Abstract
Background
Racial disparities persist in access to kidney transplantation. Racial differences in preemptive referral, or referral prior to dialysis start, may explain this discrepancy.
Methods
Patient-level data on kidney transplant referrals (2005–2012) from all Georgia transplant centers were linked to the United States Renal Data System to examine racial disparities in preemptive referral, waitlisting, and living donor transplant. Adjusted logistic regression and Cox proportional hazard models determined the associations between race (African American vs. white) and preemptive referral, and placement on the waitlist or receipt of a living donor kidney, respectively.
Results
Among 7,752 adults referred for transplant evaluation, 20.38% (n=1,580) were preemptively referred. The odds of African Americans being preemptively referred for transplant evaluation were 37% (OR=0.63; (95% CI: 0.55 0.71)) lower than whites. Among preemptively referred patients, there was no racial difference (African Americans compared to whites HR=0.96; (95% CI 0.88, 1.04)) in waitlisting. However, African Americans were 70% less likely than whites to receive a living donor transplant (HR=0.30; (95% CI: 0.21, 0.42)).
Conclusion
Racial disparities in transplant receipt may be partially explained by disparities in preemptive referral. Interventions to reduce racial disparities in kidney transplant access may need to be targeted earlier in the disease process.
Keywords: kidney failure, kidney transplantation, referral, consultation
INTRODUCTION
Kidney transplantation is the optimal treatment for most of the >700,000 adults being treated for end stage renal disease (ESRD) in the United States 1–3. ESRD Network 6 (North Carolina, South Carolina, and Georgia) has the highest prevalence of ESRD in the United States 4 and the lowest kidney transplantation rates 5,6 with African Americans being 59% less likely to receive a kidney transplant compared to whites 7.
It has been shown that the sooner an ESRD patient receives a transplant, the better the patient’s health outcomes following transplantation 8,9. Particularly, preemptive deceased donor or living donor transplantation, or receipt of a transplant prior to dialysis start, has been shown to improve graft function, as well as graft and patient survival 10–13. African Americans are less likely to be placed on the deceased donor waitlist 14,15, have longer time on dialysis before accessing the deceased donor waitlist 16, and are less likely receive a preemptive kidney transplant 17,18 compared to white patients, however it is unknown if the disparity is caused by delayed referral for transplant evaluation, or something else. Research has shown that even after controlling for clinical factors and availability of living donors, the racial disparity in kidney transplantation, between African Americans and whites, persists 19,20. Kasiske et al 14 hypothesized that the racial disparity in waitlisting could be attributed to poor pre-dialysis care, although they did not have access to pre-dialysis patient data to test this hypothesis.
Data on key steps early in the transplantation process, including when a patient is referred for transplant 21, are not collected in national surveillance data. Several smaller, regional studies have found differing results with respect to racial differences in dialysis facility level referral for kidney transplantation. Alexander et al. found that African Americans in Indiana, Kentucky, and Ohio had a ~44% lower odds of transplant referral and completion of the transplant evaluation to achieve waitlisting. However, these distinct steps were not analyzed separately 22. More recently, we reported that, in Georgia, although African American ESRD patients were surprisingly 20% more likely to be referred for kidney transplantation within one year of starting dialysis, these referred patients were 27% less likely to be waitlisted within 1 year, compared to white patients 23. Similar to Kasiske et al, we hypothesized that this surprising finding could be due to a greater proportion of white patients being preemptively referred for transplant evaluation, compared to African Americans. Thus, the primary aim of this study was to describe whether there were racial disparities in preemptive referral for kidney transplantation evaluation in Georgia. In addition, we sought to investigate whether racial disparities in waitlisting and living donor transplantation persisted even among patients who were preemptively referred for kidney transplant evaluation.
MATERIALS AND METHODS
Data Sources
Patient-level data on referrals for kidney transplant evaluation were collected from all three adult transplantation centers in Georgia between January 1, 2005, and December 31, 2012 as previously described 21,23. In brief, patient-level referral data from each transplant center were securely sent to ESRD Network 6, which served as the data coordinating center. We used deterministic record linkage between the Georgia referral data and the publicly available data from the United States Renal Data System (USRDS) to obtain more detailed patient characteristics on patients referred and not referred for kidney transplantation evaluation, and to obtain outcome information on waitlisting and transplantation. The USRDS analytic files include patient characteristics (such as demographics, dialysis start date, primary cause of ESRD, and treatment modality) with follow-up through September 2, 2015. We linked these data, via patient’s residential zip code, to data from the publicly available 2007–2011 American Community Survey (http://www.census.gov/programs-surveys/acs/) to obtain neighborhood poverty information. Adult (18–69 years) patients within USRDS who were referred at least once for their first transplant in Georgia in 2005–2012 (n=7,752), and were either African American (n=5,547) or white (n=2,205) were included in our analysis. Collection of Georgia referral data was retrospective and our study was approved by institutional review boards at each of the three transplant centers.
Study Variables
Race
Patient race was recorded by providers on the CMS-2728 form, and is derived from the medical record. Race was dichotomized as African American or white. Patients with missing (n=47) or other race/ethnicity were excluded due to small numbers (white Hispanic=204; Other=291).
Outcome
The primary outcome examined was preemptive referral for kidney transplant evaluation. A patient was considered to have a preemptive referral for transplant evaluation if the date of first referral (defined as the date the referral was received by fax at one of the three transplant centers) occurred before the patient’s dialysis start date. Secondary outcomes among preemptively referred patients included placement on the deceased donor waiting list or receipt of a living donor transplant, evaluated separately. For the secondary outcomes, time from transplant evaluation referral to event was defined as the number of days from referral date to either placement on the deceased donor waiting list or receipt of a living donor transplantation, censoring for death or end of follow-up (09/02/2015).
Other Patient- and Facility-Level Characteristics
Patient characteristics included demographic and clinical data reported on the Medicare ESRD medical eligibility form (CMS-2728). The CMS-2728 form is completed for every dialysis patient within 45 days of starting of dialysis, or by the transplant center at the time of transplant. Baseline patient demographics captured on the CMS-2728 form included age, sex, race, ethnicity, attributed cause of ESRD, and health insurance at baseline. Insurance status was a categorical variable used as a proxy for socioeconomic status. Multiple insurance coverages were defined in to a single-response categorical variable, prioritizing first Medicaid coverage, then employer-based coverage, Medicare coverage, other insurance, and then no insurance status. (For example, if a patient reported both Medicaid and employer-based insurance, the patient was categorized as Medicaid since it may to better represents the patient’s socioeconomic status.) Time from referral to event was not normally distributed, and medians were reported. Patients’ neighborhood poverty level data were obtained from the American Community Survey data. Clinical factors at ESRD start, from the CMS-2728, were body mass index (dichotomized at >/≤35 kg/m2) and history of congestive heart failure, cardiovascular disease, hypertension, diabetes, chronic obstructive pulmonary disease, tobacco use, and cancer.
Statistical Analysis
Descriptive statistics were calculated for patient- and neighborhood-level factors for the total referred population and stratified by race. We also tested for significant difference between patient- and neighborhood-level factors and race using Chi-Square or Kruskal-Wallis tests. Bivariable analysis was performed to determine the association between patient- and neighborhood-level characteristics, including race, and preemptive referral for transplant evaluation. Crude and adjusted Cox proportional hazards models were used to determine the association between preemptive referral, and waitlisting and living donor transplantation. . We used adjusted logistic regression to determine the association between African American vs. white race and preemptive referral. Demographic and clinical characteristics were considered for potential inclusion in the model a priori if they had been previously described as a factor associated with the outcome of interest. To assess racial disparities in access to kidney transplantation among patients preemptively referred for transplant evaluation, we used Cox proportional hazards models for waitlisting and living donor transplantation. Adjusted Cox proportional hazards models were determined using a four-step process: 1) bivariable association between race and transplant outcomes, 2) adjustment for age (continuous variable) and sex, 3) adjustment for patient- and neighborhood-level socioeconomic characteristics, and 4) additional adjustment for patient comorbidities. Log-log curves confirmed modeling assumptions were met for each outcome. SAS version 9.4 (SAS Institute Inc) and Stata version 14.1 (StataCorp) were used for data management and statistical analysis. We used a fully conditional specification method to obtain multiple imputed data sets (n=5) to handle missing data (5.08% of observations), and used likelihood-based methods for inference. Multiple imputation was completed for all adjusted, logistic regression and survival analysis with missing data. Multiple imputation procedures took in to account the variance within and between imputed cohorts 24. Sensitivity analysis was performed to confirm previous literature findings that report preemptively referred patients are more likely to be placed on the deceased donor waiting list or receive a living donor kidney transplant. A sensitivity analysis also assessed death as a competing risk for our secondary outcomes.
RESULTS
Study Population
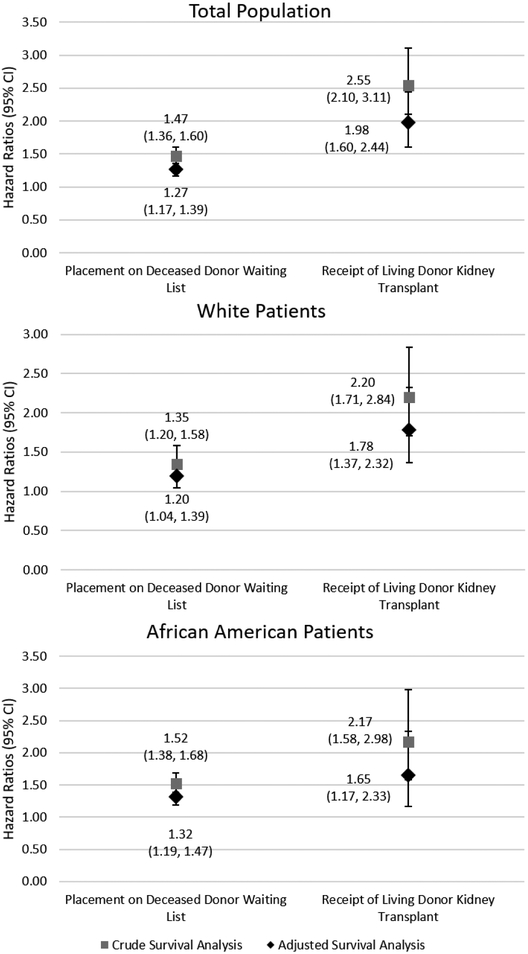
As previously described 23, 90.3% of the 17,224 patients referred to a Georgia transplant center (2005–2012) were successfully identified and linked with a USRDS identifier. A total of 7,752 adults met the study inclusion criteria and were referred to a Georgia transplant center between January 1, 2005 and December 31, 2012. A total of 1,580 (20.4%) patients were preemptively referred for transplant evaluation. Among all patients referred, there was a significant difference between insurance status (Chi-sq=148.5; p<0.001), and a higher percentage of white referred patients had diabetes (Chi-sq=30.1; p<0.001) and cardiovascular disease (Chi-sq=68.5; p<0.001) compared to African American patients (Table 1). Among waitlisted and living donor transplant patients, the median time from referral to event for African Americans were 474 days (interquartile range (IQR): 258, 1,110 days) and 874 days (IQR: 507, 1,434 days), respectively, significantly longer than the median follow-up time for whites from referral to waitlisting (332 days; IQR: 200, 646days; H=88.8, p<0.001), and living donor transplantation (574 days; IQR: 367, 1,023 days; H=22.7, p<0.001). Figure 1 reports the crude and adjusted survival analysis between preemptive referral for transplant evaluation and either placement on the deceased donor waiting list or receipt of a living donor kidney transplant. In the adjusted, Cox proportional hazard model within the total population (Figure 1) and compared to patients referred for transplant evaluation after starting dialysis, preemptively referred patients were 27% (HR=1.27; (95% CI: 1.17, 1.39)) more likely to be waitlisted and about twice (HR=1.98; (95% CI: 1.60, 2.44)) as likely to receive a living donor kidney transplantation. Preemptively referred white patients and African Americans were 20% (HR=1.20; (95% CI: 1.04, 1.39)) and 32% (HR=1.32; (95% CI: 1.19, 1.47)) more likely to be waitlisted, respectively, compared to their counterparts referred after starting dialysis.
Table 1.
Patient-level characteristics of patients in the U.S. state of Georgia referred for kidney transplantation evaluation from 2005 to 2012 (n=7,752), overall and stratified by race
| Characteristic | Study Population | White | African American | Test Statistica | p-valuea |
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | |||
| N=7,752 | N=2,205 (28.4) | N=5,547 (71.6) | |||
| Patient-level characteristics at ESRD start | |||||
| Referral Status | |||||
| Preemptively Referred | 1580 (20.4) | 608 (27.6) | 972 (17.5) | 98.2 | <0.001 |
| Median days (IQR) to event: | |||||
| Placement on the deceased donor waiting list | 425(240,980) | 332 (200, 646) | 474(258, 1110) | 88.8 | <0.001 |
| Receipt of living donor kidney transplant | 669(421, 1149) | 574 (367, 1023) | 874 (507, 1434) | 22.7 | <0.001 |
| Age, mean (SD) | 49.6(11.8) | 51.8(11.6) | 48.8(11.8) | 10.3 | <0.001 |
| Age group | 116.2 | <0.001 | |||
| 18–30 | 506 (6.5) | 115 (5.2) | 391 (7.0) | ||
| 30–39 | 1118 (14.4) | 241 (10.9) | 877 (15.8) | ||
| 40–49 | 1839 (23.7) | 461 (20.9) | 1378 (24.8) | ||
| 49–59 | 2477 (32.0) | 710 (32.2) | 1767 (31.9) | ||
| 60–69 | 1812 (23.4) | 678 (30.7) | 1134 (20.4) | ||
| Sex | 32.3 | <0.001 | |||
| Male | 4406 (56.8) | 1365 (61.9) | 3041 (54.8) | ||
| Female | 3346 (43.2) | 840 (38.1) | 2506 (45.2) | ||
| Attributed cause of end stage renal disease, N (%) | 208.6 | <0.001 | |||
| Diabetes | 3245 (41.9) | 1060 (48.1) | 2185 (39.4) | ||
| Hypertension | 2782 (35.9) | 521 (23.6) | 2261 (40.8) | ||
| Glomerulonephritis | 575 (7.4) | 214 (9.7) | 361 (6.5) | ||
| Other | 1150 (14.8) | 410 (18.6) | 740 (13.3) | ||
| Clinical measures at ESRD start | |||||
| Body Mass Index > 35 kg/m2 | 1841 (24.3) | 495 (22.8) | 1346 (24.9) | 3.4 | 0.09 |
| Congestive heart failure | 1518 (19.6) | 432 (19.6) | 1086 (19.6) | 0.0002 | 0.99 |
| Cardiovascular disease | 1791 (23.1) | 648 (29.4) | 1143 (20.6) | 68.5 | <0.001 |
| Hypertension | 6965 (89.8) | 1923 (87.2) | 5042 (90.9) | 23.5 | <0.001 |
| Diabetes | 4026 (51.9) | 1254 (56.9) | 2772 (50.0) | 30.1 | <0.001 |
| Chronic Obstructive Pulmonary Disease | 251 (3.2) | 137 (6.2) | 114 (2.1) | 87.1 | <0.001 |
| Tobacco use | 609 (7.9) | 223 (10.1) | 386 (7.0) | 21.7 | <0.001 |
| Cancer | 188 (2.4) | 80 (3.6) | 108 (1.9) | 18.8 | |
| Socioeconomic characteristics at ESRD Start | |||||
| Health insurance | 148.5 | <0.001 | |||
| Medicare | 1203 (15.5) | 413 (18.7) | 790 (14.3) | ||
| Medicaid | 1455 (18.8) | 293 (13.3) | 1162 (21.0) | ||
| Employer group | 3198 (41.3) | 1059 (48.0) | 2139 (38.6) | ||
| Other coverage | 373 (4.8) | 122 (5.5) | 251 (4.5) | ||
| No coverage | 1510 (19.5) | 317 (14.4) | 1193 (21.6) | ||
| Neighborhood Characteristics | |||||
| Neighborhood poverty (% ZIP below poverty) | 212.7 | <0.001 | |||
| 0–19% of ZIP below poverty | 3159 (41.9) | 623 (28.8) | 2536 (47.1) | ||
| >20% of ZIP below poverty | 4383 (58.1) | 1539 (71.2) | 2844 (52.9) |
Abbreviations: IQR, interquartile range
The test statistic and p-value was calculated using Chi-Square or Kruskal-Wallis test for categorical or continuous variables, respectively.
The proportion of missing values for health insurance is 0.17%, for poverty category is 2.78%, for body mass index is 2.36%. Observations with missing values were excluded from the descriptive statistics for each variable.
Figure 1.
Crude and adjusted adjusted* survival analysis determined the association between preemptive referral status and either placement on the deceased donor waiting list or receipt of a living donor kidney transplantation, overall and stratified by race (white and African American)
Preemptive Referral and Patient Characteristics
The majority of the patients referred to a Georgia transplant center for transplant evaluation were African American (71.6%), although only 17.5% were preemptively referred and were significantly less likely to be preemptively referred (Chi-sq=98.2; p<0.001) compared to whites. Table 2 reports the descriptive statistics and bivariable association between patient characteristics and preemptive referral. Among those referred for transplant evaluation, there were slightly more women (21.3%) than men (19.7%) preemptively referred, and among those referred for transplant evaluation with employer-group insurance, 28.7% were preemptively referred; approximately 80% of patients referred for transplant evaluation and diagnosed with diabetes (79.0%) or hypertension (79.6%) were not preemptively referred. The median time from referral to dialysis start was 150 days (IQR 60, 320 days). Whites had a significantly shorter time from referral to dialysis start (median=126 days; IQR 56, 288 days) compared to African Americans (median=165 days; IQR 62, 343 days) (p=0.01). In the multivariable logistic regression adjusted for patient demographics, clinical factors, socioeconomic characteristics, and neighborhood poverty, African Americans had lower odds (OR=0.63; (95% CI: 0.55, 0.71)) of being preemptively referred compared to whites.
Table 2.
Characteristics of patients preemptively referred for transplant evaluation to a Georgia transplant center from 2005 to 2012 and the odds ratios estimating the association between patient-level characteristics and preemptive referral
| Characteristic | Preemptive Referral Population N=1580 (20.48 %) | Non preemptive Referral Population N=6172 (79.6)% | Odds Ratios determining the association between preemptive (reference=non-preemptively) transplant evaluation referral and reported characteristics | |
|---|---|---|---|---|
| Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratioa (95% CI) | |||
| Patient-level characteristics at ESRD start | ||||
| Race/Ethnicity, N (%) | ||||
| Non-Hispanic White | 608 (27.6) | 1597 (72.4) | Reference | Reference |
| African American | 972 (17.5) | 4575 (82.5) | 0.56 (0.50–0.62) | 0.63 (0.55–0.71) |
| Ageb, mean ± SD | 50.9 ± 11.6 | 49.3 ± 11.8 | 1.01 (1.01–1.02) | 1.00 (1.00–1.01) |
| Age, N (%)b | ||||
| 18–30 | 77 (15.2) | 429 (84.8) | ||
| 30–39 | 213 (19.1) | 905 (80.9) | ||
| 40–49 | 371 (20.2) | 1468 (79.8) | ||
| 49–59 | 484 (19.5) | 1993 (80.5) | ||
| 60–69 | 435 (24.0) | 1377 (76.0) | ||
| Sex, N (%) | ||||
| Male | 866 (19.7) | 3540 (80.3) | Reference | Reference |
| Female | 714 (21.3) | 2632 (78.7) | 1.11 (0.99–1.24) | 1.16 (1.04–1.31) |
| Attributed cause of end stage renal disease, N (%) | ||||
| Diabetes | 679 (20.9) | 2566 (79.1) | Reference | Reference |
| Hypertension | 456 (16.4) | 2326 (83.6) | 0.74 (0.65–0.84) | 0.77 (0.69–0.85) |
| Glomerulonephritis | 193 (33.6) | 382 (66.4) | 1.91 (1.58–2.32) | 1.64 (1.41–1.91) |
| Other | 252 (21.9) | 898 (78.1) | 1.04 (0.89–1.23) | 0.94 (0.83–1.07) |
| Clinical measures at ESRD start, N (%) | ||||
| Body Mass Index > 35 kg/m2 | 370 (20.1) | 1471 (79.9) | 0.99 (0.87–1.13) | 0.94 (0.82–1.08) |
| Congestive heart failure | 226 (14.9) | 1292 (85.1) | 0.63 (0.54–0.74) | 0.67 (0.57–0.79) |
| Hypertension | 1423 (20.4) | 5542 (79.6) | 1.06 (0.88–1.27) | 1.14 (0.93–1.39) |
| Diabetes | 844 (21.0) | 3182 (79.0) | 1.08 (0.97–1.21) | 1.09 (0.93–1.28) |
| Chronic Obstructive Pulmonary Disease | 34 (13.5) | 217 (86.5) | 0.61 (0.42–0.87) | 0.55 (0.38–0.82) |
| Tobacco use | 94 (15.4) | 515 (84.6) | 0.70 (0.56–0.87) | 0.83 (0.65–1.05) |
| Cancer | 35 (18.6) | 153 (81.4) | 0.89 (0.62–1.30) | 0.68 (0.46–0.98) |
| Socioeconomic characteristics at ESRD Start | ||||
| Health insurance, N (%) | ||||
| Medicare | 261 (21.7) | 942 (78.3) | 0.69 (0.59–0.81) | 1.37 (1.19–1.58) |
| Medicaid | 241 (16.6) | 1214 (83.4) | 0.49 (0.42–0.58) | 1.06 (0.92–1.21) |
| Employer group | 917 (28.7) | 2281 (71.3) | Reference | Reference |
| Other coverage | 73 (19.6) | 300 (80.4) | 0.61 (0.46–0.79) | 1.16 (0.93–1.44) |
| No coverage | 83 (5.5) | 1427 (94.5) | 0.15 (0.11–0.18) | 0.31 (0.26–0.38) |
| Neighborhood Characteristics | ||||
| Neighborhood poverty (% ZIP below poverty), N (%) | ||||
| 0–19% of ZIP below poverty | 565 (17.9) | 2594 (82.1) | Reference | Reference |
| >20% of ZIP below poverty | 977 (22.3) | 3406 (77.7) | 0.76 (0.68–0.86) | 0.97 (10.91–1.03) |
The adjusted logistic regression model is adjusted for the covariates with ORs (95% CI) are reported.
Age was presented as a categorical variable for descriptive statistics and treated as a continuous variable for crude and adjusted logistic regression analyses. Observations with missing values were excluded from the descriptive statistics for each variable.
For the adjusted logistic regression analysis, multiple imputation handled missing data. The proportion of missing values for health insurance is 0.17%, for poverty category is 2.78%, for bod mass index is 2.36%.
Preemptive Referral and Placement on Deceased Donor Waiting List or Receipt of Living Donor Kidney Transplantation
Among patients preemptively referred to a Georgia transplant center for transplant evaluation from 2005–2012 (n=1,580), 836 (52.9%) and 161 (10.2%) were waitlisted or received a living donor kidney transplant, respectively, over the median 3.1 year study follow-up. A total of 40.4% of preemptively referred white patients and 40.0% of preemptively referred African American patients were waitlisted over the study period (p=0.70), and 10.9% of white patients received a living donor transplant vs. 3.1% of African Americans (p<0.001). In our crude and fully adjusted Cox proportional hazard models (Table 3), we found no significant association between race and placement on the deceased donor waitlist (crude HR=1.07; (95% CI: 0.93, 1.23); fully adjusted HR=0.96; (95% CI: 0.88, 1.04)). African Americans were less likely to receive a living donor transplant (crude HR=0.29; (95% CI: 0.21, 0.40)) compared to whites. These associations remained throughout adjustment, with African Americans 70% (HR=0.30; (95% CI: 0.21, 0.42)) less likely to receive a living donor kidney transplant compared to whites. Our sensitivity survival analysis accounting for death as a competing risk (Supplemental Table) for our secondary outcomes showed similar effects, although the effect estimates for living donor kidney transplantation were attenuated in the competing risk sensitivity analysis (fully adjusted HR=0.46; (95% CI: 0.38, 0.57)). However, based on our prognostic research question, we followed the recommendation of Noordzij et al 25, and have maintained our multivariable survival analysis coefficients as our main analysis.
Table 3.
Hazard ratios reporting the association between race and access to transplant among patients preemptively referred for kidney transplantation evaluation, 2005–2015
| Model 1: Unadjusted | Model 2: Adjusting for Model 1 + Agea, Sex | Model 3: Adjusting for Model 2 + patient insurance and neighborhood poverty | Model 4: Adjusting for Model 3 + patient comorbiditiesb | |
|---|---|---|---|---|
| HR for African American vs white (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Placement on the Deceased Donor Waitlist | 1.07 (0.93–1.23) | 1.07 (0.93–1.24) | 1.13 (0.98–1.31) | 0.96 (0.88–1.04) |
| Receipt of Living Donor Kidney Transplantation | 0.29 (0.21–0.40) | 0.28 (0.20–0.39) | 0.29 (0.21–0.40) | 0.30 (0.21–0.42) |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Age was treated as a continuous variable for Cox regression analysis
Comorbid conditions include: attributed cause of end stage renal disease, body mass index, hypertension, diabetes, chronic obstructive pulmonary disease, tobacco, cancer and congestive heart failure
Multiple imputation handled missing data within Model 4 for missing body mass index data (2.36%). Observations with missing values were excluded from the descriptive statistics for each variable.
DISCUSSION
In this study, after covariate adjustment, we found that 20% of patients referred to a Georgia transplant center between January 1, 2005 and December 31, 2012 were referred for transplant evaluation prior to ever starting dialysis. African Americans comprise the majority (65.7%) of ESRD patients in the Southeast 4 and the majority (71.6%) of patients referred for a transplant evaluation to Georgia transplant centers. However, among referred patients, African Americans had 37% lower odds of being preemptively referred for transplant evaluation, compared to whites, after adjusting for patient-, clinical-, socioeconomic-, and neighborhood-characteristics. Among preemptively referred patients, African Americans were 70% less likely to receive a living donor transplant compared to whites. There was no significant difference between African American and white preemptively referred patients and placement on the waiting list. Results from our study suggest that the higher referral of white vs. African American patients to kidney transplant evaluation prior to dialysis start may explain at least some of the racial disparities in transplant access in Georgia. Interventions to reduce racial disparities in kidney transplant access may need to target the early ESRD process, such as among late-stage chronic kidney disease (CKD) patients.
Our previous report on the variation of transplant referral among dialysis patients in Georgia showed African Americans were 1.22 times more likely to be referred within 1-year of starting dialysis 23. This study supports our hypothesis that the increased likelihood of African Americans being referred for kidney transplant evaluation after starting dialysis may be at least partially due to a selection bias of the sample, where whitesare more likely to be referred for transplant evaluation before starting dialysis (preemptively referred). Our previous report also showed that, although African Americans were more likely to be referred for transplant within 1-year of starting dialysis, they were 23% less likely to be waitlisted within 1 year of referral 23. Myaskovsky et al’s longitudinal study of 127 adults referred to the University of Pittsburgh Starzl Transplant Institute and completed two rounds of interviews 20 reported that when controlling for income, perceived discrimination, and transplant knowledge, race was no longer significantly associated with being eligible for a transplant; and patients with more transplant knowledge taking less time to be accepted for a kidney transplant 20. Kasiske et al investigated the variables associated with placement on the kidney transplant waitlist and found that African Americans were 53% less likely (OR=0.47) to be preemptively waitlisted compared to whites 14. Kasiske hypothesized the racial disparity in preemptive listing was partially caused by the lack of adequate healthcare and pre-dialysis care by a nephrologist 14. As identified by Kalantar-Zadeh et al (2017), there is an urgency to examine the transition of care from advanced CKD to renal replacement therapy, including kidney transplantation 26. They identified this ‘prelude’ period and report major knowledge gaps that need to be addressed by primary care and nephrologists that include the best timing for, and optimal type of renal replacement therapy, which could vary across demographics. The management of CKD and kidney failure requires a multidisciplinary, patient-centered approach and possible integration of a comprehensive scoring system 27 across specialties which may help determine the best timing for renal replacement therapy and referral for kidney transplant evaluation 26. A multidisciplinary approach could also improve patient education and provide patients more knowledge about treatment options, including transplant, and could significantly shorten their time to transplant 20.
Importantly, our results focusing only on the group of patients who were preemptively referred for transplant evaluation showed no significant difference in waitlisting between preemptively referred African Americans and whites, supporting the hypothesis that when patients are referred to a nephrologist prior to dialysis, the racial disparity in waitlisting is mitigated. Given our findings that show early referral is associated with improved probability of placement on the deceased donor waiting list or receipt of living donor kidney transplantation, primary care physicians should regularly screen their high-risk patients for kidney failure and refer patients to a nephrologist to consider transplant options as early, in the course of disease, as possible. Public policy that improves or maintains health insurance coverage could assist high risk adult patients to complete annual screenings and detect decreasing kidney function that could be monitored by either a primary care physician or nephrologist.
Literature shows that the longer a patient is on dialysis, the worse the post-transplant outcomes 8,11,28,29. With the national median time-to-transplant for newly listed candidates of 3.5 years 4, timeliness of referral is important. Late referral may limit opportunities for patient education 30 about transplantation, particularly living donor transplant as a treatment option, as well as the option for preemptive transplantation, while increasing the risk of death on dialysis 4,31 and the risk of graft failure or patient death after transplant 11,28. While some literature shows that 6 months on dialysis may not be associated with poor outcomes 29, single studies have reported that the average time from first referral to placement on the waiting list is longer than this 7,16. We report that preemptively referred patients were more likely to be placed on the deceased donor waiting list or receive a living donor kidney transplantation, compared to patients referred after they started dialysis. Joshi et al’s single center study of 1,910 waitlisted patients reported the average time from first referral to their transplant center to waitlisting was 18.8 months, with African Americans having the longest time from referral to waitlist (16.0 months) compared to whites (12.0 months) 16. While our study reported the median time from referral to event (instead of the average) due to the distribution of follow-up days, we found a similar association to Joshi et al; time from referral to waitlisting or living donor transplantation was >1 year and African Americans had significantly longer time from referral to waitlisting as well as living donor transplant. Our findings may indicate that African American patients may take longer to complete the transplant evaluation to be placed on the waiting list, or may take longer to find a living kidney donor, compare to whites. Healthcare providers throughout the transplant process, including CKD clinic nephrologists and transplant center clinicians, should provide special assistance to patients that may need additional support to complete the transplant process.
Although living kidney donation has been shown to improve patient survival 32,33 and graft survival 34, African Americans are less likely to speak to their families about living kidney donation 35,36, and engage in living donation 36–38. Our study found that among African Americans preemptively referred for kidney transplant evaluation, they were 70% less likely to receive a living donor kidney transplant, compared to whites. This suggests that, in addition to early referral for transplant evaluation, additional interventions to promote living donor transplant may be needed for this population. African Americans may have less knowledge about transplantation 36, be less accepting of their health status 39, have lower medical trust 35,40, have more challenges identifying and approaching potential donors 41, and be more likely to have suboptimal discussions about living donor kidney transplantation within their social network and with health care providers 42 which may affect their pursuit of living donor transplant. Referring healthcare providers should recognize these significant differences in their patients and provide patient-centered educational information and interventions that are culturally tailored to their patients’ needs 43,44.
Our study interpretations are limited. Although our study is, to our knowledge, the largest regional study of preemptive kidney transplant referral to date, it is limited to Georgia and does not capture transplant evaluation referrals made to centers outside of Georgia or prior to 2005. While a strength of our study was the successful linkage of 90.3% (n=15,561) of transplant evaluation referrals to a Georgia transplant center to the USRDS data, we were unable to match 1,663, possibly due to a survival bias and we did not capture patients who were preemptively referred to a Georgia transplant center and died prior to the CMS-2728 form being completed. Although our referral data source was unable to provide descriptive characteristics on the patients that were not linked to USRDS, our future studies and data collection efforts (grant # U01MD010611) aim to collect more patient-level information (such as race, sex, and evaluation start date) from transplant centers to better describe the patient population not linked to USRDS. Our analysis on the association between race and preemptive referral was limited to logistic regression, as opposed to time-to-event analysis due to the lack of patient information (such as pre-ESRD care start date) prior to referral. Although the data represents eight years of transplant evaluation referral data, and many of the preemptive referrals may have been referred by CKD clinics, we do not have data on the patient populations within CKD clinics. Additionally, patient demographics and characteristics (such as insurance status and zip code) were collected at the time the CMS-2728 form was completed and may have changed. These constraints may limit the generalizability of the results; national data on transplant referral and preemptive referral data are needed to confirm our findings. Collecting transplant referral data from CKD clinics and dialysis facilities on a national level would allow complete tracking of patients with ESRD and address this limitation. Due to a small number of Hispanics and other races within our population, we limited our study cohort to African Americans and whites. Associations between patient characteristics and preemptive transplant referrals for other race/ethnicities and other populations should be made cautiously. Future research should focus on replicating the analysis using a more diverse population. Through linking our transplant referral data to USRDS data, all patients included in our analysis had a minimum of 2.5 years of potential follow-up since referral, providing sufficient power to measure short-term outcomes such as waitlisting and living donor transplantation. However, updated USRDS data are needed to determine the association of preemptive referral and longer-term outcomes such as deceased donor transplant and post-transplant outcomes. Variability in transplant center evaluation and contraindications may influence waitlisting and living donor kidney transplantation and is unaccounted for in our study. However, in a sensitivity analysis we found no significant difference in follow-up time for referral to waitlisting or living donor transplant between transplant centers. Future studies should expand on our analyses and examine how transplant center-level factors influence these outcomes.
Duration of dialysis is a strong modifiable risk factor for patient survival and post-transplant outcomes, and early referral for transplant evaluation is important. In Georgia, only about one in five patients referred for kidney transplantation evaluation were preemptively referred, and African Americans were less likely to be preemptively referred for transplantation evaluation, compared to whites. Among preemptively referred patients, African American race was not associated with decreased access to the waitlist, though African Americans were still less likely to receive a living donor transplant compared to whites. Racial disparity in kidney transplant rates may be partially explained by African Americans not being preemptively referred as often as whites. Interventions to ensure early transplant referral as well as targeted interventions to promote living donor transplantation may be warranted.
Supplementary Material
Acknowledgements
The study was supported by National Institute on Minority Health and Health Disparities grant R24MD008077 and the National Institute of Diabetes and Digestive and Kidney Diseases grant F32DK107191.
Abbreviations:
- CI
confidence interval
- CMS
Centers for Medicare and Medicaid Services
- CKD
chronic kidney disease
- ESRD
end stage renal disease
- HR
hazard ratio
- USRDS
United States Renal Data System
Footnotes
Disclaimer
The data reported here have been supplied by the United States Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the US government. The collection of transplant referral data from the 3 Georgia transplant centers reported here was performed on part under contract HHSM-500–2013-NW006C, funded by the Centers for Medicare & Medicaid Services, an agency of the U.S. Department of Health and Human Services. The content of this publication does not necessarily reflect the policies or positions of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government. The authors assume full responsibility for the accuracy and completeness of the ideas presented.
Dr. Pastan reported being a minority shareholder in Fresenius Dialysis (College Park, Georgia). The authors have no other disclosures to report.
References
- 1.Tonelli M, Wiebe N, Knoll G, et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 2011;11(10):2093–2109. [DOI] [PubMed] [Google Scholar]
- 2.Laupacis A, Keown P, Pus N, et al. A study of the quality of life and cost-utility of renal transplantation. Kidney international. 1996;50(1):235–242. [DOI] [PubMed] [Google Scholar]
- 3.(USRDS) USRDS. USRDS 2017. Annual Data Report. Ann Arbor, MI2017. [Google Scholar]
- 4.System USRD. USRDS 2016. Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States National Institute of Health. 2016;National Institute of Diabetes and Digestive and Kidney Diseases. [Google Scholar]
- 5.Patzer R, Plantinga L, Krisher J, Pastan S. Dialysis facility and network factors associated with low kidney transplantation rates among United States dialysis facilities. American Journal of Transplantation. 2014;14(7):1562–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patzer R, Pastan S. Kidney transplant access in the Southeast: view from the bottom. American Journal of Transplantation. 2014;14(7):1499–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patzer RE, Perryman JP, Schrager JD, et al. The role of race and poverty on steps to kidney transplantation in the southeastern United States. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(2):358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roake JA, Cahill AP, Gray CM, Gray DW, Morris PJ. Preemptive cadaveric renal transplantation-clinical outcome. Transplantation. 1996;62(10):1411–1416. [DOI] [PubMed] [Google Scholar]
- 9.Asderakis A, Augustine T, Dyer P, et al. Pre-emptive kidney transplantation: the attractive alternative. Nephrology Dialysis Transplantation. 1998;13(7):1799–1803. [DOI] [PubMed] [Google Scholar]
- 10.Friedewald JJ, Reese PP. The kidney-first initiative: what is the current status of preemptive transplantation? Advances in chronic kidney disease. 2012;19(4):252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meier-Kriesche H-U, Port FK, Ojo AO, et al. Effect of waiting time on renal transplant outcome. Kidney international. 2000;58(3):1311–1317. [DOI] [PubMed] [Google Scholar]
- 12.Abecassis M, Bartlett ST, Collins AJ, et al. Kidney transplantation as primary therapy for end-stage renal disease: a National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQI™) conference. Clinical Journal of the American Society of Nephrology. 2008;3(2):471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mange KC, Weir MR. Preemptive renal transplantation: why not? American Journal of Transplantation. 2003;3(11):1336–1340. [DOI] [PubMed] [Google Scholar]
- 14.Kasiske BL, London W, Ellison MD. Race and socioeconomic factors influencing early placement on the kidney transplant waiting list. J Am Soc Nephrol. 1998;9(11):2142–2147. [DOI] [PubMed] [Google Scholar]
- 15.Kutner NG, Zhang R, Huang Y, McClellan WM, Lea J, Soltow QA. Risk factors for frailty in a large prevalent cohort of hemodialysis patients. The American journal of the medical sciences. 2014;348(4):277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joshi S, Gaynor JJ, Bayers S, et al. Disparities among Blacks, Hispanics, and Whites in time from starting dialysis to kidney transplant waitlisting. Transplantation. 2013;95(2):309–318. [DOI] [PubMed] [Google Scholar]
- 17.Grams ME, Chen BP-H, Coresh J, Segev DL. Preemptive deceased donor kidney transplantation: considerations of equity and utility. Clinical Journal of the American Society of Nephrology. 2013:CJN. 05310512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasiske BL, Snyder JJ, Matas AJ, Ellison MD, Gill JS, Kausz AT. Preemptive kidney transplantation: the advantage and the advantaged. Journal of the American Society of Nephrology. 2002;13(5):1358–1364. [DOI] [PubMed] [Google Scholar]
- 19.Weng FL, Joffe MM, Feldman HI, Mange KC. Rates of completion of the medical evaluation for renal transplantation. American journal of kidney diseases. 2005;46(4):734–745. [DOI] [PubMed] [Google Scholar]
- 20.Myaskovsky L, Doebler DA, Posluszny DM, et al. Perceived discrimination predicts longer time to be accepted for kidney transplant. Transplantation. 2012;93(4):423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patzer RE, Gander J, Sauls L, et al. The RaDIANT community study protocol: community-based participatory research for reducing disparities in access to kidney transplantation. BMC nephrology. 2014;15(1):171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alexander GC, Sehgal AR. Barriers to cadaveric renal transplantation among blacks, women, and the poor. Jama. 1998;280(13):1148–1152. [DOI] [PubMed] [Google Scholar]
- 23.Patzer RE, Plantinga LC, Paul S, et al. Variation in Dialysis Facility Referral for Kidney Transplantation Among Patients With End-Stage Renal Disease in Georgia. JAMA. 2015;314(6):582–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berglund PA. An introduction to multiple imputation of complex sample data using SAS v9. 2. Paper presented at: SAS Global Forum 2010. [Google Scholar]
- 25.Noordzij M, Leffondré K, van Stralen KJ, Zoccali C, Dekker FW, Jager KJ. When do we need competing risks methods for survival analysis in nephrology? Nephrology Dialysis Transplantation. 2013;28(11):2670–2677. [DOI] [PubMed] [Google Scholar]
- 26.Kalantar-Zadeh K, Kovesdy CP, Streja E, et al. Transition of care from pre-dialysis prelude to renal replacement therapy: the blueprints of emerging research in advanced chronic kidney disease. Nephrology Dialysis Transplantation. 2017;32(suppl_2):ii91–ii98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rambod M, Bross R, Zitterkoph J, et al. Association of Malnutrition-Inflammation Score with quality of life and mortality in hemodialysis patients: a 5-year prospective cohort study. American Journal of Kidney Diseases. 2009;53(2):298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meier-Kriesche H-U, Kaplan B. Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: A Paired Donor Kidney Analysis1. Transplantation. 2002;74(10):1377–1381. [DOI] [PubMed] [Google Scholar]
- 29.Goldfarb-Rumyantzev A, Hurdle JF, Scandling J, et al. Duration of end-stage renal disease and kidney transplant outcome. Nephrology Dialysis Transplantation. 2005;20(1):167–175. [DOI] [PubMed] [Google Scholar]
- 30.Hays R, Waterman AD. Improving preemptive transplant education to increase living donation rates: reaching patients earlier in their disease adjustment process. Progress in Transplantation. 2008;18(4):251–256. [DOI] [PubMed] [Google Scholar]
- 31.Bloembergen WE, Port FK, Mauger EA, Wolfe RA. Causes of death in dialysis patients: racial and gender differences. Journal of the American Society of Nephrology. 1994;5(5):1231–1242. [DOI] [PubMed] [Google Scholar]
- 32.Medin C, Elinder CG, Hylander B, Blom B, Wilczek H. Survival of patients who have been on a waiting list for renal transplantation. Nephrology Dialysis Transplantation. 2000;15(5):701–704. [DOI] [PubMed] [Google Scholar]
- 33.Tarantino A Why should we implement living donation in renal transplantation? Clinical nephrology. 2000;53(4):55–63. [PubMed] [Google Scholar]
- 34.Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. New England Journal of Medicine. 2000;342(9):605–612. [DOI] [PubMed] [Google Scholar]
- 35.Minniefield WJ, Yang J, Muti P. Differences in attitudes toward organ donation among African Americans and whites in the United States. Journal of the National Medical Association. 2001;93(10):372. [PMC free article] [PubMed] [Google Scholar]
- 36.Morgan SE, Miller JK, Arasaratnam LA. Similarities and Differences Between African Americans’ and European Americans’ Attitudes, Knowledge, and Willingness to Communicate About Organ Donation1. Journal of Applied Social Psychology. 2003;33(4):693–715. [Google Scholar]
- 37.Siminoff LA, Sturm CMS. African-American reluctance to donate: beliefs and attitudes about organ donation and implications for policy. Kennedy Institute of Ethics Journal. 2000;10(1):59–74. [PubMed] [Google Scholar]
- 38.Boulware L, Ratner LE, Cooper LA, Sosa JA, LaVeist TA, Powe NR. Understanding disparities in donor behavior: race and gender differences in willingness to donate blood and cadaveric organs. Medical care. 2002. [DOI] [PubMed] [Google Scholar]
- 39.Lunsford SL, Simpson KS, Chavin KD, et al. Racial differences in coping with the need for kidney transplantation and willingness to ask for live organ donation. American Journal of Kidney Diseases. 2006;47(2):324–331. [DOI] [PubMed] [Google Scholar]
- 40.McDonald EL, Powell CL, Perryman JP, Thompson NJ, Jacob Arriola KR. Understanding the relationship between trust in health care and attitudes toward living donor transplant among African Americans with end-stage renal disease. Clinical transplantation. 2013;27(4):619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burroughs TE, Waterman AD, Hong BA. One organ donation, three perspectives: experiences of donors, recipients, and third parties with living kidney donation. Progress in Transplantation. 2003;13(2):142–150. [DOI] [PubMed] [Google Scholar]
- 42.Boulware L Ebony LAM, Fink Nancy E., Parekh Rulan S., Kao W.H. Linda, Klag Michael J., Powe Neil R.. Preferences, knowledge, communication and patient-physician discussion of living kidney transplantation in African American Families. American Journal of Transplantation. 2005;5:1503–1512. [DOI] [PubMed] [Google Scholar]
- 43.Arriola K, Robinson DH, Thompson NJ, Perryman JP. Project ACTS: an intervention to increase organ and tissue donation intentions among African Americans. Health Education & Behavior. 2010;37(2):264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boulware LE, Hill-Briggs F, Kraus ES, et al. Effectiveness of educational and social worker interventions to activate patients’ discussion and pursuit of preemptive living donor kidney transplantation: a randomized controlled trial. American Journal of Kidney Diseases. 2013;61(3):476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.