Abstract
OBJECTIVE:
To evaluate the association between gestational weight gain and maternal and neonatal outcomes in a large, geographically-diverse cohort.
METHODS:
Trained chart abstractors at 25 hospitals obtained maternal and neonatal data for all deliveries on randomly selected days over 3 years (2008–2011). Gestational weight gain was derived using weight at delivery minus pre-pregnancy or first-trimester weight and categorized as below, within or above the Institute of Medicine (IOM) guidelines in this retrospective cohort study. Maternal (primary or repeat cesarean birth, 3rd or 4th degree lacerations, severe postpartum hemorrhage, hypertensive disease of pregnancy) and neonatal (preterm birth, shoulder dystocia, macrosomia, hypoglycemia) outcomes were compared among women in the gestational weight gain categories in unadjusted and adjusted analyses with ORs and 95%CI reported. Covariates included age, race-ethnicity, tobacco use, insurance type, parity, prior cesarean birth, pregestational diabetes, hypertension, and hospital type.
RESULTS:
Of the 29,861 women included, 51% and 21% had gestational weight gain above and below the guidelines, respectively. There was an association between gestational weight gain above the IOM guidelines and cesarean birth in both nulliparous women (aOR 1.44, 95%CI 1.31–1.59) and multiparous women (aOR 1.26, 95%CI 1.13–1.41) and hypertensive diseases of pregnancy in nulliparous and multiparous women combined (aOR 1.84, 95%CI 1.66–2.04). For the neonatal outcomes, gestational weight gain above the IOM guidelines was associated with shoulder dystocia (aOR 1.74, 95%CI 1.41-.2.14), macrosomia (aOR 2.66, 95%CI 2.03–3.48), and neonatal hypoglycemia (aOR 1.60, 95%CI 1.16–2.22). Gestational weight gain below the guidelines was associated with spontaneous (aOR 1.50, 95%CI 1.31–1.73) and indicated (aOR 1.34, 95%CI 1.12–1.60) preterm birth.
CONCLUSIONS:
In a large, diverse cohort with prospectively collected data, gestational weight gain below or above guidelines is associated with a variety of adverse pregnancy outcomes.
PRECIS
Gestational weight gain below or above the Institute of Medicine guidelines is associated with adverse pregnancy outcomes in a large geographically diverse cohort
INTRODUCTION
The weight gain that occurs in pregnancy has the potential to influence a woman’s long-term health by increasing the risk for weight retention and obesity, as well as related co-morbidities such as chronic hypertension or type 2 diabetes mellitus. Gestational weight gain during pregnancy also is associated with offspring health, as the risk for childhood obesity increases when gestational weight gain is excessive. The 2009 Institute of Medicine (IOM) publication, “Weight Gain in Pregnancy, Re-examining the Guidelines”, established guidelines for gestational weight gain goals.(1) Nonetheless, according to a national study, only 32% of all women met these goals and 47% had gestational weight gain above these goals in 2010–2011.(2)
Several observational studies, which have evaluated the relationship between gestational weight gain and short-term maternal and neonatal outcomes such as gestational hypertension, cesarean birth, and macrosomia, have demonstrated positive associations between gestational weight gain above the guidelines and these outcomes.(3–8) The limitations of these individual studies include small sample sizes, single sites, restricted reporting of outcomes, and a lack of racial-ethnic diversity. Furthermore, gestational age at delivery, a key determinant in the interpretation of the adequacy of gestational weight gain, was not considered when the gestational weight gain values were analyzed.(8)
The objective of this study was to evaluate maternal and neonatal outcomes in relation to gestational weight gain from a large group of women with directly-abstracted data by trained personnel from 25 hospitals located in multiple geographic regions in the United States.
METHODS
This was a secondary analysis of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network’s Assessment of Perinatal Excellence study. The methodology has been previously published, but it is described briefly as follows.(9) Trained chart abstractors at 25 hospitals obtained data for patients delivered within the institution on randomly selected days over a 3-year period (2008–2011) who were ≥ 23 weeks gestation with a live fetus on admission. During the abstraction, explicit definitions for data fields and standardized data collection tools were used after pilot testing of data collection methods. Collected data included maternal demographics, medical and obstetrical history, and intrapartum and postpartum events. The period of data collection spanned from the time of hospital admission for delivery until discharge for women and up until discharge or until 120 days of age, whichever came first, for neonates.
In this secondary analysis, women were eligible if they had a height, either self-reported pre-pregnancy or first trimester weight (≤ 13 weeks), and a weight at delivery available from a singleton gestation. Those with either an extreme weight gain (>100 pounds) or loss (>50 pounds) were excluded as these values were likely biologically implausible, similar to the methods of Beyerlein et al.(10) Gestational weight gain was derived using a delivery weight minus a pre-pregnancy or first prenatal visit weight at ≤13 weeks. If both weight values were available, the pre-pregnancy weight was chosen for the analysis.
In order to account for gestational age at delivery and baseline body mass index (BMI), the observed weight gain was compared to the expected weight gain based on Institute of Medicine 2009 guidelines for weekly gestational weight gain. These values were categorized as below, within or above IOM guidelines (1–1.3lbs/week for BMI<18.5 kg/m2, 0.8–1.0lbs/week for BMI 18.5–24.9 kg/m2, 0.5–0.7lbs/week for BMI 25.0–29.9 kg/m2, and 0.4–0.6lbs/week for BMI ≥ 30kg/m2) for the second and third trimester (1) according to a BMI calculated from the pre-pregnancy or first prenatal visit weight at ≤ 13 weeks. This calculation assumed that women gained a range of 1.1–4.4 pounds in the first trimester. A 3rd or 4th degree laceration was reported only for women who had a vaginal delivery. Severe postpartum hemorrhage was defined as an estimated blood loss ≥ 1500 mL at delivery or in the immediate postpartum period, a blood transfusion, or hysterectomy for hemorrhage. Postpartum infection was defined as the occurrence of any of the following: endometritis, wound cellulitis requiring antibiotics, wound reopened for fluid collection or infection, or wound dehiscence during the delivery hospitalization. The outcome of hypertension included gestational hypertension, preeclampsia, HELLP (hemolysis elevated liver enzymes and low platelets syndrome), or eclampsia. Preterm birth, defined as a birth < 37 weeks, was considered for both spontaneous and indicated deliveries. Macrosomia was defined as a birth weight ≥ 4500g for term neonates or a large for gestational age neonate (i.e., > 90% percentile) for preterm neonates.
Maternal demographics and characteristics were compared between gestational weight gain below, within, and above the guidelines using Chi-square or Kruskal-Wallis tests as appropriate. Univariable and multivariable analyses were performed to determine the relationship between the gestational weight gain category (below, within, above) and maternal (cesarean birth, 3rd or 4th degree lacerations, severe postpartum hemorrhage, postpartum infections, hypertensive disease of pregnancy) and neonatal (preterm birth < 37 weeks, shoulder dystocia in vaginal deliveries, macrosomia, hypoglycemia requiring treatment) outcomes whereby “within” the guidelines was the reference category. Occurrences of gestational diabetes mellitus were reported, but not evaluated as outcomes due to the nutritional counseling and dietary adjustments that occur after such a diagnosis and the consequent effect on gestational weight gain. Unadjusted and adjusted odds ratios (OR) with 95% CI intervals were reported. Multivariable analyses were adjusted for maternal age, race-ethnicity, tobacco use, insurance type, parity, prior delivery route for multiparas (for the outcomes of 3rd or 4th degree lacerations and cesarean birth), chronic hypertension, pre-gestational diabetes, and hospital type (private for profit vs. public or non-profit). Statistical significance was set at p < 0.05. No imputation for missing data was performed. Data were analyzed using SAS software (SAS Institute, Cary, NC). Institutional review board approval was obtained at all centers under a waiver of informed consent for the initial study.
RESULTS
For the 29,861 women included in this study, 16,053 had a pre-pregnancy weight whereas 13,808 had only a first trimester weight.(Figure 1) Overall, 27.8% of women gained within the IOM guidelines whereas 21.2% and 51.0% gained below and above the guidelines, respectively. Of the women with gestational weight gain below the guidelines, 13.5% of them had a net weight loss. Non-Hispanic whites (8835/16220, 54.5%) had the highest proportion with gestational weight gain above the IOM guidelines. Between the three gestational weight gain categories, there were differences in age, race-ethnicity, parity, baseline BMI, prior cesarean birth, chronic hypertension, pre-gestational diabetes, tobacco use, and insurance type (p≤0.025 for all comparisons). (Table 1)
Figure 1.
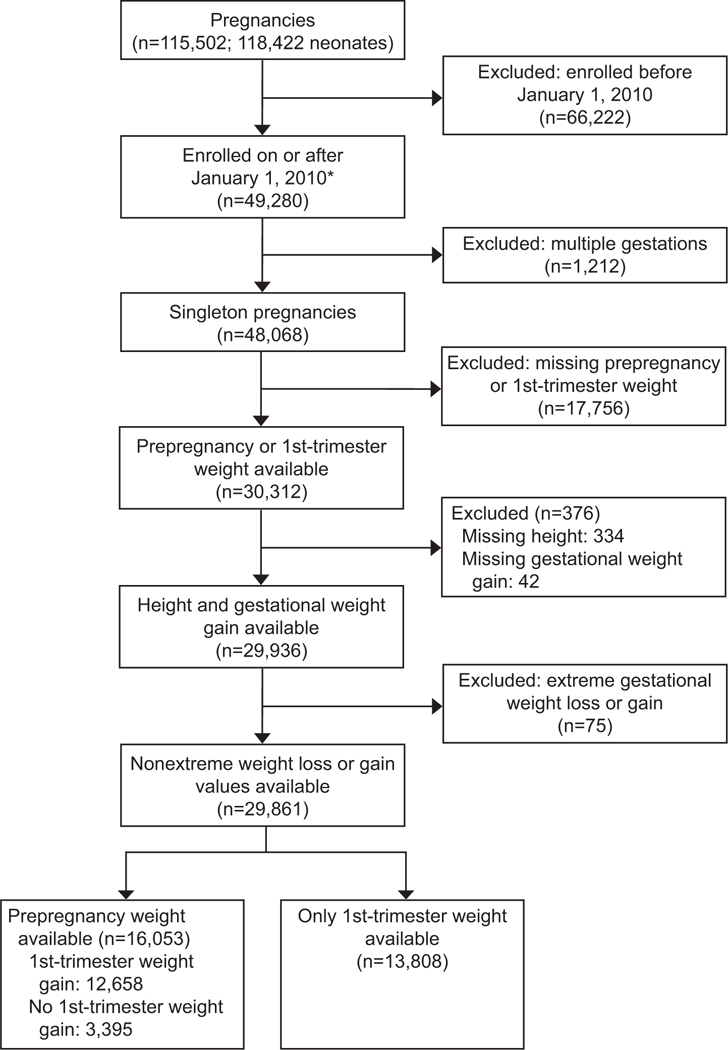
Flow diagram of participant selection. *The Assessment of Perinatal Excellence Study did not collect information on prepregnancy weight until 2010, approximately 2 years after the study began. Therefore, the cohort for this secondary analysis began in 2010 and is smaller compared to the original cohort.
Table 1:
Maternal demographics and characteristics.
| Characteristic | Gestational Weight Gain |
p-value | ||
|---|---|---|---|---|
| Below N=6,338 |
Within N=8,296 |
Above N=15,227 |
||
| Age (years) | 29.0 ± 6.1 | 29.4 ± 5.9 | 28.8 ± 5.8 | <0.001 |
| Race-Ethnicity | <0.001 | |||
| Non-Hispanic white | 2867 (45.2) | 4518 (54.5) | 8835 (58.0) | |
| Non-Hispanic black | 1213 (19.1) | 1085 (13.1) | 2490 (16.4) | |
| Non-Hispanic Asian | 408 (6.4) | 611 (7.4) | 737 (4.8) | |
| Hispanic | 1542 (24.3) | 1684 (20.3) | 2540 (16.7) | |
| Other or Unknown | 308 (4.9) | 398 (4.8) | 625 (4.1) | |
| Nulliparous | 2114 (33.4) | 3258 (39.3) | 7081 (46.5) | <0.001 |
| Pre-pregnancy Body mass | 27.3 ± 7.8 | 25.3 ± 6.0 | 26.4 ± 5.8 | <0.001 |
| index (kg/m2) | ||||
| Underweight < 18.5 kg/m2 | 422 (6.7) | 393 (4.7) | 185 (1.2) | <0.001 |
| Normal 18.5–24.9 kg/m2 | 2928 (46.2) | 4707 (56.7) | 6864 (45.1) | |
| Overweight 25.0–29.9 kg/m2 | 1129 (17.8) | 1685 (20.3) | 4898 (32.2) | |
| Obese ≥ 30 kg/m2 | 1859 (29.3) | 1511 (18.2) | 3280 (21.5) | |
| Prior cesarean birth | 1124 (17.7) | 1342 (16.2) | 2498 (16.4) | 0.025 |
| Chronic hypertension | 268 (4.2) | 219 (2.6) | 514 (3.4) | <0.001 |
| Pre-gestational diabetes | 99 (1.6) | 104 (1.3) | 274 (1.8) | 0.006 |
| Gestational diabetes | 578 (9.1) | 498 (6.0) | 753 (5.0) | <0.001 |
| Tobacco use | 625 (9.9) | 580 (7.0) | 1433 (9.4) | <0.001 |
| Insurance type | <0.001 | |||
| Private | 3218 (51.1) | 5161 (62.6) | 9412 (62.1) | |
| Government Assisted | 2533 (40.2) | 2429 (29.5) | 4825 (31.8) | |
| Uninsured | 544 (8.6) | 658 (8.0) | 929 (6.1) | |
| Gestational age at delivery (weeks) | 38.7 ± 2.4 | 39.0 ± 1.9 | 39.0 ± 2.0 | <0.001 |
Data are n (%) or mean ± standard deviation unless otherwise specified.
In unadjusted analysis, there was an association between gestational weight gain above the IOM guidelines and cesarean birth in both nulliparas (34.2% vs 27.1%) and multiparas (34.4% vs. 29.1%) compared with gestational weight gain within the guidelines whereas gestational weight gain below the IOM guidelines was associated with an increased postpartum hemorrhage (2.3% vs. 1.7%) and decreased perineal lacerations (2.8% vs. 4.0%). (Table 2) In multivariable analysis, gestational weight gain above the IOM guidelines remained associated with cesarean birth in nulliparous patients (aOR 1.44, 95%CI 1.31–1.59) and multiparous patients (aOR 1.26, 95%CI 1.13–1.41) compared with gestational weight gain within the guidelines. (Table 2) Gestational weight gain below the guidelines was no longer significantly associated with postpartum hemorrhage or perineal lacerations in the adjusted analyses. Gestational weight gain above the IOM guidelines was associated with hypertensive diseases of pregnancy in unadjusted (OR 2.00, 95%CI, 1.81–2.22) and adjusted models (aOR 1.84, 95%CI 1.66–2.04) in women of all parity.
Table 2:
Maternal Outcomes According to the Institute of Medicine Gestational Weight Gain Categories
| Outcome | Gestational Weight Gain | ||||||
|---|---|---|---|---|---|---|---|
| Below N=6,338 |
Within (referent) N=8,296 |
Above N=15,226 |
|||||
| N (%) | Unadjusted OR (95%CI) p-value |
Adjusted OR (95%CI) p-value |
N (%) | N (%) | Unadjusted OR (95%CI) p-value |
Adjusted OR (95%CI) p-value |
|
| Hypertensive disease of pregnancy* | 431 (6.8%) | 1.09 (0.95–1.24) p=0.22 |
1.02 (0.89–1.16) p=0.83 |
522 (6.3%) | 1806 (11.9%) |
2.00 (1.81–2.22) p<0.001 |
1.84 (1.66–2.04) p<0.001 |
| Cesarean birth for nulliparas† |
541 (25.6%) | 0.93 (0.82–1.05) p=0.22 |
0.90 (0.79–1.02) p=0.11 |
883 (27.1%) | 2422 (34.2%) |
1.40 (1.28–1.53) p<0.001 |
1.44 (1.31–1.59) p<0.001 |
| Cesarean birth for multiparas‡‡ | 1210 (28.7%) | 0.98 (0.90–1.07) p=0.65 |
0.94 (0.83–1.07) p=0.37 |
1465 (29.1%) | 2802 (34.4%) |
1.28 (1.19–1.38) p<0.001 |
1.26 (1.13–1.41) p<0.001 |
| Postpartum hemorrhage*§ | 141 (2.3%) |
1.38 (1.09–1.76) p=0.008 |
1.27 (1.00–1.62) p=0.05 |
134 (1.7%) | 280 (1.9%) | 1.14 (0.92–1.40) p=0.22 |
1.12 (0.91–1.38) p=0.29 |
| 3rd or 4th degree laceration in vaginal delivery only* | 128 (2.8%) |
0.70 (0.56–0.87) p=0.001 |
0.81 (0.65–1.01) p=0.06 |
236 (4.0%) | 410 (4.1%) | 1.04 (0.88–1.22) p=0.68 |
0.96 (0.81–1.14) p=0.63 |
| Postpartum infection * || | 38 (0.60%) | 1.28 (0.82–2.00) p=0.29 |
-- | 39 (0.47%) | 97 (0.64%) | 1.36 (0.94–1.97) p=0.11 |
-- |
The analysis was adjusted for maternal age, race-ethnicity, tobacco use, parity (or prior delivery type for the outcome of 3rd or 4th degree lacerations), insurance type, chronic hypertension, pre-gestational diabetes, and hospital type.
The analysis of cesarean birth for nulliparas was restricted to nulliparas only (N=2,114 below; N=3,258 within; N=7,081 above) and adjusted for maternal age, race-ethnicity, tobacco use, insurance type, chronic hypertension, pre-gestational diabetes, and hospital type.
The analysis of cesarean birth for multiparas was restricted to multiparas only (N=4,224 below; N=5,038 within; N=8,145 above) and adjusted for maternal age, race-ethnicity, tobacco use, prior delivery type, insurance type, chronic hypertension, pre-gestational diabetes, and hospital type.
The denominator for postpartum hemorrhage varies from the total sample size due to missing data for blood loss volume (N=6,190 below; N=8,090 within; N=14,895 above).
The denominator for postpartum infection varies from the total sample size due to missing data for infection (N=4,584 below; N=5,947 within; N=10,000 above). Due to the low frequency of this outcome, adjusted ORs were not reported.
The bolded values indicate statistical significance.
OR odds ratio
CI Confidence interval
For the neonatal outcomes, gestational weight gain above the IOM guidelines was associated with shoulder dystocia in vaginal deliveries (3.6% vs. 2.1%), macrosomia (2.08% vs. 0.81%), and neonatal hypoglycemia (0.98% vs. 0.61%) compared with gestational weight gain within the IOM guidelines. Gestational weight gain below and above the guidelines was associated with preterm births overall and both spontaneous and indicated preterm births in unadjusted analysis.(Table 3) In adjusted analysis, gestational weight gain above the IOM guidelines remained associated with shoulder dystocia (aOR 1.74, 95%CI 1.41–2.14), macrosomia (aOR 2.66, 95%CI 2.03–3.48), and neonatal hypoglycemia (aOR 1.60, 95%CI 1.16–2.22). Gestational weight gain outside of the guidelines also remained associated with preterm birth. Gestational weight gain below the guidelines was associated with all preterm birth (aOR 1.47, 95%CI 1.31–1.64), spontaneous preterm birth (aOR 1.50, 95%CI 1.31–1.73), and indicated preterm birth (aOR 1.34, 95%CI 1.12–1.60), whereas above the guidelines was associated only with indicated preterm birth (aOR 1.24, 95%CI 1.07–1.45).
Table 3:
Neonatal Outcomes According to the Institute of Medicine Gestational Weight Gain Categories
| Outcome | Gestational Weight Gain | ||||||
|---|---|---|---|---|---|---|---|
| Below N=6,338 |
Within (referent) N=8,296 |
Above N=15,227 |
|||||
| N (%) | Unadjusted OR (95%CI) p-value |
Adjusted OR (95%CI) p-value |
N (%) | N (%) | Unadjusted OR (95%CI) p-value |
Adjusted OR (95%CI) p-value |
|
| Preterm birth* | 751 (11.9%) |
1.59 (1.42–1.77) p<0.001 |
1.47 (1.31–1.64) p<0.001 |
648 (7.8%) | 1324 (8.7%) |
1.12 (1.02–1.24) p=0.02 |
1.05 (0.95–1.16) p=0.32 |
| Spontaneous preterm birth* | 471 (7.4%) |
1.59 (1.39–1.82) p<0.001 |
1.50 (1.31–1.73) p<0.001 |
399 (4.8%) | 712 (4.7%) | 0.97 (0.86–1.10) p=0.64 |
0.93 (0.82–1.06) p=0.27 |
| Indicated preterm birth* | 280 (4.4%) |
1.49 (1.26–1.78) p<0.001 |
1.34 (1.12–1.60) p=0.002 |
249 (3.0%) | 612 (4.0%) |
1.35 (1.17–1.57) p<0.001 |
1.24 (1.07–1.45) p=0.006 |
| Shoulder dystocia in vaginal delivery only *† |
85 (1.9%) | 0.87 (0.66– 1.15) p=0.34 |
0.86 (0.65–1.13) p=0.28 |
126 (2.1%) | 357 (3.6%) |
1.71 (1.39–2.10) p<0.001 |
1.74 (1.41–2.14) p<0.001 |
| Macrosomia * ‡‡ | 35 (0.55%) | 0.68 (0.45–1.03) p=0.07 |
-- | 67 (0.81%) | 317 (2.08%) |
2.61 (2.00–3.40) p<0.001 |
2.66 (2.03–3.48) p<0.001 |
| Treatment for neonatal hypoglycemia * ‡‡ | 45 (0.71%) | 1.16 (0.77–1.73) p=0.48 |
-- | 51 (0.61%) | 149 (0.98%) |
1.60 (1.16–2.20) p=0.004 |
1.60 (1.16–2.22) p=0.005 |
The analysis was adjusted for maternal age, race-ethnicity, tobacco use, parity, insurance type, chronic hypertension, pre-gestational diabetes, and hospital type.
The denominator for the shoulder dystocia outcome varies from the total sample size because it was limited to women with vaginal deliveries (N=4,587 below; N=5,948 within; N=10,002 above).
Due to the low frequency of macrosomia and neonatal hypoglycemia and gestational weight gain below the guidelines, the adjusted ORs were not reported.
The bolded values indicate statistical significance.
OR odds ratio
CI Confidence interval
DISCUSSION
In this study, we found associations between not achieving gestational weight gain goals and several adverse maternal and neonatal outcomes including cesarean birth, hypertensive disease of pregnancy, preterm birth, shoulder dystocia, macrosomia, and neonatal hypoglycemia.
Our findings support those of other studies including a recent systematic review and meta-analysis of gestational weight gain and maternal and neonatal outcomes that included 1,309,136 women.(11) Specifically, the investigators of that study found that gestational weight gain below the guidelines was associated with small for gestational age neonates (OR 1.53, 95%CI 1.44–1.64) and preterm birth (OR 1.70, 95%CI 1.32–2.20) whereas gestational weight gain above the guidelines was associated with large for gestational age neonates (OR 1.85, 95%CI 1.76–1.95), macrosomia (OR 1.95, 95%CI 1.79–2.11), and cesarean birth (OR 1.30, 95%CI 1.25–1.35). Of note, however, is that gestational age at delivery was not considered in some of the individual studies and also in the meta-analysis itself, potentially explaining the relationship between gestational weight gain and outcomes such as preterm birth (i.e., women who deliver earlier have less time to gain weight).(11) A similar study from a large multicenter obstetrical database evaluated gestational weight gain among 20,950 women with obesity only.(12) In that analysis, high gestational weight gain was associated with cesarean birth in nulliparas and multiparas, large for gestational age neonates, and macrosomia, but not with operative vaginal delivery, postpartum hemorrhage, or shoulder dystocia compared with women who met gestational weight gain goals.
In devising the final guidelines for gestational weight gain, the IOM omitted outcomes such as gestational hypertension or preeclampsia due to the lack of evidence to support that gestational weight gain was associated with these conditions.(1) Given the known vascular permeability and decreased plasma oncotic pressure that accompanies preeclampsia and its association with rapid weight gain (13), it is challenging to determine whether excessive gestational weight gain is a cause or effect of preeclampsia. Among the maternal outcomes, gestational weight gain above the guidelines had a nearly two-fold increase in odds of having gestational hypertension or preeclampsia in our study. In our data, serial weight gain measurements were not available to assess gestational weight gain prior to the diagnosis of preeclampsia to sort out the effects of weight gain not related to hypertension. Of note, in a study of 702 women with chronic hypertension, the timing of gestational weight gain and diagnosis of 173 women with superimposed preeclampsia was evaluated. Of the 100 women who had gestational weight gain above the IOM guidelines for the entire pregnancy, 92% had gestational weight gain above the guidelines in the 7–14 days prior to the diagnosis of superimposed preeclampsia whereas only 8% had gestational weight gain within the guidelines prior to the diagnosis.(14) These findings suggest that the relationship between gestational weight gain and hypertension may be a consequence of weight gain that occurs before, instead of after the onset of hypertensive disease of pregnancy, but the relationship is complex and further study is indicated.
We acknowledge several limitations to this study. We used either a self-reported pre-pregnancy weight or a measured weight at the first prenatal visit in the determination of gestational weight gain. These values have limitations, though they typically correlate well with each other and no other options are available unless weight data are collected prospectively prior to pregnancy.(15, 16) Therefore, we opted to combine the two variables for initial weight into one analysis. Data for this study were collected during 2010–2011, which encompasses a time when two different publications of gestational weight gain guidelines (1990 and 2009) may have influenced provider counseling practices. Given that the primary change in the guidelines was related to ranges for women with obesity, and the time it typically takes for providers to incorporate new guidelines into clinical practice, we do not suspect this alteration significantly influenced our findings. Noted strengths include analysis of 29,861 women representative of the United States with rigorous ascertainment of outcomes and calculation of gestational weight gain to account for the wide range of gestational ages at delivery.
Gestational weight gain is a potentially modifiable risk factor for a number of adverse maternal and neonatal outcomes.(17) Capitalizing on a window of opportunity during pregnancy has been challenging with respect to intensive lifestyle interventions that target diet, exercise and other health behaviors, though the effectiveness and safety of these interventions has been demonstrated with a 20% reduction in excessive gestational weight gain in a meta-analysis.(17) Given the risks of a gestational weight gain outside of the guidelines, as documented in this large geographically diverse cohort whereby only 27.8% of women had a gestational weight gain within the guidelines, further study is indicated on how to assist women in meeting their gestational weight gain goals.
Supplementary Material
ACKNOWLEDGMENTS:
The authors thank Cynthia Milluzzi, RN and Joan Moss, RNC, MSN for protocol development and coordination between clinical research centers; and William A. Grobman, MD, MBA, Elizabeth Thom, PhD, Madeline M. Rice, PhD, Brian M. Mercer, MD, and Catherine Y. Spong, MD for protocol development and oversight.
The project described was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) [HD21410, HD27869, HD27915, HD27917, HD34116, HD34208, HD36801, HD40500, HD40512, HD40544, HD40545, HD40560, HD40485, HD53097, HD53118] and the National Center for Research Resources [UL1 RR024989; 5UL1 RR025764]. This study was also supported by NICHD K23HD076010 (Dr. Kominiarek). Comments and views of the authors do not necessarily represent views of the National Institutes of Health
Footnotes
See Appendix 1, available online at http://links.lww.com/xxx, for a list of other members of the NICHD MFMU Network.
Financial Disclosure
The authors did not report any potential conflicts of interest.
Each author has indicated that he or she has met the journal’s requirements for authorship.
REFERENCES
- 1.Institute of Medicine. Weight gain during pregnancy: reexamining the guidelines. Washington, DC; 2009. [PubMed] [Google Scholar]
- 2.Deputy NP, Sharma AJ, Kim SY, Hinkle SN. Prevalence and characteristics associated with gestational weight gain adequacy. Obstet Gynecol 2015;125(4):773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durst JK, Sutton AL, Cliver SP, Tita AT, Biggio JR. I mpact of Gestational Weight Gain on Perinatal Outcomes in Obese Women. Am J Perinatol 2016;33(9):849–55. [DOI] [PubMed] [Google Scholar]
- 4.Badon SE, Dyer AR, Josefson JL, Group HSCR. Gestational weight gain and neonatal adiposity in the hyperglycemia and adverse pregnancy outcome study-north american region. Obesity (Silver Spring) 2014. July;22(7):1731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li N, Liu E, Guo J, Pan L, Li B, Wang P, et al. Maternal prepregnancy body mass index and gestational weight gain on pregnancy outcomes. PLoS One 2013;8(12):e82310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simas TA, Waring ME, Liao X, Garrison A, Sullivan GM, Howard AE, et al. Prepregnancy weight, gestational weight gain, and risk of growth affected neonates. J Womens Health 2012;21(4):410–7. [DOI] [PubMed] [Google Scholar]
- 7.Vesco KK, Sharma AJ, Dietz PM, Rizzo JH, Callaghan WM, England L, et al. Newborn size among obese women with weight gain outside the 2009 Institute of Medicine recommendation. Obstet Gynecol 2011;117(4):812–8. [DOI] [PubMed] [Google Scholar]
- 8.Blomberg M Maternal and neonatal outcomes among obese women with weight gain below the new Institute of Medicine recommendations. Obstet Gynecol 2011;117(5):1065–70. [DOI] [PubMed] [Google Scholar]
- 9.Bailit JL, Grobman WA, Rice MM, Spong CY, Wapner RJ, Varner MW, et al. Risk-adjusted models for adverse obstetric outcomes and variation in risk-adjusted outcomes across hospitals. Am J Obstet Gynecol 2013;209(5):446 e1–e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beyerlein A, Schiessl B, Lack N, von Kries R. Optimal gestational weight gain ranges for the avoidance of adverse birth weight outcomes: a novel approach. Am J Clin Nutr 2009;90(6):1552–8. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, et al. Association of gestational weight gain with maternal and infant outcomes: A systematic review and meta-analysis. JAMA 2017;317(21):2207–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kominiarek MA, Seligman NS, Dolin C, Gao W, Berghella V, Hoffman M, et al. Gestational weight gain and obesity: is 20 pounds too much? Am J Obstet Gynecol 2013;209(3):214 e1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Obstet Gynecol 2013;122(5):1122–31. [DOI] [PubMed] [Google Scholar]
- 14.Siegel AM, Tita AT, Machemehl H, Biggio JR, Harper LM. Evaluation of institute of medicine guidelines for gestational weight gain in women with chronic hypertension. AJP Rep 2017;7(3):e145–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunner Huber LR. Validity of self-reported height and weight in women of reproductive age. Matern Child Health J 2007;11(2):137–44. [DOI] [PubMed] [Google Scholar]
- 16.Johnson J, Clifton RG, Roberts JM, Myatt L, Hauth JC, Spong CY, et al. Pregnancy outcomes with weight gain above or below the 2009 Institute of Medicine guidelines. Obstet Gynecol 2013;121(5):969–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muktabhant B, Lawrie TA, Lumbiganon P, Laopaiboon M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst Rev 2015;6:CD007145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


