Abstract
Growing evidence suggests a possible link between hyperpolarization-activated cyclic nucleotide-gated nonselective cation (HCN) channels and depression. In a recent study published in Molecular Psychiatry, we first demonstrate that Ih (the membrane current mediated by HCN channels) and HCN1 protein expression were increased in dorsal, but not in ventral, CA1 region following chronic, but not acute stress. This upregulation of Ih was restricted to the perisomatic region of CA1 neurons and contributed to a reduction of neuronal excitability. A reduction of HCN1 protein expression in dorsal CA1 region before the onset of chronic unpredictable stress-induced depression was sufficient to provide resilient effects to chronic unpredictable stress. Furthermore, in vivo block of the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA) pumps, a manipulation known to increase intracellular calcium levels and upregulate Ih, produced anxiogenic-like behavior and an increase in Ih, similar to that observed in chronic unpredictable stress model of depression. Here, we share our view on (1) how the function and expression of HCN1 channels are changed in the brain in a subcellular region-specific manner during the development of depression and (2) how a reduction of HCN1 protein expression provides resilience to chronic stress.
Keywords: hippocampus, HCN1, Ih, chronic unpredictable stress, resilience
Commentary on: Kim CS, Brager DH, Johnston D. Perisomatic changes in h-channels regulate depressive behaviors following chronic unpredictable stress. Mol Psychiatry. 2018; 23: 892–903. DOI: 10.1038/mp.2017.28.
The limbic–cortical system is an integral brain region underlying the action of depression and antidepressants.1,2 Major depression is a mental disorder characterized by an all-encompassing low mood, low self-esteem, and loss of interest or pleasure in enjoyable activities. Current existing antidepressants work mainly on targeting the monoamine system and increasing serotonin levels in the limbic brain areas including hippocampus. The hippocampus is involved in the symptoms of depression such as behavior despair and anhedonia as well as a site of action for antidepressant responses. About 30% of depressed patients do not respond to available antidepressant drugs (i.e., treatment-resistant depression (TRD)).3 This TRD requires trial-and-error combinations of multiple different medication regimens and electrical stimulation therapies.4 Although electrical stimulation therapies (e.g., electro convulsive therapy, deep brain stimulation, and transcranial magnetic stimulation) are often effective to treat TRD through a change in brain activity, they also cause unwanted severe side effects such as memory loss and can be cost prohibitive for patients. Recent groundbreaking clinical findings for TRD demonstrate that a single intravenous injection with a subanesthetic dose of ketamine (0.5 mg/kg over 40 min) provides a rapid and sustained antidepressant effect.5 Given that the U.S. Food and Drug Administration has only approved ketamine, a noncompetitive N-methyl-D-aspartate receptor antagonist, as an anesthetic agent, treatment of depression by ketamine is not covered by insurance. Furthermore, ketamine has the potential for side effects such as psychotomimetic effects and drug misuse.
Growing evidence suggests a possible link between hyperpolarization-activated cyclic nucleotide-gated nonselective cation (HCN) channels and depression. Whole-brain knockout mice in which either the pore-forming (HCN1 and HCN2) protein or an auxiliary channel subunit, tetratricopeptide repeat-containing Rab8b interacting protein (TRIP8b) of HCN channels were deleted, all result in a reduction of functional Ih (the membrane current mediated by HCN channels) showed antidepressant-like behaviors.6 The HCN channels are members of the voltage-gated cation channel superfamily. The HCN1 is a main isoform of HCN channels (HCN1–HCN4) and is highly expressed in the hippocampal CA1 region, showing a gradient of increasing channel expression along the somatodendritic axis.7 HCN channels are active at the resting membrane potential leading to the generation of a noninactivating inward current that contributes to the intrinsic membrane properties, neuronal excitability, synaptic integration, and synaptic plasticity.8 Because there is no specific HCN channel blockers or genetic animal models that provide region-specific manipulation of HCN1 channels, we developed a lentiviral shRNA system, which provides sequence-specific manipulation with spatial and temporal control and high transduction efficiency.9 When HCN1 protein was reduced (about 40%–50%) in dorsal CA1 region (which corresponds to the posterior hippocampus in human) in normal rats, we observed antidepressant- and anxiolytic-like effects in specific behavioral tests (e.g., open-field test, elevated plus maze test, and forced swim test). Given the limited lentiviral shRNA infection in dorsal CA1 region (about 30% of the dorsal CA1 region), it was quite surprising that a reduction of functional Ih made such significant changes in behaviors. Furthermore, the ventral hippocampus (which corresponds to anterior hippocampus in humans) is known to be heavily involved in emotion-related behaviors such as fear and anxiety, so we assumed that we might see similar behavioral effects following a reduction of HCN1 protein expression in ventral CA1 region. However, in spite of a reduction of Ih in ventral CA1 neurons from the viral shRNA infection, there were no effects on anxiolytic- or antidepressant-like behaviors. These results suggest that a reduction of functional Ih in dorsal versus ventral CA1 region produced differential behavioral outputs.
The rodent hippocampus can be separated into dorsal and ventral regions based on the functional differentiation.10 In particular, intrinsic membrane properties of CA1 neurons between dorsal and ventral hippocampus are quite different.11 For example, dorsal CA1 neurons have more hyperpolarized resting membrane potential and a lower input resistance at rest compared with ventral CA1 neurons. In addition, higher resting G-protein-coupled inwardly rectifying K+ channels conductance activated by A1 adenosine receptors are present in dorsal compared with ventral CA1 neurons. Because we observed antidepressant- and anxiolytic-like effects following a reduction of HCN1 protein in dorsal but not ventral CA1 region in normal rats, the important questions are (1) whether function and expression of HCN1 channels are altered during the development of depression, (2) whether changes in functional Ih and HCN1 protein expression occur in dorsal or ventral or both region of hippocampus, and (3) whether a reduction of HCN1 protein expression can prevent the development of depression (i.e., resilient effects to chronic unpredictable stress (CUS)). In a recent study published in Molecular Psychiatry,12 we addressed these issues.
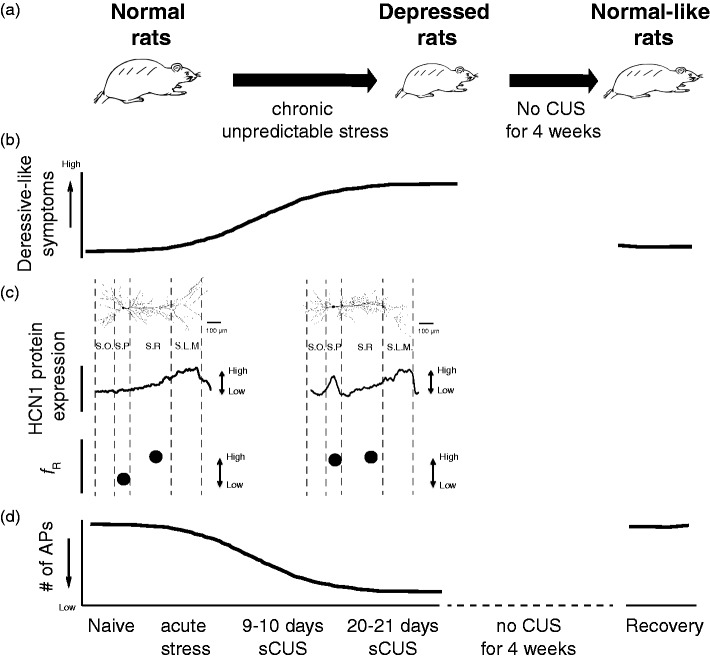
Chronic stress can be a risk factor for the development of depression. Depressed patients show relatively high cortisol levels due to CUS-induced impairment of hypothalamus–pituitary–adrenal axis.13 In this regard, CUS protocol has been widely used as an experimental tool to examine the development of psychopathology, including depression.14 When rats are exposed to random, intermittent, and unpredictable stress daily for two to four weeks, rats show a decreased sucrose preference (i.e., anhedonia), increased anxiety, and increased passive activity in an extremely stressful environment (i.e., behavioral despair) (Figure 1(b)), which are all core symptoms of human depression. Using the CUS model of depression, we found that functional Ih and HCN1 protein expression are increased in dorsal but not ventral CA1 neurons. This is consistent with the results shown by the differential behavioral effects observed through a reduction of HCN1 protein in dorsal versus ventral CA1 region in normal rats. In addition, biochemical assays revealed that HCN2 protein expression is also increased in dorsal but not in ventral CA1 region. Therefore, upregulation of functional Ih stems from increases in both HCN1 and HCN2 protein expression in dorsal but not in ventral CA1 region, and also there is a correlation in the time courses for upregulation of Ih and depression-like symptoms during the development of depression. In addition, when depressed rats were not exposed to further CUS for four weeks (i.e., recovery period), an increase in passive activity (in forced swim test) and upregulation of Ih were back to normal levels (Figure 1(b)).
Figure 1.
Perisomatic upregulation of functional Ih regulate depressive behaviors following CUS. (A) Representative illustration of development of depression following CUS and recovery. (B) Depressive-like symptoms gradually increased with increasing duration of CUS. These depressive-like symptoms decreased following four-week recovery period. (C) Neuronal excitability is gradually decreased with increasing duration of CUS. This CUS-induced a decrease in neuronal excitability do not occur after a four-week recovery period. (D) Perisomatic increases in Ih-sensitive electrophysiological measurement (i.e., resonance frequency, fR) and HCN1 protein expression along the somatodendritic region of dorsal CA1. AP: action potential; CUS: chronic unpredictable stress; HCN: hyperpolarization-activated cyclic nucleotide-gated nonselective cation; SLM: stratum lacunosum moleculare; SR: stratum radiatum; SO: stratum oriens; SP: stratum pyramidale.
One of the key features of HCN1 (and HCN2) channels in the hippocampus is a distant-dependent increase in channel expression along the somatodendritic axis.7 Given the contributions of HCN1 channels to neuronal excitability and synaptic integration, a change in subcellular expression of HCN1 channels along the somatodendritic axis of CA1 neurons has been implicated in the neuropathology of psychiatric disorders such as epilepsy. Interestingly, we found that upregulation of functional Ih is limited to the perisomatic but not dendritic region of dorsal CA1 neurons in CUS model of depression (Figure 1(d)). This abnormal perisomatic upregulation of Ih might be due to (1) the increases in cytoplasmic calcium levels, which in turn activate inositol 1,4,5-trisphosphate receptors and store-operated channels15 or (2) the involvement of specific TRIP8b isoforms (e.g., an increase in Ih by isoform A4 and a decrease in Ih by isoform B2).16 Major depression is the most frequent psychiatric comorbidity in patients with temporal lobe epilepsy (TLE). Because treatment for patients with comorbid epilepsy and major depression is difficult due to the fact that (1) conventional antidepressant drugs can lower seizure threshold and (2) antiepileptic drugs can cause mood instability. When HCN1 protein was reduced in dorsal CA1 region, we did not observe any abnormal epileptic-like activity in voltage-sensitive dye imaging and extracellular field potential recording.9 Interestingly, a reduction of dendritic Ih in CA1 region of hippocampus was observed in the kainic acid-induced poststatus epilepticus model of TLE.17 Therefore, changes in subcellular expression of functional Ih might be a cellular and molecular consequences of major depression and TLE.
Major depressive disorder (MDD) is a disorder of neural circuitry in limbic–cortical areas. Functional brain imaging of major depressed patients shows increased or decreased regional metabolic activities in different regions of these areas, which can be reversed by clinical antidepressant treatment or chronic electrical stimulation.2,4 There is a strong positive correlation between brain energy metabolism and neuronal excitability.18 Neuronal excitability can be determined by measurements of input resistance and action potential firing of neurons at rest. Interestingly, bimodal, abnormal changes in regional metabolic activities in different regions of limbic–cortical areas, increased neuronal excitability of lateral amygdala neurons,19 or decreased neuronal excitability of dorsal CA1 neurons12 following chronic stress have all been reported (Figure 1(c)). In addition, functional Ih and HCN2 protein expression are reduced in ventral tegmental area following CUS.20 Given the neural circuitry disorder of MDD, a change in either HCN1 (in dorsal hippocampal CA1 region) or HCN2 (in ventral tegmental area (VTA)) protein expression might provide therapeutic effects. Brain-derived neurotrophic factor (BDNF) can be a biological marker for depression.21 Along with the differential changes in functional Ih from depression and antidepression in dorsal CA1 region versus VTA, BDNF expression also shows opposing effects in those brain regions. A reduction of BDNF expression in hippocampus produces depressive-like effects,22 whereas antidepressant-like effects in VTA.23 In addition, Yang et al., reported that changes in action potential firing mode (i.e., tonic firing to burst firing) in the lateral habenula (LHb) occurred in the animal model of depression.24 Because LHb region is known as an antireward center, a hyperactive neuronal excitability of LHb neurons can effectively inhibit downstream reward-related brain regions such as VTA. In a similar way, a hypoactive neuronal excitability of dorsal CA1 neurons in the animal model of depression might offer difficulties to carry spatial-related information to downstream brain regions. Indeed, impaired spatial learning and memory was often observed in depressed patients.25 Furthermore, forebrain-specific HCN1 knockout mice showed enhanced short- and long-term spatial learning and memory.26
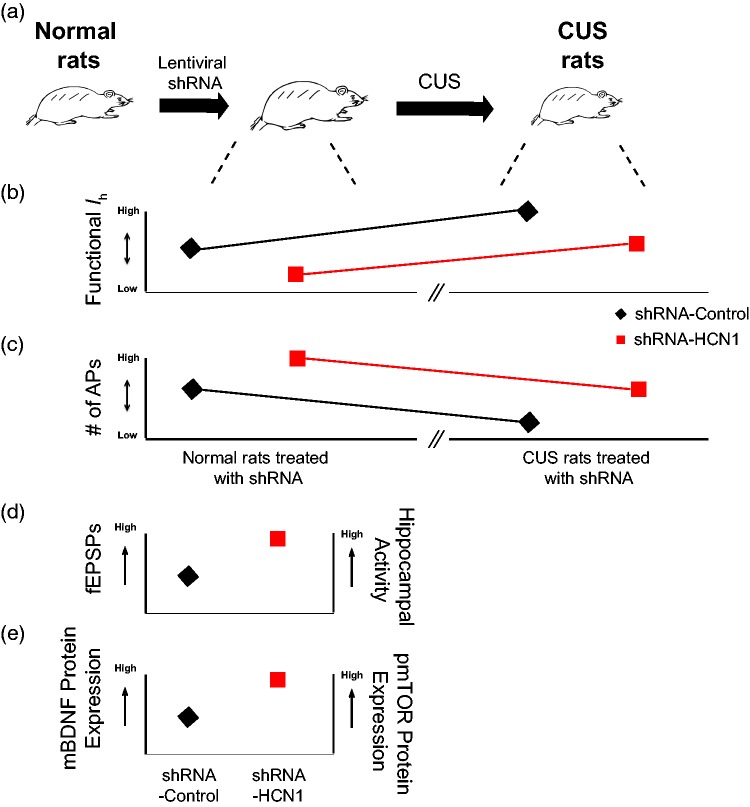
Most of the depression researches to date have been focused on finding the mechanisms underlying the action of depression and antidepressants. Recently, there is a growing interest in the concept of the “susceptibility to depression” and “resilience to depression” in stressful environments.27 Why do some people become depressed (i.e., susceptibility to depression), while others are not (i.e., resiliency to depression) following stressful life events? Resilience is considered to be a positive coping process from adverse stress. In a recent report, a single or multiple injections of neuropeptide Y (NPY) into basolateral amygdala produced resilience to stress in rats through a reduction of functional Ih.28 Importantly, we also found that a reduction of Ih in dorsal CA1 region before the CUS-induced onset of depression is sufficient to provide resilient effects to CUS (Figure 2). Before onset of CUS-induced depression, a reduction of functional Ih in dorsal CA1 region/neurons led to (1) changes in intrinsic membrane properties, (2) an increase in neuronal excitability, (3) an enhancement of the BDNF-mTOR signaling pathways, (4) an increase in field excitatory postsynaptic potentials, and (5) an increase in dorsal hippocampal activity9 (Figure 2). These cellular changes might prime the brain to prevent the development of depression (i.e., resilience to depression).
Figure 2.
A reduction of functional Ih in dorsal CA1 region/neurons confers resiliency to CUS. (a) Representative illustration of development of resilience to CUS. (b and c) Changes in functional Ih and neuronal excitability of lentiviral-shRNA-infected dorsal CA1 neurons (i.e., shRNA-control and shRNA-HCN1) before and after development of depression. (D and E) A reduction of HCN1 protein expression leads to increases in fEPSPs, hippocampal activity, mature BDNF, and pmTOR protein expression. AP: action potential; BDNF: brain-derived neurotrophic factor; CUS: chronic unpredictable stress; fEPSP: field excitatory postsynaptic potential; HCN: hyperpolarization-activated cyclic nucleotide-gated nonselective cation; pmTOR phosphorylation of mTOR.
It is quite amazing that a reduction of a single ion channels (i.e., HCN1) in a certain brain region (i.e., dorsal CA1 region) can change physiological, biochemical, and behavioral outputs. However, targeting HCN1 channels might not be a viable clinical approach for major depression due to (1) the lack of a subunit-specific inhibitor of HCN channels, (2) the fact that the HCN1 channel is also expressed in the heart and thus inhibitors could have effects on cardiac function, and (3) the changes in opposing directions in different brain regions (e.g., dorsal CA1 region vs. VTA). In this regard, targeting a brain region-specific manipulation of HCN1 channels using a viral delivery system (i.e., adeno-associated virus system) might be a possible clinical approach. Alternatively, the auxiliary subunit of HCN channels, TRIP8b, could also be an important and viable target for treatment of major depression.
Author Contributions
C.S.K. and D.J. developed the structure and arguments for the paper and wrote the paper. C.S.K. designed and produced the figures. All authors approved the final version of the paper.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interests with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: C.S.K. received 2017 Young Investigator award (#26382) from Brain & Behavior Research Foundation. D.J. received National Institutes of Health grant (NS084473).
References
- 1.Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997; 9: 471–481. [DOI] [PubMed] [Google Scholar]
- 2.Mayberg HS, Brannan SK, Tekell JL, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000; 48: 830–843. [DOI] [PubMed] [Google Scholar]
- 3.Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003; 53: 649–659. [DOI] [PubMed] [Google Scholar]
- 4.Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005; 45: 651–660. [DOI] [PubMed] [Google Scholar]
- 5.Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000; 47: 351–354. [DOI] [PubMed] [Google Scholar]
- 6.Lewis AS, Vaidya SP, Blaiss CA, et al. Deletion of the hyperpolarization-activated cyclic nucleotide-gated channel auxiliary subunit TRIP8b impairs hippocampal Ih localization and function and promotes antidepressant behavior in mice. J Neurosci. 2011; 31: 7424–7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lorincz A, Notomi T, Tamas G, Shigemoto R, Nusser Z. Polarized and compartment-dependent distribution of HCN1 in pyramidal cell dendrites. Nat Neurosci. 2002; 5: 1185–1193. [DOI] [PubMed] [Google Scholar]
- 8.Biel M, Wahl-Schott C, Michalakis S, Zong X. Hyperpolarization-activated cation channels: from genes to function. Physiol Rev. 2009; 89: 847–885. [DOI] [PubMed] [Google Scholar]
- 9.Kim CS, Chang PY, Johnston D. Enhancement of dorsal hippocampal activity by knockdown of HCN1 channels leads to anxiolytic- and antidepressant-like behaviors. Neuron. 2012; 75: 503–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998; 8: 608–619. [DOI] [PubMed] [Google Scholar]
- 11.Kim CS, Johnston D. A1 adenosine receptor-mediated GIRK channels contribute to the resting conductance of CA1 neurons in the dorsal hippocampus. J Neurophysiol. 2015; 113: 2511–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim CS, Brager DH, Johnston D. Perisomatic changes in h-channels regulate depressive behaviors following chronic unpredictable stress. Mol Psychiatry. 2018; 23: 892–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sachar EJ, Hellman L, Fukushima DK, Gallagher TF. Cortisol production in depressive illness. A clinical and biochemical clarification. Arch Gen Psychiatry. 1970; 23: 289–298. [DOI] [PubMed] [Google Scholar]
- 14.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl). 1997; 134: 319–329. [DOI] [PubMed] [Google Scholar]
- 15.Narayanan R, Dougherty KJ, Johnston D. Calcium store depletion induces persistent perisomatic increases in the functional density of h channels in hippocampal pyramidal neurons. Neuron. 2010; 68: 921–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis AS, Schwartz E, Chan CS, et al. Alternatively spliced isoforms of TRIP8b differentially control h channel trafficking and function. J Neurosci. 2009; 29: 6250–6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin M, Brager D, Jaramillo TC, Johnston D, Chetkovich DM. Mislocalization of h channel subunits underlies h channelopathy in temporal lobe epilepsy. Neurobiol Dis. 2008; 32: 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shetty PK, Galeffi F, Turner DA. Cellular links between neuronal activity and energy homeostasis. Front Pharmacol. 2012; 3: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenkranz JA, Venheim ER, Padival M. Chronic stress causes amygdala hyperexcitability in rodents. Biol Psychiatry. 2010; 67: 1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong P, Vickstrom CR, Liu X, et al. HCN2 channels in the ventral tegmental area regulate behavioral responses to chronic stress. Elife. 2018; 7: e32420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002; 34: 13–25. [DOI] [PubMed] [Google Scholar]
- 22.Taliaz D, Loya A, Gersner R, Haramati S, Chen A, Zangen A. Resilience to chronic stress is mediated by hippocampal brain-derived neurotrophic factor. J Neurosci. 2011; 31: 4475–4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berton O, McClung CA, Dileone RJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006; 311: 864–868. [DOI] [PubMed] [Google Scholar]
- 24.Yang Y, Cui Y, Sang K, et al. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature. 2018; 554: 317–322. [DOI] [PubMed] [Google Scholar]
- 25.Gould NF, Holmes MK, Fantie BD, et al. Performance on a virtual reality spatial memory navigation task in depressed patients. Am J Psychiatry. 2007; 164: 516–519. [DOI] [PubMed] [Google Scholar]
- 26.Nolan MF, Malleret G, Dudman JT, et al. A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. Cell. 2004; 119: 719–732. [DOI] [PubMed] [Google Scholar]
- 27.Krishnan V, Han MH, Graham DL, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007; 131: 391–404. [DOI] [PubMed] [Google Scholar]
- 28.Silveira Villarroel H, Bompolaki M, Mackay JP, et al. NPY induces stress resilience via downregulation of Ih in principal neurons of rat basolateral amygdala. J Neurosci. 2018; 38: 4505–4520. [DOI] [PMC free article] [PubMed] [Google Scholar]