Abstract
Ibrutinib is an irreversible inhibitor of Bruton’s tyrosine kinase (Btk) that has proven to be an effective therapeutic agent for multiple B-cell mediated lymphoproliferative disorders. Ibrutinib, however, carries an increased bleeding risk compared to standard chemotherapy. Bleeding events range from minor mucocutaneous bleeding to life-threatening hemorrhage, due in large part to the effects of ibrutinib on several distinct platelet signaling pathways. There is currently minimal data to guide clinicians regarding the use of ibrutinib in patients at high risk for bleeding or on anticoagulant or antiplatelet therapy. In addition, the potential cardiovascular protective effects of ibrutinib monotherapy in patients at risk for vascular disease is unknown. Patients should be cautioned against using nonsteroidal anti-inflammatory drugs, fish oils, vitamin E, and aspirin-containing products, and consider replacing ibrutinib with a different agent if dual antiplatelet therapy is indicated. Patients should not take vitamin K antagonists concurrently with ibrutinib; direct oral anticoagulant should be used if extended anticoagulation is strongly indicated. In this review, we describe the pathophysiology of ibrutinib-mediated bleeding and suggest risk reduction strategies for common clinical scenarios associated with ibrutinib.
Keywords: Ibrutinib, Hemorrhage, Blood Platelets, Lymphoma, Bruton type agammaglobulinemia
Introduction:
Ibrutinib is an irreversible inhibitor of Bruton’s tyrosine kinase (Btk), which is approved for the treatment of several B-cell malignancies.[1] A side effect of ibrutinib is an increased rate of clinically significant bleeding as compared to standard chemotherapy. This has been attributed in large part to the platelet-specific effects of inhibiting the non-receptor tyrosine kinases Btk and Tec, as well as drug induced thrombocytopenia. The relatively recent introduction of ibrutinib and lack of precise data on its risk in combination with other antiplatelet agents and anticoagulants has created a lack of consensus on the proper use of ibrutinib in patients at risk of bleeding and in those treated with other drugs with adverse bleeding effects. Guidelines on the management of ibrutinib in the setting of active bleeding or surgery are also poorly defined. Lastly, ibrutinib is associated with an increased risk of atrial fibrillation which itself often mandates anticoagulation, leading to complex decisions regarding stroke versus bleeding risk.[2] In this review we describe the pathophysiology of ibrutinib-mediated platelet inhibition and suggest management and risk-reduction strategies for common clinical scenarios associated with ibrutinib.
The history of Btk and ibrutinib:
The discovery of Btk dates back to the 1950s. In 1952 Colonel Ogden Bruton, a pediatrician at Walter Reed Army Hospital, reported the first case of congenital agammaglobulinemia in an 8 year-old boy.[1] This X-linked disease was later coined X-linked agammaglobulinemia (XLA) or Bruton’s agammaglobulinemia.[3] In 1993, the molecular mechanisms of the disease were discovered to be related to mutations in a specific member of the Tec family of protein tyrosine kinases, later named Bruton’s tyrosine kinase.[1] Btk is responsible for a variety of intracellular signaling pathways in B-lymphocytes and is required for B-cell precursor maturation; the absence of Btk in those with XLA leads to a paucity of peripheral B-cells and immunoglobulins.[4] Btk is also necessary for B-cell receptor signaling in the adaptive immune response and lymphocyte trafficking, among other functions.[5] In 2009, the first human Btk inhibitor, ibrutinib, was developed.[6] Since then, several clinical trials have shown efficacy in treating B-cell malignancies, leading to FDA approval for the treatment of chronic lymphocytic leukemia (CLL), mantle cell lymphomas and lymphoplasmacytic lymphoma, otherwise known as Waldenström’s macroglobulinemia.[1]
Current clinical data on ibrutinib-associated bleeding.
After three years of follow up, more than half of patients on ibrutinib will have a bleeding event. [7] The majority of reported bleeding events are low grade (Common Terminology Criteria for Adverse Events 4.0 (CTCAE) Grade I-II bleeding) (Table 1). The most common bleeding phenotypes are subcutaneous or mucosal bleeding, including contusions, epistaxis, petechial bleeding, hematuria or ecchymosis.[7] Major hemorrhage (Grade III-IV) has been reported with rates varying from 4% to 8% in trials that followed patients for over a year,[7–11] with fatal hemorrhage occurring in less than 1% of patients (0.6–0.7%).[9, 11] Subdural hematoma is the most commonly reported form of central nervous system (CNS) bleeding,[7, 10–12] though hemorrhagic conversion of ischemic stroke, subarachnoid hemorrhage after a fall and vitreous hemorrhage have also been reported.[12]
Table 1.
Incidence of bleeding events with ibrutinib.*
| Trial | Phase | Comparison | Median follow up |
Any bleeding (%) |
Grade
1–2 hemorrhage (%) |
Major hemorrhage Grade III-IV (%) |
Fatal hemorrhage (%) |
Major hemorrhage on concomitant AC, antiplatelet, or Vitamin E (%) |
Median time to major hemorrhage |
Thrombocytopenia Grade 1–2 (%) |
Thrombocytopenia Grade 3–4 (%) |
Held
for procedures /surgery |
Excluded warfarin? |
ASA during study (%) |
Clopidogrel during study (%) |
AC during study (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Chanan- Kahn, Lancet 2016 |
Phase III |
Ibrutinib
R- Benda (n=287) Vs. R-Benda (N=287), CLL |
17 mo | 31%
vs 15% |
28% vs 9% | 4% vs. 2% | 0.6% vs 0 | 54% (6/11) vs 60% (3/5) | 4.2 mo vs
2.3 mo |
16% vs 9% | 15 vs 15 | Not reported |
YES | |||
|
Burger, NEJM 2015 |
Phase III |
Ibrutinb (N=136 )Vs. Chlorambacil (N=133), CLL |
18.4 mo | Not Reported |
Not Reported | 4% vs. 2% | 0 vs 0 | 50% (3/6) vs not reported | Not Reported | Not Reported | 2% vs 6% | YES (14 days) |
YES | |||
|
Byrd, NEJM 2014 |
Phase III |
Ibrutinib (N=195) Vs. Ofatumumab (N= 196), CLL |
9.4 mo | 44%
vs 12% |
27% vs 10% | 1% vs 2% | 0 vs 0 | Not Reported | Not Reported | 11% vs 8% | 6% vs 4% | YES | YES | |||
|
Dreyling, Lancet 2016 |
Phase III |
Ibrutinib (N=139) Vs. temsirolimus (N= 141), MCL |
20 mo | Not Reported |
Not Reported | 10%vs 6% | 0 vs 0 | Not Reported | Not Reported | 9% vs 14% | 9% vs 42% | Not reported |
YES | |||
|
Byrd, Blood 2015 |
Phase II |
Ibrutinib (N=132) CLL |
36 mo | 61% | 53% | 8% | 0.7%(1) | Not Reported | Not Reported | Not Reported | 8% | Later
in protocol. 3 days for minor, 7 days for major |
Later
in protocol |
33% | 2% | 22% |
|
Wang, Blood 2015 |
Phase II |
Ibrutinib (N= 111 ) MCL |
26.7 mo | 50% | 44% | 6% | 0 | 85% (6/7) (includes NSAID) Bleeding events were higher in those on AC/anti plt: (69% any grade, 8% grade 3–4) vs those not receiving these treatments (28% any grade, 4% grade 3–4). |
Not Reported (41% were in the first 6mo) |
9% | 13% | Not reported | Later in protocol |
26% | 6% | 24% (14% LMWH, 10% Warfari n) |
Phase III and selected phase II trials with over 100 patients, reporting bleeding events.
Post-procedural bleeding accounted for a large amount of bleeding events in initial clinical trials,[11, 13] leading to amendments requiring discontinuation of the drug for a period of time before and after invasive procedures. A protocol of holding ibrutinib for three days prior to minor procedures and seven days prior to major surgery was used in some clinical trials, with re-initiation of the drug 1–3 days later.[11, 13] Other trials held the drug for 14 days (one week prior and one week after the procedure).[10]
Initial ibrutinib clinical trials allowed the inclusion of patients on warfarin. These were later amended, however, to exclude vitamin K antagonists given evidence of excessive bleeding.[7, 11] Ibrutinib is currently contraindicated in the setting of warfarin use. Clinical trials did allow other anticoagulants including heparin, low molecular weight heparin (LMWH) and direct oral anticoagulants (DOACs).[14] Clinical trials generally did not restrict the use of other antiplatelet agents concurrently with ibrutinib including aspirin, clopidogrel, and nonsteroidal anti-inflammatory drugs (NSAIDs). At least one clinical trial reported that concurrent use of antiplatelet agents or anticoagulants resulted in notably increased rates of bleeding (69% any grade bleeding, 8% grade 3–4 in those on concurrent anticoagulation/antiplatelet vs 28% any grade, 4% grade 3–4 in those on ibrutinib alone).[7] Bleeding has also been reported in association with other drugs known to inhibit platelet function including vitamin E[10] and fish oil.[13]
Lastly, drug-induced thrombocytopenia does occur with ibrutinib and may also increase bleeding risk, although only to a mild degree. Grade III-IV thrombocytopenia has been reported at rates from 2% to 13% when the drug is used as a single agent.[7, 10]
The pathophysiology of ibrutinib-associated bleeding.
While Btk is important in platelet signaling, individuals with XLA do not have a bleeding phenotype, suggesting that both on- and off-target effects of ibrutinib contribute to aggravated bleeding.[15] Besides irreversibly inhibiting Btk, ibrutinib inhibits several other intracellular molecules important for platelet signaling including Tec (tyrosine kinase expressed in hepatocellular carcinoma), the first discovered kinase of the Tec family of protein-tyrosine kinases.[16] Both Btk and Tec are irreversibly inhibited by ibrutinib at clinically relevant concentrations with the IC50 of 0.5 and 78 nM, respectively.[6] Btk and Tec function in the downstream signaling of several platelet transmembrane receptors, including the platelet collagen receptor glycoprotein VI (GPVI) and C-type lectin-like receptor 2 (CLEC-2). GPVI is responsible for platelet activation secondary to collagen and collagen-related peptide exposure.[17] (Figure 1) Platelets from patients with XLA have mildly diminished, but not abolished, collagen activation.[17, 18] It has been shown that in the absence of Btk, Tec signaling regulates platelet activation through GPVI resulting in only a mild deficit in collagen-mediated activation. Mouse platelets deficient in both Btk and Tec, however, are significantly inhibited and fail to undergo Ca2+ mobilization, aggregation, granular secretion, and spreading in response to collagen or the collagen analog, collagen related peptide (CRP), though they continue to aggregate normally in response to adenosine diphosphate (ADP).[18] This effect appears to be dose dependent, [19, 20] and has been confirmed clinically, as patients on ibrutinib have reductions in collagen-mediated platelet aggregation that correlates with the occurrence of clinical bleeding.[20–22] The effect was found to be partially reversed after holding the drug for 2.5 days and fully reversed after one week.[20, 21] Interestingly, there are several reports of individuals with congenital or acquired defects in GPVI, many of whom have a similar bleeding phenotype to patients on ibrutinib.[23].
Fig. 1.
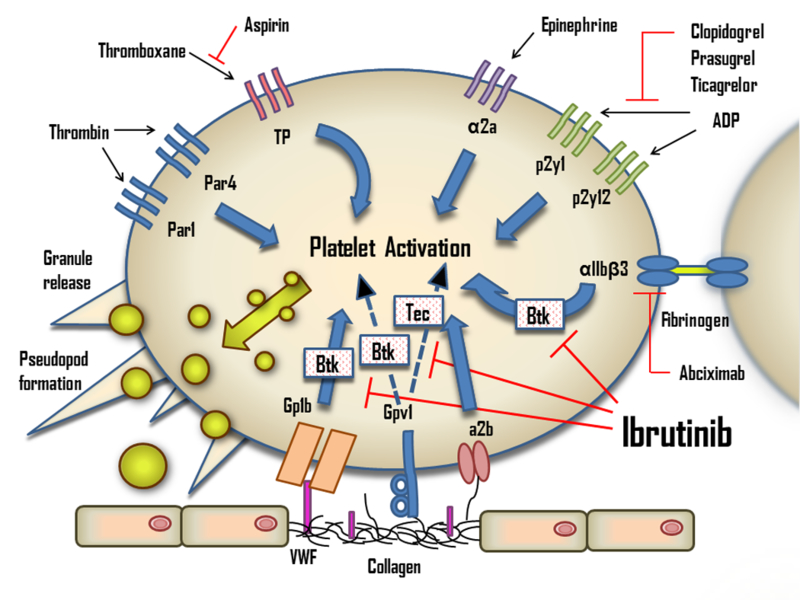
The effects of ibrutinib on platelets. ADP, adenosine diphosphate; Btk, Bruton’s tyrosine kinase; Tec, tyrosine kinase expressed in hepatocellular carcinoma; TP, Thromboxane A2 receptor; VWF, von Willebrand factor.
Ibrutinib also inhibits downstream signaling of CLEC-2, a platelet transmembrane receptor reliant on Btk and Tec signaling.[24] CLEC-2 has been shown to mediate thrombus stability after platelet adhesion to damaged endothelium.[25] Mouse models show that ibrutinib abolishes platelet aggregation in the setting of CLEC-2 stimulation by the exogenous agonist rhodocytin.[26] The only known endogenous ligand of CLEC-2, podoplanin, is notably absent in vascular endothelial cells and platelets, yet is abundant in the lymphatic system.[27] Along these lines, podoplanin has been shown to be highly expressed in type I pneumocytes, renal podocytes and lymphatic endothelial cells, and podoplanin-platelet interactions have been shown to be required for the maintenance of high endothelial venule integrity.[28] Mice deficient in CLEC-2 signaling proteins developed disorganized lymphatics and petechia in utero.[27] It is unknown what effects ibrutinib-mediated inhibition of CLEC-2 signaling might have on pulmonary, renal or lymphatic systems in humans.
Besides the GPVI and CLEC-2-mediated pathways, ibrutinib also interferes with platelet GPIb-mediated platelet functions. Btk has been found to be essential in Von Willebrand factor (vWF)-induced signaling and GPIb-dependent thrombus formation in vivo.[29] GPIb binds to vWF, effectively tethering platelets to injured vessel walls, and stimulates multiple platelet pathways and platelet cytoskeletal reorganization.[30] In vitro data found that pre-incubation of blood from healthy donors with ibrutinib decreased the firm platelet adhesion with vWF under high shear stress while sparing platelet rolling and expression of GPIb.[20] This effect has been correlated clinically, as platelets from patients on ibrutinib with a bleeding phenotype minimally adhered to vWF under flow compared with patients with no bleeding symptoms.[20] It has been suggested that the inhibitory effect of ibrutinib on vWF-GPIb interactions may partially explain the clinical phenotype of bleeding in the microvasculature where shear stress is elevated.[20]
Lastly, in vitro experiments have suggested that ibrutinib also inhibits platelet adhesion to fibrinogen. Ordinarily, binding of fibrinogen to integrin αIIbβ3 promotes platelet adhesion, spreading and clot retraction by evoking outside-in signaling as positive feedback for platelet activation.[31]. In vitro data suggests ibrutinib inhibits the αIIbβ3 outside-in signaling pathway, which has been shown to involve Btk.[32] It has recently been shown that irreversible inhibition of Btk with two ibrutinib analogs in vitro decreased human platelet activation, phosphorylation of Btk, P-selectin exposure, spreading on fibrinogen, and aggregation under shear flow conditions.[33] Moreover, short-term studies of ibrutinib analogs administered to non-human primates also showed abrogation of platelet aggregation in vitro.[33]
Important questions include why some patients do not bleed on ibrutinib and whether we can predict who is at risk for bleeding. Several factors may explain the variability of reported bleeding events, including differences in dosing, disease type, use of other antiplatelet and anticoagulant agents, severity of thrombocytopenia, and across-trial variability in the reporting of bleeding events. Also plausible are individual differences in platelet responsiveness to ibrutinib. Small studies have found that the degree of inhibition of either collagen- or ristocetin-mediated platelet aggregation in patients on ibrutinib correlates with bleeding.[20, 21, 34] Variability in disease-associated platelet defects may also play a role. Patients with CLL, for instance, often have varying degrees of mild platelet dysfunction which is likely exacerbated by ibrutinib. Some authors have suggested that interactions between potential baseline platelet abnormalities and ibrutinib-related effects may determine the risk of bleeding.[22]
One study compared markers of coagulation in patients with CLL at baseline and directly after starting ibrutinib, including platelet function assays (PFA-110™), vWF activity and antigen, factor VIII (FVIII) levels, and platelet aggregation studies.[22] Increased baseline PFA-100 Epinephrine closure time was predictive of bleeding. Baseline levels of FVIII and vWF activity were generally high in CLL patients, and subsequently decreased on ibrutinib. Baseline levels of FVIII and vWF activity in the lower range of normal were also predictive of bleeding. The authors suggested that a pro-inflammatory state associated with CLL may drive increased levels of FVIII and vWF, with baseline elevations conferring some protection against bleeding.[22]
Does ibrutinib have potential cardiovascular protective effects?
While ibrutinib is not an ideal agent for cardiovascular protection given its off-target and side effects, the question of what cardiovascular benefit it offers is relevant to patients already on antiplatelet agents planning to start ibrutinib. The possibility to safely discontinue antiplatelet agents in these patients without increasing their risk of cardiovascular events is appealing and would likely result in less bleeding. Current use of antiplatelet agents in patients not on ibrutinib include aspirin or P2Y12-antagonist (clopidogrel, prasugrel, ticagrelor) monotherapy for primary and secondary prevention of cardiovascular disease, and dual antiplatelet therapy (DAPT) in the settings of acute stroke, myocardial infarction, or coronary artery stenting. [35] The true risks and benefits of stopping antiplatelet agents in patients taking ibrutinib at high risk of cardiovascular events is unknown, though suggestions of its potential cardioprotective efficacy can be drawn from studies targeting the specific platelet receptor pathways affected by ibrutinib.
Experimental models of thrombosis and atherosclerosis have suggested that GPVI blockade may reduce thrombotic risk. GPVI-deficient animal models suggest protection against platelet adhesion, thrombus propagation, leukocyte recruitment and neointimal hyperplasia after mechanical arterial injury.[36, 37] [38] Subsequent animal models have shown that GPVI deficiency significantly impairs thrombus formation caused by ultrasound or needle-induced plaque rupture [39, 40] and reduces neointimal proliferation after carotid artery injury.[41, 42] Lastly, antibody blockade of GPVI inhibits plaque-induced thrombus formation.[43, 44] Several drugs including direct blocking molecules, antibodies, and signal transduction blockers against GPVI have been explored, though none have been evaluated for safety or efficacy in humans to date.[45] As Btk and Tec inhibition affects multiple platelet signaling pathways, ibrutinib cannot be directly compared to agents that inhibit the GPVI receptor alone, particularly in terms of safety. The impact on the GPVI pathway of newer inhibitors more specific to Btk is also unclear. Alternative BTK inhibitors are discussed separately below.
Inhibiting the vWF-GPIb axis is less well-studied, but has been suggested as a potential antithrombotic target in vascular disease. [46]. Animal models show that arterial thrombus formation is inhibited by antibodies targeting the GPIb. [47, 48] Human studies have evaluated inhibition of the vWF-GPIb interaction as a potential therapy for thrombotic thrombocytopenic purpura, though no studies have examined GPIb inhibition as a potential target for cardiovascular risk reduction. [49]
Despite both these pathophysiologic suggestions and animal data, there is insufficient data addressing ibrutinib’s efficacy or safety for primary or secondary cardiovascular risk reduction. With the collection of more clinical data, it may be possible to better clarify the role of discontinuing other cardiovascular protective agents in patients taking ibrutinib.
Clinical management of ibrutinib in patients at risk for bleeding, and the management of ibrutinib-associated bleeding, based on the current body of evidence.
Ibrutinib is generally used in an older patient population with inherently higher cardiovascular and bleeding risks. These risks can be mitigated with forethought and an understanding of the pathophysiology of ibrutinib-associated bleeding. In the following sections, we outline risk reduction strategies to prevent bleeding in patients with indications for antiplatelet agents, anticoagulation, pain management needs, and atrial fibrillation, based on the current understandings of the pathophysiology and risks of ibrutinib-associated bleeding. The evidence behind these recommendations is limited by the currently available data and emphasizes the need for further investigation.
Management with antiplatelet agents.
Many patients treated with ibrutinib have a history of, or risk factors for, cardiovascular disease, warranting antiplatelet therapy for primary or secondary prevention. Likewise, patients may have cardiovascular events during therapy requiring coronary artery stenting and DAPT for extended periods. In making decisions about antiplatelet therapy concurrently with ibrutinib, particularly for primary or secondary prevention of cardiovascular events, it is important to consider patients’ overall life expectancy along with their bleeding and cardiovascular risks. While some patients may have very indolent disease, patients with a short life expectancy are unlikely to benefit from primary risk reduction, and may already be at a sufficiently decreased risk on ibrutinib.
Reported clinical trial data suggest that the addition of antiplatelet agents to ibrutinib increases the risk of bleeding.[7] DAPT with aspirin and a P2Y12-antagonist raises the risk of major bleeding by 40–50% compared to single antiplatelet therapy;[50] adding ibrutinib to DAPT is therefore likely to further increase this risk. Indeed, in vitro data has suggested ibrutinib combined with P2Y12 antagonists has an additive antiplatelet effect.[31] For these reasons, we are cautious about concurrent use of ibrutinib with other antiplatelet agents. Based on the available data, we recommend the following in patients taking ibrutinib:
Patients should be cautioned against the use of NSAIDs, fish oils, vitamin E, and inadvertent use of aspirin-containing products.
Consider stopping aspirin in patients on ibrutinib who have low or moderate cardiovascular risk.
For patients at high cardiovascular risk that may compromise their survival, including those with recent MI or stroke, consider ibrutinib plus 81 mg of aspirin. We recommend against higher doses of aspirin in light of data suggesting increased bleeding with no benefit.[51]
For patients with recent bare metal cardiac stent placement, consider delaying or holding ibrutinib while on DAPT. After the required DAPT period, consider ibrutinib plus 81 mg of aspirin.
For patients with recent drug eluting stent placement, consider replacing ibrutinib with an alternative treatment strategy given the extended duration of recommended DAPT.
Some authors initially trial ibrutinib at a lower dose (280 mg/day) in patients on other antiplatelet agents or anticoagulants and slowly increase to treatment dose if bleeding does not occur. This dose escalation strategy is based on in vitro data suggesting that the antiplatelet effects of ibrutinib are dose-dependent.[19, 20] It is important to note, however, that there is no clinical data to endorse this practical strategy, and that studies have shown significant bleeding rates at both lower (420mg/day)[7, 10–12] and higher (560mg/day)[8, 9] doses of ibrutinib.
Management with anticoagulants:
Ibrutinib has been associated with unacceptable bleeding rates in combination with vitamin K antagonists and thus should not be given to patients on warfarin.[7, 11] Other anticoagulants were allowed in clinical trials and are used concurrently with ibrutinib in some studies.[14] DOACs have been shown to cause fewer bleeding events than warfarin in multiple phase III trials, and are likely a safer class of anticoagulant to combine with ibrutinib.[52] However, current data regarding the combination of DOACs and ibrutinib is insufficient to draw strong conclusions on the associated bleeding risk, which is presumed higher than either agent alone. The risks and benefits of anticoagulation should be considered on a case by case basis and relayed to the patient. It is important to use the appropriate duration of anticoagulation and to avoid anticoagulation in combination with ibrutinib for extended periods without an appropriate indication. Contemporary guidelines recommend three months of anticoagulation for patients with venous thromboembolism provoked by surgery or an alternative transient risk factor, and consideration of indefinite anticoagulation for patients with unprovoked thrombosis who are not at a high risk of bleeding.[53]
When considering the combination of ibrutinib and DOACs, it is also important to note that ibrutinib primarily undergoes CYP3A4-mediated metabolism, and CYP3A4 inhibitors will increase ibrutinib exposure.[54] Rivaroxaban and apixaban in particular undergo CYP3A4-mediated metabolism (33% and 25%, respectively), while dabigatran and edoxaban do not.[55] It is not known how the presence of both substrates (ibrutinib and a CYP3A4-metabolized DOAC) affects their respective metabolism, particularly as DOAC levels are not routinely monitored. Clinicians should be conscious of medications which impact the CYP3A4 pathway in patients taking ibrutinib, especially in those taking ibrutinib and DOACs concurrently. Importantly, concurrent administration of additional CYP3A4 inhibitors (e.g. fluconazole) will likely increase plasma concentrations of both drugs, potentially leading to a significantly increased risk of bleeding.
For patients potentially requiring ibrutinib concurrent with anticoagulation we propose the following:
For patients who require short courses of anticoagulation for a provoked VTE, consider postponing ibrutinib therapy if feasible and using an alternative agent in the interim. If overlapping with ibrutinib, we recommend using a DOAC such as apixaban given the smaller bleeding rates as compared to warfarin.[56] Avoid concurrent antiplatelet therapy unless there is a strong indication.
For patients requiring extended anticoagulation for unprovoked VTE, consider using another agent or using a DOAC such as apixaban, again avoiding other antiplatelet agents unless strongly indicated. Half-dose apixaban can be safely used after 6 months of therapy, likely decreasing bleeding risk.[57]
Patients should be cautioned against the use of NSAIDs, fish oils, vitamin E, and inadvertent use of aspirin-containing products.
Some authors initially trial ibrutinib at a lower dose (280 mg/day) in patients on other antiplatelet or anticoagulants and slowly increase to treatment dose if bleeding does not occur. This dose escalation recommendation is based on in vitro data which suggest that the antiplatelet effects of ibrutinib are dose dependent.[19, 20] It is important to note however that there is no clinical data to confirm the usefulness of this practice, and that studies have shown significant bleeding rates regardless of lower (420mg/day)[9–12] or higher (560mg/day)[7, 8] treatment doses.
Management of bleeding
Low-grade bleeding can be managed with supportive care or by holding ibrutinib for a short period of time. For high grade (III-IV) bleeding, we recommend the following:
Non-CNS bleeding:
For patients admitted to the hospital for non-CNS bleeding, or those requiring transfusions, we recommend stopping ibrutinib and transfusing platelets. In vitro data suggests that transfusion of platelets to achieve 50% fresh platelets should correct hemostasis.[20] The time since the last dose of ibrutinib should be considered if transfusing platelets, as the drug may still inhibit platelets within the initial 3–4 hour half-life.[21] If holding ibrutinib alone, the antiplatelet effects should be partially reversed after 2.5 days.[20, 21]
CNS Bleeding:
Although there have been reports of ibrutinib-associated CNS bleeding effectively treated with platelet transfusion, [58] recent data suggest that platelet transfusion may have some detrimental effects which must be considered. In the PATCH trial, investigators compared platelet transfusion verses supportive care in patients who presented with spontaneous antiplatelet agent-associated intraparenchyma bleeding. The platelet transfusion group, which was mostly composed of patients taking aspirin alone, was significantly more likely to have adverse advents, and had a significantly higher mortality (adjusted common odds ratio 2·05, 95% CI 1·18–3·56; p=0·0114).[59] The authors concluded that platelet transfusion cannot be recommended in clinical practice as a hemostatic measure in patients with intraparenchyma hemorrhage taking antiplatelet agents.[59] Although no explanation is readily available, the data is compelling, and since the publication of this study it has been our practice to not offer platelet transfusion to patients with antiplatelet agent-associated CNS bleeding. There is however, no data to determine if this finding holds true in patients with ibrutinib-associated CNS bleeding. Ibrutinib has distinctly different antiplatelet effects and bleeding rates compared to other antiplatelet agents, and as such, the risk/benefit of transfusion in the setting of ibrutinib-associated CNS bleeding is uncertain. We recommend the decision to transfuse platelets in this setting be individualized on a case by case basis. No strong recommendations can be made due to a lack of specific data.
Management of elective and unplanned procedures.
Ibrutinib should be held for 7 days prior to major procedures, as the antiplatelet effects of ibrutinib have been shown to be fully reversed after a week off therapy.[20, 21] Ibrutinib can be restarted 1–3 days postoperatively.[11, 13] The risk of bleeding during urgent, unplanned procedures can be mitigated with platelet transfusion to achieve 50% fresh platelets.[20] The time since the last dose of ibrutinib should be considered if transfusing platelets, as outlined above.[21]
Management of atrial fibrillation
Though ibrutinib may have cardioprotective effects via platelet inhibition, an additional, less-desirable side effect is an increased risk of atrial fibrillation. This was shown in a systematic review and meta-analysis which found a pooled relative risk of 3.5 (95% CI 1.8–6.9, p<0.0001) for developing atrial fibrillation in those treated with ibrutinib versus alternative therapies.[2] Experimental mouse models have suggested a potential culprit for this association: the phosphoinositide 3-kinase (PI3K)-Akt pathway, critical for protecting cardiac myocytes under stress and itself closely tied to Btk and Tec pathways.[60] This risk appears to be ongoing for as long as patients take the drug, with rates up to 16% reported after 2 years of therapy with ibrutinib.[61]
Atrial fibrillation in patients taking ibrutinib poses a unique challenge in balancing thrombosis and bleeding risks. A major cause of morbidity and mortality in patients with atrial fibrillation is cardioembolic stroke, the risk of which can be estimated by models such as the widely-used CHA2DS2VASc system.[62] Contemporary guidelines recommend therapeutic anticoagulation for individuals at sufficiently elevated stroke risk (CHA2DS2VASc score of 2 or greater).[62] Individuals with a CHA2DS2VASc score of zero can safely omit antithrombotic therapy, while those with a CHA2DS2VASc score of 1 may be managed with no antithrombotic therapy, anticoagulation, or aspirin, based on physician and patient preference.[62]
In patients with atrial fibrillation receiving ibrutinib, however, the cumulative bleeding risk from both antiplatelet and anticoagulant effects can be dangerously high. As noted above, warfarin has been associated with serious bleeding prompting exclusion of patients taking this drug from subsequent trials.[7, 11, 22] Clinical data examining the safety of ibrutinib taken in conjunction with DOACs is lacking, though small studies have not reported excess bleeding when using these agents together; as previously noted, the number of patients on DOACs in these trials was limited.[14] In addition, it is currently unknown whether the multiple antiplatelet effects of ibrutinib itself confer any meaningful protection against cardioembolic stroke in atrial fibrillation. Statistically significant reductions in stroke have been found by adding clopidogrel to aspirin, speaking to the additive effects of inhibiting multiple platelet signaling pathways;[63] however, this combination is still inferior to anticoagulation with warfarin alone.[64] Additional studies are necessary to clarify how ibrutinib might fit into atrial fibrillation stroke prophylaxis strategies.
We recommend the following management of patient on ibrutinib who develop atrial fibrillation.
If patents have a high bleeding risk, including mandated use of DAPT, or a history of significant bleeding, consider using an alternative agent to ibrutinib and managing their atrial fibrillation per conventional guidelines.
In patients who are to continue ibrutinib and have a sufficiently elevated stroke risk per standard models (CHA2DS2VASc score of 2 or greater), we recommend a DOAC be used as opposed to a vitamin K antagonist, without additional antiplatelet agents. We at our institution, as well as our colleagues across the United States, have successfully combined ibrutinib with DOACs in patients who develop atrial fibrillation. As discussed above, in this setting we use a dose escalation strategy for ibrutinib (often starting at 280 mg/day), slowly increasing to standard treatment doses if bleeding does not occur.
For patients at low risk for cardioembolic stroke (CHA2DS2VASc score of zero), we recommend continuing ibrutinib alone without the addition of anticoagulation or alternative antiplatelet agents.
For patients at intermediate risk for cardioembolic stroke (CHA2DS2VASc score of one), we recommend continuing ibrutinib alone, or ibrutinib plus aspirin, based on patient-physician preferences and consideration of individualized risks.
In patients requiring DAPT (e.g. those with recent coronary artery stenting) who develop atrial fibrillation and have a high stroke risk, we recommend discontinuing ibrutinib due to the significant bleeding risk and switching to alternative therapy for lymphoproliferative disorders.
In patients with an indication for aspirin for cardioprotective benefit who develop atrial fibrillation with elevated stroke risk (CHA2DS2VASc score of 2 or greater), we recommend treating with a DOAC plus ibrutinib alone and stopping other antiplatelet agents. This is based on prospective data showing that single antiplatelet therapy with anticoagulation had equal efficacy and superior safety to dual antiplatelet therapy with anticoagulation.[65]
Pain management on ibrutinib
Ibrutinib’s antiplatelet effects also demand careful consideration when addressing patient’s pain symptoms. NSAIDs inhibit platelet function through non-selective inhibition of the COX-1 enzyme constitutive in platelets, leading to reduced synthesis of the platelet-stimulatory molecule thromboxane A2.[66] Use of non-selective NSAIDs in conjunction with ibrutinib has been shown to increase the risk of both minor and major bleeding and should therefore be avoided.[14, 22] Celecoxib, the only available COX-2 selective NSAID, has been shown to have no effect on platelet function, and therefore should not further increase the risk of bleeding associated with ibrutinib.[67] Likewise, meloxicam has minimal to no effects on platelets.[68] Selective serotonin reuptake inhibitors (SSRI) do have some documented antiplatelet effects and increased risk for bleeding;[69] via similar mechanisms, serotonin-norepinephrine reuptake inhibitors (SNRIs) used for neuropathic pain may be just as likely to cause bleeding, although current clinical data is conflicting.[70] Acceptable analgesics with no major adverse platelet effects include acetaminophen and opioids, as well as anti-epileptic drugs such as gabapentin, pregabalin, and tricyclic antidepressants.
Are providers conscious of bleeding risk?
There is limited data describing physician and patient awareness of the bleeding risks associated with ibrutinib. One group performed a medication review of 96 CLL patients beginning ibrutinib and found that 64% of the patients were concurrently taking medications that increased risk of ibrutinib toxicity, including CYP3A4 inhibitors carbamazepine, rifampin, and rifabutin.[71] When beginning ibrutinib, 9% of patients were taking an anticoagulant and 30% were on aspirin.[71] The data showed that 10% of the patients had clinically significant bleeding, with 4% (4 patients: 1 on enoxaparin, 2 on a SSRI, 1 on a NSAID) requiring hospitalization.[71]
Future Btk inhibitors
Newer Btk inhibitors are more selective for Btk with less off-target kinase inhibition and may reduce bleeding as compared to ibrutinib.[72] Phase 1 data for ONO/GS-4059, a “second generation” Btk inhibitor, shows clinical activity in CLL and non-Hodgkin lymphoma.[73] This study did not exclude patients based on anticoagulant use, and the use of anticoagulant therapy was not associated with increased risk of bleeding for the 31.1% of patients receiving anticoagulation.[73] Of these 28 patients who were receiving anticoagulation, 18 were treated with only prophylactic doses, and 5 were being anticoagulated for atrial fibrillation (4 of 5 cases were pre-existing before initiation of the study drug). Grade III-IV hematologic toxicities recovered spontaneously and included neutropenia in 10%, anemia in 13.3%, and thrombocytopenia in 13.3%.[73] One episode of grade III hemorrhage was reported (psoas hematoma) in a patient who did not receive anticoagulants, resulting in withdrawal from the trial. [73]
Acalabrutinib is another novel second-generation Btk inhibitor with increased selectivity for Btk. Whereas ibrutinib completely suppressed Tec activity, an equivalent dose of acalbrutinib had minimal effect in vitro on Tec.[74] Btk suppression alone is not likely to lead to a strong bleeding phenotype, just as individuals with XLA generally do not bleed. Assessment of in vivo platelet function in samples from human subjects treated with acalabrutinib showed no reduction in platelet-vessel wall interactions, in contrast to control patients treated with ibrutinib.[74] In a phase I-II study of acalabrutinib in 61 relapsed CLL patients that excluded patients requiring warfarin therapy but allowed other anticoagulants, no major hemorrhage was noted. However, Grade 1–2 petechia were noted in 16% of patients, and contusion in 18%, which in summation represents low grade bleeding not dissimilar to that reported with ibrutinib. [74]
More data is needed for the second-generation Btk inhibitors (acalabrutinib, ONO/GS-4059, BGB-3111) to understand their long-term safety and efficacy. A phase III trial comparing acalabrutinib with ibrutinib is underway for high-risk CLL patients.[75]
Conclusion
Ibrutinib is an effective agent for the treatment of several lymphoproliferative disorders but results in increased rates of low and high-grade bleeding through several platelet inhibitory mechanisms. With careful planning and recognition, these risks can be mitigated. While data is lacking to accurately define the risks of combining ibrutinib with other antiplatelet agents and anticoagulants, educated risk assessment can be made with the current body of knowledge. Future studies are needed to determine the appropriate role of other antiplatelet agents in combination with ibrutinib and the safest means of combining the drug with anticoagulants.
References:
- 1.Ponader S, Burger JA. Bruton’s Tyrosine Kinase: From X-Linked Agammaglobulinemia Toward Targeted Therapy for B-Cell Malignancies. Journal of Clinical Oncology 2014; 32: 1830–9. 10.1200/jco.2013.53.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leong DP, Caron F, Hillis C, Duan A, Healey JS, Fraser G, Siegal D. The risk of atrial fibrillation with ibrutinib use: a systematic review and meta-analysis. Blood 2016. 10.1182/blood-2016-05-712828. [DOI] [PubMed] [Google Scholar]
- 3.Buckley RH. Primary immunodeficiency diseases due to defects in lymphocytes. N Engl J Med 2000; 343: 1313–24. 10.1056/nejm200011023431806. [DOI] [PubMed] [Google Scholar]
- 4.Conley ME, Dobbs AK, Farmer DM, Kilic S, Paris K, Grigoriadou S, Coustan-Smith E, Howard V, Campana D. Primary B cell immunodeficiencies: comparisons and contrasts. Annu Rev Immunol 2009; 27: 199–227. 10.1146/annurev.immunol.021908.132649. [DOI] [PubMed] [Google Scholar]
- 5.Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B, Kolibaba KS, Furman RR, Rodriguez S, Chang BY, Sukbuntherng J, Izumi R, Hamdy A, Hedrick E, Fowler NH. Bruton Tyrosine Kinase Inhibitor Ibrutinib (PCI-32765) Has Significant Activity in Patients With Relapsed/Refractory B-Cell Malignancies. Journal of Clinical Oncology 2013; 31: 88–94. 10.1200/jco.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, Li S, Pan Z, Thamm DH, Miller RA, Buggy JJ. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A 2010; 107: 13075–80. 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang ML, Blum KA, Martin P, Goy A, Auer R, Kahl BS, Jurczak W, Advani RH, Romaguera JE, Williams ME, Barrientos JC, Chmielowska E, Radford J, Stilgenbauer S, Dreyling M, Jedrzejczak WW, Johnson P, Spurgeon SE, Zhang L, Baher L, Cheng M, Lee D, Beaupre DM, Rule S. Long-term follow-up of MCL patients treated with single-agent ibrutinib: updated safety and efficacy results. Blood 2015; 126: 739–45. 10.1182/blood-2015-03-635326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dreyling M, Jurczak W, Jerkeman M, Silva RS, Rusconi C, Trneny M, Offner F, Caballero D, Joao C, Witzens-Harig M, Hess G, Bence-Bruckler I, Cho S-G, Bothos J, Goldberg JD, Enny C, Traina S, Balasubramanian S, Bandyopadhyay N, Sun S, Vermeulen J, Rizo A, Rule S. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. The Lancet 387: 770–8. 10.1016/S0140-6736(15)00667-4. [DOI] [PubMed] [Google Scholar]
- 9.Chanan-Khan A, Cramer P, Demirkan F, Fraser G, Silva RS, Grosicki S, Pristupa A, Janssens A, Mayer J, Bartlett NL, Dilhuydy M-S, Pylypenko H, Loscertales J, Avigdor A, Rule S, Villa D, Samoilova O, Panagiotidis P, Goy A, Mato A, Pavlovsky MA, Karlsson C, Mahler M, Salman M, Sun S, Phelps C, Balasubramanian S, Howes A, Hallek M. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. The Lancet Oncology 2016; 17: 200–11. 10.1016/S1470-2045(15)00465-9. [DOI] [PubMed] [Google Scholar]
- 10.Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, Bairey O, Hillmen P, Bartlett NL, Li J, Simpson D, Grosicki S, Devereux S, McCarthy H, Coutre S, Quach H, Gaidano G, Maslyak Z, Stevens DA, Janssens A, Offner F, Mayer J, O’Dwyer M, Hellmann A, Schuh A, Siddiqi T, Polliack A, Tam CS, Suri D, Cheng M, Clow F, Styles L, James DF, Kipps TJ. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. New England Journal of Medicine 2015; 373: 2425–37. 10.1056/NEJMoa1509388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byrd JC, Furman RR, Coutre SE, Burger JA, Blum KA, Coleman M, Wierda WG, Jones JA, Zhao W, Heerema NA, Johnson AJ, Shaw Y, Bilotti E, Zhou C, James DF, O’Brien S. Three-year follow-up of treatment-naive and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood 2015; 125: 2497–506. 10.1182/blood-2014-10-606038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byrd JC, Brown JR, O’Brien S, Barrientos JC, Kay NE, Reddy NM, Coutre S, Tam CS, Mulligan SP, Jaeger U, Devereux S, Barr PM, Furman RR, Kipps TJ, Cymbalista F, Pocock C, Thornton P, Caligaris-Cappio F, Robak T, Delgado J, Schuster SJ, Montillo M, Schuh A, de Vos S, Gill D, Bloor A, Dearden C, Moreno C, Jones JJ, Chu AD, Fardis M, McGreivy J, Clow F, James DF, Hillmen P. Ibrutinib versus Ofatumumab in Previously Treated Chronic Lymphoid Leukemia. New England Journal of Medicine 2014; 371: 213–23. 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Treon SP, Tripsas CK, Meid K, Warren D, Varma G, Green R, Argyropoulos KV, Yang G, Cao Y, Xu L, Patterson CJ, Rodig S, Zehnder JL, Aster JC, Harris NL, Kanan S, Ghobrial I, Castillo JJ, Laubach JP, Hunter ZR, Salman Z, Li J, Cheng M, Clow F, Graef T, Palomba ML, Advani RH. Ibrutinib in Previously Treated Waldenström’s Macroglobulinemia. New England Journal of Medicine 2015; 372: 1430–40. 10.1056/NEJMoa1501548. [DOI] [PubMed] [Google Scholar]
- 14.Jones JA, Hillmen P, Coutre S, Tam C, Furman RR, Barr PM, Schuster SJ, Kipps TJ, Flinn IW, Jaeger U, Burger JA, Cheng M, Lee D, James DF, Byrd JC, O’Brien S. Pattern of Use of Anticoagulation and/or Antiplatelet Agents in Patients with Chronic Lymphocytic Leukemia (CLL) Treated with Single-Agent Ibrutinib Therapy. Blood 2014; 124: 1990–. [Google Scholar]
- 15.Futatani T, Watanabe C, Baba Y, Tsukada S, Ochs HD. Bruton’s tyrosine kinase is present in normal platelets and its absence identifies patients with X-linked agammaglobulinaemia and carrier females. Br J Haematol 2001; 114: 141–9. [DOI] [PubMed] [Google Scholar]
- 16.Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, Li S, Pan Z, Thamm DH, Miller RA, Buggy JJ. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proceedings of the National Academy of Sciences 2010; 107: 13075–80. 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quek LS, Bolen J, Watson SP. A role for Bruton’s tyrosine kinase (Btk) in platelet activation by collagen. Curr Biol 1998; 8: 1137–40. [DOI] [PubMed] [Google Scholar]
- 18.Atkinson BT, Ellmeier W, Watson SP. Tec regulates platelet activation by GPVI in the absence of Btk. Blood 2003; 102: 3592–9. 10.1182/blood-2003-04-1142. [DOI] [PubMed] [Google Scholar]
- 19.Ysebaert L, Levade M, Cedric G, Michallet A-S, Tam C, Pierre S, Payrastre B. Elucidation of Mild Bleeding Disorders Reported Under Ibrutinib (Imbruvica(R)) Therapy: Implications for Optimal Clinical Management. Blood 2014; 124: 3296–. [Google Scholar]
- 20.Levade M, David E, Garcia C, Laurent P-A, Cadot S, Michallet A-S, Bordet J-C, Tam C, Sié P, Ysebaert L, Payrastre B. Ibrutinib treatment affects collagen and von Willebrand factor-dependent platelet functions. Blood 2014; 124: 3991–5. 10.1182/blood-2014-06-583294. [DOI] [PubMed] [Google Scholar]
- 21.Kamel S, Horton L, Ysebaert L, Levade M, Burbury K, Tan S, Cole-Sinclair M, Reynolds J, Filshie R, Schischka S, Khot A, Sandhu S, Keating MJ, Nandurkar H, Tam CS. Ibrutinib inhibits collagen-mediated but not ADP-mediated platelet aggregation. Leukemia 2015; 29: 783–7. 10.1038/leu.2014.247. [DOI] [PubMed] [Google Scholar]
- 22.Lipsky AH, Farooqui MZH, Tian X, Martyr S, Cullinane AM, Nghiem K, Sun C, Valdez J, Niemann CU, Herman SEM, Saba N, Soto S, Marti G, Uzel G, Holland SM, Lozier JN, Wiestner A. Incidence and risk factors of bleeding-related adverse events in patients with chronic lymphocytic leukemia treated with ibrutinib. Haematologica 2015; 100: 1571–8. 10.3324/haematol.2015.126672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arthur JF, Dunkley S, Andrews RK. Platelet glycoprotein VI-related clinical defects. British Journal of Haematology 2007; 139: 363–72. 10.1111/j.1365-2141.2007.06799.x. [DOI] [PubMed] [Google Scholar]
- 24.Manne BK, Badolia R, Dangelmaier C, Eble JA, Ellmeier W, Kahn M, Kunapuli SP. Distinct pathways regulate Syk protein activation downstream of immune tyrosine activation motif (ITAM) and hemITAM receptors in platelets. The Journal of biological chemistry 2015; 290: 11557–68. 10.1074/jbc.M114.629527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navarro-Nunez L, Langan SA, Nash GB, Watson SP. The physiological and pathophysiological roles of platelet CLEC-2. Thromb Haemost 2013; 109: 991–8. 10.1160/th13-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manne BK, Badolia R, Dangelmaier CA, Kunapuli SP. C-type lectin like receptor 2 (CLEC-2) signals independently of lipid raft microdomains in platelets. Biochem Pharmacol 2015; 93: 163–70. 10.1016/j.bcp.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watson SP, Herbert JMJ, Pollitt AY. GPVI and CLEC-2 in hemostasis and vascular integrity. Journal of Thrombosis and Haemostasis 2010; 8: 1456–67. 10.1111/j.1538-7836.2010.03875.x. [DOI] [PubMed] [Google Scholar]
- 28.Herzog BH, Fu J, Wilson SJ, Hess PR, Sen A, McDaniel JM, Pan Y, Sheng M, Yago T, Silasi-Mansat R, McGee S, May F, Nieswandt B, Morris AJ, Lupu F, Coughlin SR, McEver RP, Chen H, Kahn ML, Xia L. Podoplanin maintains high endothelial venule integrity by interacting with platelet CLEC-2. Nature 2013; 502: 105–9. 10.1038/nature12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J, Fitzgerald ME, Berndt MC, Jackson CW, Gartner TK. Bruton tyrosine kinase is essential for botrocetin/VWF-induced signaling and GPIb-dependent thrombus formation in vivo. Blood 2006; 108: 2596–603. 10.1182/blood-2006-01-011817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan Y, Kulkarni S, Ulsemer P, Cranmer SL, Yap CL, Nesbitt WS, Harper I, Mistry N, Dopheide SM, Hughan SC, Williamson D, de la Salle C, Salem HH, Lanza F, Jackson SP. The von Willebrand Factor-Glycoprotein Ib/V/IX Interaction Induces Actin Polymerization and Cytoskeletal Reorganization in Rolling Platelets and Glycoprotein Ib/V/IX-transfected Cells. Journal of Biological Chemistry 1999; 274: 36241–51. 10.1074/jbc.274.51.36241. [DOI] [PubMed] [Google Scholar]
- 31.Bye AP, Unsworth AJ, Vaiyapuri S, Stainer AR, Fry MJ, Gibbins JM. Ibrutinib Inhibits Platelet Integrin αIIbβ3 Outside-In Signaling and Thrombus Stability But Not Adhesion to Collagen. Arteriosclerosis, Thrombosis, and Vascular Biology 2015; 35: 2326–35. 10.1161/atvbaha.115.306130. [DOI] [PubMed] [Google Scholar]
- 32.Soriani A, Moran B, De Virgilio M, Kawakami T, Altman A, Lowell C, Eto K, Shattil SJ. A role for PKCθ in outside-in αIIbβ3 signaling. Journal of Thrombosis and Haemostasis 2006; 4: 648–55. 10.1111/j.1538-7836.2006.01806.x. [DOI] [PubMed] [Google Scholar]
- 33.Rigg RA, Aslan JE, Healy LD, Wallisch M, Thierheimer ML, Loren CP, Pang J, Hinds MT, Gruber A, McCarty OJ. Oral administration of Bruton’s tyrosine kinase inhibitors impairs GPVI-mediated platelet function. American journal of physiology Cell physiology 2016; 310: C373–80. 10.1152/ajpcell.00325.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kazianka L, Drucker C, Skrabs C, Staber PB, Porpaczy EA, Einberger C, Heinz M, Hauswirth A, Pabinger I, Quehenberger P, Jilma B, Jaeger U. Ristocetin-Induced Platelet Aggregation for Monitoring of Bleeding Tendency in Ibrutinib-Treated Patients with Chronic Lymphocytic Leukemia. Blood 2015; 126: 718–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yousuf O, Bhatt DL. The evolution of antiplatelet therapy in cardiovascular disease. Nat Rev Cardiol 2011; 8: 547–59. 10.1038/nrcardio.2011.96. [DOI] [PubMed] [Google Scholar]
- 36.Konishi H, Katoh Y, Takaya N, Kashiwakura Y, Itoh S, Ra C, Daida H. Platelets activated by collagen through immunoreceptor tyrosine-based activation motif play pivotal role in initiation and generation of neointimal hyperplasia after vascular injury. Circulation 2002; 105: 912–6. [DOI] [PubMed] [Google Scholar]
- 37.Massberg S, Gawaz M, Grüner S, Schulte V, Konrad I, Zohlnhöfer D, Heinzmann U, Nieswandt B. A Crucial Role of Glycoprotein VI for Platelet Recruitment to the Injured Arterial Wall In Vivo. The Journal of Experimental Medicine 2003; 197: 41–9. 10.1084/jem.20020945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bender M, Hagedorn I, Nieswandt B. Genetic and antibody-induced glycoprotein VI deficiency equally protects mice from mechanically and FeCl3-induced thrombosis. Journal of Thrombosis and Haemostasis 2011; 9: 1423–6. 10.1111/j.1538-7836.2011.04328.x. [DOI] [PubMed] [Google Scholar]
- 39.Hechler B, Gachet C. Comparison of two murine models of thrombosis induced by atherosclerotic plaque injury. Thromb Haemost 2011; 105 Suppl 1: S3–12. 10.1160/ths10-11-0730. [DOI] [PubMed] [Google Scholar]
- 40.Kuijpers MJE, Gilio K, Reitsma S, Nergiz-Unal R, Prinzen L, Heeneman S, Lutgens E, Van Zandvoort MAMJ, Nieswandt B, Oude Egbrink MGA, Heemskerk JWM. Complementary roles of platelets and coagulation in thrombus formation on plaques acutely ruptured by targeted ultrasound treatment: a novel intravital model. Journal of Thrombosis and Haemostasis 2009; 7: 152–61. 10.1111/j.1538-7836.2008.03186.x. [DOI] [PubMed] [Google Scholar]
- 41.Bultmann A, Li Z, Wagner S, Peluso M, Schonberger T, Weis C, Konrad I, Stellos K, Massberg S, Nieswandt B, Gawaz M, Ungerer M, Munch G. Impact of glycoprotein VI and platelet adhesion on atherosclerosis--a possible role of fibronectin. J Mol Cell Cardiol 2010; 49: 532–42. 10.1016/j.yjmcc.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 42.Schonberger T, Siegel-Axel D, Bussl R, Richter S, Judenhofer MS, Haubner R, Reischl G, Klingel K, Munch G, Seizer P, Pichler BJ, Gawaz M. The immunoadhesin glycoprotein VI-Fc regulates arterial remodelling after mechanical injury in ApoE−/− mice. Cardiovasc Res 2008; 80: 131–7. 10.1093/cvr/cvn169. [DOI] [PubMed] [Google Scholar]
- 43.Cosemans JM, Kuijpers MJ, Lecut C, Loubele ST, Heeneman S, Jandrot-Perrus M, Heemskerk JW. Contribution of platelet glycoprotein VI to the thrombogenic effect of collagens in fibrous atherosclerotic lesions. Atherosclerosis 2005; 181: 19–27. 10.1016/j.atherosclerosis.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 44.Reininger AJ, Bernlochner I, Penz SM, Ravanat C, Smethurst P, Farndale RW, Gachet C, Brandl R, Siess W. A 2-step mechanism of arterial thrombus formation induced by human atherosclerotic plaques. J Am Coll Cardiol 2010; 55: 1147–58. 10.1016/j.jacc.2009.11.051. [DOI] [PubMed] [Google Scholar]
- 45.Zahid M, Mangin P, Loyau S, Hechler B, Billiald P, Gachet C, Jandrot-Perrus M. The future of glycoprotein VI as an antithrombotic target. Journal of Thrombosis and Haemostasis 2012; 10: 2418–27. 10.1111/jth.12009. [DOI] [PubMed] [Google Scholar]
- 46.Denorme F, De Meyer SF. The VWF-GPIb axis in ischaemic stroke: lessons from animal models. Thrombosis and Haemostasis 2016. 10.1160/TH16-01-0036. [DOI] [PubMed] [Google Scholar]
- 47.Deckmyn H, Cauwenberghs N, Wu D, Depraetere H, Vanhoorelbeke K. Development of antibodies that interfere with the collagen-VWF-GPIb axis as new antithrombotics. Verh K Acad Geneeskd Belg 2005; 67: 55–65. [PubMed] [Google Scholar]
- 48.Maurer E, Tang C, Schaff M, Bourdon C, Receveur N, Ravanat C, Eckly A, Hechler B, Gachet C, Lanza F, Mangin PH. Targeting Platelet GPIbβ Reduces Platelet Adhesion, GPIb Signaling and Thrombin Generation and Prevents Arterial Thrombosis. Arteriosclerosis, Thrombosis, and Vascular Biology 2013; 33: 1221–9. 10.1161/atvbaha.112.301013. [DOI] [PubMed] [Google Scholar]
- 49.Peyvandi F, Scully M, Kremer Hovinga JA, Cataland S, Knöbl P, Wu H, Artoni A, Westwood J-P, Mansouri Taleghani M, Jilma B, Callewaert F, Ulrichts H, Duby C, Tersago D. Caplacizumab for Acquired Thrombotic Thrombocytopenic Purpura. New England Journal of Medicine 2016; 374: 511–22. 10.1056/NEJMoa1505533. [DOI] [PubMed] [Google Scholar]
- 50.Serebruany VL, Malinin AI, Ferguson JJ, Vahabi J, Atar D, Hennekens CH. Bleeding risks of combination vs. single antiplatelet therapy: a meta-analysis of 18 randomized trials comprising 129,314 patients. Fundam Clin Pharmacol 2008; 22: 315–21. 10.1111/j.1472-8206.2008.00582.x. [DOI] [PubMed] [Google Scholar]
- 51.Peters RJ, Mehta SR, Fox KA, Zhao F, Lewis BS, Kopecky SL, Diaz R, Commerford PJ, Valentin V, Yusuf S. Effects of aspirin dose when used alone or in combination with clopidogrel in patients with acute coronary syndromes: observations from the Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) study. Circulation 2003; 108: 1682–7. 10.1161/01.cir.0000091201.39590.cb. [DOI] [PubMed] [Google Scholar]
- 52.Chai-Adisaksopha C, Crowther M, Isayama T, Lim W. The impact of bleeding complications in patients receiving target-specific oral anticoagulants: a systematic review and meta-analysis. Blood 2014; 124: 2450–8. 10.1182/blood-2014-07-590323. [DOI] [PubMed] [Google Scholar]
- 53.Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, Stevens SM, Vintch JRE, Wells P, Woller SC, Moores L. Antithrombotic therapy for vte disease: Chest guideline and expert panel report. Chest 2016; 149: 315–52. 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 54.de Zwart L, Snoeys J, De Jong J, Sukbuntherng J, Mannaert E, Monshouwer M. Ibrutinib Dosing Strategies Based on Interaction Potential of CYP3A4 Perpetrators Using Physiologically Based Pharmacokinetic Modeling. Clinical pharmacology and therapeutics 2016; 100: 548–57. 10.1002/cpt.419. [DOI] [PubMed] [Google Scholar]
- 55.Burnett AE, Mahan CE, Vazquez SR, Oertel LB, Garcia DA, Ansell J. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. Journal of Thrombosis and Thrombolysis 2016; 41: 206–32. 10.1007/s11239-015-1310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Granger CB, Alexander JH, McMurray JJV, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FWA, Zhu J, Wallentin L. Apixaban versus Warfarin in Patients with Atrial Fibrillation. New England Journal of Medicine 2011; 365: 981–92. 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 57.Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, Porcari A, Raskob GE, Weitz JI. Apixaban for Extended Treatment of Venous Thromboembolism. New England Journal of Medicine 2013; 368: 699–708. 10.1056/NEJMoa1207541. [DOI] [PubMed] [Google Scholar]
- 58.Seiter K, Stiefel MF, Barrientos J, Shaikh A, Ahmed N, Baskind P, Liu D. Successful treatment of ibrutinib-associated central nervous system hemorrhage with platelet transfusion support. Stem Cell Investigation 2016; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baharoglu MI, Cordonnier C, Salman RA-S, de Gans K, Koopman MM, Brand A, Majoie CB, Beenen LF, Marquering HA, Vermeulen M, Nederkoorn PJ, de Haan RJ, Roos YB. Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): a randomised, open-label, phase 3 trial. The Lancet 387: 2605–13. 10.1016/S0140-6736(16)30392-0. [DOI] [PubMed] [Google Scholar]
- 60.McMullen JR, Boey EJH, Ooi JYY, Seymour JF, Keating MJ, Tam CS. Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood 2014; 124: 3829–30. 10.1182/blood-2014-10-604272. [DOI] [PubMed] [Google Scholar]
- 61.Farooqui M, Valdez J, Soto S, Bray A, Tian X, Wiestner A. Atrial Fibrillation in CLL/SLL Patients on Ibrutinib. Blood 2015; 126: 2933–. [Google Scholar]
- 62.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JJC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: Executive SummaryA Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Journal of the American College of Cardiology 2014; 64: 2246–80. 10.1016/j.jacc.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 63.Connolly SJ, Pogue J, Hart RG, Hohnloser SH, Pfeffer M, Chrolavicius S, Yusuf S. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med 2009; 360: 2066–78. 10.1056/NEJMoa0901301. [DOI] [PubMed] [Google Scholar]
- 64.Connolly S, Pogue J, Hart R, Pfeffer M, Hohnloser S, Chrolavicius S, Pfeffer M, Hohnloser S, Yusuf S. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet 2006; 367: 1903–12. 10.1016/s0140-6736(06)68845-4. [DOI] [PubMed] [Google Scholar]
- 65.Dewilde WJ, Oirbans T, Verheugt FW, Kelder JC, De Smet BJ, Herrman JP, Adriaenssens T, Vrolix M, Heestermans AA, Vis MM, Tijsen JG, van ‘t Hof AW, ten Berg JM. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 2013; 381: 1107–15. 10.1016/s0140-6736(12)62177-1. [DOI] [PubMed] [Google Scholar]
- 66.Patrono C Aspirin as an antiplatelet drug. N Engl J Med 1994; 330: 1287–94. 10.1056/nejm199405053301808. [DOI] [PubMed] [Google Scholar]
- 67.Leese PT, Hubbard RC, Karim A, Isakson PC, Yu SS, Geis GS. Effects of celecoxib, a novel cyclooxygenase-2 inhibitor, on platelet function in healthy adults: a randomized, controlled trial. J Clin Pharmacol 2000; 40: 124–32. [DOI] [PubMed] [Google Scholar]
- 68.Rinder HM, Tracey JB, Souhrada M, Wang C, Gagnier RP, Wood CC. Effects of meloxicam on platelet function in healthy adults: a randomized, double-blind, placebo-controlled trial. Journal of clinical pharmacology 2002; 42: 881–6. [DOI] [PubMed] [Google Scholar]
- 69.de Abajo FJ. Effects of Selective Serotonin Reuptake Inhibitors on Platelet Function. Drugs & Aging 2011; 28: 345–67. 10.2165/11589340-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 70.Perahia DG, Bangs ME, Zhang Q, Cheng Y, Ahl J, Frakes EP, Adams MJ, Martinez JM. The risk of bleeding with duloxetine treatment in patients who use nonsteroidal anti-inflammatory drugs (NSAIDs): analysis of placebo-controlled trials and post-marketing adverse event reports. Drug, Healthcare and Patient Safety 2013; 5: 211–9. 10.2147/DHPS.S45445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Finnes HD, Chaffee KG, Call TG, Ding W, Bowen DA, Conte M, McCullough KB, Merten JA, Bartoo GT, Smith MD, Schwager SM, Slager SL, Kay NE, Shanafelt TD, Parikh SA. The Importance of Pharmacovigilance during Ibrutinib Therapy for Chronic Lymphocytic Leukemia (CLL) in Routine Clinical Practice. Blood 2015; 126: 717–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu J, Zhang M, Liu D. Acalabrutinib (ACP-196): a selective second-generation BTK inhibitor. J Hematol Oncol 2016; 9: 21 10.1186/s13045-016-0250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Walter HS, Rule SA, Dyer MJ, Karlin L, Jones C, Cazin B, Quittet P, Shah N, Hutchinson CV, Honda H, Duffy K, Birkett J, Jamieson V, Courtenay-Luck N, Yoshizawa T, Sharpe J, Ohno T, Abe S, Nishimura A, Cartron G, Morschhauser F, Fegan C, Salles G. A phase 1 clinical trial of the selective BTK inhibitor ONO/GS-4059 in relapsed and refractory mature B-cell malignancies. Blood 2016; 127: 411–9. 10.1182/blood-2015-08-664086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Byrd JC, Harrington B, O’Brien S, Jones JA, Schuh A, Devereux S, Chaves J, Wierda WG, Awan FT, Brown JR, Hillmen P, Stephens DM, Ghia P, Barrientos JC, Pagel JM, Woyach J, Johnson D, Huang J, Wang X, Kaptein A, Lannutti BJ, Covey T, Fardis M, McGreivy J, Hamdy A, Rothbaum W, Izumi R, Diacovo TG, Johnson AJ, Furman RR. Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med 2016; 374: 323–32. 10.1056/NEJMoa1509981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Registry NCT. Elevate CLL R/R: Study of Acalabrutinib (ACP-196) Versus Ibrutinib in Previously Treated Subjects With High Risk Chronic Lymphocytic Leukemia. ClinicalTrials.gov, 2016.


