Abstract
Problem
IgG is the only antibody class that is actively transferred from the mother to the fetus across the placenta by an active, neonatal Fc receptor (FcRn) mediated process during pregnancy, conferring passive immunity and protection against infections to the newborn during the first months of life. Preterm infants may not receive sufficient titers of protective antibodies, as most of them are transferred only after the 34th week of gestation. Because of the great importance of this process, we investigated in a clinical setting the placental transmission of IgG antibodies in term and preterm newborns.
Method of Study
This work was conducted in 85 woman and their newborns, divided into four groups according to their clinical gestational age (≤37 weeks were considered as preterm). Blood samples were collected from the mothers and their newborns’ umbilical cords to analyze total serum IgG concentrations, and a subgroup of 32 placentas were analyzed by immunohistochemistry to quantify the expression of the FcRn receptor.
Results
Total IgG levels in both mothers and neonates increased significantly through the third trimester of gestation. Regarding the newborns, in all groups IgG levels exceeded their mother’s values by a ~2.4%. A higher expression of FcRn was detected in placentas from newborns at week 36 of gestation onwards.
Conclusions
Our results obtained from clinical samples, were in line with previous descriptions in model systems and confirmed that the IgG transfer from maternal serum to the fetus is positively correlated with FcRn expression in placental tissue throughout gestation.
Keywords: neonates, FcRn, placenta, IgG, preterm
1. INTRODUCTION
Protection against pathogens is achieved through the coordinated actions of the innate and the adaptive arms of the immune system. Newborns rely heavily on their innate immune defenses as their “educated” adaptive immunity fully develops only later, in the early years of life1. Of the five antibody classes, IgG is the most prevalent in serum and certain mucosal tissues such as the airways, distal gastrointestinal tract and genitourinary tract, and has long been known to be the only class that is actively transferred from the mother to the fetus across the placenta by an active, neonatal Fc receptor (FcRn) mediated process during pregnancy, thus conferring short-term passive immunity and protection against infections to the newborn during the first months of life2,3,4. Indeed, at the 32nd week of gestation, detectable IgG levels in newborns are only ~400 mg/dL (reference values for adults is 548–1768 mg/dL and in term newborns is 631–1431 mg/dL). Therefore, preterm infants may not receive sufficient titers of protective antibodies, as most of them are transferred only after the 34th week of gestation5.
Neonates are one of the highest risk age groups for mortality and morbidity from infection. According to the World Health Organization, 3.7 million neonates less than 28 days of age died in 2010 — 37% of these deaths were due to infectious causes6. Eleven percent of all infants are born premature, representing about 12.9 million infants born prematurely each year worldwide, a population exposed undoubtedly to a higher risk of death7.
FcRn is expressed mainly in the syncytiotrophoblast, where it transports IgG from the maternal circulation to the fetal capillaries of the placental villi by bidirectionall transcytosis across a polarized cell layer8,9,10. The syncytiotrophoblast internalizes fluid containing maternal IgG into endosomes, which are then gradually acidified thereby allowing IgG to bind tightly to the FcRn in a strictly pH-dependent manner and in a 2:1 stoichiometry2,3,11,12. The endosome then fuses with the membrane on the fetal side of the syncytiotrophoblast, where the physiological pH promotes the dissociation of IgG from FcRn releasing the antibody to the fetal circulation. The FcRn molecule may then be recycled to the maternal membrane to perform additional rounds of transcytosis13. Therefore, the pH-dependent binding of IgG to FcRn allows for IgG transport through a cell layer and down a concentration gradient of IgG14.
In humans, the active role of this receptor starts early in fetal development and increases progressively from the early second trimester until the end of pregnancy5. Because of the great importance of acquiring maternal antibodies that can provide protection early in life, investigations into the mechanisms behind this pattern have been reported several times since the early 1980s. However, partly because of the very limited access to fetal samples (blood/placenta) in the first and second trimester, the vast majority of these reports were detailed reviews about the chemistry and functional physiology of the FcRn receptor, or studies using the ex-vivo placental transfer model, but that were not verified in human clinical samples15. In fact, there are only limited number of reports of FcRn expression in early human life but have not been correlated with IgG levels16.
We now report on the placental transmission of IgG antibodies in clinical samples. Our specific objectives were to: 1) investigate if there is a relationship between maternal and the neonatal IgG levels, and 2) compare serum IgG levels and FcRn expression in placental tissue in preterm and term neonates according to gestational age (third trimester of pregnancy).
2. MATERIALS AND METHODS
2.1 Patients
The present work was conducted in 85 woman between 19 and 40 years of age and their 90 offspring (10 twins), admitted to the Obstetrics Department at the Clínica Universitaria Reina Fabiola, Córdoba, Argentina, between April and October 2013. All women that agreed to participate in this study signed an informed consent. Patients presenting prenatal diagnosis of placental pathology, immunological deficiencies or autoimmunity were excluded from the study as were newborns presenting any genetic syndrome diseases.
The mothers and their respective newborns were divided into four groups according to their clinical gestational age as determined by the Capurro method17: Group I, 32–33 weeks (n=8), Group II, 34–35 weeks (n=15), Group III, 36–37 weeks (n=9) and Group IV, 38–41 weeks (n=58). Those infants with gestational ages ≤37 weeks were considered as preterm18.
2.2 Serum IgG levels determination
Immediately after birth, blood samples were collected from the mothers and their respective newborns’ umbilical cords. The samples were centrifuged at 3000 rpm for 10 min and stored at −20°C until assayed. Total serum IgG concentrations (mg/dL) were measured by the quantitative radial immunodiffusion technique, using a commercial kit with anti-human IgG antibody (Diffuplate, Biocientífica SA, Buenos Aires, Argentina). Briefly, 3 μl of sera were incubated at 4 °C for 48 hours in a humidified chamber to allow diffusion of the immunoglobulin. Following incubation, diameters of the precipitation rings of antigen-antibody complexes were measured and the concentration of IgG was calculated by generating a dose–response curve against a reference serum of pre-determined IgG concentration19.
2.3 Placental FcRn receptor
The placentas were obtained after birth and immediately fixed using a 10% buffered formaldehyde solution until further processing. Some placentas were lost during the process because of technical problems during urgency of a preterm delivery. Therefore, a subgroup of 31 placentas was analyzed.
To quantify the expression of the FcRn receptor, the placental tissue was mounted on positively charged slides and stained with the EnVision G2 Doublestain System, rabbit/mouse (DAB+/Permanent Red) Kit from Dako, following heat-induced epitope retrieval in antigen retrieval buffer (10 mM citrate, 1 mM EDTA, 0.05% Tween-20, pH 6.0). Slides were stained with anti-hFCGRT (HPA012122, Sigma Aldrich) at 1/50 dilution overnight at 4°C. Initially, a counterstain was also used (hemotoxilin) in the slides; however, because the counterstain was observed to interfere with the DAB reaction when analyzing the images, its use was eliminated from the process.
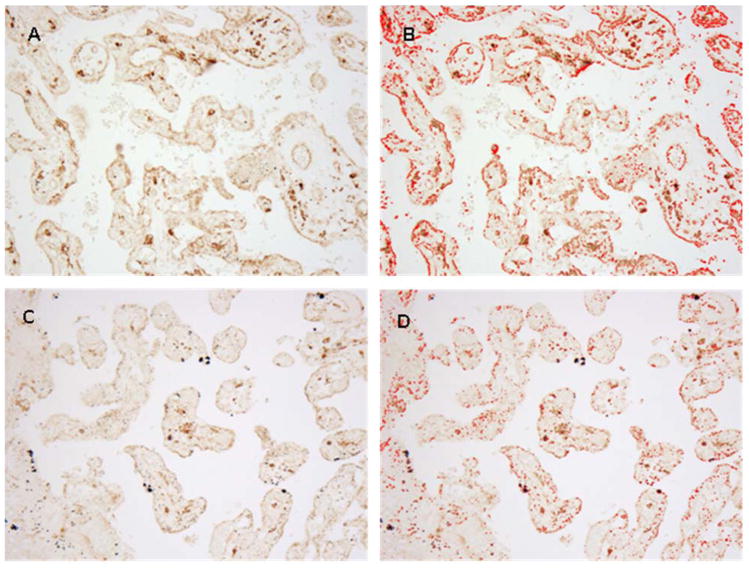
For the quantification of the placental area expressing FcRn, digital pictures were taken using an Olympus optical microscope under a 20× magnification. Two pictures were taken from each slide in representative parts of the tissue and analyzed using the Image J software (W. Rastband, Research Services Brach, National Institute of Mental Health, Bethesda, MD, USA). The images were adjusted by brightness and saturation before using the “analyze particles” tool (Figure 1). The results were expressed as percentage of the total area expressing the receptor.
Figure 1.
Representative figure of the FcRn expression in human placental tissue (20× magnification) in term and pre-term newborns before and after ImageJ processing (A vs. B and C vs. D, respectively).
2.4 Ethical approval
This research protocol was approved by the Institutional Ethics Committee of the Clínica Universitaria Reina Fabiola, Facultad de Ciencias de la Salud, Universidad Católica de Córdoba, Argentina, and in accordance to the guidelines of the Helsinki Declaration, the Administración Nacional de Medicamentos Alimentos y Tecnología Médica Good Clinical Practices and in agreement with the Province of Córdoba (Argentina) laws for Research in humans (9694/09). Informed consent for all interventions and the use of data was acquired from all patients, and confidentiality is guaranteed under national laws for the protection of personal data (25326/00). The authors declare that there are no conflicts of interest.
2.5 Data analysis
Data analysis was performed using the Infostat statistical software (2013 version)20. Values were expressed as median and range (Q1–Q3). To check the assumptions of normality and variance homogeneity, diagnostic techniques based in the residuals were applied. Accordingly, the data was analyzed using the Wilcoxon Mann–Whitney test or Kruskal-Wallis test and Dunn’s post-hoc test for multiple comparisons. In the case of categorical variables, the Chi Square test was applied. The significance level for all statistical tests was set at 0.05.
3. RESULTS
A total of 85 blood samples were obtained from the mothers, and 90 samples were obtained from the newborns umbilical cord. All newborns had a gestational age greater than 32 weeks; ten newborns (11.1%) were twins, 41 (45.6%) were born from primiparous mothers and 49 (54.4%) from multiparous mothers. The full clinical and biochemical data from maternal and cord blood IgG levels in the four groups are shown in Table 1.
Table 1.
Clinical data and IgG levels in mothers and their newborns during the third trimester of gestation.
| Mothers | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|
| n | 7 (8.2%) | 12 (14.1%) | 8 (9.4%) | 58 (68.2%) |
| Age (years) | 31 (26–37) | 33 (31–37) | 30 (30–32) | 31 (29–34) |
| IgG levels (mg/dL) | 998.4 (995.2–1003.5) a | 1001.8 (992.2–1005.3) a | 1003.5 (996.8–1007) a | 1013.9 (1007.2–1031.3) b |
|
| ||||
| Neonates | Group I | Group II | Group III | Group IV |
|
| ||||
| n | 8 (8.9%) | 15 (16.7%) | 9 (10%) | 58 (64.4%) |
| Weight (g) | 2032.5 (1585–2115) | 2250 (2065–2460) | 2800 (2725–3050) | 3367.5 (3105–3680) |
| IgG level (mg/dL) | 1014.55 (1007–1022.6) a | 1029.1 (1003.5–1035.8) a | 1022.6 (1016.5–1031.3) a | 1046.35 (1035.8–1057.9) b |
Values are expressed as median and range (Q1–Q3). Weeks of gestation: Group I: 32–33 weeks; Group II: 34–35 weeks; Group III: 36–37 weeks; Group IV: 38–41 weeks. a vs b: p<0.001.
The total IgG levels in both mothers and neonates were found to be within the normal range as published in the literature.
In the mothers, a slight increase in IgG levels was observed towards the end of gestation, reaching significantly higher levels in Group IV (38–41 weeks of gestation; p<0.0001; Table 1); additionally a linear regression analysis of the data also showed a positive and significant correlation between increased IgG levels and gestational age (p<0.001; slope: 2.38; r2= 0.21).
No significant differences were found when analyzing the influence of the mothers’ age or number of previous gestations on maternal IgG levels (p=0.85 and 0.07 respectively).
In the newborns, the pre-term groups (I, II and III) showed a significantly lower IgG level than those individuals born at full term (p<0.0001; Table 1). IgG levels increased by 2.44 mg/dL per week throughout gestation.
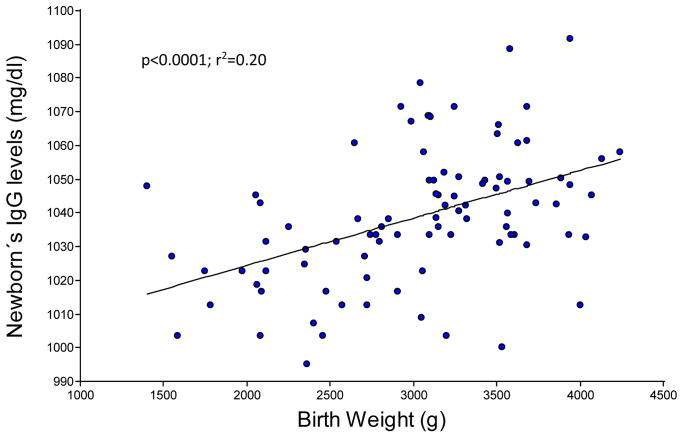
With respect to the impact of the newborn weight at birth, a regression analysis revealed that IgG levels increased significantly by 0.014 mg/dL per gram of body weight during the third trimester of gestation (p<0.001; Figure 2).
Figure 2.
Newborn IgG levels and body weight increase during third trimester of gestation (n=90).
Neither sex of the individual, age of the mother, or number of previous gestations influenced the newborns’ IgG levels (p= 0.98; p=0.53 and p= 0.47 respectively).
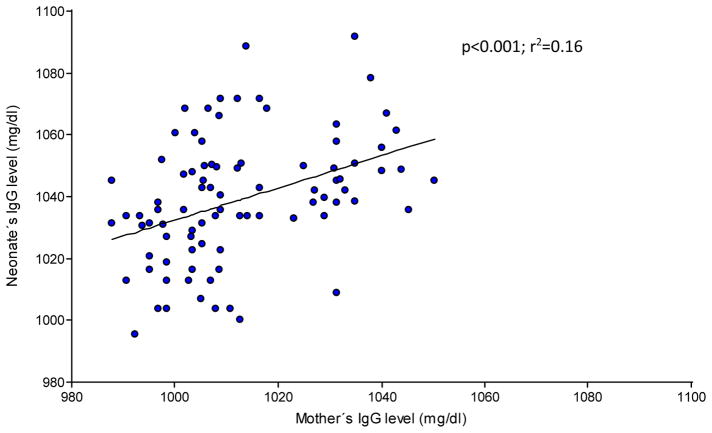
The total IgG levels detected in the newborns correlated with their mothers IgG levels, increasing progressively by 0.40 mg/dl for every 1.0 mg/dl of increase in the mother, and reached a significantly higher value towards the end of gestation in week 38 (Group IV) (p<0.0001; Figure 3). Finally, IgG levels were significantly higher in the newborns than in their mothers in all four groups studied (p≤0.05, Table 1).
Figure 3.
Relationship between mother’s (n=85) and neonate’s IgG levels (n=90).
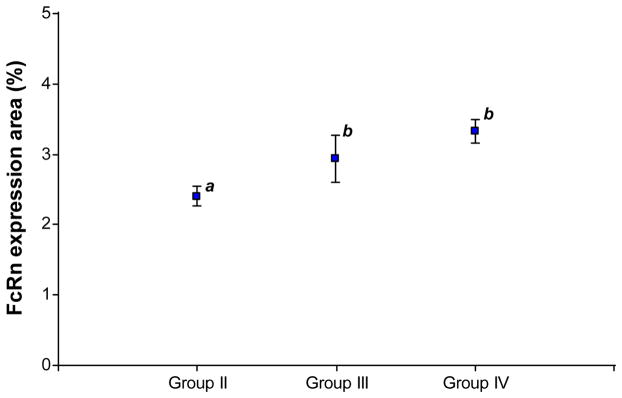
A total of 32 placental tissue samples were analyzed for the expression of FcRn (Group I n=1; Group II n=8; Group III n=5; Group IV n=18). When analyzing the percentage of the total area of the placental tissue expressing FcRn, we observed higher expression of the receptor in placentas obtained from term vs. preterm newborns (3.25% (2.8–3.6) (n=18) vs. 2.69% (2.4–3.0) (n=14), respectively, p=0.01). In a further analysis, we observed that expression of the receptor increased significantly from week 36 of gestation onwards (Groups III and IV, p=0.004, Figure 4).
Figure 4.
FcRn expression in human placental tissue during the third trimester of gestation. Group II: 34–35 weeks (n=7); Group III: 36–37 weeks (n=5); Group IV: 38–41 weeks (n=18). a vs b: p=0.004. Group I is excluded from the figure because it consists of a single sample of placental tissue.
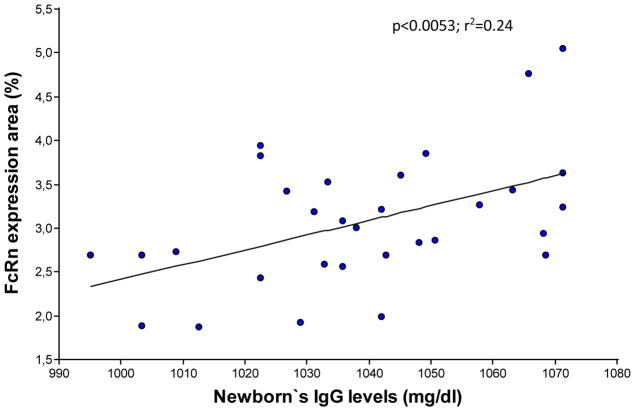
Finally, an increase in the area of placental tissue expressing FcRn was associated with an increase in IgG levels in the newborns (p<0.0053; slope= 0.02; r2= 0.24) (Figure 5).
Figure 5.
Relationship between percentage of the total area in the placental tissue with FcRn expression and the newborn’s IgG levels (n=31).
4. DISCUSION
Newborns, especially preterm infants, have an immature immune system that makes them a vulnerable population with high infection-associated morbidities and mortality rates21,22. Premature neonates are even more susceptible to infections due to several factors, including immature mechanical barriers, limited neutrophils function, low plasma concentrations of specific antibodies, low activities of the complement system proteins and poor cooperation between T and B lymphocytes. This favors bacterial translocation across the intestinal epithelium and dissemination to other organs and tissues, thereby increasing the risk of systemic infections and potential for sepsis23,24,25.
As previously mentioned, the immunoglobulins of the fetus consist almost entirely of maternal immunoglobulin G (IgG) transferred across the placenta. Such transplacental transfer of immunoglobulins provides temporary protection to the newborn against antigens that the mother had been exposed to during her own life24.
Because of the great importance of this process, we investigated in a clinical setting the placental transmission of IgG antibodies in term and preterm newborns.
In the present work we found that through the third trimester of gestation, total IgG levels in both mothers and neonates showed a significant increase towards the end of gestation.
Surprisingly in the mothers, both statistical tests applied indicated a mild, yet significant increase in IgG levels over the course of the last trimester. A large number of studies have been conducted on the relationship between IgG levels during pregnancy in the first, second and the third trimester, reporting a significant drop throughout gestation26. This gradual decline in IgG was described as a consequence of the physiological expansion of maternal blood volume (hemodilution) and is consistent with a similar change in i.e. albumin levels26. However, little is known about the subtle changes developed exclusively during the third trimester. To our knowledge only a few studies have investigated this relationship and they reported no significant variations in maternal IgG concentrations between weeks 28 and 42 of gestation26, or during the last 8 weeks of pregnancy27. One report in Brazilian mothers suggested that although not statistically significant, mothers of preterm babies had lower IgG levels as compared with term babies’ mothers24. The reason for the slightly higher IgG levels found in our study remains unclear, although we might speculate a higher incidence of (subclinical) infections. We should also note that the higher data variation in groups III and IV could factor in to the trend we observed (Table I).
Regarding the newborns, our results were consistent with the scarce literature available. As early as 1967, Hobbs et al.28 found an exponential relationship between total IgG and gestational age in preterm infants. In their review, Van den Berg et al.29 clearly demonstrated that in the first trimester, very little IgG is transported to the fetus. In the second trimester, the fetal IgG rises from approximately 10% of the maternal concentration at 17–22 weeks of gestation to 50% at 28–32 weeks of gestation. In the third trimester, the increase of fetal IgG concentration between 29 and 41 weeks of gestation is two times as high as between 17 and 28 weeks of gestation30. Similarly, other authors asserts that the immune transfer begins slightly earlier at 16 weeks of gestation, and that between the 22nd and 26th week, the level of fetal IgG increases rapidly doubling the IgG concentration at the end of gestation31. The greatest rate of antibody transfer to the fetus is after 34 weeks of pregnancy5.
Also, in all four groups studied in our work, the newborn’s IgG levels exceeded their mother’s values by a ~2.4%. Several studies have described similar findings. As human pregnancy progresses, fetal/newborn IgG levels continuously increase to reach and even surpass maternal levels at the end of the third trimester, resulting in a fetal:maternal ratio higher than one and sometimes reaching more than twice the maternal concentrations at the time of delivery32,33,34,35. Assuming that these levels of IgG in the fetus are entirely of maternal origin, this would remarkably suggest that FcRn is able to transport this macromolecule against a concentration gradient.
On the other hand, our results agree with those reported by Palmeira et al.25, who found that maternal age and parity do not seem to influence placental antibody transfer.
In our study, IgG was observed to increase significantly with the newborns weight at birth; it is likely that this association is due to the fact that infants delivered at later gestational weeks usually present a higher body weight27. However, some, but not all investigators also have noted reduced circulating IgG concentrations in low birth weight infants, compared to those of similar gestational age with normal birth weight36. This suggests that for a given gestational age, placental insufficiency may limit IgG transfer to the fetus37.
Finally, the present work also demonstrated that a higher expression of the placental neonatal receptor FcRn was detected in placentas obtained from newborns at week 36 of gestation onwards. This is consistent with previous observations that expression of the FcRn receptor is dependent on gestational age and is at its highest in the third trimester of human pregnancy38,25. In this regard, it is known that in placental tissues these receptors are mainly located in the apical side and cytoplasm of the syncytiotrophoblast39. At early stages of pregnancy, the inner cytotrophoblast forms a continuous layer between the syncytotrophoblast and the stromal cells. Cytotrophoblast cells neither express FcRn9, nor contain IgG, and may prevent further penetration of the villi by IgG40. However, late in this period the FcRn is already expressed9. As gestation progresses and after the forth month of pregnancy, the cytotrophoblast layer slowly disappears from the walls of the tertiary villi and becomes discontinuous, when the chorionic villi surface increments and expands, therefore exposing the syncytotrophoblast cells to maternal blood vessels41. It is in this stage of gestation when the receptor can be exposed and the active transport of IgG begins. Experimental data obtained in rats indicated that the increasing total IgG levels during gestation coincides with an increase in FcRn expression in the placental tissue throughout pregnancy42.
To summarize, our results obtained from clinical samples, were in line with previous descriptions in model systems and confirmed that the amount of IgG transfer from maternal serum to the fetus is positively correlated with FcRn expression in placental tissue throughout gestation. The elucidation and full understanding of the physiological processes involved in the placental transfer of IgG antibodies is crucial to establish therapeutic protocols to treat immunodeficient mothers, as well as to allow comprehensive clinical care and management of the preterm newborn.
Acknowledgments
This work was performed with the technical collaboration of Maria Jose Viola MD, Esteban Ceballos MD, Eugenia Concari Tech. and Cristina Trezza MD. NAL was supported by Fundacion Florencio Fiorini, Argentina. RSB was supported by NIH DK53056. KB current affiliation is the Department of Oncology, University of Alberta, Canada.
Footnotes
6. Declarations of interest: none.
References
- 1.Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 2009;9:185–194. doi: 10.1038/nri2508. [DOI] [PubMed] [Google Scholar]
- 2.Brambell FW. The transmission of immunity from mother to young and the catabolism of immunoglobulins. Lancet. 1966;2:1087–1093. doi: 10.1016/s0140-6736(66)92190-8. [DOI] [PubMed] [Google Scholar]
- 3.Morphis LG, Gitlin D. Maturation of the maternofoetal transport system for human γ-globulin in the mouse. Nature. 1970;228(5271):573. doi: 10.1038/228573a0. [DOI] [PubMed] [Google Scholar]
- 4.Simister NE, Rees AR. Isolation and characterization of an Fc receptor from neonatal rat small intestine. Eur J Immunol. 1985;15:733–738. doi: 10.1002/eji.1830150718. [DOI] [PubMed] [Google Scholar]
- 5.Landor M. Maternal–fetal transfer of immunoglobulins. Ann Allerg Asthma Immunol. 1995;74:279–283. [PubMed] [Google Scholar]
- 6.WHO. World Health Statistics: 2010. World Health Organization; 2010. [Google Scholar]
- 7.Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, Rubens C, Menon R, Van Look PFA. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bulletin of the World Health Organization. 2010;88:31–38. doi: 10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leach JL, Sedmak DD, Osborne JM, Rahill B, Lairmore MD, Anderson CL. Isolation from human placenta of the IgG transporter, FcRn, and localization to the syncytiotrophoblast: implications for maternal–fetal antibody transport. J Immunol. 1996;157:3317–3322. [PubMed] [Google Scholar]
- 9.Simister NE, Story CM, Chen HL, Hunt JS. An IgG-transporting Fc receptor expressed in the syncytiotrophoblast of human placenta. Eur J Immunol. 1996;26:1527–1531. doi: 10.1002/eji.1830260718. [DOI] [PubMed] [Google Scholar]
- 10.Kristoffersen EK. Human placental Fc γ-binding proteins in the maternofetal transfer of IgG. APMIS. 1996;64:5–36. doi: 10.1111/j.1600-0463.1996.tb05583.x. [DOI] [PubMed] [Google Scholar]
- 11.Raghavan M, Chen MY, Gastinel LN, Bjorkman PJ. Investigation of the interaction between the class I MHC-related Fc receptor and its immunoglobulin G ligand. Immunity. 1994;1:303–315. doi: 10.1016/1074-7613(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 12.Raghavan M, Bonagura VR, Morrison SL, Bjorkman PJ. Analysis of the pH dependence of the neonatal Fc receptor/immunoglobulin G interaction using antibody and receptor variants. Biochemistry. 1995;34:14649–14657. doi: 10.1021/bi00045a005. [DOI] [PubMed] [Google Scholar]
- 13.Ober RJ, Martinez C, Lai X, Zhou J, Ward ES. Exocytosis of IgG as mediated by the receptor, FcRn: an analysis at the single-molecule level. Proc Natl Acad Sci USA. 2004;101:11076–11081. doi: 10.1073/pnas.0402970101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 15.Stapleton NM, Einarsd_ottir HK, Stemerding AM, Vidarsson G. The multiple facets of FcRn in immunity. Immunol Rev. 2015;268:253–268. doi: 10.1111/imr.12331. [DOI] [PubMed] [Google Scholar]
- 16.Shah U, Dickinson BL, Blumberg RS, Simister NE, Lencer WI, Walker WA. Distribution of the IgG Fc receptor, FcRn, in the human fetal intestine. Pediatr Res. 2003;53(2):295–301. doi: 10.1203/01.PDR.0000047663.81816.E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capurro H, Konichezky S, Fonseca D, Caldeyro-Barcia R. A simplified method for diagnosis of gestational age in the newborn infant. J Pediatr. 1978;93:120–122. doi: 10.1016/s0022-3476(78)80621-0. [DOI] [PubMed] [Google Scholar]
- 18.Engle WA. A recommendation for the definition of “late preterm” (near-term) and the birth weight-gestational age classification system. Semin Perinatol. 2006;30:2–7. doi: 10.1053/j.semperi.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Mancini G, Carbonara AG, Heremans JF. Immunochemical quantitation of antigens by single radial immunodifusion. Immunochemistry. 1965;2:235. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- 20.Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW. InfoStat version 2012. Grupo InfoStat, FCA, Universidad Nacional de Córdoba; Argentina: http://www.infostat.com.ar. [Google Scholar]
- 21.Van den Berg JP, Westerbeek EA, Berbers GA, et al. Transplacental transport of IgG antibodies specific for pertussis, diphtheria, tetanus, Haemophilus influenzae type b, and neisseria meningitidis serogroup C is lower in preterm compared with term infants. Pediatr Infect Dis J. 2010;29(9):801–805. doi: 10.1097/inf.0b013e3181dc4f77. [DOI] [PubMed] [Google Scholar]
- 22.Sharma AA, Jen R, Butler A, Lavoie PM. The developing human preterm neonatal immune system: A case for more research in this area. Clin Immunol. 2012;145:61–68. doi: 10.1016/j.clim.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mussi-Pinhata MM, Rego MAC. Immunological peculiarities of extremely preterm infants: a challenge for the prevention of nosocomial sepsis. J Pediatr (Rio J) 2005;81:59–68. doi: 10.2223/1301. [DOI] [PubMed] [Google Scholar]
- 24.Silveira Lessa AL, Jornada Krebs VL, Braga Brasil T, Neres Pontes G, Carneiro-Sampaio M, Palmeira P. Pretermand term neonates transplacentally acquire IgG antibodies specific to LPS from Klebsiella pneumoniae, Escherichia coli and Pseudomonas aeruginosa. FEMS Immunol Med Microbiol. 2011;62:236–243. doi: 10.1111/j.1574-695X.2011.00807.x. [DOI] [PubMed] [Google Scholar]
- 25.Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG Placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. 2012;2012:1–13. doi: 10.1155/2012/985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malek A, Sager R, Kuhn P, Nicolaides KH, Schneider H. Evolution of Maternofetal Transport of Immunoglobulins During Human Pregnancy. Am J Reprod Immunol. 1996;36:248–255. doi: 10.1111/j.1600-0897.1996.tb00172.x. [DOI] [PubMed] [Google Scholar]
- 27.Boersma ER. Serum immunoglobulins IgG, IgM, and IgA in maternal cord blood pairs from infants of normal and low birthweights in Tanzania. Arch Dis Child. 1981;56:31–35. doi: 10.1136/adc.56.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hobbs JR, Davis JA. Serum gamma-G-globulin levels and gestational age in premature babies. Lancet. 1967;1:757–9. doi: 10.1016/s0140-6736(67)91369-4. [DOI] [PubMed] [Google Scholar]
- 29.Van den Berg JP, Westerbeek EAM, van der Klis FRM, Berbers GAM, van Elburg RM. Transplacental transport of IgG antibodies to preterm infants: A review of the literature. Early Hum Dev. 2011;87:67–72. doi: 10.1016/j.earlhumdev.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Simister NE. Placental transport of immunoglobulin G. Vaccine. 2003;21:3365–3369. doi: 10.1016/s0264-410x(03)00334-7. [DOI] [PubMed] [Google Scholar]
- 31.Chucri TM, Monteiro JM, Lima AR, Salvadori ML, Kfoury JR, Jr, Miglino MA. A review of immune transfer by the placenta. J Reprod Immunol. 2010;87:14–20. doi: 10.1016/j.jri.2010.08.062. [DOI] [PubMed] [Google Scholar]
- 32.Pitcher-Wilmott RW, Hindocha P, Wood CBS. The placental transfer of IgG subclasses in human pregnancy. Clin Exp Immunol. 1980;41:303–308. [PMC free article] [PubMed] [Google Scholar]
- 33.Malek A, Sager R, Schneider H. Maternal-Fetal Transport of Immunoglobulin G and Its Subclasses During the Third Trimester of Human Pregnancy. Am J Reprod Immunol. 1994;32:8–14. doi: 10.1111/j.1600-0897.1994.tb00873.x. [DOI] [PubMed] [Google Scholar]
- 34.Garty BZ, Ludomirsky A, Danon YL, Peter JB, Douglas SD. Placental transfer of immunoglobulin G subclasses. Clin Diagn Lab Immunol. 1994;1:667–669. doi: 10.1128/cdli.1.6.667-669.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hashira S, Okitsu-Negishi S, Yoshino K. Placental transfer of IgG subclasses in a Japanese population. Pediatr Int. 2000;42:337–342. doi: 10.1046/j.1442-200x.2000.01245.x. [DOI] [PubMed] [Google Scholar]
- 36.Catty D, Drew R, Seger R. Transmission of IgG subclasses to the human fetus. In: Hemmings WA, editor. Protein transmission though living membranes. Amsterdam: Elsevier/North-Holland; 1979. pp. 37–43. [Google Scholar]
- 37.Sierig G, Labitzke B, Diez U, Kiess W, Borte M. Natural history of serum immunoglobulin concentrations in low birth weight infants and association with respiratory tract infections. Biol Neonate. 2002;82(3):159–165. doi: 10.1159/000063614. [DOI] [PubMed] [Google Scholar]
- 38.Okoko BJ, Wesumperuma HL, Fern J, Yamuah LK, Hart CA. The transplacental transfer of IgG subclasses: influence of prematurity and low borthweight in the Gambian population. Ann Trop Paediatr. 2002;22(4):325–332. doi: 10.1179/027249302125001985. [DOI] [PubMed] [Google Scholar]
- 39.Rath T, Baker K, Pyzik M, Blumberg RS. Regulation of immune responses by the neonatal Fc receptor and its therapeutic implications. Front Immunol. 2015;5(664):1–8. doi: 10.3389/fimmu.2014.00664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bright NA, Ockleford CD. Cytotrophoblast cells: a barrier to maternofetal transmission of passive immunity. J Histochem Cytochem. 1995;43:933–44. doi: 10.1177/43.9.7642966. [DOI] [PubMed] [Google Scholar]
- 41.Simpson RA, Mayhew TM, Barnes PR. From 13 weeks to term, the trophoblast of human placenta grows by the continuous recruitment of new proliferative units: a study of nuclear number using the disector. Placenta. 1992;13(5):501–512. doi: 10.1016/0143-4004(92)90055-x. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Jiang X, He J, Diraviyam T, Zhang X. Quantitative Investigation on Correlation Between IgG and FcRn During Gestation and Lactating Periods in Rat. Am J Reprod Immunol. 2016;75:81–85. doi: 10.1111/aji.12465. [DOI] [PubMed] [Google Scholar]