Abstract
Despite decades of research, the induction and maintenance of long-term allograft tolerance without immunosuppression remains an elusive goal in the field of solid organ and cell transplantation. Immunosuppressive medications frequently prevent or minimize acute cellular rejection but have failed to halt anti-donor antibody production and chronic organ rejection. Past efforts aimed at promoting lasting allograft tolerance have focused primarily on peripheral T cell depletion, augmentation of regulatory T cells, or induction via simultaneous hematopoietic stem cell transplantation and facilitation of donor chimerism. So far, none of these methods have led to consistently safe, feasible and long lasting donor organ acceptance. Over the course of the past 4 decades, the study of a unique population of antigen-presenting cells known as dendritic cells (DCs) has shown promise for breaking new ground in achieving indefinite allograft survival without immunosuppression and its associated adverse effects. In this review, we discuss the discovery and early investigations of DCs and chronicle some of the key studies demonstrating their role in transplantation, particularly in indirect allorecognition, the immunologic pathway thought to drive chronic rejection and perhaps tolerance induction.
I. Identification and Characterization of Dendritic Cells
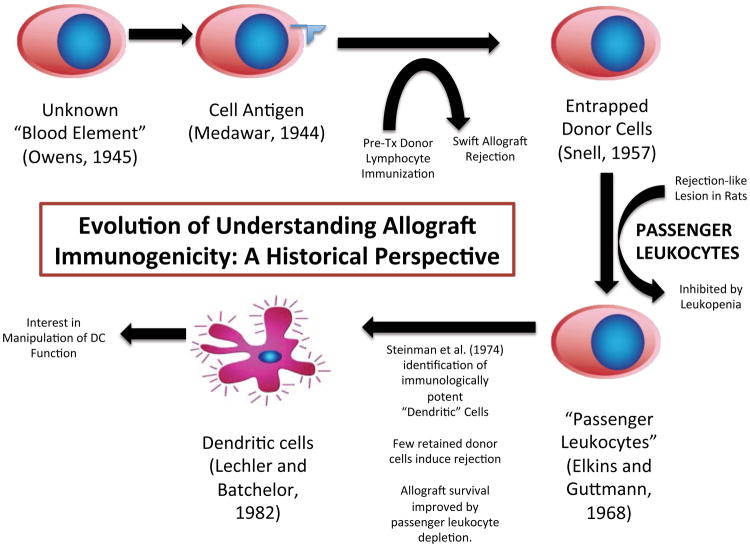
For many years, immunologists speculated that some unknown blood element, transferred in tandem with the donor allograft, likely played a crucial role in transplant outcomes (Figure 1). In 1944, Medawar argued that donor-specific tissue antigen acted as a key element in promoting rejection.1 Owen’s seminal finding published in 1945, that fraternal twin calves’ placental cross-circulation leads to the production of chimerism, where each twin has the blood cells of both,2 laid the foundation for future studies that form the basis of immunologists’ understanding of tolerance induction for solid organ allografts. The extent of chimerism required, the precise reason for its establishment, as well as the feasibility of cellular depletion techniques used to achieve it, however, remain problematic.3
Figure 1.
Timeline represen;ng changes in the understanding of visceral allograO immunogenicity. (Tx, transplant; DC, dendri;c cell)
Twelve years later, Snell observed that in fact, recipient pretransplant immunization with donor lymphoid cells sensitized the allograft to rejection more effectively than tissue antigen alone, suggesting that entrapped donor leukocytes significantly contribute to graft immunogenicity.4 Later, on encountering a renal allograft lesion in a rat model that histologically mimicked acute rejection but was inhibited by leukopenia, Elkins and Guttmann hypothesized that the lesion had been stimulated not by antigen, but by the interaction between “passenger leukocytes,” incidentally transferred with the allograft and host (recipient) leukocytes. The same authors soon after provided the first evidence of a graft-versus host response in a nonlymphoid organ, verifying the importance of donor-to-recipient leukocyte transfer as one mechanism central to the immunogenicity of a transplanted allograft.5,6
In the early 1970s, Ralph Steinman and his colleagues identified a morphologically and functionally distinct, previously uncharacterized population of cells in the mouse spleen while investigating normal immune responses to transplanted tissue. Steinman called the cells “dendritic cells” (DCs) due to their low density and stellate structure.7,8,9 By 1980, he had demonstrated that DCs not only participated in immune responses, but that they were also uniquely potent immunologic stimulators, 100 times more so than macrophages.10 These cells, which led to the award of a Nobel Prize in 2011 to Dr. Steinman, have been and continue to be the subject of intense interest to immunologists and clinicians, especially in the fields of transplantation and oncology.
II. Passenger lymphocytes are the primary barrier to prolonged allograft acceptance—Brief historical perspective
In light of the work of Steinman and others, Lafferty et al immunologically altered donor tissue by pretransplant tissue culture or irradiation to deplete passenger leukocytes11,12 as a possible method of prolonging allograft survival. Their findings suggested that elimination of donor-derived, antigen-presenting “stimulator cells,” rather than the depletion of donor tissue antigen, which remained intact in treated cells, was responsible for producing tolerance by effectively preventing the timely signaling and mobilization of a pro-active T cell response.12,13 Similar experiments by Sollinger and his team using cultured and uncultured thyroid allografts found that even in presensitized mice, tissue cultured in both a high oxygen and high pressure environment to deplete passenger leukocytes had prolonged allograft survival by rendering the original graft alloantigen unrecognizable by the histoincompatible host.14
Lechler and Batchelor were the first to suggest that passenger leukocytes, the so-called immunologic “stimulator cells,” may in fact be Steinman’s DCs. They restored immunogenicity to a syngeneic re-transplanted, passenger cell-depleted donor allograft by re-introducing donor leukocytes, confirming that both mismatched tissue antigen and the presence of passenger lymphocytes (presumed DCs) were required to stimulate allograft rejection.15,12 Our group inactivated passenger cells using low-dose UVB irradiation with brief peritransplant immunosuppression to induce tolerance, initially to pancreatic islet allografts in a diabetic rat model16 and then to cardiac allografts. However, the presence of retained donor DCs, even at a density as low as 1%, was enough to stimulate a robust in vitro response; macrophages at 10 times the cell concentration fell short of producing this effect.17,18 UVB irradiation above a specified dose, which varied based on species, affected the viability of host progenitor cells and was thus too toxic to apply clinically for cellular transplants other than platelets.19
III. Dendritic cells’ functionality varies by subtype
The broad array of immune cells characterized as DCs can be subdivided into several categories, including monocyte (peripheral blood)-derived (moDC) and “classical,” myeloid (bone marrow)-derived (cDC), which either migrate from tissues or permanently reside in lymph nodes and are known as immunologic “sentinels,” the most effective activators of the naïve T cell response.20,21,22 In the late 1980s and early 1990s, several studies revealed that mature DCs (mDCs) accelerate the early sensitization phase of the immune response and play a central role in transporting antigen from nonlymphoid organs to lymphoid tissue. DCs were shown to be professional antigen-presenting cells (APCs) with the capacity to produce an antigen-specific immune response, resulting in the expansion of an antigen-specific effector T cell population in the host.23
Consistent with earlier findings, Inaba et al demonstrated that the presence of even a small number of antigen-primed mDCs resulted in robust, antigen-specific CD4+ T cell reactivity, suggesting that DCs’ efficacy depends on their ability to home to a site that permits them to bind and activate large numbers of T cells relative to alloantigen, rather than primarily on the number of primed, autologous DCs present.24 Above all, other studies repeatedly showed that standard, peripheral DCs acted as in vivo instigators and amplifiers of an antigen-specific T cell response, disruption of which could potentially lead to tolerance induction.24
Understanding the in vitro and in vivo generation of DC subsets, their unique mechanisms of antigen presentation, their migratory properties, the nature of their contact with native and donor T cells—and how to manipulate these interactions—became essential to the development of strategies to induce allograft tolerance.25
IV. The thymus is the site of immunologic education: Central deletion and regulatory T cell development are key mechanisms of tolerance induction by cell therapy
As early as 1959, Burnet proposed that immune cells fail to launch an anti-self reaction due to programmed deletion of autoreactive lymphocytes in the thymus.3 In the 1960s, Miller’s mouse studies of early neonatal thymectomy showed that unlike their healthy counterparts, neonatally thymectomized mice were deficient in germinal centers of lymphocyte proliferation and were both more susceptible to infection and developed prolonged tolerance to allogeneic skin grafts.26 Good and colleagues confirmed Miller’s findings of a quantitative deficiency of mature lymphoid structures in thymectomized mice, which resulted in loss of their splenic cells’ capacity to induce a graft-versus-host reaction.27 Transplantation of allogeneic thymic grafts into thymectomized mice resulted in development of chimerism, co-existence of both donor- and recipient-derived blood cells, such that the animal readily accepted skin grafts from both the donor and the host.28 More than twenty years later, the thymus was definitively identified as the site of T cell maturation and the anatomic location at which self-reactive T cells are commanded to self-destruct or are rendered anergic.29,30,31
In order to stimulate lymphocyte production in the absence of a normally functioning thymus, as in the case of the inherited human equivalent of neonatal thymectomy, complete Di George’s Syndrome (also known as 22q11.2 deletion), Goldstein and White pioneered efforts to isolate and purify a thymus-derived lymphocytopoietic factor called thymosin.32 We found it to enhance regeneration of lymphoid tissue in mice and to restore immunological competence to neonatally thymectomized rodents.33,34 Our group also showed that thymosin combined with anti-thymocyte serum (ATS) potentiated prolongation of skin allograft survival in mice compared to ATS alone, while large, daily doses of thymosin resulted in accelerated rejection. We speculated that the augmented effectiveness of ATS was due to its more effective depleting action on the increased number of peripheral thymocytes mobilized by thymosin.33,35
Years later, Markert performed postnatal allogeneic thymic transplant on infant patients with complete Di George anomaly (absence of the thymus) after trials of thymosin a1 proved to be only partially effective.36 After several months, recipients developed a diverse and functional T cell repertoire genetically matched to them. This outcome was thought to be due to migration of CD34+ recipient cells from the recipient marrow to the transplanted thymic tissue, a concept that supported the thymic role in T cell production and education and stimulated investigation into the development of tolerance following bone marrow transplant (BMT).36
V. Stage of maturation further determines DC function: Immaturity of DCs is essential to the development of tolerance
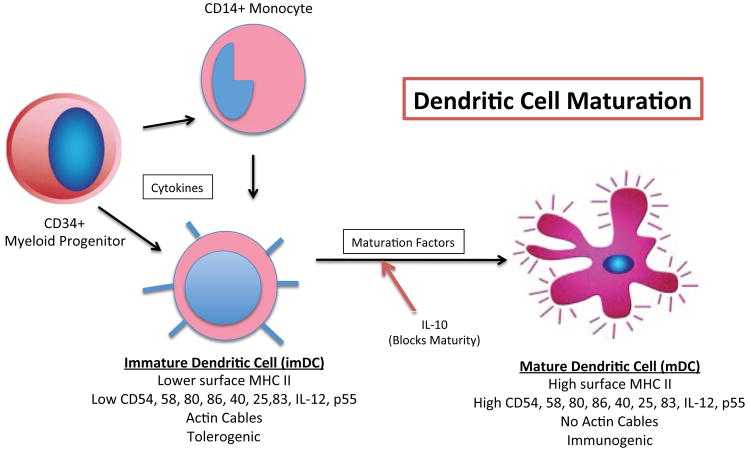
To more clearly define the immunologic role of various subsets of DCs and their relationships with the thymus, Kyewski et al, who had previously shown that DCs function as APCs within the thymic medulla37 established that intrathymic (IT) DCs could be distinguished from those in the periphery by their uniformly immature cell surface markers.38 The discovery of persistent, multilineage donor chimerism among allograft recipients decades after transplantation, coupled with the revelation that a leukocyte-rich donor liver is most likely to be accepted by a recipient, convinced Thomson et al that donor-derived progenitor cells migrated and survived in the host, facilitating immunologic nonreactivity to the allograft.39 These findings supported the critical role of immature dendritic cells (imDCs) in tolerance induction and posited on how a single cell type, depending on its maturational state, could be so effective at initiating two, seemingly opposite immune processes, allograft rejection and allograft tolerance (Figure 2).38
Figure 2.
Schema;c representa;on of myeloid- (bone marrow) and monocyte-derived dendri;c cell development, including a simplified comparison of surface markers and other characteris;cs present at different stages of matura;on. (imDC, immature dendri;c cell; mDC, mature dendri;c cell; MHC, major histocompa;bility complex; CD, cluster of differen;a;on; IL, interleukin
Thompson et al subsequently discovered that activation and differentiation of DCs, leading to T cell stimulation, depends on nuclear translocation of nRelB, a member of the NFkB family of transcription factors, whereas their immature DC precursors present in noninflamed tissue lack this protein.40 In this study, mDCs were found only in the presence of T lymphocytes, suggesting that cross talk between these subpopulations of cells, via high levels of accessory and co-stimulatory molecules, which are not present in imDC, is necessary to facilitate their migration and functionality.41,42,43
Segovia et al described another type of recipient-derived myeloid DCs (which they termed “autologous tolerogenic dendritic cells,” or ATDC), which possessed the cell membrane gene TMEM176B, directing them to home first to the transplanted allograft for donor antigen exposure to prolong skin allograft survival in mice when paired with short-term, anti-CD3 immunosuppression. When treated synergistically with anti-CD3+, ATDCs produce CD8+ regulatory T cells (Tregs), which prevent skin allograft rejection. Mice who lacked the TMEM176B gene rejected the allograft outright. The authors point out that human monocyte-derived DCs are known to lack the TMEM176B gene, suggesting that a different mechanism may be responsible for the development of tolerance using monocyte-derived DCs.44
The stark differences between IT and extrathymic encounters with antigen, where the latter reliably produces T cell responsiveness while the former seemingly curtails it, inspired Matzinger and Guerder to investigate the phenotype of DCs at various anatomical sites since they found out that the cells’ disparate functions were reflected in their different stages of maturation.45,29,46,47 While peripheral, splenic DCs were excellent initiators of mature T cell immune responses, those within the thymus were conversely the most effective inactivators of young, developing T cells.45 These findings led to the development of various methods of tolerance induction that rely on halting DC maturation long enough so that a critical number of imDC can be collected and manipulated. Conversion of cells originally destined to potentiate and amplify recognition of nonself to those with the ability to promote nonself antigen acceptance by misrepresentation of nonself antigens in the thymus (therefore “fooling Mother Nature”) may constitute effective tolerance induction clinically.
VI. Several pathways of allorecognition influence rejection and tolerance
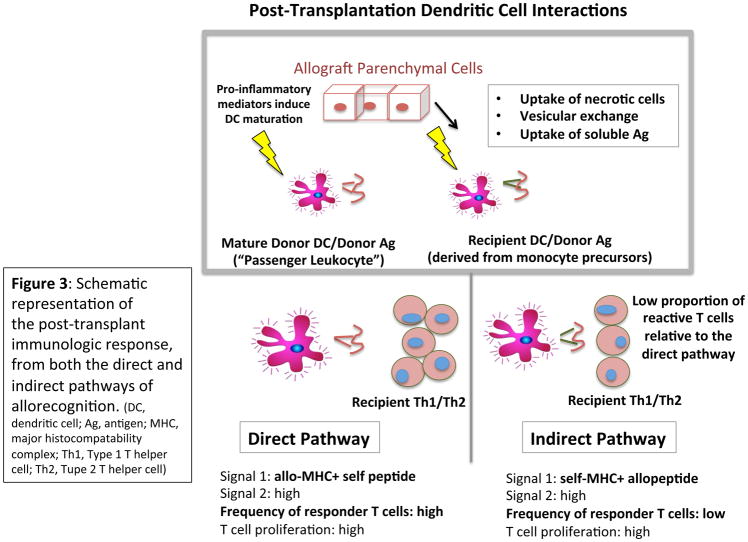
Direct and indirect allorecognition by host T cells have been the primary focus of efforts to manipulate cell interactions in favor of tolerance (Figure 3). Direct allorecognition, which is thought to dominate early immunologic responses following allografting, occurs when host T cells recognize intact, nonself major histocompatibility complex (MHC) molecules (disparate to that of the host) on the surface of foreign APCs. Indirect recognition occurs when host CD4+ T cells recognize nonself allopeptides (derived from allograft MHC proteins) processed and presented by self MHC II molecules on native APCs; this process is also crucial to alloantibody production, in which direct responsiveness plays a supporting role.48,49,50 Indirect allorecognition can also partially account for the initial alloresponse.51 According to one model, the kinetics of graft rejection by the direct and indirect pathways are nearly identical, suggesting that differences noted in other models could be due to alloreactive T cell precursor frequencies, not an intrinsic disparity between pathways.50
Figure 3.
Schema;c representa;on of the post-transplant immunologic response, from both the direct and indirect pathways of allorecogni;on. (DC, dendri;c cell; Ag, an;gen; MHC, major histocompatability complex; Th1, Type 1 T helper cell; Th2, Tupe 2 T helper cell)
As donor “passenger” APCs inevitably become less numerous over time, indirect recognition appears to “take over,” leading to chronic rejection. Indeed, years following heart transplantation, patients have been shown to be hyporesponsive to directly, but not indirectly presented donor HLA antigens.52,53,54,55 Recent studies also suggest the existence of a third, “semi-direct” pathway relying on the transfer of allo-MHC-peptide complexes (MHC “cross-dressing”) to recipient APCs via cell-cell interactions and/or excretion of extracellular vesicles, which may also contribute to persistent recipient T cell activation by perpetuating the direct pathway for the life span of the transplanted allograft.56,57,58
VII. Central intrathymic deletion and regulatory T cell development depend on intrathymic antigen presentation: Key mechanisms of tolerance induction by DCs
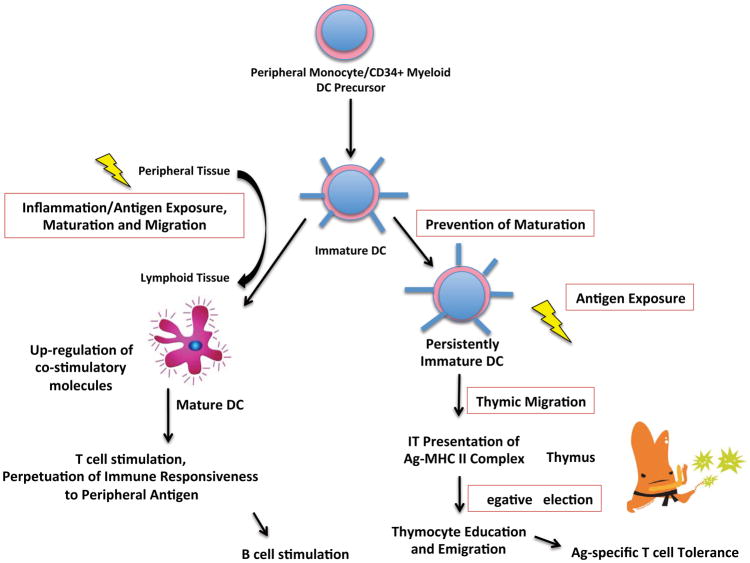
Based on mounting evidence regarding the central role of the thymus, we and others hypothesized that the mechanism of tolerance induction relies on IT antigen presentation (Figure 4).59,60,61 Several studies demonstrated that direct IT inoculation with donor immunodominant allopeptide or with donor allopeptide-pulsed, myeloid-derived imDCs led to long term, donor-specific tolerance in transiently immunosuppressed rats, which was disrupted by early, but not late, thymectomy.62 Intravenous (IV) injection of donor allopeptide-primed imDC with or without a host T cell-depleting agent, but not allopeptide alone, also led to prolonged allograft survival, strongly supporting the idea that manipulation of T cell education in the thymus is needed to initiate systemic allograft tolerance.63 This is further supported by the finding that intravenously administered, allopeptide-primed imDC (but not mDC) tagged with indium-111 radiotracer home primarily to the thymus on single-photon emission computed tomography (SPECT) imaging studies.64,65 It has been shown that systemic, intravenous treatment with allopeptide alone, without immunosuppression, results in tolerance induction to cardiac allografts in rats via indirect interactions between CD8+ CD40Ig Tregs and dominant donor MHC class II molecules. These interactions lead to CD8+ Treg expansion in the presence of plasmacytoid DCs (pDCs), thus augmenting their immunosuppressive effect. The possible role of the thymus in this interaction is not discussed.66
Figure 4.
Flow chart comparing the basic func;ons of mature versus immature dendri;c cells and their contribu;ons to immunologic s;mula;on versus tolerance. (DC, dendri;c cell)
Lee et al investigated the role of indirect recognition on chronic rejection of swine leukocyte antigen (SLA) class I-incompatible cardiac allografts in miniature swine and verified the significance of pretransplant intrathymic versus extrathymic donor allopeptide exposure. Consistent with our experience, recipient swine primed intravenously with donor-specific, immunodominant allopeptide alone rapidly developed the cardiac lesion emblematic of chronic rejection, unlike those that underwent direct IT injection of bare allopeptide or primed imDC.67
Tran et al studied the effectiveness of IT donor antigen inoculation paired with short-term anti-lymphocyte serum (ALS) with regards to producing sustained tolerance to rat islet xenografts. Unlike control mice, which received identical treatment and an allograft, the experimental xenograft recipients demonstrated no improvement in survival compared to treatment with ALS alone, despite a maximized donor inoculum and an increased interval between inoculation and transplantation. This study highlights the inherent differences in the immunologic response to allograft versus xenograft in transplantation.68
Odorico et al examined factors that prevent the tolerogenic effect of IT donor antigen on allograft prolongation. Failure to inoculate all the thymic tissue present (including both lobes or any aberrant thymic tissue) resulted in allograft rejection. They concluded that thymic tissue free of donor allopeptide is capable of nurturing alloreactive cells to maturity and thus, could directly sabotage the development of tolerance despite the presence of donor allopeptide in other—even adjacent—thymic tissue where deletion and/or inactivation of alloreactive cells is taking place. This is particularly important in many species, including humans, in which thymic tissue is anatomically less well defined than in other animals.69
VIII. Timing of thymic manipulation and the need for peritransplant immunosuppression
The timing of DC inoculation and immunosuppression in relation to transplantation proved critical in the development of host unresponsiveness. We found that simultaneous allopeptide or donor allopeptide-primed imDC inoculation and islet or cardiac transplantation in rats using only peritransplant ATG resulted in acute allograft rejection, while inoculation at 7 days prior to allografting led to indefinite histoincompatible islet (>200d) or cardiac graft (>150d) acceptance and rejection of third party allografts without any posttransplant immunosuppression. Replacement of imDC with peptide-pulsed, mDC resulted in accelerated allograft rejection, as did IV injection of immunodominant allopeptide alone.70,71
These studies suggest that the timing of IT donor allopeptide or of primed imDC exposure was critical to clinical outcomes. The “learning curve” in the host following IT or IV administration of allopeptide-primed, recipient-derived, imDCs, during which they either directly modulate T cell reactivity or effectively “teach” host thymocytes to delete reactive T cell clones and positively select migratory, donor-specific suppressor T cells, influences the nature of peripheral lymphoid responses to donor tissue over the course of several days. This process does not occur rapidly enough to protect simultaneously transplanted allograft and thus, indirect, IT presentation is the likely mechanism by which tolerance is induced (Table 1a &1b).70
Table 1a.
Studies of Intrathymic (IT) Injection (1965–1995)
| STUDY | INJECTION | IS | ALLOGRAFT | OUTCOME | CONTROL |
|---|---|---|---|---|---|
| Vojtiskova & Lengerora 1965 & 1968 | IT allogeneic spleen cells into syngeneic thymus in mice | Sublethal irradiation | Skin | Prolonged survival | Without irradiation & Allogeneic thymus |
| Posselt & Naji 1990 | IT allogeneic islets & subsequent 2nd set donor specific islets in diabetic rats | ALS 1 dose | Islets | Tolerance | Renal subcapular Cultured islets 3rd party islets |
| Remuzzi & Perico 1991 | IT renal glomeruli in rats | Cyclosporine X2 & steroids | Kidney | Tolerance | IT medium |
| Oluwole & Hardy 1992 | IT UVB donor spleen cells or bone marrow in rats | ALS 1 dose | Cardiac Islets |
Tolerance Acceptance of 2nd set allografts |
Rejection of 3rd party allografts |
| Ohzato & Monaco 1992 | IT spleen cells in mice –day +7 | ALS day −1 & +2 | Skin | Prolonged survival –Tolerance in majority -2nd set survival |
Rejection of 3rd party skin |
| Oluwole & Hardy 1993 | IT class I MHC immunodominant Allopeptide day −7 |
ALS day −7 & 0 | Cardiac | Tolerance | IV injections – NO Thymectomy –NO 3rd Party -reject |
| Thomson & Starzl 1995 | imDC IV day −7 in mice | None | Cardiac Islets |
Allograft Prolongation | IV Medium IV Mature DCs 3rd party allograft |
Table 1b.
Studies of Intrathymic (IT) Injection (2000–2017)
| STUDY | INJECTION | IS | ALLOGRAFT | OUTCOME | CONTOL |
|---|---|---|---|---|---|
| Garrovillo & Oluwole 1999 Ali & Hardy 2000 |
IT & IV host immature DCs primed with donor immunodominant MHC I Allopeptide | ALS day −7 & 0 | Cardiac in rats Islets in diabetic rats |
Tolerance Acceptance of 2nd set allografts |
Rejection of 3rd party allografts abrogated by thymectomy |
| Yamada & Sachs 2003 | Composite thymo/kidneys in mini swine | Tacrolimus 28 days | Kidney with thymus under the capsule | Prolonged survival | Kidney alone or with Thymocyte injection |
| Nabori & Yamada 2006 | Donor Heart and donor vascularized thymus (VTA) in mini swine | Tacrolimus 28 days | Heart Transplant | Prolonged survival | Heart alone Thymocyte infusion |
| Ezzelarab & Thomson 2013 | Reg DCs (donor) infusion day −7 in NHP (Rhesus) | CTLA4Ig, Rapamycin taper | Kidney allograft | Prolonged survival | No reg DCs |
| Leventhal & Ildstad 2015 | Facilitating cell (FC) (donor precursor cells- plasmacytoid dendritic cells) infusion, Human Recipient (Mismatch) | Day-4 to 0 Fludarabine, Cytoxan; SOC Tacrolimus and Myfortic |
Mismatched living donor kidney | Prolonged survival in 18 patients off immuno-suppression with biopsies negative for rejection | In vitro studies strongly suggestive of donor-specific tolerance |
| Ezzelarab & Thomson 2017 | Reg DCs (recipient) pulsed with donor antigen day −7 in NHP (Rhesus) | CTLA4Ig Rapamycin taper | Kidney allograft | Prolonged survival (mild and not significant) | No reg DCs |
The T cell-depleting agent most commonly used by transplantation investigators is ATG. ATG is needed to deplete the periphery of most T cells before the periphery is populated by new, tolerogenic T cells generated in the thymus. It is best used just after exposure of the thymus to the foreign allopeptide. Once the emigration of the newly “educated” T cells that recognize foreign as self occurs, at the time of allografting, immunosuppression may also be used to shift the balance in favor of donor-specific regulatory cells as compared to anti-donor effector cells that may persist.
IX. Potential clinical utility and challenges of delivery of immature DCs to the thymus
The major contributions of Steinman, Thompson and others, including our group, stimulated further investigations of the therapeutic potential for tolerance induction of imDC and of the accessory cell interactions that explain DCs’ tolerogenic effect.72 Aware of the tendency of the aged human thymus to involute and therefore, to theoretically limit the applicability of tolerance induction in adult subjects by indirect IT exposure of alloantigens via the IV route, several groups have examined tolerance induction in thymectomized animals. Their models rely on passive co-transfer of donor immunodominant allopeptide-primed, syngeneic T cells and naïve, unmodified thymocytes. When combined with a single dose of ALS, this approach produced permanent graft acceptance in 70% of rodent subjects. Tested with simultaneous transplantation or a renal subcapsular thymic graft, rather than free thymocytes, the acceptance rate was 100%.73,74,63
X. Critical role of donor-specific T cell development, migration and usefulness of passive transfer
Since cultured imDC express MHC II but not many other co-stimulatory molecules seen on the surface of mDCs, imDCs, in contrast to mDCs, fail to stimulate a robust immune response in mixed lymphocyte reaction (MLR) assay in vitro. Likewise, T cells harvested after transplantation from tolerant animals are hyporesponsive to donor antigen and after secondary transfer to untreated syngeneic recipients lead to tolerance despite consistent rejection of a third party allograft. These data suggest that imDCs not only induce, but also maintain tolerance by promoting expansion of a specialized, donor-specific, regulatory T cell population.75,76
Given the evidence that IV or direct IT immunization with donor-specific, immunodominant allopeptide-primed imDCs induces tolerance that is disrupted by thymectomy,73,74,63 we studied thymic emigration of Tregs produced as a result of intrathymic exposure to antigen. Such Tregs appear not just to induce but also to maintain tolerance in this model. When combined with ALS-induced depletion of mature, directly alloreactive T cells in the periphery, such cells, which contain responses from reactive T cells, can effectively prevent an early “attack” on the allograft, and thus maintain lasting tolerance.77
Passive transfer of various subsets of specific T cells obtained from tolerant recipients into a syngeneic, naïve, recipient prior to cardiac transplantation induced reliable allograft tolerance, depending on the type and dose of the infused T cells, with particular emphasis on the CD4+ ratio.77 Syngeneic CD4+CD25+ Treg cells completely suppressed the normal, inflammatory immune response of specific peptide-primed T cells whereas CD4+CD25− cells failed to halt in vitro proliferation in response to the same peptide in MLR assays. In vivo, co-transfer of “CD4+CD25+ but not CD4+CD25− thymic T cells simultaneously with immunodominant allopeptide-primed syngeneic T cells restored tolerance to thymectomized recipients.”77 When these tolerized rats received a second, syngeneic cardiac transplant, allograft acceptance remained uninterrupted while they were able to acutely reject third party cardiac transplants. This type of allograft tolerance induction has a very high specificity, as it relies on indirect allorecognition.77 Though the molecular mechanisms accounting for tolerance induction in this model remain incompletely understood, donor–specific CD4 and CD8 Tregs are strongly implicated as playing a critical role.78
Once the importance of regulatory CD4+ cells was established, the role and importance of CD8+CD28− suppressor T cells, which are known to share the transcription factor FOXP3 with CD4+ regulatory cells but are antigen- and MHC I-specific, needed to be clarified. In another rat cardiac allograft tolerance model using transfusions of UVB-irradiated donor blood rich in modified DCs, we found that the CD8+ cells of tolerant recipients express FOXP3. When transferred to a naïve, secondary sygeneic host, those same cells induced tolerance only to tissue of the identical donor haplotype.78
In 1994, Ildstad and colleagues discovered a novel, bone marrow-derived cell population capable of facilitating reliable engraftment of purified stem cells without causing Graft-versus-Host Disease (GVHD). These “facilitator cells” (FCs), as they were called, raised the possibility that BMT could be applied to treatment of nonmalignant diseases and perhaps be useful in transplantation.79 These cells have been successfully used clinically in a passive fashion by Leventhal et al in renal transplantation to induce tolerance in a small number of patients.80,81 Interestingly, the primary sub-population of FCs found to be necessary for stem cell engraftment and tolerance induction has been recently identified as being composed of plasmacytoid precursor DCs.82,83
Gregori et al investigated the proliferation of regulatory T cells and identified and characterized a novel subset of DCs, known as DC-10, which produce the immune-modulating cytokine interleukin-10 (IL-10). They further outlined the pathway through which these unique cells induce differentiation of the Type 1 regulatory T cells (Tr1) responsible for suppressing immune responses.84 These and other studies using indirect antigen presentation to induce tolerance focused on the importance of the maturational state of DCs, their interactions with other immune cells and their ability to stimulate the development of specialized T and B suppressor populations to perpetuate long-term transplantation tolerance.
XI. Safety and reliability of dendritic cell therapies in large animal studies
Despite much success in rodent transplantation models, the application of DC to clinical therapies has remained slow. In 1994, Granger et al used donor DC infusions combined with simultaneous Rapamycin immunosuppression in SLA class I-mismatched swine to induce allograft prolongation.85 To test the role of the thymus in establishing and maintaining allograft tolerance, Yamada et al transplanted a donor thymo-kidney (recipient thymus tissue vascularized under the donor kidney capsule) weeks after recipient thymectomy of histoincompatible miniature swine. The presence of allogeneic DCs facilitated the education of recipient T cells recirculating to the donated thymus, leading to prolonged renal acceptance and stable organ function.86
Weiss performed combined cardiac and renal transplants in SLA class I-mismatched swine immunized with donor-derived “bare” MHC peptides 21 days prior to or 100 days after transplantation, where controls received 3rd party MHC peptides and all groups received the same peri-transplant immunosuppression. All recipients pretreated with donor-derived peptide acutely rejected the allograft while all other peptide-treated animals had the same outcomes as the untreated controls. In swine, as in rodents, T cells stimulated via indirect recognition with “bare” donor antigen act as a barrier to induction of long-term tolerance but cannot break maintenance of already-established tolerance.87
As recently as 2013, Ezzelarab et al tested the influence of imDC-induced T regulatory cells on nonhuman primate (NHP) organ transplant survival. Extrapolating from previous studies, they immunosuppressed the animals with a combination of costimulation-blocking agents and were able to propagate a population of donor monocyte-derived, stably hyporesponsibe imDCs low in MHC class II and costimulatory molecule expression and resistant to cytokine-induced maturation. The primates received infusions of these cells 7 days prior to allograft transplantation, with either a short- or long-term costimulation blockade and a 6-month Rapamycin taper. Compared to control animals, which received no DC infusion, cells from experimental primates demonstrated higher levels of markers of immaturity, lower levels of proteins facilitating homing to lymphoid tissue, maturation resistance, weaker stimulation of CD4+ and CD8+ T cells and fewer CD8+CD95+ memory cells, both in vitro and in vivo. This study was the first to demonstrate the effectiveness of imDC infusion in prolonging renal allograft survival.88 The authors concluded, despite practical limitations, that infusion with unprimed, donor-derived imDC was of superior therapeutic value compared to the use of donor antigen-primed, recipient imDC.89 Given the challenge of obtaining immature, donor, myeloid-derived DCs prior to transplantation in the clinical setting and their proven inefficiency in comparison to recipient-derived cells,90 the successful use of donor allopeptide-primed, recipient imDC in large animals has become of critical importance, since they have the potential to be clinically applicable in both living and deceased donor transplantation and are relatively safe for the patient. Peche et al also tried injecting unprimed recipient imDC on the day of transplantation, thus precluding the danger of recipient immunization against donor antigen.90 Though they did achieve some success at allograft prolongation in rats with this method, the results fell short of inducing specific tolerance with indefinite survival or acceptance of second set allografts.90
XII. Discussion
As data regarding these DCs and their unique potency has accumulated, so, too, have questions regarding the conditions under which they hold the most promise in organ transplantation. Debates persist about the usefulness of donor- versus recipient-derived DCs, whether to rely on those of marrow or monocyte origin, the ideal stage of maturation for their various functions, the need for priming with a single immunodominant allopeptide versus several donor allopeptides, the timing of donor immunopeptide-primed imDC infusion, the protocol for administration and withdrawal of simultaneous and/or repeated dose(s) of peritransplant immunosuppression, and whether these should be lymphocyte depleting agents, co-stimulatory blockers, mTOR inhibitors, calcineurin inhibitors or perhaps multiple medications. Solutions are needed to identify the most effective, yet least toxic method for lymphocyte depletion, evaluation of age/thymic regression on outcomes and whether thymic hormones or donated thymus could contribute to thymic regeneration.
Despite reported success of tolerance induction with monocyte-derived donor DCs, the comparative benefits and similar safety profile of DC harvested from the recipient periphery, rather than from the donor or the bone marrow, is the next frontier for a clinically relevant large animal model that takes advantage of cell mobilization and leukapheresis techniques already well-developed in humans. The unpredictability of deceased donor transplantation makes adequate harvesting and preparation of donor-derived DC difficult, if not impossible. Recipient-derived imDCs would be readily accepted and give recipients of deceased donor allografts a similar opportunity for long-term, immunosuppression-free tolerance as those who receive allografts from living donors. Preparation of recipient imDCs broadly primed with the most common donor Class I and II MHC allopeptides in a given population (for example, see reference)91 within a few weeks of a projected transplant could allow a large number of individuals to benefit from this technique of tolerance induction.
The age of the thymus may be critical in tolerance induction. In patients who lack a critical volume of healthy thymic tissue, the development of tolerance may be very difficult, unless the thymus can be rejuvenated, perhaps with hormones or cellular replacement. The effect of the status of the reticuloendothelial scaffold that forms the thymic wall and the lower concentrations of thymic hormones secreted by the aging thymus on the education of naïve thymic T cells by the imDCs is unknown, as is the threshold for the critical mass of thymic tissue or the thymic elements that serve as a “schoolhouse” for delivered allopeptides. Studies are in progress on the use of co-transplantation of vascularized donor thymus or donor thymocytes as well as the possible use of thymic hormones for host thymus “reconstitution” in aging patients.
DCs have been repeatedly shown to be unique and potent participants in the immune response, both in rejection and in tolerance induction. Why, then, are we not yet uniformly and effectively using cell therapy in the field of vascularized organ transplantation? An ongoing, early clinical trial of cell therapy, the ONE Study in Europe and in the United States focuses on passive infusion of various hematopoietic, regulatory cell types, including dendritic cells, regulatory T cells and macrophages to “strengthen regulatory immune responses against donor alloantigens.”92 The hypothesis proposes that this will facilitate tolerance and lower or eliminate the need for commonly-prescribed immunosuppressive medications associated with toxicity, malignancy and infection.93 This approach does not emphasize donor specificity or address the transient effect of passive infusions. There is still somewhat of a paucity of consistent experimental data in large animals using tolerogenic DCs in transplantation and thus, human clinical trials in this area have lagged. The ONE Study currently underway in Europe is the first to conduct a phase I clinical trial examining the effect of recipient-derived DCs on renal transplant patients.93 Our failure to precisely identify tolerance in patients, allowing us to safely and confidently attempt discontinuation of immunosuppression, which itself may interfere with tolerance induction and/or maintenance, is another important obstacle.
Although large animal models such as swine and NHP are helpful prototypes for assessing the possible use of protocols in humans, better defined and goal-oriented large animal studies to address the critical questions raised in this review are still needed to more comprehensively evaluate the immunologic potential of DCs, their safety and efficacy. DCs will be deemed appropriate for testing in the context of transplantation in humans when reproducible conditions are established to induce long-term, donor-specific tolerance with minimal adverse effects.
Acknowledgments
Funding for this work was provided by the National Institutes for Health 2T32HL007854-21.
Abbreviations (in alphabetical order)
- ALS
anti-lymphocyte serum
- APC
antigen presenting cell
- ATS
anti-thymocyte serum
- DC(s)
dendritic cell(s)
- FC(s)
Facilitator cell(s)
- imDC
immature dendritic cell
- IT
intrathymic
- mDC
mature dendritic cell
- MHC
major histocompatibility complex
- MLR
mixed lymphocyte reaction
- NHP
nonhuman primate
- SLA
swine leukocyte antigen
- Treg
regulatory T cell
Footnotes
The authors declare no conflicts of interest.
Sarah J. Rosen participated in the writing of the paper.
Paul E. Harris participated in the writing of the paper.
Mark A. Hardy participated in the writing of the paper.
References
- 1.Medawar PB. The behaviour and fate of skin autografts and skin homografts in rabbits: A report to the War Wounds Committee of the Medical Research Council. J Anat. 1944;78(Pt 5):176–199. [PMC free article] [PubMed] [Google Scholar]
- 2.Owen RD. IMMUNOGENETIC CONSEQUENCES OF VASCULAR ANASTOMOSES BETWEEN BOVINE TWINS. Science. 1945;102(2651):400–401. doi: 10.1126/science.102.2651.400. [DOI] [PubMed] [Google Scholar]
- 3.Burnet FM. The Clonal Selection of Acquired Immunity. New York: Cambridge University; 1959. [Google Scholar]
- 4.Snell GD. The genetics of transplantation. Ann N Y Acad Sci. 1957;69(4):555–560. doi: 10.1111/j.1749-6632.1957.tb49695.x. [DOI] [PubMed] [Google Scholar]
- 5.Elkins WL, Guttmann RD. Pathogenesis of a local graft versus host reaction: immunogenicity of circulating host leukocytes. Science. 1968;159(3820):1250–1251. doi: 10.1126/science.159.3820.1250. [DOI] [PubMed] [Google Scholar]
- 6.Billingham RE. The passenger cell concept in transplantation immunology. Cell Immunol. 1971;2(1):1–12. doi: 10.1016/0008-8749(71)90022-0. [DOI] [PubMed] [Google Scholar]
- 7.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137(5):1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. II. Functional properties in vitro. J Exp Med. 1974;139(2):380–397. doi: 10.1084/jem.139.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinman RM, Lustig DS, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. 3. Functional properties in vivo. J Exp Med. 1974;139(6):1431–1445. doi: 10.1084/jem.139.6.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nussenzweig MC, Steinman RM. Contribution of dendritic cells to stimulation of the murine syngeneic mixed leukocyte reaction. J Exp Med. 1980;151(5):1196–1212. doi: 10.1084/jem.151.5.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lafferty KJ, Bootes A, Dart G, Talmage DW. Effect of organ culture on the survival of thyroid allografts in mice. Transplantation. 1976;22(2):138–149. doi: 10.1097/00007890-197608000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Lafferty KJ, Prowse SJ, Simeonovic CJ, Warren HS. Immunobiology of tissue transplantation: a return to the passenger leukocyte concept. Annu Rev Immunol. 1983;1:143–173. doi: 10.1146/annurev.iy.01.040183.001043. [DOI] [PubMed] [Google Scholar]
- 13.Talmage DW, Dart G, Radovich J, Lafferty KJ. Activation of transplant immunity: effect of donor leukocytes on thyroid allograft rejection. Science. 1976;191(4225):385–388. doi: 10.1126/science.1082167. [DOI] [PubMed] [Google Scholar]
- 14.Hullett DA, Landry AS, Leonard DK, Sollinger HW. Enhancement of thyroid allograft survival following organ culture. Alteration of tissue immunogenicity. Transplantation. 1989;47(1):24–27. doi: 10.1097/00007890-198901000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Lechler RI, Batchelor JR. Restoration of immunogenicity to passenger cell-depleted kidney allografts by the addition of donor strain dendritic cells. J Exp Med. 1982;155(1):31–41. doi: 10.1084/jem.155.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lau H, Reemtsma K, Hardy MA. Pancreatic islet allograft prolongation by donor-specific blood transfusions treated with ultraviolet irradiation. Science. 1983;221(4612):754–756. doi: 10.1126/science.6410509. [DOI] [PubMed] [Google Scholar]
- 17.Lindahl-Kiessling K, Säfwenberg J. Inability of UV-irradiated lymphocytes to stimulate allogeneic cells in mixed lymphocyte culture. Int Arch Allergy Appl Immunol. 1971;41(5):670–678. doi: 10.1159/000230559. [DOI] [PubMed] [Google Scholar]
- 18.Lau H, Reemtsma K, Hardy MA. The use of direct ultraviolet irradiation and cyclosporine in facilitating indefinite pancreatic islet allograft acceptance. Transplantation. 1984;38(6):566–569. doi: 10.1097/00007890-198412000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Noizat-Pirenne F, Greenfeld JI, Hardy MA, Oluwole SF, De Groote D, Franchimont P. UVB-irradiation of human bone marrow: potential for donor specific tolerance. J Surg Res. 1996;61(1):267–274. doi: 10.1006/jsre.1996.0115. [DOI] [PubMed] [Google Scholar]
- 20.Segura E, Soumelis V. Of Human DC Migrants and Residents. Immunity. 2017;46(3):342–344. doi: 10.1016/j.immuni.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Belz GT, Nutt SL. Transcriptional programming of the dendritic cell network. Nat Rev Immunol. 2012;12(2):101–113. doi: 10.1038/nri3149. [DOI] [PubMed] [Google Scholar]
- 22.Granot T, Senda T, Carpenter DJ, et al. Dendritic Cells Display Subset and Tissue-Specific Maturation Dynamics over Human Life. Immunity. 2017;46(3):504–515. doi: 10.1016/j.immuni.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sertl K, Takemura T, Tschachler E, Ferrans VJ, Kaliner MA, Shevach EM. Dendritic cells with antigen-presenting capability reside in airway epithelium, lung parenchyma, and visceral pleura. J Exp Med. 1986;163(2):436–451. doi: 10.1084/jem.163.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inaba K, Metlay JP, Crowley MT, Steinman RM. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J Exp Med. 1990;172(2):631–640. doi: 10.1084/jem.172.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 26.Miller JF. Immunological function of the thymus. Lancet Lond Engl. 1961;2(7205):748–749. doi: 10.1016/s0140-6736(61)90693-6. [DOI] [PubMed] [Google Scholar]
- 27.Good RA, Dalmasso AP, Martinez C, Archer OK, Pierce JC, Papermaster BW. The role of the thymus in development of immunologic capacity in rabbits and mice. J Exp Med. 1962;116:773–796. doi: 10.1084/jem.116.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalmasso AP, Martinez C, Sjodin K, Good RA. STUDIES ON THE ROLE OF THE THYMUS IN IMMUNOBIOLOGY; RECONSTITUTION OF IMMUNOLOGIC CAPACITY IN MICE THYMECTOMIZED AT BIRTH. J Exp Med. 1963;118:1089–1109. doi: 10.1084/jem.118.6.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kappler JW, Staerz U, White J, Marrack PC. Self-tolerance eliminates T cells specific for Mls-modified products of the major histocompatibility complex. Nature. 1988;332(6159):35–40. doi: 10.1038/332035a0. [DOI] [PubMed] [Google Scholar]
- 30.MacDonald HR, Lees RK, Schneider R, Zinkernagel RM, Hengartner H. Positive selection of CD4+ thymocytes controlled by MHC class II gene products. Nature. 1988;336(6198):471–473. doi: 10.1038/336471a0. [DOI] [PubMed] [Google Scholar]
- 31.Kisielow P, Teh HS, Blüthmann H, von Boehmer H. Positive selection of antigen-specific T cells in thymus by restricting MHC molecules. Nature. 1988;335(6192):730–733. doi: 10.1038/335730a0. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein AL, Slater FD, White A. Preparation, assay, and partial purification of a thymic lymphocytopoietic factor (thymosin) Proc Natl Acad Sci U S A. 1966;56(3):1010–1017. doi: 10.1073/pnas.56.3.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hardy MA, Quint J, Goldstein AL, State D, White A. Effect of thymosin and an antithymosin serum on allograft survival in mice. Proc Natl Acad Sci U S A. 1968;61(3):875–882. doi: 10.1073/pnas.61.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldstein AL, Asanuma Y, Battisto JR, Hardy MA, Quint J, White A. Influence of thymosin on cell-mediated and humoral immune responses in normal and in immunologically deficient mice. J Immunol Baltim Md 1950. 1970;104(2):359–366. [PubMed] [Google Scholar]
- 35.Wara DW, Goldstein AL, Doyle NE, Ammann AJ. Thymosin activity in patients with cellular immunodeficiency. N Engl J Med. 1975;292(2):70–74. doi: 10.1056/NEJM197501092920204. [DOI] [PubMed] [Google Scholar]
- 36.Markert ML, Devlin BH, Chinn IK, McCarthy EA. Thymus transplantation in complete DiGeorge anomaly. Immunol Res. 2009;44(1–3):61–70. doi: 10.1007/s12026-008-8082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kyewski BA, Fathman CG, Kaplan HS. Intrathymic presentation of circulating non-major histocompatibility complex antigens. Nature. 1984;308(5955):196–199. doi: 10.1038/308196a0. [DOI] [PubMed] [Google Scholar]
- 38.Kyewski BA, Fathman CG, Rouse RV. Intrathymic presentation of circulating non-MHC antigens by medullary dendritic cells. An antigen-dependent microenvironment for T cell differentiation. J Exp Med. 1986;163(2):231–246. doi: 10.1084/jem.163.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomson AW, Lu L, Murase N, Demetris AJ, Rao AS, Starzl TE. Microchimerism, dendritic cell progenitors and transplantation tolerance. Stem Cells Dayt Ohio. 1995;13(6):622–639. doi: 10.1002/stem.5530130607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson AG, Pettit AR, Padmanabha J, et al. Nuclear RelB+ cells are found in normal lymphoid organs and in peripheral tissue in the context of inflammation, but not under normal resting conditions. Immunol Cell Biol. 2002;80(2):164–169. doi: 10.1046/j.1440-1711.2002.01070.x. [DOI] [PubMed] [Google Scholar]
- 41.Thompson AG, O’Sullivan BJ, Beamish H, Thomas R. T cells signaled by NF-kappa B- dendritic cells are sensitized not anergic to subsequent activation. J Immunol Baltim Md 1950. 2004;173(3):1671–1680. doi: 10.4049/jimmunol.173.3.1671. [DOI] [PubMed] [Google Scholar]
- 42.Young JW, Koulova L, Soergel SA, Clark EA, Steinman RM, Dupont B. The B7/BB1 antigen provides one of several costimulatory signals for the activation of CD4+ T lymphocytes by human blood dendritic cells in vitro. J Clin Invest. 1992;90(1):229–237. doi: 10.1172/JCI115840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu L, McCaslin D, Starzl TE, Thomson AW. Bone marrow-derived dendritic cell progenitors (NLDC 145+, MHC class II+, B7-1dim, B7-2-) induce alloantigen-specific hyporesponsiveness in murine T lymphocytes. Transplantation. 1995;60(12):1539–1545. doi: 10.1097/00007890-199560120-00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Segovia M, Louvet C, Charnet P, et al. Autologous dendritic cells prolong allograft survival through Tmem176b-dependent antigen cross-presentation. Am J Transplant. 2014;14(5):1021–1031. doi: 10.1111/ajt.12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matzinger P, Guerder S. Does T-cell tolerance require a dedicated antigen-presenting cell? Nature. 1989;338(6210):74–76. doi: 10.1038/338074a0. [DOI] [PubMed] [Google Scholar]
- 46.MacDonald HR, Schneider R, Lees RK, et al. T-cell receptor V beta use predicts reactivity and tolerance to Mlsa-encoded antigens. Nature. 1988;332(6159):40–45. doi: 10.1038/332040a0. [DOI] [PubMed] [Google Scholar]
- 47.Kisielow P, Blüthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333(6175):742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 48.Sayegh MH, Turka LA. The role of T-cell costimulatory activation pathways in transplant rejection. N Engl J Med. 1998;338(25):1813–1821. doi: 10.1056/NEJM199806183382506. [DOI] [PubMed] [Google Scholar]
- 49.Steele DJ, Laufer TM, Smiley ST, et al. Two levels of help for B cell alloantibody production. J Exp Med. 1996;183(2):699–703. doi: 10.1084/jem.183.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sauvé D, Baratin M, Leduc C, Bonin K, Daniel C. Alloantibody production is regulated by CD4+ T cells’ alloreactive pathway, rather than precursor frequency or Th1/Th2 differentiation. Am J Transplant. 2004;4(8):1237–1245. doi: 10.1111/j.1600-6143.2004.00520.x. [DOI] [PubMed] [Google Scholar]
- 51.Benichou G, Thomson AW. Direct versus indirect allorecognition pathways: on the right track. Am J Transplant. 2009;9(4):655–656. doi: 10.1111/j.1600-6143.2009.02572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Z, Sun YK, Xi YP, et al. Contribution of direct and indirect recognition pathways to T cell alloreactivity. J Exp Med. 1993;177(6):1643–1650. doi: 10.1084/jem.177.6.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ciubotariu R, Liu Z, Colovai AI, et al. Persistent allopeptide reactivity and epitope spreading in chronic rejection of organ allografts. J Clin Invest. 1998;101(2):398–405. doi: 10.1172/JCI1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hornick PI, Mason PD, Baker RJ, et al. Significant frequencies of T cells with indirect anti-donor specificity in heart graft recipients with chronic rejection. Circulation. 2000;101(20):2405–2410. doi: 10.1161/01.cir.101.20.2405. [DOI] [PubMed] [Google Scholar]
- 55.Vella JP, Spadafora-Ferreira M, Murphy B, et al. Indirect allorecognition of major histocompatibility complex allopeptides in human renal transplant recipients with chronic graft dysfunction. Transplantation. 1997;64(6):795–800. doi: 10.1097/00007890-199709270-00001. [DOI] [PubMed] [Google Scholar]
- 56.Smyth LA, Lechler RI, Lombardi G. Continuous Acquisition of MHC:Peptide Complexes by Recipient Cells Contributes to the Generation of Anti-Graft CD8(+) T Cell Immunity. Am J Transplant. 2017;17(1):60–68. doi: 10.1111/ajt.13996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marino J, Paster J, Benichou G. Allorecognition by T Lymphocytes and Allograft Rejection. Front Immunol. 2016;7:582. doi: 10.3389/fimmu.2016.00582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Breman E, van Miert PP, van der Steen DM, et al. HLA monomers as a tool to monitor indirect allorecognition. Transplantation. 2014;97(11):1119–1127. doi: 10.1097/TP.0000000000000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oluwole SF, Chowdhury NC, Jin MX. The relative contribution of intrathymic inoculation of donor leukocyte subpopulations in the induction of specific tolerance. Cell Immunol. 1994;153(1):163–170. doi: 10.1006/cimm.1994.1014. [DOI] [PubMed] [Google Scholar]
- 60.Fiedor P, Jin MX, Hardy MA, Oluwole SF. Dependence of acquired systemic tolerance to rat islet allografts induced by intrathymic soluble alloantigens on host responsiveness, MHC differences, and transient immunosuppression in the high responder recipient. Transplantation. 1997;63(2):279–283. doi: 10.1097/00007890-199701270-00018. [DOI] [PubMed] [Google Scholar]
- 61.Ali A, Garrovillo M, Jin MX, Hardy MA, Oluwole SF. Major histocompatibility complex class I peptide-pulsed host dendritic cells induce antigen-specific acquired thymic tolerance to islet cells. Transplantation. 2000;69(2):221–226. doi: 10.1097/00007890-200001270-00005. [DOI] [PubMed] [Google Scholar]
- 62.Oluwole SF, Chowdhury NC, Jin MX, Hardy MA. Induction of transplantation tolerance to rat cardiac allografts by intrathymic inoculation of allogeneic soluble peptides. Transplantation. 1993;56(6):1523–1527. doi: 10.1097/00007890-199312000-00046. [DOI] [PubMed] [Google Scholar]
- 63.Oluwole OO, Depaz HA, Gopinathan R, et al. Indirect allorecognition in acquired thymic tolerance: induction of donor-specific permanent acceptance of rat islets by adoptive transfer of allopeptide-pulsed host myeloid and thymic dendritic cells. Diabetes. 2001;50(7):1546–1552. doi: 10.2337/diabetes.50.7.1546. [DOI] [PubMed] [Google Scholar]
- 64.Oluwole SF, Fawwaz RA, Engelstad K, Wang TS, Hardy MA. Migration patterns of indium-111 labeled dendritic cells in the rat. Prog Clin Biol Res. 1990;355:247–256. [PubMed] [Google Scholar]
- 65.Lutz MB, Suri RM, Niimi M, et al. Immature dendritic cells generated with low doses of GM-CSF in the absence of IL-4 are maturation resistant and prolong allograft survival in vivo. Eur J Immunol. 2000;30(7):1813–1822. doi: 10.1002/1521-4141(200007)30:7<1813::AID-IMMU1813>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 66.Picarda E, Bézie S, Venturi V, et al. MHC-derived allopeptide activates TCR-biased CD8+ Tregs and suppresses organ rejection. J Clin Invest. 2014;124(6):2497–2512. doi: 10.1172/JCI71533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee RS, Yamada K, Houser SL, et al. Indirect recognition of allopeptides promotes the development of cardiac allograft vasculopathy. Proc Natl Acad Sci U S A. 2001;98(6):3276–3281. doi: 10.1073/pnas.051584498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tran HM, Patel A, Allen RD, O’Connell PJ. Intrathymic inoculation of donor antigen: an ineffective strategy for prolonging xenograft survival. Xenotransplantation. 1999;6(2):147–154. doi: 10.1034/j.1399-3089.1999.00018.x. [DOI] [PubMed] [Google Scholar]
- 69.Odorico JS, O’Connor T, Campos L, Barker CF, Posselt AM, Naji A. Examination of the mechanisms responsible for tolerance induction after intrathymic inoculation of allogeneic bone marrow. Ann Surg. 1993;218(4):525–531. doi: 10.1097/00000658-199310000-00012. discussion 531–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oluwole SF, Jin MX, Chowdhury NC, Engelstad K, Ohajekwe OA, James T. Induction of peripheral tolerance by intrathymic inoculation of soluble alloantigens: evidence for the role of host antigen-presenting cells and suppressor cell mechanism. Cell Immunol. 1995;162(1):33–41. doi: 10.1006/cimm.1995.1048. [DOI] [PubMed] [Google Scholar]
- 71.Garrovillo M, Ali A, Oluwole SF. Indirect allorecognition in acquired thymic tolerance: induction of donor-specific tolerance to rat cardiac allografts by allopeptide-pulsed host dendritic cells. Transplantation. 1999;68(12):1827–1834. doi: 10.1097/00007890-199912270-00001. [DOI] [PubMed] [Google Scholar]
- 72.Lu L, Khoury S, Sayegh M, Thomson A. Dendritic cell tolerogenicity and prospects for dendritic cell-based therapy of allograft rejection and autoimmunity. In: Lotze M, Thomson A, editors. Dendritic Cells: Biology and Clinical Applications. San Diego: Academic Press; 1999. pp. 487–511. [Google Scholar]
- 73.Garrovillo M, Ali A, Depaz HA, et al. Induction of transplant tolerance with immunodominant allopeptide-pulsed host lymphoid and myeloid dendritic cells. Am J Transplant. 2001;1(2):129–137. [PubMed] [Google Scholar]
- 74.Ali A, Garrovillo M, Oluwole OO, et al. Mechanisms of acquired thymic tolerance: induction of transplant tolerance by adoptive transfer of in vivo allomhc peptide activated syngeneic T cells. Transplantation. 2001;71(10):1442–1448. doi: 10.1097/00007890-200105270-00015. [DOI] [PubMed] [Google Scholar]
- 75.DePaz HA, Oluwole OO, Adeyeri AO, et al. Immature rat myeloid dendritic cells generated in low-dose granulocyte macrophage-colony stimulating factor prolong donor-specific rat cardiac allograft survival. Transplantation. 2003;75(4):521–528. doi: 10.1097/01.TP.0000048380.84355.4A. [DOI] [PubMed] [Google Scholar]
- 76.Cobbold S, Waldmann H. Infectious tolerance. Curr Opin Immunol. 1998;10(5):518–524. doi: 10.1016/s0952-7915(98)80217-3. [DOI] [PubMed] [Google Scholar]
- 77.Oluwole OO, DePaz HA, Adeyeri A, Jin M-X, Hardy MA, Oluwole SF. Role of CD41CD251 regulatory T cells from naive host thymus in the induction of acquired transplant tolerance by immunization with allo-major histocompatibility complex peptide. Transplantation. 2003;75(8):1136–1142. doi: 10.1097/01.TP.0000062842.47597.13. [DOI] [PubMed] [Google Scholar]
- 78.Liu J, Liu Z, Witkowski P, et al. Rat CD8+ FOXP3+ T suppressor cells mediate tolerance to allogeneic heart transplants, inducing PIR-B in APC and rendering the graft invulnerable to rejection. Transpl Immunol. 2004;13(4):239–247. doi: 10.1016/j.trim.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 79.Kaufman CL, Colson YL, Wren SM, Watkins S, Simmons RL, Ildstad ST. Phenotypic characterization of a novel bone marrow-derived cell that facilitates engraftment of allogeneic bone marrow stem cells. Blood. 1994;84(8):2436–2446. [PubMed] [Google Scholar]
- 80.Leventhal JR, Elliott MJ, Yolcu ES, et al. Immune reconstitution/immunocompetence in recipients of kidney plus hematopoietic stem/facilitating cell transplants. Transplantation. 2015;99(2):288–298. doi: 10.1097/TP.0000000000000605. [DOI] [PubMed] [Google Scholar]
- 81.Chhabra AY, Leventhal J, Merchak AR, Ildstad S. HSCT-Based Approaches for Tolerance Induction in Renal Transplant. Transplantation. 2017;101(11):2682–2690. doi: 10.1097/TP.0000000000001837. [DOI] [PubMed] [Google Scholar]
- 82.Fugier-Vivier IJ, Rezzoug F, Huang Y, et al. Plasmacytoid precursor dendritic cells facilitate allogeneic hematopoietic stem cell engraftment. J Exp Med. 2005;201(3):373–383. doi: 10.1084/jem.20041399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang Y, Bozulic LD, Miller T, Xu H, Hussain L-R, Ildstad ST. CD8α+ plasmacytoid precursor DCs induce antigen-specific regulatory T cells that enhance HSC engraftment in vivo. Blood. 2011;117(8):2494–2505. doi: 10.1182/blood-2010-06-291187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gregori S, Tomasoni D, Pacciani V, et al. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood. 2010;116(6):935–944. doi: 10.1182/blood-2009-07-234872. [DOI] [PubMed] [Google Scholar]
- 85.Granger DK, Matas AJ, Jenkins MK, Moss AA, Chen SC, Almond PS. Prolonged survival without posttransplant immunosuppression in a large animal model. Surgery. 1994;116(2):236–241. [PubMed] [Google Scholar]
- 86.Yamada K, Shimizu A, Utsugi R, et al. Thymic transplantation in miniature swine. II. Induction of tolerance by transplantation of composite thymokidneys to thymectomized recipients. J Immunol Baltim Md 1950. 2000;164(6):3079–3086. doi: 10.4049/jimmunol.164.6.3079. [DOI] [PubMed] [Google Scholar]
- 87.Weiss MJ, Guenther DA, Mezrich JD, et al. The indirect alloresponse impairs the induction but not maintenance of tolerance to MHC class I-disparate allografts. Am J Transplant. 2009;9(1):105–113. doi: 10.1111/j.1600-6143.2008.02494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ezzelarab MB, Zahorchak AF, Lu L, et al. Regulatory dendritic cell infusion prolongs kidney allograft survival in nonhuman primates. Am J Transplant. 2013;13(8):1989–2005. doi: 10.1111/ajt.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ezzelarab MB, Raich-Regue D, Lu L, et al. Renal Allograft Survival in Nonhuman Primates Infused With Donor Antigen-Pulsed Autologous Regulatory Dendritic Cells. Am J Transplant. 2017;17(6):1476–1489. doi: 10.1111/ajt.14182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pêche H, Trinité B, Martinet B, Cuturi MC. Prolongation of heart allograft survival by immature dendritic cells generated from recipient type bone marrow progenitors. Am J Transplant. 2005;5(2):255–267. doi: 10.1111/j.1600-6143.2004.00683.x. [DOI] [PubMed] [Google Scholar]
- 91.Liu Z, Colovai AI, Tugulea S, et al. Indirect recognition of donor HLA-DR peptides in organ allograft rejection. J Clin Invest. 1996;98(5):1150–1157. doi: 10.1172/JCI118898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hutchinson JA, Ahrens N, Riquelme P, et al. Clinical management of patients receiving cell-based immunoregulatory therapy. Transfusion (Paris) 2014;54(9):2336–2343. doi: 10.1111/trf.12641. [DOI] [PubMed] [Google Scholar]
- 93.The ONE Study. The ONE Study; [Accessed December 22, 2017]. http://www.onestudy.org/index.html. Published 2011. [Google Scholar]