Abstract
With increasing survival of patients infected with human immunodeficiency virus type 1 (HIV-1), the manifestation of heterogeneous neurological complications is also increasing alarmingly in these patients. Currently, more than 30% of about 40 million HIV-1 infected people worldwide develop central nervous system (CNS)-associated dysfunction, including dementia, sensory, and motor neuropathy. Furthermore, the highly effective antiretroviral therapy has been shown to increase the prevalence of mild cognitive functions while reducing other HIV-1-associated neurological complications. On the contrary, the presence of neurological disorder frequently affects the outcome of conventional HIV-1 therapy. Although, both the children and adults suffer from the post-HIV treatment-associated cognitive impairment, adults, especially depending on the age of disease onset, are more prone to CNS dysfunction. Thus, addressing neurological complications in an HIV-1-infected patient is a delicate balance of several factors and requires characterization of the molecular signature of associated CNS disorders involving intricate cross-talk with HIV-1-derived neurotoxins and other cellular factors. In this review, we summarize some of the current data supporting both the direct and indirect mechanisms, including neuro-inflammation and genome instability in association with aging, leading to CNS dysfunction after HIV-1 infection, and discuss the potential strategies addressing the treatment or prevention of HIV-1-mediated neurotoxicity.
Keywords: AIDS, cognitive dysfunction, dementia, genome instability, human immunodeficiency virus type 1, neurodegeneration
HUMAN IMMUNODEFICIENCY VIRUS 1 EPIDEMIOLOGY
Despite advances in knowledge, treatment, and awareness, human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS) remains a pandemic with a global prevalence of 0.8%. Since the start of the epidemic, an estimated 78 million people have become infected with HIV-1, and 35 million people have died as a result of HIV-1-related illnesses. In 2015, 36.7 million people (including 1.8 million children) were living with HIV-1. Sub-Saharan Africa remains the most affected region, with over 25 million HIV-1 infected individuals. In 2015, 2.1 million new HIV-1 infections were identified, of which 150,000 were children. Most newly infected children live in Sub-Saharan Africa and were infected via their HIV-positive mothers during pregnancy, childbirth, or breastfeeding (http://www.unaids.org/en/resources/fact-sheet).
The global epidemic of HIV-1 infection has changed significantly after the introduction of antiretroviral therapy (ART). As of June 2016, 18.6 million people (46% of all adults and 49% of all children) have had access to combination ART (cART) globally. The global prevalence of HIV-1 infection increased from 31.8 million in 2005 to 36.7 million by the end of 2015, because patients on cART live much longer. The number of new HIV-1 infections in children decreased by 67% between 2005 and 2015, because of increased access to cART, resulting in prevention of mother-to-child HIV-1 transmission. However, despite advances in treatment, HIV-1 remains a major contributor to global deaths. AIDS-related deaths peaked at 2 million in 2005 and decreased to 1.1 million in 2015. Tuberculosis remains the leading cause of death among HIV-1 patients, accounting for one in three AIDS-related deaths. However, some progress has been made, with a 32% drop in tuberculosis-related deaths among people with HIV since 2004 (http://www.unaids.org/en/resources/fact-sheet).
In 1984, after the first discovery of HIV-1, a second type of the virus was discovered in AIDS patients in West Africa in 1986. Both HIV-1 and HIV-2 use the same modes of transmission and involve similar opportunistic secondary infections upon development of AIDS. However, unlike HIV-1, HIV-2 infections progress slowly. Although, the number of HIV-2-infected patients is growing slowly globally and spreading from Africa to Europe, India, and the United States, HIV-1 infection affects the majority of the population more aggressively, leading to death. In the present review, we will focus on the HIV-1 infection particularly and its disease mechanism and pathology. Hereafter, HIV-1 will be followed as HIV unless and otherwise mentioned.
HIV-ASSOCIATED NEUROPATHOLOGICAL COMPLICATIONS
Most HIV-infected patients suffer from neurological dysfunctions collectively known as HIV- associated neurocognitive disorder (HAND). HAND is typically mild in most cases and, thus, is unrecognizable at the beginning. Cognitive impairments in an HIV-affected CNS can be broadly categorized into three groups.
Asymptomatic neurocognitive impairment (ANI)
ANI, which was first recognized as a key neuropathological disorder in HIV-infected patients in 2007 [1], is more prevalent than HIV-associated dementia (HAD) among infected individuals. As it is not an overt pathology, it is often difficult to clinically distinguish ANI from mild neurocognitive disorder (MND). According to the criteria for HAND diagnosis, ANI is characterized by cognitive impairment involving at least two cognitive domains without any functional deficits in everyday performance. To date, it remains controversial whether ANI can be considered an early sign of developing MND or HAD among HIV-infected patients. However, a longitudinal study led by Grant et al. for the CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) Group concluded that individuals suffering from ANI exhibita 2-to 6-fold higher risk of developing early symptomatic cognitive decline, suggesting an urgent need to identify treatment avenues for those at highest risk of MND [2]. Furthermore, about16–19% of HIV-negative individuals over 35 years of age were characterized as having ANI based on American Academy of Neurology criteria, suggesting that further scrutiny of the parameters of test batteries for diagnosing ANI is required [3].
The major bottleneck in finding predictive biomarkers and/or definitive neuropsychological parameters for characterizing ANI is the identification of actual disease onset and progression. ANI can develop from multiple secondary factors, such as genetic susceptibility toward neurodegeneration, substance abuse, other infectious diseases, and neurotoxicity of ART. Moreover, at the initial stage of highly active ART (HAART), most cognitive deficits (both asymptomatic and symptomatic) are reversed, making it difficult to pinpoint the natural history of disease origin [4, 5]. Furthermore, the symptoms of ANI may fluctuate over time depending on the age of the infected individual, rendering it difficult to directly correlate progression of HIV infection with MND or HAD.
To date, ANI diagnosis as a disease is challenging, requiring a very stringent approach for characterizing the disease. However, recent advances in brain imaging techniques may have much higher potential to identify the disease than other previously employed criteria. Because asymptomatic decline is not associated with anatomic alterations in brain architecture, it is difficult to utilize structural imaging tools. However, molecular imaging techniques, such as proton magnetic resonance spectroscopy (MRS), show increases in the levels of choline, a cell proliferation and inflammation marker, and myo-inositol, a tissue glial marker, in almost all cases of HIV infection, even in totally asymptomatic individuals, N-acetyl-aspartate (NAA), a neuronal marker of injury and an indirect measure for brain metabolism, is found to be closely related to the degree of cognitive dysfunction. Also, blood oxygen level-dependent functional magnetic resonance imaging, which can measure the blood flow rate in the brain associated with specific cognitive functions to correlate and understand the extent of damage due to HIV infection in comparison with age-matched healthy individuals [6], could be a promising tool in the diagnosis and treatment of ANI.
Mild Neurocognitive Disorder (MND)
MND is defined as an impairment of at least two cognitive domains associated with learning, memory, or thinking with detectable impairment/aberration in motor skills and other daily life activities [7, 8]. Unlike ANI, MND involves mild to moderate anatomic changes associated with the affected cognitive domains, including substantial volumetric reductions in cortical and subcortical brain regions [9]. As mild to moderate neurocognitive disorder can even occur in HIV-negative individuals depending on their age, genetic factors, dementia, delirium, alcohol or drug abuse, or unattended infectious diseases, extremely cautious and stringent diagnostic approaches should be taken to confirm that MND is only attributable to HIV infection. Although still under debate, a growing body of evidence shows that early onset of MND increases the risk of developing HAD by several fold, specifically fueled by chronic HIV and persistent immunosuppression, in the later stages of HIV infection predominantly among individuals genetically predisposed to neurodegenerative diseases [10, 11].
HIV-Associated Dementia (HAD)
HAD, or AIDS dementia complex, is a late-onset neurological complication observed in nearly 30% of HIV-infected individuals and diagnosed based on changes in neuropsychological behaviors of the infected person [12, 13]. Continuing viral load, combined with immunosuppression and prolonged neurotoxicity of ART drugs, causes severe anatomic deformity in the brain architecture, leading to HAD. Although it is beyond the scope of this review, it is worth mentioning that secondary pathogenic infections in the CNS can lead to HAD in AIDS patients with longer lifespans following HAART. HAD symptoms and severity widely vary from patient to patient, depending most importantly on patient age at onset of the disease. HAD is primarily characterized by cognitive impairments involving short-term memory loss combined with lack of concentration, difficulty in learning new things, difficulty in finding words, confusion in following instructions, longer response time, inability to perform daily tasks, and/or abrupt psychological changes such as social withdrawal, personality changes,and depression. Advanced stages of HAD are associated with more severe systemic motor dysfunctions, such as movement disorder, muscle weakness, vision problems, speech problems,and balance disturbances. The severity and stage of dementia are usually assessed by the International HIV Dementia Scale (IHDS), for which a score of ≤10 out of 12 is considered the upper limit for further evaluation of possible dementia [14]. Despite the well-established IHDS screening methods routinely used for American and African populations, recent evaluations of the clinical utility of the IHDS, compared with the HIV Dementia Scale (HDS), reveal divergent outcomes. In one study among Spanish-speaking adults, the HDS showed more appropriate screening results and clinical utility in terms of time and cost compared with the IHDS[15]. Conversely,a separate study among German-speaking people recommended the IHDS as a useful screening tool [16], suggesting that the pattern of HAD symptoms and their severity could be linked to the genetic background and lifestyle of individual patients.
Due to the lack of predictive biomarkers and early physical diagnosis, HAD diagnosis largely relies on the exclusion of other diseases. Although earlier studies in primate models suggest the possibility of separating simian immunodeficiency viruses (SIV)/AIDS from SIV encephalitis by identifying novel metabolites using MRS scanning, its clinical application in human patients has not been tested [17]. However, recent advances in in vivo molecular and structural brain imaging techniques for patients with dementia, or dementia-associated diseases like Alzheimer’s disease (AD) or Parkinson’s disease (PD), offer a great opportunity to design precise therapeutic strategies to combat the disease from its early onset [18].
NEURO-MUSCULAR COMPLICATIONS IN HIV-ASSOCIATED SENSORY NEUROPATHY
HIV-associated sensory neuropathy (HIV-SN), which involves both myelinated and unmyelinated axons of distal nerves, is another common neurological complication in the majority of HIV-infected elderly people. HIV-SN is thought to be the outcome of chronic HIV-infection along with opportunistic secondary infections and/or antiretroviral drug toxicity. Peripheral sensory neuropathy can be categorized into four groups based on their possible origins: (1) Painful Sensory Neuropathy (PSN) includes distal sensory polyneuropathy and toxic neuropathy from antiretroviral drugs, with undistinguishable neuropathological symptoms; (2) Inflammatory demyelinating polyradiculoneuropathies (IDP) including both acute and chronic forms occur with high frequency at the early stage of HIV-infection, possibly due to autoimmune phenomena. Clinically and electrophysiologically,IDP sin HIV-infected people are indistinguishable than that of non-HIV people [19]; (3) Mononeuropathy multiplex can occur at both early stage due to immune dysfunction and advanced stage due to secondary opportunistic pathogens like cytomegalovirus,Varicella-Zostervirus,andhepatitis B or C virus in immunocompromised conditions [20, 21]; (4) Progressive polyradiculopathy causes due to progressive loss of sensory and motor neurons involving the lumbar and sacral roots, characterized by lumbosacral pain, saddle anesthesia, and impairment of urinary retention and rectal sphincter control as early signs. Cytomegalovirus and Varicella-Zoster virus are considered to be primary pathogens to develop this disease at the advanced stage of AIDS [22–24].
Among these different subtypes, PSN is the most commonly occurring form, affecting ~60% of HIV-infected population. The clinical symptoms of PSN includes reduced sharp sensation for vibration in the legs and feet, and delayed ankle reflexes in a distal symmetrical pattern. Pain symptoms are characterized by gradual onset of bilateral pain, tingling, and numbness with a burning or aching sensation. Pain generally starts in toes, gradually spreads to proximal regions, and worsens in soles. In advanced stages, pain spreads to hands.
Distal sensory polyneuropathy has been found to occur at the early stage of primary HIV infection due to high rate of activated macrophage, cytokines, and chemokines infiltration in the CNS resulting from systemic and nervous system immune responses [25]. Studies in the SIV-infected primate models have shown that primary HIV-infection induces sensory neuropathy by damaging dorsal root ganglia nerve fibers and intra-epidermal nerve fiber density [26, 27]. It has been found that sCD133 and regulated on activation normal T cell expressed and secreted (RANTES) can serve as potential biomarkers for SN. Additionally,CD137 signaling regulates trafficking of activated macrophage to dorsal root ganglia, leading to severe loss of intra-epidermal nerve fiber densit [28]. Recently, as a potential therapeutic approach, treatment with α4-integrin antibody has been found to rescue dorsal root ganglia fiber damage by block-ingthetraffickingofactivatedmonocyte/macrophage, but not T-lymphocyte [29]. Similar antibody treatment in another study has been found to decrease cardiac pathology by regulating monocyte/macrophage trafficking to the heart in SIV-infected primate model of HIV-AIDS [30]. CHARTER Group studies on a large cohort of HIV-infected people receiving cART have shown that ~50% of the patients suffer from PSN, causing physical disability and reduced quality of life despite successful cART treatment [31]. A cross-sectional deep profiling study revealed that patients with HIV-SN presented metabolic dysfunction (higher plasma triglyceride), lack of concentrations, depression and anxiety, higher pain catastrophizing scores, and insomnia compared to HIV participants without peripheral sensory neuropathy [32].
In this context, it is important to mention that although majority of the HIV-infected patients suffer from PSN, the extent of severity differs among different races and genders, even some HIV-infected patients showing resistance to PSN. This suggests the underlying roles of human genetic variations toward disease susceptibility. A study on large cohort of US women has revealed that HIV-PSN has been less prevalent in women than previously reported. They have also found that African-Americans are highly predisposed to HIV-PSN than other racial groups, and particularly patients with comorbid hepatitis C virus infection, older age and diabetes mellitus type 2, and metabolic syndrome in terms of higher triglyceride levels are at highest risk [33, 34]. In the context of genetic variations, mitochondrial DNA (mtDNA) undergoes spontaneous mutagenesis generating different mitochondrial haplogroups, leading to variable disease susceptibility across the races. It has been observed from the very beginning of the implementation of HAART treatment that the HAART receiving HIV-infected patients develop similar neuromuscular diseases like people with inherited mitochondrial dysfunction. Following that observation, it was discovered that the most common clinically effective nucleoside-analogue reverse transcriptase inhibitors (NRTI) used to block the activity of mitochondria specific DNA polymerase γ, required for mtDNA replication, led to mitochondrial dysfunction [35–37]. In recent years, the association between HIV-sensory neuropathy and mtDNA variation has further been established by the CHARTER Group Cohort Study, where they identified the two most significant single-nucleotide polymorphisms, namely A12810G and T489C corresponding to the African haplogroup L1c and European haplogroup J, respectively, with decreased occurrences of HIV-linked sensory neuropathy compared to all other haplogroups [38]. However, these incidences of PSN have been inevitable because of the widespread clinical applications of NRTIs. Recent findings have established a link between genetic variation in iron-metabolism and PSN in HIV +ve patients on cART treatment [39]. They have found that polymorphisms in transferrin, transferrin receptor, bone morphogenetic protein 6, aconitase 1, solute carrier family 11 member 2, and frataxin genes confer reduced risk; other variants of transferrin,aconitase 1, bone morphogenetic protein 6, beta-2-microglobulin, and ceruloplasmin genes confer increased risk to peripheral neuropathy.
HAND IN THE ERA OF cART
The introduction of cART in the 1990 s dramatically improved the clinical outcome of HIV-infected patients in terms of morbidity and mortality. cART significantly reduced the incidence of CNS-associated opportunistic infections in patients. Also, the prevalence of HAD, the most severe form of HAND, declined from 20% to 5% [40, 41]. However, progress toward eliminating HAND has been discouraging, as 20–50% of patients continue to suffer from a milder form of HAND, namely ANI and MND [40, 42]. In a cross-sectional study conducted in Switzerland, HAND was present in 84% of patients with evidence of cognitive decline and in 64% of patients without cognitive decline. In the first group of patients, 24% had ANI, 52% had MND, and 8% had HAD, whereas in the second group, 60% had ANI, 4% had MND, and 0% had HAD [43]. In another cross-sectional study performed by the CHARTER Group including 1,555 HIV patients, HAND was prevalent in 52% of patients, of which 33% had ANI, 12% had MND, and 2% had HAD [3]. These milder forms of HAND not only impact quality of life [44, 45] but also affect cART adherence [46]. There have been three characteristic changes in HAND in the cART era. First, as previously mentioned, HAND has become less severe, with ANI and MND becoming the predominant subtypes among patients [42, 47, 48]. Second, cortical deficits can develop in HAND patients, with executive function and learning ability being the most frequently affected domains [47–49]. The development of vascular cognitive impairment also poses a substantial concern, but the evidence for this is preliminary and largely obtained from neuroimaging studies [50–52]. Third, HAND can be associated with extrapyramidal features that overlap with PD [53, 54]. Emerging evidence shows that HAND shares some common features with other neurodegenerative diseases in terms of pathogenesis, including dysfunction in autophagy [55, 56] and the ubiquitin-proteasome system [57, 58].
ART-induced neurotoxicity
Various mechanisms have been implicated in the pathogenesis of HAND, including ongoing viral replication [59, 60], the presence of activated CD8-positive T cells in the brain [61–64], and indirect neurotoxicity caused by viral proteins such as Gp120 [65–71] and Tat [72–77]. As several ART drugs, including NRTIs and protease inhibitors (PIs), can attain therapeutically effective concentrations in the CNS, a decline in HAND should be expected. However, increasing therapeutically effective drug concentrations in the CNS by cART does not always result in improved cognition and, in fact, can increase the risk of HAND by more than 50% [78]. Emerging evidence now demonstrates direct neurotoxic effects of ART drugs. Nucleoside reverse transcriptase inhibitors have been reported to reduce neuronal axon length [79, 80] and mitochondrial DNA content [81, 82]. Recently, efavirenz which was found to promote amyloid-β (Aβ) production in vitro and in vivo [83], abrogate neural stem cell proliferation [84],and alter mitochondrial dynamics(i.e., increased mitochondrial depolarization, decreased mitochondrial DNA, and altered mitochondrial respiratory function) [81, 85, 86]. In addition, raltegravir was shown to enhance IL-8 production, providing further evidence for cART-mediated neurotoxicity [87]. ART drugs can also compromise the structural integrity of the corpus callosum [88] and worsen neurocognitive function in clinical [89] and experimental [90] models. Moreover, PIs (i.e., saquinavir, indinavir, and ritonavir), efavirenz, and zidovudine disrupt the blood-brain barrier (BBB) by decreasing the expression of tight junction proteins (TJPs), causing oxidative and endoplasmic reticulum (ER) stress as well as mitochondrial dysfunction [91–93]. The regulation of TJPs is controlled by signaling factors originating from endothelial cells and is also influenced by astrocytes and microglia. Dysfunction in the BBB and increased BBB permeability are hallmarks of several acute and chronic CNS pathologies, including HIV-infection [94]. ART drugs significantly diminish HIV burden but fail to restore damage to BBB integrity caused by HIV proteins. Therefore, it can be safely assumed that in the presence of ongoing HIV-infection, the neurotoxic effects of ART drugs on the BBB could be further worsened, as both HIV proteins and ART drugs induce oxidative stress, disrupt normal mitochondrial function, alter gene expression, and activate various cell signaling cascades [95]. The studies described above highlight the possible role of cART-induced neurotoxicity in HAND. Further stud-iesexaminingtheeffectsofARTdrugs, withorwithout HIV or the role of viral proteins in combination with ARTdrugs, may provide novel insights into the underlying mechanisms. The outcomes of these studies will be of vital importance to developing better HIV treatment approaches.
ROLE OF HIV-1 PROTEINS IN NEUROTOXICITY
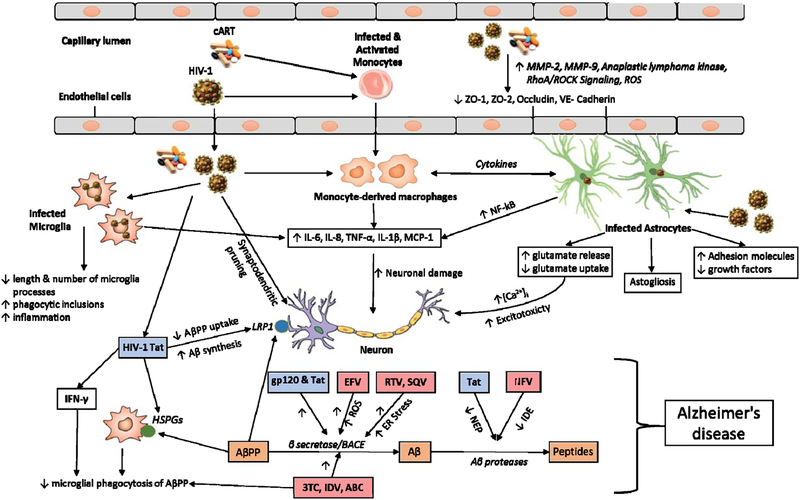
HIV crosses the BBB soon after infection, leading toCNS infection and eventually causing HAND.Neurotoxicity is mediated by the direct effects of the virus and/or viral particles on neurons and glial cells through the release of a myriad of toxic factors. Viral particles shed from the virus include Gp120, HIV-1 Tat, Nef, and Vpr. Fig. 1 provides an illustration of multifaceted nature of HIV mediated neurotoxicity.
Fig. 1.
Schematic overview of HIV-1 effects in CNS. The figure illustrates the mechanisms by which HIV-1, HIV-1 proteins, and cART mediate neurotoxicity; act on endothelial cells to decrease the expression of tight junction proteins; act on microglia to promote the release of various inflammatory factors and increasing phagocytic inclusions; act on monocyte derived macrophages and astrocytes to promote the release of various cytokines that act on neurons directly. HIV-1 infected astrocytes also promote neurotoxicity by decreasing the production of growth factors and altering the glutamate homeostasis. HIV-1 viral proteins (HIV-1 Tat and gp120) and cART promote the formation of Aβ by decreasing AβPP uptake or increasing the activity of BACE or decreasing the activity of NEP and IDE. HIV-1 viral proteins are indicated in blue boxes and various antiretroviral drugs are indicated in light pink boxes. Also included are cART, combination antiretroviral therapy; MMP, matrix metalloproteinase; ROS, reactive oxygen species; ZO, zona occludens; RhoA, Ras homolog gene family, member A; ROCK, Rho-associated protein kinase; IL, interleukin; TNF, tumor necrosis factor; MCP-1, monocyte chemotactic protein 1; AβPP, amyloid-β protein precursor; LRP1, low density lipoprotein receptor-related protein 1; Aβ, amyloid-β; IFN, interferon; HSPGs, heparan sulfate proteoglycans; BACE, beta-site amyloid precursor protein cleaving enzyme; ER, endoplasmic reticulum; EFV, Efavirenz; RTV, Ritonavir; SQV, Saquinavir; 3TC, Lamivudine; IDV, Indinavir; ABC, Abacavir; NFV, Nelfinavir; NEP, Neprilysin; IDE, Insulin degrading enzyme.
Role of HIV-1 Tat
HIV-Tat is a functional protein that is produced early during virus replication. Depending on the viral subtype, it consists of 86–101 amino acids. The cysteine-rich region of Tat is responsible for the transcriptional activity of the virus (reviewed in [96]). Tat is secreted from HIV-infected cells and acts on surrounding cells, affecting their function and causing toxicity. Various in vitro and in vivo studies show that Tat administration increases the apoptosis of neurons [97, 98]. Various mechanisms have been implicated in Tat-mediated neurotoxicity. Tat toxicity affects the integrity of the BBB by inducing oxidative stress and lowering the expression of various TJPs and adhesion proteins, including occludin, ZO-1, and VE-cadherin, in brain endothelial cells [99–101]. Disruption of BBB integrity occurs through activation of matrix metalloproteinase (MMP)-9 and the RhoA/ROCK signaling pathway, resulting in the buildup of various immune cells in the CNS. Tat acts on different cell types in the CNS to promote the release of various soluble mediators. Tat increases the expression of various pro-inflammatory cytokines such as IL-6, IL-8, TNF-α, IL-1β, and MCP-1 from astrocytes, microglia, and monocytes [102–106]. Tat-mediated induction of cytokines is mediated through activation of the NF-NF-κBB pathway and different MAPKs as well as elevated levels of intracellular calcium. Micereceiving stereotactic Tat injections exhibit shorter and fewer microglial processes than vehicle-treated mice. Furthermore, the number of dendritic spines is reduced in Tat-exposed mice [107]. Tat also reduces the number of synapses between hippocampal neurons, indicated by a lower number of post-synaptic density-95 clusters [108]. Administration of Tat increases the number of microglial phagocytic inclusions that contains axon terminals, dendritic spines, and post-synaptic densities, indicating increased proteolytic degradation [109].
Tat interacts with synaptosomes to induce oxidative stress, resulting in increased protein and lipid oxidation and decreased mitochondrial membrane potential [110]. Tat injection increases levels of malondialdehyde in the caudate-putamen of rat brains, indicating elevated oxidative stress [98]. Tat decreases levels of antioxidant enzymes, including glutathione peroxidase and superoxide dismutase, and administration of these antioxidant enzymes protects neurons against Tat-induced apoptosis [111, 112].
In an initial study by Cheng et al., Tat induced depolarization of human fetal neurons, indicating its direct excitatory effect [113]. Tat promotes toxicity by affecting the release of various neurotransmitters. Tat facilitates the exocytosis of excitatory neurotransmitters such as glutamate and NMDA and decreases the release of GABA, an inhibitory neurotransmitter, from the cortex and hippocampal neurons [114, 115]. Furthermore,Tat-mediated glutamate toxicity is exacerbated by phosphorylation of NMDA receptors. Tat also promotes the release of acetylcholine through the activation of metabotropic glutamate receptors and mobilization of intracellular calcium stores [116]. Tat potentiates the toxic effect of glutamate by impairing its reuptake by astrocytes [117].
Tat increases levels of intracellular calcium by interacting with NMDA receptors and promoting the release of inositol triphosphate-sensitive intracellular calcium stores [118–120]. Increased intracellular calcium overload promotes toxicity by inducing oxidative stress and producing pro-inflammatory cytokines such as TNF - α [121, 122].
Role of Gp120
Gp120 is expressed on the surface of HIV and facilitates its interaction with various surface receptors [123]. Free protein, shed from the virus, promotes neuronal toxicity by directly interacting with neurons and increasing the release of toxic mediators from surrounding immune cells. Gp120 induces neuronal apoptosis by increasing the expression of Fas ligand [124]. By interacting with neurons, Gp120 disrupts calcium homeostasis and causes significant intracellular calcium accumulation [125]. Furthermore, Gp120 acts as a partial agonist to CXC4 receptors expressed onCajal-Retzius neurons and increases their excitability via calcium-dependent chloride channels [126]. Activation of calcium channels by Gp120 also triggers the expression of various pro-inflammatory cytokines, including TNF-α and IL-6, through activation of the wnt5a/CaMKII pathway [127]. Up-regulation of IL-1β by Gp120 from glial cells induces the expression of ferritin heavy chain and mediates the phosphorylation of NMDA receptors in neurons, resulting in the loss of spines and subsequent neuronal death [128, 129]. Furthermore, Gp120 directly interacts with neurons and reduces neurite outgrowth [130].
Gp120-mediated production of cytokines, including IL-1β and TNF-α, from microglia and astrocytes, promotes glutamate-associated toxicity by increasing its release. Furthermore, glutamate release promotes calcium influx in astrocytes, and thereby decreases the expression of Na+-dependent glutamate/aspartate transporter, whichplaysanimportantroleinglutamate uptake [131].
Gp120 decreases permeability of the BBB by various mechanisms [132, 133]. It decreases the expression of various TJPs, including occludin, ZO-1,and ZO-2,and increases the migration of monocytes into the CNS[134,135]. Gp 120 induces the production of reactive oxygen species (ROS) through NADPH to upregulate the expression of MMP2 and MMP9 in endothelial cells [136, 137]. Gp120 also interacts with neurons to decrease mitochondrial movement, resulting in mitochondrial dysfunction [125, 138]. Furthermore, Gp120 increases the induction of ROS by neurons and glial cells by various mechanisms. It decreases the expression of various antioxidant enzymes such as SOD2, glutathione peroxidase, and glutathione synthase in neurons and glial cells [139, 140]. It increases the production of ROS in microglia by increasing the expression of voltage-gated potassium channels, which is reversed by pretreatment with curcumin [141,142]. It also increases ROS production in astrocytes by increasing the expression of CYP2E1 and activating NADPH oxidase enzymes [143].
Role of Nef
HIV Nef is an accessory viral protein found in post-mortem brains of HIV-infected patients [144, 145]. Nef is also expressed in astrocytes from post-mortem brains of SIV-infected macaques[146]. Mutations in the Nef open reading frame affect disease progression [147, 148]. The presence of Nef protein is favorable for HIV-infection of astrocytes [149]. Nef alone plays a role in the development of HAND by various mechanisms. Recombinant Nef decreases the viability of neurons and astrocytes and induces astrogliosis [150, 151]. Nef induces the production of various pro-inflammatory cytokines such as IL-6, IL-8, CCL5, TNF-α, and IFN- γ from astrocytes and microglia. Nef-mediated up-regulation of cytokines is promoted through the activation of MAPKs and calmodulin signaling pathways [152–155]. Nefserves as a chemotactic agent by promoting the infiltration of leucocytes and monocytes into the CNS [152, 156]. Nef also induces the production of quinolinic acid from macrophages, a neurotoxin that is detected in the brain and cerebrospinal fluid (CSF) of HIV-infected individuals [157, 158]. A study by Van Marle and colleagues showed that overexpression of brain-derived Nef in neurons promotes astrocytic cell death. Furthermore, supernatants from Nef-expressing astrocytic cultures induce the death of neurons mainly through the actions of IP-10 [159]. Nef expression alone can induce cognitive deficits in rat models, as rats that are injected with Nef-expressing astrocytes show impaired object and spatial recognition memory [160, 161].
Nef affects the transcriptional level of anaplastic lymphoma kinase, which activates MMPs that disrupt the integrity of the BBB[162]. Treatment of astrocytes with Nef increases the production of complement factor C3 and nitric oxide and induces lipid peroxidation [163]. Expression of Nef in astrocytes triggers autophagy and inhibits the fusion of autophagosomes to lysosomes [164]. Furthermore, by colocalizing with an important autophagic protein, Beclin 1, Nef affects autophagic maturation and protects HIV from degradation [165]. Nef is released from primary human fetal astrocytes in extracellular vesicles, and this release is increased by regulators that influence the autophagic pathway. Neurons treated with purified Nef-enriched extracellular vesicles exhibit symptoms of toxicity, as shown by a decrease in levels of the antioxidant enzyme glutathione and degeneration of neurites and axons [166].
Role of Vpr
Vpr is one of six auxiliary proteins produced by HIV and is detected in the serum and CSF of HIV-infected patients [167]. Vpr plays an important role in the pathogenesis of SIV in macaques (reviewed in [168]). It induces viral reactivation by promoting the degradation of histone deacetylase 1 and 3 and inducing the production of IL-6 [169, 170]. It promotes activation of HIV promoter by inducing the expression of hypoxia-inducible factor 1-α through increased production of ROS [171, 172]. Loss of Vpr decreases HIV antigen production by more than 1,000-fold in macrophages [173]. Vpr and its fragments permeabilize and result in the apoptosis of various CD4-positive and non-CD4-positive cell types [174, 175]. Vpr protein treatment induces apoptosis of human neurons by activating caspase-8 [176]. Furthermore, intracellular Vpr expression induces apoptosis in human neurons, indicating that both extracellular and intracellular Vpr are toxic to cells [177]. The region of Vpr between 70–96 amino acids is essential for inducing apoptosis of neurons [178]. Vpr elevates intracellular calcium concentration in neurons [179, 180]. Infection of monocytederivedmacrophageswithVpr-deletedHIVdecreases the production of pro-inflammatory cytokines, such as IL-1β, IL-8, and TNF-α, as compared with infection with wild-type HIV. Vpr alone induces the production of pro-inflammatory cytokines, such as IL-6, IL-8, MCP-1, and CCL5, from astrocytes and macrophages [169, 181, 182]. Induction of cytokines involves the activation of p38 MAPK, JNK MAPK, TLR4/MyD88, and NF-0κB signaling pathways [169, 183]. Infusion of Vpr-transfected astrocytes into the hippocampus impairs spatial and recognition memory in rats and induces morphological changes in neurons with reduced expression of synaptophysin [184]. Furthermore, supernatant from Vpr-deleted HIV results in decreased neurotoxicity compared with wild-type HIV [183]. Addition of extracellular Vpr to human astrocytes affects the glycolysis pathway and decreases production of ATP, resulting in an increased amount of oxidative stress. An elevation of oxidative stress is induced partly by decreased levels of the antioxidant enzyme glutathione [181]. Vpr affects the expression of miRNAs, particularly miR-34a, which alters the expression of various genes associated with neuronal dysfunction [179].
Role of Aβ
With the advent of cART, the lifespan of people infected with HIV-1 has significantly increased. According to Joint United Nations Programme on HIV and AIDS, there are 4.2 million people over the age of 50 who are living with HIV (http://www.unaids.org/sites/default/files/media_asset/12 Peoplea ged50yearsandolder.pdf). According to the Centers for Disease Control and Prevention, in the United States alone, the number of people over the age of 50 who were living with HIV increased by 42% by the end of 2013, whereas the number of new cases of HIV in 2014 decreased by 17% (https://www.cdc.gov/hiv/ group/age/olderamericans/). With the widespread reach of cART, the number of older patients with HIV is projected to increase in the future, which increases their risk of developing AD-like pathology. Post-mortem analysis of brain sections from HIV-infected people over 50 years of age showed an increase in the deposition of Aβplaques[185–187].
Furthermore, elevated hippocampal deposition of hyperphosphorylated Tau has been noted in HIV-infected patients on cART [188]. All accumulating evidence suggests a role of Aβ in the development of HAND in the HIV-infected population.
CONTRIBUTION OF HIV VIRAL PROTEINS AND CART TO THE DEVELOPMENT OF AD-LIKE PATHOLOGY
Increasing evidence suggests a role of HIV viral proteins in Aβ production and the development of HIV-associated neurotoxicity.
Role of HIV-1 Tat
Silver staining analysis of the frontal cortex in post-mortem brains of HIV-infected patients with HAD shows an increased presence of neuritic plaques, which is a common feature in AD patients [187]. Furthermore, immunostaining of SIV-infected monkeys shows elevated deposition of Aβ precursor protein (AβPP) in neurons in close proximity to cells stained forTat[189].Tat increases Aβ secretion in neuron cultures [187]. Injection of lentiviral Tat construct into the hippocampus of mice results in elevated levels of Aβ production and increases the size and number of Aβ plaques [190]. Moreover, Tat further increases the burden of Aβ in AβPP/PS1 mice crossed with HIV-1Tattransgenicmice[191].Severalmechanisms are implicated in the Tat-mediated increased burden of Aβ. Tat binds to lipoprotein receptor protein 1 (LRP1) on the surface of neurons, which inhibits the uptake of AβPP (a precursor molecule for the generation of Aβ) and results in an elevated burden of Aβ. LRP1 plays a critical role in the clearance of Aβ, and binding of Tat to LRP1 decreases its clearance [189, 192]. Tat also binds to heparan sulfate proteoglycan receptors on the microglia surface and decreases the transfer of Aβ to LRP1, thereby inhibiting phagocytosis [193]. Tat enhances Aβ burden by promoting the accumulation of AβPP into lipid rafts [190]. Tat and peptides derived from Tat decrease the activity of neprisylin, a major Aβ-degrading enzyme [187, 194].
Tat also promotes the production of proinflammatory cytokines, such as IFN-γ, which act together to decrease the microglial uptake of Aβ. By interacting with endolysosomes, Tat increases their pH and the activity of beta secretase (BACE1), thereby increasing the accumulation of Aβ [195]. Tat further increases the activity of BACE1 by promoting the release of glutamate [196]. Tat-mediated toxicity is potentiated by the production of Aβ [197, 198]. Caffeine has been shown to be a therapeutic intervention that overcomes Tat-mediated endosomal dysfunction [199].
Role of Gp120
The addition of Gp120 to hippocampal cell cultures and brain microvascular endothelial cell cultures increases the production of Aβ in cell culture supernatants [200, 201]. Zhang et al. (2011) showed that Gp120 treatment of rat brain slices increases the accumulation of AβPP in the corpus callosum. Furthermore, they showed that the Gp120-mediated increase in AβPP accumulation was decreased by the addition of CXCR4 antagonist but not NMDA receptor antagonist, implying an important role of CXCR4 [202]. Generation of Gp120/AβPP/PS1 triple-knockout mice revealed an increase in the number and size of Aβ deposits in the hippocampus and cortex. These mice also showed elevated levels of lipofuscin, a characteristic protein associated with decreased lysosomal function, and enlarged Aβ accumulated lysosomes. Furthermore, experiments with these mice also showed that Gp120 alters the trafficking of Aβ distribution in neurons and increases the activities of BACE1 and γ-secretase,which affects Aβ clearance [203]. Gp120-mediated deposition and transport of Aβ are attenuated by an α7 nicotinic acetylcholine receptor antagonist [201].
A recent study by Khan et al. showed that exosomes containing Nef mRNA, isolated from patients with HAD, exhibit increased production and secretion of Aβ when added to a SH-SY5Y neuroblastoma cell line [204].
Role of ART drugs in AD progression
Different ART drugs have distinct capabilities of BBB penetration as demonstrated by CNS penetration-effectiveness scores. Patients on cART with higher CNS penetration-effectiveness scores are expected to benefit from a higher bioavailability of these drugs in the brain, resulting in better control of HIV replication. However, several reports show that there is lack of substantial neurocognitive improvement with cART. Evidence also indicates a shifting pattern of neurocognitive impairment in HIV patients, from deficits in motor ability, speed of information processing, and verbal speed in the pre-cART era to deficits in memory and executive function in the post-cART era [47]. There are several potential explanations for this clinically meaningful finding. One is ART-induced neurotoxicity, which is now known to result in part by dysregulated A[H9252]PP processing and subsequent deposition of Aβ plaques in the brain. Based on neuropathological data [11, 205], genetic screening [206, 207], and CSF markers [208], researchers have raised the possibility that AD may be becoming more common in HIV patients on cART. Although the exact mechanisms through which cART contributes to Aβ deposition in the brain are unknown, neurotoxic effects of ART drugs such as mitochondrial dysfunction [81, 91, 209], increased oxidative and ER stress [82, 92, 93, 210, 211], and neuronal damage and synaptic loss [212, 213] are now linked to altered AβPP processing and Aβ deposition in the brains of HIV patients.
In the first study of its kind, Giunta and colleagues [214] examined the effect of various ART drugs on neuronal Aβ production and clearance by microglial phagocytosis. ART drugs, especially in combination, were observed to increase the synthesis of Aβ1–40,42 from SweAβPP N2a neurons (i.e., murine N2a cells transfected with the human ‘Swedish’ mutant form of AβPP) and decrease the clearance of Aβ1–42 peptides by preventing their phagocytosis by N9 microglial cells. The combination of lamivudine, indinavir, and abacavir was found to have the most significant amyloidogenic effects, with indinavir and abacavir also having additive damaging effects on Aβ microglial clearance. In another study, Lan et al. [215] examined the effect of several PIs on the clearance of Aβ42 in macrophages. They observed that ritonavir, saquinavir, and atazanavir slightly suppressed Aβ42 clearance, whereas lopinavir, nelfinavir, and ritonavir increased the number of undegraded Aβ peptides. Additionally, Lan et al. found that all aforementioned PIs except atazanavir reduced Aβ40 synthesis in neurons through the inhibition of BACE1 and γ-secretase. However, these PIs slightly suppressed purified BACE1 enzyme activity in in vitro studies, indicating that ART drugs may have an indirect action on BACE1 activity in neurons. The authors also studied the effect of nelfinavir and lopinavir/ritonavir on Aβ production in immunodeficient AβPP SCID mice (with a double-mutant form of AβPP695 (KM670/671NL + V717F) in homozygosity for the SCID allele of Prkdc). Although nelfinavir achieved a significant concentration in the mouse brain, no changes in Aβ accumulation were observed. In general, PIs do not readily cross the BBB because of high levels of protein binding. Therefore, the interpretation of these effects of PIs on amyloidosis in the brain should consider their low infiltration into the CNS.
In another study, Brown et al. [83] found that efavirenz, in combination with lamivudine and zidovudine, induces mitochondrial dysfunction as evidenced by reduced cellular ATP stores, diminished mitochondrial membrane potential, and enhanced release of ROS in SweAβPP N2a neurons. Also, efavirenz increases Aβ production via BACE1 activation in vitro and in vivo. Combination treatment also inhibits microglial phagocytosis of Aβ1–42. The authors propose that an efavirenz-induced high ROS microenvironment in the CNS of HIV patients promotes BACE1-mediated AβPP processing and also inhibits phagocytic clearance, leading to the production of Aβ. In a very recent study [211], ritonavir and saquinavir were found to induce the expression of classical ER stress markers BiP, p-eIF2α, and XBP-1 in neuroglial cultures, leading to activation of the unfolded protein response. Induction of ER stress is associated with PERK-dependent BACE1 activation. PIs also increase Aβ in multiple cell types, which is dose-dependently blocked by BACE1 inhibitor. These findings were also validated in macaques and rodents that received chronic PI treatment.
Disruption of the BBB by ongoing HIV infection and ART drugs can also decrease the brain-to-blood clearance of Aβ, leading to its accumulation [216]. A balance between two transporters, (LRP1, which transports Aβ from brain to blood) and the receptor for advanced glycation end products (RAGE, which transports Aβ into the brain), have been found to regulate levels of Aβ in the brain [217]. LRP shares a 63% homology with the catalytic region of HIV-1 pro-tease[218]. Inonestudy,PIswerereportedtodecrease LRP levels in HepG2 cells [219]. LRP mediates the endocytosis and degradation of AβPP. Therefore, decreasing LRP levels by PIs could increase Aβ accumulation in the brain. Further conclusive studies are needed to delineate the effects of ART drugs on LRP1 and RAGE and their role in Aβ accumulation in the brain.
Accumulating evidence shows that Aβ proteases, such as neprilysin (NEP), insulin-degrading enzyme (IDE), and endothelin-converting enzymes (ECE) 1 and 2, play an important role in regulating the level of A[H9252]peptides in the brain. IDE degrades Aβ in neuronal and microglial cell cultures[220–223].Similarly,NEP and ECE degrade Aβ in vivo [224, 225]. In a study by Hamel et al. [226], nelfinavir was found to inhibit IDE in vitro. Hypofunction of IDE by PIs might inhibit Aβ degradation, promoting its accumulation in the brain. However, more studies are needed to gather conclusiveevidencefortheroleofART-mediatedproteolytic activity in the development of amyloidogenesis.
OTHER NEURODEGENERATIVE DISEASES LINKED TO HIV INFECTION
Parkinson’s disease
PD is primarily characterized by the loss of pigmented dopaminergic neurons from the midbrain substantia nigra pars compacta (SNpc) region due to the formation of α-synuclein inclusion bodies (also called Lewy bodies). However, there are occurrences of PD without Lewy bodies called secondary Parkinsonism that are thought to involve viral infections. A large subset of HIV-infected patients present akinetic Parkinsonian syndromes at the early stage of disease onset, but PD-like syndromes are mostly reversed by HAART depending on the age and genetic predisposition of the patient [227, 228]. Patients who survive beyond 50 years of age most often exhibit accelerated degradation of neural networks compared with age-matched controls, mostly in the basal ganglia and hippocampal regions, due to the synergistic effects of immune-senescence and sustained viral load, despite beingundercontrol[229].Multiplestudies,inpatients and animal models, show that dopaminergic neurons are the most vulnerable to HIV-infection. Interestingly, increased levels of α-synuclein are found in the substantia nigra of HIV-infected patients compared with that of normal age-matched controls[229],which is one major cause of the depletion of dopaminergic neurons in α-synuclein gene triplication-associated PD. In a separate study, Riederer and colleagues showed in a primate model that HIV infection can decrease dopamine levels by at least 44% within only 2 months of infection, which is consistent with observations of HIV-infected patients [230]. This reduction in the dopamine level could be attributed to aproportional decrease in the level of dopamine transporter, which is further consistent with the lower neuropsychological performance of HIV-infected patients [231, 232]. In this context, it is important to mention that not only HIV, but also several other viruses, cause neurological complications[233].For example,infection of rats with Japanese encephalitis B virus, the most common encephalitis-causing agent in Asia, induces marked gliosis in the SNpc similar to that observed in PD, and behavioral studies show that bradykinesia is recovered by treatment with L-DOPA and MAO inhibitor, suggesting that the virus induces typical symptoms of PD in infected animals [234]. Furthermore, other evidence that viruses can cause PD-linked pathology comes from a risk assessment of people living in “Parkinsonian clusters” who share common lifestyles and environments and who exhibit at least two times more disease susceptibility than people living outside the cluster [235, 236].
Amyotrophic Lateral Sclerosis (ALS)
Although classical ALS-like symptoms largely differ from HIV-associated ALS-related neurological symptoms, the viral etiology of ALS could be a significant concern due to the selective vulnerability of motor neurons to certain viruses, including HIV. The expression of human endogenous retroviral (HERV)-linked sequences in motor neurons in the CNS dramatically increases in ALS patients [237], suggesting an undetermined link between viral infection and motor neurodegenerative disease. Transactive response DNA binding protein of 43 Kd (TDP-43), which was earlier discovered as a regulator of HIV infection in humans, also contributes to a major ALS disease subtype called ALS-TDP-43. Interestingly, recent findings by Nath and colleagues show that expression of HERV-K or its envelope protein is controlled by TDP-43, leading to neurodegeneration and muscular atrophy [238]. After the first discovery of human retrovirus T-lymphotrophic virus-1 in postmortem brain tissue of two ALS patients in Guam in the 1970 s [239], exhaustive survey and medical evidence collected to date shows that only a small subset of retrovirus-infected patients develop symptoms like those in ALS. However, at the same time, it is critical to note that neuropathological symptoms in patients infected with different types of retroviruses exhibit distinct patterns of disease phenotypes similar to classical ALS phenotypes. Particularly, HIV-infected patients with a median age of ~40 years exhibit neuropatho-logical symptoms very similar to those of classical ALS patients with a median age of ~55 years for sporadic disease onset, but their recovery is quite common following ART, unlike the invariable deterioration in sporadic ALS. HIV-associated ALS can also develop at any stage of HIV disease progression, with the majority of patients affected by both upper and lower motor neurodegenerative disorder. However,inmostcases, following ART, even a considerably improved immunosuppression condition and almost undetectable viral load may cause mild neuropathological symptoms that are mostly ignored at the beginning. Because the expression of viral infectious proteins depends on the expression of reverse transcriptases in the human body, the level of reverse transcriptases in body fluids primarily serves as a confirmatory indicator of viral load in the body. Based on earlier studies, patients with HIV or other retrovirus-related neurological complications show serum reverse transcriptase activity levels 53% higher than those of non-HIV control patients.
RELATIONSHIP BETWEEN BRAIN STRUCTURE ALTERATIONS AND AGING IN HIV INFECTION
Although brain aging is associated with mild volu-metric alterations, comparative analysis of total brain volume between aging HIV-positive and HIV-negative individuals may not be useful for advanced diagnosis, as cognitive or neuropsychological deficits are mainly related to microstructural alterations in specific brain regions. Instead, because of fluid-filled spaces in the brain,imaging the diffusion or movement of molecular water could increase our understanding of brain degeneration. Unlike gray matter (GM), diffusion of water molecules is more directional (i.e., anisotropic) in white matter (WM) due to its compact microstructure consisting of axonal cell membranes, myelin sheaths, and neurofilaments. MND or dementia is most frequently associated with WM abnormalities in elderly people. Despite successful cART/HAART treatment in HIV-positive patients, they continue to have persistent untraceable viral load in the CNS, resulting in WM abnormalities and neuro-inflammation, and thus leading to mild to severe cognitive impairment and brain atrophy at older ages [240, 241]. Multiple studies show a significant interaction between age and HIV infection, as HIV-infected patients frequently exhibit a greater extent of structural deformation in cognitive regions of the brain with aging, including abnormalities in whole-brain WM hyperintensities and fronto-subcortical WM integrity, compared with age-matched HIV-negative individuals [242–245]. Diffusion tensor imaging (DTI) revealed the marked degeneration of WM microstructures, expressed as large differences between fractional anisotropy and mean diffusivity, that is highest in the corpus callosum and projection fibers of the corona radiata in HIV-positive elderly patients compared with age-matched controls, which is consistent with earlier studies suggesting that WM-associated microstructural alterations lead to severe dementia and motor dysfunction [246, 247]. Interestingly, recent DTI studies reveal that loss of WM integrity correlates with duration of HIV infection. Chronic HIV-infected patients present both the loss of WM integrity as well as disruption of the BBB. On the other hand, HIV-negative and early HIV-infected (≤1 year of viral exposure) patients exhibit only disruption of the BBB and no significant alterations in WM integrity [248]. Moreover, aging HIV-infected patients exhibit greater WM hyper-intensities and lower fractional anisotropy in the anterior corona radiata due to hepatitis C virus coinfection, the more likely development of AIDS, and higher CD4-positive cell counts as a marker of hyper-activation of inflammatory responses [245, 249].
However, the normalization of DTI diffusivity parameters within specific regions of the corpus callosum and centrum semiovale is possible in HIV-positive patients who receive long-term cART or merely initiate treatment [250]. This same study suggests that the initial modulation in DTI parameters could be due to alterations in inflammatory responses rather than cognitive performance, as neuropsycho-logical testing reveals similar scoring before and after receiving cART regimens. However, to better understand the underlying pathogenesis, DTI scanning of the corpus callosum should be performed. Interestingly, the Hawaii Aging with HIV Cohort Study revealedthatWMhyper-intensitiesareassociatedwith reduced frontal GM volume in aging HIV-infected patients, suggesting that the frontal lobes may be more susceptible to small vessel ischemic vascular disease [251]. With CD4-positive cell count acting as an index for immune suppression or activation in HIV-infected patients, a CHARTER group study showed that extent of CD4-positive T-cell recovery is associated with increased abnormal WM and sub-cortical GM volumes, suggesting the involvement of neuro-inflammation in CNS pathogenesis [252]. As supported by other studies, an increase in subcortical GM volume could be attributed to a proportional increase in tissue water content and/or infiltration of inflammatory cells [253, 254]. These alterations in brain structure might be the outcome of diverse inflammatory responses from recovering immune systems across the brain. On rare occasions, severe immune reconstitution inflammatory syndrome can influence WM abnormalities in the CNS in settings of secondary opportunistic infections [255, 256]. Moreover, even with successful cART treatment of HIV-infected patients, neuropathological evidence of inflammatory responses has been observed in the basal ganglia [257].
Substance abuse is a serious threat to brain functions related to cognitive performance, which significantly reduces quality of life [258–263]. Substance abusers, particularly psychostimulant users, are most susceptible to infectious diseases like HIV acquired by intravenous drug abuse and unsafe sexual practices [264, 265]. Recent findings show that frontal WM and the corpus callosum are the most vulnerable brain regions to psychostimulant abuse as measured by DTI diffusivity parameters, leading to drastic cognitive impairment [266].
Another common type of substance abuse is alcoholism. A growing body of evidence indicates that long-term alcohol consumption causes significant metabolic dysfunction and cognitive impairment in middle-aged to older people. Chemical shift imaging analysis of brain metabolites reveals that levels of NAA, a marker of living neurons, decrease in alcoholics or short-term abstinent alcoholics, whereas NAA reaches normal levels in long-term abstinent alcoholics [267]. Notably, individuals with comorbid alcoholism and HIV infection show a drastic decrease in NAA levels in the parietal-occipital cortex,whereas neither HIV infection nor alcoholism alone exerts the same magnitude of effect, suggesting that alcoholics with comorbid HIV infection have a higher risk of neuronal compromise [268, 269].
DNA DAMAGE AND HIV-1 INFECTION: A CAUSAL CONNECTION?
HIV-infected patients develop multiple types of cancerous neoplasms depending on co-infection by secondary viral agents in the context of AIDS. However, non-AIDS HIV-patients also have a higher incidence of cancers, indicating that HIV has the potential to induce cancerous lesions before the development of AIDS. Multiple studies provide mechanistic insights into how viral agents interfere with host cell cycle signaling pathways and induce genomic instabilities that facilitate incorporation of viral gene elements into the host genome primarily through Vpr and integrase. Vpr is capable of inducing DNA strand breaks via both direct and indirect mechanisms. Vpr itself can bind to chromosomal DNA, inducing double-strand breaks (DSBs) by recruiting yet unknown nuclear factors with endonuclease activity, possibly through its carboxy-terminal domain [270]. Previously, it was assumed that Vpr interacts with the SLX4 protein complex to recruit structure-specific endonucleases for inducing the DNA damage response (DDR) in infected cells. However, a recent study shows that HIV-1, HIV-2, and other Vpr orthologs have inherent abilities to activate the DDR and cell cycle arrest without interacting with the SLX4 complex [271]. On the other hand, Vpr can interfere with the cell cycle by arresting infected cells at G2-phase by activating ataxia-telangiectasia-mutated and Rad3-related kinase (ATR)-Chk1-Wee1 DNA damagesignaling[272–274]. Vpr modulates this signaling pathway by activating ataxia-telangiectasia-mutated (ATM)to induce phosphorylation of Chk2 and histone H2A.X. Homologous recombination (HR)-mediated DNA repair machinery is involved in the integration of viral genomic elements into the host genome, which is marked by the foci formation of HR-related proteins breast cancer susceptibility protein 1 (BRCA1) and Rad51 [272, 275]. Taken together, these results show that the ATM-mediated DDR pathway and HR play crucial roles in introducing a higher viral copy number into the host genome. This is further supported by evidence that treatment with caffeine (an inhibitor of both ATR and ATM) or the ATM inhibitor KU55933 significantly decreases viral copy number in infected cells [275, 276].
Integrase (Int), a 32Kd HIV protein, promotes the integration of reverse-transcribed double-strand DNA into the host genome [277]. During the transfer of viral DNA elements, the catalytic activity of Int, depending on the presence of the D,D(35)E motif in the central domain, generates two-nucleotide gaps in a single strand, which are presumably repaired by host repair machinery [277–279]. The involvement of different repair pathways has been proposed to repair single-strand gaps for efficient viral transduction. However, Daniel and colleagues suggest that the efficient repair of gaps by the post-integration repair mechanism, which geometrically differs from conventional DSB repair, prevents Int-dependent apoptosis through the association of DSB sensor protein Nijmegen breakage syndrome 1 protein (NBS1) and ATM kinase [280]. Surprisingly, this kind of repair recruits ATR independently of NBS1 and ATM, suggesting a distinct DSB repair pathway separate from conventional DSB repair as a potential therapeutic target [281]. However, HIV DNA can be integrated in the presence of DSB-inducing agents, including Vpr, in an Int-independent manner in Int catalysis-defective virus. Also, this virus has been shown to be resistant against raltegravir, an inhibitor of Int catalytic activity. These findings provide crucial insights into possible underlying mechanisms of uninterrupted viral replication in monocyte-derived macrophages, a persistent reservoir of viral particles [282].
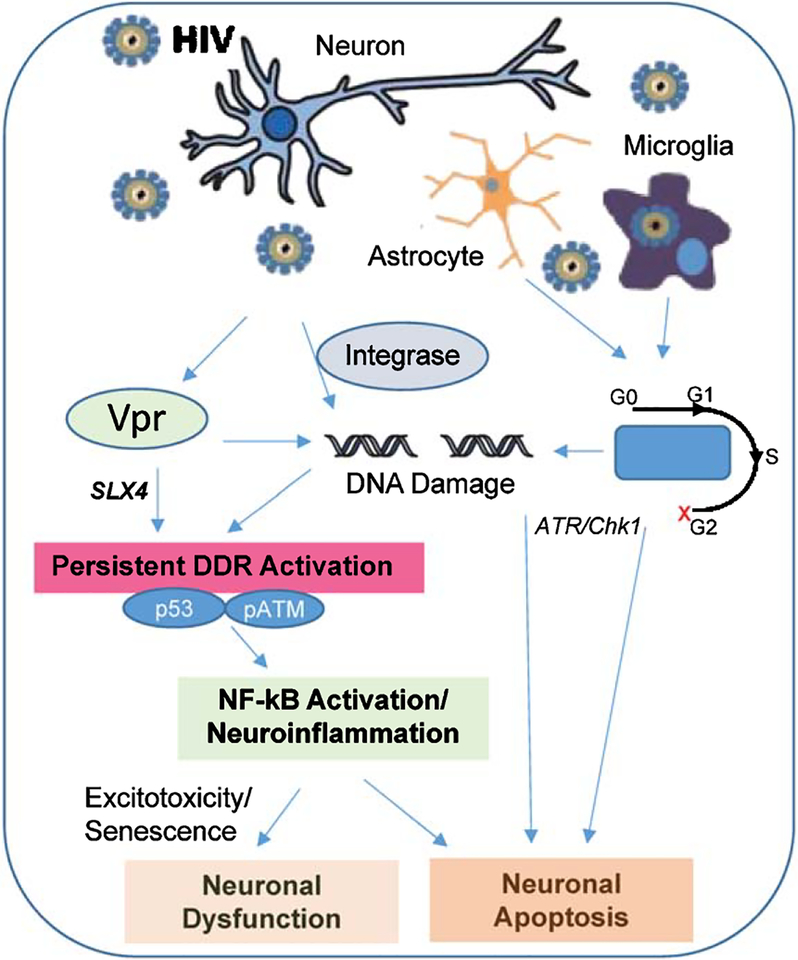
Sakurai et al. showed that both HR and nonhomologous end joining proteins are involved in the process of viral DNA integration into the host genome for efficient transduction [283]. Also, several other studies report controversial roles of DSB sensor proteins, such as poly(ADP-ribose) polymerase 1, ATR, ATM, and DNA-dependent protein kinase, in retroviral host integration [284–287], warranting further investigation into the mechanistic aspects of viral DNA integration to develop potential therapeutic strategies (Fig. 2).
Fig.2.
Schematicpresentation of HIV-associatedCNSdysfunction via genome damage. The major CNS cell populations, neurons, astrocytes and microglia, are differentially affected by HIV factors, but may involve genome damage signaling. HIV-secreted Vpr protein can cause DNA strand breaks by directly binding to the chromosome along with other endonuclease partners. Vpr can also lead to constitutive DDR (p53 and ATM) activation through SLX4, which leads to NF-kB-activation, leading to neuroinflammation and which could contribute to neuronal dysfunction and apoptosis. Another HIV protein Integrase also causes neural loss by apoptosis viaitsinteractionwithNBS1andATM.Furthermore,Vprinterferes with cell cycle in replicating pool of brain cells including neural stem cells and cycling glia arresting them at G2-phase of cell cycle, finally leading to cell death by ATR/Chk1 activation in the infected brain cells.
CONCLUDING REMARKS
Diverse neurological complications are routinely associated with HIV-infection. As comprehensively described in this review, several mechanisms could cumulatively contribute to the neurotoxicity of HIV, which is also affected by factors such as aging and nature of HIV treatment regime. These complications include dementia, sensory dysfunction, motor function deficits, and overlap with other neurodegenerative diseases like PD, ALS, and AD. The treatment of HIV-associated neuropathy remains difficult, due to a multitude of challenges including a complex diagnosis that largely depends on clinical symptoms without the availability of a definitive biomarker. The success of treating neurocognitive impairment in aged HIV-patients relies on the early detection of the CNS dysfunctions, which most often remains undetectable due to their mild nature in the beginning. The rate of progression of motor and cognitive dysfunctions like dementia largely depends on the patient’s age and genetic make-up. The viral neurotoxins can also induce promote proteinopathies including TDP-43, α-synuclein and tau in an aggressive presentation. Thus, the cognitive test standards to clearly distinguish between age-related and viral toxin-induced neuronal dysfunction is critical to diagnose and prevent cART or HAART- associated neurological complications in the elderly patients. Further research is required to elucidate the signature profile of HIV-associated neurological manifestation in different populations and to understand the molecular insight in to the specific as well as generic crosstalk of HIV and CNS cells, which would allow for development of mechanism-based treatment strategies.
ACKNOWLEDGMENTS
The research in authors’ laboratories is supported by USPHS grants R01DA025528, DA025011 and AA020806 (AK) and R01 NS088645, Muscular Dystrophy Association, Melo Foundation and Houston Methodist (MLH).
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/17-0473r1).
REFERENCES
- [1].Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE (2007) Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69, 1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Grant I, Franklin DR Jr, Deutsch R, Woods SP, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Collier AC, Marra CM, Clifford DB, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Smith DM, Heaton RK; CHARTER Group (2014) Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology 82, 2055–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Heaton R, Clifford D, Franklin DR Jr, Woods S, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA,Abramson I,Gamst A,Fennema-Notestine C, Jernigan TL, Wong J, Grant I; CHARTER Group (2010) HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75, 2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cohen RA, Boland R, Paul R, Tashima KT, Schoenbaum EE, Celentano DD, Schuman P, Smith DK, Carpenter CC (2001) Neurocognitive performance enhanced by highly active antiretroviral therapy in HIV-infected women. AIDS 15, 341–345. [DOI] [PubMed] [Google Scholar]
- [5].Habib AG, Yakasai AM, Owolabi LF, Ibrahim A, Habib ZG, Gudaji M, Karaye KM, Ibrahim DA, Nashabaru I (2013) Neurocognitive impairment in HIV-1-infected adults in Sub-Saharan Africa: A systematic review and meta-analysis. Int J Infect Dis 17, e820–e831. [DOI] [PubMed] [Google Scholar]
- [6].Ances BM, Hammoud DA (2014) Neuroimaging of HIV-associated neurocognitive disorders (HAND). Curr Opin HIV AIDS 9, 545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wilson TW, Proskovec AL, Heinrichs-Graham E, O’Neill J, Robertson KR, Fox HS, Swindells S (2017) Aberrant neuronal dynamics during working memory operations in the aging HIV-infected brain. Sci Rep 7, 41568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ernst T, Chang L, Arnold S (2003) Increased glial metabolites predict increased working memory network activation in HIV brain injury. Neuroimage 19, 1686–1693. [DOI] [PubMed] [Google Scholar]
- [9].Sanford R, Fernandez Cruz AL, Scott SC, Mayo NE, Fellows LK,Ances BM,Collins DL (2017) Regionally specific brain volumetric and cortical thickness changes in HIV-infected patients in the HAART era. JAcquir Immune Defic Syndr 74, 563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cysique LA,Hewitt T,Croitoru-Lamoury J,Taddei K,Mar-tins RN, Chew CS, Davies NN, Price P, Brew BJ (2015) APOE e4 moderates abnormal CSF-abeta-42 levels, while neurocognitive impairment is associated with abnormal CSF tau levels in HIV+ individuals - a cross-sectional observational study. BMC Neurol 15, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Soontornniyomkij V, Moore DJ, Gouaux B, Soontornniyomkij B,Tatro ET,Umlauf A,Masliah E,Levine AJ,Singer EJ, Vinters HV, Gelman BB, Morgello S, Cherner M, Grant I, Achim CL (2012) Cerebral β-amyloid deposition predicts HIV-associated neurocognitive disorders in APOE e4 carriers. AIDS 26, 2327–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Janssen RS, Nwanyanwu OC, Selik RM, Stehr-Green JK (1992) Epidemiology of human immunodeficiency virus encephalopathy in the United States. Neurology 42, 1472–1476. [DOI] [PubMed] [Google Scholar]
- [13].Reger M, Welsh R, Razani J, Martin DJ, Boone KB (2002) A meta-analysis of the neuropsychological sequelae of HIV infection. J Int Neuropsychol Soc 8, 410–424. [DOI] [PubMed] [Google Scholar]
- [14].Sacktor NC, Wong M, Nakasujja N, Skolasky RL, Selnes OA, Musisi S, Robertson K, McArthur JC, Ronald A, Katabira E (2005) The International HIV Dementia Scale: A new rapid screening test for HIV dementia. AIDS 19, 1367–1374. [PubMed] [Google Scholar]
- [15].Lopez E, Steiner AJ, Smith K, Thaler NS, Hardy DJ, Levine AJ, Al-Kharafi HT, Yamakawa C, Goodkin K (2016) Diagnostic utility of the HIV dementia scale and the international HIV dementia scale in screening for HIV-associated neurocognitive disorders among Spanish-speaking adults Appl Neuropsychol Adult. doi: 10.1080/23279095.2016.1214835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Marin-Webb V, Jessen H, Kopp U, Jessen AB, Hahn K (2016) Validation of the International HIV Dementia Scale as a screening tool for HIV-associated neurocognitive disorders in a German-speaking HIV outpatient clinic. PLoS One 11, e0168225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lentz MR, Lee V, Westmoreland SV, Ratai EM, Halpern EF,González RG (2008) Factor analysis reveals differences in brain metabolism in macaques with SIV/AIDS and those with SIV-induced encephalitis. NMR Biomed 21, 878–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Narayanan L, Murray AD (2016) What can imaging tell us about cognitive impairment and dementia? World J Radiol 8, 240–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cornblath DR, McArthur JC, Kennedy PG, Witte AS, Griffin JW (1987) Inflammatory demyelinating peripheral neuropathies associated with human T-cell lymphotropic virus type III infection. Ann Neurol 21, 32–40. [DOI] [PubMed] [Google Scholar]
- [20].Said G, Lacroix C, Chemouilli P, Goulon-Goeau C, Roullet E, Penaud D, de Broucker T, Meduri G, Vincent D, Torchet M, Vittcoq D, Leport C, Vildé JL (1991) Cytomegalovirus neuropathy in acquired immunodeficiency syndrome: A clinical and pathological study. Ann Neurol 29, 139–146. [DOI] [PubMed] [Google Scholar]
- [21].Caniatti LM, Tugnoli V, Eleopra R, Tralli G, Bassi R, De Grandis D (1996) Cryoglobulinemic neuropathy related to hepatitis C virus infection. Clinical, laboratory and neurophysiological study. J Peripher Nerv Syst 1, 131–138. [PubMed] [Google Scholar]
- [22].Eidelberg D, Sotrel A, Vogel H, Walker P, Kleefield J, Crumpacker CS 3rd (1986) Progressive polyradiculopathy in acquired immune deficiency syndrome. Neurology 36, 912–916. [DOI] [PubMed] [Google Scholar]
- [23].So YT, Olney RK (1994) Acute lumbosacral polyradiculopathy in acquired immunodeficiency syndrome: Experience in 23 patients. Ann Neurol 35, 53–58. [DOI] [PubMed] [Google Scholar]
- [24].Miller RF, Fox JD, Thomas P, Waite JC, Sharvell Y, Gazzard BG, Harrison MJ, Brink NS (1996) Acute lumbosacral polyradiculopathy due to cytomegalovirus in advanced HIV disease: CSF findings in 17 patients. J Neurol Neurosurg Psychiatry 61, 456–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang SX, Ho EL, Grill M, Lee E, Peterson J, Robertson K, Fuchs D, Sinclair E, Price RW, Spudich S (2014) Peripheral neuropathy in primary HIV infection associates with systemic and central nervous system immune activation. J Acquir Immune Defic Syndr 66, 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Burdo TH, Orzechowski K, Knight HL, Miller AD, Williams K (2012) Dorsal root ganglia damage in SIV-infected rhesus macaques: An animal model of HIV-induced sensory neuropathy. Am J Pathol 180, 1362–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lakritz JR,Bodair A,Shah N,O’Donnell R,Polydefkis MJ, Miller AD, Burdo TH (2015) Monocyte traffic, dorsal root ganglion histopathology, and loss of intraepidermal nerve fiber density in SIV peripheral neuropathy. Am J Pathol 185, 1912–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lakritz JR,Robinson JA,Polydefkis MJ,Miller AD, Burdo TH (2015) Loss of intraepidermal nerve fiber density during SIV peripheral neuropathy is mediated by monocyte activation and elevated monocyte chemotactic proteins. J Neuroinflammation 12, 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lakritz JR, Thibault DM, Robinson JA, Campbell JH, Miller AD, Williams KC, Burdo TH (2016) α4-integrin antibody treatment blocks monocyte/macrophage traffic to, vascular cell adhesion molecule-1 expression in, and pathology of the dorsal root ganglia in an SIV macaque model of HIV-peripheral neuropathy. Am J Pathol 186, 1754–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Walker JA, Beck GA, Campbell JH, Miller AD, Burdo TH, Williams KC (2015) Anti-[H9251]4 integrin antibody blocks monocyte/macrophage traffic to the heart and decreases cardiac pathology in a SIV infection model of AIDS. J Am Heart Assoc doi: 10.1161/JAHA.115.001932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ellis RJ, Rosario D, Clifford DB, McArthur JC, Simpson D, Alexander T, Gelman BB, Vaida F, Collier A, Marra CM, Ances B, Atkinson JH, Dworkin RH, Morgello S, Grant I, CHARTER Study Group (2010) Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: The CHARTER Study. Arch Neurol 67, 552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Phillips TJ, Brown M, Ramirez JD, Perkins J, Woldeamanuel YW, Williams AC, Orengo C, Bennett DL, Bodi I, Cox S, Maier C, Krumova EK, Rice AS (2014) Sensory, psychological, and metabolic dysfunction in HIV-associated peripheral neuropathy: A cross-sectional deep profiling study. Pain 155, 1846–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Anziska Y, Helzner EP, Crystal H, Glesby MJ, Plankey M, Weber K, Golub E, Burian P (2012) The relationship between race and HIV-distal sensory polyneuropathy in a large cohort of US women. J Neurol Sci 315, 129–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ances BM, Vaida F, Rosario D, Marquie-Beck J, Ellis RJ, Simpson DM, Clifford DB, McArthur JC, Grant I, McCutchan JA; CNS HIV Antiretroviral Therapy Effects Research (CHARTER) Metabolic Study Group (2009) Role of metabolic syndrome components in HIV-associated sensory neuropathy. AIDS 23, 2317–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lewis W, Dalakas MC (1995) Mitochondrial toxicity of antiviral drugs. Nat Med 1, 417–422. [DOI] [PubMed] [Google Scholar]
- [36].Brinkman K, ter Hofstede HJ, Burger DM, Smeitink JA, Koopmans PP (1998) Adverse effects of reverse transcriptase inhibitors: Mitochondrial toxicity as common pathway. AIDS 12, 1735–1744. [DOI] [PubMed] [Google Scholar]
- [37].Brinkman K, Smeitink JA, Romijn JA, Reiss P (1999) Mitochondrial toxicity induced by nucleoside-analogue reverse-transcriptase inhibitors is a key factor in the pathogenesis of antiretroviral-therapy-related lipodystrophy. Lancet 354, 1112–1115. [DOI] [PubMed] [Google Scholar]
- [38].Holzinger ER, Hulgan T, Ellis RJ, Samuels DC, Ritchie MD,Haas DW,Kallianpur AR,Bloss CS,Clifford DB,Collier AC,Gelman BB,Marra CM,Mc Arthur JC, Mc Cutchan JA, Morgello S, Simpson DM, Franklin DR, Rosario D, Selph D, Letendre S, Grant I; CHARTER Group (2012) Mitochondrial DNA variation and HIV-associated sensory neuropathy in CHARTER. J Neurovirol 18, 511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kallianpur AR, Jia P, Ellis RJ, Zhao Z, Bloss C, Wen W, Marra CM,Hulgan T,Simpson DM,Morgello S, Mc Arthur JC, Clifford DB, Collier AC, Gelman BB, McCutchan JA, Franklin D, Samuels DC, Rosario D, Holzinger E, Mur-dock DG, Letendre S, Grant I; CHARTER Study Group (2014) Genetic variation in iron metabolism is associated with neuropathic pain and pain severity in HIV-infected patients on antiretroviral therapy. PLoS One 9, e103123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].McArthur JC, Brew BJ, Nath A (2005) Neurological complications of HIV infection. Lancet Neurol 4, 543–555. [DOI] [PubMed] [Google Scholar]
- [41].Burdo TH, Ellis RJ, Fox HS (2008) Osteopontin is increased in HIV-associated dementia. J Infect Dis 198, 715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, McArthur JC, Collier AC, Evans SR, Ellis RJ (2007) The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS 21, 1915–1921. [DOI] [PubMed] [Google Scholar]
- [43].Simioni S, Cavassini M, Annoni JM, Rimbault Abraham A, Bourquin I, Schiffer V, Calmy A, Chave JP, Giacobini E, Hirschel B, Du Pasquier RA (2010) Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS 24, 1243–1250. [DOI] [PubMed] [Google Scholar]
- [44].Marcotte TD, Heaton RK, Wolfson T, Taylor MJ, Alhas-soon O, Arfaa K, Grant I (1999) The impact of HIV-related neuropsychological dysfunction on driving behavior. The HNRC Group. J Int Neuropsychol Soc 5, 579–592. [DOI] [PubMed] [Google Scholar]
- [45].Albert SM, Marder K, Dooneief G, Bell K, Sano M, Todak G, Stern Y (1995) Neuropsychologic impairment in early HIV infection:A risk factor for work disability. ArchNeurol 52, 525–530. [DOI] [PubMed] [Google Scholar]
- [46].Hinkin CH, Castellon SA, Durvasula RS, Hardy DJ, Lam MN,Mason KI,Thrasher D,Goetz MB,Stefaniak M (2002) Medication adherence among HIV+ adults: Effects of cognitive dysfunction and regimen complexity. Neurology 59, 1944–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I; CHARTER Group; HNRC Group (2011) HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: Differences in rates, nature, and predictors. JNeurovirol 17, 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Cysique LA, Brew BJ (2011) Prevalence of non-confounded HIV-associated neurocognitive impairment in the context of plasma HIV RNA suppression. J Neurovirol 17, 176–183. [DOI] [PubMed] [Google Scholar]
- [49].Cysique LA, Maruff P, Brew BJ (2004) Prevalence and pattern of neuropsychological impairment in human immunodeflciency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre-and post-highly active antiretroviral therapy eras: A combined study of two cohorts. J Neurovirol 10, 350–357. [DOI] [PubMed] [Google Scholar]
- [50].Cysique LA, Moffat K, Moore DM, Lane TA, Davies NW, Carr A, Brew BJ, Rae C (2013) HIV, vascular and aging injuries in the brain of clinically stable HIV-infected adults: A (1)H MRS study. PloS One 8, e61738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].McCutchan JA, Marquie-Beck JA, Fitzsimons CA, Letendre SL, Ellis RJ, Heaton RK, Wolfson T, Rosario D, Alexander TJ, Marra C, Ances BM, Grant I; CHARTER Group (2012) Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology 78, 485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Soontornniyomkij V, Umlauf A, Chung SA, Cochran ML, Soontornniyomkij B, Gouaux B, Toperoff W, Moore DJ, Masliah E,Ellis RJ,Grant I,Achim CL (2014) HIV protease inhibitor exposure predicts cerebral small vessel disease. AIDS 28, 1297–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Valcour V, Watters MR, Williams AE, Sacktor N, McMurtray A, Shikuma C (2008) Aging exacerbates extrapyramidal motor signs in the era of highly active antiretroviral therapy. J Neurovirol 14, 362–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Tisch S, Brew B (2009) Parkinsonism in HIV-infected patients on highly active antiretroviral therapy. Neurology 73, 401–403. [DOI] [PubMed] [Google Scholar]
- [55].Zhou D, Masliah E, Spector SA (2011) Autophagy is increased in postmortem brains of persons with HIV-1-associated encephalitis. J Infect Dis 203, 1647–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Alirezaei M, Kiosses WB, Flynn CT, Brady NR, Fox HS (2008) Disruption of neuronal autophagy by infected microglia results in neurodegeneration. PLoSOne 3, e2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ardley HC, Scott GB, Rose SA, Tan NG, Robinson PA (2004) UCH-L1 aggresome formation in response to proteasome impairment indicates a role in inclusion formation in Parkinson’s disease. J Neurochem 90, 379–391. [DOI] [PubMed] [Google Scholar]
- [58].McNaught KS, Belizaire R, Isacson O, Jenner P, Olanow CW (2003) Altered proteasomal function in sporadic Parkinson’s disease. Exp Neurol 179, 38–46. [DOI] [PubMed] [Google Scholar]
- [59].Marra CM, Zhao Y, Clifford DB, Letendre S, Evans S, Henry K, Ellis RJ, Rodriguez B, Coombs RW, Schifitto G, McArthur JC, Robertson K; AIDS Clinical Trials Group 736 Study Team (2009) Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS 23, 1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Valcour V, Sithinamsuwan P, Letendre S, Ances B (2011) Pathogenesis of HIV in the central nervous system. Curr HIV/AIDS Rep 8, 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Langford D, Letendre S (2013) Editorial commentary: Severe HIV-associated CD8+ T-cell encephalitis: Is it the tip of the iceberg? Clin Infect Dis 57 109–111. [DOI] [PubMed] [Google Scholar]
- [62].Marcondes MC, Burdo TH, Sopper S, Huitron-Resendiz S, Lanigan C, Watry D, Flynn C, Zandonatti M, Fox HS (2007) Enrichment and persistence of virus-specific CTL in the brain of simian immunodeficiency virus-infected monkeys is associated with a unique cytokine environment. J Immunol 178, 5812–5819. [DOI] [PubMed] [Google Scholar]
- [63].Marcondes MC, Burudi E, Huitron-Resendiz S, Sanchez-Alavez M, Watry D, Zandonatti M, Henriksen SJ, Fox HS (2001) Highly activated CD8(+) T cells in the brain correlate with early central nervous system dysfunction in simian immunodeficiency virus infection. J Immunol 167, 5429–5438. [DOI] [PubMed] [Google Scholar]
- [64].Schrier RD, Hong S, Crescini M, Ellis R, Pérez-Santiago J, Spina C, Letendre S; HNRP Group (2015) Cerebrospinal fluid (CSF) CD8+ T-cells that express interferon-gamma contribute to HIV associated neurocognitive disorders (HAND). PLoS One 10, e0116526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Shah A, Kumar A (2010) HIV-1 gp120-mediated increases in IL-8 production in astrocytes are mediated through the NF-κB pathway and can be silenced by gp120-specific siRNA. J Neuroinflammation 7, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Shah A,Singh DP,Buch S,Kumar A (2011) HIV-1 envelope protein gp120 up regulates CCL5 production in astrocytes which can be circumvented by inhibitors of NF-κB pathway. Biochem Biophys Res Commun 414, 112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Kaul M, Lipton SA (1999) Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc Natl Acad Sci U S A 96, 8212–8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Hesselgesser J, Taub D, Baskar P, Greenberg M, Hoxie J, Kolson DL, Horuk R (1998) Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1 alpha is mediated by the chemokine receptor CXCR4. Curr Biol 8, 595–598. [DOI] [PubMed] [Google Scholar]
- [69].Nath A, Padua RA, Geiger JD (1995) HIV-1 coat protein gp120-induced increases in levels of intrasynaptosomal calcium. Brain Res 678, 200–206. [DOI] [PubMed] [Google Scholar]
- [70].Bardi G, Sengupta R, Khan MZ, Patel JP, Meucci O (2006) Human immunodeficiency virus gp120-induced apoptosis of human neuroblastoma cells in the absence of CXCR4 internalization. J Neurovirol 12, 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Meucci O, Miller RJ (1996) gp120-induced neurotoxicity in hippocampal pyramidal neuron cultures: Protective action of TGF-β1. J Neurosci 16, 4080–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Gandhi N, Saiyed ZM, Napuri J, Samikkannu T, Reddy PV, Agudelo M, Khatavkar P, Saxena SK, Nair MP (2010) Interactive role of human immunodeficiency virus type 1 (HIV-1) clade-specific Tat protein and cocaine in blood-brain barrier dysfunction: Implications for HIV-1-associated neurocognitive disorder. J Neurovirol 16, 294–305. [DOI] [PubMed] [Google Scholar]
- [73].Haughey NJ, Holden CP, Nath A, Geiger JD (1999) Involvement of inositol 1,4,5-trisphosphate-regulated stores of intracellular calcium in calcium dysregulation and neuron cell death caused by HIV-1 protein Tat. J Neurochem 73, 1363–1374. [DOI] [PubMed] [Google Scholar]
- [74].Haughey NJ, Nath A, Mattson MP, Slevin JT, Geiger JD (2001) HIV-1 Tat through phosphorylation of NMDA receptors potentiates glutamate excitotoxicity. J Neurochem 78, 457–467. [DOI] [PubMed] [Google Scholar]
- [75].Hui L, Chen X, Haughey NJ, Geiger JD (2012) Role of endolysosomes in HIV-1 Tat-induced neurotoxicity. ASN Neuro 4, 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Paris JJ, Singh HD, Carey AN, McLaughlin JP (2015) Exposure to HIV-1 Tat in brain impairs sensorimotor gating and activates microglia in limbic and extralimbic brain regions of male mice. Behav Brain Res 291, 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Stevens PR, Gawryluk JW, Hui L, Chen X, D Geiger JD (2014) Creatine protects against mitochondrial dysfunction associated with HIV-1 Tat-induced neuronal injury. Curr HIV Res 12, 378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Smurzynski M, Wu K, Letendre S, Robertson K, Bosch RJ, Clifford DB, Evans S, Collier AC, Taylor M, Ellis R (2011) Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort. AIDS 25, 357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Liu H,Liu Z,Yang X,Huang F, Ma C, Li Z (2008) Neurotoxicity caused by didanosine on cultured dorsal root ganglion neurons. Cell Biol Toxicol 24, 113–121. [DOI] [PubMed] [Google Scholar]
- [80].Robinson B, Li Z, Nath A (2007) Nucleoside reverse transcriptase inhibitors and human immunodeficiency virus proteins cause axonal injury in human dorsal root ganglia cultures. J Neurovirol 13, 160–167. [DOI] [PubMed] [Google Scholar]
- [81].Purnell PR, Fox HS (2014) Efavirenz induces neuronal autophagy and mitochondrial alterations. J Pharmacol Exp Ther 351, 250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Zhang X, Cao R, Liu R, Zhao R, Huang Y, Gurley EC, Hylemon PB, Pandak WM, Wang G, Zhang L, Li X, Zhou H (2014) Reduction of the HIV protease inhibitor-induced ER stress and inflammatory response by raltegravir in macrophages. PLoS One 9, e90856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Brown LA, Jin J, Ferrell D, Sadic E, Obregon D, Smith AJ, Tan J, Giunta B (2014) Efavirenz promotes β-secretase expression and increased A[H9252]1–40,42 via oxidative stress and reduced microglial phagocytosis :Implications for HIV associated neurocognitive disorders (HAND). PLoSOne 9, e95500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Jin J, Grimmig B, Izzo J, Brown LA, Hudson C, Smith AJ, Tan J, Bickford PC, Giunta B (2016) HIV non-nucleoside reverse transcriptase inhibitor efavirenz reduces neural stem cell proliferation in vitro and in vivo. Cell Transplant 25, 1967–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Apostolova N, Funes HA, Blas-Garcia A, Alegre F, Polo M, Esplugues JV (2015) Involvement of nitric oxide in the mitochondrial action of efavirenz: A differential effect on neurons and glial cells. J Infect Dis 211, 1953–1958. [DOI] [PubMed] [Google Scholar]
- [86].Funes HA, Blas-Garcia A, Esplugues JV, Apostolova N (2015) Efavirenz alters mitochondrial respiratory function in cultured neuron and glial cell lines. J Antimicrob Chemother 70, 2249–2254. [DOI] [PubMed] [Google Scholar]
- [87].Tatro ET, Soontornniyomkij B, Letendre SL, Achim CL (2014) Cytokine secretion from brain macrophages infected with human immunodeficiency virus in vitro and treated with raltegravir. BMC Infect Dis 14, 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kelly SG, Taiwo BO, Wu Y, Bhatia R, Kettering CS, Gao Y, Li S, Hutten R, Ragin AB (2014) Early suppressive antiretroviral therapy in HIV infection is associated with measurable changes in the corpus callosum. J Neurovirol 20, 514–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Ma Q, Vaida F, Wong J, Sanders CA, Kao YT, Croteau D, Clifford DB, Collier AC, Gelman BB, Marra CM, McArthur JC, Morgello S, Simpson DM, Heaton RK, Grant I,Letendre SL;CHARTERGroup(2016)Long-term efavirenz use is associated with worse neurocognitive functioning in HIV-infected patients. J Neurovirol 22, 170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Vivithanaporn P, Asahchop EL, Acharjee S, Baker GB, Power C (2016) HIV protease inhibitors disrupt astrocytic glutamate transporter function and neurobehavioral performance. AIDS 30, 543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Manda KR, Banerjee A, Banks WA, Ercal N (2011) Highly active antiretroviral therapy drug combination induces oxidative stress and mitochondrial dysfunction in immortalized human blood-brain barrier endothelial cells. Free Radic Biol Med 50, 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Bertrand L, Toborek M (2015) Dysregulation of endoplasmic reticulum stress and autophagic responses by the antiretroviral drug efavirenz. Mol Pharmacol 88, 304–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Wang X, Chai H, Yao Q, Chen C (2007) Molecular mechanisms of HIV protease inhibitor-induced endothelial dysfunction. J Acquir Immune Defic Syndr 44, 493–499. [DOI] [PubMed] [Google Scholar]
- [94].Kanmogne GD, Schall K, Leibhart J, Knipe B, Gendelman HE, Persidsky Y (2007) HIV-1 gp120 compromises blood–brain barrier integrity and enhance monocyte migration across blood-brain barrier: Implication for viral neuropathogenesis. J Cereb Blood Flow Metab 27, 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Kline ER,Sutliff RL(2008)The roles of HIV-1 proteins and antiretroviral drug therapy in HIV-1-associated endothelial dysfunction. J Investig Med 56, 752–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Bagashev A, Sawaya BE (2013) Roles and functions of HIV-1 Tat protein in the CNS: An overview. Virol. 10, 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Agrawal L, Louboutin JP, Strayer DS (2007) Preventing HIV-1 Tat-induced neuronal apoptosis using antioxidant enzymes: Mechanistic and therapeutic implications. Virology 363, 462–472. [DOI] [PubMed] [Google Scholar]
- [98].Pinto AC, Wachholz PA, Masuda PY, Freitas FB, Cavalcante ML, Soares CT (2015) Preliminary results of the application of a disease activity score in a patient with relapsing polychondritis. G Ital Dermatol Venereol 20. [DOI] [PubMed] [Google Scholar]
- [99].Mishra R, Singh SK (2013) HIV-1 Tat C modulates expression of miRNA-101 to suppress VE-cadherin in human brain microvascular endothelial cells. J Neurosci 33, 5992–6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Pu H, Tian J, Andras IE, Hayashi K, Flora G, Hennig B,Toborek M (2005) HIV-1 Tat protein-induced alterations of ZO-1 expression are mediated by redox-regulated ERK 1/2 activation. J Cereb Blood Flow Metab 25, 1325–1335. [DOI] [PubMed] [Google Scholar]
- [101].Xu R, Feng X, Xie X, Zhang J, Wu D, Xu L (2012) HIV-1 Tat protein increases the permeability of brain endothelial cells by both inhibiting occludin expression and cleaving occludin via matrix metalloproteinase-9. Brain Res 1436, 13–19. [DOI] [PubMed] [Google Scholar]
- [102].Ben Haij N, Planès R, Leghmari K, Serrero M, Delobel P, Izopet J, BenMohamed L, Bahraoui E (2015) HIV-1 Tat protein induces production of proinflammatory cytokines by human dendritic cells and monocytes/macrophages through engagement of TLR4-MD2-CD14 complex and activation of NF-κB pathway. PLoS One 10, e0129425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].El-Hage N, Bruce-Keller AJ, Yakovleva T, Bazov I,Bakalkin G, Knapp PE, Hauser KF (2008) Morphine exacerbates HIV-1 Tat-induced cytokine production in astrocytes through convergent effects on [Ca(2+)](i), NF-kappaB trafficking and transcription. PLoS One 3, e4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Kiebala M, Polesskaya O, Yao Z, Perry SW, Maggirwar SB (2010) Nuclear factor-kappa B family member RelB inhibits human immunodeficiency virus-1 Tat-induced tumor necrosis factor-alpha production. PLoS One 5, e11875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Nath A, Conant K, Chen P, Scott C, Major EO (1999) Transient exposure to HIV-1 Tat protein results in cytokine production in macrophages and astrocytes. A hit and run phenomenon. J Biol Chem 274, 17098–17102. [DOI] [PubMed] [Google Scholar]
- [106].Nookala AR, Kumar A (2014) Molecular mechanisms involved in HIV-1 Tat-mediated induction of IL-6 and IL-8 in astrocytes. J Neuroinflammation 11, 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Marker DF, Tremblay ME, Puccini JM, Barbieri J, Gantz Marker MA, Loweth CJ, Muly EC, Lu SM, Goodfellow VS, Dewhurst S, Gelbard HA (2013) The new small-molecule mixed-lineage kinase 3 inhibitor URMC-099 is neuroprotective and anti-inflammatory in models of human immunodeficiency virus-associated neurocognitive disorders. J Neurosci 33, 9998–10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Kim HJ, Martemyanov KA, Thayer SA (2008) Human immunodeficiency virus protein Tat induces synapse loss via a reversible process that is distinct from cell death. J Neurosci 28, 12604–12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Tremblay ME, Marker DF, Puccini JM, Muly EC, Lu SM, Gelbard HA (2013) Ultrastructure of microglia-synapse interactions in the HIV-1 Tat-injected murine central nervous system. Commun Integr Biol 6, e27670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Carroll AM, Porter RK (2004) Starvation-sensitive UCP 3 protein expression in thymus and spleen mitochondria. Biochim Biophys Acta 1700, 145–150. [DOI] [PubMed] [Google Scholar]
- [111].Louboutin JP, Agrawal L, Reyes BA, Van Bockstaele EJ, Strayer DS (2014) Oxidative stress is associated with neuroinflammation in animal models of HIV-1 Tat neurotoxicity. Antioxidants (Basel) 3, 414–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Willmoth F (2007) Rumblings in the air: Understanding earthquakes in the 1690s. Endeavour 31, 24–29. [DOI] [PubMed] [Google Scholar]
- [113].Cheng J, Nath A, Knudsen B, Hochman S, Geiger JD, Ma M, Magnuson DS (1998) Neuronal excitatory properties of human immunodeficiency virus type 1 Tat protein. Neuro-science 82, 97–106. [DOI] [PubMed] [Google Scholar]
- [114].Haughey NJ, Nath A, Mattson MP, Slevin JT, Geiger JD (2001) HIV-1 Tat through phosphorylation of NMDA receptors potentiates glutamate excitotoxicity. J Neurochem 78, 457–467. [DOI] [PubMed] [Google Scholar]
- [115].Musante V,Summa M,Neri E,Puliti A,Godowicz TT,Severi P, Battaglia G, Raiteri M, Pittaluga A (2010) The HIV-1 viral protein Tat increases glutamate and decreases GABA exocytosis from human and mouse neocortical nerve endings. Cereb Cortex 20, 1974–1984. [DOI] [PubMed] [Google Scholar]
- [116].Feligioni M, Raiteri L, Pattarini R, Grilli M, Bruzzone S, Cavazzani P, Raiteri M, Pittaluga A (2003) The human immunodeficiency virus-1 protein Tat and its discrete fragments evoke selective release of acetylcholine from human and rat cerebrocortical terminals through species-specific mechanisms. J Neurosci 23, 6810–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Kleinfeld AM, Kampf JP, Lechene C (2004) Transport of 13C-oleate in adipocytes measured using multi imaging mass spectrometry. J Am Soc Mass Spectrom 15, 1572–1580. [DOI] [PubMed] [Google Scholar]
- [118].Haughey NJ, Holden CP, Nath A, Geiger JD (1999) Involvement of inositol 1,4,5-trisphosphate-regulated stores of intracellular calcium in calcium dysregulation and neuron cell death caused by HIV-1 protein tat. J Neurochem 73, 1363–1374. [DOI] [PubMed] [Google Scholar]
- [119].Hu XT (2016) HIV-1 Tat-mediated calcium dysregulation and neuronal dysfunction in vulnerable brain regions. Curr Drug Targets 17, 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Krogh KA, Lyddon E, Thayer SA (2015) HIV-1 Tat activates a RhoA signaling pathway to reduce NMDA-evoked calcium responses in hippocampal neurons via an actin-dependent mechanism. J Neurochem 132, 354–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Kruman II, Nath A, Mattson MP (1998) HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp Neurol 154, 276–288. [DOI] [PubMed] [Google Scholar]
- [122].Mayne M, Holden CP, Nath A, Geiger JD (2000) Release of calcium from inositol 1,4,5-trisphosphate receptor-regulated stores by HIV-1 Tat regulates TNF-alpha production in human macrophages. J Immunol 164, 6538–6542. [DOI] [PubMed] [Google Scholar]
- [123].Wilen CB, Tilton JC, Doms RW (2012) HIV: Cell binding and entry. Cold Spring Harb Perspect Med 2, a006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Thomas S, Mayer L, Sperber K (2009) Mitochondria influence Fas expression in gp120-induced apoptosis of neuronal cells. Int J Neurosci 119, 157–165. [DOI] [PubMed] [Google Scholar]
- [125].Meeker RB, Poulton W, Clary G, Schriver M, Longo FM (2016) Novel p75 neurotrophin receptor ligand stabilizes neuronal calcium, preserves mitochondrial movement and protects against HIV associated neuropathogenesis. Exp Neurol 275, 182–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Marchionni I, Beaumont M, Maccaferri G (2012) The chemokine CXCL 12 and the HIV-1 envelope protein gp120 regulate spontaneous activity of Cajal-Retzius cells in opposite directions. J Physiol 590, 3185–3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Wang WY, Pan L, Su SC, Quinn EJ, Sasaki M, Jimenez JC, Mackenzie IRA, Huang EJ, Tsai LH (2013) Interaction of FUS and HDAC1 regulates DNA damage response and repair in neurons. Nat Neurosci 16, 1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Festa L,Gutoskey CJ,Graziano A,Waterhouse BD,Meucci O (2015) Induction of interleukin-1β by Human Immunodeficiency Virus-1 viral proteins leads to increased levels of neuronal ferritin heavy chain, synaptic injury, and deficits in flexible attention. J Neurosci 35, 10550–10561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Viviani B, Gardoni F, Bartesaghi S, Corsini E, Facchi A, Galli CL, Di Luca M, Marinovich M (2006) Interleukin-1 beta released by gp120 drives neural death through tyrosine phosphorylation and trafficking of NMDA receptors.JBiol Chem 281, 30212–30222. [DOI] [PubMed] [Google Scholar]
- [130].Moss PJ, Huang W, Dawes J, Okuse K, McMahon SB, Rice AS (2015) Macrophage-sensory neuronal interaction in HIV-1 gp120-induced neurotoxicity‡. Br J Anaesth 114,499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Liu YP, Yang CS, Chen MC, Sun SH, Tzeng SF (2010) Ca(2+)-dependent reduction of glutamate aspartate transporter GLAST expression in astrocytes by P2X(7) receptor-mediated phosphoinositide 3-kinase signaling. J Neurochem 113, 213–227. [DOI] [PubMed] [Google Scholar]
- [132].Louboutin JP, Strayer DS (2012) Blood-brain barrier abnormalities caused by HIV-1 gp120: Mechanistic and therapeutic implications. ScientificWorldJournal 2012, 482575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].McRae M (2016) HIV and viral protein effects on the blood brain barrier. Tissue Barriers 4, e1143543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Kanmogne GD, Primeaux C, Grammas P (2005) HIV-1 gp120 proteins alter tight junction protein expression and brain endothelial cell permeability: Implications for the pathogenesis of HIV-associated dementia. J Neuropathol Exp Neurol 64, 498–505. [DOI] [PubMed] [Google Scholar]
- [135].Kanmogne GD, Schall K, Leibhart J, Knipe B, Gendelman HE, Persidsky Y (2007) HIV-1 gp120 compromises blood-brain barrier integrity and enhances monocyte migration across blood-brain barrier: Implication for viral neuropathogenesis. J Cereb Blood Flow Metab 27, 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Louboutin JP, Reyes BA, Agrawal L, Van Bockstaele EJ, Strayer DS (2011) HIV-1 gp120 upregulates matrix metalloproteinases and their inhibitors in a rat model of HIV encephalopathy. Eur J Neurosci 34, 2015–2023. [DOI] [PubMed] [Google Scholar]
- [137].Shiu C, Barbier E, Di Cello F, Choi HJ, Stins M (2007) HIV-1 gp120 as well as alcohol affect blood-brain barrier permeability and stress fiber formation:Involvementof reactive oxygen species. Alcohol Clin Exp Res 31,130–137. [DOI] [PubMed] [Google Scholar]
- [138].Avdoshina V, Fields JA, Castellano P, Dedoni S, Palchik G, Trejo M, Adame A, Rockenstein E, Eugenin E, Masliah E, Mocchetti I (2016) The HIV protein gp120 alters mitochondrial dynamics in neurons. Neurotox Res 29, 583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Saha RN, Pahan K (2007) Differential regulation of Mnsuperoxide dismutase in neurons and astroglia by HIV-1 gp120: Implications for HIV-associated dementia. Free Radic Biol Med 42, 1866–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Samikkannu T, Ranjith D, Rao KV, Atluri VS, Pimentel E, El-Hage N, Nair MP (2015) HIV-1 gp120 and morphine inducedoxidativestress:Roleincellcycleregulation.Front Microbiol 6, 614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Guo L, Xing Y, Pan R, Jiang M, Gong Z, Lin L, Wang J, Xiong G, Dong J (2013) Curcumin protects microglia and primary rat cortical neurons against HIV-1 gp 120-mediated inflammation and apoptosis. PLoS One 8, e70565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Liu J, Xu C, Chen L, Xu P, Xiong H (2012) Involvement of Kv1.3 and p38 MAPK signaling in HIV-1 glycoprotein 120-induced microglia neurotoxicity. Cell Death Dis 3, e254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Shah A, Kumar S, Simon SD, Singh DP, Kumar A (2013) HIV gp120- and methamphetamine-mediated oxidative stress induces astrocyte apoptosis via cytochrome P450 2E1. Cell Death Dis 4, e850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Fiala M, Singer EJ, Commins D, Mirzapoiazova T, Verin A, Espinosa A, Ugen K, Bernas M, Witte M, Weinand M, Lossinsky AS (2008) HIV-1 antigens in neurons of cocaine-abusing patients. Open Virol J 2, 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Ranki A,Nyberg M,Ovod V,Haltia M,Elovaara I,Raininko R, Haapasalo H, Krohn K (1995) Abundant expression of HIV Nef and Rev proteins in brain astrocytes in vivo is associated with dementia. AIDS 9, 1001–1008. [DOI] [PubMed] [Google Scholar]
- [146].Overholser ED, Coleman GD, Bennett JL, Casaday RJ, Zink MC, Barber SA, Clements JE (2003) Expression of simian immunodeficiency virus (SIV) nef in astrocytes during acute and terminal infection and requirement of nef for optimal replication of neurovirulent SIV in vitro. J Virol 77, 6855–6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Brambilla A, Turchetto L, Gatti A, Bovolenta C, Veglia F, Santagostino E,Gringeri A,Clementi M,Poli G,Bagnarelli P, Vicenzi E (1999) Defective nef alleles in a cohort of hemophiliacs with progressing and nonprogressing HIV-1 infection. Virology 259, 349–368. [DOI] [PubMed] [Google Scholar]
- [148].Rhodes DI, Ashton L, Solomon A, Carr A, Cooper D, Kaldor J, Deacon N (2000) Characterization of three nef-defective human immunodeficiency virus type 1 strains associated with long-term nonprogression. Australian Long-Term Nonprogressor Study Group.JVirol 74,10581–10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Bencheikh M, Bentsman G, Sarkissian N, Canki M, Volsky DJ (1999) Replication of different clones of human immunodeficiency virus type 1 in primary fetal human astrocytes: Enhancement of viral gene expression by Nef. J Neurovirol 5, 115–124. [DOI] [PubMed] [Google Scholar]
- [150].Mordelet E, Kissa K, Cressant A, Gray F, Ozden S, Vidal C, Charneau P, Granon S (2004) Histopathological and cognitive defects induced by Nef in the brain. FASEB J 18, 1851–1861. [DOI] [PubMed] [Google Scholar]
- [151].Trillo-Pazos G, McFarlane-Abdulla E, Campbell IC, Pilkington GJ, Everall IP (2000) Recombinant nef HIV-IIIB protein is toxic to human neurons in culture. Brain Res 864, 315–326. [DOI] [PubMed] [Google Scholar]
- [152].Lehmann MH, Masanetz S, Kramer S, Erfle V (2006) HIV-1 Nef upregulates CCL2/MCP-1 expression in astrocytes in a myristoylation- and calmodulin-dependent manner. J Cell Sci 119, 4520–4530. [DOI] [PubMed] [Google Scholar]
- [153].Liu X, Kumar A (2015) Differential signaling mechanism for HIV-1 Nef-mediated production of IL-6 and IL-8 in human astrocytes. Sci Rep 5, 9867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Liu X,Shah A,Gangwani MR,Silverstein PS,Fu M,Kumar A (2014) HIV-1 Nef induces CCL5 production in astrocytes through p38-MAPK and PI3K/Akt pathway and utilizes NF-kB, CEBP and AP-1 transcription factors. Sci Rep 4, 4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Si Q, Kim MO, Zhao ML, Landau NR, Goldstein H, Lee S (2002) Vpr- and Nef-dependent induction of RANTES/CCL5 in microglial cells. Virology 301, 342–353. [DOI] [PubMed] [Google Scholar]
- [156].Moffat T, Herring A (1999) The historical roots of high rates of infant death in Aboriginal communities in Canada in the early twentieth century: The case of Fisher River, Manitoba. Soc Sci Med 48, 1821–1832. [DOI] [PubMed] [Google Scholar]
- [157].Heyes MP, Saito K, Lackner A, Wiley CA, Achim CL, Markey SP (1998) Sources of the neurotoxin quinolinic acid in the brain of HIV-1-infected patients and retrovirus-infected macaques. FASEB J 12, 881–896. [DOI] [PubMed] [Google Scholar]
- [158].Smith DG, Guillemin GJ, Pemberton L, Kerr S, Nath A, Smythe GA, Brew BJ (2001) Quinolinic acid is produced by macrophages stimulated by platelet activating factor, Nef and Tat. J Neurovirol 7, 56–60. [DOI] [PubMed] [Google Scholar]
- [159].van Marle G, Henry S, Todoruk T, Sullivan A, Silva C, Rourke SB, Holden J, McArthur JC, Gill MJ, Power C (2004) Human immunodeficiency virus type 1 Nef protein mediates neural cell death: A neurotoxic role for IP-10. Virology 329, 302–318. [DOI] [PubMed] [Google Scholar]
- [160].Bessette EE, Fasco MJ, Pentecost BT, Kaminsky LS (2005) Mechanisms of arsenite-mediated decreases in benzo[k]fluoranthene-induced human cytochrome P4501A1 levels in HepG2 cells. Drug Metab Dispos 33, 312–320. [DOI] [PubMed] [Google Scholar]
- [161].Chompre G, Cruz E, Maldonado L, Rivera-Amill V, Porter JT, Noel RJ Jr (2013) Astrocytic expression of HIV-1 Nef impairs spatial and recognition memory. Neurobiol Dis 49, 128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Bergonzini V, Calistri A, Salata C, Del Vecchio C, Sartori E, Parolin C, Palù G (2009) Nef and cell signaling transduction: A possible involvement in the pathogenesis of human immunodeficiency virus-associated dementia. J Neurovirol 15, 238–248. [DOI] [PubMed] [Google Scholar]
- [163].Acheampong EA, Roschel C, Mukhtar M, Srinivasan A, Rafi M, Pomerantz RJ, Parveen Z (2009) Combined effects of hyperglycemic conditions and HIV-1 Nef: A potential model for induced HIV neuropathogenesis. Virol J 6, 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Saribas AS, Khalili K, Sariyer IK (2015) Dysregulation of autophagy by HIV-1 Nef in human astrocytes. Cell Cycle 14, 2899–2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Kyei GB, Dinkins C, Davis AS, Roberts E, Singh SB, Dong C, Wu L, Kominami E, Ueno T, Yamamoto A, Federico M, Panganiban A, Vergne I, Deretic V (2009) Autophagy pathway intersects with HIV-1 biosynthesis and regulates viral yields in macrophages. J Cell Biol 186, 255–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Sami Saribas A, Cicalese S, Ahooyi TM, Khalili K, Amini S, Sariyer IK (2017) HIV-1 Nef is released in extra-cellular vesicles derived from astrocytes: Evidence for Nef-mediated neurotoxicity. Cell Death Dis 8, e2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Levy DN, Refaeli Y, MacGregor RR, Weiner DB (1994) Serum Vpr regulates productive infection and latency of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A 91, 10873–10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Kogan M,Rappaport J (2011) HIV-1 accessory protein Vpr: Relevance in the pathogenesis of HIV and potential for therapeutic intervention. Retrovirology 8, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Hoshino S, Konishi M, Mori M, Shimura M, Nishitani C, Kuroki Y, Koyanagi Y, Kano S, Itabe H, Ishizaka Y (2010) HIV-1 Vpr induces TLR4/MyD88-mediated IL-6 production and reactivates viral production from latency. J Leukoc Biol 87, 1133–1143. [DOI] [PubMed] [Google Scholar]
- [170].Romani B, Kamali Jamil R, Hamidi-Fard M, Rahimi P, Momen SB, Aghasadeghi MR, Allahbakhshi E (2016) HIV-1 Vpr reactivates latent HIV-1 provirus by inducing depletionofclassIHDACsonchromatin. Sci Rep 6,31924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Deshmane SL, Amini S, Sen S, Khalili K, Sawaya BE (2011) Regulation of the HIV-1 promoter by HIF-1α and Vpr proteins. Virol J 8, 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Deshmane SL, Mukerjee R, Fan S, Del Valle L, Michiels C, Sweet T, Rom I, Khalili K, Rappaport J, Amini S, Sawaya BE (2009) Activation of the oxidative stress pathway by HIV-1 Vpr leads to induction of hypoxia-inducible factor 1alpha expression. J Biol Chem 284, 11364–11373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Balliet JW, Kolson DL, Eiger G, Kim FM, McGann KA, Srinivasan A, Collman R (1994) Distinct effects in primary macrophages and lymphocytes of the human immunodeficiency virus type 1 accessory genes vpr, vpu, and nef: Mutational analysis of a primary HIV-1 isolate. Virology 200, 623–631. [DOI] [PubMed] [Google Scholar]
- [174].Ayyavoo V, Mahboubi A, Mahalingam S, Ramalingam R, Kudchodkar S, Williams WV, Green DR, Weiner DB (1997) HIV-1 Vpr suppresses immune activation and apoptosis through regulation of nuclear factor kappa B. Nat Med 3, 1117–1123. [DOI] [PubMed] [Google Scholar]
- [175].Azad AA (2000) Could Nef and Vpr proteins contribute to disease progression by promoting depletion of bystander cells and prolonged survival of HIV-infected cells? Biochem Biophys Res Commun 267, 677–685. [DOI] [PubMed] [Google Scholar]
- [176].Patel CA, Mukhtar M, Pomerantz RJ (2000) Human immunodeficiency virus type 1 Vpr induces apoptosis in human neuronal cells. J Virol 74, 9717–9726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Patel CA, Mukhtar M, Harley S, Kulkosky J, Pomerantz RJ (2002) Lentiviral expression of HIV-1 Vpr induces apoptosis in human neurons. J Neurovirol 8, 86–99. [DOI] [PubMed] [Google Scholar]
- [178].Sabbah EN, Roques BP (2005) Critical implication of the (70–96) domain of human immunodeficiency virus type 1 Vpr protein in apoptosis of primary rat cortical and striatal neurons. J Neurovirol 11, 489–502. [DOI] [PubMed] [Google Scholar]
- [179].Mukerjee R, Chang JR, Del Valle L, Bagashev A, Gayed MM, Lyde RB, Hawkins BJ, Brailoiu E, Cohen E, Power C, Azizi SA, Gelman BB, Sawaya BE (2011) Deregulation of microRNAs by HIV-1 Vpr protein leads to the developmentofneurocognitivedisorders.J Biol Chem 286, 34976–34985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Na H,Acharjee S,Jones G,Vivithanaporn P,Noorbakhsh F,Mc Farlane N,Maingat F,Ballanyi K,Pardo CA,Cohen EA, Power C (2011) Interactions between human immunodeficiency virus (HIV)-1 Vpr expression and innate immunity influence neurovirulence. Retrovirology 8, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Ferrucci A, Nonnemacher MR, Wigdahl B (2013) Extra-cellular HIV-1 viral protein R affects astrocytic glyceralde-hyde 3-phosphate dehydrogenase activity and neuronal survival. J Neurovirol 19, 239–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Gangwani MR, Kumar A (2015) Multiple protein kinases via activation of transcription factors NF-κB, AP-1 and C/EBP-δ regulate the IL-6/IL-8 production by HIV-1 Vpr in astrocytes. PLoS One 10, e0135633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Guha D, Nagilla P, Redinger C, Srinivasan A, Schatten GP, Ayyavoo V (2012) Neuronal apoptosis by HIV-1 Vpr: Contributionofproinflammatorymolecularnetworksfrom infected target cells. J Neuroinflammation 9, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Torres L, Noel RJ Jr (2014) Astrocytic expression of HIV-1 viral protein R in the hippocampus causes chromatolysis, synaptic loss and memory impairment. J Neuroinflammation 11, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Achim CL, Adame A, Dumaop W, Everall IP, Masliah E; Neurobehavioral Research Center (2009) Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. J Neuroimmune Pharmacol 4, 190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL (2005) Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS 19, 407–411. [DOI] [PubMed] [Google Scholar]
- [187].Rempel HC,Pulliam L (2005) HIV-1 Tat inhibits neprilysin and elevates amyloid beta. AIDS 19, 127–135. [DOI] [PubMed] [Google Scholar]
- [188].Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE (2006) Accelerated Tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active anti-retroviral therapy. Acta Neuropathol 111, 529–538. [DOI] [PubMed] [Google Scholar]
- [189].Liu Y, Jones M, Hingtgen CM, Bu G, Laribee N, Tanzi RE, Moir RD, Nath A, He JJ (2000) Uptake of HIV-1 tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nat Med 6, 1380–1387. [DOI] [PubMed] [Google Scholar]
- [190].Kim J, Yoon JH, Kim YS (2013) HIV-1 Tat interacts with and regulates the localization and processing of amyloid precursor protein. PLoS One 8, e77972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Giunta B, Hou H, Zhu Y, Rrapo E, Tian J, Takashi M, Commins D, Singer E, He J, Fernandez F, Tan J (2009) HIV-1 Tat contributes to Alzheimer’s disease-like pathology in PSAPP mice. Int J Clin Exp Pathol 2, 433–443. [PMC free article] [PubMed] [Google Scholar]
- [192].Deane R, Bell RD, Sagare A, Zlokovic BV (2009) Clearance of amyloid-beta peptide across the blood-brain barrier: Implication for therapies in Alzheimer’s disease. CNS Neurol Disord Drug Targets 8, 16–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Giunta B, Zhou Y, Hou H, Rrapo E, Fernandez F, Tan J (2008) HIV-1 TAT inhibits microglial phagocytosis of Abeta peptide. Int J Clin Exp Pathol 1, 260–275. [PMC free article] [PubMed] [Google Scholar]
- [194].Daily A, Nath A, Hersh LB (2006) Tat peptides inhibit neprilysin. J Neurovirol 12, 153–160. [DOI] [PubMed] [Google Scholar]
- [195].Noguchi Y, Asayama K, Staessen JA, Inaba M, Ohkubo T, Hosaka M, Satoh M, Kamide K, Awata T, Katayama S, Imai Y, HOMED-BP study group (2013) Predictive power of home blood pressure and clinic blood pressure in hyper-tensive patients with impaired glucose metabolism and diabetes. J Hypertens 31, 1593–1602. [DOI] [PubMed] [Google Scholar]
- [196].Lesné S, Ali C, Gabriel C, Croci N, MacKenzie ET, Glabe CG, Plotkine M, Marchand-Verrecchia C, Vivien D, Buisson A (2005) NMDA receptor activation inhibits alpha-secretase and promotes neuronal amyloid-beta production. J Neurosci 25, 9367–9377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Chen L, Choi JJ, Choi YJ, Hennig B, Toborek M (2012) HIV-1 Tat-induced cerebrovascular toxicity is enhanced in mice with amyloid deposits. Neurobiol Aging 33, 1579–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Chen Y, Huang W, Jiang W, Wu X, Ye B, Zhou X (2016) HIV-1 Tat regulates occludin and Aβ transfer receptor expression in brain endothelial cells via Rho/ROCK signaling pathway. Oxid Med Cell Longev 2016, 4196572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Soliman ML, Geiger JD, Chen X (2017) Caffeine blocks HIV-1 Tat-induced Amyloid Beta production and Tauphosphorylation. J Neuroimmune Pharmacol 12, 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Aksenov MY, Aksenova MV, Mactutus CF, Booze RM (2010) HIV-1 protein-mediated amyloidogenesis in rat hippocampal cell cultures. Neurosci Lett 475, 174–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Liu L, Yu J, Li L, Zhang B, Liu L, Wu CH, Jong A, Mao DA, Huang SH (2017) Alpha7 nicotinic acetylcholine receptor is required for amyloid pathology in brain endothelial cells induced by Glycoprotein 120, methamphetamine and nicotine. Sci Rep 7, 40467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Zhang J, Liu J, Katafiasz B, Fox H, Xiong H (2011) HIV-1 gp120-induced axonal injury detected by accumulation of β-amyloid precursor protein in adult rat corpus callosum. J Neuroimmune Pharmacol 6, 650–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Bae M, Patel N, Xu H, Lee M, Tominaga-Yamanaka K, Nath A, Geiger J, Gorospe M, Mattson MP, Haughey NJ (2014) Activation of TRPML1 clears intraneuronal Aβ in preclinical models of HIV infection. J Neurosci 34, 11485–11503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Khan MB, Lang MJ, Huang MB, Raymond A, Bond VC, Shiramizu B, Powell MD (2016) Nef exosomes isolated fromtheplasmaofindividualswithHIV-associateddementia (HAD) can induce Aβ(1–42) secretion in SH-SY5Y neural cells. J Neurovirol 22, 179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Everall I, Vaida F, Khanlou N, Lazzaretto D, Achim C, Letendre S, Moore D, Ellis R, Cherner M, Gelman B, Morgello S, Singer E, Grant I, Masliah E; National NeuroAIDS Tissue Consortium (NNTC) (2009) Cliniconeuropathologic correlates of human immunodeficiency virus in the era of antiretroviral therapy. J Neurovirol 15, 360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Chang L, Jiang C, Cunningham E, Buchthal S, Douet V, Andres M, Ernst T (2014) Effects of APOE e4, age, and HIV on glial metabolites and cognitive deficits. Neurology 82, 2213–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Schrier RD, Gupta S, Riggs P, Cysique LA, Letendre S, Jin H, Spector SA, Singh KK, Wolfson T, Wu Z, Hong KX, Yu X, Shi C, Heaton RK; HNRC Group (2012) The influence of HLA on HIV-associated neurocognitive impairment in Anhui, China. PloS One 7, e32303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Gisslén M, Krut J, Andreasson U, Blennow K, Cinque P, Brew BJ, Spudich S, Hagberg L, Rosengren L, Price RW, Zetterberg H (2009) Amyloid and tau cerebrospinal fluid biomarkers in HIV infection. BMC Neurol 9, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Blas-García A, Polo M, Alegre F, Funes HA, Martínez E, Apostolova N, Esplugues JV (2014) Lack of mitochondrial toxicity of darunavir, raltegravir and rilpivirine in neurons and hepatocytes: A comparison with efavirenz. J Antimicrob Chemother 69, 2995–3000. [DOI] [PubMed] [Google Scholar]
- [210].Jensen BK, Monnerie H, Mannell MV, Gannon PJ, Espinoza CA, Erickson MA, Bruce-Keller AJ, Gelman BB, Briand LA, Pierce RC, Jordan-Sciutto KL, Grinspan JB (2015) Altered oligodendrocyte maturation and myelin maintenance: The role of antiretrovirals in HIV-associated neurocognitive disorders. J Neuropathol Exp Neurol 74, 1093–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Gannon PJ, Akay-Espinoza C, Yee AC, Briand LA, Erickson MA, Gelman BB, Gao Y, Haughey NJ, Zink MC, Clements JE, Kim NS, Van De Walle G, Jensen BK, Vassar R, Pierce RC, Gill AJ, Kolson DL, Diehl JA, Mankowski JL, Jordan-Sciutto KL (2017) HIV protease inhibitors alter amyloid precursor protein processing via β-site amyloid precursor protein cleaving enzyme-1 translational up-regulation. Am J Pathol 187, 91–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Akay C, Cooper M, Odeleye A, Jensen BK, White MG, Vassoler F, Gannon PJ, Mankowski J, Dorsey JL, Buch AM, Cross SA, Cook DR, Pena MM, Andersen ES, Christofidou-Solomidou M,Lindl KA,Zink MC,Clements J, Pierce RC, Kolson DL, Jordan-Sciutto KL (2014) Antiretroviral drugs induce oxidative stress and neuronal damage in the central nervous system. J Neurovirol 20, 39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Robertson K, Liner J, Meeker RB (2012) Antiretroviral neurotoxicity. J Neurovirol 18, 388–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Giunta B, Ehrhart J, Obregon DF, Lam L, Le L, Jin J, Fernandez F, Tan J, Shytle RD (2011) Antiretroviral medications disrupt microglial phagocytosis of β-amyloid and increase its production by neurons: Implications for HIV-associated neurocognitive disorders. Mol Brain 4, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Lan X, Kiyota T, Hanamsagar R, Huang Y, Andrews S, Peng H, Zheng JC, Swindells S, Carlson GA, Ikezu T (2012) The effect of HIV protease inhibitors on amyloid-β peptide degradation and synthesis in human cells and Alzheimer’s disease animal model. J Neuroimmune Pharmacol 7, 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Zlokovic BV (2005) Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci 28, 202–208. [DOI] [PubMed] [Google Scholar]
- [217].Deane R, Wu Z, Zlokovic BV (2004) RAGE (Yin) versus LRP (Yang) balance regulates Alzheimer amyloid beta-peptide clearance through transport across the blood-brain barrier. Stroke 35, 2628–2631. [DOI] [PubMed] [Google Scholar]
- [218].Kotler DP (2008) HIV and antiretroviral therapy: Lipid abnormalities and associated cardiovascular risk in HIV-infected patients. J Acquir Immune Defic Syndr 49, S79–S85. [DOI] [PubMed] [Google Scholar]
- [219].Tran H, Robinson S, Mikhailenko I, Strickland DK (2003) Modulation of the LDL receptor and LRP levels by HIV protease inhibitors. J Lipid Res 44, 1859–1869. [DOI] [PubMed] [Google Scholar]
- [220].Vekrellis K, Ye Z, Qiu WQ, Walsh D, Hartley D, Chesneau V, Rosner MR, Selkoe DJ (2000) Neurons regulate extra-cellular levels of amyloid beta-protein via proteolysis by insulin-degrading enzyme. J Neurosci 20, 1657–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Qiu WQ, Ye Z, Kholodenko D, Seubert P, Selkoe DJ (1997) Degradation of amyloid beta-protein by a metalloprotease secreted by microglia and other neural and non-neuralcells. J Biol Chem 272, 6641–6646. [DOI] [PubMed] [Google Scholar]
- [222].Qiu WQ, Walsh DM, Ye Z, Vekrellis K, Zhang J, Podlisny MB, Rosner MR, Safavi A, Hersh LB, Selkoe DJ (1998) Insulin-degrading enzyme regulates extracellular levels of amyloid beta-protein by degradation. J Biol Chem 273, 32730–32738. [DOI] [PubMed] [Google Scholar]
- [223].Sudoh S, Frosch MP, Wolf BA (2002) Differential effects of proteases involved in intracellular degradation of amyloid beta-protein between detergent-soluble and -insoluble pools in CHO-695 cells. Biochemistry 41, 1091–1099. [DOI] [PubMed] [Google Scholar]
- [224].Iwata N, Tsubuki S, Takaki Y, Shirotani K, Lu B, Gerard NP, Gerard C, Hama E, Lee HJ, Saido TC (2001) Metabolic regulation of brain Abeta by neprilysin. Science 292, 1550–1552. [DOI] [PubMed] [Google Scholar]
- [225].Eckman EA, Watson M, Marlow L, Sambamurti K, Eckman CB (2003) Alzheimer’s disease beta-amyloid peptide is increased in mice deficient in endothelin-converting enzyme. J Biol Chem 278, 2081–2084. [DOI] [PubMed] [Google Scholar]
- [226].Hamel FG, Fawcett J, Tsui BT, Bennett RG, Duckworth WC (2006) Effect of nelfinavir on insulin metabolism, proteasome activity and protein degradation in HepG2 cells. Diabetes, Obes Metab 8, 661–668. [DOI] [PubMed] [Google Scholar]
- [227].Mirsattari SM, Power C, Nath A (1998) Parkinsonism with HIV infection. Mov Disord 13, 684–689. [DOI] [PubMed] [Google Scholar]
- [228].Thomas JB, Brier MR, Snyder AZ, Vaida FF, Ances BM (2013) Pathways to neurodegeneration: Effects of HIV and aging on resting-state functional connectivity. Neurology 80, 1186–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].DeVaughn S, Muller-Oehring EM, Markey B, Bronte-Stewart HM, Schulte T (2015) Aging with HIV-1 infection: Motor functions, cognition, and attention–A comparison with Parkinson’s disease. Neuropsychol Rev 25, 424–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Koutsilieri E, Sopper S, Scheller C, ter Meulen V, Riederer P (2002) Parkinsonism in HIV dementia. J Neural Transm (Vienna) 109, 767–775. [DOI] [PubMed] [Google Scholar]
- [231].Wang GJ, Chang L, Volkow ND, Telang F, Logan J, Ernst T, Fowler JS (2004) Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain 127, 2452–2458. [DOI] [PubMed] [Google Scholar]
- [232].Kumar AM, Ownby RL, Waldrop-Valverde D, Fernandez B, Kumar M (2011) Human immunodeficiency virus infection in the CNS and decreased dopamine availability: Relationship with neuropsychological performance. J Neurovirol 17, 26–40. [DOI] [PubMed] [Google Scholar]
- [233].Jang H, Boltz DA, Webster RG, Smeyne RJ (2009) Viral parkinsonism. Biochim Biophys Acta 1792, 714–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Ogata A, Tashiro K, Nukuzuma S, Nagashima K,Hall WW (1997) A rat model of Parkinson’s disease induced by Japanese encephalitis virus. J Neurovirol 3, 141–147. [DOI] [PubMed] [Google Scholar]
- [235].Goldsmith JR, Herishanu YO, Podgaietski M, Kordysh E (1997) Dynamics of parkinsonism-Parkinson’s disease in residents of adjacent kibbutzim in Israel’s Negev. Environ Res 73, 156–161. [DOI] [PubMed] [Google Scholar]
- [236].Kumar A, Calne SM, Schulzer M, Mak E, Wszolek Z, Van Netten C, Tsui JK, Stoessl AJ, Calne DB (2004) Clustering of Parkinson disease: Shared cause or coincidence? Arch Neurol 61, 1057–1060. [DOI] [PubMed] [Google Scholar]
- [237].Alfahad T, Nath A (2013) Retroviruses and amyotrophic lateral sclerosis. Antiviral Res 99, 180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Li W, Lee MH, Henderson L, Tyagi R, Bachani M, Steiner J, Campanac E, Hoffman DA, von Geldern G, Johnson K, Maric D, Morris HD, Lentz M, Pak K, Mammen A, Ostrow L, Rothstein J, Nath A (2015) Human endogenous retrovirus-K contributes to motor neuron disease. Sci Transl Med 7, 307ra153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Viola MV, Frazier M, White L, Brody J, Spiegelman S (1975) RNA-instructed DNA polymerase activity in a cytoplasmic particulate fraction in brains from Guamanian patients. J Exp Med 142, 483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Narciso P,Galgani S,DelGrosso B,DeMarco M,De Santis A, Balestra P, Ciapparoni V, Tozzi V (2001) Acute disseminated encephalomyelitis as manifestation of primary HIV infection. Neurology 57, 1493–1496. [DOI] [PubMed] [Google Scholar]
- [241].Cardenas VA,Meyerhoff DJ,Studholme C,Kornak J,Roth-lind J, Lampiris H, Neuhaus J, Grant RM, Chao LL, Truran D, Weiner MW (2009) Evidence for ongoing brain injury in human immunodeficiency virus-positive patients treated with antiretroviral therapy. J Neurovirol 15, 324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Chang L, Wong V, Nakama H, Watters M, Ramones D, Miller EN, Cloak C, Ernst T (2008) Greater than age-related changes in brain diffusion of HIV patients after 1 year. J Neuroimmune Pharmacol 3, 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Cloak CC, Chang L, Ernst T (2004) Increased frontal white matter diffusion is associated with glial metabolites and psychomotor slowing in HIV. J Neuroimmunol 157, 147–152. [DOI] [PubMed] [Google Scholar]
- [244].Haddow LJ, Dudau C, Chandrashekar H, Cartledge JD, Hyare H, Miller RF, Jager HR (2014) Cross-sectional study of unexplained white matter lesions in HIV positive individuals undergoing brain magnetic resonance imaging. AIDS Patient Care STDS 28, 341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Seider TR, Gongvatana A, Woods AJ, Chen H, Porges EC, Cummings T, Correia S, Tashima K, Cohen RA (2016) Age exacerbates HIV-associated white matter abnormalities. J Neurovirol 22, 201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Nir TM, Jahanshad N, Busovaca E, Wendelken L, Nicolas K, Thompson PM, Valcour VG (2014) Mapping white matter integrity in elderly people with HIV. Hum Brain Mapp 35, 975–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Wu Y, Storey P, Cohen BA, Epstein LG, Edelman RR, Ragin AB (2006) Diffusion alterations in corpus callosum of patients with HIV. AJNR Am J Neuroradiol 27, 656–660. [PMC free article] [PubMed] [Google Scholar]
- [248].Wright PW, Vaida FF, Fernández RJ, Rutlin J, Price RW, Lee E, Peterson J, Fuchs D, Shimony JS, Robertson KR, Walter R, Meyerhoff DJ, Spudich S, Ances BM (2015) Cerebral white matter integrity during primary HIV infection. AIDS 29, 433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Gongvatana A, Cohen RA, Correia S, Devlin KN, Miles J, Kang H, Ombao H, Navia B, Laidlaw DH, Tashima KT (2011) Clinical contributors to cerebral white matter integrity in HIV-infected individuals. J Neurovirol 17, 477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Wright PW, Heaps JM, Shimony JS, Thomas JB, Ances BM (2012) The effects of HIV and combination antiretroviral therapy on white matter integrity. AIDS 26, 1501–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].McMurtray A, Nakamoto B, Shikuma C, Valcour V (2008) Cortical atrophy and white matter hyperintensities in HIV: The Hawaii Aging with HIV Cohort Study. J Stroke Cerebrovasc Dis 17, 212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Fennema-Notestine C, Ellis RJ, Archibald SL, Jernigan TL, Letendre SL, Notestine RJ, Taylor MJ, Theilmann RJ, Julaton MD, Croteau DJ, Wolfson T, Heaton RK, Gamst AC, Franklin DR Jr, Clifford DB, Collier AC, Gelman BB, Marra C, McArthur JC, McCutchan JA, Morgello S, Simpson DM, Grant I; CHARTER Group (2013) Increases in brain white matter abnormalities and subcortical gray matter are linked to CD4 recovery in HIV infection. J Neurovirol 19, 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Chang L, Cloak C, Patterson K, Grob C, Miller EN, Ernst T (2005) Enlarged striatum in abstinent methamphetamine abusers: A possible compensatory response. Biol Psychiatry 57, 967–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TD, Heaton RK, Ellis RJ, Grant I (2005) Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry 162, 1461–1472. [DOI] [PubMed] [Google Scholar]
- [255].Johnson T, Nath A (2010) Neurological complications of immune reconstitution in HIV-infected populations. Ann N Y Acad Sci 1184, 106–120. [DOI] [PubMed] [Google Scholar]
- [256].Sidhu N, McCutchan JA (2010) Unmasking of PML by HAART: Unusual clinical features and the role of IRIS. J Neuroimmunol 219, 100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE (2005) Influence of HAART on HIV-related CNS disease andneuroinflammation.JNeuropatholExpNeurol 64,529–536. [DOI] [PubMed] [Google Scholar]
- [258].Lane SD, Steinberg JL, Ma L, Hasan KM, Kramer LA, Zuniga EA, Narayana PA, Moeller FG (2010) Diffusion tensor imaging and decision making in cocaine dependence. PLoS One 5, e11591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Kim IS, Kim YT, Song HJ, Lee JJ, Kwon DH, Lee HJ, Kim MN, Yoo DS, Chang Y (2009) Reduced corpus callosum white-matter microstructural integrity revealed by diffusion tensor eigenvalues in abstinent methamphetamine addicts. Neurotoxicology 30, 209–213. [DOI] [PubMed] [Google Scholar]
- [260].Tobias MC, O’Neill J, Hudkins M, Bartzokis G, Dean AC, London ED (2010) White-matter abnormalities in brain during early abstinence from methamphetamine abuse. Psychopharmacology (Berl) 209, 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, Valdes I, Swann AC, Barratt ES,Narayana PA (2005) Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: Diffusion tensor imaging. Neuropsychopharmacology 30, 610–617. [DOI] [PubMed] [Google Scholar]
- [262].Bernardin F, Maheut-Bosser A, Paille F (2014) Cognitive impairments in alcohol-dependent subjects. Front Psychiatry 5, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Schmidt KS, Gallo JL, Ferri C, Giovannetti T, Sestito N, Libon DJ, Schmidt PS (2005) The neuropsychological profile of alcohol-related dementia suggests cortical and subcortical pathology. Dement Geriatr Cogn Disord 20, 286–291. [DOI] [PubMed] [Google Scholar]
- [264].Colfax G, Shoptaw S (2005) The methamphetamine epidemic: Implications for HIV prevention and treatment. Curr HIV/AIDS Rep 2, 194–199. [DOI] [PubMed] [Google Scholar]
- [265].Rajasingham R, Mimiaga MJ, White JM, Pinkston MM, Baden RP, Mitty JA (2012) A systematic review of behavioral and treatment outcome studies among HIV-infected men who have sex with men who abuse crystal methamphetamine. AIDS Patient Care STDS 26, 36–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Tang VM, Lang DJ, Giesbrecht CJ, Panenka WJ, Willi T, Procyshyn RM, Vila-Rodriguez F, Jenkins W, Lecomte T, Boyda HN, Aleksic A, MacEwan GW, Honer WG, Barr AM (2015) White matter deficits assessed by diffusion tensor imaging and cognitive dysfunction in psychostimulant users with comorbid human immunodeficiency virus infection. BMC Res Notes 8, 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Durazzo TC, Gazdzinski S, Rothlind JC, Banys P, Meyerhoff DJ (2006) Brain metabolite concentrations and neurocognition during short-term recovery from alcohol dependence: Preliminary evidence of the effects of concurrent chronic cigarette smoking. Alcohol Clin Exp Res 30, 539–551. [DOI] [PubMed] [Google Scholar]
- [268].Pfefferbaum A, Adalsteinsson E, Sullivan EV (2005) Cortical NAA deficits in HIV infection without dementia: Influence of alcoholism comorbidity. Neuropsychopharmacology 30, 1392–1399. [DOI] [PubMed] [Google Scholar]
- [269].Rosenbloom MJ,Sullivan EV,Pfefferbaum A (2010) Focus on the brain: HIV infection and alcoholism: Comorbidity effects on brain structure and function. Alcohol Res Health 33, 247–257. [PMC free article] [PubMed] [Google Scholar]
- [270].Tachiwana H, Shimura M, Nakai-Murakami C, Tokunaga K, Takizawa Y, Sata T, Kurumizaka H, Ishizaka Y (2006) HIV-1Vprinduces DNA double-strand breaks.CancerRes 66, 627–631. [DOI] [PubMed] [Google Scholar]
- [271].Fregoso OI, Emerman M (2016) Activation of the DNA damage response is a conserved function of HIV-1 and HIV-2V pr that is independent of SLX4 recruitment.MBio 7.doi: 10.1128/mBio.01433-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [272].Zimmerman ES, Chen J, Andersen JL, Ardon O, Dehart JL, Blackett J, Choudhary SK, Camerini D, Nghiem P, Planelles V (2004) Human immunodeficiency virus type 1 Vpr-mediated G2 arrest requires Rad17 and Hus1 and induces nuclear BRCA1 and gamma-H2AX focus formation. Mol Cell Biol 24, 9286–9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Roshal M, Kim B, Zhu Y, Nghiem P, Planelles V (2003) Activation of the ATR-mediated DNA damage response by the HIV-1 viral protein R. J Biol Chem 278, 25879–25886. [DOI] [PubMed] [Google Scholar]
- [274].Yuan H, Kamata M, Xie YM, Chen IS (2004) Increased levels of Wee-1 kinase in G(2) are necessary for Vpr- and gamma irradiation-induced G(2) arrest. J Virol 78, 8183–8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Nakai-Murakami C, Shimura M, Kinomoto M, Takizawa Y, Tokunaga K, Taguchi T, Hoshino S, Miyagawa K, Sata T, Kurumizaka H, Yuo A, Ishizaka Y (2007) HIV-1 Vpr induces ATM-dependent cellular signal with enhanced homologous recombination. Oncogene 26, 477–486. [DOI] [PubMed] [Google Scholar]
- [276].Daniel R, Marusich E, Argyris E, Zhao RY, Skalka AM, Pomerantz RJ (2005) Caffeine inhibits human immunodeficiency virus type 1 transduction of nondividing cells. J Virol 79, 2058–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Engelman A, Mizuuchi K, Craigie R (1991) HIV-1 DNA integration: Mechanism of viral DNA cleavage and DNA strand transfer. Cell 67, 1211–1221. [DOI] [PubMed] [Google Scholar]
- [278].Engelman A, Craigie R (1992) Identification of conserved amino acid residues critical for human immunodeficiency virus type 1 integrase function in vitro. J Virol 66, 6361–6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Daniel R, Katz RA, Skalka AM (1999) A role for DNA-PK in retroviral DNA integration. Science 284, 644–647. [DOI] [PubMed] [Google Scholar]
- [280].Lau A, Swinbank KM, Ahmed PS, Taylor DL, Jackson SP, Smith GC, O’Connor MJ (2005) Suppression of HIV-1 infection by a small molecule inhibitor of the ATM kinase. Nat Cell Biol 7, 493–500. [DOI] [PubMed] [Google Scholar]
- [281].Smith JA, Wang FX, Zhang H, Wu KJ, Williams KJ, Daniel R (2008) Evidence that the Nijmegen breakage syndrome protein, an early sensor of double-strand DNA breaks (DSB), is involved in HIV-1 post-integration repair by recruiting the ataxia telangiectasia-mutated kinase in a process similar to, but distinct from, cellular DSB repair. Virol J 5, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Koyama T, Sun B, Tokunaga K, Tatsumi M, Ishizaka Y (2013) DNA damage enhances integration of HIV-1 into macrophages by overcoming integrase inhibition. Retrovirology 10, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Sakurai Y, Komatsu K, Agematsu K, Matsuoka M (2009) DNA double strand break repair enzymes function at multiple steps in retroviral infection. Retrovirology 6, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Siva AC, Bushman F (2002) Poly(ADP-ribose)polymerase 1 is not strictly required for infection of murine cells by retroviruses. J Virol 76, 11904–11910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Dehart JL, Andersen JL, Zimmerman ES, Ardon O, An DS, Blackett J, Kim B, Planelles V (2005) The ataxia telangiectasia-mutated and Rad3-related protein is dispensable for retroviral integration. J Virol 79, 1389–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Baekelandt V, Claeys A, Cherepanov P, De Clercq E, De Strooper B, Nuttin B, Debyser Z (2000) DNA-dependent protein kinase is not required for efficient lentivirus integration. J Virol 74, 11278–11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287].Ariumi Y, Turelli P, Masutani M, Trono D (2005) DNA damage sensors ATM, ATR, DNA-PKcs, and PARP-1 are dispensableforhumanimmunodeficiencyvirustype1integration. J Virol 79, 2973–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]