Abstract
Background:
It is well established that as a blood unit ages, fewer of the unit’s red blood cells (RBCs) remain in circulation post transfusion. The mechanism for clearance is not well defined. Phosphatidylethanolamine (PE) is a phospholipid that is primarily found on the inner leaflet of healthy cells, and is an important ligand for phagocytosis of dead cells when exposed.
Objectives:
The objective of the present study was to measure the change in PE surface exposure on donor RBCs over increasing storage ages using the novel PE-specific probe, duramycin.
Methods/Materials:
Five AS-1-preserved RBC units were sampled weekly for 6 weeks and were labeled with duramycin. The percent of PE exposing red cells on each sample was determined by flow cytometry. Surface phosphatidylserine (PS) was evaluated for comparison.
Results:
We found that RBCs in AS-preserved donor units increasingly exposed PE, from less than 1% in freshly processed RBCs, to nearly 20% at the time of storage outdate of 42 days and correlated with increased relative vesiculation or microparticle concentration and release of cell-free hemoglobin. By comparison, only 5% of cells exposed PS at 42 days.
Conclusion:
We conclude that exposure of PE at the RBC outer membrane increased more than PS during 42 days of storage and correlated significantly with increased vesiculation and release of hemoglobin.
Keywords: Phosphatidylethanolamine, Transfusion Practice, Red cell storage age
Introduction
Red blood cell (RBC) transfusions are a mainstay of modern medicine. However differences in unit efficacy and safety during storage exist (Rohde et al., 2014; Glynn et al., 2016). Although prospective randomized trials have been performed, there remains some debate on the specific clinical contexts where red cell storage age influences patient outcomes (Glynn et al., 2016).
Currently, additive solutions preserve RBCs such that if transfused just prior to the expiration date, about 75% of RBCs are recovered 24 hours post transfusion (Glynn et al., 2016). The mechanism mediating the rapid removal of roughly 25% of stored RBCs is not entirely understood. Well-documented intracellular changes with stored RBCs include depletion of glucose, ATP and 2,3 DPG (Hess, 2014). Changes to the overall make-up of the unit also occur, including increases in cell-free hemoglobin (both soluble and microparticle-encapsulated), potassium, and acids (Donadee et al., 2011). More recent studies have highlighted a critical role for nitric oxide (NO) in the changes that occur during RBC storage, including depletion of s-nitrosohemoglobin (Bennett-Guerroro et al., 2007) and enhanced scavenging of NO (Donadee et al., 2011). These aberrations at the molecular level are further associated with cellular-level defects including red cell morphology changes, decreased red cell deformability, and membrane bilayer disruption including the formation of microparticles (MP) (Berezina et al., 2002).
The red cell lipid bilayer is composed of phosphatidylcholine, sphingomyelin, phosphatidylserine (PS), phosphatidylinositol, phosphatidic acid, and phosphatidylethanolamine (PE)(Virtanen et al., 1998). Under normal conditions, enzymes known as flippases and floppases maintain a controlled lipid asymmetry, with phosphatidylcholine and sphingomyelin predominantly on the outer leaflet, and PS, phosphatidylinositol, phosphatidic acid, and PE predominantly on the inner leaflet (Virtanen et al., 1998). Multiple studies have established that the normal red cell membrane asymmetry decreases with storage, with significant increases in PS exposure on the outer leaflet of the cell membrane having been reported (Berezina et al., 2002). In quantifying membrane disturbance over the storage duration, studies variably report up to 6% of RBCs exposed PS on the outer leaflets of the lipid bilayer at 42 days (Koshkaryev et al., 2009). This association is of interest, as both human and murine macrophages can bind to and engulf symmetric red cell ghosts, red cells with PS inserted externally, oxidized red cells, and sickled red cells, all of which expressed PS on the outer leaflet (Fadok et al., 2001; Styles et al., 1997; Zenarruzabeitia et al., 2015). While promising, it remains unclear whether PS outer leaflet exposure can entirely explain the rapid phagocytosis of nearly one-quarter of the red cells in an aged stored unit post-transfusion.
We recently found that PE is also exposed on the outer leaflet of crenated/damaged erythrocytes in aged RBCs (Larson et al., 2012). Unlike PS, which makes up only 3–15% of total lipids, PE is the second most abundant phospholipid in mammalian cells, making 20–45% of total lipids (Zhao, 2011). Thus, significantly increased outer leaflet PE exposure may also contribute to the extensive removal of older stored red cells.
To determine whether storage age is associated with increased PE RBC surface exposure, we performed a longitudinal evaluation of PE using the novel PE-probe, duramycin. Duramycin is a well described lantibiotic that binds to phosphatidylethanolamine with high affinity (Zhao, 2011), and, as reported previously (Larson et al., 2012), can be used as a sensitive and specific marker for PE content on the red cell outer leaflet. The objectives of this study were to use duramycin to: 1) define the distribution of PE exposure over the shelf-life of a RBC unit, and 2) determine the association between PE exposure and other biological markers of storage age, MPs and free hemoglobin.
Methods
Five donor RBC units (BloodCenter of Wisconsin, Milwaukee, WI) were obtained through a protocol approved by the Medical College of Wisconsin Internal Review Board. Stored RBC units contained either AS-1 (serially and single sampled units) or AS-3 (single sampled units only) preservative, and were leukocyte reduced per established donor center protocols. Units were stored flat in a cold room that maintained at a temperature of 1–6°C for the duration of the study; each unit was gently inverted 10 times prior to each sampling. Sampling occurred weekly for 6 weeks. To ensure that the serial sampling process did not alter PE exposure, nine other regularly outdated (AS-1 and −3 preserved) units (43–46 days old) were also examined. Lastly, to compare the PE content of fresh blood to the sampled stored units, blood from 3 healthy donors was drawn in accordance with approved institutional protocols into acid-citrate-dextrose, and allowed to cool to room temperature before diluting as described below.
Direct detection of PE and PS exposure
All blood samples were diluted repeatedly 10 fold to a final 1:1000 dilution in pH 7–7.2 sterile-filtered normal saline with 12.5 nM streptavidin Alexafluor-647 (SA647, Invitrogen, Carlsbad, CA) only as a fluorescent control, or with 1 µM duramycin-biotin (gift from Ming Zhao, Northwestern University) followed 7–10 minutes later by SA647 to examine PE exposure. Optimization and validation experiments for this assay and MP detection using flow cytometry were done as reported previously (Larson et al., 2012, Larson et al., 2013); duramycin-induced cytotoxicity was avoided by using a sub-hemolytic concentration and using cold incubation with an optimized incubation time (validation of this assay revealed that cytotoxicity was avoided when incubation time was less than 60 minutes given the selected concentrations and storage temperature, validation data not shown). We further co-labeled MPs with glycophorin A to confirm that these small particles were distinct from electronic noise (Larson et al., 2012, Larson et al., 2013). Samples were incubated on ice, and examined within 30 minutes of dilution on an Accuri C6 (BD biosciences, San Jose, CA, USA) using the default fast setting with a forward scatter-height (FSC-H) threshold of 1000; this setting allowed for detection of actual MPs with acceptable noise that could be gate-excluded post-hoc similarly as previously reported (Larson et al., 2012; Larson et al., 2013). For comparison, lactadherin-FITC (Haematologic Tech, Essex Junction, VT, USA) per manufacturer’s recommendation was added to identically-diluted and treated cells to concurrently measure PS. Co-labeling experiments demonstrated duramycin inhibited lactadherin binding (data not shown). C6 Plus analytical software (BD biosciences, San Jose, CA, USA) was used to analyze the flow cytometry data. Of note, autofluorescence has been reported to increase in RBCs with storage in the UV to visible green wavelengths (Mérian et al., 2012), but there is no report of increased autofluorescence in the near-to-infrared region (around the spectral region of the fluorochrome used). To control for possible autofluorescence, unstained controls were assessed for changes in near-infrared region (the Accuri C6 excitation laser is near-infrared at 640 nm and the fluorescence channel used detects the infrared at around 675nm) over the course of storage (for which there was no difference, data not shown).
Cell-free hemoglobin measurements
Cell-free hemoglobin (CFHb) was measured by isolation of sample cell-free supernatant using centrifugation (15 min at 500 × g, 4°C for stored blood, room temperature for fresh blood; the supernatant was analyzed by flow cytometry and did not contain RBCs, data not shown). Supernatant was then examined by UV/visible spectroscopy on a HP/Agilent 8453 UV/Vis spectrometer (Santa Clara, CA, USA). Met-heme concentration was then determined from absorbance at 631 nm (using extinction coefficient of 5030 M−1 cm-1) and added to the oxy-heme concentration approximated from absorbances at 405, 577, and 561 nm (averaged using extinction coefficients of 3.31 ×105, 5.51 ×104, and 3.26 ×104 M−1 cm-1, respectively) (Antonini & Brunori 1971).
Statistical Analysis
All continuous numeric data was evaluated using standard parametric tests. Data are summarized as means ± standard error. A p-value less than 0.05 was considered statistically significant throughout.
Results
Leukoreduced RBC units (RBCs) were examined for 42 days from initial donation/processing. Figure 1A shows the flow cytometry light scatter gating of intact cells from the sampled RBC units. Events with smaller side and forward scatter, outside of the cell gate, but larger than the background cytometer noise, were considered to be MPs. Figure 1B demonstrates representative cell-gate histograms of duramycin-stained, serially-sampled, AS-1 preserved RBCs at various time points. RBCs stored for 1 week post-donation showed minimal duramycin staining, indicating a minimal number of RBCs with substantial outer leaflet PE exposure (left panel of Figure 1B). With increasing storage age, there were an increased number of PE-outer-leaflet-positive cells at 3 and 6 weeks of storage, respectively (Figure 1B).
Figure 1: Cellular PE exposure increases steadily over duration of storage.
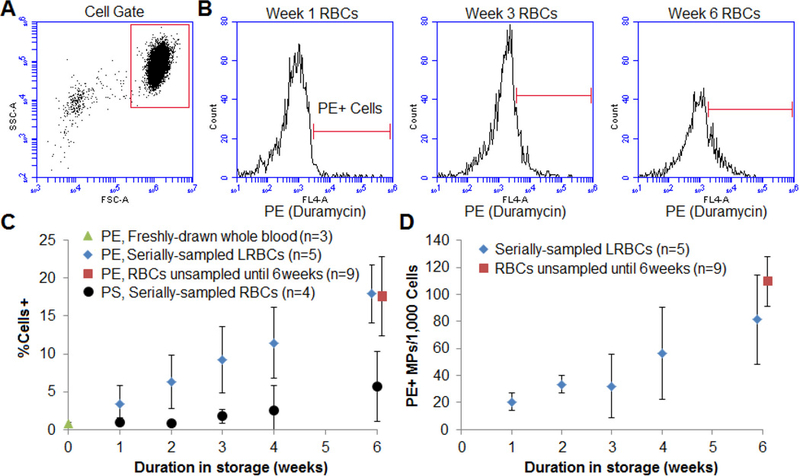
A) Representative dotplot gating of cells and B) histogram of PE exposure in (diluted, leukocyte-reduced RBC [RBC]) donor units at week 1, week 3, and week 6. Gating was based on fluorescent streptavidin-only control (data not shown). C) The percentage of PE-positive cells increased steadily over time (N=5). PS staining using lactadherin-FITC was also measured and displayed for comparison (black circles, N=4). Also shown are data from unsampled recently-outdated RBC units (n=9), and whole blood from healthy controls (week 0) for comparison (N=3). D) To account for possible dilution artifact, the number of PE-positive microparticles (MPs, cell-gate excluded events) per cell were also recorded.
Over the course of storage (Figure 1C), there was a steady and significant increase in the percent of PE positive cells (n=5, R2=0.93, p=0.001), and nearly 20% of sampled red cells had outer leaflet PE at 6 weeks. Freshly-drawn whole blood (green triangle, n=3) and recently-expired RBC units (red square, n=9) were also examined for comparison, and the percentage of PE-positive RBCs from serially-sampled units at the time of expiration was comparable to that of the unsampled expired units (p>0.8). As a comparison, we also stained for PS using lactadherin, which is a more sensitive flow cytometer marker than Annexin V (Larson et al., 2012). While there also was a steady increase in PS positive cells, only about 5% of sampled cells had outer leaflet PS at 6 weeks (black circle, N=4). Lastly, there was also a steady and significant increase in PE-positive MPs with increased storage duration when normalized to cells (Figure 1D).
CFHb is a marker of hemolysis (Donadee et al, 2011). Figure 2A shows the average CFHb concentration in each RBC unit over its storage duration as determined by UV-visible spectrosctopy, again with a comparison to freshly-drawn blood (which expectedly had nearly-undetectable concentrations of CFHb) and unsampled expired units. In agreement with previously published studies, free hemoglobin increased with storage age, and both serially and single sampled RBC units contained roughly 0.1 mM (0.16g/dL) of free hemoglobin (in terms of total heme) by 6 weeks of storage (Donadee et al., 2011). Lastly, there was a strong linear positive correlation between the percent of PE-positive cells detected and the average concentration of CFHb (R2=0.93, p=0.001) in each unit (Figure 2B).
Figure 2: Cell-free hemoglobin over duration of storage.
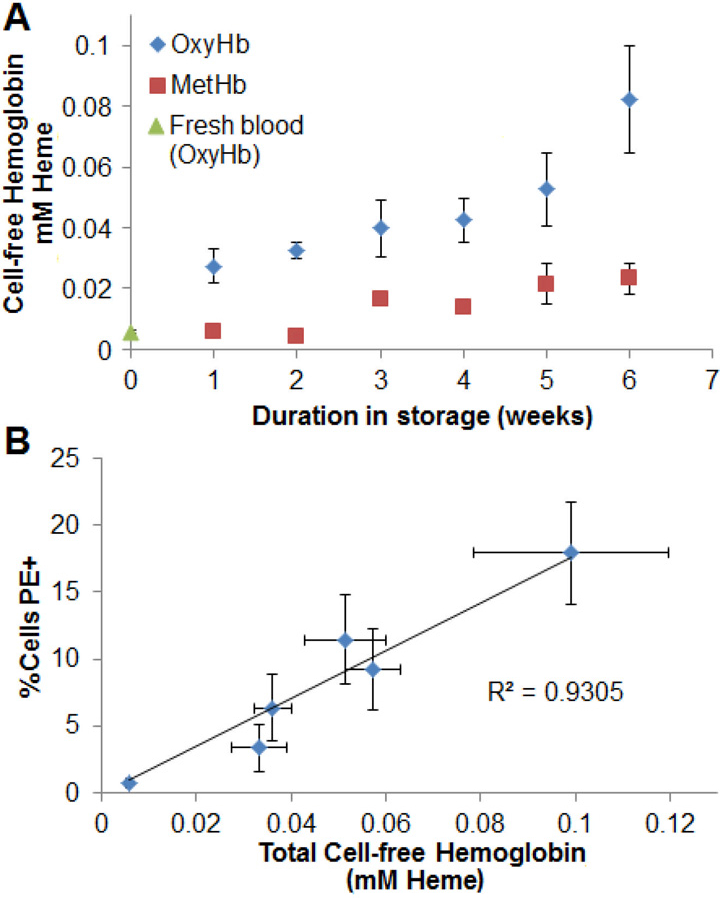
A) Cell-free hemoglobin increased through the duration of storage (N=5). B) There was a linear correlation between the percent of PE-positive cells and total cell-free hemoglobin by week (R2=0.93, p=0.001).
Discussion
The loss of phospholipid asymmetry is considered to be a key signal for erythrophagocytosis of senescent red cells (Fadok et al., 2001). Therefore, changes in red cell surface lipids during red cell storage may alter how the recipient immune system reacts to these transfusions. An increase in PS exposure on red cells during storage has been reported, but the overall concentration of PS on red cells is small as a fraction of total phospholipids (Koshkaryev et al., 2009), even when using sensitive PS markers such as lactadherin. PE is a much more abundant phospholipid (Vance, 2008), and may therefore act as another, potentially more robust and/or dynamic marker of lipid asymmetry priming red cells for phagocytosis. This study evaluated the changes in surface PE exposure during standard RBC storage using the novel PE-dependent binding properties of duramycin. We observed a significant positive correlation between increasing storage age and PE outer-leaflet exposure as demonstrated by duramycin binding both in intact red cells and in MPs. Moreover, we found that these changes parallel the established increases in free hemoglobin in those same older units.
This pilot study has found evidence supporting the association between the percent of red cells with outer-leaflet PE exposure and the surrogate markers of red cell decay and hemolysis such as the level of free hemoglobin and MPs in the red cell unit over time. Several studies have previously associated increased hemolysis with the duration of storage (Hess, 2014). Similar to previous reports (Hess, 2014), we found that free hemoglobin increased about 4-fold from 7 days of storage to 42 days of storage. External leaflet PS has previously been established as a marker for damaged or aged red cells, which, due to the lack of phagocytosis in a stored unit, leads to cell lysis and the release of MPs and free hemoglobin over time (Hess, 2014). Like PS, PE may be a marker of increasing red cell damage during storage. However, unlike PS, PE is much more abundant and therefore possibly a more dynamic marker of cell membrane derangement.
Previous studies have suggested that outer leaflet PE may serve a similar immune function to PS, but their immune and clinical effects are not yet established for red cells. PE and PS are both known ligands involved in the clearance of dying cells via their direct interaction with TIM1 and CD300a receptors of phagocytic cells (Simhadri et al., 2012; Jemielity et al., 2013; Richard et al., 2015; Kobayashi et al., 2007). However, to date only PS, and not PE, has been found to be involved in the clearance specifically of red cells (Kobayashi et al., 2007). In addition to inducing phagocytosis in multiple experimental models, activation of TIM1 and CD300a receptors by PS and PE interaction induce secretion of anti-inflammatory interleukin-10 and TGF-β, and simultaneously decrease the secretion of the pro-inflammatory cytokines TNF-α, IL-1β, and IL-12 (Huynh et al., 2002), modulating the immune system. In contrast, activation of CD300C by PE in vitro increases inflammatory/immune activity, and may act in a synergistic fashion with toll like receptor 4 signaling (Takahashi et al., 2013). In specific clinical populations, the increased external PS and PE content of red cells may be harmful, as outer leaflet aminophospholipid exposure (specifically PS) has been associated with worsened red cell endothelial adhesion in individuals with sickle cell disease, a contributor to vaso-occlusion (Manodori et al., 2000; Guchhait et al., 2007).
We found that close to 20% of cells were outer leaflet PE-positive at 42 days. This percentage mirrors the observed RBC clearance of 42 day-old units seen 24 hours after transfusion (Glynn et al., 2016) in comparison to the percentage of PS-positive cells as reported by our own work and others (Koshkaryev et al., 2009, Berezina et al., 2002). Consequently, we speculate that PE may provide a plausible contributor to the abrupt clearance of transfused old RBCs and their downstream clinical effects. Future studies will be required to establish any causal relationship between surface red cell PE and in vivo immune clearance. Mechanistic hypotheses such as these, however, are increasingly important, as clinical outcomes such as increased circulating non-transferrin bound (free) iron and infections from transfusion (Hod et al., 2010, Rohde et al., 2014) may be modulated by blocking or altering these factors.
This study has several limitations. First, the donor RBC units were gently agitated prior to sampling, which is a deviation from what is practiced clinically. Second, the additive solutions were not identical between the serially-sampled (all AS-1) and single-sampled units (AS-1 and 3). However, we found no statistically significant difference in the PE surface content of single-sampled expired AS-1 and AS-3 stored RBCs (p>0.3, data not shown). Lastly, while a non-trivial percent of total PE is normally found in a lattice on the extracellular leaflet (an estimated 11% of total extracellular phospholipid is PE) using other methods (Virtanen et al., 1998), the conditions and parameters used (including duramycin-biotin and fluorescent streptavidin concentrations, RBC dilution, flow cytometer settings) in our technique resulted in less than 1% of freshly-drawn cells appreciably binding duramycin.
In conclusion, our study provides initial evidence suggesting a potential role for external leaflet PE in the red cell storage lesion. We show that PE, like PS, appears to be correlated with increasing red cell damage and hemolysis during storage. Increased PE exposure on stored RBCs may become an important biomarker in future studies of red cell storage age efficacy and safety due to its increased abundance in comparison to PS. Additional studies are now needed to further establish the relationship between changes in the membrane orientation of PE during RBC storage and red cell hemolysis, phagocytosis, and their downstream clinical effects.
Acknowledgments:
MCL was a member of the Medical Scientist Training Program at MCW where much of this work was done, which is partially supported by a training grant from NIGMS T32-GM080202.
Funding sources
This study was funded by the Midwest Athletes against Childhood Cancer (MACC) fund and R01 NS070711 (to CAH), Midwest Basic and Translational Program U54 HL090503 (to NH and CAH), and ML was a member of the MCW-MSTP, which is partially supported by a T32 grant from NIGMS, GM080202.
Footnotes
Conflicts of Interest
MCL is a co-inventor of a donor blood cleaning device. CAH. is a consultant for Bayer. MSK and NH have no conflicts to declare.
References
- Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS (2007). Evolution of adverse changes in stored RBCs. Proceedings of the National Academy of Sciences, 104(43), 17063–17068. doi: 10.1073/pnas.0708160104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezina TL, Zaets SB, Morgan C, Spillert CR, Kamiyama M, Spolarics Z, Deitch EA and Machiedo GW (2002). Influence of Storage on Red Blood Cell Rheological Properties. Journal of Surgical Research, 102(1), 6–12. doi: 10.1006/jsre.2001.6306 [DOI] [PubMed] [Google Scholar]
- Donadee C, Raat NJ, Kanias T, Tejero J, Lee JS, Kelley EE, Zhao X, Liu C, Reynolds H, Azarov I, Frizzell S, Gladwin MT (2011). Nitric Oxide Scavenging by Red Blood Cell Microparticles and Cell-Free Hemoglobin as a Mechanism for the Red Cell Storage Lesion. Circulation, 124(4), 465–476. doi: 10.1161/circulationaha.110.008698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok VA, Cathelineau AD, Daleke DL, Henson PM, & Bratton DL (2000). Loss of Phospholipid Asymmetry and Surface Exposure of Phosphatidylserine Is Required for Phagocytosis of Apoptotic Cells by Macrophages and Fibroblasts. Journal of Biological Chemistry, 276(2), 1071–1077. doi: 10.1074/jbc.m003649200 [DOI] [PubMed] [Google Scholar]
- Glynn SA, Klein HG, & Ness PM (2016). The red blood cell storage lesion: The end of the beginning. Transfusion, 56(6), 1462–1468. doi: 10.1111/trf.13609 [DOI] [PubMed] [Google Scholar]
- Guchhait P, Dasgupta SK, Le A, Yellapragada S, Lopez JA, & Thiagarajan P (2007). Lactadherin mediates sickle cell adhesion to vascular endothelial cells in flowing blood. Haematologica, 92(9), 1266–1267. doi: 10.3324/haematol.11379 [DOI] [PubMed] [Google Scholar]
- Hess JR (2014). Measures of stored red blood cell quality. Vox Sanguinis Vox Sang, 107(1), 1–9. doi: 10.1111/vox.12130 [DOI] [PubMed] [Google Scholar]
- Hod EA, Zhang N, Sokol SA, Wojczyk BS, Francis RO, Ansaldi D, … Spitalnik SL (2010). Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood, 115(21), 4284–4292. doi: 10.1182/blood-2009-10-245001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemielity S, Wang JJ, Chan YK, Ahmed AA, Li W, Monahan S, … Choe H (2013). TIM-family Proteins Promote Infection of Multiple Enveloped Viruses through Virion-associated Phosphatidylserine. PLoS Pathog PLoS Pathogens, 9(3). doi: 10.1371/journal.ppat.1003232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi N, Karisola P, Peña-Cruz V, Dorfman DM, Jinushi M, Umetsu SE, … Freeman GJ (2007). TIM-1 and TIM-4 Glycoproteins Bind Phosphatidylserine and Mediate Uptake of Apoptotic Cells. Immunity, 27(6), 927–940. doi: 10.1016/j.immuni.2007.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshkaryev A, Zelig O, Manny N, Yedgar S, & Barshtein G (2009). Rejuvenation treatment of stored red blood cells reverses storage-induced adhesion to vascular endothelial cells. Transfusion, 49(10), 2136–2143. doi: 10.1111/j.1537-2995.2009.02251.x [DOI] [PubMed] [Google Scholar]
- Larson MC, Woodliff JE, Hillery CA, Kearl TJ, & Zhao M (2012). Phosphatidylethanolamine is externalized at the surface of microparticles. Biochimica Et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids, 1821(12), 1501–1507. doi: 10.1016/j.bbalip.2012.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson MC, Luthi MR, Hillery CA, Hogg N (2013). Calcium-phosphate microprecipitates mimic microparticles when examined with flow cytometry. Cytometry Part A, 83(2), 242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manodori AB, Barabino GA, Lubin BH and Kuypers FA (2000). Adherence of phosphatidylserine-exposing erythrocytes to endothelial matrix thrombospondin. Blood, 95(4), 1293–1300. [PubMed] [Google Scholar]
- Mérian J, Julien G, Navarro F, Texier I (2012) Fluorescent nanoprobes dedicated to in vivo imaging: from preclinical validations to clinical translation. Molecules, 17(5), 5564–5591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichlin M (1972). Hemoglobin and Myoglobin in Their Reactions with Ligands. Science, 178(4058), 296–296. doi: 10.1126/science.178.4058.296 [DOI] [Google Scholar]
- Richard AS, Zhang A, Park S, Farzan M, Zong M, & Choe H (2015). Virion-associated phosphatidylethanolamine promotes TIM1-mediated infection by Ebola, dengue, and West Nile viruses. Proceedings of the National Academy of Sciences Proc Natl Acad Sci USA, 112(47), 14682–14687. doi: 10.1073/pnas.1508095112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde JM, Dimcheff DE, Blumberg N, Saint S, Langa KM, Kuhn L, Rogers MA (2014). Health Care–Associated Infection After Red Blood Cell Transfusion. Jama, 311(13), 1317. doi: 10.1001/jama.2014.2726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simhadri VR, Andersen JF, Calvo E, Choi S, Coligan JE, & Borrego F (2012). Human CD300a binds to phosphatidylethanolamine and phosphatidylserine, and modulates the phagocytosis of dead cells. Blood, 119(12), 2799–2809. doi: 10.1182/blood-2011-08-372425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styles L, de Jong K, Vichinsky E, Lubin L, Adams R, Kuypers FA (1997). Increased RBC phosphatidylserine exposure in sickle cell disease patients at risk for stroke by transcranial Doppler screening. Blood, 90, 604a. [Google Scholar]
- Takahashi M, Izawa K, Kashiwakura J, Yamanishi Y, Enomoto Y, Kaitani A, … Kitaura J (2013) Human CD300C delivers an Fc receptor-y-dependent activating signal in mast cells and monocytes and differs from CD300A in ligand recognition. J. Biol. Chem, 288(11), 7662–7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance JE (2008). Thematic Review Series: Glycerolipids. Phosphatidylserine and phosphatidylethanolamine in mammalian cells: Two metabolically related aminophospholipids. The Journal of Lipid Research, 49(7), 1377–1387. doi: 10.1194/jlr.r700020-jlr200 [DOI] [PubMed] [Google Scholar]
- Virtanen JA, Cheng KH, & Somerharju P (1998). Phospholipid composition of the mammalian red cell membrane can be rationalized by a superlattice model. Proceedings of the National Academy of Sciences, 95(9), 4964–4969. doi: 10.1073/pnas.95.9.4964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RH, Phillips G, Medof ME, & Mold C (1993). Activation of the alternative complement pathway by exposure of phosphatidylethanolamine and phosphatidylserine on erythrocytes from sickle cell disease patients. Journal of Clinical Investigation J. Clin. Invest, 92(3), 1326–1335. doi: 10.1172/jci116706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenarruzabeitia O, Vitalle J, Eguizabal C, Simhadri VR, & Borrego F (2015). The Biology and Disease Relevance of CD300a, an Inhibitory Receptor for Phosphatidylserine and Phosphatidylethanolamine. The Journal of Immunology, 194(11), 5053–5060. doi: 10.4049/jimmunol.1500304 [DOI] [PubMed] [Google Scholar]
- Zhao M (2009). Lantibiotics as probes for phosphatidylethanolamine. Amino Acids, 41(5), 1071–1079. doi: 10.1007/s00726-009-0386-9 [DOI] [PMC free article] [PubMed] [Google Scholar]


