding sites promote ROS production. Metallomics. 2016;8:1081–1089. doi: 10.1039/C6MT00150E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Kowalik-Jankowska T, Rajewska A, Jankowska E, Grzonka Z. Products of Cu(II)-catalyzed oxidation of α-synuclein fragments containing M 1 -D 2 and H 50 residues in the presence of hydrogen peroxide. Dalton Trans. 2008:832–838. doi: 10.1039/B714440G. [DOI] [PubMed] [Google Scholar]
- [221].Barnham KJ, Bush AI. Biological metals and metal-targeting compounds in major neurodegenerative diseases. Chem Soc Rev. 2014;43:6727–6749. doi: 10.1039/c4cs00138a. [DOI] [PubMed] [Google Scholar]
- [222].Smith DG, Cappai R, Barnham KJ. The redox chemistry of the Alzheimer’s disease amyloid β peptide. Biochim Biophys Acta - Biomembr. 2007;1768:1976–1990. doi: 10.1016/j.bbamem.2007.02.002. [DOI] [PubMed] [Google Scholar]
- [223].Al-Hilaly YK, Williams TL, Stewart-Parker M, Ford L, Skaria E, Cole M, Bucher WG, Morris KL, Sada AA, Thorpe JR, Serpell LC. A central role for dityrosine crosslinking of Amyloid-β in Alzheimer’s disease. Acta Neuropathol Commun. 2013;1:83. doi: 10.1186/2051-5960-1-83. [DOI] [PMC free article] [PubMed] [Google Scholar]