Introduction
Charcot-Marie-Tooth disease (CMT) is an umbrella term for more than 90 different genetic causes of inherited peripheral neuropathies (http://hihg.med.miami.edu/code/http/cmt/public_html/index.html#/) and has an estimated prevalence of 1 in 2500. Most cases present within the first two decades of life but increasing axonal genetic subtypes (CMT2) first present in adulthood. Identifying additional genes causing CMT2 is important because these can identify molecular pathways involved in axonal degeneration and enable development of rational therapies for these and related disorders.
We have used whole exome sequencing (WES) to identify two families with CMT2 caused by mutations in the Bcle-associated athanogene 3 (BAG3). Mutations in BAG3 have previously been shown to cause a myofibrillar myopathy (1, 2) often associated with cardiomyopathy that usually presents in childhood. Children have been reported with peripheral neuropathy in addition to myopathy (3) and/or cardiomyopathy (4).
Genetic testing
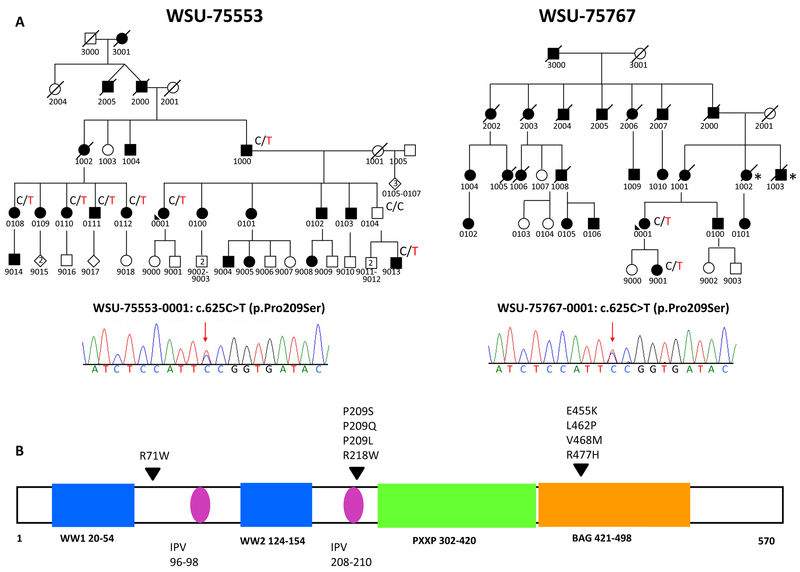
Genomic analysis was focused on rare and conserved variants that followed an autosomal dominant inheritance pattern in whole exome sequencing (WES) of both probands using the GEM.app/GENESIS software. We only considered variants that met the following criteria: 1) non-synonymous change; 2) minor allele frequency in ExAC database < 0.0001 out of ~120,000 chromosomes; 3) not present in more than two families within the GEM.app/GENESIS database of 12,500 chromosomes, 4) GERP > 3 OR PhastCons > 0.6, and 5) Genotype Quality > 75. This analysis resulted in the identification of a variant in BAG3 (c.625C>T; p.Pro209Ser) that completely co-segregated with the presence of neuropathy in both large families (Figure). Segregation testing of family members was by Sanger Sequencing. The nucleotide is highly conserved (phastcons 1, GERP score 6.170) and predicted to be damaging (LRT - damaging, SIFT - damaging, Mutationtaster - disease causing). We calculated the simple probability that the observed variant segregates in family 75553 by chance and found that probability N equals (1/2)8 (affected meiosis) = 1/256. According to ACMG Pathogenicity Classification N<1/16, identified in more than one family, supports strong evidence for pathogenicity.
Clinical Summary
We evaluated five affected members from two families; four from one family (Table 1A). Institutional Review Boards at the participating institutions approved the study and all participating individuals signed informed consents that were approved by the IRBs. Symptoms were first noted in adulthood although several patients retrospectively recognized earlier symptoms. Sensory abnormalities predominated. All affected subjects had normal birth histories and early milestones. Nerve conduction studies demonstrated reduced motor and/or sensory responses in all patients (Table 1B). Extensive needle EMG on proximal and distal muscles in four subjects demonstrated chronic denervation but no acute or chronic myopathic findings. No subject developed symptoms or signs of proximal weakness. Creatine kinase (CK) levels were normal or at most mildly elevated at levels of 112, 341, and 389 for subject 75553–0108, who is not included in the table as we did not evaluate him (Table 1). One subject we evaluated (75767–0001) had a history of cardiac issues (palpitations). However, in family 75767, four additional family members died of a cardiac death, one as early as 46 years of age. There were no known cases of cardiac disease in the larger family 75553; however, at least 4 family members developed shortness of breath starting around 40–50 years of age. We cannot exclude congestive heart disease as a cause of this shortness of breath.
Table 1:
CLINICAL FEATURES OF FAMILY WITH BAG3 Mutation
| Patient Age (years) |
CMTNSv2 | Distal Weakness LL |
Proximal Weakness LL |
Distal Weakness UL |
Proximal Weakness UL |
Vibration LL |
Vibration UL |
Cutaneous LL |
Cutaneous UL |
C K |
AOO | Cardiac | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
WSU-75767–0001 KH |
61 | 12 (moderate) |
+ (4,5) | − (5,5) | +(5,4,5) | − (5,5,5) | Abs Toes, ankles, red knees | Normal | Abs toes | Normal | 40s | − | |
| WSU-75767-0101 | 52 | 5 (mild) |
− (5,5) | − (5,5) | − (5,5,5) | − (5,5,5) | Abs toes | Normal | Abs toes, ankles | Normal | 40s | − | |
| 0103 | 66 | 16 (moderate) |
− (5,5) | − (5,5) | − (5,5,5) | − (5,5,5) | Abs toes, Red ankles, knees | Red fingers | Abs toes, ankles, knees | Normal | |||
| 0108 | 48 | 5 (mild) |
+ (4,5) | − (5,5) | + (4+,4+,4+) | − (5,5,5) | Abs toes | Normal | Absent toes | Normal | |||
| 0104 | 66 | 7 (mild) |
+ (0,3) | + (4,4−) | + (4+,5, 4) | − (5,5,5) | Abs toes, Red. ankles | Normal | Red toes | Normal |
Motor weakness based on MRC scale (0–5): “+” = weakness present, “−” = no weakness detected. LL Distal weakness assessed by Anterior Tibialis and Gastrocnemius, LL Proximal weakness assessed by Ilio Psoas and Quadriceps; UL Distal weakness assessed by First Dorsal Interosseous, Abductor Pollicis Brevis, and Adductor Digiti Minimi, UL Distal weakness assessed by Deltoids, Biceps Brachii, and Triceps. Vibration based on Rydell tuning fork with “5” on scale of “8” being considered normal and Cutaneous based on Pinprick sensation: Normal is no decrease compared to the examiner, Red is reduced, and abs is absent up to level indicated. Both motor and sensory evaluations were based on worst score observed of the two limbs. CMTNSv2 scores are separable into <10 (mild), 11–20 (moderate) or >20 (severe) impairment (22003934).
DISCUSSION
BAG3 is a member of the BAG family that act as co-chaperones, and is known to interact with the ATPase domains of both heat shock protein 70 (Hsp70) and heat shock cognate protein 70 (Hsc70) (5). Several mutations in BAG3 cause a myofibrillar myopathy (1, 2) through mechanisms probably related to interactions between BAG3 and HspB8, a member of the HspB family of molecular chaperones. Mutations in HsbP8 also cause axonal CMT2 (6). BAG3 binding to HspB8 is mediated by two conserved Ile-Pro-Val (IPV) motifs, one of which includes Pro209 (7), the amino acid that is mutated in our patients. Deletion of IPV suppresses BAG3-HspB8 binding and fails to promote clearance of huntingtin mutant aggregates(8). In skeletal muscles BAG3 plays a role in cell resilience to mechanical stress in a process known as CASA (chaperone-assisted selected autophagy) (9). Peripheral nerves are constantly subjected to mechanical tension and prone to protein misfolding/aggregation potentially involving this mechanism of action.
Distinct mutations at Pro209 appear to cause distinct phenotypes, both in the cellular target and severity. Pro209Leu BAG3 mutation causes a severe distal myopathy and cardiomyopathy in children (1). In addition, three children with Pro209Leu BAG3 mutations were shown to have giant axonal neuropathies, cardiomyopathy and evidence of proximal weakness (4). Alternatively, Pro209Gln mutation only caused a mild, adult onset myopathy in several families (3); one of these family members also had an axonal neuropathy (3). All affected individuals in our families with Pro209Ser BAG mutations have late onset sensorimotor axonal neuropathies with no clinical evidence of myopathy. These results demonstrate that BAG3 mutations can specifically cause classic Charcot Marie Tooth disease and further demonstrate the importance of codon 209 for normal functioning of BAG3. Finally, as this case illustrates, a clinical diagnosis of CMT2 may trigger identification of a subclinical, potentially life threatening, cardiac disease.
BAG3 is the most recent gene that can cause CMT2 with onset in adulthood. Multiple mutations in the Myelin Protein Zero gene (MPZ) cause axonal neuropathies beginning after age 40 as do some mutations in Mitofusin 2 (MFN2), Membrane Metalloendopeptidase (MME) and Leucine Rich Repeat and Sterile Alpha Motif containing 1 (LRSAM1), in some cases without a clear family history. Identification of these genes is significant because it suggests that adult patients diagnosed with idiopathic axonal neuropathy may have a genetic cause for their disease. Peripheral neuropathy affects 4% of the population over age 60 and 10% over age 80 (10). Identifying genetic causes in this population will potentially provide answers to many patients with iatrogenic diagnoses.
Figure 1: Bag3 mutation in CMT2 families.
A. Segregation of the P209S variant in two autosomal dominant CMT2 families. B. Diagram depicts position of pathogenic variants (arrow heads) in the BAG3 protein. The IPV (Ile-Pro-Val) motifs are highlighted in pink and the protein domains are represented by the colored boxes (PXXP: proline-rich repeat region, WW (Trp-Trp) region).
Acknowledgments
Study funding: This study was supported in part by the Inherited Neuropathy Consortium - Rare Disease Clinical Research Consortium (INC RDCRC - U54NS065712), NINDS/ORDR, NCATS, the Muscular Dystrophy Association (MDA), the Charcot Marie Tooth Association (CMTA) and NINDS R01NS075764 (SZ and MES).
Footnotes
Disclosures: The authors have no relevant disclosures with the exception of grant support listed above
REFERENCES
- 1.Selcen D, Muntoni F, Burton BK, Pegoraro E, Sewry C, Bite AV, et al. Mutation in BAG3 causes severe dominant childhood muscular dystrophy. Annals of neurology. 2009;65(1):83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Homma S, Iwasaki M, Shelton GD, Engvall E, Reed JC, Takayama S. BAG3 deficiency results in fulminant myopathy and early lethality. The American journal of pathology. 2006;169(3):761–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Semmler AL, Sacconi S, Bach JE, Liebe C, Burmann J, Kley RA, et al. Unusual multisystemic involvement and a novel BAG3 mutation revealed by NGS screening in a large cohort of myofibrillar myopathies. Orphanet journal of rare diseases. 2014;9:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaffer F, Murphy SM, Scoto M, Healy E, Rossor AM, Brandner S, et al. BAG3 mutations: another cause of giant axonal neuropathy. Journal of the peripheral nervous system : JPNS. 2012;17(2):210–6. [DOI] [PubMed] [Google Scholar]
- 5.Takayama S, Xie Z, Reed JC. An evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone regulators. The Journal of biological chemistry. 1999;274(2):781–6. [DOI] [PubMed] [Google Scholar]
- 6.Tang BS, Zhao GH, Luo W, Xia K, Cai F, Pan Q, et al. Small heat-shock protein 22 mutated in autosomal dominant Charcot-Marie-Tooth disease type 2L. Human genetics. 2005;116(3):222–4. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs M, Poirier DJ, Seguin SJ, Lambert H, Carra S, Charette SJ, et al. Identification of the key structural motifs involved in HspB8/HspB6-Bag3 interaction. The Biochemical journal. 2010;425(1):245–55. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs M, Poirier DJ, Seguin SJ, Lambert H, Carra S, Charette SJ, et al. Identification of the key structural motifs involved in HspB8/HspB6-Bag3 interaction. The Biochemical journal. 2009;425(1):245–55. [DOI] [PubMed] [Google Scholar]
- 9.Ulbricht A, Gehlert S, Leciejewski B, Schiffer T, Bloch W, Hohfeld J. Induction and adaptation of chaperone-assisted selective autophagy CASA in response to resistance exercise in human skeletal muscle. Autophagy. 2015;11(3):538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffman EM, Staff NP, Robb JM, St Sauver JL, Dyck PJ, Klein CJ. Impairments and comorbidities of polyneuropathy revealed by population-based analyses. Neurology. 2015;84(16):1644–51. [DOI] [PMC free article] [PubMed] [Google Scholar]