Abstract
Chromatin organization is crucial for nuclear functions such as gene regulation, DNA replication and DNA repair. Insulator binding proteins, such as the Drosophila Boundary Element-Associated Factor (BEAF), are involved in chromatin organization. To further understand the role of BEAF, we detected cis- and trans-interaction partners of four BEAF binding regions (viewpoints) using 4C (circular chromosome conformation capture) and analyzed their association with different genomic features. Previous genome-wide mapping found that BEAF usually binds near transcription start sites, often of housekeeping genes, so our viewpoints were selected to reflect this. Our 4C data show the interaction partners of our viewpoints are highly variable and generally enriched for active chromatin marks. The most consistent association was with housekeeping genes, a feature in common with our viewpoints. Fluorescence in situ hybridization indicated that the long-distance interactions occur even in the absence of BEAF. These data are most consistent with a model in which BEAF is redundant with other factors found at active promoters. Our results point to principles of long-distance interactions made by active chromatin, supporting a previously proposed model in which condensed chromatin is sticky and associates into topologically associating domains (TADs) separated by active chromatin. We propose that the highly variable long-distance interactions we detect are driven by redundant factors that open chromatin to promote transcription, combined with active chromatin filling spaces between TADs while packing of TADs relative to each other varies from cell to cell.
Introduction
Multiple studies have shown the importance of chromatin architecture for proper gene regulation (for example, [1–3]). The development of 3C (chromosome conformation capture) and 3C-related methods such as 4C, 5C, Hi-C and ChIA-PET, have helped us understand the complexity of genome organization in three-dimensional space [4, 5]. For example, such studies have found that chromosomes are organized into topologically associating domains (TADs). Insulators, or boundary elements, are a class of architectural elements necessary to maintain a healthy functioning genome [6–8]. They are defined as specialized protein-bound DNA elements known to play an important role in chromatin organization and gene regulation by influencing intra- and inter-chromosomal interactions. There are two classical assays of insulator function. One is the protection of transgenes bracketed by insulators from chromosomal position effects [9, 10]. The other is the blocking of enhancer-promoter communication by an intervening insulator, for instance by insertion of an insulator-containing gypsy retrotransposon [11, 12]. These functions are thought to be important for protecting genes from chromosomal position effects and preventing promiscuous interactions between long-distance regulatory elements and promoters, and are presumably related to their role in genome organization.
Insulators need insulator binding proteins (IBPs) to function. Of the many IBPs found in Drosophila, Boundary Element-Associated Factor-32 (BEAF) is our protein of interest [13]. There are two 32 kDa BEAF proteins (BEAF-32A and BEAF-32B, hereafter referred to as 32A and 32B) made from alternative promoters of the same gene [14]. The two isomers of BEAF differ in their N-termini by 80 amino acids which contain single DNA-binding BED zinc fingers, and interact via a BESS domain located in their C-termini [15–17]. The number of BEAF subunits in a BESS-mediated complex is unknown, and might be variable.
BEAF was discovered based on its binding to the scs’ insulator [13], one of the first insulators to be described together with scs as special chromatin structures bracketing the 87A hsp70 heat shock locus [9, 11, 18]. There are two BEAF binding sites in scs’, both with clusters of 3 CGATA motifs. The BED finger of 32B binds this motif [14], and 32B is essential while 32A is not [19]. The CGATA motifs are important for scs’ insulator function, and clusters are found in other sequences that BEAF binds to that have been shown to have insulator activity [10, 20]. This led to the model that BEAF binds 3 or more CGATA motifs clustered in a 100 bp region. A bioinformatics approach found that around 1700 clusters matching this model also have a second cluster of 2 or more CGATA motifs in a 50 bp region located 200 bp to 1 kb from the first cluster, an arrangement termed a dual-core [21]. Genome-wide mapping found 1800 to 3000 BEAF peaks and confirmed that CGATA clusters are frequent in BEAF binding regions [22–25]. However, the relative orientations and spacing of CGATA motifs in clusters is highly variable and many peak regions have only one or no CGATA motifs. Electrophoretic mobility shift assays found that CGATA clusters are not sufficient to guarantee binding by BEAF, and BEAF can bind sequences with a single CGATA [22]. In addition, less than one-third of dual-cores overlap with BEAF peaks. Thus details of what constitutes a high affinity BEAF binding site remain elusive.
A particularly interesting result to emerge from the BEAF mapping studies cited above is that over 85% of centers of BEAF peaks are within 500 bp of gene transcription start sites (TSSs), often within 200 bp. In turn, we found that around 85% of these BEAF-associated genes are found in lists of housekeeping genes [26, 27]. Thus, in addition to insulator activity, BEAF could play a role in keeping associated promoters active. Around half of BEAF peaks are between divergently transcribed genes. An example is scs’, which has two BEAF binding sites, one near each TSS. Around 20% of the dual-cores described above are also between divergently transcribed genes. At least 80% of these correspond to BEAF peaks, suggesting that BEAF might frequently bind near both TSSs of divergent genes. In contrast, less than 5% of dual-cores not near TSSs correspond to BEAF peaks. This is another illustration that clusters of CGATA motifs alone are not a good predictor of BEAF binding. However, there is no other known predictor and sequences with CGATA clusters near TSSs are often bound by BEAF.
To explore the role of BEAF in genome organization and gene regulation, we mapped long-distance interactions of four BEAF-binding regions using 4C. We chose high confidence BEAF binding sites that correspond to dual-cores located between divergently transcribed genes. The results were complicated, with one viewpoint in particular differing from the other three in terms of histone modifications, chromatin states and insulator proteins associated with interacting DNA. The strongest correlation was with housekeeping genes. BEAF is not essential for the observed interactions to occur. We conclude that long-distance interactions of active chromatin such as our viewpoints are highly variable and enriched for active chromatin. This is consistent with the model that condensed, largely inactive chromatin is sticky and associates into TADs that are separated by inter-TAD regions of active chromatin [27–29]. It is possible that phase-separation helps drive condensed chromatin into TADs [30, 31]. Redundant factors that open chromatin and promote transcription, perhaps including BEAF, would facilitate exclusion from TADs. The less sticky, active inter-TAD chromatin that fills the spaces between TADS could allow variable packing of TADs relative to each other, resulting in the variable long-distance interactions between active chromatin that we observe.
Materials and methods
4C library preparation
4C libraries were prepared using published methods with modifications (S1 Fig) [32–34]. Two biological replicates were performed for each viewpoint. Drosophila Kc cells (Drosophila Genomics Resource Center Kc167) were grown at 25°C in Shields and Sang M3 medium (Sigma) with 1x Pen-Strep (Gibco) and 5% fetal bovine serum (Gibco). Cells (500 ml at ~9x106 cells/ml) were spun down (400g, 4°C, 10 minutes) two times and resuspended in 30 ml PBS both times. Protein-DNA complexes were cross-linked by adding 37% formaldehyde (final concentration 1%) to the cells and incubating at room temperature for 10 minutes. Formaldehyde was quenched by adding 1.25 M glycine (final concentration 125 mM). The cells were washed with 30 ml PBS, resuspended in 10 ml lysis buffer (10 mM Tris-HCl pH 8.0, 10 mM NaCl, 0.2% NP40), and incubated on ice for 15 minutes. The cells were dounced 15 times on ice with a B pestle, incubated on ice for another 15 minutes, then dounced 15 times again. The nuclei were pelleted at 2000g at 4°C for 5 minutes.
Depending on the viewpoint, 5x108 nuclei were resuspended in 500 μl DpnII (scs’, hts, snf) or NlaIII (RpS6) restriction enzyme buffer and distributed into 50 μl aliquots in 10 tubes. Nuclei were lysed by adding 10% SDS (final concentration: 0.3%) and shaking at 37°C overnight. SDS was diluted to 0.1% with DpnII or NlaIII restriction enzyme buffer, and the SDS was quenched by adding 20% Triton X-100 (final concentration 1.1%) and shaking at 37°C overnight. The following day 22.5 units of DpnII or 15 units of NlaIII (New England Biolabs) was added to each of the appropriate 10 tubes and shaken at 37°C overnight. The same amount of restriction enzyme was added the next day and shaken again at 37°C overnight. Restriction digestion efficiency was determined by isolating DNA from 5% of the digested samples to determine the DNA concentration and perform PCR using primers spanning viewpoint restriction sites (S1 Table), as described [34], and found to be approximately 90% cut.
Restriction digestions were stopped by adding SDS to 1.6% and heating at 65°C for 30 minutes. Samples cut with the same restriction enzyme were pooled, 30 μg was diluted to 2 ng/μl with ligation buffer, and 20% Triton X-100 was added to a final concentration of 1.1%. Samples were incubated at 37°C for 4 hours, then 6000 units T4 DNA ligase (New England Biolabs) was added and incubated at 4°C for 72 hours. After ligation, Proteinase-K was added to 0.1 mg/ml and incubated overnight at 65°C. Next samples were treated with 0.22 ng/ml RNAse A at 37°C for one hour, and DNA was extracted using phenol chloroform and ethanol precipitated. The DNA pellet was resuspended in 100 μl TE buffer and quantified by Qubit (Thermo-Fisher). We then set up second restriction digestions, using 3 μg per viewpoint. For DNA first cut with DpnII, we used CviQI (scs’) or NlaIII (snf and hts); for DNA first cut with NlaIII we used DpnII (RpS6). After the second digestion samples were phenol chloroform extracted, ethanol precipitated, resuspended, and 1 μg was ligated at 0.8 ng/μl at 16°C overnight. The final ligated DNA was again extracted, precipitated and quantified by Qubit. This DNA was the input for making 4C libraries.
The four 4C libraries were made by inverse PCR using Phusion polymerase (New England Biolabs) and primers with Ion Torrent PGM barcodes and adapters incorporated into the primer sequences (S1 Table). Two libraries were made for each of the four viewpoints, one for each end of a viewpoint. PCR products were size selected on a 2% agarose gel (100–500 bp). DNA from the gel was extracted with a Wizard PCR clean-up kit (Promega) and submitted to the LSU Genomics Facility for Ion Torrent sequencing. Data are available at NCBI GEO accession number GSE118013.
Calculating significant cis- and trans-interactions using fourSig
Viewpoint sequences at the 5’ and 3’ ends were trimmed using Btrim [35]. The remaining sequences were aligned to the Drosophila Release 6.01 genome using bowtie2 [35, 36]. The resulting sam files were converted to bam files, filtering out sequences with Q-scores < 30, for use in the fourSig analysis suite [37]. Reads from both ends of viewpoints were combined for 4C analysis. In fourSig, bamToReTab.pl converted bam files to tab files, which are tables of reads mapped to restriction fragments generated by the first 4C restriction enzyme. The tab files were used by fourSig.R to calculate significant cis- and trans-interaction intervals. Additional information required for the script to run are: chromosomal location of the viewpoint, sliding window size, and FDR cutoff value. Significance thresholds for calculating FDR values were generated using 1000 random shuffling steps. We excluded ±2 kb from the viewpoint while calculating significant interactions. Sliding window sizes of 5 and 20 were used for cis- and trans-interactions, respectively, with an FDR value of 0.001 and an FDR probability value of 0.01. We used only Category 1 (broad) and Category 2 (intermediate) peaks in our analysis. Category 3 (narrow) peaks were excluded because they represent viewpoint interactions with a single restriction fragment, rather than also neighboring fragments. Therefore, they are more likely to be false positives. Overlap of cis- and trans-interactions from two biological replicates were calculated using a custom Python script.
Performing virtual 4C using Hi-C data
To evaluate the quality of our 4C data, we performed virtual 4C on Hi-C data from DpnII-cut DNA. S2 cell data from GSE99104 [38] and GSE101317 [39], and Kc cell data from GSE85503 [28] and GSE80701 [40], were analyzed using HOMER (Hypergeometric Optimization of Motif EnRichment) software [41]. The analyzeHiC script was run with the -4C option using the coordinates of our 4C viewpoints with 2000 bp resolution and binning windows. Virtual 4C replicates were analyzed for reproducibility using BEDTools intersect (S2 Table) [42].
Calculating overlap of significant cis- and trans-interactions with genomic features using GAT
Data for the genomic features used in Genomic Association Tester (GAT) analyses [43] were from published sources. Unless otherwise noted, all data are for Drosophila Kc cells. For data not available from Kc cells, we used data from S2 cells or embryos. These are reasonable substitutes since Kc and S2 cells are similar and are derived from embryos, and M1BP, Zw5 and TADs are more or less constitutive. The ChIP-seq files for H3K4me1, H3K4me3 and H3K27ac were obtained from GSE36374 [40]. BEAF, dCTCF, Su(Hw) and CP190 peaks were obtained from GSE15661 [24]. H3K27me3 depleted D and enriched E domains were obtained from GSE85504 [28], and the five chromatin states interval file was from GSE22069 [44]. We used housekeeping gene lists from the Akhtar and Razin labs [26, 27] and obtained S2 cell M1BP binding sites from GSE46630 [45]. Chromator (Chro), Zw5 (S2 cells), GAGA factor (GAF), H2Av, H3K9me2, H3K9me3 and H3K27me3 peaks were downloaded from modENCODE (datasets 277, 3803, 2568, 3282, 938, 3013 and 5136) [46]. Embryo TAD sites were from GSE34453 [47]. Genomic files in Drosophila genome Release 5 were converted to Drosophila genome Release 6 using the FlyBase Coordinate Converter [48]. For motif analysis, we used the FIMO program from the MEME suite to find motif occurrences in our viewpoint interactions [49] with dm6 release 6.01 as the reference sequence. We used consensus motifs curated from the literature by Mourad and Cuvier [50] and a p-value of 0.001. From the GAT output, we used fold enrichment (observed/expected nucleotide overlap) and Benjamini-Hochberg corrected p-values to generate heatmaps showing enrichment or depletion of features in 4C interaction partners of our viewpoints.
Testing 4C interactions by 3C
To make 3C libraries, the 4C library protocol through the first ligation and purification of ligated DNA was followed. The 5’ primers were designed from the viewpoint sequence and the 3’ primers were designed from the interacting partner sequences (S3 Table). PCR was done using Phusion polymerase. PCR products were run on 1.5% agarose gels with a 50 bp DNA ladder, and the presence of a correct-sized PCR product indicated a positive 3C result. The interaction between scs’ and scs was used as a positive control [51].
Testing 4C interactions by FISH
We used Fluorescence In Situ Hybridization (FISH) to test our 4C interactions [52]. A list of primers used to make FISH probes are in S4 Table. For each probe, we designed 4 or 5 primer pairs that give PCR fragments of 1.6–1.8 kb over 8–10 kb of the region of interest (viewpoint and partner). The PCR products were purified using a Wizard PCR clean-up kit (Promega). The PCR products for each probe were pooled in equimolar amounts and labelled with either Alexa Fluor 555 or Alexa Fluor 488 (Invitrogen FISH tag DNA kit). We used 90 ng of each labelled probe in 30 μl of FISH hybridization buffer per hybridization. For each hybridization, 10 wild-type BEAF (y1 w67c23) and 10 null BEAFAB-KO [19] third instar larvae were dissected and processed as described [52]. BEAF-32B is essential, so the BEAFAB-KO larvae were from heterozygous mothers that provided maternal BEAF to eggs. The FISH slides were viewed on a Leica TCS SP8 confocal microscope with a white light laser using a 63X water immersion lens, capturing Alexa Fluor 555, Alexa Fluor 488 and DAPI (DNA) images. Wing discs and brains were viewed.
FISH image analysis using Fiji
FISH Z-stack images were analyzed using ImageJ Fiji [53]. Z-stacks were split into green (viewpoint), red (interacting partner) and blue (DNA) channels. The red and green channels were merged. Background noise was reduced by processing with the filter Gaussian Blur (value: 2.00) and removing noise using Despeckle. We then used the plugin ComDet, which marks colocalized red and green signals (yellow) with a yellow square [54]. A signal was considered colocalized if the red and green signals overlapped by ≥ 0.5 pixels. These marked images were again split into red, green and blue channels. The blue channel images were discarded, while images in the red and green channels were merged with the blue channel images from the original Z-stack images (before removing background noise). The merged images allow FISH signals to be assigned to specific nuclei. Finally, the percentage of colocalization of a viewpoint and its interacting partner was calculated after counting from 130 to 525 nuclei in wing discs and brains, in the presence and absence of BEAF.
Results
Selection of 4C viewpoints and initial characterization of interactions
Insulator binding proteins are also called architectural proteins because they are thought to play a role in 3D genome architecture by mediating long-distance chromatin looping interactions [55]. We wanted to determine the nature of long-distance interactions made by regions that BEAF binds to, and whether BEAF is necessary for these looping interactions to occur. For this purpose we chose 4 BEAF binding regions from our previous embryo mapping results to use as 4C viewpoints [22]. Approximately 85% of the 1818 regions we mapped were within 300 bp of TSSs, and half were between divergently transcribed genes. The 4 regions we chose are between divergently transcribed genes (Fig 1, S2 Fig) and have CGATA motifs that fit the dual-core model [21]. In addition, BEAF binds to these regions in datasets generated by other labs, including another embryo dataset, Kc cells, Mbn2 cells, and S2 cells [23–25]. Kc cell data from modENCODE indicates that Chromator and CP190 are also found at these viewpoints, and ZIPIC is found at the hts and RpS6 viewpoints [56].
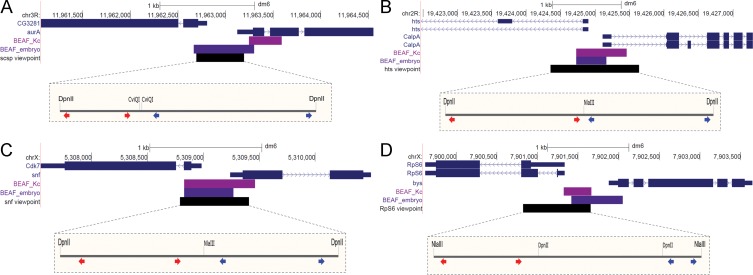
Fig 1. Locations of the 4C viewpoints.
(A) Chr3R scs’ viewpoint at the divergent CG3281 and aurA genes. (B) Chr2R hts viewpoint at the divergent hts and CalpA genes. (C) ChrX snf viewpoint at the divergent Cdk7 and snf genes. (D) ChrX RpS6 viewpoint at the divergent RpS6 and bys genes. The UCSC Genome Browser snapshots show the gene models (blue), BEAF peak limits from Kc cell (magenta) or embryo (purple) mapping, and the viewpoint region (black). The expanded views show the sites for the two restriction enzymes used, with the positions of inverse-PCR primers for the left and right sides of the viewpoints indicated by red and blue arrow pairs respectively.
We obtained 1.4 million to 32 million filtered, aligned reads per viewpoint per replicate (median 2.5 million), of which around 30% mapped to the viewpoint chromosome arm (S5 Table). This level of inter-chromosomal ligation is reasonable for open chromatin, such as our viewpoints, when using a 4-cutter restriction enzyme [57]. The number of reads mapping to significant 4C interactions by fourSig varied between replicates and viewpoints from 13% to 56% for both cis and trans-interactions. Hundreds of cis-interactions were identified (Fig 2A–2D). In line with other 4C results, there were high densities of interactions near the viewpoints (S3 Fig). Most interactions that overlapped between replicates were found in a window of 1 Mb centered on the viewpoints, which we refer to as near-cis-interactions (Table 1). Low levels of overlap of cis-interactions between replicates outside of this window, which we refer to as far-cis-interactions, were only observed for the hts and RpS6 viewpoints. Although the snf and Rps6 viewpoints are both on the X chromosome, none of their interactions overlapped with each other even though they are only separated by around 2.5 Mb and both had longer-distance cis-interactions. Similarly, hundreds of trans-interactions were identified (Fig 3A–3D, Table 1). There was around 15% overlap for replicates of the hts viewpoint, but less than 1% overlap of trans-interactions for replicates of the other viewpoints. Additionally, only a few trans-interactions for any viewpoint overlapped with interactions for other viewpoints.
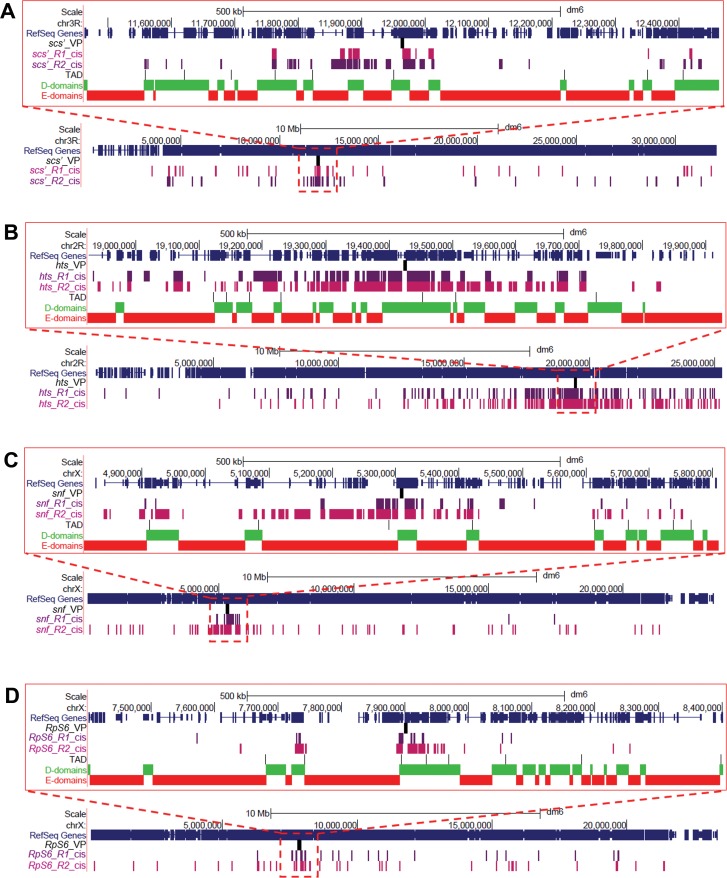
Fig 2. Distribution of cis-interactions along the chromosome arms of the viewpoints.
Shown are UCSC genome browser snapshots of near-cis-interactions (1 Mb centered on the viewpoints, top of each panel) and all interactions along the chromosome arm containing the viewpoint (bottom of each panel) for replicates R1 (purple) and R2 (magenta) for the (A) scs’, (B) hts, (C) snf, and (D) RpS6 viewpoints, with the viewpoint location indicated (VP). Near-cis-interactions also show the gene distribution (blue), embryo TAD boundaries, and H3K27me3-depleted D domains (green) and enriched E domains (red). Most interactions common between replicates are in the near-cis regions, which also contain many unique interactions.
Table 1. 4C interaction data.
| Viewpoint | Number of cis-interactions | Common cis-interactions (%) | Common interactions that are near-cis (%) | Unique interactions that are near-cis (%) | Number of trans-interactions |
|---|---|---|---|---|---|
| scs'_R1 | 270 | 22 | 100 | 59 | 386 |
| scs'_R2 | 148 | 33 | 100 | 29 | 257 |
| hts_R1 | 876 | 58 | 74 | 15 | 2110 |
| hts_R2 | 1216 | 42 | 76 | 30 | 1418 |
| snf_R1 | 140 | 79 | 100 | 80 | 190 |
| snf_R2 | 423 | 27 | 100 | 53 | 951 |
| RpS6_R1 | 76 | 45 | 94 | 17 | 517 |
| RpS6_R2 | 206 | 21 | 95 | 53 | 374 |
Cis-interactions occur on the same chromosome arm as the viewpoint; trans-interactions are on any other chromosome arm. Common interactions are interactions that overlap in both biological replicates (R1 and R2) for a viewpoint. Near-cis-interactions are interactions that occur within 500 kb on either side of a viewpoint.
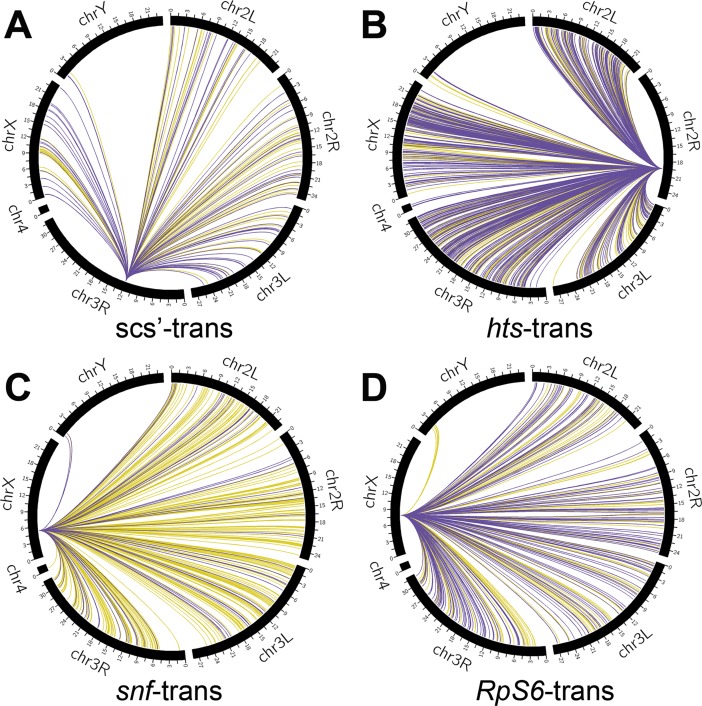
Fig 3. Circos-plots of significant trans-interactions.
Interactions of replicates R1 (yellow) and R2 (purple) occurring on chromosome arms other than the one containing the viewpoint are shown for the (A) scs’, (B) hts, (C) snf, and (D) RpS6 viewpoints. There were 295 trans-interactions that overlapped for the hts replicates, while there was only 1 overlapping trans-interaction for the replicates of the scs’, snf and RpS6 viewpoints.
Virtual 4C and 3C analysis of 4C interactions
There was little overlap between viewpoint replicates outside of the near-cis region. The low level of reproducibility could reflect the complexity of long-distance interactions that occur if chromosome packaging is highly variable between nuclei. Alternatively, it is possible that our viewpoints make a limited number of preferred long-distance cis- and trans-interactions either due to affinity for particular regions or limited variability in nuclear organization. In the latter case, the variability in 4C interactions that we detected would indicate problems with our 4C libraries. We took two approaches to explore this. In one, we performed virtual 4C using Hi-C data. In the other, we performed 3C analysis of selected interactions.
We performed virtual 4C on four Hi-C datasets. All were from DpnII-cut DNA, with two from Kc cells and two from S2 cells. One Kc cell dataset only gave near-cis interactions, and was not analyzed further [40]. The other datasets [28, 38, 39] were similar to our 4C data in that many viewpoint interactions, especially interactions further than 500 kb away, were unique to one replicate (S2 Table). This indicates that the lack of reproducibility of distant interactions in our 4C data is a common feature of these types of experiment.
Interactions found in the 4C analyses were confirmed by 3C for all viewpoints (Table 2, S3 Table). This included the previously reported interaction between the scs’ and scs insulators [51]. Between 13 and 16 cis-interactions were tested for each viewpoint, and over half gave a positive 3C result for each. Over 80% of the tested interactions closer than 1 Mb were positive by 3C (24 of 29), while over 50% of the tested interactions ranging from 1 Mb to 17.1 Mb from viewpoints were positive (15 of 28). Most of the negative 3C results (72%) were for interactions over 1 Mb distant from viewpoints (S3 Table). In addition, 3 viewpoints had 3 trans-interactions tested, and 56% were positive by 3C. Similar to finding that many 4C interactions were detected in only one biological replicate, around 57% of the 3C interactions were detected in only one 3C replicate. Presumably this reflects the variability of nuclear organization, so no 3C or 4C library captures all interactions made by a viewpoint. In contrast, no cross-interactions were detected between viewpoints and interaction partners from a different viewpoint. Seven or eight partners were cross-tested for each viewpoint, including both cis and trans-interactions (Table 2, S3 Table). Together, this suggests that most 4C interactions called by fourSig are valid.
Table 2. Results of testing 4C interactions by 3C.
| Viewpoint | cis < 1 Mb | cis > 1 Mb | trans | Cross-check | ||||
|---|---|---|---|---|---|---|---|---|
| No. | % 3C pos. | No. | % 3C pos. | No. | % 3C pos. | No. | % 3C pos. | |
| scs' | 9 | 67 | 4 | 75 | 0 | na | 8 | 0 |
| hts | 5 | 100 | 10 | 30 | 3 | 67 | 8 | 0 |
| snf | 7 | 86 | 6 | 50 | 3 | 33 | 7 | 0 |
| RpS6 | 8 | 88 | 8 | 75 | 3 | 67 | 7 | 0 |
The number (No.) of tested cis-interactions within 1 Mb on either side of the viewpoints (cis < 1 Mb), further than 1 Mb (cis > 1 Mb), and trans-interactions are indicated together with the percent that gave a positive 3C result (% 3C pos.). Cross-check: testing a viewpoint with an interaction from a different viewpoint; na: not applicable. See S3 Table for details.
FISH analysis of 4C interactions
Fluorescence in situ hybridization (FISH) was also used to examine interactions found by 4C. Interactions verified by 3C were selected for analysis by FISH. Two interactions were tested for the scs’ viewpoint, and one for each of the others. Interactions ranged from 200 kb to 3 Mb away from viewpoints. Three negative controls were included. One used a chromosome 3R partner of scs’ with the hts viewpoint, representing a potential trans-interaction. The other two tested for cis-interactions on the X chromosome, switching the snf and RpS6 viewpoints and partner sequences. Hybridization was done with third instar larval wing discs and brains. To determine if BEAF is required for the detected 4C interactions to occur, we used wild-type larvae and larvae homozygous for the null BEAFAB-KO allele [19]. Null females are nearly sterile, so the null larvae were from heterozygous mothers. BEAF is maternally provided, but is depleted to undetectable levels in null third instar larvae [19]. Between 130 and 525 nuclei were counted for each of the conditions (S6 Table). The expected cis-interactions were confirmed by FISH for all four viewpoints, while the negative controls gave obviously lower colocalization of FISH signals (Fig 4). However, the results indicate that BEAF is not required for the interactions to occur. The lack of dependence on BEAF is consistent with a recent report that RNAi knockdown of BEAF has minimal effects on TAD organization detected by Hi-C [58].
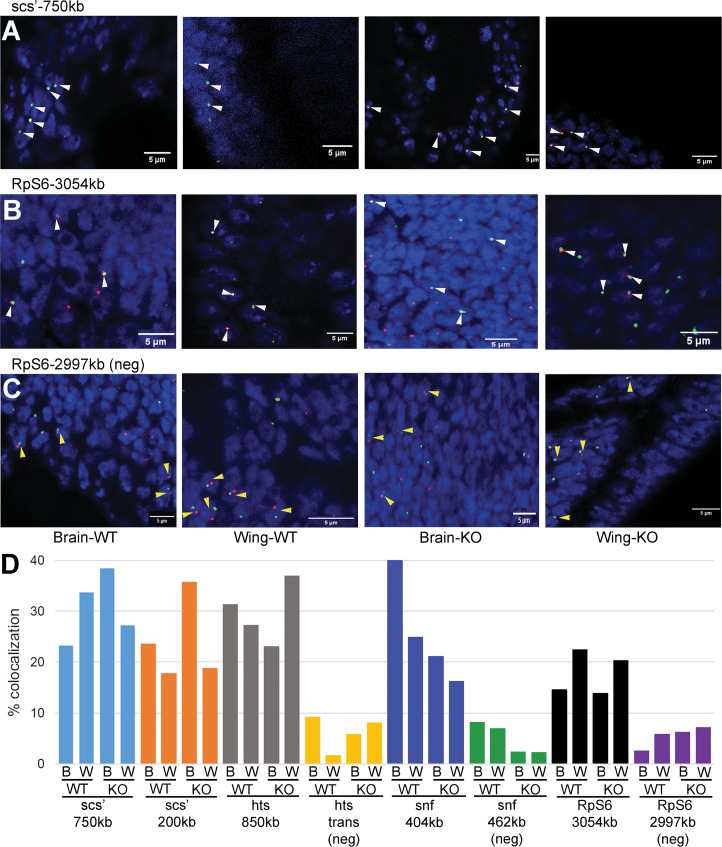
Fig 4. Testing 4C interactions by FISH.
Representative confocal micrographs of hybridizations to third instar larval brains and wing discs from BEAF wild-type (WT) or null (KO; BEAFAB-KO) animals are shown, as labeled. DNA is labeled with DAPI to identify nuclei. White and yellow arrowheads indicate representative nuclei with colocalizing or non-colocalizing FISH signals, respectively. (A) Hybridization to the scs’ viewpoint (green) and a sequence around 750 kb away (red). Overlapping signals are yellow. (B) Hybridization to the RpS6 viewpoint (green) and a sequence around 3054 kb away (red). (C) Negative control hybridization to the RpS6 viewpoint (green) and a sequence around 2997 kb away (red) that does not represent a 4C interaction. (D) Graph of all FISH results, indicating % of counted nuclei showing colocalization of signals (n = 131 to 525, see S6 Table). The viewpoint probe and distance to the second probe are indicated, as well as if the second probe was a negative control (neg) that did not represent a 4C interaction. W: third instar larval wing disc; B: third instar larval brain; WT: wild-type BEAF animal; KO: null BEAFAB-KO animal. The percent colocalization is comparable between WT and KO for both wing discs and brains, but is lower for the three negative controls.
Feature analysis of 4C interactions for epigenetic marks and DNA binding motifs
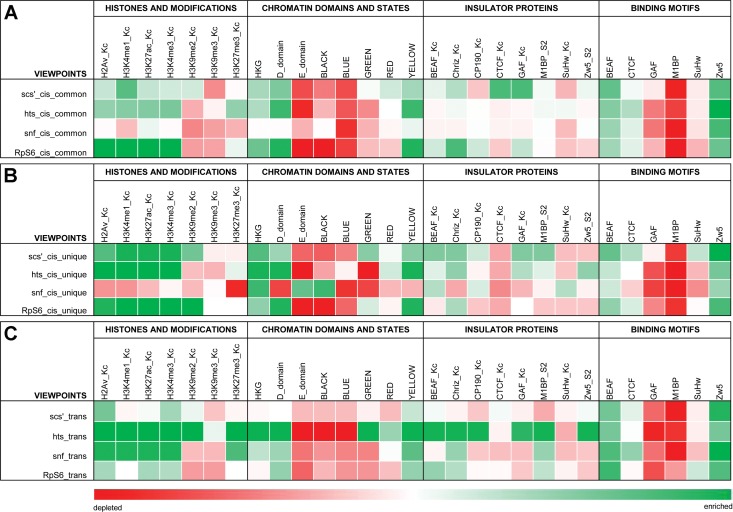
We wanted to know if interactions with our viewpoints were enriched for particular genomic features. BEAF is mostly present near TSSs and most associated genes are actively transcribed [22]. We chose our viewpoints based on this. Therefore we postulated the interaction partners of our viewpoints would also be enriched for active histone marks, especially the active promoter mark H3K4me3. We focused on this mark, H3K4me1 (enhancers), H3K27ac (active enhancers), H2Av (active chromatin), and for inactive chromatin we used the repressive histone marks H3K9me2, H3K9me3 and H3K27me3. We split interactions into three categories. Cis-common refers to cis-interactions present in both 4C replicates (mostly near-cis as described above, Fig 2); cis-unique refers to cis-interactions present in only one replicate; and trans-interactions, nearly all of which were unique. As shown in Fig 5, we found that all interactions for the scs’, hts and RpS6 viewpoints are generally enriched for active histone marks and depleted for inactive histone marks. On the other hand, the snf viewpoint follows this pattern for trans-interactions but cis-interactions are generally depleted of active as well as inactive marks used in our analysis. It should be noted that cis-common and cis-unique interactions had similar associations for a given viewpoint. Like our virtual 4C and 3C results, this similarity suggests that unique interactions, many of which are far from viewpoints, represent genuine interactions.
Fig 5. Feature analysis shows the 4C interactions have a preference for active chromatin and housekeeping genes.
The 4C interactions were categorized as (A) overlapping between 4C replicates (cis-common) or (B) unique cis-interactions or (C) trans-interactions (nearly all unique) for each viewpoint. Genomic Association Tester (GAT) analysis was done for the indicated histones and modifications, chromatin states and domains, housekeeping genes, and insulator proteins plus M1BP. The FIMO program from the MEME suite was used to find DNA binding motif occurrences in viewpoint interactions. Enrichment is indicated in green, depletion in red. See text for details.
Interactions were also characterized with respect to a five chromatin state model based on various histone marks and chromatin proteins [44]. Interactions for all viewpoints were enriched for the active yellow chromatin state, and sometimes to a lesser extent also the red state (Fig 5). Yellow chromatin is enriched for broadly expressed (housekeeping) genes, while the red state is enriched for genes that show more restricted expression patterns (developmental). Conversely, interactions were mainly depleted for repressed black (devoid of histone modifications) and Polycomb group (PcG)-associated blue (H3K27me3) states. Cis-interactions for the snf viewpoint are an exception to this, particularly the cis-unique interactions. They are enriched for black chromatin and depleted for yellow and red, consistent with the depletion for histone modifications. The repressive HP1-associated green chromatin state (H3K9me2/3) is more complicated. Trans-interactions for the hts viewpoint are enriched for this state, as well as for yellow and red, while cis-unique interactions for the scs’ viewpoint and to a lesser extent the RpS6 viewpoint also show some enrichment for green chromatin. A possible explanation for this rather unexpected association could be that active genes in repressive green chromatin are kept active in part by long-distance interactions with active genes [59].
As another way to view the chromatin in our 4C interactions, we analyzed it with respect to D and E domains [28] and housekeeping genes [26, 27]. D domains are depleted for the PcG-associated mark H3K27me3, are largely transcriptionally active, and are enriched for housekeeping genes. In contrast, E domains are enriched for H3K27me3, are largely transcriptionally inactive, and are enriched for regulated genes. Consistent with usually being found near TSSs of housekeeping genes, we found that 95% of BEAF peaks in embryos and Kc cells are in D domains [22, 24]. Our 4C interactions are also associated with D domains and housekeeping genes, and depleted for E domains (Figs 2 and 5). These associations are weakest for scs’ trans-interactions. Also, once again snf cis-interactions varied by having a stronger association with E domains and weaker association with D domains than other viewpoints, although it is similarly associated with housekeeping genes.
Finally, we checked to see if our 4C interactions were enriched for insulator proteins or their DNA binding motifs (Fig 5). We used publicly available Kc cell datasets for BEAF, dCTCF, Su(Hw), CP190, Chro and GAF, and an S2 cell dataset for Zw5. In addition, we used an S2 cell dataset for M1BP because, like BEAF, it often localizes near TSSs of housekeeping genes [45]. As explained in Methods, Kc datasets were not available for Zw5 or M1BP. We found a 28% overlap of Kc cell BEAF peaks with M1BP peaks (45% overlap of M1BP with BEAF), suggesting that BEAF and M1BP independently bind near housekeeping gene TSSs.
Interactions were enriched for M1BP and insulator proteins other than Su(Hw), although there is no clear pattern. In general, the most consistent associations were with BEAF and Chro, followed by M1BP, GAF and finally CP190. However, for each viewpoint there were differences in association profiles between cis-common, cis-unique and trans-interactions. Once again, association profiles for interaction partners of the snf viewpoint differed the most from those of the other viewpoints. Analysis for DNA binding motifs was more consistent, with all viewpoints showing a general enrichment for BEAF and Zw5 motifs, a depletion for GAF, M1BP and Su(Hw) motifs, and being fairly neutral for dCTCF motifs. The significance of the difference between the ChIP and motif analyses is unclear.
We conclude that rather than any particular protein or histone modification, interactions with our viewpoints are driven by redundant factors that lead to open chromatin conducive to active transcription, particularly of housekeeping genes. On the surface, it appears the snf viewpoint often interacts with different chromatin environments than the other viewpoints. Yet snf viewpoint interactions are enriched for housekeeping genes. This suggests that active transcription is a unifying theme even if surrounded by a generally repressive environment.
Discussion
Early Hi-C mapping of chromosome interactions found that chromatin is organized into TADs [47, 60, 61]. There is strong evidence that the insulator protein CTCF, together with Cohesin, plays a role in defining boundaries between TADs in vertebrates by binding oriented binding sites [1, 3, 62, 63]. Drosophila has several insulator proteins in addition to a homolog of vertebrate CTCF, and it has been proposed that insulator proteins often cluster at Drosophila TAD boundaries with boundary strength correlating with the number of clustered proteins [64]. Other evidence suggests that rather than TAD boundaries, Drosophila has inter-TADs that are regions of clustered housekeeping genes [27, 40]. This is reflected in polytene chromosome organization [29]. The short-range interactions within these inter-TADs presumably limits interactions between adjacent TADs. It is possible that housekeeping genes also form some TAD boundaries or inter-TADs in vertebrates [60, 65].
BEAF was originally identified as an insulator binding protein. Genome-wide mapping revealed that it usually binds near TSSs, mainly of housekeeping genes. Our intention was to determine how BEAF contributes to nuclear architecture by mapping looping interactions made by BEAF-associated sequences. Rather than focus only on near-cis-interactions, we were interested to characterize longer distance interactions to determine if there were any shared genomic features that might drive these interactions. Our FISH results indicate that BEAF is not essential for the interactions that we detect, consistent with recently observed minimal effects of BEAF RNAi knockdown on TAD organization [58]. There could be residual maternal BEAF present in third instar larvae, although our efforts to detect it were not successful. There is certainly some BEAF left in cells after RNAi, although in the same study a similar knockdown of M1BP had drastic effects on chromosome organization [58]. It is possible that residual BEAF could be sufficient to maintain chromatin organization, or that there is an epigenetic memory established by BEAF. For example, maternal BEAF could play a role in zygotic activation of associated housekeeping genes and afterwards not be necessary to keep the genes active. In our view, it is more likely that BEAF is one of several redundant factors working in various combinations to open and keep associated promoters active. In fact, rather than a direct role in long range nuclear architecture, the major role of BEAF might be to help open and keep promoter regions in an active state. Regardless of the role of BEAF, our results point to principles of long-distance interactions made by active chromatin.
Viewpoints were selected that were typical of many BEAF binding regions in that they had multiple 32B binding motifs between divergently transcribed genes. Based on high-throughput expression data, seven of the eight genes are classified as housekeeping [26, 27]. The exception is the aurA gene in the scs’ insulator; aurA is active in cultured cells. As such, the viewpoints are in active chromatin. Interactions made by three of the viewpoints are enriched for active chromatin marks, the active yellow chromatin state that is enriched for housekeeping genes [44], H3K27me3-depleted D domains that are enriched for housekeeping genes [28], and housekeeping genes. This indicates active chromatin preferentially interacts with active chromatin. Trans-interactions for the snf viewpoint follow this pattern, but cis-interactions are generally depleted of histone modifications and are generally enriched for the inactive black chromatin state and repressive H3K27me3-enriched E domains. Yet interactions are also enriched for housekeeping genes. One interpretation is that active chromatin in generally repressive environments is on the surface of inactive domains, available for long-distance interactions with other active chromatin such as our viewpoints. This could also explain the enrichment of interactions for other viewpoints with the HP1-associated repressive green chromatin state, in addition to their association with the active yellow chromatin state and D domains.
Although we found a tendency for interactions to occur with active chromatin enriched for housekeeping genes, we did not find evidence that a particular insulator protein drives the interactions. This suggests that redundant factors involved in promoting active transcription participate in establishing the long-distance interactions we found. These factors are likely to include BEAF, Chro, M1BP, CP190 and GAF. Of these, GAF is surprising because it is thought to be associated with regulated gene expression and pausing by RNA polymerase II, although a correlation with BEAF was previously noted [66].
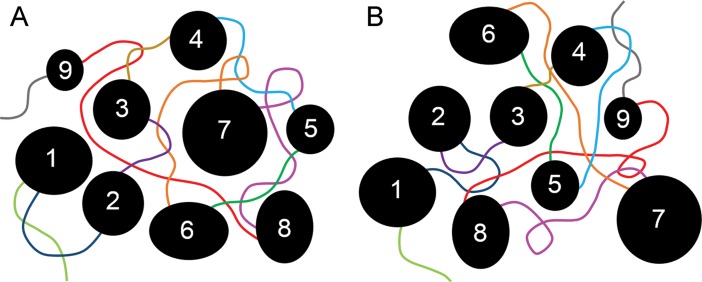
Few of the cis-interactions further than a few hundred kilobases from our viewpoints or trans-interactions were reproducible between replicates. Yet our 4C interactions represent multiple restriction fragments in the neighborhood ligating to our viewpoints, and 3C and FISH results suggest that most are likely genuine interactions. This is supported by virtual 4C analysis of our viewpoints using Hi-C data, which also found a tendency for long-distance interactions to be unique to one replicate. This indicates that long-distance interactions are highly variable, and individual 4C libraries do not capture all interactions. Why would TADs be fairly reproducible between cells, cell types and species while long-distance interactions of active chromatin are not? Repressive chromatin is thought to be sticky, and to be largely responsible for generating TADs [27, 28]. It is possible that these sticky interactions could cause phase separation of heterochromatin from decondensed, active chromatin [30, 31]. Packing TADS together could be less reproducible than folding within TADs, resulting in active chromatin, which is less sticky and between TADs, having different long-distance neighbors in different cells (Fig 6). Highly transient interactions in this environment could keep active chromatin mobile, increasing the number of contacts that can be captured by cross-linking.
Fig 6. Model for variable long-distance interactions between active chromatin.
In this model, TADs (numbered black circles and ovals) are regions of mainly condensed, inactive chromatin that are largely reproducible between cells and cell types. They are separated by inter-TAD regions (lines of different colors) of open, active chromatin such as housekeeping genes. While interactions within TADs are fairly reproducible, packing of TADs relative to each other is highly variable as indicated by the two examples in (A) and (B). Inter-TADs fill the space between TADs, and make variable long-distance interactions (crossing lines of different colors) depending on how TADs are arranged relative to each other. Active chromatin within TADs could be mainly on the surface of TADs (for simplicity, not shown) and so also participate in active chromatin interactions. Highly transient interactions could keep active chromatin mobile, increasing the variability of contacts. This model is particularly appealing if condensed chromatin has a tendency to phase-separate from active chromatin.
Although our viewpoints share BEAF binding, divergent transcription and housekeeping genes, interactions of the snf viewpoint showed some differences from the other three. This raises the question of whether there are different subtypes of active chromatin interactions involving housekeeping genes. For instance, many of the original TADs defined in embryos have BEAF binding near their borders [47]. None of our viewpoints are included in that set. However, higher resolution analyses of Drosophila TADs have been done. One found over 2800 TADs [58]. Using their Chorogenome Navigator, we found that two of our viewpoints, snf and hts, correspond to TAD boundaries (S2 Fig). Looking at the Hi-C heatmaps, these regions could also be considered inter-TADs. Another found over 4000 TADs [39]. All of our viewpoints are near TAD boundaries in that analysis. Thus as resolution increases, more of our viewpoints are placed at TAD boundaries (or TADs and inter-TADs are divided into micro-TADs). This dependence on resolution could reflect some aspect of chromatin that is different between different regions where BEAF is found. At this point it is not clear why the snf viewpoint interacts with chromatin with some different characteristics than the other three viewpoints. It remains for future studies to determine what drives the complex folding of genomes beyond the level of TADs. Our results suggest that one factor could be the preference for active chromatin containing housekeeping genes to interact, perhaps driven in part by exclusion from sticky, condensed chromatin.
Supporting information
Drosophila Kc cells were cross-linked with formaldehyde, lysed, and digested using a restriction enzyme recognizing 4 bp. The protein-DNA complexes were diluted so that the sticky DNA ends were ligated in conditions that favor intramolecular ligations. Cross-links were reversed and DNA was isolated. Because multiple DNA fragments could be ligated into large circles, a second digestion was done with another restriction enzyme recognizing 4 bp. Ligation was again performed under dilute conditions to favor intramolecular ligations. Resulting circles were subjected to inverse PCR using primers based on DNA sequences in viewpoints of interest, to co-amplify unknown sequences from interaction partners. Our primers included Ion Torrent A and P1 adapters and barcode sequences. Amplified 4C libraries were size selected and used for high-throughput sequencing.
(PDF)
Shown are the Hi-C maps, genome-wide mapping information for various insulator and other chromatin proteins (BEAF, M1BP, ZIPIC, dCTCF, Su(Hw), Ibf1/2, Pita, GAF, Zw5, condensin Cap-H2, CP190, Chromator, cohesion Rad21, Pol II) and histone modifications (H3K36me3, H3K79me3, H3K27me3, H4K16ac, H3K4me1, H3K4me3), binding site motif locations (BEAF, M1BP, motif-6, motif-8, ZIPIC, dCTCF, Su(Hw), Ibf) and gene models around the (A) scs’ viewpoint (CG3281 and aur genes); (B) hts viewpoint (hts and CalpA genes); (C) snf viewpoint (Ckd7 and snf genes); and (D) RpS6 viewpoint (RpS6 and bys genes). See Chorogenome Navigator (http://chorogenome.ie-freiburg.mpg.de/) [52] for details.
(PDF)
The highest read densities are around the (A) scs’ viewpoint, (B) hts viewpoint, (C) snf viewpoint, and (D) RpS6 viewpoint for both biological replicates, as is typical of 4C studies.
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We thank Dr. Scott Herke of LSU Genomics Facility for helping us sequence 4C libraries; Dr. Joshua Starmer of University of North Carolina at Chapel Hill for helping us with fourSig; Dr. David Burk of the Socolofsky Microscopy Centre for assisting us with confocal microscopy and ImageJ; Dr. David Donze, Dr. Joomeyeong Kim and Dr. Patrick DiMario of the LSU Department of Biological Sciences for their helpful suggestions during the course of this project. Finally, we wish to thank the reviewers for their valuable comments that helped us improve this manuscript.
Data Availability
4C fastq files are available from NCBI GEO accession number GSE118013. All other relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Science Foundation [1244100 to CMH; 1616827 to DHO and MD]; Louisiana Board of Regents [LEQSF-EPS(2012)-PFUND-274 to CMH]; and Rural Development Administration, Republic of Korea Next-Generation BioGreen21 Program [PJ01317301 to DHO and MD]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Guo Y, Xu Q, Canzio D, Shou J, Li J, Gorkin DU, et al. CRISPR Inversion of CTCF Sites Alters Genome Topology and Enhancer/Promoter Function. Cell. 2015;162(4):900–10. 10.1016/j.cell.2015.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franke M, Ibrahim DM, Andrey G, Schwarzer W, Heinrich V, Schopflin R, et al. Formation of new chromatin domains determines pathogenicity of genomic duplications. Nature. 2016;538(7624):265–9. 10.1038/nature19800 [DOI] [PubMed] [Google Scholar]
- 3.Lupianez DG, Kraft K, Heinrich V, Krawitz P, Brancati F, Klopocki E, et al. Disruptions of topological chromatin domains cause pathogenic rewiring of gene-enhancer interactions. Cell. 2015;161(5):1012–25. 10.1016/j.cell.2015.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dekker J, Misteli T. Long-Range Chromatin Interactions. Cold Spring Harbor perspectives in biology. 2015;7(10):a019356 10.1101/cshperspect.a019356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sati S, Cavalli G. Chromosome conformation capture technologies and their impact in understanding genome function. Chromosoma. 2017;126(1):33–44. 10.1007/s00412-016-0593-6 [DOI] [PubMed] [Google Scholar]
- 6.Schwartz YB, Cavalli G. Three-Dimensional Genome Organization and Function in Drosophila. Genetics. 2017;205(1):5–24. 10.1534/genetics.115.185132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali T, Renkawitz R, Bartkuhn M. Insulators and domains of gene expression. Curr Opin Genet Dev. 2016;37:17–26. 10.1016/j.gde.2015.11.009 [DOI] [PubMed] [Google Scholar]
- 8.Chetverina D, Aoki T, Erokhin M, Georgiev P, Schedl P. Making connections: insulators organize eukaryotic chromosomes into independent cis-regulatory networks. Bioessays. 2014;36(2):163–72. 10.1002/bies.201300125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kellum R, Schedl P. A position-effect assay for boundaries of higher order chromosomal domains. Cell. 1991;64(5):941–50. Epub 1991/03/08. 0092-8674(91)90318-S [pii]. [DOI] [PubMed] [Google Scholar]
- 10.Cuvier O, Hart CM, Laemmli UK. Identification of a class of chromatin boundary elements. Mol Cell Biol. 1998;18(12):7478–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kellum R, Schedl P. A group of scs elements function as domain boundaries in an enhancer-blocking assay. Mol Cell Biol. 1992;12(5):2424–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geyer PK, Corces VG. DNA position-specific repression of transcription by a Drosophila zinc finger protein. Genes Dev. 1992;6(10):1865–73. [DOI] [PubMed] [Google Scholar]
- 13.Zhao K, Hart CM, Laemmli UK. Visualization of chromosomal domains with boundary element-associated factor BEAF-32. Cell. 1995;81(6):879–89. [DOI] [PubMed] [Google Scholar]
- 14.Hart CM, Zhao K, Laemmli UK. The scs' boundary element: characterization of boundary element-associated factors. Mol Cell Biol. 1997;17(2):999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avva SV, Hart CM. Characterization of the Drosophila BEAF-32A and BEAF-32B Insulator Proteins. PLoS One. 2016;11(9):e0162906 10.1371/journal.pone.0162906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aravind L. The BED finger, a novel DNA-binding domain in chromatin-boundary-element-binding proteins and transposases. Trends Biochem Sci. 2000;25(9):421–3. [DOI] [PubMed] [Google Scholar]
- 17.Delattre M, Spierer A, Hulo N, Spierer P. A new gene in Drosophila melanogaster, Ravus, the phantom of the modifier of position-effect variegation Su(var)3-7. Int J Dev Biol. 2002;46(1):167–71. [PubMed] [Google Scholar]
- 18.Udvardy A, Maine E, Schedl P. The 87A7 chromomere. Identification of novel chromatin structures flanking the heat shock locus that may define the boundaries of higher order domains. J Mol Biol. 1985;185(2):341–58. [DOI] [PubMed] [Google Scholar]
- 19.Roy S, Gilbert MK, Hart CM. Characterization of BEAF Mutations Isolated by Homologous Recombination in Drosophila. Genetics. 2007;176(2):801–13. 10.1534/genetics.106.068056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuvier O, Hart CM, Kas E, Laemmli UK. Identification of a multicopy chromatin boundary element at the borders of silenced chromosomal domains. Chromosoma. 2002;110(8):519–31. [DOI] [PubMed] [Google Scholar]
- 21.Emberly E, Blattes R, Schuettengruber B, Hennion M, Jiang N, Hart CM, et al. BEAF regulates cell-cycle genes through the controlled deposition of H3K9 methylation marks into its conserved dual-core binding sites. PLoS Biol. 2008;6(12):2896–910. 10.1371/journal.pbio.0060327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang N, Emberly E, Cuvier O, Hart CM. Genome-wide mapping of boundary element-associated factor (BEAF) binding sites in Drosophila melanogaster links BEAF to transcription. Mol Cell Biol. 2009;29(13):3556–68. 10.1128/MCB.01748-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Negre N, Brown CD, Shah PK, Kheradpour P, Morrison CA, Henikoff JG, et al. A comprehensive map of insulator elements for the Drosophila genome. PLoS Genet. 2010;6(1):e1000814 Epub 2010/01/20. 10.1371/journal.pgen.1000814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bushey AM, Ramos E, Corces VG. Three subclasses of a Drosophila insulator show distinct and cell type-specific genomic distributions. Genes Dev. 2009;23(11):1338–50. 10.1101/gad.1798209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang J, Lacroix L, Gamot A, Cuddapah S, Queille S, Lhoumaud P, et al. Chromatin immunoprecipitation indirect peaks highlight long-range interactions of insulator proteins and Pol II pausing. Mol Cell. 2014;53(4):672–81. 10.1016/j.molcel.2013.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lam KC, Muhlpfordt F, Vaquerizas JM, Raja SJ, Holz H, Luscombe NM, et al. The NSL complex regulates housekeeping genes in Drosophila. PLoS Genet. 2012;8(6):e1002736 Epub 2012/06/23. 10.1371/journal.pgen.1002736 PGENETICS-D-11-02234 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ulianov SV, Khrameeva EE, Gavrilov AA, Flyamer IM, Kos P, Mikhaleva EA, et al. Active chromatin and transcription play a key role in chromosome partitioning into topologically associating domains. Genome Res. 2016;26(1):70–84. 10.1101/gr.196006.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Sharnouby S, Fischer B, Magbanua JP, Umans B, Flower R, Choo SW, et al. Regions of very low H3K27me3 partition the Drosophila genome into topological domains. PLoS One. 2017;12(3):e0172725 10.1371/journal.pone.0172725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eagen KP, Hartl TA, Kornberg RD. Stable Chromosome Condensation Revealed by Chromosome Conformation Capture. Cell. 2015;163(4):934–46. 10.1016/j.cell.2015.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strom AR, Emelyanov AV, Mir M, Fyodorov DV, Darzacq X, Karpen GH. Phase separation drives heterochromatin domain formation. Nature. 2017;547(7662):241–5. 10.1038/nature22989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larson AG, Elnatan D, Keenen MM, Trnka MJ, Johnston JB, Burlingame AL, et al. Liquid droplet formation by HP1alpha suggests a role for phase separation in heterochromatin. Nature. 2017;547(7662):236–40. 10.1038/nature22822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gondor A, Rougier C, Ohlsson R. High-resolution circular chromosome conformation capture assay. Nat Protoc. 2008;3(2):303–13. Epub 2008/02/16. nprot.2007.540 [pii] 10.1038/nprot.2007.540 [DOI] [PubMed] [Google Scholar]
- 33.Splinter E, de Wit E, van de Werken HJ, Klous P, de Laat W. Determining long-range chromatin interactions for selected genomic sites using 4C-seq technology: from fixation to computation. Methods. 2012;58(3):221–30. 10.1016/j.ymeth.2012.04.009 [DOI] [PubMed] [Google Scholar]
- 34.Gheldof N, Leleu M, Noordermeer D, Rougemont J, Reymond A. Detecting long-range chromatin interactions using the chromosome conformation capture sequencing (4C-seq) method. Methods in molecular biology. 2012;786:211–25. 10.1007/978-1-61779-292-2_13 [DOI] [PubMed] [Google Scholar]
- 35.Kong Y. Btrim: a fast, lightweight adapter and quality trimming program for next-generation sequencing technologies. Genomics. 2011;98(2):152–3. 10.1016/j.ygeno.2011.05.009 [DOI] [PubMed] [Google Scholar]
- 36.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357–9. 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams RL Jr., Starmer J, Mugford JW, Calabrese JM, Mieczkowski P, Yee D, et al. fourSig: a method for determining chromosomal interactions in 4C-Seq data. Nucleic Acids Res. 2014;42(8):e68 10.1093/nar/gku156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szabo Q, Jost D, Chang JM, Cattoni DI, Papadopoulos GL, Bonev B, et al. TADs are 3D structural units of higher-order chromosome organization in Drosophila. Sci Adv. 2018;4(2):eaar8082 10.1126/sciadv.aar8082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Q, Sun Q, Czajkowsky DM, Shao Z. Sub-kb Hi-C in D. melanogaster reveals conserved characteristics of TADs between insect and mammalian cells. Nature communications. 2018;9(1):188 10.1038/s41467-017-02526-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cubenas-Potts C, Rowley MJ, Lyu X, Li G, Lei EP, Corces VG. Different enhancer classes in Drosophila bind distinct architectural proteins and mediate unique chromatin interactions and 3D architecture. Nucleic Acids Res. 2017;45(4):1714–30. 10.1093/nar/gkw1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38(4):576–89. 10.1016/j.molcel.2010.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26(6):841–2. 10.1093/bioinformatics/btq033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heger A, Webber C, Goodson M, Ponting CP, Lunter G. GAT: a simulation framework for testing the association of genomic intervals. Bioinformatics. 2013;29(16):2046–8. 10.1093/bioinformatics/btt343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Filion GJ, van Bemmel JG, Braunschweig U, Talhout W, Kind J, Ward LD, et al. Systematic protein location mapping reveals five principal chromatin types in Drosophila cells. Cell. 2010;143(2):212–24. 10.1016/j.cell.2010.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li J, Gilmour DS. Distinct mechanisms of transcriptional pausing orchestrated by GAGA factor and M1BP, a novel transcription factor. EMBO J. 2013;32(13):1829–41. 10.1038/emboj.2013.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Celniker SE, Dillon LA, Gerstein MB, Gunsalus KC, Henikoff S, Karpen GH, et al. Unlocking the secrets of the genome. Nature. 2009;459(7249):927–30. 10.1038/459927a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sexton T, Yaffe E, Kenigsberg E, Bantignies F, Leblanc B, Hoichman M, et al. Three-dimensional folding and functional organization principles of the Drosophila genome. Cell. 2012;148(3):458–72. Epub 2012/01/24. S0092-8674(12)00016-5 [pii] 10.1016/j.cell.2012.01.010 [DOI] [PubMed] [Google Scholar]
- 48.Gramates LS, Marygold SJ, Santos GD, Urbano JM, Antonazzo G, Matthews BB, et al. FlyBase at 25: looking to the future. Nucleic Acids Res. 2017;45(D1):D663–D71. 10.1093/nar/gkw1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grant CE, Bailey TL, Noble WS. FIMO: scanning for occurrences of a given motif. Bioinformatics. 2011;27(7):1017–8. 10.1093/bioinformatics/btr064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mourad R, Cuvier O. TAD-free analysis of architectural proteins and insulators. Nucleic Acids Res. 2018;46(5):e27 10.1093/nar/gkx1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blanton J, Gaszner M, Schedl P. Protein:protein interactions and the pairing of boundary elements in vivo. Genes Dev. 2003;17(5):664–75. 10.1101/gad.1052003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bantignies F, Cavalli G. Topological organization of Drosophila Hox genes using DNA fluorescent in situ hybridization. Methods in molecular biology. 2014;1196:103–20. 10.1007/978-1-4939-1242-1_7 [DOI] [PubMed] [Google Scholar]
- 53.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–82. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Katrukha E. Spots colocalization (ComDet): Utrecht University; 2012 [updated 20 March cited 2017 23 August]. ComDet_v_0.3.6.1:[Available from: http://imagej.net/Spots_colocalization_(ComDet).
- 55.Gomez-Diaz E, Corces VG. Architectural proteins: regulators of 3D genome organization in cell fate. Trends Cell Biol. 2014;24(11):703–11. 10.1016/j.tcb.2014.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zolotarev N, Fedotova A, Kyrchanova O, Bonchuk A, Penin AA, Lando AS, et al. Architectural proteins Pita, Zw5,and ZIPIC contain homodimerization domain and support specific long-range interactions in Drosophila. Nucleic Acids Res. 2016;44(15):7228–41. 10.1093/nar/gkw371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kalhor R, Tjong H, Jayathilaka N, Alber F, Chen L. Genome architectures revealed by tethered chromosome conformation capture and population-based modeling. Nat Biotechnol. 2011;30(1):90–8. 10.1038/nbt.2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramirez F, Bhardwaj V, Arrigoni L, Lam KC, Gruning BA, Villaveces J, et al. High-resolution TADs reveal DNA sequences underlying genome organization in flies. Nature communications. 2018;9(1):189 10.1038/s41467-017-02525-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salzberg AC, Harris-Becker A, Popova EY, Keasey N, Loughran TP, Claxton DF, et al. Genome-wide mapping of histone H3K9me2 in acute myeloid leukemia reveals large chromosomal domains associated with massive gene silencing and sites of genome instability. PloS one. 2017;12(3):e0173723 10.1371/journal.pone.0173723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dixon JR, Selvaraj S, Yue F, Kim A, Li Y, Shen Y, et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485(7398):376–80. 10.1038/nature11082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lieberman-Aiden E, van Berkum NL, Williams L, Imakaev M, Ragoczy T, Telling A, et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326(5950):289–93. Epub 2009/10/10. 326/5950/289 [pii] 10.1126/science.1181369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vietri Rudan M, Barrington C, Henderson S, Ernst C, Odom DT, Tanay A, et al. Comparative Hi-C reveals that CTCF underlies evolution of chromosomal domain architecture. Cell reports. 2015;10(8):1297–309. 10.1016/j.celrep.2015.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gomez-Marin C, Tena JJ, Acemel RD, Lopez-Mayorga M, Naranjo S, de la Calle-Mustienes E, et al. Evolutionary comparison reveals that diverging CTCF sites are signatures of ancestral topological associating domains borders. Proc Natl Acad Sci U S A. 2015;112(24):7542–7. 10.1073/pnas.1505463112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Bortle K, Nichols MH, Li L, Ong CT, Takenaka N, Qin ZS, et al. Insulator function and topological domain border strength scale with architectural protein occupancy. Genome Biol. 2014;15(6):R82 10.1186/gb-2014-15-5-r82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tang Z, Luo OJ, Li X, Zheng M, Zhu JJ, Szalaj P, et al. CTCF-Mediated Human 3D Genome Architecture Reveals Chromatin Topology for Transcription. Cell. 2015;163(7):1611–27. 10.1016/j.cell.2015.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fuda NJ, Guertin MJ, Sharma S, Danko CG, Martins AL, Siepel A, et al. GAGA Factor Maintains Nucleosome-Free Regions and Has a Role in RNA Polymerase II Recruitment to Promoters. PLoS Genet. 2015;11(3):e1005108 10.1371/journal.pgen.1005108 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Drosophila Kc cells were cross-linked with formaldehyde, lysed, and digested using a restriction enzyme recognizing 4 bp. The protein-DNA complexes were diluted so that the sticky DNA ends were ligated in conditions that favor intramolecular ligations. Cross-links were reversed and DNA was isolated. Because multiple DNA fragments could be ligated into large circles, a second digestion was done with another restriction enzyme recognizing 4 bp. Ligation was again performed under dilute conditions to favor intramolecular ligations. Resulting circles were subjected to inverse PCR using primers based on DNA sequences in viewpoints of interest, to co-amplify unknown sequences from interaction partners. Our primers included Ion Torrent A and P1 adapters and barcode sequences. Amplified 4C libraries were size selected and used for high-throughput sequencing.
(PDF)
Shown are the Hi-C maps, genome-wide mapping information for various insulator and other chromatin proteins (BEAF, M1BP, ZIPIC, dCTCF, Su(Hw), Ibf1/2, Pita, GAF, Zw5, condensin Cap-H2, CP190, Chromator, cohesion Rad21, Pol II) and histone modifications (H3K36me3, H3K79me3, H3K27me3, H4K16ac, H3K4me1, H3K4me3), binding site motif locations (BEAF, M1BP, motif-6, motif-8, ZIPIC, dCTCF, Su(Hw), Ibf) and gene models around the (A) scs’ viewpoint (CG3281 and aur genes); (B) hts viewpoint (hts and CalpA genes); (C) snf viewpoint (Ckd7 and snf genes); and (D) RpS6 viewpoint (RpS6 and bys genes). See Chorogenome Navigator (http://chorogenome.ie-freiburg.mpg.de/) [52] for details.
(PDF)
The highest read densities are around the (A) scs’ viewpoint, (B) hts viewpoint, (C) snf viewpoint, and (D) RpS6 viewpoint for both biological replicates, as is typical of 4C studies.
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
4C fastq files are available from NCBI GEO accession number GSE118013. All other relevant data are within the paper and its Supporting Information files.