Abstract
This article describes three simple activities we presented at the 2017 FUN Faculty Workshop at Dominican University that demonstrate how proprioceptive information contributes to our mental image of physical self, and how artificially altering this information creates kinesthetic illusions. We focus on the muscle spindle contribution to limb positional sense and standing postural maintenance. We use a percussion stimulator to vibrate muscle spindles in several muscle groups, causing an artificially incorrect message to the CNS that a muscle has lengthened. This creates an illusion of limb position or standing posture change. Although descriptive data can suffice to engage students in these activities, we suggest quantitative measurements to add further depth.
These activities are open for continued student-designed exploration. They lead directly to discussions of sensory physiology, central pathways for integration of sensory information and spinal pathways to execute motor commands. A broader context for the activities could include postural adaptations at sea and upon return to land, postural illusions experienced by astronauts and the postural and locomotor problems they experience upon return to Earth, and the effects of aging and disease on the proprioceptive control of limb position and posture.
Keywords: muscle spindle, kinesthesia, posture, sensory physiology, proprioception, illusion
INTRODUCTION
We were inspired by Keating et al. (2005) to adapt their student lab exercise on the perception of body position and movement (kinesthesia) for an active learning session in a freshman physiology course at Cornell University, and to present our version of this exercise at the 2017 FUN Workshop at Dominican University. The focus of the exercise is the role muscle spindles play in providing correct positional information of body parts to the CNS for awareness of limb position and maintenance of standing posture. A distorted perception of limb position or body movement (kinesthetic illusion) can be caused by artificially altering the action potential firing rate of muscle spindles as they report muscle length information to the brain (Proske and Gandevia, 2012). Specifically, for the set of activities described here, inappropriate muscle spindle firing can produce distortions in perception of arm position and correct standing posture (Proske and Gandevia, 2012). We offer suggestions for updating and quantifying the qualitative exercises of Keating et al. (2005) to help bring these interesting, fun and pedagogically rich student activities into greater classroom and outreach use.
Proprioception is the sense that provides the feedback for our motor systems to produce appropriate movements and posture (Silverthorn, 2019). Muscle spindles are considered the principal proprioceptive sense organs, although golgi tendon organs, joint receptors and skin receptors also contribute to our body sense (Proske and Gandevia, 2012; Silverthorn, 2019). Briefly (for more details see especially Pearson and Gordon (2013), and also Silverthorn, 2019), muscle spindles are sensory organs imbedded in muscle among and parallel to the main working muscles (extrafusal), which are innervated by alpha motor neurons. Muscle spindles provide information to the CNS on muscle length in a static position (close your eyes and you still know where your limbs are), and muscle length changes that may be caused by local joint angle changes during movement (Keating et al., 2005). The muscle spindle is a muscle and nerve complex encapsulated by connective tissue. The capsule has specialized muscle fibers (intrafusal) with stretch sensitive nerve endings wrapped around them (Fig. 1). There are two types of sensory endings; larger diameter, primary sensory endings that respond to static intrafusal muscle fiber length and changes in length, and smaller diameter, secondary sensory endings that respond only to intrafusal muscle fiber length. Gamma motor neurons innervate the intrafusal muscle fibers to set the sensory sensitivity to static and dynamic changes in muscle length (Fig. 1).
Figure 1.
The muscle spindle embedded in a skeletal muscle, highlighting motor and sensory innervation of the extra- and intrafusal muscle fibers.
We describe here three sets of activities presented at the 2017 FUN Workshop that demonstrate the importance of the muscle spindle for limb position and for maintaining a balanced standing posture. When a percussion stimulator (massage or physical therapy vibrator) sends vibratory waves into a muscle, the muscle lengthens without a change in the angle of the joint the muscle crosses. The artificially lengthened muscle creates a kinesthetic illusion of limb position because the muscle spindles send length information to the CNS that does not match the actual joint angle the muscle is crossing (Keating et al., 2005; see McCloskey et al. [1983] for a key research paper).
Our objectives for these activities are:
Students will understand that proprioception is an important sense for body position (the 6th sense), and to guide appropriate movement.
Students will understand the structure and functional properties of muscle spindles.
Students will learn that body position illusions can be created when muscle length information is misinterpreted by the brain. They will understand that perceptual illusions, can be created for other senses besides vision.
Students will appreciate that multiple sensory systems support standing posture.
Students will be introduced to the sensory, integrative and motor pathways that create body perception and appropriate body movement.
Students will practice quantitative experimental design.
Students will practice working collaboratively in teams.
Students will have fun learning.
MATERIALS AND METHODS
For all three exercises, a Wahl Deep-Tissue Percussion Therapeutic Massager (model number 4290-300) with the deep tissue attachment was used to vibrate muscle tissue. Blindfolds for students ensure that the test subject keeps their eyes closed during the activity but are not necessary. Vision is of course an additional, important source of information for proper limb positioning and postural control.
The qualitative data collection for these activities is by itself extremely powerful for student engagement. However, for the first two activities, results are easily quantified to add depth to the exercise. Quantitative student data reported for the first two activities were gathered from the Cornell University class “BioG 1440 Introductory Biology: Comparative Physiology” during Spring Semester, 2018. Simple statistics were used to generalize the effect of each variable tested in the first two activities. At least 633 Cornell students over several semesters have experienced the activities reported here.
Activity 1: Role of the muscle spindle in adjusting limb position (see Goodwin et al. [1972] for a key research paper)
In this activity, students worked in pairs with one student serving as the test subject and the other as the investigator. The investigator moved the subject’s right arm, stopping periodically at random positions. The subject was instructed to match the position of their left arm with their right under three different conditions (eyes open no spindle stimulation, eyes closed no stimulation, and eyes closed with stimulation).
Test subjects sat with their elbows placed on a table, palms up (Fig. 2). First, with the subject’s eyes open, the investigator held the subject’s left wrist and moved the subject’s arm slowly up and down so that the arm flexed and extended about the elbow. The investigator stopped and started left arm movement periodically at random positions while the subject attempted to match the position of their right arm with their left (Fig. 2).
Figure 2.
Activity 1: Application of the percussion stimulator to the biceps muscle of test subject to alter arm position information. The test subject attempts to mimic the left arm position with the right arm while the observer applies percussion stimulation to the left arm biceps muscle and moves the left arm up and down.
Next the subject closed their eyes (or applied a blindfold). The investigator continued to move the subject’s left arm in the same manner, stopping and starting periodically at random positions. Finally, the subject’s eyes remained closed and the investigator set the percussion stimulator to the highest vibration speed (3,350 pulses per minute) and placed it firmly against the belly of the subject’s left biceps (Fig. 2). The investigator then continued to move the subject’s left arm, stopping and starting at random positions again instructed the subject to match the position of their left arm with their right.
Under each set of conditions, the investigator qualitatively assessed how well the subject matched the position of their right arm with their left. In addition, 14 student investigators used chalkboard protractors to measure the angle formed by the humerus and radius of their test subjects. The angles formed by the right and left arms were compared to determine how well the subject mimicked the position of their left arm with their right at each step of the activity sequence.
A more sophisticated quantitative analysis can be performed by attaching goniometers (joint angle sensors) to the elbows of the subject to collect highly precise angle differences. This allows measurement of tiny changes in the angle between each limb. Goniometers, supplied by ADInstruments, were tested by participants at the 2017 FUN Workshop and worked well. This equipment was not used in the Cornell classes because of the cost for the large number of students.
Activity 2: Using spindle information to actively hold limb position
In this activity, students investigated how spindles contribute to maintaining one’s internal representation of a limb in space. Again, students worked in pairs with one serving as the test subject and the other as the investigator. The subject was instructed to hold one arm out from their body and touch their nose with the index finger of that arm (Fig. 3). Then the subject was told to pull their finger back several centimeters away from their nose, close their eyes, and hold the position of the active arm.
Figure 3.

Activity 2: Application of the percussion stimulator to the triceps muscle of test subject to alter arm position information. Subject attempts to maintain finger position during triceps muscle vibration.
With the percussion stimulator set to the highest speed of vibration, the observer applied stimulation to the triceps muscle of that arm (Fig. 3) and observed the finger position. Next, the subject was instructed to slowly move their finger forward to touch their nose. Student investigators noted any position changes when the percussion massager was applied to the triceps muscle, as well as the accuracy of the test subject’s touch to their nose. Subjects were asked to describe their perception of finger distance during triceps vibration. Nineteen student pairs used a ruler to measure the distance of the finger tip from subject’s nose before and during triceps stimulation.
Activity 3: Role of spindle information in balanced, standing posture
In activity 3, students explored a variety of cues (kinesthetic, visual, and vestibular) that contribute to upright standing posture, and the consequences when one or more of these cues is compromised. Students worked in teams of four, with one student serving as the subject, one as the observer, and two as spotters. The subject was required to adjust their socks and/or pants so that their Achilles tendons were accessible for stimulation (Fig. 4).
Figure 4.
Activity 3: Application of percussion stimulators to both Achilles tendons of a test subject to alter postural position information.
The subject was instructed to stand with arms folded and feet together. The spotters stood on either side of the subject, ready to catch them should they fall. The observer knelt behind the subject and applied two percussion stimulators simultaneously, one to each Achilles tendon, to muscles stimulate the spindles in the gastrocnemius (Fig. 4). The observer and spotters noted the subject’s steadiness and any changes in their posture upon stimulus application.
Next, the observer instructed the subject to close their eyes and the observer/spotters noted how the subject’s steadiness was affected by application of gastrocnemius stimulation during the loss of visual information. Depending on how severely the subject reacted to the percussion stimulation with eyes closed, groups determined whether or not to proceed. If the subject was swaying dramatically at this point, the observer stopped the activity here. If not, the subject was instructed to keep their eyes closed and tilt their head back as far as possible (to compromise vestibular signals for posture). The observer/spotters now observed the subject’s steadiness with gastrocnemius stimulation, eyes closed, and head tilted back. To end this session, the subject was told to open their eyes and look straight ahead before removing the percussion stimulators. This is an important procedure to avoid a sudden loss of balance when muscle stimulation ceases. In our experience, when the gastrocnemius muscle stimulation stops with visual and vestibular information compromised, the subject will sometimes lunge forward, perhaps in an attempt to overcompensate for a sudden change in muscle length information.
At the 2017 FUN Workshop, we used electromyography (EMG) to record leg muscle activity during standing postural control under the different conditions. Surface EMG electrodes were placed on the gastrocnemius and tibialis leg muscles, digitized with PowerLab® and recorded and displayed with LabChart8® (ADInstruments). In addition, a force plate (Wii balance board) connected to the PowerLab measured body sway as the test subject experienced the loss of different sources of postural information.
Participants in our FUN Workshop sessions on kinesthetic illusions (n=26) evaluated these activities by answering six questions, provided by the workshop organizers, on a Likert scale of 1–5, with 1-strongly agree to 5-strongly disagree.
RESULTS
The general qualitative observations reported below for the muscle spindle activities were commonly seen with both the freshman physiology students and the participants in our FUN Workshop sessions. The entire sequence of the three activities were completed in a 50-minute session with the Cornell students and in 1.5 hour sessions at the FUN Workshop.
Activity 1
Qualitatively, students observed that in the absence of muscle stimulation, the test subject was able to very closely match the position of their left arm with the right while the left arm was moved, both with their eyes open and closed (Fig. 5). Upon applying stimulation to the biceps with eyes closed, the subject’s coordination between right and left arms was compromised. Movements of the right arm became delayed and sometimes jerky compared to the left. When the observer stopped the test subject’s stimulated left arm from moving, the subject had difficulty matching the angle of the right arm with the left, and almost always overextended the right arm.
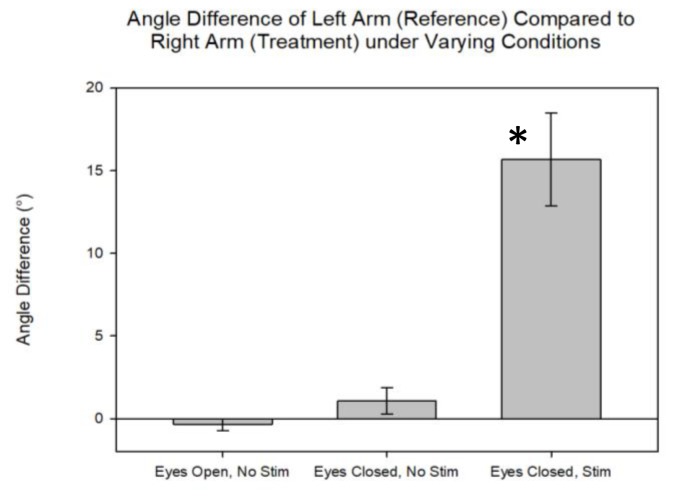
Figure 5.
Right and left arm angle difference for activity 1 under different biceps stimulation conditions. There was no significant difference between left and right arm angles upon stopping left arm movement with eyes open or closed and no percussion stimulation of left biceps muscle (p = 0.11). Adding biceps stimulation with eyes closed significantly increased the final angle difference between the two arms (asterisk, p < 0.001).
These observations were quantitated in a group of Cornell physiology students (n=14). No significant difference was found between the arm elbow angles with eyes open or closed without stimulation (t = 1.77, P = 0.11). A statistically significant mismatch between right and left arm angles was seen with eyes closed and bicep stimulation (t = 1.77, P < 0.001; Fig. 5).
Activity 2
When triceps stimulation started with eyes closed, test subjects were no longer able to maintain the exact position of their finger in front of their nose. Their finger, originally positioned a few centimeters away from their noise, drifted an average of 3.0 cm (SD +− 2.67) further away (Fig. 6). When now asked to touch their nose, subjects moved their arm awkwardly as if unsure of the distance between finger and nose. They frequently missed the nose, sometimes poking their cheek after a longer move forward than they predicted. They often expressed surprise at how far their finger moved away from their nose with triceps stimulation, experiencing the mirage that their finger was closer to their face than it was.
Figure 6.
Average distance of finger from nose before and during stimulation in activity 2. During triceps percussion stimulation, the subject’s finger drifted significantly away from the nose (asterisk, p < 0.001), indicating altered sense of finger position.
These observations were also quantitated in a group of Cornell physiology students (n=19). After triceps stimulation and eyes closed, the finger distance from the nose was significantly greater than before stimulation (t = 1.73, P < 0.001; Fig. 6).
Activity 3
When stimulation was applied to the Achilles tendon of standing subjects, they swayed and reported feeling as though they were falling and needed to catch their balance (similar to how one might react when trying to balance on one leg). Generally, the swaying and balance correction became more pronounced as additional sensory cues were removed (first proprioception, then visual, and finally vestibular). There was variability in how pronounced the effect was with Achilles tendon stimulation. In some cases, obvious swaying was noticed immediately upon applying the percussion stimulators, while in others, there was not a noticeable effect of stimulation until the subject closed their eyes or additionally tilted their head. Subjects reported activating their leg muscles to try to maintain posture once the stimulation was applied—this was the case even when the subject was not noticeably swaying.
EMG recordings can also display and quantitate leg muscle activity changes during attempts to balance posture as sensory feedback for upright standing is disturbed. As an example, in the FUN Workshop we recorded EMGs from a test subjects’ gastrocnemius and tibialis muscles (Fig. 7). These recordings were converted to root mean square (RMS) signals for more quantitative analysis (see Crisp et al., 2016 and Crisp (2018). The RMS signals in Figure 7 show activity in the gastrocnemius and tibialis muscles, occasionally alternating, while the subject swayed during Achilles tendon stimulation alone, then with eyes closed and then adding the head tilted back. At the end of the RMS traces in Figure 7, the increased activity of both muscles represents a balance overcompensation as stimulation is removed and eyes are opened and looking ahead. It was especially important to have spotters ready at this stage because the subjects often lunged forward.
Figure 7.
Root mean square (RMS) of the EMG recorded from the gastrocnemius (purple) and tibialis (Green) muscles during Activity 3. Note increased muscle activity beginning with muscle spindle stimulation as test subject sways, and very active, compensatory muscle activity when eyes are reopened with head facing forward.
A Wii balance board can be used to measure postural swaying as sensory cues for upright standing are manipulated. At the FUN Workshop we recorded the center of a test subject’s vertical pressure (Macpherson and Horak, 2013) with a Wii balance board simultaneously with the EMG signal. Figure 8 shows XY plots of a subject’s center of pressure under sequential conditions of no Achilles tendon stimulation and eyes open looking forward, tendon stimulation and eyes open looking forward, tendon stimulation, eyes closed looking forward, and tendon stimulation, eyes closed and head tilted back. The XY plots of Figure 8 show increasing variability in the subject’s center of pressure as muscle spindle, vision, and equilibrium information for standing posture are sequentially taken away, and the subject sways more.
Figure 8.
XY plot of four 5-second selections of force plate data showing changes in test subject’s center of pressure as they attempt to maintain standing posture. Recorded using LabChart 8 and the Wii balance board. A. No Achilles tendon stimulation, eyes open, looking forward. B. Tendon stimulation, eyes open, looking forward. C. Tendon stimulation, eyes closed, looking forward. D. Tendon stimulation, eyes closed and head tilted back.
Activity Assessment
Qualitatively, most Cornell students were continuously engaged with the activities presented here. They often expressed that they were having fun with the activities and with their small group interactions. Many were curious about the physiological mechanisms underlying body position, especially for the third activity. They expressed surprise at how large and easily observable the effects were. The clear effects of muscle spindle stimulation in all three activities were a hook to get students thinking and talking about body sense. Many pairs of students asked to switch subject and observer roles to either experience the kinesthetic mirages or observe them.
The responses of the FUN Workshop faculty participants (n = 26) to the short questionnaire expressed that our session was pedagogically useful, well organized, and they thought that the material was a good resource for teaching neuroscience. Participants rated our workshop session with the following questions on a scale of 1 (strongly agree) to 5 (strongly disagree) (mean responses after each question): 1) this workshop met my expectations (1.31); 2) this workshop increased my understanding of the topic material (1.65); 3) this workshop was well organized with clear objectives (1.27); 4) the exercise presented at this workshop is a good vehicle for teaching undergraduates principles of neuroscience (1.46); 5) I am likely to incorporate this material as a classroom activity (1.73); 6) I am likely to use the material in this workshop as a resource for my class lectures and my own neuroscience background (1.62). Unfortunately, we lost the original raw data with participant written comments, but mean responses for the questions fall between 1, strongly agree and 2, agree, which suggests low variability in the participant ratings (R. Calin-Jageman, personal communication).
DISCUSSION
The most striking qualitative feature of the student and FUN faculty responses to these series of activities was the expression of having engaged fun. Test subjects and observers were surprised that percussion stimulation of specific muscle groups distorted the sense of limb position, and that leg muscle stimulation distorted standing postural control. The idea that illusions could be created through other sensory systems besides vision (Wyttenbach, 2006, 2012) was new to the biology freshmen. Faculty participant responses to the short questionnaire about our sessions indicated that the activities were also useful neuroscience teaching materials.
These activities demonstrating the importance of the muscle spindles in the construction of our mental image of physical self can easily be adapted to meet the needs of specific courses. The level of detail students should know about muscle spindle physiology, central spinal and brain integration of the spindle information and the motor commands for muscle activation, as well as the level of sophistication of student explanations for the observations made during the activity can be adjusted for class objectives. For example, muscle spindle physiology can be a platform to introduce the general properties of sensory systems such as adequate stimulus, transduction, adaptation, range fractionation and efferent control of sensory sensitivity (Hill et al., 2012; Silverthorn, 2019).
Basic muscle spindle physiology could be examined in more detail in a concurrent Crawdad student lab exercise. The action potential firing of the crayfish muscle receptor organs, which are functionally and anatomically similar to the muscle spindle, is easily recorded in undergraduate neuroscience laboratory classes (Wyttenbach et al., 2014).
Discussions of the central integration of sensory information and motor output for simple muscle and postural reflexes can also be part of these activities. The muscle spindles influence motor firing directly and indirectly through spinal pathways to participate in important spinal reflexes, and through projections to the brain (Silverthorn, 2019). The control of standing posture by spindle, visual and vestibular information through cerebellar and brainstem pathways is complex with both reactive and anticipatory components (Macpherson and Horak, 2013; Colgan, 2015). All three sources of information are required for the CNS to determine if the head alone or the environment around the head is moving (Ghez, 1991). Thus, there is much educational depth that could be explored in these activities.
We prefer to teach these exercises as an inquiry-based learning by invention activity in which students are asked to solve a problem or generate an explanation on their own before receiving instruction on the accepted explanation or solution. Learning by invention activities have been found to increase understanding compared to traditional instruction (Schwartz and Martin, 2004; Jarosz et al., 2017). Students participate in the lab activity as an introduction to sensory systems before being exposed to the conceptual material. They first complete the three activities to observe the kinesthetic mirages, then work in small groups to generate an explanation for their observations. Students are not expected to come up with the term “muscle spindle” or the details of how they function at this point. A reasonable expectation is for students to say that we must have sense organs/systems that give us information about where our limbs and body are, and that the percussion stimulators cause these organs to malfunction/send incorrect information to the brain. After students have had time to work in small groups, the instructor facilitates a class discussion, allowing each group the opportunity to share their ideas. Students are then instructed on the details of muscle spindle physiology, and perhaps central sensory and motor pathways, and asked to make connections between this material and their lab observations. Although we do not have quantitative assessment to present now, we experienced that having such post-activity discussions with the student groups helped them make connections between the role of the muscle spindles in kinesthetic illusions and the neural pathways facilitating them.
These activities can also be extended with student driven experiments. For example, students will note variability in responses of their peers to triceps, biceps and Achilles stimulation. This may result from differences in the reliance of limb position sense on a variety of sensory input types that they could explore (Proske and Gandvia, 2012). Effects of different stimulation intensities and muscle fatigue on limb position and posture could be examined. Fine tuning of the posture activity protocols could determine the relative importance of spindle, visual and vestibular input for postural control, and the relative intensity of sensory input needed in each modality: For example, comparing stimulation of 1 vs two legs, 1 eye closed or 2, and different angles of head tilt. The review of Proske and Gandevia (2012) notes that cutaneous touch receptors are not as important as muscle spindles for position sense. To test this, a perceptive reviewer suggested an important control experiment. Students could simply press the turned off percussion stimulator against the skin in each activity to determine the contribution of cutaneous receptors to these positional illusions.
Finally, these activities can lead to interesting and lively class discussions on a variety of topics. These can include postural adaptations at sea and upon return to land, postural illusions experienced in space and postural and locomotor problems when astronauts adapt back to gravity on Earth (Reschke et al.,1998; Keating et al., 2005), and the effects of aging and disease on the proprioceptive control of limb position and posture (Proske and Gandevia, 2012; see Cole and Sedgwick [1992] for an interesting case study of disease-induced loss of proprioception). We feel we have only scratched the surface of the educational experimental and conceptual depth of these series of student activities.
Footnotes
We thank Dr. Vicki Tu for sharing her student handouts describing similar student exercises, Dr. Robert Calin-Jageman for statistical advice, anonymous reviewers for excellent suggestions of a further experiment and deeper background citations, ADInstruments for providing the muscle spindle figure and Ryan Ludwig for drawing Figures 2–4.
Lab Chart® and PowerLab® are trademarks of ADInstruments Pty Ltd.
REFERENCES
- Cole JD, Sedgwick EM. The perceptions of force and of movement without large myelinated sensory afferents below the neck. J Physiol. 1992;449:503–515. doi: 10.1113/jphysiol.1992.sp019099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan W., III Student friendly technique to demonstrate coordination between postural (involuntary) and voluntary muscle contractions. J Undergrad Neurosci Educ. 2015;13:A244–A246. [PMC free article] [PubMed] [Google Scholar]
- Crisp KM. Recording EMG signals on a computer sound card. J Undergrad Neurosci Educ. 2018;16:A210–A216. [PMC free article] [PubMed] [Google Scholar]
- Crisp KM, Lin H, Prosper I. Breadboard amplifier: building and using simple electrophysiology equipment. J Undergrad Neurosci Educ. 2016;14:A124–A131. [PMC free article] [PubMed] [Google Scholar]
- Ghez C. Posture. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of neuroscience. 3th edition. chapter 39. Norwalk, CT: Appleton & Lange; 1991. [Google Scholar]
- Goodwin GM, McCloskey DI, Matthews PBC. The contribution of muscle afferents to keslesthesia shown by vibration induced illusions of movement and by effects of paralyzing afferents. Brain. 1972;4:705–748. doi: 10.1093/brain/95.4.705. [DOI] [PubMed] [Google Scholar]
- Hill RW, Wyse GA, Anderson M. Animal Physiology. Chapter 14. Sinauer Associates, Inc Publishers; Sunderland MA: 2012. [Google Scholar]
- Jarosz AF, Goldenberg O, Wiley J. Learning by invention: small group discussion activities that support learning in statistics. Discourse Processes. 2017;54:285–302. [Google Scholar]
- Keating JG, Martin TA, Flez JA, Buckner RL. Altered limb position sense by muscle vibration. In: Silverthorn, Johnson, Mills, editors. Laboratory Manual for Physiology. Pearson Education; 2005. [Google Scholar]
- Macpherson JM, Horak FB. Posture. In: Kandel ER, Schwartz JH, Jessell TM, Siegelbaum SA, Hudspeth AJ, editors. Principles of Neuroscience. 5th edition. chapter 41. New York: McGraw Hill Medical; 2013. [Google Scholar]
- McCloskey DI, Cross MJ, Honner R, Potter EK. Sensory effects of pulling or vibrating exposed tendons in man. Brain. 1983;106(Pt 1):21–37. doi: 10.1093/brain/106.1.21. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Gordon JE. Spinal Reflexes. In: Kandel ER, Schwartz JH, Jessell TM, Siegelbaum SA, Hudspeth AJ, editors. Principles of neuroscience. 5th edition. chapter 13. New York: McGraw Hill Medical; 2013. [Google Scholar]
- Proske U, Gandevia SC. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol Rev. 2012;92:1651–1697. doi: 10.1152/physrev.00048.2011. [DOI] [PubMed] [Google Scholar]
- Reschke MF, Bloomberg JJ, Harm DL, Paloski WH, Layne C, McDonald V. Posture, locomotion, spatial orientation, and motion sickness as a function of space flight. Brain Res Brain Rev. 1998;28:102–117. doi: 10.1016/s0165-0173(98)00031-9. [DOI] [PubMed] [Google Scholar]
- Schwartz DL, Martin T. Inventing to prepare for future learning: the hidden efficiency of encouraging original student production in statistics instruction. Cognit Instruct. 2004;22(2):129–184. [Google Scholar]
- Silverthorn DU. Chapters 10, equilibrium and 13, skeletal muscle reflexes. 8th edition. Pearson Education; 2019. Human physiology: an integrated approach. [Google Scholar]
- Wyttenbach RA. PsyCog: explorations in perception and cognition. New York: Oxford University Press; 2006. [Google Scholar]
- Wyttenbach RA. Exploring sensory neuroscience through experience and experiment. J Undergrad Neurosci Educ. 2012;11:A126–A131. [PMC free article] [PubMed] [Google Scholar]
- Wyttenbach RA, Johnson BR, Hoy RR. Crawdad: a CD-ROM lab manual for neurophysiology. New York: Oxford University Press; 2014. [Google Scholar]