Abstract
Background
Childhood brain tumours have some of the longest time to diagnosis. A timely diagnosis may have a role in reducing anxiety in waiting for a diagnosis and subsequent morbidity and mortality. We investigated where the opportunities for an earlier diagnosis were, and for which anatomical locations this strategy will most likely to be effective.
Methods
A record-linkage cohort study of patients diagnosed aged 0–24 years with a primary intracranial tumour between 1989 and 2006 in England, using records from the National Cancer Registry linked to hospital admission records from Hospital Episode Statistics (HES, 1997–2006) and primary care consultation records from Clinical Practice Research Datalink (CPRD, 1989–2006). Relevant neurological presentations were extracted from HES and CPRD. Temporal changes in presentation rates were estimated in generalised additive models.
Results
Frequency of presentation began to increase six months before diagnosis in primary care and three months before diagnosis in hospital. Supratentorial and midline tumours had the longest presentation history before diagnosis. Peri-ventricular tumours presented frequently in hospital (rate ratio = 1.29 vs supratentorial tumours; 95% CI = 1.12–1.48) or as an emergency (1.24; 1.01–1.51), and in primary care (1.12; 0.62–1.85).
Conclusions
Opportunities for an earlier diagnosis are greater in supratentorial, midline or cranial nerve tumours, which have a longer presentation history than peri-ventricular, cerebellar or brainstem tumours. Common features before diagnosis include headache, convulsions, and growth or endocrine disorders. Focal neurological deficits are uncommon and emerge late in the pre-diagnosis period.
MeSH keywords: brain neoplasms, signs and symptoms, early diagnosis, symptom assessment, oncology, epidemiology
1. Introduction
Primary intracranial tumours account for 25% of all childhood cancers, and are associated with the greatest number of cancer deaths.1 This has generated substantial interests in improving the prognosis of intracranial tumours through earlier detection,2–8 and culminated in the identification of early diagnosis as one of the top 10 priorities for clinical research in neuro-oncology by The James Lind Alliance and by the National Cancer Research Institute Brain Supportive and Palliative Care subgroup in the United Kingdom.9 The James Lind Alliance is a non-profit making initiative bringing together patients, carers and clinicians to identify unanswered questions that they agree are most important.10
Evidence on early diagnosis of intracranial tumours in children and young adults, and particularly its relationship with survival, is scarce because of the logistical cost in recruiting sufficient patients to create a traditional cohort for identifying earlier diagnostic opportunities. Advances in statistical methodology and computing power in linking routinely collected patient care records have enabled creation of a population-based cohort with histologically verified intracranial tumours for examining temporal changes in the symptoms and signs at each primary care or hospital visit, thus allowing us to investigate if an earlier diagnosis of an intracranial tumour would have been possible.11–14 Our aims are to investigate if such opportunities were limited to tumours in certain locations or existed uniformly for tumours in any location to tailor recommendations on early diagnosis for specific intracranial neoplasms. This will provide evidence for a more focused approach in developing guidelines and evaluating interventions on early diagnosis to achieve the maximum possible effect in the population.
2. Patients and Methods
We identified patients aged 0–24 years when diagnosed in England with a benign, borderline or malignant primary intracranial tumour from the National Cancer Registry. We have included patients up to the age of 24 years as those patients are often managed in specialist teenage cancer units in the United Kingdom.
Intracranial tumours were defined as those with a relevant morphology (diagnostic groups III, IX.b.2, IX.d.8 and X.a in the third edition of the International Classification of Childhood Cancer15) and arising from one of the following sites (the 9th or 10th revision of the International Classification of Diseases, ICD16, 17): the supratentorial compartment, midline, cerebellum, brainstem, ventricular system, meninges, cranial nerves and other intracranial sites. Records were excluded if they contained invalid dates or unknown sex or vital status. Records which failed Office for National Statistics validity checks, those of secondary or metastatic tumours, synchronous or multiple primary tumours were also excluded.18
We obtained records of primary care consultations between 1989 and 2006 from Clinical Practice Research Datalink (CPRD), a database of longitudinal records of primary care consultations from over 600 practices from anywhere in the UK.19, 20 Patient data in CPRD are representative of the UK population in age, sex and ethnicity (compared with UK Census 2011), with high level of validity in data on diagnoses (over 95% of cases confirmed in internal and external validations for neoplasms).20, 21 We also obtained records of admissions between 1997 and 2006 from Hospital Episode Statistics (HES), which collates data on in-patient stays in National Health Service (NHS) hospitals in England.22 CPRD and HES records were linked to the National Cancer Registry by matching on NHS number, sex, date of birth and postcode.23, 24
2.1. Presentation rates
Over 800 clinical features relevant to an intracranial tumour presentation were identified from manually searching the list of Read and ICD-10 codes.25 Read coding is a hierarchical system for coding symptoms, signs, diagnoses, interventions and administrative events in primary care. We retained for analysis records of primary care or hospital visits containing one or more coded features that may be explained by the presence of an underlying intracranial tumour. Each episode of hospital stay was also classified as “non-emergency” or “emergency” based on how the patient was admitted. An emergency admission came from any of the following sources: the Accident and Emergency department, general practice (direct admission or after consulting the duty hospital doctor), outpatient clinics, or by urgent transfer from another hospital.
We calculated presentation rate, which was the unit of analysis, by dividing the number of visits by observation time. Changes in the pattern of hospital presentations were estimated using a cohort of patients with linked HES records, and changes in the pattern of primary care presentations were estimated from a separate cohort of patients with linked CPRD records. The observation time for each patient in HES began on the later of the date of birth or the start date of the HES data and ended with the earlier of the date of death or the end date of HES data. The observation time in CPRD began on the date of registration with the general practice (most took place within a few weeks after birth) and ended with the earliest of the date of death, transferring out (if a patient had moved to a practice not contributing data to CPRD) or last collection date (when a practice last submitted data to CPRD). Presentation rates may thus be interpreted as the number of visits per month in a cohort of 100 patients. We described temporal changes in the presentation rates from the date of diagnosis in the National Cancer Registry for 0–1, 1–3, 3–6, 6–12 and over 12 months in the main text.26, 27 But since time from diagnosis when patients presented is continuous in nature, we have also illustrated changes in presentation rate graphically (in supplemental materials) to overcome the arbitrariness of dividing time into intervals, especially for presentations that took place exactly at the boundary of those intervals. Although we are primarily interested in presentations before the diagnosis of an intracranial tumour, presentations after diagnosis have been included for two reasons: (a) to demonstrate, rather than to assume, that the intensity of healthcare use falls after a diagnosis and thus emphasise the importance of reaching a correct diagnosis; and (b) to reduce statistical uncertainty in estimating rates around the time of diagnosis by placing the important observations at the centre of the dataset. Estimation of rate ratios and their 95% confidence intervals was carried out in generalised linear models, with smoothing in the time domain using generalised additive models with locally weighted regression (LOESS) to highlight the underlying trend.28–31
We analysed our data in the statistical language R, with functions from the ‘gam’ package.32, 33 Computationally intensive calculations were carried out on the High Performance Computing cluster at the London School of Hygiene and Tropical Medicine.
3. Results
Among 9,799 brain tumour patients diagnosed aged 0–24 years between 1989 and 2006 and registered in the National Cancer Registry, we linked 181 individuals to 3,787 primary care records from CPRD. Of the 5,061 patients diagnosed in the period 1997–2006, we linked 3,959 patients to 60,351 in-patient admission records from HES. The characteristics of patients with linked records have been discussed elsewhere.13 Briefly, the distributions in age and sex were similar between patients with and without linked records when compared with the National Cancer Registry, in which the data came from the general population. Tumours in the supratentorial compartment or the midline were more likely to be linked to CPRD and peri-ventricular tumours less so. For HES linkage, cerebellar and brainstem tumours were more likely to have linked records whereas midline tumours were less likely.
3.1. Primary care consultations
Consultation rates varied between tumour locations, after accounting for the effects of age and year of diagnosis (likelihood ratio test statistic = 122.8, P < 0.001). Patients with a peri-ventricular tumour had the highest pre-diagnosis presentation frequency (mean = 11.5 consultations per 100 persons each month, Table 1), and was 1.12 times (95% CI = 0.62–1.85) higher than in patients with a supratentorial tumour. Patients with a tumour in the midline (rate ratio = 0.52; 95% CI = 0.42–0.64), brainstem (0.48; 0.35–0.64), cerebellum (0.47; 0.37–0.59), the meninges (0.24; 0.14–0.38) or the cranial nerves (0.87; 0.58–1.27) presented less often than supratentorial tumours, that is, between 2.4 and 6.5 consultations per 100 persons each month in those patients.
Table 1. Presentation rate (per 100 person-months) before an intracranial tumour diagnosis from CPRD (1989–2006) and HES-linked registrations (1997–2006) in patients aged 0 to 24 years in England.
| Primary care presentations | Hospital presentations | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All admissions | Emergency only | |||||||||||
| Count | Rate | Rate ratio* | 95% CI | Count | Rate | Rate ratio* | 95% CI | Count | Rate | Rate ratio* | 95% CI | |
| Supratentorial | 382 | 9.8 | 1.00 | reference | 1,981 | 3.7 | 1.00 | reference | 951 | 1.8 | 1.00 | reference |
| Midline | 144 | 5.2 | 0.52 | 0.42 – 0.64 | 668 | 2.7 | 0.81 | 0.74 – 0.88 | 304 | 1.2 | 0.79 | 0.69 – 0.90 |
| Cerebellum | 85 | 4.4 | 0.47 | 0.37 – 0.59 | 967 | 3.5 | 0.84 | 0.77 – 0.91 | 623 | 2.3 | 1.10 | 0.99 – 1.22 |
| Brainstem | 47 | 4.8 | 0.48 | 0.35 – 0.64 | 585 | 3.2 | 0.80 | 0.73 – 0.88 | 352 | 1.9 | 0.98 | 0.87 – 1.11 |
| Ventricles | 14 | 11.5 | 1.12 | 0.62 – 1.85 | 227 | 5.4 | 1.29 | 1.12 – 1.48 | 105 | 2.5 | 1.24 | 1.01 – 1.51 |
| Meninges | 17 | 2.4 | 0.24 | 0.14 – 0.38 | 168 | 2.4 | 0.78 | 0.66 – 0.91 | 74 | 1.1 | 0.73 | 0.57 – 0.92 |
| Cranial nerves | 32 | 6.5 | 0.87 | 0.58 – 1.27 | 147 | 1.9 | 0.58 | 0.48 – 0.68 | 44 | 0.6 | 0.37 | 0.27 – 0.50 |
| Other | 234 | 5.0 | 0.49 | 0.42 – 0.58 | 2,230 | 3.5 | 0.91 | 0.85 – 0.96 | 1,187 | 1.8 | 1.00 | 0.92 – 1.09 |
Estimated rate ratios were adjusted for age and year of diagnosis.
CPRD = Clinical Practice Research Datalink, HES = Hospital Episode Statistics.
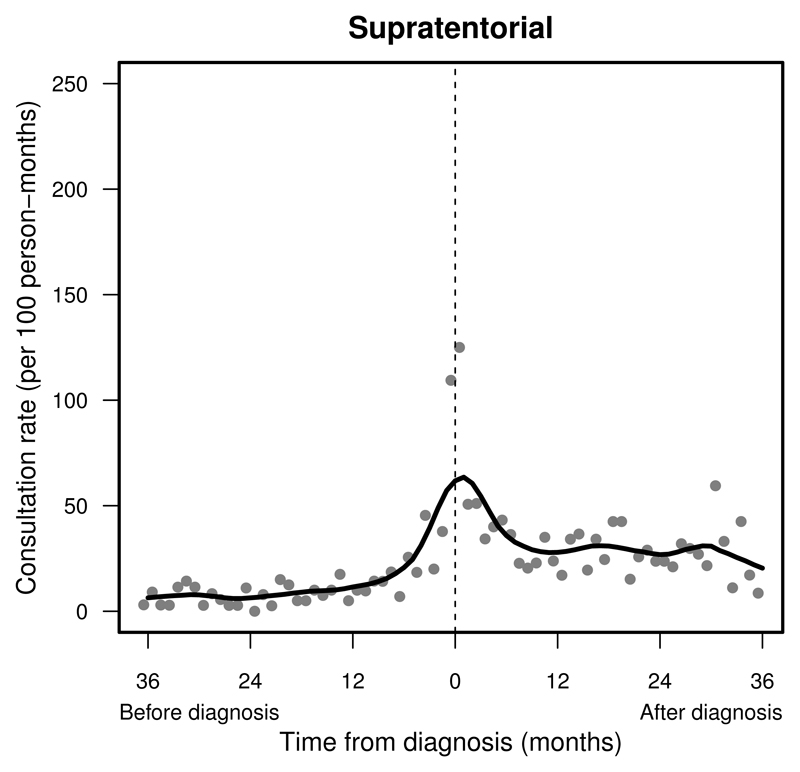
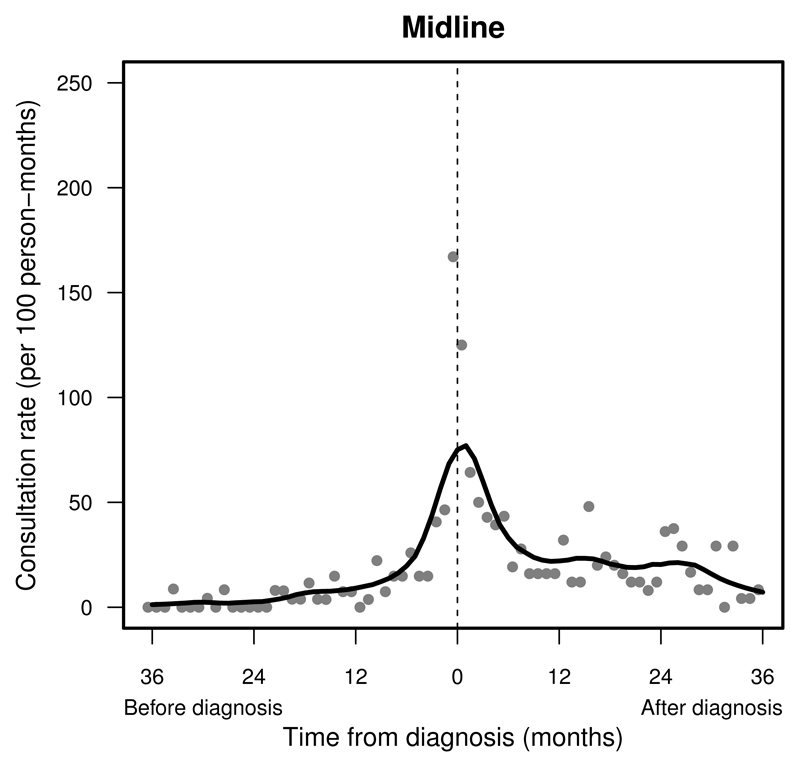
Consultation rates peaked in the final month before diagnosis at rates over 100 consultations per 100 persons (Supplemental Table S.1). Patients with a tumour in the supratentorial compartment, the midline or cranial nerves were seen with steadily increasing frequency before diagnosis (Figures 1 and 2). Patients with a tumour in the cerebellum, brainstem, ventricular system or the meninges seldom presented until about six months before diagnosis (Supplemental Figure S.3–S.6, left). The frequency of consultations remained raised 12 months after a brain tumour diagnosis (Supplemental Table S.1).
Figure 1.
Pattern of primary care presentations in children and young adults with a supratentorial tumour before and after diagnosis (time = 0): England, 1989–2006. Change in monthly presentation rates (grey dots) after LOESS smoothing (solid line).
Figure 2.
Pattern of primary care presentations in children and young adults with a midline tumour before and after diagnosis (time = 0): England, 1989–2006. Change in monthly presentation rates (grey dots) after LOESS smoothing (solid line).
Convulsion was the commonest feature of tumours in the supratentorial compartment (8.7% of consultations), ventricular system (28.0%) and meninges (27.3%). Disorders of growth or endocrine functions were common in midline tumours (15.5%). Headaches were the dominant feature of tumours in the brainstem (7.9%), cerebellum (6.2%), midline (11.5%) and ventricles (10.0%). Dysfunction of cranial nerves II, III, IV or VI was also commonly seen in tumours in the supratentorial compartment (6.9%), midline (9.5%), ventricular system (10.0%) and cranial nerves (13.4%). Non-specific symptoms were common in brainstem (6.8%) or meninges (7.8%) tumours. Focal neurological deficits were rarely seen.
3.2. Hospital presentations
Similar to the pattern in primary care, the presentation rates varied between tumour sites after accounting for age and year of diagnosis (likelihood ratio test statistic = 106.5, P < 0.001; and for emergency presentations only: 87.2; P < 0.001). Patients with a peri-ventricular tumour were most frequently admitted to hospitals (rate = 5.4 admissions per 100 persons each month, which was 1.29 times (95% CI = 1.12–1.48) that of supratentorial tumours, Table 1), and often as an emergency (2.5 admissions per 100 persons each month, which was 1.24 times (95% CI = 1.01–1.51) that of supratentorial tumours). Tumours outside the ventricular system presented less frequently than supratentorial tumours, with the exception of cerebellar tumours which presented as emergency with a rate 10% higher than supratentorial tumours (rate ratio = 1.10, 95% CI = 0.99–1.22).
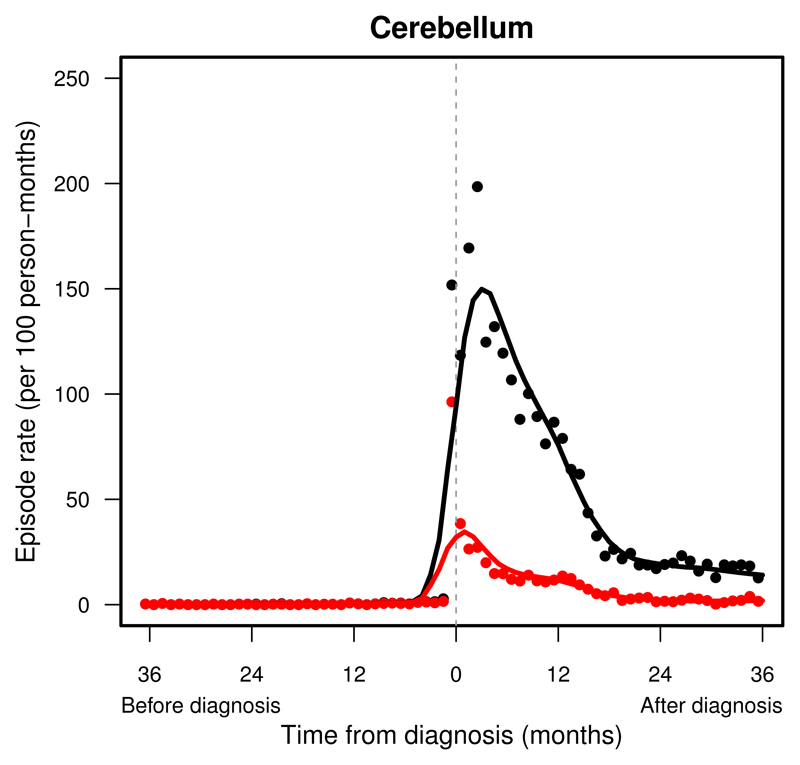
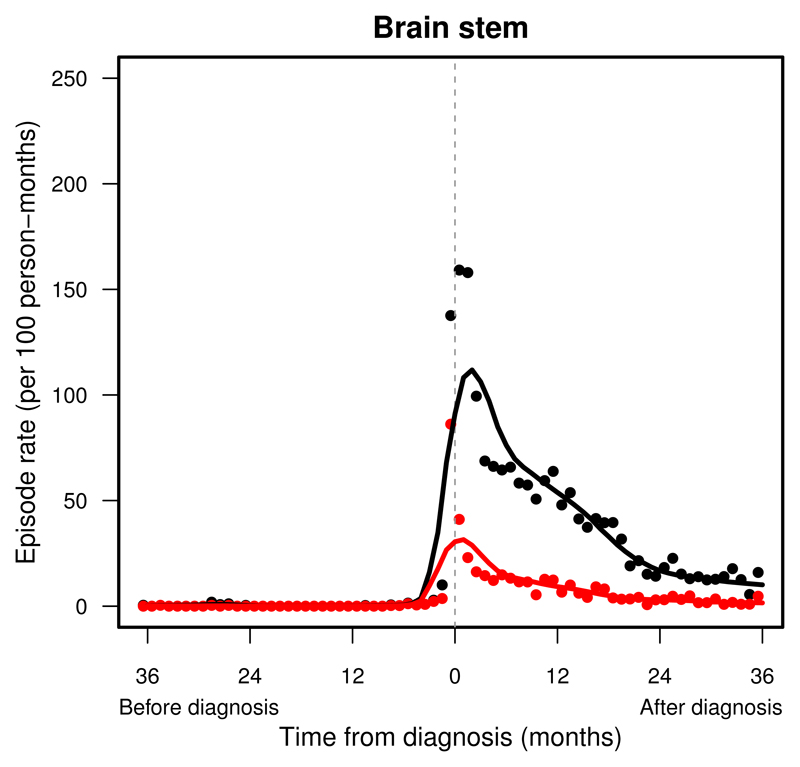
Admissions were the most frequent within one month around the diagnosis of an intracranial tumour, with rates over 100 admissions per 100 persons each month (Supplemental Table S.2). Patients were seldom admitted urgently with any relevant symptoms until the final 3–6 months before diagnosis (Supplemental Table S.3). A sudden rise in urgent admissions was seen in patients with tumours in the cerebellum (Figure 3), brainstem (Figure 4) or supratentorial compartment than in peri-ventricular, midline, meninges or cranial nerve tumours (Supplemental Figure S.5-S.7, right).
Figure 3.
Pattern of hospital presentations in children and young adults with a cerebellar tumour before and after diagnosis (time = 0): England, 1997–2006. Change in monthly rates of all presentations (black dots) after LOESS smoothing (black line), and of emergency presentations (red dots) after LOESS smoothing (red line).
Figure 4.
Pattern of hospital presentations in children and young adults with a brainstem tumour before and after diagnosis (time = 0): England, 1997–2006. Change in monthly rates of all presentations (black dots) after LOESS smoothing (black line), and of emergency presentations (red dots) after LOESS smoothing (red line).
The commonest reason for admission was convulsion in patients with a tumour in the supratentorial compartment (11.3% of admissions) or meninges (21.6%). Raised intracranial pressure was the commonest reason in cerebellar (16.1%), brainstem (16.5%) or peri-ventricular tumours (18.0%), and the second commonest reason for tumours in the supratentorial compartment (10.0%), midline (12.8%), meninges (14.0%) or cranial nerves (11.6%). Growth or endocrine disorders were the commonest in midline tumours (24.5%). Focal neurological deficits were uncommon (fewer than in 5% of admissions).
4. Discussion
Children and young adults with an intracranial tumour begin presenting six months before diagnosis in primary care and three months before diagnosis in hospital. These contacts with healthcare professionals in primary care and in hospitals represent opportunities for an earlier diagnosis.
Patients may visit Accident and Emergency with very few primary care consultations (as in cerebellum or brainstem tumours, Figures S.3 and S.4), or may have a greater number of such consultations before referral to a specialist (supratentorial or midline tumours, Figures S.1 and S.2). A consultation rate of over 100 per 100 person-months in the final month implies each patient may, on average, be seen at least once in primary care before the diagnosis of an intracranial tumour. Hospital admissions, especially for emergency presentations, also occur most frequently in the final month. These patterns imply a definitive diagnosis may be brought forward by at least one month for many intracranial tumours, or even earlier for tumours arising in the supratentorial compartment, in the midline or in cranial nerves. Many midline and cranial nerve tumours are craniopharygiomas (ICD-Oncology morphology code: 9350), germ cell tumours (9064), pineal tumours (9362) or Schwannomas (9560), which are slow-growing and likely to cause multiple presentations of less acute symptoms, offering opportunities for earlier detection in their natural history to reduce morbidity associated with their symptoms.34 Consistent with earlier studies, we found convulsion to be the commonest symptom of supratentorial tumours in primary care and in hospitals, followed by ophthalmic signs in primary care and features of raised intracranial pressure in secondary care.6, 35 Midline tumours are likely to present with loss of visual field and acuity, endocrinopathy or growth and developmental disorders,6, 36–38 features that are more likely to trigger a visit to the general practitioner early in the course of their natural history. Since focal neurological deficits are uncommon, particularly early in the natural history of intracranial tumours,13 examining for their presence plays very little role in bringing forward the diagnosis of a brain tumour. Early clinical features to watch out for, in both primary care and hospital settings, include convulsions, growth or endocrine disorders and recurrent headaches. First presentation of a seizure, especially a non-febrile seizure, should be evaluated by a paediatrician or a physician with training and expertise in epilepsy, and referred to tertiary service for any doubtful diagnosis or treatment failure.39
Cerebellar or brainstem tumours are expected to present commonly with loss of balance and coordination, cranial nerve dysfunction, focal neurological deficits and long-tract signs.36–38 We found tumours in the cerebellum or the brainstem, parts of the brain close to vital structures, rarely present in primary care much earlier than when the tumours are currently diagnosed when raised intracranial pressure develops to the extent that an urgent hospital visit becomes necessary. This means the diagnosis time for posterior fossa tumours has already reached an optimal level under current technology, and the scope for further reduction is limited.
4.1. Strengths and limitations
Many studies about symptoms and signs were limited to examining a snapshot close to a diagnosis date from interviews or review of medical notes after the researchers and the patients (or their parents) were fully aware of the true diagnosis.2–5, 40, 41 We were able to use the official diagnosis date in the National Cancer Registry, which is derived under international standards, as our end-point for the pre-diagnosis period to ensure consistency across individuals in the measurement of timing of primary care consultations and hospital visits.26, 27 We also developed a pre-defined list of symptoms and signs to search for relevant presentations in primary care (CPRD) and hospital (HES) records to ensure comparability between different cohorts and reproducibility of our findings in future studies, especially for investigating trends in clinical care.
We were unable to examine more closely the temporal pattern of symptoms and signs specific to each tumour location because of the small number of patients with linked primary care records: the population coverage of CPRD was 5–10% when our study was carried out. These have limited the certainty of our findings, but we have analysed primary care and hospital use in the largest population-based cohort of children with a histologically verified brain tumour available in England. This cohort of patients would otherwise be logistically difficult to create and follow up prospectively due to low incidence of childhood brain tumours.
Variations in the proportion of patients with linked records are due to differences in the probability of healthcare use. Tumours with a greater chance of requiring urgent interventions in hospitals (e.g. cerebellar, brainstem or peri-ventricular tumours) are found to be less likely to have presented in primary care, and therefore a linked record. The behaviour of those tumours are more fast-growing and they also originate in locations close to vital areas in the brain (brainstem) or in strategic sites that may cause life-threatening complications such as raised intracranial pressure (peri-ventricular). These two factors, in combination, reduced the chances for those tumours to be detected in primary care and contributed to the development of complications requiring urgent intervention.
The presence of a CPRD or HES record implies one or more clinical features have caused sufficient concern to the patient (or parents) to seek medical advice, and the symptoms and signs on record are those that were interpreted as important by the clinician who saw the patient. Although they may not represent the complete picture of presenting features, decisions on investigations and treatment were often made on the basis of medical records.42, 43 Thus, CPRD and HES data are reasonably representative of the information that were used in deciding diagnosis and treatment strategy in cancer patients.
4.2. Conclusion and implications
Many patients present to primary care and hospitals with increasing frequency until the eventual diagnosis of their brain tumours. The gradual increase in the frequency of primary care visits by patients with supratentorial, midline or cranial nerve tumours in the six-month period before diagnosis provides opportunities for an earlier diagnosis. The scope for earlier detection of cerebellar, brainstem or peri-ventricular tumours is more limited since the frequency of their presentation in primary care or in hospitals was very low before their diagnosis.
Common features of intracranial tumours in primary care are headaches, convulsions, growth and endocrine disorders, and cranial nerve II, III, IV or VI dysfunction.5, 13, 28, 35, 44 Presentations of these clinical features, especially when occurring repeatedly, should trigger further investigations into the possibility of an intracranial tumour. Features of raised intracranial pressure or focal neurological deficits are late signs more likely seen in emergency hospital admissions.
Supplementary Material
Highlights.
Peri-ventricular tumours have the highest presentation intensity.
Cerebellar or brainstem tumours are often of late sudden onset.
Non-localising features (convulsion, headaches, vomiting) predominate.
Focal neurological deficits are rarely seen.
Acknowledgements
We thank Children with Cancer UK for funding this work through the Jane Davidson and Paul O’Gorman Scholarship. We thank colleagues at the Cancer Research UK Cancer Survival Group, London School of Hygiene and Tropical Medicine for their guidance on data management and statistical analysis.
Funding: This work was supported by Children with Cancer UK through their Jane Davidson and Paul O’Gorman Scholarship.
Footnotes
Ethics approval
This study was approved by the Research Ethics Committee at the London School of Hygiene and Tropical Medicine (reference 5566). Use of patient information was approved by the Patient Information Advisory Group (succeeded by the National Health Service Health Research Authority Confidentiality Advisory Group) under Section 60 of the Health and Social Care Act 2001 and Section 251 of the National Health Service Act 2006 in England and Wales (reference PIAG 1-05(c)/2007 and PIAG 3-06(f)/2008).
Contributions
TPCC searched the literature, designed the study, carried out data management and statistical analysis, interpreted the findings and drafted the manuscript. AS helped with literature search, study design and contributed to interpretation of findings. DW helped with literature search. MPC helped with literature search, secured data access, advised on study design, data analysis and interpretation of findings. All authors contributed to revision of the manuscript.
TPCC and MPC have full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest
TPCC’s doctoral fellowship was funded by Children with Cancer UK. DW and TPCC are funded by The Brain Tumour Charity to evaluate the impact of raising awareness of brain tumour symptoms. DW receives funding from the Health Foundation and is a member of the Children with Cancer UK Scientific Advisory Panel. MPC and AS do not have any conflict of interest.
The funder does not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1.Stiller C. Childhood Cancer in Britain: Incidence, survival, mortality. Oxford, UK: Oxford University Press; 2007. [Google Scholar]
- 2.Coserria Sanchez JF, Garrido Ocana AI, Quiroga Cantero E, Reina Gonzalez AM, Amadeu Da Costa AP, Garcia Zarza N. [Presenting signs and symptoms of central nervous system tumors according to age] An Pediatr (Barc) 2007 Feb;66(2):115–20. doi: 10.1157/13098927. [DOI] [PubMed] [Google Scholar]
- 3.Dommett RM, Redaniel MT, Stevens MC, Hamilton W, Martin RM. Features of childhood cancer in primary care: a population-based nested case-control study. Br J Cancer. 2012 Feb 28;106(5):982–7. doi: 10.1038/bjc.2011.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dommett RM, Redaniel MT, Stevens MC, Hamilton W, Martin RM. Features of cancer in teenagers and young adults in primary care: a population-based nested case-control study. Br J Cancer. 2013 Jun 11;108(11):2329–33. doi: 10.1038/bjc.2013.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vazquez Roman S, Martinez Anton A, Llorente Otones L, Rojo Conejo P, Hinojosa Bernal J. [Initial signs and symptoms of brain tumors in children] Neurologia. 2008 May;23(4):215–9. [PubMed] [Google Scholar]
- 6.Wilne S, Collier J, Kennedy C, Koller K, Grundy R, Walker D. Presentation of childhood CNS tumours: a systematic review and meta-analysis. Lancet Oncol. 2007 Aug;8(8):685–95. doi: 10.1016/S1470-2045(07)70207-3. [DOI] [PubMed] [Google Scholar]
- 7.Wilne S, Koller K, Collier J, Kennedy C, Grundy R, Walker D. The diagnosis of brain tumours in children: a guideline to assist healthcare professionals in the assessment of children who may have a brain tumour. Arch Dis Child. 2010 Jul;95(7):534–9. doi: 10.1136/adc.2009.162057. [DOI] [PubMed] [Google Scholar]
- 8.National Collaborating Centre for Cancer. Suspected cancer: recognition and referral. National Institute for Health and Care Excellence; 2015. [PubMed] [Google Scholar]
- 9.Neuro-Oncology Group, James Lind Alliance. Top 10 priorities for clinical research in primary brain and spinal cord tumours: Final report of the James Lind Alliance Priority Setting Partnership in Neuro-Oncology [Google Scholar]
- 10.The James Lind Alliance. James Lind Alliance: Priority Setting Partnerships 2016. 2016 May 16; Available from: http://www.jla.nihr.ac.uk.
- 11.Fellegi IP, Sunter AB. A Theory for Record Linkage. Journal of the American Statistical Association. 1969;64(328):1183–210. [Google Scholar]
- 12.Gill L. Methods for Automatic Record Matching and Linkage and their Use in National Statistics. Norwich, UK: Her Majesty's Stationery Office; 2001. [Google Scholar]
- 13.Chu TP, Shah A, Walker D, Coleman MP. Pattern of symptoms and signs of primary intracranial tumours in children and young adults: a record linkage study. Arch Dis Child. 2015 Dec;100(12):1115–22. doi: 10.1136/archdischild-2014-307578. [DOI] [PubMed] [Google Scholar]
- 14.Kariyawasam DS, McShane T. Brain tumours in paediatrics: when should they be suspected? Arch Dis Child. 2015 Dec;100(12):1102–3. doi: 10.1136/archdischild-2015-308729. [DOI] [PubMed] [Google Scholar]
- 15.Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International Classification of Childhood Cancer, third edition. Cancer. 2005 Apr 1;103(7):1457–67. doi: 10.1002/cncr.20910. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Manual of the international statistical classification of diseases, injuries, and causes of death. Geneva, Switzerland: World Health Organization; 1977. [Google Scholar]
- 17.World Health Organization. International statistical classification of diseases and related health problems. Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]
- 18.Coleman MP, Babb P, Damiecki P, Grosclaude P, Honjo S, Jones J, et al. Cancer survival trends in England and Wales, 1971-1995: deprivation and NHS region. London, UK: The Stationery Office; 1999. [Google Scholar]
- 19.GPRD. The General Practice Research Database. London, UK: GPRD; 2011. [Google Scholar]
- 20.Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van Staa T, et al. Data Resource Profile: Clinical Practice Research Datalink (CPRD) Int J Epidemiol. 2015 Jun;44(3):827–36. doi: 10.1093/ije/dyv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol. 2010 Jan;69(1):4–14. doi: 10.1111/j.1365-2125.2009.03537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Health and Social Care Information Centre. The processing cycle and HES data quality Leeds. UK: Health and Social Care Information Centre; 2016. Mar 1, Available from: http://www.hscic.gov.uk/article/1825/The-processing-cycle-and-HES-dataquality. [Google Scholar]
- 23.Hanchett N. ONS to HES Linkage Release 1: Core Documentation. London, UK: Thames Cancer Registry, King's College London; 2008. [Google Scholar]
- 24.Thames Cancer Registry. National Cancer Data Repository - 1990 to 2008: Matching Registry Records to Hospital Episodes Version 1.2. London, UK: Thames Cancer Registry, King's College London; 2011. [Google Scholar]
- 25.General Practice Research Database. GPRD Medical Browser, version 1.3.1. London, UK: Medicines and Healthcare products Regulatory Agency; 2010. [Google Scholar]
- 26.Jensen OM, Parkin DM, MacLennan R, Muir CS, Skeet RG, editors. International Agency for Research on Cancer, International Association of Cancer Registries. Cancer Registration: Principles and Methods. Lyon, France: International Agency for Research on Cancer; 1991. [Google Scholar]
- 27.United Kingdom Association of Cancer Registries. Po/99/03 Definition of Diagnosis Date. United Kingdom Association of Cancer Registries; 2011. [Google Scholar]
- 28.Ansell P, Johnston T, Simpson J, Crouch S, Roman E, Picton S. Brain tumor signs and symptoms: analysis of primary health care records from the UKCCS. Pediatrics. 2010 Jan;125(1):112–9. doi: 10.1542/peds.2009-0254. [DOI] [PubMed] [Google Scholar]
- 29.Chambers J, Hastie T. Statistical Models in S. London, UK: Chapman & Hall; 1993. [Google Scholar]
- 30.Cleveland WS. Robust Locally Weighted Regression and Smoothing Scatterplots. Journal of the American Statistical Association. 1979;74(368):829–36. [Google Scholar]
- 31.Cleveland WS, Devlin SJ. Locally Weighted Regression - an Approach to Regression-Analysis by Local Fitting. Journal of the American Statistical Association. 1988 Sep;83(403):596–610. [Google Scholar]
- 32.Hastie T. gam: Generalized Additive Models. 2011 doi: 10.1177/096228029500400302. [DOI] [PubMed] [Google Scholar]
- 33.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: 2011. [Google Scholar]
- 34.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007 Aug;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilne SH, Ferris RC, Nathwani A, Kennedy CR. The presenting features of brain tumours: a review of 200 cases. Arch Dis Child. 2006 Jun;91(6):502–6. doi: 10.1136/adc.2005.090266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crossman AR, Neary D. Neuroanatomy: an illustrated colour text. Edinburgh, UK: Churchill Livingstone; 2005. [Google Scholar]
- 37.Sinnatamby C. Last's Anatomy - Regional and Applied. Edinburgh, UK: Churchill Livingstone; 1999. [Google Scholar]
- 38.Ogilvie C, Evans C. Chamberlain's Symptoms and Signs in Clinical Medicine: An Introduction to Medical Diagnosis. Oxford, UK: Butterworth-Heinemann; 1997. [Google Scholar]
- 39.National Institute for Health and Clinical Excellence. Epilepsies: diagnosis and management. London, UK: National Institute for Health and Care Excellence; 2011. [Google Scholar]
- 40.Hamilton W, Kernick D. Clinical features of primary brain tumours: a case-control study using electronic primary care records. Br J Gen Pract. 2007 Sep;57(542):695–9. [PMC free article] [PubMed] [Google Scholar]
- 41.Weller D, Vedsted P, Rubin G, Walter FM, Emery J, Scott S, et al. The Aarhus statement: improving design and reporting of studies on early cancer diagnosis. Br J Cancer. 2012 Mar 27;106(7):1262–7. doi: 10.1038/bjc.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davies E, Clarke C. Early symptoms of brain tumours. J Neurol Neurosurg Psychiatry. 2004 Aug;75(8):1205–6. doi: 10.1136/jnnp.2003.033308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dixon-Woods M, Findlay M, Young B, Cox H, Heney D. Parents' accounts of obtaining a diagnosis of childhood cancer. Lancet. 2001 Mar 3;357(9257):670–4. doi: 10.1016/S0140-6736(00)04130-1. [DOI] [PubMed] [Google Scholar]
- 44.Gordon GS, Wallace SJ, Neal JW. Intracranial tumours during the first two years of life: presenting features. Arch Dis Child. 1995 Oct;73(4):345–7. doi: 10.1136/adc.73.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.