Abstract
We report a fungal pan-genome study involving Parastagonospora spp., including 21 isolates of the wheat (Triticum aestivum) pathogen Parastagonospora nodorum, 10 of the grass-infecting Parastagonospora avenae, and 2 of a closely related undefined sister species. We observed substantial variation in the distribution of polymorphisms across the pan-genome, including repeat-induced point mutations, diversifying selection and gene gains and losses. We also discovered chromosome-scale inter and intraspecific presence/absence variation of some sequences, suggesting the occurrence of one or more accessory chromosomes or regions that may play a role in host–pathogen interactions. The presence of known pathogenicity effector loci SnToxA, SnTox1, and SnTox3 varied substantially among isolates. Three P. nodorum isolates lacked functional versions for all three loci, whereas three P. avenae isolates carried one or both of the SnTox1 and SnTox3 genes, indicating previously unrecognized potential for discovering additional effectors in the P. nodorum-wheat pathosystem. We utilized the pan-genomic comparative analysis to improve the prediction of pathogenicity effector candidates, recovering the three confirmed effectors among our top-ranked candidates. We propose applying this pan-genomic approach to identify the effector repertoire involved in other host–microbe interactions involving necrotrophic pathogens in the Pezizomycotina.
Keywords: Parastagonospora nodorum, Pan-genome, plant pathogen, crop disease, host–microbe interactions
Introduction
The Parastagonospora Pathosystems
Parastagonospora (teleomorph: Phaeosphaeria) nodorum (Berk.) is an economically important necrotrophic fungal pathogen that causes septoria nodorum blotch (SNB) in wheat (Triticum aestivum) (Solomon et al. 2006) and is also a model organism for the fungal order Pleosporales and for necrotrophic phytopathogenicity (Oliver and Solomon 2010; Tan et al. 2010; Oliver et al. 2012). Significant experimental resources are available for P. nodorum, including a high-quality genome assembly of the reference strain SN15 (Hane et al. 2007; Syme et al. 2016), microarray analyses of gene expression (Ipcho et al. 2012), proteomics and proteogenomics, metabolomic profiling, and comparative genomics versus two contrasting P. nodorum strains: Sn4 and Sn79-1087 (Syme et al. 2013; Richards et al. 2018). SN15 and Sn4 are highly aggressive isolates on wheat, whereas Sn79-1087 (hereafter referred to as Sn79) was isolated from the wild grass Agropyron, is avirulent on wheat, and serves as a useful negative control for comparative genomics in a disease context (Friesen et al. 2006; Richards et al. 2018).
In this study we resequenced the genomes of 18 new strains of P. nodorum as well as 10 strains representing closely related species within the Parastagonospora genus (table 1). Parastagonospora avenae is a species associated with SNB-like symptoms in various Poaceae spp. (Quaedvlieg et al. 2013). P. avenae was further subdivided into two formae speciales. P. avenae f. sp. avenaria (Paa) infects oats (Avena spp.) and P. avenae f. sp. triticea (Pat) infects wheat and some other grasses (Cunfer 2000; Ueng et al. 2003; Malkus et al. 2005). Restriction fragment length polymorphism patterns were used to further differentiate Pat strains into distinct subgroups named Pat1-Pat6 (Ueng and Chen 1994; Ueng et al. 1995; Ueng et al. 1998). Two isolates of a sister species, provisionally named P2 (Phaeosphaeria 2) were collected from Iranian wheat fields. The placement of P2 is not yet clear, but sequences of the β-tubulin and β-xylosidase loci were similar to P. nodorum and P. avenae (McDonald et al. 2012).
Table 1.
Summary of Source Material and Resequencing Data for Strains of (A) P. nodorum, (B) P. avenae, and (C) the P2 clade
| Isolate ID | Source | Collection Year | Total Length of Error-corrected Reads (Mb) | Estimated Genome Coverage (X) | Isolation Source | Contributor |
|---|---|---|---|---|---|---|
| (A) P. nodorum strains | ||||||
| B2.1b | Iran | 2005 | 686.1 | 9.2 | T. aestivum | Mohammad Razavi, Iranian Research Institute of Plant Protection |
| C1.2a | 886.1 | 11.9 | ||||
| IR10_9.1a | 2010 | 528.4 | 7.1 | |||
| FIN-2 | Finland | unknown | 1,998.7 | 26.9 | Andrea Ficke, Nibio, As, Norway | |
| SWE-3 | Sweden | 1,517.8 | 20.4 | |||
| Sn Cp2052 | 1,031.9 | 13.9 | Hans Jorgensen, University of Copenhagen | |||
| BRSn9870 | Brazil | 3,316.9 | 44.6 | Flavio Santana, EMBRAPA | ||
| Sn99CH 1A7a | Switzerland | 1999 | 3,888.2 | 52.3 | Bruce McDonald, ETH Zurich | |
| SnChi01 40a | China | 2001 | 2,796.4 | 37.6 | R. Wu, Louyuan, Fujian Province | |
| SnSA95.103 | South Africa | 1994 | 1,019 | 13.7 | Pedro Crous, University of Stellenbosch, South Africa | |
| AR1-1 | Arkansas, USA | 2011 | 1,986.2 | 26.7 | Christina Cowger, USDA-ARS | |
| GA9-1 | Georgia, USA | 4,296.5 | 57.8 | |||
| MD4-1 | Maryland, USA | 2012 | 4,325.8 | 58.2 | ||
| VA 5-2 | Virginia, USA | 2,288.3 | 30.8 | |||
| OH03 Sn-1501 | Ohio, USA | 2003 | 2,229.8 | 30 | Pat Lipps, Ohio State University | |
| SNOV92X D1.3 | Texas, USA | 1992 | 2,524.6 | 34 | Bruce McDonald, Texas A&M University | |
| SnOre11-1 | Oregon, USA | 2011 | 4,763.4 | 64.1 | – | |
| WAC8410 | Australia | 2010 | 6,032.4 | 81.2 | Department of Agriculture and Food, Western Australia | |
| (B) P. avenae strains | ||||||
| (i) P. avenae f. sp. triticea 1 | ||||||
| IR10_5.2b | Iran | 2010 | 850.5 | 11.4 | T. aestivum | Mohammad Razavi, Iranian Research Institute of Plant Protection |
| Hartney99 | Canada | 2005 | 1,132.5 | 15.2 | T. aestivum (spring wheat) | – |
| Jansen #4_55 | 480.1 | 6.5 | – | |||
| (ii) P. avenae f. sp. triticea 4 | ||||||
| SN11IR_2_1.1 | Iran | 2011 | 1,415.3 | 19 | Dactylis glomerata | Mohammad Razavi, Iranian Research Institute of Plant Protection |
| (iii) P. avenae f. sp. triticea 5 | ||||||
| 82-4841 | North Dakota, USA | 1982 | 1,207.7 | 16.2 | Unknown grass | Joe Krupinsky, USDA-ARS |
| 83-6011-2 | 1983 | 1,136.3 | 15.3 | Bromus (Brome grass) | ||
| iv) P. avenae f. sp. triticea 6 | ||||||
| SN11IR_6_1.1 | Iran | 2011 | 2,150.2 | 28.9 | Agropyron tauri | Mohammad Razavi, Iranian Research Institute of Plant Protection |
| SN11IR_7_2.3 | Iran | 2011 | 1,039.4 | 14 | Dactylis glomerata | |
| (v) P. avenae f. sp. Avenaria | ||||||
| Mt. Baker | Washington, USA | 2009 | 585.8 | 7.9 | Avena sativa | – |
| s258 | Netherlands | 2005 | 1,163.2 | 15.6 | – | |
| (C) P2 strains (McDonald et al. 2012) | ||||||
| A1 3.1a | Iran | 2005 | 1,813.1 | 24.4 | T. aestivum | Mohammad Razavi, Iranian Research Institute of Plant Protection |
| H6.2b | 2005 | 3,030 | 40.8 | |||
Many of the recent studies of Parastagonospora spp. are oriented around an important class of genes encoding effector molecules that interact with the host to determine the outcome of specific host–pathogen interactions (Vleeshouwers and Oliver 2014). A class of effectors called necrotrophic effectors (NEs) interacts with host dominant susceptibility genes to form an inverse gene-for-gene interaction (Oliver and Solomon 2010; Tan et al. 2010). A compatible interaction results in plant cell death and subsequently, infection. Parastagonospora spp. were already shown to produce several NEs (Friesen et al. 2007), which are thought to maximize the likelihood of interacting with a corresponding susceptibility protein in the host. So far, three well characterized NE genes have been identified in P. nodorum: SnToxA (SNOG_16571) (Friesen et al. 2006), SnTox1 (SNOG_20078) (Liu et al. 2004; Liu et al. 2012), and SnTox3 (SNOG_08981) (Friesen et al. 2008; Liu et al. 2009). These three effectors are already used in breeding programs to accelerate development of disease-resistant cereal cultivars (Vleeshouwers and Oliver 2014), but several NE loci have not yet been identified to the gene level, including SnTox2 (Friesen et al. 2007), SnTox4 (Abeysekara et al. 2009), SnTox5 (Friesen et al. 2012), SnTox6 (Gao et al. 2015), and SnTox7 (Shi et al. 2015). Although continued laboratory testing may yield new effectors, further advances in genome sequencing technologies and bioinformatic methods may also improve effector discovery (Jones et al. 2018). This study provides enhancements to the P. nodorum SN15 reference strain assembly and its gene annotations, but also explores features of the Parastagonospora fungal genomes that are relevant for effector discovery, including repeat-induced point mutation (RIP), presence–absence variation (PAV), and diversifying selection.
RIP is a fungal-specific form of mutation that targets repetitive sequences and introduces cytosine to thymine (C → T) transitions, or the reverse complement G → A. In the filamentous Ascomycota (syn. Pezizomycotina) where RIP is commonly observed (Testa et al. 2016), there is a strong bias for mutations at cytosine bases adjacent to adenine (CpA → TpA). RIP provides a mechanism of genome defence against transposon invasion, by disabling transposable elements through introduction of premature stop codons into their open reading frames and/or through silencing of the RIP-mutated sequence through further DNA methylation (Galagan and Selker 2004; Hane and Oliver 2008; Clutterbuck 2011; Hane 2015; Hane et al. 2015). RIP has also been linked to mutation of avirulence effector genes AvrLm1, AvrLm6, and AvrLm4-7 in Leptosphaeria maculans through leakage into nonrepetitive regions flanking repeats (Fudal et al. 2009; Van de Wouw et al. 2010). Fungal genes most likely to be affected by RIP leakage can be identified by annotation of AT-rich regions with OcculterCut (Testa et al. 2016).
Recent resequencing studies have repeatedly shown that PAV in gene content is common in Fungi (McDonald et al. 2013; Gao et al. 2015; Golicz et al. 2015). PAV patterns have been observed at both the gene cluster level (Plissonneau et al. 2016) and at whole (or partial) chromosome level (Ma et al. 2010; Goodwin et al. 2011), and may indicate variable effector loci when applied across multiple isolates of a single species with a range of virulence phenotypes. Sectional absences of small groups of genes were previously reported between the reference strain SN15 and other strains of P. nodorum (Syme et al. 2013). PAVs related to known effectors vary in length, with SnTox1 and SnTox3 absent from the wheat-avirulent strain Sn79 in small 2 and 4 kb stretches, respectively, whereas SnToxA is part of a much larger 72 kb absence in Sn79 (Syme et al. 2013). The PAV pattern of known effectors also varies in field populations of Parastagonospora spp. (fig. 1), which may indicate multiple, independent horizontal gene transfer (HGT) events (McDonald et al. 2013). Notably, there does not appear to be a significant fitness penalty incurred by the pathogen harboring an effector when growing on a host that lacks the corresponding sensitivity gene (McDonald et al. 2013). Genes within repeat-rich regions may be more prone to loss due to mesosyntenic recombination (Hane et al. 2011) or breakage fusion bridge cycles (Bertazzoni et al. 2018).
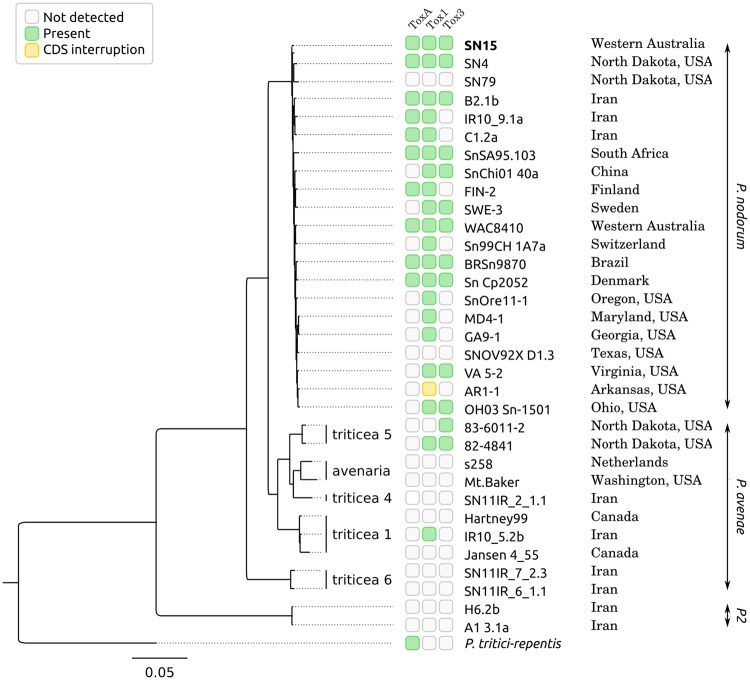
Fig. 1.
—A phylogeny of the P. nodorum, P. avenae, and P2 strains used in this study and the presence or absence of known effector loci: ToxA, Tox1, and Tox3. Green boxes indicate the presence of a known effector locus in that strain and yellow box indicates the presence of a psuedogenized version.
The genomes of some pathogenic species are compartmentalized into regions with “two speeds” of evolution: “core” gene content—which tends to be well-conserved among strains and under purifying selection, and “accessory” gene content—which typically is repeat-rich and exhibits both PAV and evidence for diversifying selection. For some species, such as L. maculans, these variable regions are interspersed throughout the genome. In other species, accessory and core gene contents are divided among separate chromosomes (Bertazzoni et al. 2018). Fungal accessory (syn. supernumerary or dispensable) chromosomes have been observed to be gene sparse, repeat-rich, and exhibit higher rates of mutation, positive selection and pathogenicity-associated loci relative to the “core” chromosomes (Bertazzoni et al. 2018). The P. nodorum isolates in this study may also contain accessory sequences, as wheat- and barley-infecting isolates have been previously reported to possess an accessory chromosome of approximately 400 kb (Zolan 1995). Furthermore, P. nodorum SN15 scaffold_46 was previously predicted to be dispensable based on sharing common characteristics with Z. tritici accessory chromosomes, including low GC content, high repeat content, low gene density and a low percentage of predicted genes with conserved functional domains (Ohm et al. 2012).
Recent advances in understanding fungal genome evolution coupled with improved criteria for identifying candidate effector genes provide new opportunities to understand the role of intraspecies genome diversity in the context of pathogenicity. Here, we compare 19 isolates of P. nodorum, 10 isolates of P. avenae, and 2 isolates of the closely related sister group P2.
Materials and Methods
Strain Sampling, DNA Extraction and Sequencing
Illumina paired-end libraries were constructed for each haploid strain. P. nodorum WAC8410 was sequenced from a TruSeq 500 bp library on an Illumina 2000 to produce 150 bp paired-end reads. All other strains were sequenced from 300 bp NexteraXT libraries on an Illumina 2500 multiplexed over two lanes.
Reference-Alignment and de novo Assembly of Strain Sequences
Raw Illumina reads were filtered for PCR duplicates, then trimmed using cutadapt v1.7.1 (Martin 2011), removing the adapter sequences, bases with quality scores less than 25, and any reads shorter than 50 bp. P. nodorum strain SN15 (Hane et al. 2007) was used as the reference against which each of the newly sequenced strains was compared. The annotated gene set of SN15 has been improved with transcriptomic and proteomic data, and manual curation of gene models (Syme et al. 2016). Trimmed reads were aligned to the reference genome using bowtie2 (Langmead and Salzberg 2012) (“very-sensitive”). Trimmed reads were also assembled de novo with SPAdes v.3.5.0 (Bankevich et al. 2012) (kmers 21, 33, 55, and 77) using mismatch and short indel correction via BWA mapping (Li 2013). Assembly quality was assessed with QUAST v2.3 (Gurevich et al. 2013). Genome assemblies were aligned to the reference with MUMmer v3.0 (nucmer) (Kurtz et al. 2004) and repetitive matches were filtered with delta-filter (–r and –q). SNP and indel variants from genome alignments were determined with MUMmer (show-snps). PAV profiles were generated by calculating the coverage of each of the SN15 reference scaffolds by nucmer matches to each isolate assembly using the genomecov function of BEDtools (Quinlan and Hall 2010). Homologs of known effectors were extracted from orthologous clusters. Each effector absence was manually confirmed by blasting the reference effector against the new strain’s genome assembly.
Genome Annotation
Manually curated annotations from the reference genome SN15 (Syme et al. 2016) were used to train CodingQuarry (Testa et al. 2015) and these parameters were applied to each of the alternate strains. A database of repeats was generated using RepeatModeler v1.0.8 (Smit and Hubley 2010) augmented with full copies of the known P. nodorum repeats Molly (AJ488502.1), Pixie (AJ488503.1), and Elsa (AJ277966.1). The RepeatModeler repeats were combined with known “DeRIPped” (predicted pre-RIP consensus) (Hane and Oliver 2010) and repbase (Jurka et al. 2005) repeats using RepeatMasker v4.0.5 (Smit et al. 2015). A final annotation set for each alternate strain (supplementary file S1, Supplementary Material online) was generated using Augustus v3.3 (Stanke et al. 2006) using annotation hints provided CodingQuarry annotations, exonerate v2.2.0 protein matches (Slater and Birney 2005) to the SN15 predicted proteome and from RepeatMasker matches described above. Secretion signals were detected using SignalP v4.1 (Bendtsen et al. 2004) and transmembrane domains by TMHMM v2.0 (Krogh et al. 2001). Secondary metabolite clusters were predicted in nonreference strains by antismash 2.1.1 (Medema et al. 2011). Orthofinder v2.2.3 (Emms and Kelly 2015) using Diamond v0.9.21 (Buchfink et al. 2015) for distance estimation was used to predict clusters of orthologous proteins and to generate a phylogeny of the isolates used in this study, using the closely related species Pyrenophora tritici-repentis as an outgroup (Moolhuijzen et al. 2018). In the case of known effector loci SnToxA, SnTox1 and SnTox3, their ortholog cluster membership was manually inspected by tblastn (Altschul et al. 1990) (e-value cutoff 1 × 10−5) queries of their translated sequences versus each of the alternate strain de novo assemblies.
Identification of Genes under Diversifying Selection
Coding sequences from all strains that were orthologous to protein-coding genes from the reference strain were extracted, translated, and aligned using ClustalW (Larkin et al. 2007). Protein truncations due to incorrect annotation in a novel strain can limit detection of diversifying selection, so only codons present in all strains were considered. Short proteins with lengths more than 1 standard deviation from the mean were excluded from these alignments to reduce the frequency of false-positive results.
Codon alignments were generated with PAL2NAL v14 (Suyama et al. 2006). The M1a and M2a site models were applied to codon-aligned transcripts of orthologous proteins to generate a maximum likelihood (ML) estimation of ω. The H0 model (PAML model M1a) confines codon membership to one of two classes where ω < 1 (purifying selection) or ω = 1 (neutral/drift). The H1 model (PAML model M2a) extends H0 to allow codon membership to a third possible class where ω > 1 (diversifying selection). Loci with sites under diversifying selection were identified where the χ2-distributed likelihood ratio of the two models exceeded the 1% significance level (2 degrees of freedom). Local patterns of selection were identified by stepping a 100 kbp window over the reference assembly in 1 kbp increments and counting the number of transcripts under diversifying selection as a percentage of the total number of transcripts in each window.
Prediction of Effector Candidates
For each reference transcript sequence, a number of tests were applied and a cumulative score was generated based on the number of passed tests. Protein scores were increased if they had a molecular mass less than 30 kDa, had a cysteine percentage greater than 4%, were longer than 200 bp, did not have tblastn hits to the wheat-avirulent Sn79 genome assembly (e-value ≤ 1 × 10−30), were in regions of low gene density (no genes predicted within the 2 kb region(s) up/downstream), were predicted to be secreted by SignalP v4.1 (Bendtsen et al. 2004), were predicted to be under positive diversifying selection pressure as described above, were not part of the core proteome, or were not predicted to encode a transmembrane domain by TMHMM (Krogh et al. 2001), were less than 5 kb from the nearest AT-rich region as predicted by OcculterCut, or had an EffectorP score greater than 0.9.
Results
Pan-Genome Resources Generated for Multiple Isolates of P. nodorum, P. avenae and P2
The Parastagonospora strains originated from a broad geographical range with a focus on the Fertile Crescent and the United States (table 1 and fig. 1) and included three previously sequenced P. nodorum strains (Syme et al. 2016; Richards et al. 2018), 18 newly sequenced P. nodorum strains, 10 newly sequenced P. avenae strains, and 2 newly sequenced strains of the closely related P2 group (McDonald et al. 2012) (BioProject: PRJNA476481). The estimated genome coverage for each strain, relative to the SN15 reference assembly, ranged between 7–64X for strains sequenced using the NexteraXT libraries and 81X for the WAC8410 isolate sequenced using a separate TruSeq library (table 1). Total genome assembly length ranged from 33.5 to 49.9 Mb (table 2).
Table 2.
Summary of Genome Assembly Metrics for Resequenced Strains of (A) P. nodorum, (B) P. avenae, and (C) the P2 clade
| Isolate ID | № Scaffolds | Largest Scaffold (kb) | Total Length (Mb) | N50 (kb) | Whole SN15 Gene Count by QUAST | Partial SN15 Gene Count by QUAST |
|---|---|---|---|---|---|---|
| (A) P. nodorum strains | ||||||
| B2.1b | 2,906 | 386.4 | 37.38 | 60.3 | 12,628 | 784 |
| C1.2a | 1,557 | 325.3 | 37.43 | 80.4 | 12,751 | 649 |
| IR10_9.1a | 3,673 | 140.6 | 37.28 | 20.7 | 11,442 | 1,920 |
| FIN-2 | 1,381 | 451.6 | 38.41 | 117.9 | 12,952 | 479 |
| SWE-3 | 1,714 | 335.5 | 37.85 | 58.8 | 12,734 | 727 |
| Sn Cp2052 | 3,026 | 190.2 | 37.32 | 38.2 | 12,310 | 1,148 |
| BRSn9870 | 4,911 | 309.9 | 41.23 | 52.4 | 12,342 | 1,148 |
| Sn99CH 1A7a | 853 | 521 | 37.9 | 180.7 | 13,062 | 361 |
| SnChi01 40a | 779 | 1,268.10 | 37.88 | 206.3 | 13,118 | 332 |
| SnSA95.103 | 11,772 | 48 | 49.94 | 6.5 | 9,545 | 3,882 |
| AR1-1 | 882 | 748.5 | 36.61 | 135.6 | 12,999 | 412 |
| GA9-1 | 664 | 938.6 | 36.53 | 215.3 | 13,074 | 356 |
| MD4-1 | 701 | 599.9 | 36.53 | 191 | 13,045 | 384 |
| VA 5-2 | 1,115 | 485.6 | 36.48 | 89.1 | 12,787 | 606 |
| OH03 Sn-1501 | 1,281 | 349.9 | 37.1 | 85 | 12,844 | 577 |
| SNOV92X D1.3 | 785 | 524.1 | 36.66 | 145.6 | 13,009 | 442 |
| SnOre11-1 | 748 | 875.4 | 37.42 | 249.6 | 13,103 | 352 |
| WAC8410 | 384 | 1,060.50 | 40.27 | 316.8 | 13,223 | 277 |
| (B) P. avenae strains | ||||||
| (i) P. avenae f. sp. triticea 1 | ||||||
| IR10_5.2b | 1,681 | 267 | 35.51 | 57.3 | 18 | 14 |
| Hartney99 | 3,381 | 124.3 | 36.58 | 27.6 | 47 | 20 |
| Jansen 4_55 | 10,109 | 83.1 | 32.06 | 4.3 | 20 | 166 |
| (ii) P. avenae f. sp. triticea 4 | ||||||
| SN11IR_2_1.1 | 5,762 | 168.2 | 41.54 | 38.6 | 12 | 15 |
| (iii) P. avenae f. sp. triticea 5 | ||||||
| 82-4841 | 2,444 | 218.3 | 38.53 | 50.5 | 21 | 13 |
| 83-6011-2 | 2,367 | 193.8 | 37.52 | 43.9 | 21 | 13 |
| (iv) P. avenae f. sp. triticea 6 | ||||||
| SN11IR_6_1.1 | 1,174 | 737.1 | 33.51 | 102.8 | 3 | 7 |
| SN11IR_7_2.3 | 2,215 | 244.5 | 33.6 | 44.8 | 6 | 12 |
| (v) P. avenae f. sp. Avenaria | ||||||
| Mt. Baker | 8,309 | 38.5 | 34.14 | 6.2 | 15 | 81 |
| s258 | 4,090 | 183.1 | 39.49 | 32.8 | 16 | 21 |
| (C) P2 strains | ||||||
| H6.2b | 1,613 | 411.9 | 38.68 | 74.6 | 2 | 6 |
| A1 3.1a | 1,764 | 419.9 | 39.05 | 68.3 | 3 | 6 |
Note.—P. avenae and P2 strains show a low number of P. nodorum gene matches from QUAST due to dissimilarities in the coding sequence relative to the P. nodorum SN15 reference.
SnToxA, SnTox1, and SnTox3 Effector Loci Vary in Their Distribution Across Both Taxonomic Groups and Geographic Locations
The ortholog cluster containing the SnToxA effector gene was found in 10 P. nodorum strains (48%) but no P. avenae strains. Intact SnTox1 gene sequences were detected in 18 P. nodorum strains (86%) and 2 P. avenae strains (17%). The SnTox3 gene was detected in 11 P. nodorum strains (52%) and the 2 P. avenae Pat5 strains (figs. 1 and 2).
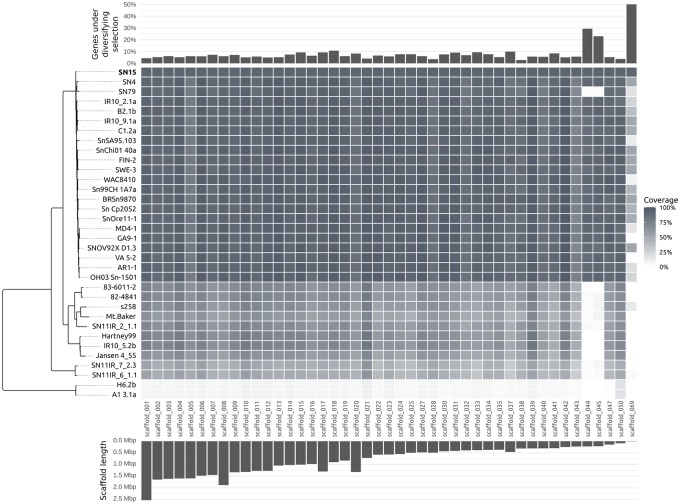
Fig. 2.
—Coverage of reference P. nodorum scaffolds by nucmer matches from the alternate strains. Scaffolds 44 and 45 (putatively dispensable) and Scaffold 51 (contains ToxA) are absent from the wheat-avirulent P. nodorum strain SN79, and all P. avenae and P2 strains.
Presence/Absence Patterns Across the Parastagonospora Pan-Genome Indicate Putative Lineage-Specific Accessory Sequences
There were differing outcomes for sectional absence based on the read-mapping methods employed in previous studies (Croll and McDonald 2012; Croll et al. 2013; McDonald et al. 2015; Syme et al. 2016) compared with de novo assembly and subsequent alignment via nucmer used here. For comparisons between P. nodorum strains and the SN15 reference, the read-mapping method was able to genotype 90.3% of the genome. In contrast, the nucmer method found fewer sectional absences and was able to identify variants over 93.5% of the genome. In more distant comparisons of P. avenae strains to the SN15 reference, read-mapping was able to genotype only 16.2% of the genome and nucmer was able to find variants over 61.9% of the genome.
Of the 51 P. nodorum SN15 reference scaffolds that contained genes, most had consistent nucmer match coverage by sequences of the resequenced strains. Scaffolds 44, 45, 51, 57, 69, 97, 99, and 101 had very low coverage by alignment of P. avenae and P2 isolates (fig. 3). These scaffolds are composed largely of repeats, except scaffolds 44 and 45 which respectively encode 75 and 61 genes and contain only 1.9% and 2.4% repetitive sequence. Scaffolds 44, 45 also had a high percentage of genes under diversifying selection (29.3% and 23.0%). The wheat-avirulent P. nodorum strain SN79 also covered scaffolds 44 and 45 over 6.7% and 5.0% of their lengths, respectively, compared with 94.4% and 96.3% coverage by the other P. nodorum strains.
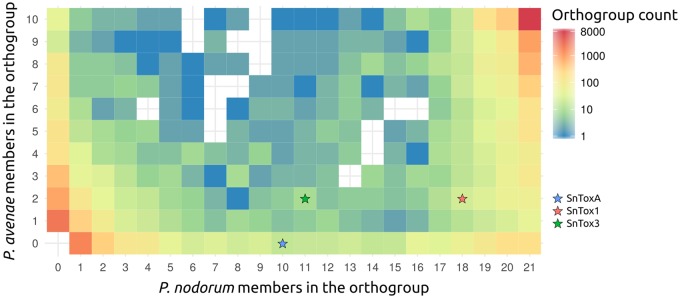
Fig. 3.
—Presence and absence of protein orthologs between P. nodorum and P. avenae strains. The number of P. nodorum strains that have contributed a protein to the cluster determines the x-axis location and the number of P. avenae strains that have contributed a protein to the cluster determines the y-axis location. Core conserved genes with members from all strains are at the top-right and strain-specific genes are at the bottom-left. About 10,798 (53.8%) of clusters are missing from at most 2 strains. About 6,229 clusters (29.0%) are present in at most 3 strains. The three known P. nodorum effectors (red, blue, and green stars) are present in only some of the isolates.
Patterns of Strain-Specificity and Positive Selection Across the Pan-Genome Refines Effector Candidate Predictions
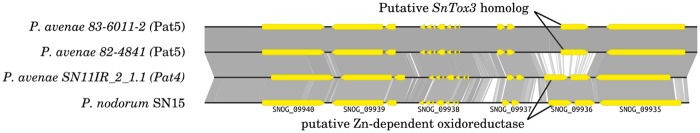
The distribution of conserved proteins between the P. avenae and P. nodorum strains shows most proteins are either strain-specific or are well conserved, but known effectors are present in the sparsely populated middle-ground (fig. 4). Of the 21,470 orthologous protein groups, 6,229 (29.0%) were observed in fewer than four strains, 12,473 (58.1%) were missing from at most one strain, and 6,620 (30.8%) were between the two extremes (table 3 and fig. 4). P. nodorum strain WAC8410 has 184 strain-specific loci (supplementary file S1 and table S2, Supplementary Material online). This strain was sequenced at higher coverage (table 1) and is a more complete assembly (table 2) than the other strains, enabling a more detailed study of its strain-specific loci. The mean intergenic flanking distance for all WAC8410 genes is 1,162 bp. The mean intergenic flanking distance for the strain-specific subset of WAC8410 genes is 6,558 bp (fig. 5). WAC8410 scaffolds 50 and 55 are rich in strain-specific genes, with 18 of 20 and 11 of 23, respectively. At least 8 of the 18 strain-specific genes on scaffold 55 appear to be pseudogenes with high rates of mutation and premature stop codons. Included in the scaffold 55 strain-specific set are a pseudogene which shows similarity to the P. nodorum PnPf2 transcription factor (Rybak et al. 2017), an indole-diterpene biosynthesis protein, and a pseudogene which shows similarity to SNOG_08983 which, in SN15, is notably near to the SnTox3 locus. Effector candidates were predicted from the SN15 reference protein set by using a series of criteria based on the expected characteristics of NEs (table 4). Scores were calculated for 13,949 SN15 proteins, of which: 616 were cysteine-rich; 451 were associated with AT-rich regions; 798 were absent from Sn79; 2,414 resided in regions of low gene density; 1,475 were predicted as secreted; and 945 were under positive selection. Effector candidate scores are presented in full in supplementary table S3, Supplementary Material online, and 63 candidates with scores of 5 and above are presented in table 5. Known effector genes SnToxA, SnTox1, and SnTox3 all scored highly using this system (table 5). Of the P. avenae isolates sequenced, only the wheat-avirulent Pat5 genomes (McDonald et al. 2012) contained putative SnTox3 orthologs (fig. 1). Interestingly, the genomic context of this gene in P. avenae is different compared with the SnTox3 positive P. nodorum strains. The putative SnTox3 ortholog in P. avenae Pat5 is in the position of a Zn-dependent oxidoreductase in P. nodorum and the other P. avenae genomes (fig. 5).
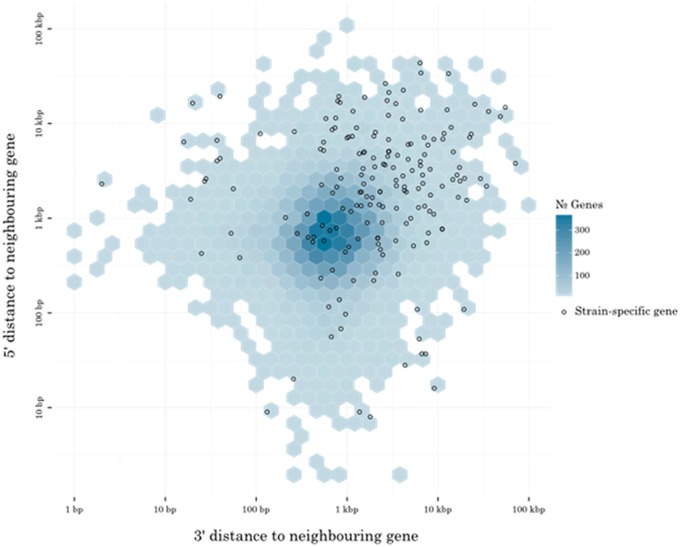
Fig. 4.
—Intergenic distances at the 3′ and 5′ ends for all genes of P. nodorum WAC8410. Intergenic distances at 3′ and 5′ for all WAC8410 genes. Hexagonal cells are colored to indicate the number of genes that have flanking intergenic distances that place it within the cell’s bounds. Strain-specific genes (highlighted as hollow circles) generally have higher distances to the neighboring gene, placing them in the top-right quadrant. The mean intergenic flank distance is 6,558 bp for strain specific genes and 1,162 bp for all other genes.
Table 3.
Summary of Protein Conservation Across the P. nodorum and P. avenae Strains
| Reference Protein Set | 13,836 proteins |
|---|---|
| Core Phaeosphaeria protein set | |
| Missing from 0 strains | 8,660 clusters |
| Missing from at most 1 strain | 9,921 clusters |
| Missing from at most 2 strains | 10,798 clusters |
| Core P. nodorum protein set | |
| Missing from 0 P. nodorum strains | 11,366 clusters (11,821 SN15 proteins) |
| Missing from at most 1 P. nodorum strain | 12,473 clusters (12,877 SN15 proteins) |
| Missing from at most 2 P. nodorum strains | 13,049 clusters (13,139 SN15 proteins) |
| Strain-specific protein set | |
| Observed in only 1 strain | 3,995 clusters (108 SN15 proteins) |
| Observed in at most 2 strains | 5,438 clusters (240 SN15 proteins) |
| Observed in at most 3 strains | 6,229 clusters (291 SN15 proteins) |
| Dispensable protein set (effector-containing set) | |
| Observed in between 4 and 30 strains (inclusive) | 6,620 clusters (321 SN15 proteins) |
| P. nodorum-specific proteins | |
| Present in all nodorum, absent from all P. avenae | 216 |
| P. avenae-specific proteins | |
| Present in all P. avenae, absent from all P. nodorum | 44 |
Note.—About 8660 protein clusters are observed in all strains. There are 204 protein clusters containing members of only one species. The set of “dispensable” proteins is defined here as proteins that are not species-specific (observed in fewer than four isolates) and not well conserved (missing in fewer than three isolates). This “dispensable” set of 2192 proteins contains 213 SN15 proteins, including all of the known effectors.
Fig. 5.
—Alignment of genes surrounding the P. avenae Pat5 Tox3 homolog with the corresponding loci from the P. nodorum SN15 reference and a representative P. avenae Pat4 region. The Tox3 gene is absent from the Pat4 genome completely, and present in the SN15, but on a different scaffold to the one shown here.
Table 4.
Counts of the Numbers of SN15 Reference Proteins That Match Each Effector Prediction Criteria
| Criteria | № proteins |
|---|---|
| Positive scores | |
| Small—less than 30 kDa | 4,362 |
| Cysteine-rich—encodes an amino acid with >4% cysteine residues | 616 |
| Near repeats—less than 5 kb from repetitive sequence | 3,417 |
| Absent from SN79—no blast hits to the avirulent strain | 798 |
| Low gene density—encoded in a region with large intergenic space | 2,414 |
| Secreted—includes a signal peptide | 1,475 |
| Under positive selection | 945 |
| EffectorP (subset of secreted) | 288 |
| OcculterCut proximity to GC-AT border | 451 |
| Negative scores | |
| Core Set—missing in at most one strain | 10,294 |
| Strain specific—only found in SN15 | 108 |
| Membrane bound—not predicted to encode a transmembrane domain | 2,381 |
Note.—Each predicted protein is assessed against each of these criteria and assigned a total score calculated as the sum of the criteria scores (table 5).
Table 5.
Top Effector Candidates with Scores ≥5
| Locus | Predicted Secreted | Absent in SN79 | <1 Gene/2 kb | ≤30 kDa | Positive Selection | ≥4% Cys | AT-Rich Regions | Effector P Score ≥0.9 | Candidate Score ≥5 |
|---|---|---|---|---|---|---|---|---|---|
| SNOG_20078 (SnTox1) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | – | ✓ | 7 |
| SNOG_01124 | ✓ | – | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 7 |
| SNOG_11452 | ✓ | ✓ | ✓ | ✓ | – | ✓ | ✓ | ✓ | 7 |
| SNOG_11453 | ✓ | ✓ | ✓ | ✓ | – | ✓ | ✓ | ✓ | 7 |
| SNOG_30077 | ✓ | ✓ | ✓ | ✓ | – | ✓ | ✓ | ✓ | 7 |
| SNOG_30343 | ✓ | – | ✓ | ✓ | – | ✓ | ✓ | – | 7 |
| SNOG_30466 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | – | ✓ | 7 |
| SNOG_30828 | ✓ | ✓ | ✓ | ✓ | – | ✓ | ✓ | ✓ | 7 |
| SNOG_16571 (SnToxA) | ✓ | ✓ | ✓ | ✓ | – | – | ✓ | ✓ | 6 |
| SNOG_08981 (SnTox3) | ✓ | ✓ | ✓ | ✓ | – | – | ✓ | ✓ | 6 |
| SNOG_01097 | ✓ | – | ✓ | ✓ | ✓ | – | ✓ | ✓ | 6 |
| SNOG_07039 | ✓ | ✓ | ✓ | ✓ | – | ✓ | – | ✓ | 6 |
| SNOG_12811 | ✓ | – | ✓ | ✓ | ✓ | ✓ | – | ✓ | 6 |
| SNOG_16520 | ✓ | ✓ | – | ✓ | ✓ | – | ✓ | ✓ | 6 |
| SNOG_20100 | ✓ | ✓ | ✓ | ✓ | – | ✓ | – | ✓ | 6 |
| SNOG_30802 | ✓ | ✓ | – | ✓ | – | ✓ | ✓ | ✓ | 6 |
| SNOG_30888 | ✓ | ✓ | ✓ | ✓ | – | ✓ | – | ✓ | 6 |
| SNOG_00726 | ✓ | – | ✓ | ✓ | ✓ | – | – | ✓ | 5 |
| SNOG_03114 | ✓ | – | – | ✓ | – | ✓ | ✓ | ✓ | 5 |
| SNOG_04279 | ✓ | ✓ | ✓ | ✓ | – | – | – | ✓ | 5 |
| SNOG_04353 | ✓ | – | ✓ | ✓ | – | ✓ | – | ✓ | 5 |
| SNOG_05030 | ✓ | ✓ | ✓ | ✓ | – | ✓ | – | – | 5 |
| SNOG_06202 | ✓ | – | ✓ | ✓ | – | ✓ | – | ✓ | 5 |
| SNOG_06459 | ✓ | ✓ | – | ✓ | – | ✓ | – | ✓ | 5 |
| SNOG_07292 | ✓ | – | ✓ | ✓ | – | ✓ | – | ✓ | 5 |
| SNOG_08206 | ✓ | – | ✓ | ✓ | – | ✓ | – | ✓ | 5 |
| SNOG_08469 | – | – | ✓ | ✓ | – | – | ✓ | – | 5 |
| SNOG_08606 | ✓ | ✓ | – | ✓ | – | ✓ | – | ✓ | 5 |
| SNOG_09147 | ✓ | – | ✓ | ✓ | – | ✓ | – | ✓ | 5 |
| SNOG_09446 | – | – | ✓ | ✓ | ✓ | ✓ | ✓ | – | 5 |
| SNOG_09672 | ✓ | ✓ | – | ✓ | – | ✓ | – | ✓ | 5 |
| SNOG_09738 | ✓ | ✓ | – | ✓ | – | ✓ | – | ✓ | 5 |
| SNOG_10135 | ✓ | – | ✓ | ✓ | – | – | – | – | 5 |
| SNOG_10664 | – | ✓ | ✓ | ✓ | ✓ | – | ✓ | – | 5 |
| SNOG_11632 | ✓ | – | – | ✓ | ✓ | ✓ | – | ✓ | 5 |
| SNOG_12350 | ✓ | ✓ | – | ✓ | – | ✓ | – | ✓ | 5 |
| SNOG_12382 | ✓ | ✓ | ✓ | ✓ | – | ✓ | – | – | 5 |
| SNOG_12564 | ✓ | – | ✓ | ✓ | – | ✓ | – | ✓ | 5 |
| SNOG_12748 | ✓ | – | ✓ | ✓ | – | ✓ | – | ✓ | 5 |
| SNOG_13126 | ✓ | ✓ | – | ✓ | – | ✓ | – | ✓ | 5 |
| SNOG_13993 | ✓ | ✓ | ✓ | ✓ | – | – | – | ✓ | 5 |
| SNOG_14135 | ✓ | – | ✓ | ✓ | ✓ | – | – | ✓ | 5 |
| SNOG_14618 | ✓ | – | ✓ | ✓ | – | – | ✓ | ✓ | 5 |
| SNOG_14826 | ✓ | – | ✓ | ✓ | – | ✓ | – | ✓ | 5 |
| SNOG_14955 | – | ✓ | ✓ | ✓ | – | ✓ | ✓ | – | 5 |
| SNOG_15074 | ✓ | – | – | ✓ | ✓ | ✓ | – | ✓ | 5 |
| SNOG_15150 | ✓ | – | ✓ | ✓ | – | ✓ | – | ✓ | 5 |
| SNOG_15417 | ✓ | – | ✓ | ✓ | – | – | – | – | 5 |
| SNOG_15989 | ✓ | – | ✓ | ✓ | – | ✓ | – | ✓ | 5 |
| SNOG_16091 | ✓ | – | ✓ | ✓ | – | ✓ | – | ✓ | 5 |
| SNOG_16131 | ✓ | – | ✓ | ✓ | – | ✓ | – | ✓ | 5 |
| SNOG_16166 (scaffold 44) | – | ✓ | ✓ | ✓ | – | ✓ | ✓ | – | 5 |
| SNOG_16226 (scaffold 44) | ✓ | ✓ | – | ✓ | ✓ | – | – | ✓ | 5 |
| SNOG_16236 (scaffold 44) | ✓ | ✓ | – | ✓ | – | – | ✓ | ✓ | 5 |
| SNOG_16237 (scaffold 44) | ✓ | ✓ | – | ✓ | ✓ | – | ✓ | – | 5 |
| SNOG_16270 (scaffold 45) | – | ✓ | ✓ | ✓ | ✓ | – | ✓ | – | 5 |
| SNOG_16345 (scaffold 45) | – | ✓ | ✓ | ✓ | ✓ | ✓ | – | – | 5 |
| SNOG_20011 | ✓ | ✓ | – | ✓ | – | ✓ | – | ✓ | 5 |
| SNOG_30026 | ✓ | – | – | ✓ | ✓ | ✓ | – | ✓ | 5 |
| SNOG_30316 | ✓ | – | ✓ | ✓ | – | ✓ | – | ✓ | 5 |
| SNOG_30334 | ✓ | – | ✓ | ✓ | – | ✓ | ✓ | – | 5 |
| SNOG_30645 | ✓ | – | ✓ | ✓ | – | ✓ | – | ✓ | 5 |
| SNOG_30701 | ✓ | – | ✓ | ✓ | – | ✓ | – | ✓ | 5 |
Discussion
This comparative analysis of the pan-genomes of P. nodorum, P. avenae, and P2 identified dynamic genome regions in the SN15 reference relative to isolates derived from diverse locations and hosts. Analyses of PAV identified several sequences that may be dispensable or lineage specific. Among these, P. nodorum SN15 scaffolds 44 and 45 are predicted to collectively encode 136 genes and have distinctive profiles of gene density and positive selection that resemble the “two-speed” genome compartmentalization observed in the genomes of other fungal phytopathogens (Testa et al. 2016), including L. maculans (Fudal et al. 2009; Van de Wouw et al. 2010; Rouxel et al. 2011), Zymoseptoria tritici (Croll et al. 2015; Goodwin et al. 2011), and Fusarium oxysporum (Ma et al. 2010). We speculate that these scaffolds may comprise one or more accessory chromosomes or regions (Covert 1998; Croll and McDonald 2012). Their absence from Sn79 demonstrates that the genes on these scaffolds are not strictly required for survival, and their retention in more virulent strains suggests that they may confer some advantages in some host environments. The strong positive selection observed in SN15 scaffolds 44 and 45 also suggests that the localized accumulation of mutations in these putative accessory regions may promote genetic innovation (Croll and McDonald 2012; Oliver 2012; Croll et al. 2013). Interestingly, 4 of the top 63 effector candidate loci (score ≥ 5, table 5) resided on SN15 scaffold 44 (SNOG_16166, SNOG_16226, SNOG_16236, and SNOG_16237) and 2 more (SNOG_16270 and SNOG_16345) resided on SN15 scaffold 45 (supplementary file S1 and table S3, Supplementary Material online). Scaffolds 50 and 55 in isolate WAC8410 also exhibited the low gene density typical of accessory chromosomes (Covert 1998; Goodwin et al. 2011), as well as an extremely high frequency of pseudogenes with identifiable functional paralogs. Collectively, these anomalous scaffolds share many of the features typically associated with accessory chromosomes of other plant pathogens (Goodwin et al. 2011; Croll et al. 2015).
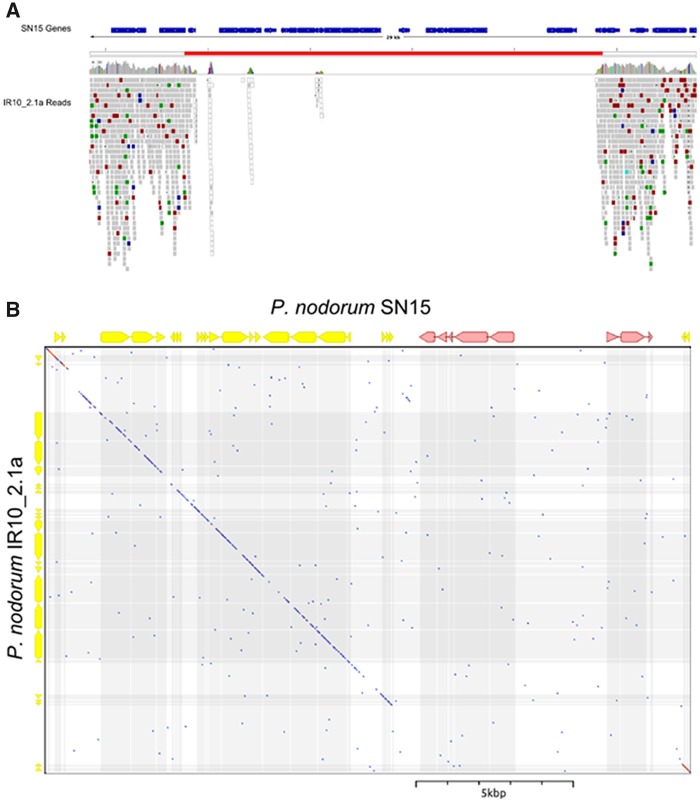
By surveying the SNP distribution and density, we identified an oversight associated with previously used short-read sequencing approaches. Previous genome comparisons between P. nodorum species used the depth of reads mapped to the reference genome to infer gene absence in the alternate strain (Syme et al. 2013). Regions that are highly differentiated relative to the reference genome can prevent reads from mapping to the reference, inflating the count of genes absent in the alternate strain. Comparisons based on de novo assemblies of the alternate strain produced a greater coverage of the reference genome for both P. nodorum and P. avenae. Figure 6 illustrates this using a region in the reference genome where reads from P. nodorum IR10_2.1a failed to map. The region without mapped reads covers 20 kb and includes eight reference genes. A protocol that calculates gene absence from mapping data alone would describe this as an 8-gene sectional absence. However, using nucmer to align de novo assembled sequences of this strain to the reference reveals that the absence is only 8 kb and that only two genes are absent from the alternate strain. The surrounding regions are sufficiently variable to prevent the read mapping algorithms from mapping reads and providing coverage. In variable genomic regions that exceed the tolerances of read mapping algorithms, a hybrid mapping/assembly approach to variant detection is likely to provide a more complete picture of sequence differences.
Fig. 6.
—Presence–absence detection is more accurate using alignments of de novo assembled sequences than read mappings. (A) Mapped read depth of a region on scaffold_004 in the SN15 reference assembly shows a putative sectional absence of seven genes. (B) Dotplot of the alternate strain’s (P. nodorum IR10_2.1a) de novo assembly at the region (marked red in A) shows that only two of the reference genes (marked in pink) are absent in the alternate strain. Highly variable regions around sectional absences can frustrate mapping algorithms leading to an inflated estimation of absent genes.
The Parastagonospora spp. pan-genome data set made it possible to assess positive selection for almost every locus of the reference isolate SN15. The frequency distribution of genes under diversifying selection allowed us to divide the genome into three categories: (1) a background rate of 5–10% genes under diversifying selection, which represented the majority of the genome; (2) large regions where 20–30% of genes are under diversifying selection. Examples include the putative accessory sequences on scaffolds 44 and 45; and (3) small islands of genes adjacent to repetitive regions under diversifying selection, such as scaffolds 7, 15, and 20 (supplementary tables S3 and S5, and fig. S4, Supplementary Material online).
We hypothesize that these categories may relate to the biological function of the genes they contain. The first category is likely to contain the bulk of the stable genome and the housekeeping genes undergoing long term and continuous adaptation to the environment. The second category represents accessory genomic regions involved in rapid changes such as adaptation to new hosts species or genotypes. Genes contained in regions of the third category may represent coordinately regulated gene clusters such as those involved in secondary metabolism. These category 3 regions are adjacent to repeat-rich stretches of the genome, which in other species are associated with secondary metabolite synthesis gene clusters (Dallery et al. 2017). RIP is known to encroach from repetitive regions into nearby single-copy genes, in some cases disabling recognized effector genes (Fudal et al. 2009; Rouxel et al. 2011). In the case of genes encoding avirulence effectors, mutation can be beneficial to the pathogen by enabling evasion of recognition on a host carrying the corresponding resistance gene. Islands of positive selection were observed to often be adjacent to AT-rich regions (Testa et al. 2016), which suggests there may be an association between RIP and effector loci in P. nodorum.
The known P. nodorum effector genes are all positioned near repetitive sequences (Syme et al. 2013; Kellner et al. 2014). It has been suggested that repetitive elements provide a mechanism for effector mobilization within genomes and HGT between species, while also increasing diversity at these loci (Croll and McDonald 2012; Oliver 2012; Croll et al. 2013). Notably, a putative SnTox3 ortholog was observed in both P. avenae Pat5 isolates, within a gene-dense region that is otherwise syntenic to the other strains sequenced and shows no sign of nearby transposon activity (fig. 5). The evolutionary history of these homologs at two different loci is unclear. Possible explanations include independent acquisition of the gene by P. nodorum and Pat5 ancestors, or lateral transfer between these taxa. Another possibility is that the location in Pat5 is the ancestral version, and subsequent translocation in P. nodorum adjacent to a repeat-rich region may provide an adaptive advantage in the form of RIP-mediated accelerated mutation rates (Fudal et al. 2009; Van De Wouw and Howlett 2011; Testa et al. 2016). The absence of any isolate with a copy in both locations likely indicates either direct translocation or transduplication with subsequent loss of the original paralog, likely due to selection pressures imposed by RIP on duplicated sequences (Galagan and Selker 2004; Hane and Oliver 2008).
The addition of new genome assemblies allowed us to expand the set of criteria used to identify candidate effector genes. The presence/absence allele frequency in a P. nodorum population differs for each effector, and was hypothesized to reflect the prevalence of each effector’s susceptibility gene in the corresponding host population (McDonald et al. 2013). SnToxA, SnTox1 and SnTox3 all showed presence/absence polymorphisms, and each were individually observed in 50–85% of the P. nodorum isolates studied (fig. 1). A more accurate determination of core and strain-specific genes (fig. 3 and table 3) allows us to better identify genes that are neither perfectly conserved nor infrequent. Based on updated criteria for ranking effector candidate loci, SnTox1 is among the eight equally top-ranked candidates and SnTox3 and SnToxA are among the nine 2nd-ranked candidates (table 5 and supplementary table S3, Supplementary Material online). The 63 top-scoring candidates will be prioritized for purification in a heterologous expression system and screened against wheat lines to test for each candidate effector’s ability to produce disease symptoms. We anticipate that P. nodorum effectors that have not yet been identified to the gene level (SnTox2, SnTox4, SnTox5, and SnTox6) may be able to be matched to these candidates using PAV profiles across the sequenced isolates (e.g., SN4, SN79, and Sn99CH 1A7a) to help unmask these recalcitrant loci. Once validated, effector molecules can be applied as tools to accelerate disease resistance breeding programs (Vleeshouwers and Oliver 2014).
Analyses of the P. nodorum and P. avenae pan-genomes allowed us to quantify different types of genomic variation across a variety of scales. De novo assembly comparisons highlighted the large number of strain-specific loci and the extent of PAV within the two species. Accessory regions were identified, suggesting the possibility of one or more lineage-specific dispensable chromosomes with potential roles in pathogenicity (Zolan 1995; Covert 1998). At the smallest scale of resolution, the pan-genomic comparisons identified loci under diversifying selection. These observations collectively deepen our understanding of the genomic history of Parastagonospora spp. and improve the prediction of potential effector sequences in P. nodorum. We expect that our approach can be broadly applied to other species in the Pezizomycotina, particularly to those which are necrotrophic pathogens.
Supplementary Material
Supplementary data are available at Genome Biology and Evolution online.
Supplementary Material
Acknowledgments
This work was supported by access to facilities managed by Bioplatforms Australia; and funded by the Australian Government National Collaborative Research Infrastructure Strategy; Education Investment Fund Super Science Initiative; and Grains Research and Development Corporation research [grant numbers CUR00012, CUR00023, and GRS10061 to R.A.S.]. This work was supported by resources provided by the NCI Specialized Facility in Bioinformatics at The University of Queensland through the National Computational Merit Allocation Scheme; and the advanced computing resources located at the Pawsey Supercomputing Centre supported by the Australian Government.
Literature Cited
- Abeysekara NS, Friesen TL, Keller B, Faris JD.. 2009. Identification and characterization of a novel host–toxin interaction in the wheat–Stagonospora nodorum pathosystem. Theor Appl Genet. 1201:117–126. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ.. 1990. Basic local alignment search tool. J Mol Biol. 2153:403–410. [DOI] [PubMed] [Google Scholar]
- Bankevich A, et al. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Computat Biol. 195:455–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S.. 2004. Improved prediction of signal peptides: signalP 3.0. J Mol Biol. 3404:783–795. [DOI] [PubMed] [Google Scholar]
- Bertazzoni S, et al. 2018. Accessories make the outfit: accessory chromosomes and other dispensable DNA regions in plant-pathogenic Fungi. Mol Plant Microbe Interact. 318:779–788. [DOI] [PubMed] [Google Scholar]
- Buchfink B, Xie C, Huson DH.. 2015. Fast and sensitive protein alignment using DIAMOND. Nat Methods. 121:59.. [DOI] [PubMed] [Google Scholar]
- Clutterbuck AJ. 2011. Genomic evidence of repeat-induced point mutation (RIP) in filamentous ascomycetes. Fungal Genet Biol. 483:306–326. [DOI] [PubMed] [Google Scholar]
- Covert SF. 1998. Supernumerary chromosomes in filamentous fungi. Curr Genet. 335:311–319. [DOI] [PubMed] [Google Scholar]
- Croll D, Lendenmann MH, Stewart E, McDonald BA.. 2015. The impact of recombination hotspots on genome evolution of a fungal plant pathogen. Genetics 2013:1213–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croll D, McDonald BA.. 2012. The accessory genome as a cradle for adaptive evolution in pathogens. PLoS Pathog. 84:e1002608.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croll D, Zala M, McDonald BA.. 2013. Breakage-fusion-bridge cycles and large insertions contribute to the rapid evolution of accessory chromosomes in a fungal pathogen. PLoS Genet. 96:e1003567.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunfer BM. 2000. Stagonospora and Septoria diseases of barley, oat, and rye. Can J Plant Pathol. 224:332–348. [Google Scholar]
- Dallery J-F, et al. 2017. Gapless genome assembly of Colletotrichum higginsianum reveals chromosome structure and association of transposable elements with secondary metabolite gene clusters. BMC Genom. 181:667.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emms DM, Kelly S.. 2015. OrthoFinder: solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biol. 161:157.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen TL, Chu C, Xu SS, Faris JD.. 2012. SnTox5–Snn5: a novel Stagonospora nodorum effector–wheat gene interaction and its relationship with the SnToxA–Tsn1 and SnTox3–Snn3–B1 interactions. Mol Plant Pathol. 139:1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen TL, Meinhardt SW, Faris JD.. 2007. The Stagonospora nodorum‐wheat pathosystem involves multiple proteinaceous host‐selective toxins and corresponding host sensitivity genes that interact in an inverse gene‐for‐gene manner. Plant J. 514:681–692. [DOI] [PubMed] [Google Scholar]
- Friesen TL, et al. 2006. Emergence of a new disease as a result of interspecific virulence gene transfer. Nat Genet. 388:953–956. [DOI] [PubMed] [Google Scholar]
- Friesen TL, Zhang Z, Solomon PS, Oliver RP, Faris JD.. 2008. Characterization of the interaction of a novel Stagonospora nodorum host-selective toxin with a wheat susceptibility gene. Plant Physiol. 1462:682–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudal I, et al. 2009. Repeat-induced point mutation (RIP) as an alternative mechanism of evolution toward virulence in Leptosphaeria maculans. Mol Plant Microbe Interact. 228:932–941. [DOI] [PubMed] [Google Scholar]
- Galagan JE, Selker EU.. 2004. RIP: the evolutionary cost of genome defense. Trends Genet. 209:417–423. [DOI] [PubMed] [Google Scholar]
- Gao Y, et al. 2015. Identification and characterization of the SnTox6-Snn6 interaction in the Parastagonospora nodorum–wheat pathosystem. Mol Plant Microbe Interact. 285:615–625. [DOI] [PubMed] [Google Scholar]
- Golicz AA, et al. 2015. Gene loss in the fungal canola pathogen Leptosphaeria maculans. Funct Integr Genom. 152:189–196. [DOI] [PubMed] [Google Scholar]
- Goodwin SB, et al. 2011. Finished genome of the fungal wheat pathogen Mycosphaerella graminicola reveals dispensome structure, chromosome plasticity, and stealth pathogenesis. PLoS Genet. 76:e1002070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich A, Saveliev V, Vyahhi N, Tesler G.. 2013. QUAST: quality assessment tool for genome assemblies. Bioinformatics 298:1072–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hane JK. 2015. Calculating RIP Mutation in Fungal Genomes Using RIPCAL In: van den Berg M, Maruthachalam K, editors. Genetic transformation systems in fungi, Vol. 2 Springer; p. 69–78. [Google Scholar]
- Hane JK, et al. 2007. Dothideomycete–plant interactions illuminated by genome sequencing and EST analysis of the wheat pathogen Stagonospora nodorum. Plant Cell 1911:3347–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hane JK, Oliver RP.. 2010. In silico reversal of repeat-induced point mutation (RIP) identifies the origins of repeat families and uncovers obscured duplicated genes. BMC Genom. 111:655.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hane JK, Oliver RP.. 2008. RIPCAL: a tool for alignment-based analysis of repeat-induced point mutations in fungal genomic sequences. BMC Bioinformatics 9:478.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hane JK, et al. 2011. A novel mode of chromosomal evolution peculiar to filamentous Ascomycete fungi. Genome Biol. 125:R45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hane JK, Williams AH, Taranto AP, Solomon PS, Oliver RP.. 2015. Repeat-induced point mutation: a fungal-specific, endogenous mutagenesis process In: van den Berg M, Maruthachalam K, editors. Genetic transformation systems in fungi, Vol. 2 Springer; p. 55–68. [Google Scholar]
- Ipcho SVS, et al. 2012. Transcriptome analysis of Stagonospora nodorum: gene models, effectors, metabolism and pantothenate dispensability. Mol Plant Pathol. 136:531–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DA, Bertazzoni S, Turo CJ, Syme RA, Hane JK.. 2018. Bioinformatic prediction of plant–pathogenicity effector proteins of fungi. Curr Opin Microbiol. 46:43–49. [DOI] [PubMed] [Google Scholar]
- Jurka J, et al. 2005. Repbase update, a database of eukaryotic repetitive elements. Cytogenet Genome Res. 110(1-4):462–467. [DOI] [PubMed] [Google Scholar]
- Kellner R, et al. 2014. Expression profiling of the wheat pathogen Zymoseptoria tritici reveals genomic patterns of transcription and host-specific regulatory programs. Genome Biol Evolut. 66:1353–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh A, Larsson B, Von Heijne G, Sonnhammer EL.. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 3053:567–580. [DOI] [PubMed] [Google Scholar]
- Kurtz S, et al. 2004. Versatile and open software for comparing large genomes. Genome Biol. 52:R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL.. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods. 94:357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin MA, et al. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 2321:2947–2948. [DOI] [PubMed] [Google Scholar]
- Li H. 2013. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv Preprint arXiv:1303:3997. [Google Scholar]
- Liu Z, et al. 2009. SnTox3 acts in effector triggered susceptibility to induce disease on wheat carrying the Snn3 gene. PLoS Pathog. 59:e1000581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, et al. 2012. The cysteine rich necrotrophic effector SnTox1 produced by Stagonospora nodorum Triggers Susceptibility of Wheat Lines Harboring Snn1. PLoS Pathog. 81:e1002467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZH, et al. 2004. Genetic and physical mapping of a gene conditioning sensitivity in wheat to a partially purified host-selective toxin produced by Stagonospora nodorum. Phytopathology 9410:1056–1060. [DOI] [PubMed] [Google Scholar]
- Ma L-J, et al. 2010. Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature 4647287:367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkus A, et al. 2005. Sequence diversity of β-tubulin (tubA) gene in Phaeosphaeria nodorum and P. avenaria. FEMS Microbiol Lett. 2491:49–56. [DOI] [PubMed] [Google Scholar]
- Martin M. 2011. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 171:10–12. p [Google Scholar]
- McDonald MC, Oliver RP, Friesen TL, Brunner PC, McDonald BA.. 2013. Global diversity and distribution of three necrotrophic effectors in Phaeosphaeria nodorum and related species. New Phytol. 1991:241–251. [DOI] [PubMed] [Google Scholar]
- McDonald MC, Razavi M, Friesen TL, Brunner PC, McDonald BA.. 2012. Phylogenetic and population genetic analyses of Phaeosphaeria nodorum and its close relatives indicate cryptic species and an origin in the Fertile Crescent. Fungal Genet Biol. 4911:882–895. [DOI] [PubMed] [Google Scholar]
- McDonald MC, et al. 2015. Next-generation re-sequencing as a tool for rapid bioinformatic screening of presence and absence of genes and accessory chromosomes across isolates of Zymoseptoria tritici. Fungal Genet Biol. 79:71–75. [DOI] [PubMed] [Google Scholar]
- Medema MH, et al. 2011. antiSMASH: rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Res. 39(suppl_2):W339–W346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolhuijzen P, et al. 2018. Comparative genomics of the wheat fungal pathogen Pyrenophora tritici-repentis reveals chromosomal variations and genome plasticity. BMC Genom. 191:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohm RA, et al. 2012. Diverse lifestyles and strategies of plant pathogenesis encoded in the genomes of eighteen Dothideomycetes fungi. PLoS Pathog. 812:e1003037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver R. 2012. Genomic tillage and the harvest of fungal phytopathogens. New Phytol. 1964:1015–1023. [DOI] [PubMed] [Google Scholar]
- Oliver RP, Friesen TL, Faris JD, Solomon PS.. 2012. Stagonospora nodorum: from pathology to genomics and host resistance. Annu Rev Phytopathol. 501:23–43. [DOI] [PubMed] [Google Scholar]
- Oliver RP, Solomon PS.. 2010. New developments in pathogenicity and virulence of necrotrophs. Curr Opin Plant Biol. 134:415–419. [PubMed] [Google Scholar]
- Plissonneau C, Stürchler A, Croll D.. 2016. The evolution of orphan regions in genomes of a fungal pathogen of wheat. mBio 75:e01231–e01216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaedvlieg W, et al. 2013. Sizing up Septoria. Stud Mycol. 751:307–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan AR, Hall IM.. 2010. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 266:841–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JK, Wyatt NA, Liu Z, Faris JD, Friesen TL.. 2018. Reference quality genome assemblies of three parastagonospora nodorum isolates differing in virulence on wheat. G3: Genes, Genomes, Genetics 8:393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouxel T, et al. 2011. Effector diversification within compartments of the Leptosphaeria maculans genome affected by Repeat-Induced Point mutations. Nat Commun. 2:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak K, et al. 2017. A functionally conserved Zn2Cys6 binuclear cluster transcription factor class regulates necrotrophic effector gene expression and host‐specific virulence of two major Pleosporales fungal pathogens of wheat. Mol Plant Pathol. 183:420–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi G, et al. 2015. The wheat gene confers susceptibility on recognition of the necrotrophic effector SnTox7. Plant Genome 82:0. [DOI] [PubMed] [Google Scholar]
- Slater GSC, Birney E.. 2005. Automated generation of heuristics for biological sequence comparison. BMC Bioinformatics 6:31.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit A, Hubley R.. 2010. RepeatModeler Open-1.0. Repeat Masker Website.
- Smit A, Hubley R, Green P.. 2015. RepeatMasker Open-4.0. 2013–2015. Institute for Systems Biology. http://repeatmasker. org.
- Solomon PS, Lowe RG, TAN KC, Waters OD, Oliver RP.. 2006. Stagonospora nodorum: cause of stagonospora nodorum blotch of wheat. Mol Plant Pathol. 73:147–156. [DOI] [PubMed] [Google Scholar]
- Stanke M, et al. 2006. AUGUSTUS: ab initio prediction of alternative transcripts. Nucleic Acids Res. 34(Web Server):W435–W439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suyama M, Torrents D, Bork P.. 2006. PAL2NAL: robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res. 34(Web Server):W609–W612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syme RA, Hane JK, Friesen TL, Oliver RP.. 2013. Resequencing and comparative genomics of Stagonospora nodorum: sectional gene absence and effector discovery. G3: Genes| Genomes| Genetics 3:959–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syme RA, et al. 2016. Comprehensive annotation of the Parastagonospora nodorum reference genome using next-generation genomics, transcriptomics and proteogenomics. PLoS One 112:e0147221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K-C, Oliver RP, Solomon PS, Moffat CS.. 2010. Proteinaceous necrotrophic effectors in fungal virulence. Funct Plant Biol. 3710:907–912. [Google Scholar]
- Testa AC, Hane JK, Ellwood SR, Oliver RP.. 2015. CodingQuarry: highly accurate hidden Markov model gene prediction in fungal genomes using RNA-seq transcripts. BMC Genom. 16:170.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa AC, Oliver RP, Hane JK.. 2016. OcculterCut: a comprehensive survey of AT-rich regions in fungal genomes. Genome Biol Evolut. 86:2044–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueng P, et al. 1998. Intraspecific genetic variation of Stagonospora avenae and its differentiation from S. nodorum. Mycol Res. 1025:607–614. [Google Scholar]
- Ueng PP, Chen W.. 1994. Genetic differentiation between Phaeosphaeria nodorum and P. avenaria using restriction fragment length polymorphisms. Phytopathology 848:800–806. [Google Scholar]
- Ueng PP, Cunfer BM, Alano AS, Youmans JD, Chen W.. 1995. Correlation between molecular and biological characters in identifying the wheat and barley biotypes of Stagonospora nodorum. Phytopathology 851:44–52. [Google Scholar]
- Ueng PP, et al. 2003. Sequence diversity of mating-type genes in Phaeosphaeria avenaria. Curr Genet. 432:121–130. [DOI] [PubMed] [Google Scholar]
- Van de Wouw AP, et al. 2010. Evolution of linked avirulence effectors in Leptosphaeria maculans is affected by genomic environment and exposure to resistance genes in host plants. PLoS Pathog. 611:e1001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Wouw AP, Howlett BJ.. 2011. Fungal pathogenicity genes in the age of ‘omics’. Mol Plant Pathol. 125:507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleeshouwers VG, Oliver RP.. 2014. Effectors as tools in disease resistance breeding against biotrophic, hemibiotrophic, and necrotrophic plant pathogens. Mol Plant Microbe Interact. 273:196–206. [DOI] [PubMed] [Google Scholar]
- Zolan ME. 1995. Chromosome-length polymorphism in fungi. Microbiol Rev. 594:686–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.