Abstract
There is clear association between the intensity of the acute inflammatory response during acute myocardial infarction (AMI) and adverse prognosis after AMI. Interleukin‐1 (IL‐1) is a pro‐inflammatory cytokine released during AMI and involved in adverse remodeling and heart failure (HF). We describe a study to evaluate the safety and efficacy of IL‐1 blockade using an IL‐1 receptor antagonist (anakinra) during the acute phase of ST‐segment elevation myocardial infarction (STEMI). The Virginia Commonwealth University–Anakinra Remodeling Trial‐3 (VCU‐ART3; http://www.ClinicalTrials.gov NCT01950299) is a phase 2, multicenter, double‐blinded, randomized, placebo‐controlled clinical trial comparing anakinra 100 mg once or twice daily vs matching placebo (1:1:1) for 14 days in 99 patients with STEMI. Patients who present to the hospital with STEMI within 12 hours of symptom onset will be eligible for enrollment. Patients will be excluded for a history of HF (functional class III–IV), severe valvular disease, severe kidney disease (stage 4–5), active infection, recent use of immunosuppressive drugs, active malignancy, or chronic autoimmune/auto‐inflammatory diseases. We will measure the difference in the area under the curve for C‐reactive protein between admission and day 14, separately comparing each of the anakinra groups with the placebo group. The P value will be considered significant if <0.025 to adjust for multiple comparisons. Patients will also be followed for up to 12 months from enrollment to evaluate cardiac remodeling (echocardiography), cardiac function (echocardiography), and major adverse cardiovascular outcomes (cardiovascular death, MI, revascularization, and new onset of HF).
Keywords: Interleukin‐1, STEMI, study design
1. INTRODUCTION
Acute myocardial infarction (AMI) remains a major cause of morbidity and mortality in the United States and worldwide. Despite considerable advances in the treatment of AMI, >20% of AMI survivors develop heart failure (HF) within 1 year.1 This observation suggests that the current treatment paradigm misses 1 or more key pathophysiologic mechanisms. Therefore, an urgent need exists to develop additional treatments to prevent HF after AMI.
1.1. Inflammation and HF after AMI
A close interplay exists between inflammation, adverse cardiac remodeling, and HF after AMI. Acute myocardial ischemia and infarction initiate an intense inflammatory response within the myocardium.2 In experimental animal models of AMI due to surgical coronary artery ligation, the degree of the inflammatory response is a strong predictor of adverse cardiac remodeling independent of infarct size.2 Likewise, in patients with AMI, the intensity of the inflammatory response, reflected in levels of circulating biomarkers, predicts adverse cardiac remodeling, HF, and death.3 Modulation of the inflammatory response, therefore, represents an intriguing target for therapeutic intervention. Although previous attempts to target inflammation have failed, interleukin‐1 (IL‐1) blockade is a novel and substantially different approach to modulating the inflammatory response.4
1.2. IL‐1 in experimental AMI
IL‐1 is a prototypical inflammatory cytokine involved in virtually every inflammatory response in the body and plays a critical role in the pathophysiologic sequelae of AMI.5 In experimental mouse models of AMI due to surgical coronary artery ligation, genetic deletion of the IL‐1 type 1 receptor (IL‐1R1) protects against adverse cardiac remodeling, whereas genetic deletion of the naturally occurring receptor antagonist (IL‐1 receptor antagonist [IL‐1Ra]) amplifies the response to IL‐1 and promotes worse cardiac remodeling compared with wild‐type mice.6 Anakinra, a recombinant human IL‐1Ra (Kineret; Biovitrum, Stockholm, Sweden) is approved for the treatment of rheumatoid arthritis and is generally well tolerated following daily subcutaneous injection.7 Mice treated with daily injections of anakinra had improved survival at 7 days after large anterior AMI, and the survivors had evidence of more favorable cardiac remodeling (smaller left ventricular [LV] end‐diastolic and end‐systolic diameters), higher LV ejection fraction, and reduced cardiomyocyte apoptosis.6
Based on the preliminary benefits observed in the experimental AMI model and the established safety profile of anakinra, 2 pilot clinical trials were conducted with anakinra in ST‐segment elevation myocardial infarction (STEMI): VCU‐ART8 and VCU‐ART2.9 Collectively, these phase 2 pilot studies enrolled 40 patients with reperfused STEMI and randomized them (within 12 hours of coronary angiography) to daily treatment with anakinra 100 mg or placebo for 14 days. Anakinra was well tolerated and reduced serum levels of C‐reactive protein (CRP), a surrogate marker of IL‐1 activity. Anakinra‐treated patients tended to have more favorable LV remodeling and a lower incidence of HF at mid‐ and long‐term follow‐up.10
2. STUDY DESIGN
The Virginia Commonwealth University–Anakinra Remodeling Trial‐3 (VCU‐ART3; http://www.ClinicalTrials.gov NCT01950299) is a phase 2, multicenter, double‐blinded, randomized, placebo‐controlled clinical trial comparing anakinra 100 mg twice daily, 100 mg once daily, and placebo once daily or placebo twice daily (1:1:1). The study will test the hypothesis that IL‐1 blockade will quench the inflammatory response during acute STEMI, as measured by area under the curve (AUC) for CRP between admission and day 14 (Figure 1).
Figure 1.
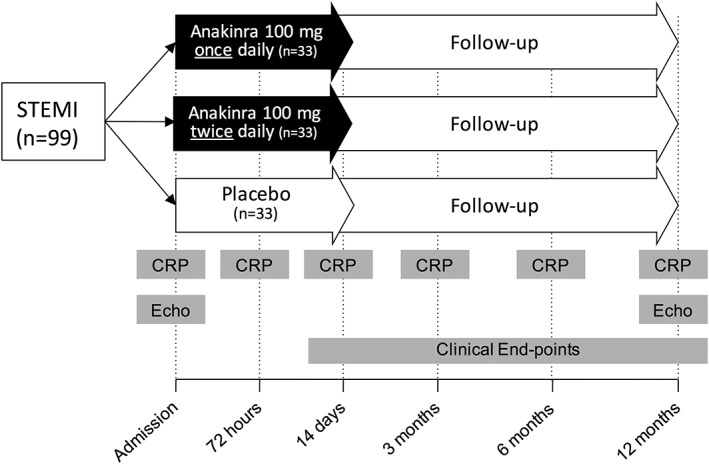
Design of the Virginia Commonwealth University–Anakinra Remodeling Trial‐3 (VCU‐ART3). The overall study design is represented as a schematic. Abbreviations: CRP, C‐reactive protein; Echo, transthoracic echocardiography; STEMI, ST‐segment elevation myocardial infarction
2.1. Screening and enrollment
Patients age ≥ 21 years presenting to the hospital with acute STEMI, defined as chest pain or equivalent with electrocardiographic evidence of new or presumably new ST‐segment elevation (>1 mm) in ≥2 anatomically contiguous leads and who undergo coronary angiography for potential intervention within 12 hours of symptoms onset will be approached by the investigators for enrollment. Potential subjects will be provided with an informed consent form in accordance with the local institutional review board oversight. Patients will be excluded from the study if they have contraindications to treatment with anakinra or preexisting structural or functional cardiac abnormalities (see Supporting Information, Table 1, in the online version of this article).
Table 1.
Baseline demographics (N = 99 patients)
| Demographics | |
|---|---|
| Age, y, mean ± SD | 56.0 ± 10.4 |
| Male sex, % | 83 |
| African American race, % | 42 |
Abbreviations: SD, standard deviation.
2.2. Randomization and allocation concealment
The manufacturer has provided anakinra and identical matching placebo syringes to the investigational pharmacy at the coordinating center. A randomization log was prepared by a consultant not involved in the conduct of the study and sent electronically to the director of the investigational pharmacy at Virginia Commonwealth University Health System, and then distributed to the other centers. Syringes were sequentially numbered from 1 to 28 to maintain allocation concealment.
2.3. Treatment with investigational drug
Patients will receive the first dose of anakinra 100 mg or placebo at time of enrollment, which will occur within 12 hours of coronary angiography. One group of patients (n = 33) will receive anakinra 100 mg twice daily for 14 days; a second group (n = 33) will receive anakinra 100 mg alternated with placebo twice daily approximately 12 hours apart for 14 days, so that anakinra will be given every 24 hours; and the third group (n = 33) will receive placebo twice daily approximately 12 hours apart for 14 days. Patients in the study will also receive guideline‐based medical treatments as indicated.
2.4. Endpoints and objectives
The primary endpoint will be the AUC for CRP between admission and day 14. The rationale for measuring AUC for CRP is that it integrates measurements across multiple time points to more accurately capture the acute inflammatory response and IL‐1 activity and is also an established prognostic marker in STEMI.3 The primary endpoint will then be used to compare the anakinra 100 mg twice‐daily group with the placebo group, and then the anakinra 100 mg daily group with the placebo group, separately.
Exploratory endpoints will include changes in structural and functional echocardiographic parameters such as LV and right ventricular dimensions, ventricular mass, and systolic and diastolic function between baseline and 12 months. Additional cardiac biomarkers will be tested including N‐terminal pro brain natriuretic peptide. These will provide mechanistic insight as to whether the hypothesized changes in the acute inflammatory response will correlate with changes in cardiac dimensions, function, or biomarkers.
Incidence of death (cardiac and noncardiac), hospitalizations (for HF and for other cardiac causes not related to HF or for noncardiac reasons), and new onset of HF (defined as hospitalization for HF or need for a new prescription for loop diuretic in the appropriate clinical setting) will be recorded at each time point throughout the study and verified by chart review or telephone interview after the last subject visit. The investigators and clinicians caring for the patients will remain blinded to treatment allocation and to the CRP data throughout the study. An ad hoc committee composed of a general cardiologist, a HF specialist, and an internal medicine hospitalist—who are also blinded to treatment allocation and to CRP data—will perform event adjudication (see Supporting Information, Table 4, in the online version of this article).
2.5. Safety assessment
All patients will undergo a complete physical examination and clinical evaluation at each visit. Disease‐related assessments will include changes in physical symptoms, exercise tolerance, vital signs (including weight), renal function, and changes in medications. Given that anakinra may mask signs of infection (eg, fever), patients will be evaluated for signs and symptoms of occult infection at each visit.
A Data and Safety Monitoring Board composed of a HF cardiologist, 2 interventional cardiologists, a general internal medicine specialist, and an infectious disease specialist will oversee the study (see Supporting Information, Table 5, in the online version of this article).
This study will be conducted in accordance with the National Institutes of Health Good Clinical Practice guidelines. An Investigational New Drug use from the Division of Cardiovascular and Renal Products, Center for Drug Evaluation and Research, US Food & Drug Administration is held by Dr. Abbate. The study protocol, consent, and Data and Safety Monitoring Plan have been approved by the VCU institutional review board.
2.6. Sample size and statistical analysis
The sample size for this study was calculated with a superiority scope according to the objective of comparing AUC for CRP with anakinra (high dose) vs placebo and anakinra (standard dose) vs placebo. Given an expected average AUC for CRP of 350 ±250 mg/L for placebo‐treated STEMI patients (based on the pilot studies) and an average AUC for CRP of 175 ±150 mg/L for anakinra (standard dose; also based on the pilot studies), 33 patients per group would provide an 85% power (2‐tailed α 0.025 considering multiple testing) to detect a further 50% reduction in the anakinra (high dose; estimated AUC for CRP of 88 ±75 mg/L) vs anakinra (standard dose) and a > 99% power (2‐tailed α 0.025) vs placebo. A conservative estimate of 20% loss to follow‐up or withdrawal would retain >80% power for all analyses.
For statistical analysis, all values will be reported as the median and interquartile range for potential deviation from Gaussian distribution. The differences between treatment groups will be computed using the Wilcoxon signed‐rank test for continuous variables or the Fisher exact test for discrete variables. The differences in interval changes between the treatments will be compared using random‐effect analysis of variance for repeated measures to analyze the effects of time and group allocation. Unadjusted P values will be reported throughout, with statistical significance set at the 2‐tailed 0.025 levels for the primary analysis, to adjust for multiplicity. Secondary analyses will account for potential confounders (eg, group imbalances at baseline, differences in post‐MI treatment regimens between groups). All analyses will be completed using SPSS, version 24.0 (IBM Corp., Armonk, NY).
2.7. Progress to date
The study began enrollment in July 2014 and completed enrollment in December 2017. A total of 99 patients were enrolled at 3 study sites in Virginia and Washington, DC (Table 1). The final follow‐up visit is scheduled for December 2018.
3. DISCUSSION
Patients presenting with STEMI are at particularly high risk for adverse cardiac remodeling and HF.11 A growing body of evidence suggests that cardiovascular disease is both a metabolic and inflammatory disorder. Whereas atherogenesis is a slow process, promoted or accelerated by chronic subclinical inflammation, acute atherothrombotic events are rather stochastic, with sudden events leading to acute coronary syndrome (ACS) facilitated by inflammatory processes.12 A recent large study of IL‐1β blockade using canakinumab in patients with prior AMI has shown that systemic inflammation is a significant driver of recurrent AMI.13 In the VCU‐ART3 study, we propose to explore the role of IL‐1 during the intense acute systemic inflammatory response observed following myocardial ischemic injury and estimate the relationship between this response and subsequent remodeling. Moreover, the use of an IL‐1 receptor antagonist (anakinra) instead of an IL‐1β antibody (canakinumab) may help to clarify the role of other IL‐1 isoforms (eg, IL‐1α) in ACS.14, 15
Three prior studies (VCU‐ART,8 VCU‐ART2,9 and the MRC ILA‐HEART Study16) of IL‐1 blockade with anakinra during ACS confirm that IL‐1 is an important mediator of inflammation at the time of ACS and that IL‐1 blockade is sufficient to provide (at least) partial inhibition of the CRP response. However, there remains some uncertainty over the benefits of IL‐1 blockade in the ACS setting and the CRP response after discontinuation of IL‐1 blockade.16 Due to observations in these prior studies that CRP levels in many patients remained elevated (>2 mg/L) after 14 days of treatment with the standard anakinra dose (100 mg daily), the VCU‐ART3 study will explore the dose–response relationship between anakinra and CRP through use of a higher anakinra dose (100 mg twice daily). Normalization of CRP levels may be particularly important for long‐term prognosis, given that on‐treatment CRP >2 mg/L in the CANTOS study was associated with reduced efficacy of canakinumab.13, 17
As with all pilot clinical trials, there are inherent limitations to the design of VCU‐ART3. In particular, the study will be underpowered for detecting differences in clinical outcomes, and therefore the results will need to be considered hypothesis‐generating.
4. CONCLUSION
Despite the increasing interest in inflammation and AMI, there are few clinical trials of anti‐inflammatory drugs in patients with AMI, and no drugs are currently approved for use in the acute setting. IL‐1 has emerged as a key pro‐inflammatory mediator in cardiovascular disease. The VCU‐ART3 study will investigate the safety of standard or high‐dose anakinra in patients with STEMI, as well as the effects on the acute inflammatory response as measured by the AUC for CRP at 14 days. The study will also explore the effects of anakinra on interval changes in LV dimensions and systolic/diastolic function at 12 months and on clinical cardiovascular outcomes, including new‐onset of HF, up to 1 year from enrollment of the study. Ultimately, the findings from this phase 2 study may be used to inform the design of future phase 3 studies.
Conflicts of interest
Dr. Abbate and Dr. Van Tassell have served as consultants to Swedish Orphan Biovitrum. The authors declare no other potential conflicts of interest.
Supporting information
Appendix S1.
Van Tassell BW, Lipinski MJ, Appleton D, et al. Rationale and design of the Virginia Commonwealth University–Anakinra Remodeling Trial‐3 (VCU‐ART3): A randomized, placebo‐controlled, double‐blinded, multicenter study. Clin Cardiol. 2018;41:1004–1008. 10.1002/clc.22988
Funding information National Heart, Lung, and Blood Institute, Grant/Award Numbers: 1R34HL121402, 1R34HL121402‐01; Swedish Orphan Biovitrum (Stockholm, Sweden) provided anakinra and placebo free of charge
REFERENCES
- 1. Velagaleti RS, Pencina MJ, Murabito JM, et al. Long‐term trends in the incidence of heart failure after myocardial infarction. Circulation. 2008;118:2057–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mezzaroma E, Toldo S, Farkas D, et al. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci U S A. 2011;108:19725–19730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roubille F, Samri A, Cornillet L, et al. Routinely‐feasible multiple biomarkers score to predict prognosis after revascularized STEMI. Eur J Intern Med. 2010;21:131–136. [DOI] [PubMed] [Google Scholar]
- 4. Seropian IM, Toldo S, Van Tassell BW, et al. Anti‐inflammatory strategies for ventricular remodeling following ST‐segment elevation acute myocardial infarction. J Am Coll Cardiol. 2014;63:1593–1603. [DOI] [PubMed] [Google Scholar]
- 5. Abbate A, Van Tassell BW, Biondi‐Zoccai GG. Blocking interleukin‐1 as a novel therapeutic strategy for secondary prevention of cardiovascular events. BioDrugs. 2012;26:217–233. [DOI] [PubMed] [Google Scholar]
- 6. Abbate A, Salloum FN, Vecile E, et al. Anakinra, a recombinant human interleukin‐1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation. 2008;117:2670–2683. [DOI] [PubMed] [Google Scholar]
- 7. Furst DE. Anakinra: review of recombinant human interleukin‐I receptor antagonist in the treatment of rheumatoid arthritis. Clin Ther. 2004;26:1960–1975. [DOI] [PubMed] [Google Scholar]
- 8. Abbate A, Kontos MC, Grizzard JD, et al. Interleukin‐1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University–Anakinra Remodeling Trial [VCU‐ART] pilot study). Am J Cardiol. 2010;105:1371.e1–1377.e1. [DOI] [PubMed] [Google Scholar]
- 9. Abbate A, Van Tassell BW, Biondi‐Zoccai G, et al. Effects of interleukin‐1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction (from the Virginia Commonwealth University–Anakinra Remodeling Trial (2) [VCU‐ART2] pilot study). Am J Cardiol. 2013;111:1394–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abbate A, Kontos MC, Abouzaki NA, et al. Comparative safety of interleukin‐1 blockade with anakinra in patients with ST‐segment elevation acute myocardial infarction (from the VCU‐ART and VCU‐ART2 pilot studies). Am J Cardiol. 2015;115:288–292. [DOI] [PubMed] [Google Scholar]
- 11. Eapen ZJ, Tang WH, Felker GM, et al. Defining heart failure endpoints in ST‐segment elevation myocardial infarction trials: integrating past experiences to chart a path forward. Circ Cardiovasc Qual Outcomes. 2012;5:594–600. [DOI] [PubMed] [Google Scholar]
- 12. Van Tassell BW, Toldo S, Mezzaroma E, et al. Targeting interleukin‐1 in heart disease. Circulation. 2013;128:1910–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ridker PM, Everett BM, Thuren T, et al; CANTOS Trial Group . Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 14. Toldo S, Austin D, Mauro AG, et al. Low‐density lipoprotein receptor–related protein‐1 is a therapeutic target in acute myocardial infarction. JACC Basic Transl Sci. 2017;2:561–74. doi: 10.1016/j.jacbts.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mauro AG, Mezzaroma E, Torrado J, et al. Reduction of myocardial ischemia‐reperfusion injury by inhibiting interleukin‐1 alpha. J Cardiovasc Pharmacol. 2017;69:156–160. [DOI] [PubMed] [Google Scholar]
- 16. Morton AC, Rothman AMK, Greenwood JP, et al. The effect of interleukin‐1 receptor antagonist therapy on markers of inflammation in non‐ST elevation acute coronary syndromes: the MRC‐ILA Heart Study. Eur Heart J. 2015;36:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ridker PM, MacFadyen JG, Everett BM, et al; CANTOS Trial Group . Relationship of C‐reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet. 2018;391:319–328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.


