Abstract
Rationale & Objective
Inflammation, cardiac remodeling, and fibrosis may explain, in part, the excess risk of cardiovascular disease (CVD) in patients with chronic kidney disease (CKD). Growth differentiation factor 15 (GDF-15), galectin 3 (Gal-3), and soluble ST2 (sST2) are possible biomarkers of these pathways in patients with CKD.
Study Design
Nonconcurrent observational cohort study
Setting and Participants
Individuals with CKD enrolled in either of two multi-center CKD cohort studies; the Seattle Kidney Study or the C-PROBE (Clinical Phenotyping and Resource Biobank Study).
Exposures
Circulating GDF-15, Gal-3, and sST-2 measured at baseline.
Outcomes
Primary outcome was all-cause mortality. Secondary outcomes included hospitalization for physician-adjudicated heart failure (HF) and the atherosclerotic CVD events of myocardial infarction and cerebrovascular accident.
Analytic Approach
Cox proportional hazards models used to test association of each biomarker with each outcome, adjusting for demographics, CVD risk factors, and renal function.
Results
Among 883 participants, the mean eGFR was 49 +/− 19 mL/min/1.73 m2. Higher GDF-15 (aHR per 1-SD higher, 1.87; 95% CI, 1.53–2.29), gal-3 (aHR per 1-SD higher, 1.51; 95% CI, 1.36–1.78), and sST-2 (aHR per 1-SD higher, 1.36; 95% CI, 1.17–1.58) concentrations were significantly associated with mortality. Only GDF-15 was also associated with HF events (HR per 1-SD higher, 1.56; 95% CI, 1.12–2.16). There were no detectable associations between GDF-15, gal-3, or sST-2 and atherosclerotic CVD events.
Limitations
Event rates for HF and atherosclerotic CVD were low.
Conclusions
Adults with CKD and higher circulating levels of GDF-15, gal-3, and sST-2 experienced greater mortality. Elevated GDF-15 was also associated with an increased rate of HF. Further work is needed to elucidate the mechanisms linking these circulating biomarkers with CVD in patients with CKD.
Keywords: growth differentiation factor 15 (GDF-15), galectin 3 (Gal-3), soluble ST2 (sST2), mortality, cardiovascular disease (CVD), CKD, cardiac biomarker, heart failure (HF), acute myocardial infarction (AMI), acute ischemic stroke, atherosclerotic CVD
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of mortality in patients with chronic kidney disease (CKD) and accounts for significant morbidity, including recurrent hospitalizations, debilitating symptoms, and low reported health-related quality of life.1–4 Substantial cardiovascular risk persists in CKD patients despite treatment of established cardiovascular risk factors, such as hypertension and dyslipidemia. Identifying new pathways of CVD is an important step toward developing potentially effective therapies.
Inflammation, cardiac remodeling, and fibrosis are potentially important pathways in the pathogenesis of cardiovascular disease. While there are numerous candidate biomarkers that may reflect alterations in these biological pathways, growth differentiation factor 15 (GDF-15), galectin 3 (Gal-3), and soluble ST2 (sST2) have emerged as some of the strongest predictors of CVD in the general population and two (galectin-3 and sST2) of these biomarkers have been approved by the FDA for clinical use.5
GDF-15 is a member of the transforming growth factor β (TGFβ) cytokine family that is widely distributed in mammalian tissues (including the prostate, intestinal mucosa, and kidney) and has been shown to play multiple roles in various pathologies, including cardiovascular disease inflammation, cancer, obesity and kidney disease.6, 7 One study found that GDF-15 plays a role in cardiomyocyte repair in response to tissue inflammation, oxidative stress and injury and is anti-apoptotic and anti-hypertrophic.8 However, in the general population and in patients with heart disease, higher concentrations of GDF-15 have been associated with all-cause mortality and increased cardiovascular events independent of traditional biomarkers and other CVD risk factors.9–21 Thus the role of GDF-15 remains controversial.
Gal-3 belongs to the β-galactoside-binding protein family. It is a ubiquitous protein expressed in epithelial cells, endothelial cells, and macrophages. It plays a role in embryonic development, and is both proinflammatory and profibrotic. It has been shown to be involved in initiating cardiac fibrosis and ventricular remodeling.22, 23 Among European populations, higher concentrations of gal-3 have been associated with increased all-cause and cardiovascular mortality.24–26 ST-2 is a member of the interleukin 1 (IL-1) receptor family. It has two forms, soluble ST2 (sST-2) and transmembrane ST-2 (ST-2L). ST-2 is a marker of cardiac stress that is upregulated with myocyte stretch similar to brain natriuretic peptide (BNP). In the large Framingham Offspring Study, elevated sST-2 was significantly associated with increased risk of death, heart failure, and major cardiovascular events.9
Circulating GDF-15, gal-3, and sST-2 concentrations are higher among people who have CKD and are associated with CVD in the general population, raising the question of whether these biomarkers are associated with mortality and CVD in CKD. Few studies have evaluated the association of GDF-15 or sST-2 with moratlity and CVD in an exclusive CKD population. Prior studies of gal-3 in CKD cohorts have had conflicting results and may not be generalizable to a U.S. CKD population.26, 27 Herein, we evaluated all three novel cardiac biomarkers for associations with mortality, heart failure, and atherosclerotic cardiovascular events in two large prospective cohort studies of CKD in the United States.
METHODS
Study Populations
The Seattle Kidney Study (SKS)
The SKS is a nephrology clinic-based cohort study of people with CKD, which began recruitment in 2004. Participants were recruited from outpatient nephrology clinics at the University of Washington Medical Center, Harborview Medical Center, and the Veterans Affairs Puget Sound Health Care Center in Seattle, Washington. Inclusion criteria are age >18 years and any stage of CKD (eGFR <90ml/min/1.73m2 or a urinary albumin-creatinine ratio of >30 mg/g). Exclusion criteria are current dialysis, current or previous kidney transplant, inability to provide informed consent, or expectation of dialysis initiation within 3 months. SKS personnel conducted annual in-person study visits, including blood collections and ascertainment of interim events. A total of 530 participants met inclusion criteria and were enrolled in the SKS. For this study, we also excluded participants who did not have follow up beyond the baseline study visit or who did not have available bio-samples at the time of study initiation date, leaving a final analytic sample of n= 304.
The Clinical Phenotyping and Resource Biobank Study (C-PROBE)
C-PROBE, the CKD cohort of the George M. O’Brien Michigan Kidney Translational Core Center at the University of Michigan, is an ongoing multicenter prospective observational cohort study that began recruitment in March 2009. Adults and children with CKD stage 1–4 are recruited from six sites in the United States, including: John H. Stroger Hospital, Chicago; Renaissance Renal Research Institute, Detroit; University of Michigan Nephrology Program, Ann Arbor; Wayne State University Nephrology Program, Detroit; Temple University Nephrology Program, Philadelphia; and Carolinas Medical Center, Levin Children’s Hospital, Charlotte. Patient demographics, socioeconomic variables, clinical information, and bio-samples are collected at enrollment and annually thereafter. Adult C-PROBE participants with polycystic kidney disease and those who underwent dialysis or transplantation are excluded. Inclusion criteria for this study were age >18 years and baseline eGFR <90ml/min/1.73m2. Participants also needed to have baseline bio-samples available including serum or plasma samples at study entry to be included in our study. We also excluded participants who did not have follow-up beyond the baseline study visit, leaving a final sample of n= 579 for analyses which included gal-3 and sST2. Of the 579 C-PROBE participants, 314 also had GDF-15 measured as part of a second ancillary study.
Our study did not require additional IRB approval at either site since no new additional data or biospeciman collection was required.
Biomarker (GDF-15, Gal-3, sST-2) Measurements in SKS and C-PROBE
Three cardiac biomarkers of interest were evaluated in both SKS and C-PROBE: GDF-15, gal-3, and sST-2. Blood samples from SKS and C-PROBE participants were stored at −80°C until shipment to the University of Washington. All samples were first thaw serum/plasma concentrations measured by ELISA (R&D Systems for GDF-15 and gal-3 [Minneapolis, MN] and Critical Diagnostics for sST-2 [San Diego, CA]). The absorbances for each analyte were measured by spectrophotometry (450 nm) and unknown concentrations determined through a 4-parameter logistic curve fit. The inter-assay coefficients of variation were 3.58% for GDF-15, 9.80% for galectin-3, and 9.58% for sST-2. We performed duplicate measures of each biomarker in 44 participants in our study which showed good repeatability (intra-assay Pearson’s correlation coefficients: 0.8381 for GDF-15, 0.9407 for gal-3, and 0.9588 for sST-2).
Outcomes
The primary outcomes was all-cause mortality. Deaths were identified from report by next of kin, retrieval of death certificates or obituaries, review of hospital records, and the Social Security Death Master File.
Secondary outcomes included heart failure events, and atherosclerotic CVD events (defined as acute myocardial infarction or acute ischemic stroke). In the SKS and the C-PROBE, hospitalized events and procedures were ascertained via telephone contacts and at in-person study examinations every 12 months. Identified hospitalized events and procedures prompted the collection of relevant medical records, which were reviewed by two physician investigators. The physicians had to agree and resolve any discordances. Adjudication of heart failure events was based on the Framingham and ALLHAT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial) criteria.28, 29 Diagnosis of probable or definite myocardial infarction was based on symptoms consistent with acute ischemia, cardiac biomarker levels, and electrocardiograms as recommended by a consensus statement on the universal definition of myocardial infarction.30 Ischemic stroke outcomes included both probable and definite ischemic stroke. The latter was determined based on sudden onset of neurologic symptoms supported with computed tomography or magnetic resonance imaging demonstration of infarction in a territory where an injury or infarction would be expected to create those symptoms. The former was defined as sudden or rapid onset of 1 major or 2 minor neurologic signs or symptoms lasting for more than 24 hours or until the patient died with no evidence of hemorrhage or infarction on computed tomography or magnetic resonance imaging performed within 24 hours of the onset of symptoms.31
Covariates
The SKS determined demographic data, prevalent medical conditions, height, and weight via in-person questionnaires and examinations. Current medications were assessed using medication inventories. Blood and urine measurements of standard chemistries were performed at in-person study examinations.
CPROBE determined demographic data and prevalent medical conditions by physician diagnosis in the medical record. Medications were ascertained from the patients’ medical records.
Diabetes in both cohorts was defined by use of an oral hypoglycemic medication or insulin, fasting blood sugar ≥126 mg/dl, non-fasting blood sugar ≥200 mg/dl, or hemoglobin A1c ≥6.5%. Urine albumin and creatinine were measured in spot morning or overnight urine collections, by a timed endpoint method and with the modified rate Jaffe method.
Statistical Analysis
We performed a pooled analysis of the SKS and the C-PROBE cohorts. We tabulated baseline participant characteristics for each cohort and overall. We compared baseline characteristics of the study population across quartiles of each biomarker. A multivariable linear regression analysis was performed to test the associations of these baseline characteristics with GDF-15, gal-3, and sST2 concentrations. We used unadjusted Spearman correlations and scatterplots to determine correlations among the cardiac biomarkers, eGFR, and urine albumin-creatinine ratio (UACR). Kaplan-Meier curves were generated to examine the probabilities of the primary and secondary outcomes in participants above and below the median level of GDF-15, gal-3, and sST2. Unadjusted incidence rates for each outcome across quartiles of each biomarker were calculated as the number of events divided by person-years at risk. We assessed the functional forms of associations of GDF-15, gal-3, and sST-2 levels with each outcome (mortality, heart failure, and atherosclerotic CVD) using cubic spline models. There was no evidence of departure from linearity; therefore, we assessed these markers as continuous linear exposures. Cox proportional hazards regression were used to test the association of each biomarker level (modeled continuously per standard deviation increase) at baseline and time until first occurrence of mortality, heart failure, or atherosclerotic CVD. Covariates for adjustment were selected a priori based on previous literature regarding potential confounders as well as examining the covariates that differed greatly across quartiles of each biomarker. Nested models were used to progressively explore the confounding effects of demographics, known cardiovascular risk factors, and kidney function. The first model adjusted for baseline age, gender, race, and study cohort. The second model additionally adjusted for prevalent cardiovascular disease, current smoking status, diabetes, eGFR and urine albumin-creatinine ratio. Participants were censored at first event, death, or end of study period (July 31, 2013 for SKS and July 29, 2016 for C-PROBE). Two-sided probability values ≤ 0.05 were considered statistically significant. All analyses were performed using Stata version 13.1 (College Station, TX) and IBM SPSS Statistics for Windows version 23.0 (Armonk, NY).
RESULTS
Characteristics of Study Populations
The pooled study population included 883 participants with gal-3 and sST2 measures and 618 participants with GDF-15 measures. Baseline characteristics of the study population are listed in Table 1. Participants were a mean age of 57 years old, predominantly white and male. Overall, 87% of participants had prevalent hypertension, 43% had prevalent diabetes, and 40% had prevalent CVD. The mean eGFR was 49 mL/min/1.73 m2 (CKD stage 3a) and median UACR was 160 mg/g. Compared to SKS, participants in C-PROBE were younger, more racially diverse, and had lower prevalence of comorbid hypertension, diabetes, and CVD. C-PROBE participants also had higher urine albumin excretion and eGFR compared to SKS participants.
Table 1.
Baseline characteristics of the study population
| SKS | C-PROBE | Pooled | |
|---|---|---|---|
| No. of participants | 304 | 579 | 883 |
| Age, years | 62 (13) | 55 (16) | 57 (15) |
| Male | 250 (82) | 240 (42) | 490 (56) |
| Race | |||
| White | 198 (65) | 289 (50) | 487 (55) |
| Black | 76 (25) | 227 (39) | 303 (34) |
| Other | 30 (10) | 62 (11) | 92 (10) |
| Education | |||
| Less than High school | 11 (4) | 79 (14) | 90 (11) |
| Completed high school | 179 (64) | 292 (51) | 471 (55) |
| Completed college or more | 90 (32) | 203 (35) | 293 (34) |
| Current smoker | 49 (17) | 67 (12) | 116 (13) |
| Cardiovascular Disease | 177 (58) | 178 (31) | 355 (40) |
| Heart Failure | 86 (28) | 44 (8) | 130 (15) |
| Myocardial Infarction | 62 (20) | 80 (14) | 142 (16) |
| Ischemic Stroke | 46 (15) | 49(8) | 95 (11) |
| Cardiac Arrhythmia | 26 (9) | 71 (12) | |
| Diabetes | 171 (56) | 206 (35) | 377 (43) |
| Hypertension | 295 (97) | 469 (81) | 764 (87) |
| Antihypertensive use | 283 (93) | 440 (76) | 723 (82) |
| Statin use | 191 (63) | 182 (32) | 373 (42) |
| Systolic Blood Pressure, mmHg | 133 (20) | 132 (20) | 132 (20) |
| Diastolic Blood Pressure, mmHg | 75 (13) | 74 (11) | 74 (12) |
| Body Mass Index, kg/m2 | 31.4 (7.3) | 31.8 (8.1) | 31.7 (7.8) |
| eGFR, mL/min/1.73 m2 | 40 (19) | 55 (31) | 49 (28) |
| UACR, mg/g | 136 [16, 757] | 234 [16, 885] | 160 [16, 776] |
Data are displayed as mean +/− SD or median [Q1, Q3] for continuous variables and N(%) for categorical variables unless noted otherwise.
UACR, urinary albumin-creatinine ratio; eGFR, estimated glomerular filtration rate; SKS, ______; C-PROBE, ________; Q, quartile.
History of cardiovascular disease and diabetes was greater across quartiles of each biomarker (Table S1). Furthermore, eGFR was lower and UACR was higher across higher quartiles of GDF-15, gal-3 and sST-2.
Associations of GDF-15, Gal-3, and sST-2 with Baseline Characteristics and Kidney Function
In multivariable analysis, female sex, older age, current smoking, and prevalent diabetes were each independently associated with higher levels of GDF-15. Black race was inversely associated with GDF-15 concentration. Male sex and antihypertensive medication use were associated with higher levels of gal-3, and prevalent diabetes and CVD were associated with higher levels of sST-2 (Table S2).
Gal-3 and sST-2 were modestly correlated with GDF-15. Gal-3 and sST-2 were weakly correlated with each other. GDF-15, gal-3, and to a lesser extent sST-2 were inversely correlated with eGFR and modestly correlated with ACR. (Table 2 and Figure S1).
Table 2.
Correlations of GDF-15, Gal-3, sST2 with eGFR and UACR
| Scr | eGFR | UACR | GDF-15 | Gal-3 | sST2 | |
|---|---|---|---|---|---|---|
| Scr | 1.00 | −0.943** | 0.153** | 0.555** | 0.384** | 0.165** |
| eGFR | 1.00 | −0.137** | −0.587** | −0.443** | −0.113** | |
| UACR | 1.00 | 0.246** | 0.187** | 0.207** | ||
| GDF-15 | 1.00 | 0.381** | 0.331* | |||
| Gal-3 | 1.00 | 0.155** | ||||
| sST2 | 1.00 |
correlation significant at the 0.05 level
correlation significant at the 0.01 level
UACR, urinary albumin-creatinine ratio; eGFR, estimated glomerular filtration rate; Gal-3, ____; GDF-15, _____; sST2, _____; Scr, serum creatinine.
Overall Incidence of Mortality, Heart Failure, and Atherosclerotic Cardiovascular Disease
In our pooled cohort, there were 98 total deaths (mortality rate of 3.3% per year), 41 heart failure events (incidence rate of 1.42% per year), and 29 atherosclerotic cardiovascular events (incidence rate of 1% per year) over a median time of 3.10 years.
Association of GDF-15 with Mortality, Heart Failure, and Atherosclerotic Cardiovascular Disease
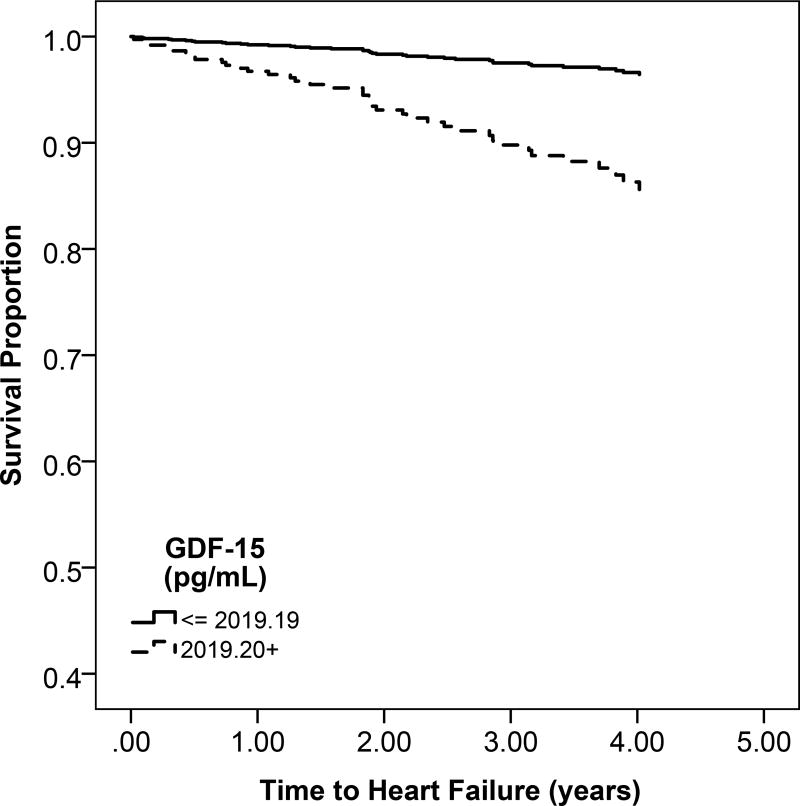
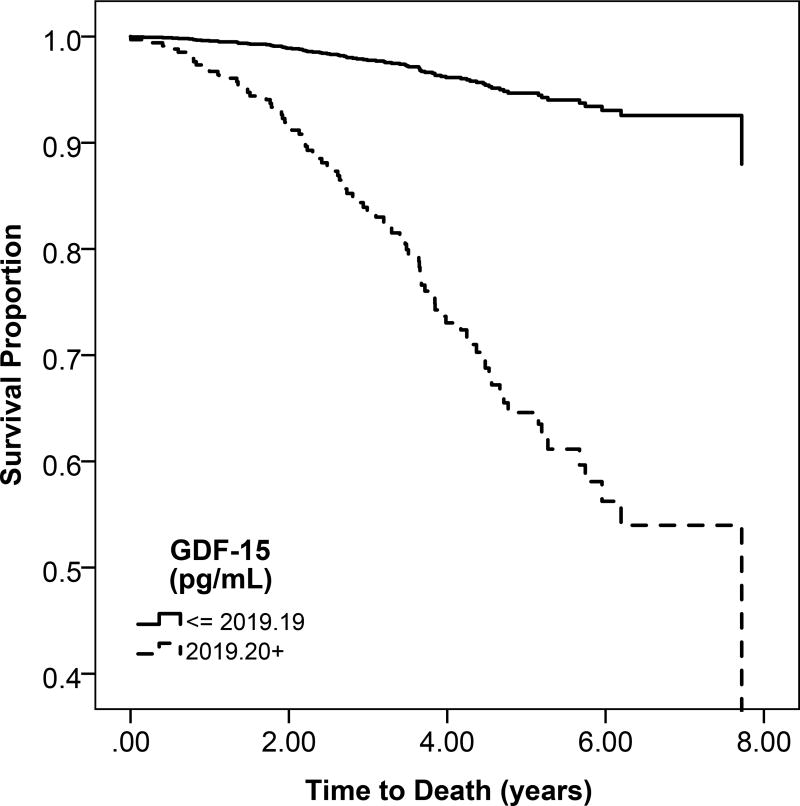
Higher circulating GDF-15 concentration was associated with greater unadjusted risk of mortality (Table 3; Figure 1a). After adjustment,. each 1-SD higher GDF-15 concentration was associated with an estimated 87% greater risk of mortality (95% confidence interval, 53%–129%).
Table 3.
Multivariable association of circulating GDF-15, Gal-3, and sST2 levels with mortality, heart failure, and atherosclerotic CVD
| SD | HR (95% CI) | |||
|---|---|---|---|---|
| Unadjusted | Model 1 | Model 2 | ||
| Mortality (N= 98) | ||||
| GDF-15, per 1-SD greater | 1607pg/mL | 2.06 (1.78, 2.38) | 1.97 (1.66, 2.34) | 1.87 (1.53, 2.29) |
| Gal-3, per 1-SD greater | 7.87ng/mL | 1.21 (1.12, 1.30) | 1.45 (1.30, 1.63) | 1.51 (1.36, 1.78) |
| sST2, per 1-SD greater | 17.49ng/mL | 1.41 (1.26, 1.58) | 1.41 (1.22, 1.63) | 1.36 (1.17, 1.58) |
| Heart Failure (N= 41) | ||||
| GDF-15, per 1-SD greater | 1607pg/mL | 1.71 (1.36, 2.14) | 1.60 (1.22, 2.10) | 1.56 (1.12, 2.16) |
| Gal-3, per 1-SD greater | 7.87ng/mL | 1.09 (0.89, 1.34) | 1.15 (0.84, 1.56) | 1.11 (0.77, 1.38) |
| sST2, per 1-SD greater | 17.49ng/mL | 1.30 (1.07, 1.56) | 1.23 (0.97, 1.56) | 1.22 (0.94, 1.60) |
| Atherosclerotic CVD (N= 29) | ||||
| GDF-15, per 1-SD greater | 1607pg/mL | 1.60 (1.21, 2.12) | 1.39 (0.99, 1.95) | 1.19 (0.77, 1.55) |
| Gal-3, per 1-SD greater | 7.87ng/mL | 1.10 (0.86, 1.39) | 1.21 (0.84, 1.74) | 1.16 (0.75, 1.78) |
| sST2, per 1-SD greater | 17.49ng/mL | 1.20 (0.92, 1.57) | 0.92 (0.60, 1.41) | 0.67 (0.36, 1.27) |
Hazard ratios are calculated based on per standard deviation elevation in biomarker level
Model 1: adjusted for age, gender, race, and study cohort
Model 2: Model 1 + prevalent cardiovascular disease, diabetes, current smoking status, eGFR and UACR
UACR, urinary albumin-creatinine ratio; eGFR, estimated glomerular filtration rate; Gal-3, ____; GDF-15, _____; sST2, _____; Scr, serum creatinine. HR, _____; CI, _______; CVD, cardiovascular dsiease.
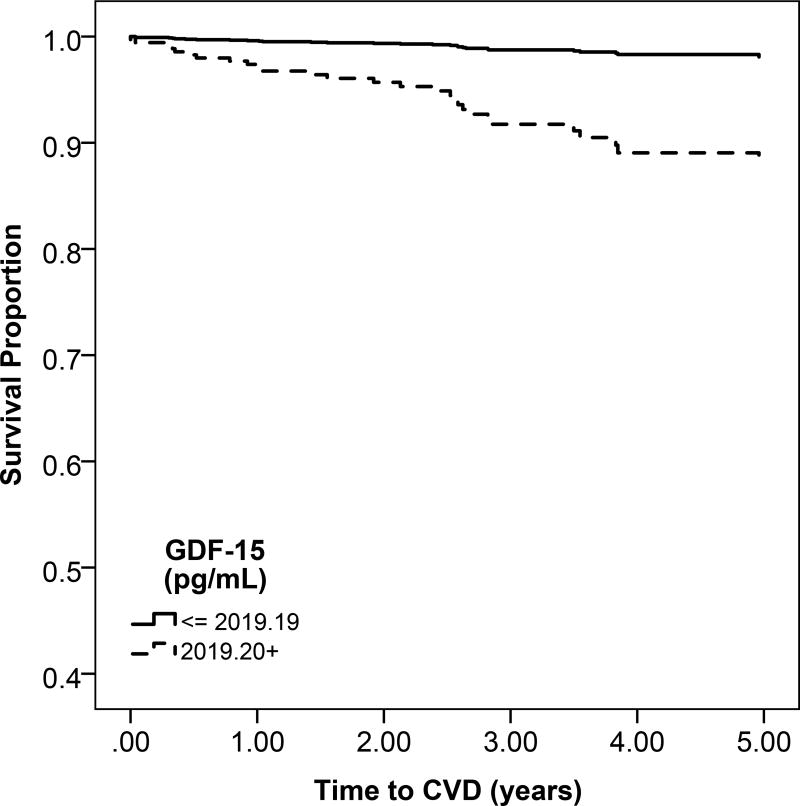
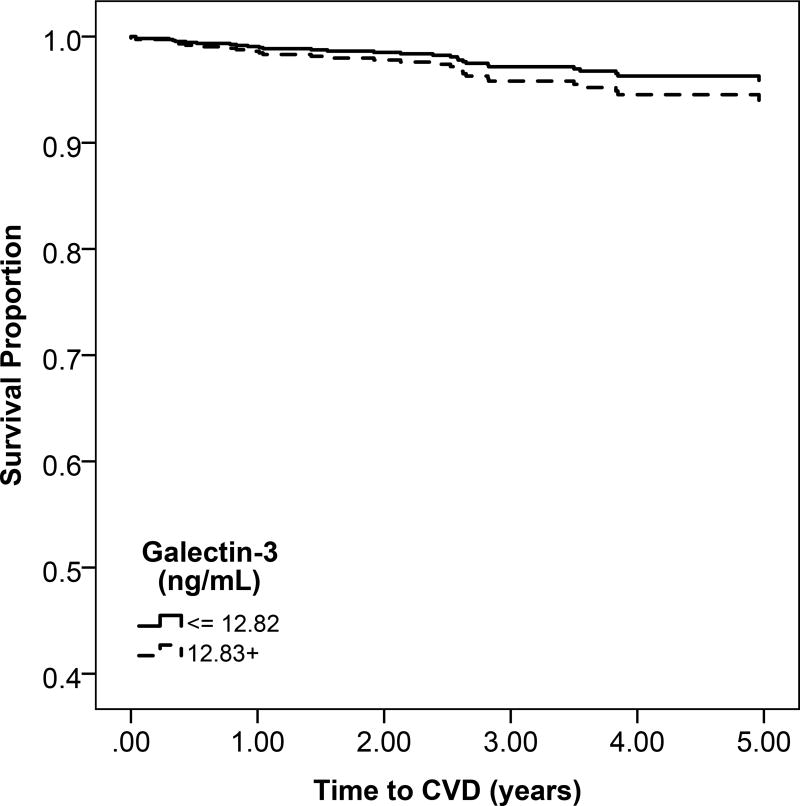
Figure 1.
a: Unadjsuted Kaplan Meier estimates of disease-free survival according to high (above the median) or low (below the median) GDF-15 levels
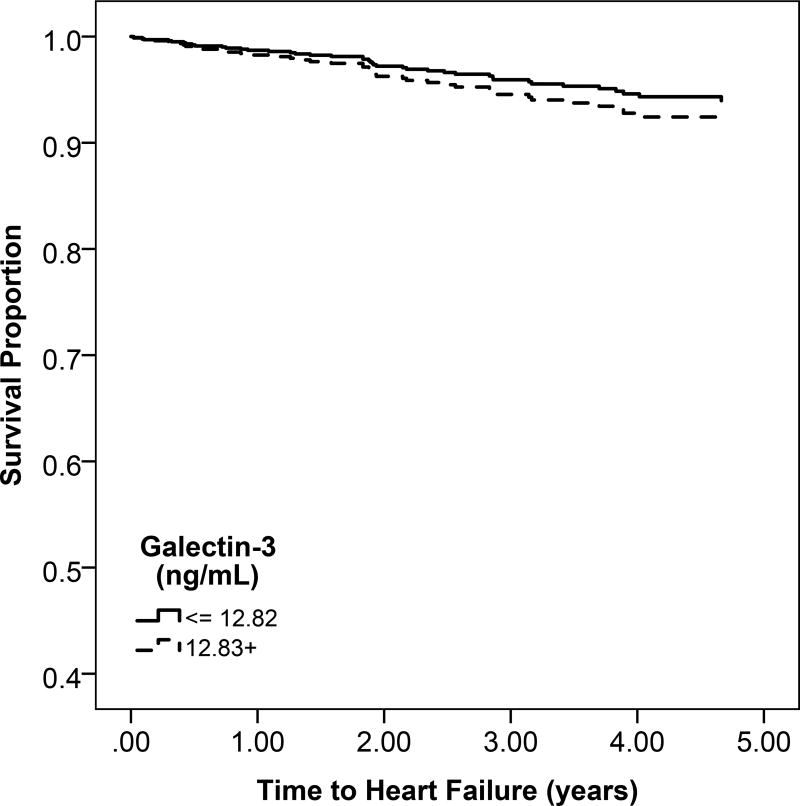
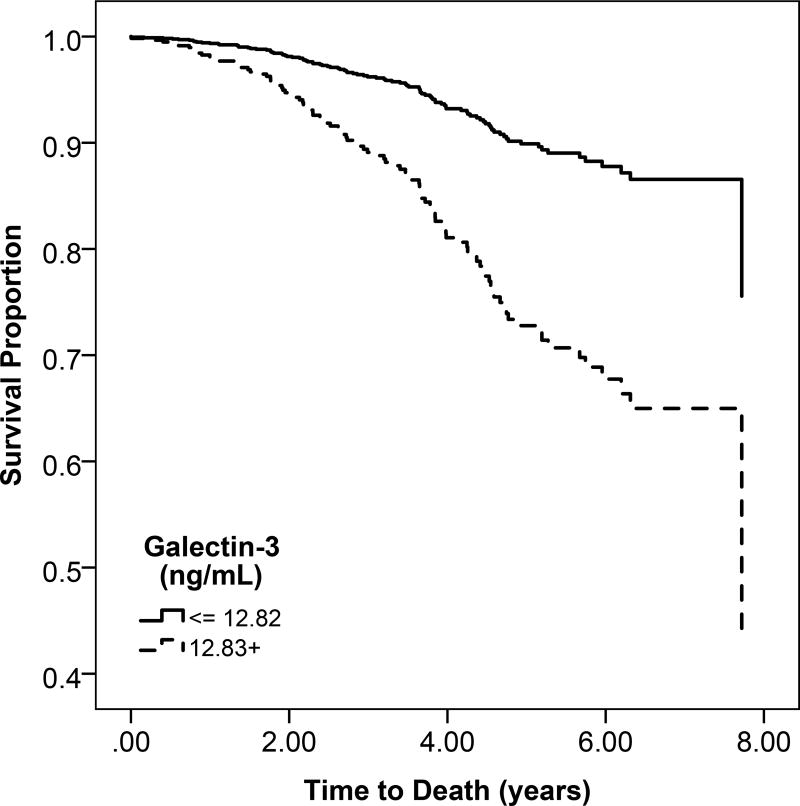
b: Unadjsuted Kaplan Meier estimates of disease-free survival according to high (above the median) or low (below the median) galectin-3 levels
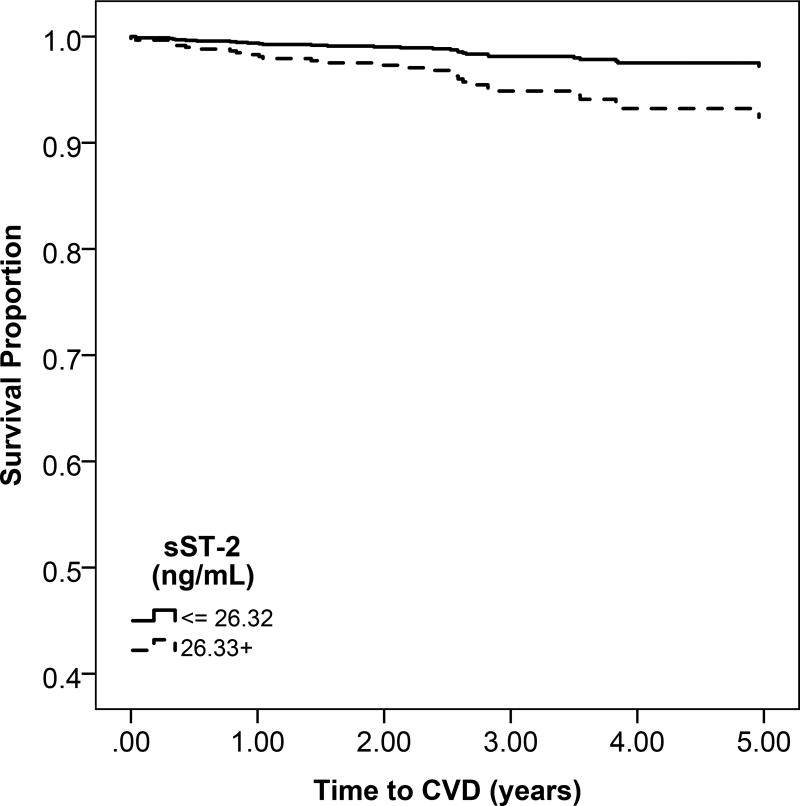
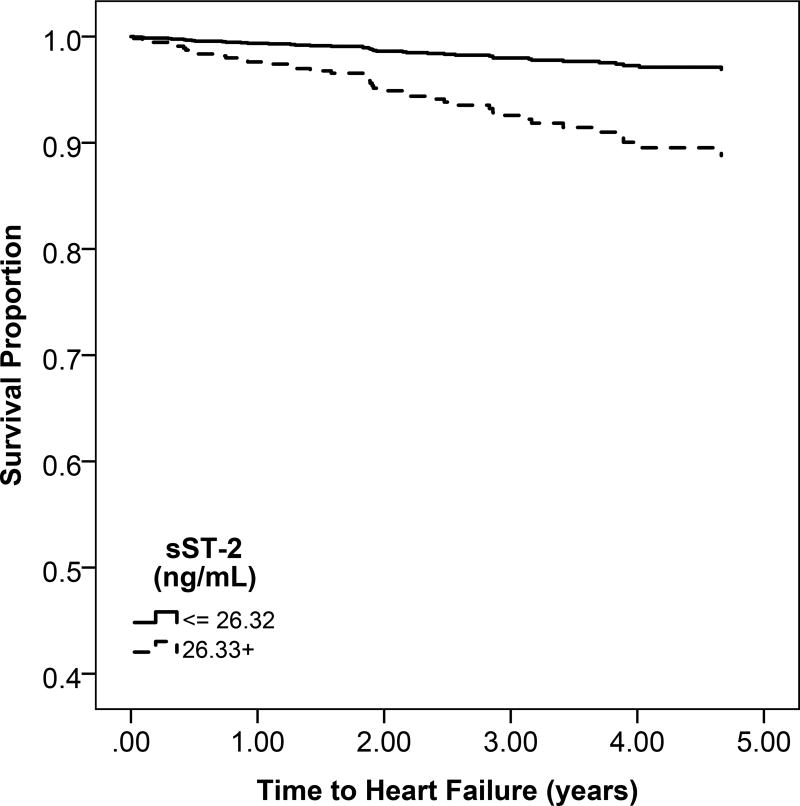
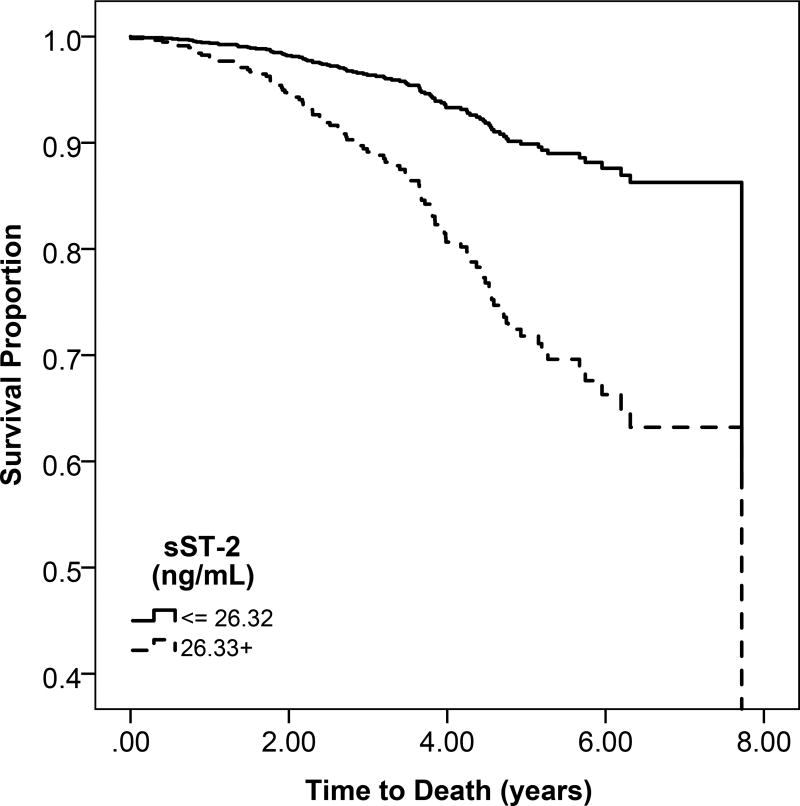
c: Unadjsuted Kaplan Meier estimates of disease-free survival according to high (above the median) or low (below the median) sST2 levels
In unadjusted models, the rates of heart failure and atherosclerotic CVD were greater across higher categories of GDF-15 (Table 3; Figure 1a). After multivariable adjustment, every 1-SD higher GDF-15 was associated with 56% greater risk of heart failure (95% CI, 12%–116%). GDF-15 was not significantly associated with increased atherosclerotic CVD risk (Table 3).
Association of Gal-3 with Mortality, Heart Failure, and Atherosclerotic Cardiovascular Disease
Participants with high gal-3 levels had a lower probability of overall survival compared to low gal-3 levels (Figure 1b). Higher quartiles of serum gal-3 were associated with progressively greater rates of mortality (Figure S2). For every 1-SD higher level of gal-3, there was a 51% increase in mortality after adjusting for confounders (95% CI, 36%–78%) (Table 3).
There was no significant difference in rates of heart failure or atherosclerotic CVD across higher categories of gal-3 (Figure 1b and Figure S2). In multivariable models, gal-3 was not associated with risk of heart failure or atherosclerotic CVD (Table 3).
Association of sST-2 with Mortality, Heart Failure, and Atherosclerotic Cardiovascular Disease
Participants with higher sST-2 levels had lower probability of overall survival compared to participants with low sST-2 levels (Figure 1c). All-cause mortality rates were higher across sST-2 quartiles, (Figure S2). When fully adjusted, higher sST-2 levels were significantly associated with a 36% increase in mortality (95% CI, 17%–58%). (Table 3).
Heart failure and atherosclerotic CVD event rates were higher across sST-2 quartiles (Figure S2). However, in multivariable models, there was no association of sST2 with heart failure or atherosclerotic CVD (Table 3).
DISCUSSION
In this pooled multi-center cohort of 883 CKD patients, we found strong associations between GDF-15, gal-3, and sST-2 with all-cause mortality. We also found a significant association of GDF-15 with risk of heart failure. Our findings both support and differ from prior studies in the general population and may indicate that pathways of inflammation and cardiac stress marked by elevations in GDF-15, gal-3, and sST-2 behave uniquely in the setting of CKD.
We observed an association of higher GDF-15 with risk of mortality and heart failure. Previous studies of GDF-15 in patients with normal eGFR have shown associations with atrial fibrillation, myocardial infarction, stroke, and heart failure.9, 10 Furthermore, GDF-15 levels have been shown to be chronically elevated in individuals with CVD, specifically in individuals with a history of myocardial infarction or heart failure.11, 12, 32 In heart failure, higher GDF-15 levels correlate with more advanced New York Heart Association (NYHA) class13 and elevations in GDF-15 are associated with more heart failure hospitalizations.14,15, 16 The mechanisms of inflammation marked by GDF-15 have not been fully elucidated, but its expression is upregulated in tissue injury. It is unknown whether GDF-15 expression is a compensatory or putative response to injury. Recent in vivo mouse studies have shown that GDF-15 interferes with chemokine-triggered integrin activation, preventing inflammatory cell extravasation at sites of cardiac injury and subsequent inflammatory damage.33, 34 Another study of mice who were exposed to coronary artery ligation, myocardial GDF-15 mRNA expression increased in the area at risk for ischemic injury. GDF-15 deficient mice developed greater infarct sizes and displayed more cardiomyocyte apoptosis in the infarct border zone, suggesting the endogenous GDF-15 limits myocardial damage in vivo.8
Our results also suggest an association between GDF-15 and heart failure events, however, we did not find an association of GDF-15 with atherosclerotic CVD in those with kidney disease (though we may have been underpowered to detect an association). Prior literature has shown that GDF-15 is expressed in various tissues beyond the kidey and heart. For example, recent data from animal models has shown that GDF-15 binds to GFRAL (GDNF family receptor α-like), which is primarily located in the central nervous system.35 GDF-15/GFRAL expression regulates food intake and body weight in mice. In animal models, overexpression of GDF-15 leads to a lean phenotype, hypophagia and improvements in metabolic parameters.36 Our study provides further data on the association of GDF-15 with cardiac outcomes in patients with kidney disease.
In our study, elevations of gal-3 were significantly associated with greater risk of mortality. Previous studies of gal-3 in patients with normal kidney function have shown that higher gal-3 levels predict all-cause mortality37, 38 and cardiovascular events. In particular, studies have shown that gal-3 is associated with heart failure,39 including new diagnosis and prognosis of both acute and chronic heart failure in the general population.40–42,43 The US Food and Drug Administration (FDA) approved clinical gal-3 testing to aid in prognosis for patients with chronic heart failure in 2010, but since then our knowledge of gal-3 has continued to evolve.44 Gal-3likely acts in a variety of different pathways, including in embryogenesis, cell proliferation, cell adhesion, and inflammation. At the molecular level, gal-3 crosslinks with glycoproteins to promote cellcell and cell-matrix interactions, ultimately leading to fibrosis and extracellular matrix stiffening.45 In the heart, gal-3 is highly expressed by cardiac macrophages and promotes cardiac fibrosis.46 Similarly, gal-3 is also present in the kidney, where it promotes tubulointerstitial fibrosis45 and its levels rise with advancing CKD and end stage renal disease (ESRD).26 In the Atherosclerosis Risk in Communities (ARIC) Study, participants free of CKD and heart failure in the highest quartile of galectin-3 had a twofold greater risk of developing incident CKD.47 Small studies of patients with moderate CKD and ESRD have shown that gal-3 levels are inversely associated with kidney function in both the presence and absence of heart failure.48–50. There have been a few studies that have examined the association of gal-3 with clinical outcomes in patients with CKD, where the pathophysiology of CVD may differ. A 2014 study of ambulatory heart failure patients in Spain showed that the prognostic value of gal-3 for CVD declined after adjusting for participant kidney function (participants had an average eGFR of 43 mL/min/1.73m2).51 In contrast, an analysis of the LURIC (LUdwigshafen RIsk and Cardiovascular Health) and 4D (Die Deutsche Diabetes Dialyse Studie) cohorts found a statistically significant increase in fatal and nonfatal combined cardiovascular endpoints (sudden cardiac death, myocardial infarction, stroke, and death due to heart failure) among those with CKD and elevated gal-3.26 Our study extends the literature by studying gal-3 in a a U.S. based racially diverse CKD cohort and demonstrating an association of galectin-3 with mortality. Further work is needed to investigate associations with heart failure and atherosclerosis in this population.
In our pooled cohort, sST-2 elevation was found to be significantly associated with increased mortality. sST-2 has also been previously studied in the general population and, to a limited extent, in those with kidney disease where it was associated with all-cause mortality, severity of heart failure, and adverse heart failure outcomes.52–56 It has also shown promise in predicting adverse cardiac remodeling in patients after myocardial infarction.57, 58 In PROTECT (Placebo-Controlled Randomized Study of the Selective A1 Adenosine Receptor Antagonist Rolofylline for Patients Hospitalized with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function), a European multicenter randomized placebo-controlled study of over 2,300 participants analyzing 44 novel cardiac biomarkers in heart failure, sST-2 was one of the strongest independent predictors of 30-day and 180-day all-cause mortality and cardiovascular or renal rehospitalization.59 Spanish investigators also demonstrated this increased mortality risk in those with elevated sST-2 and heart failure, which was still strong even in the subset of their cohort with eGFR <60mL/min/1.73m2.60 sST-2 was also approved by the FDA for use clinically, and has been subsequently studied in head to head comparison with gal-3 in chronic heart failure patients, where only sST-2 was predictive of five-year cardiovascular death, superior to gal-3.27 ST-2 has two forms, soluble ST2 (sST-2) and transmembrane ST-2 (ST-2L). Mechanistically, IL-33 binds as a ligand to ST-2L, providing cardioprotection in vivo. The interaction of ST-2L/IL-33 reduces cardiomyocyte apoptosis and prevents the adverse cardiac remodeling seen after cardiac ischemia.61 The soluble form of ST2, however, acts as a decoy and disrupts binding of ST-2L and IL-33, preventing its cardio-protective effects.62 Our study supports and extends the prior literature in that we examined sST2 and associations with clinical outcomes in a relatively large population of patients with moderate to advanced CKD.
Our study adds to the growing body of literature on GDF-15, gal-3, and sST-2 and their associations with CVD by focusing on patients with CKD, who have a disproportionate risk of CVD. While some of our findings in this unique population mirrored those results seen in the general population, some did not. For instance, gal-3 in the general population has been strongly associated with heart failure, we found that this may not be true in the kidney disease population. Likewise, GDF-15, gal-3, and sST-2 have all been associated with atherosclerotic CVD in general populations, which we did not see in our CKD cohort (which may have been affected by limited power). CKD patients have unique CVD risk factors, such as metabolic imbalances, abnormalities in mineral metabolism and accumulation of uremic solutes, which may alter the expression of these biomarkers and their underlying pathways. Our study highlights the need for more research to elucidate the pathways reflected by elevations in GDF-15, gal-3, and sST-2 specifically in the vulnerable CKD population. These biomarkers and their respective biological pathways may be future targets for therapeutic intervention to mitigate mortality and heart failure risk in CKD populations.
Our study has several strengths. It is a large multi-center study pooling two racially diverse cohorts from diverse geographic regions in the United States. Participants had a variety of kidney disease etiologies, making our results generalizable to the CKD population at large. All study outcomes were adjudicated by the same experienced physicians to ensure diagnostic accuracy, and all biomarker measurements were performed in the same laboratory to ensure measurement consistency across the pooled cohort. We also included a set of well-recognized and well-defined confounders in our study.
The limitations of our study should also be acknowledged. Overall, our event rate for heart failure and atherosclerotic CVD was low and thus we may have been underpowered to detect an association between GDF-15, gal-2 and sST-2 levels with heart failure and atherosclerotic CVD. Another limitation to our study is that we were not able to distinguish between preserved or reduced ejection fraction heart failure events, which likely have different biological mechanisms. We were not able to look at combinations of biomarkers or prediction models given the limited power in the analysis. We also did not measure tissue-specific biomarker levels; it is therefore possible that elevations in local biomarker levels in the heart or kidneys were clinically significant, but not detected in our study. However, our prior studies reported a strong positive correlation between tissue GDF-15 and circulating GDF-15.7 The molecular weight of these proteins are lower than that albumin and is likely that they are filtered in the glomerulus. While we adjusted for eGFR in our models, it is possible that elevated concentrations of GDF-15, gal-3 and sST2 reflect decreased kidney function in this population of CKD patients. Finally, this was an observational study thus we cannot establish causality.
In conclusion, elevations in GDF-15, gal-3, and sST-2 are associated with greater risk of mortality among the CKD population. Furthermore, GDF-15 may also be associated with greater heart failure risk in this population. Further studies are needed to determine whether the pathways reflected by GDF-15, gal-3 and sST-2 are possible therapeutic targets.
Supplementary Material
Figure S1. Scatterplots of eGFR and UACR with GDF-15, Gal-3, and sST2.
Figure S2. Incidence rates of study outcomes based on GDF-15, Gal-3, and sST-2 quartiles.
Table S1. Baseline characteristics of study population across quartiles of GDF-15, Gal-3, and sST2.
Table S2. Multivariable linear regression analysis of patient baseline characteristics and GDF-15, Gal-3, sST2 levels.
Acknowledgments
Support: This work was supported by R03 DK102452-01A1 (to Dr Bansal). The funder had no role in the study design; collection, analysis and interpretation of data; writing of the manuscript; and the decision to submit the report for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material Descriptive Text for Online Delivery
Supplementary Figure S1 (PDF). Scatterplots of eGFR and UACR with GDF-15, Gal-3, and sST2.
Supplementary Figure S2 (PDF). Incidence rates of study outcomes based on GDF-15, Gal-3, and sST-2 quartiles.
Supplementary Table S1 (PDF). Baseline characteristics of study population across quartiles of GDF-15, Gal-3, and sST2.
Supplementary Table S2 (PDF). Multivariable linear regression analysis of patient baseline characteristics and GDF-15, Gal-3, sST2 levels.
Authors’ Contributions: Research idea and study design: CT, RK, IdB, BK, WJ, JH, NB; data acquisition: ZB, KB, FB, CG, DG, JH, MK, SS; data analysis/interpretation: CT, RK, MA, IdB, BK, JH, CRC, NB; statistical analysis: RK, CRC; supervision or mentorship: NB. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Financial Disclosure: None
The involvement of an Acting Editor-in-Chief was to comply with AJKD’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
References
- 1.Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2016 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2017;69(3 suppl 1):A7–A8. doi: 10.1053/j.ajkd.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porter AC, Lash JP, Xie D, et al. Predictors and Outcomes of Health-Related Quality of Life in Adults with CKD. Clin J Am Soc Nephrol. 2016;11:1154–1162. doi: 10.2215/CJN.09990915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harel Z, Wald R, McArthur E, et al. Rehospitalizations and Emergency Department Visits after Hospital Discharge in Patients Receiving Maintenance Hemodialysis. J Am Soc Nephrol. 2015;26:3141–3150. doi: 10.1681/ASN.2014060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J. Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 5.de Boer RA, Daniels LB, Maisel AS, Januzzi JL., Jr State of the Art: Newer biomarkers in heart failure. Eur J Heart Fail. 2015;17:559–569. doi: 10.1002/ejhf.273. [DOI] [PubMed] [Google Scholar]
- 6.Unsicker K, Spittau B, Krieglstein K. The multiple facets of the TGF-beta family cytokine growth/differentiation factor-15/macrophage inhibitory cytokine-1. Cytokine & growth factor reviews. 2013;24:373–384. doi: 10.1016/j.cytogfr.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Nair V, Robinson-Cohen C, Smith MR, et al. Growth Differentiation Factor-15 and Risk of CKD Progression. J Am Soc Nephrol. 2017 doi: 10.1681/ASN.2016080919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kempf T, Eden M, Strelau J, et al. The transforming growth factor-beta superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circ. Res. 2006;98:351–360. doi: 10.1161/01.RES.0000202805.73038.48. [DOI] [PubMed] [Google Scholar]
- 9.Wang TJ, Wollert KC, Larson MG, et al. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation. 2012;126:1596–1604. doi: 10.1161/CIRCULATIONAHA.112.129437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rohatgi A, Patel P, Das SR, et al. Association of growth differentiation factor-15 with coronary atherosclerosis and mortality in a young, multiethnic population: observations from the Dallas Heart Study. Clin Chem. 2012;58:172–182. doi: 10.1373/clinchem.2011.171926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kempf T, Horn-Wichmann R, Brabant G, et al. Circulating concentrations of growth-differentiation factor 15 in apparently healthy elderly individuals and patients with chronic heart failure as assessed by a new immunoradiometric sandwich assay. Clin Chem. 2007;53:284–291. doi: 10.1373/clinchem.2006.076828. [DOI] [PubMed] [Google Scholar]
- 12.Bonaca MP, Morrow DA, Braunwald E, et al. Growth differentiation factor-15 and risk of recurrent events in patients stabilized after acute coronary syndrome: observations from PROVE IT-TIMI 22. Arterioscler Thromb Vasc Biol. 2011;31:203–210. doi: 10.1161/ATVBAHA.110.213512. [DOI] [PubMed] [Google Scholar]
- 13.Kempf T, von Haehling S, Peter T, et al. Prognostic utility of growth differentiation factor-15 in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1054–1060. doi: 10.1016/j.jacc.2007.04.091. [DOI] [PubMed] [Google Scholar]
- 14.Anand IS, Kempf T, Rector TS, et al. Serial measurement of growth-differentiation factor-15 in heart failure: relation to disease severity and prognosis in the Valsartan Heart Failure Trial. Circulation. 2010;122:1387–1395. doi: 10.1161/CIRCULATIONAHA.109.928846. [DOI] [PubMed] [Google Scholar]
- 15.Xanthakis V, Larson MG, Wollert KC, et al. Association of novel biomarkers of cardiovascular stress with left ventricular hypertrophy and dysfunction: implications for screening. Journal of the American Heart Association. 2013;2:e000399. doi: 10.1161/JAHA.113.000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lind L, Wallentin L, Kempf T, et al. Growth-differentiation factor-15 is an independent marker of cardiovascular dysfunction and disease in the elderly: results from the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) Study. European heart journal. 2009;30:2346–2353. doi: 10.1093/eurheartj/ehp261. [DOI] [PubMed] [Google Scholar]
- 17.Kempf T, Bjorklund E, Olofsson S, et al. Growth-differentiation factor-15 improves risk stratification in ST-segment elevation myocardial infarction. Eur Heart J. 2007;28:2858–2865. doi: 10.1093/eurheartj/ehm465. [DOI] [PubMed] [Google Scholar]
- 18.Wollert KC, Kempf T. Growth differentiation factor 15 in heart failure: an update. Current heart failure reports. 2012;9:337–345. doi: 10.1007/s11897-012-0113-9. [DOI] [PubMed] [Google Scholar]
- 19.Wollert KC, Kempf T, Lagerqvist B, et al. Growth differentiation factor 15 for risk stratification and selection of an invasive treatment strategy in non ST-elevation acute coronary syndrome. Circulation. 2007;116:1540–1548. doi: 10.1161/CIRCULATIONAHA.107.697714. [DOI] [PubMed] [Google Scholar]
- 20.Wollert KC, Kempf T, Peter T, et al. Prognostic value of growth-differentiation factor-15 in patients with non-ST-elevation acute coronary syndrome. Circulation. 2007;115:962–971. doi: 10.1161/CIRCULATIONAHA.106.650846. [DOI] [PubMed] [Google Scholar]
- 21.Wollert KC, Kempf T, Wallentin L. Growth Differentiation Factor 15 as a Biomarker in Cardiovascular Disease. Clin Chem. 2017;63:140–151. doi: 10.1373/clinchem.2016.255174. [DOI] [PubMed] [Google Scholar]
- 22.Calvier L, Miana M, Reboul P, et al. Galectin-3 mediates aldosterone-induced vascular fibrosis. Arterioscler Thromb Vasc Biol. 2013;33:67–75. doi: 10.1161/ATVBAHA.112.300569. [DOI] [PubMed] [Google Scholar]
- 23.Vergaro G, Prud'homme M, Fazal L, et al. Inhibition of Galectin-3 Pathway Prevents Isoproterenol-Induced Left Ventricular Dysfunction and Fibrosis in Mice. Hypertension. 2016;67:606–612. doi: 10.1161/HYPERTENSIONAHA.115.06161. [DOI] [PubMed] [Google Scholar]
- 24.Gleissner CA, Erbel C, Linden F, et al. Galectin-3 binding protein, coronary artery disease and cardiovascular mortality: Insights from the LURIC study. Atherosclerosis. 2017;260:121–129. doi: 10.1016/j.atherosclerosis.2017.03.031. [DOI] [PubMed] [Google Scholar]
- 25.Miró Ò, González de la Presa BB, Herrero P, et al. The GALA study: Relationship between galectin-3 serum levels and short- and long-term outcomes of patients with acute heart failure. Biomarkers. 2017:1–18. doi: 10.1080/1354750X.2017.1319421. [DOI] [PubMed] [Google Scholar]
- 26.Drechsler C, Delgado G, Wanner C, et al. Galectin-3, Renal Function, and Clinical Outcomes: Results from the LURIC and 4D Studies. J Am Soc Nephrol. 2015;26:2213–2221. doi: 10.1681/ASN.2014010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bayes-Genis A, de Antonio M, Vila J, et al. Head-to-head comparison of 2 myocardial fibrosis biomarkers for long-term heart failure risk stratification: ST2 versus galectin-3. Journal of the American College of Cardiology. 2014;63:158–166. doi: 10.1016/j.jacc.2013.07.087. [DOI] [PubMed] [Google Scholar]
- 28.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J. Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 29.Einhorn PT, Davis BR, Massie BM, et al. The Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Heart Failure Validation Study: diagnosis and prognosis. Am Heart J. 2007;153:42–53. doi: 10.1016/j.ahj.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 30.Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 31.Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke; a journal of cerebral circulation. 1999;30:736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 32.Hagstrom E, Held C, Stewart RA, et al. Growth Differentiation Factor 15 Predicts All-Cause Morbidity and Mortality in Stable Coronary Heart Disease. Clin Chem. 2017;63:325–333. doi: 10.1373/clinchem.2016.260570. [DOI] [PubMed] [Google Scholar]
- 33.Artz A, Butz S, Vestweber D. GDF-15 inhibits integrin activation and mouse neutrophil recruitment through the ALK-5/TGF-betaRII heterodimer. Blood. 2016;128:529–541. doi: 10.1182/blood-2016-01-696617. [DOI] [PubMed] [Google Scholar]
- 34.Kempf T, Zarbock A, Widera C, et al. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat. Med. 2011;17:581–588. doi: 10.1038/nm.2354. [DOI] [PubMed] [Google Scholar]
- 35.Yang L, Chang CC, Sun Z, et al. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nature medicine. 2017;23:1158–1166. doi: 10.1038/nm.4394. [DOI] [PubMed] [Google Scholar]
- 36.Emmerson PJ, Wang F, Du Y, et al. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nature medicine. 2017;23:1215–1219. doi: 10.1038/nm.4393. [DOI] [PubMed] [Google Scholar]
- 37.Daniels LB, Clopton P, Laughlin GA, Maisel AS, Barrett-Connor E. Galectin-3 is independently associated with cardiovascular mortality in community-dwelling older adults without known cardiovascular disease: The Rancho Bernardo Study. Am Heart J. 2014;167:674–682.e671. doi: 10.1016/j.ahj.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Imran TF, Shin HJ, Mathenge N, et al. Meta-Analysis of the Usefulness of Plasma Galectin-3 to Predict the Risk of Mortality in Patients With Heart Failure and in the General Population. Am J Cardiol. 2017;119:57–64. doi: 10.1016/j.amjcard.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 39.Maiolino G, Rossitto G, Pedon L, et al. Galectin-3 predicts long-term cardiovascular death in high-risk patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2015;35:725–732. doi: 10.1161/ATVBAHA.114.304964. [DOI] [PubMed] [Google Scholar]
- 40.de Boer RA, Voors AA, Muntendam P, van Gilst WH, van Veldhuisen DJ. Galectin-3: a novel mediator of heart failure development and progression. Eur J Heart Fail. 2009;11:811–817. doi: 10.1093/eurjhf/hfp097. [DOI] [PubMed] [Google Scholar]
- 41.Ho JE, Liu C, Lyass A, et al. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol. 2012;60:1249–1256. doi: 10.1016/j.jacc.2012.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Kimmenade RR, Januzzi JL, Ellinor PT, et al. Utility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failure. Journal of the American College of Cardiology. 2006;48:1217–1224. doi: 10.1016/j.jacc.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 43.de Boer RA, Lok DJ, Jaarsma T, et al. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann. Med. 2011;43:60–68. doi: 10.3109/07853890.2010.538080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Food U, Administration D. Device market approval under the federal food, drug, and cosmetic act. 21 CFR 862.1117. 2010 Nov 3; [Google Scholar]
- 45.Desmedt V, Desmedt S, Delanghe JR, Speeckaert R, Speeckaert MM. Galectin-3 in Renal Pathology: More Than Just an Innocent Bystander. Am J Nephrol. 2016;43:305–317. doi: 10.1159/000446376. [DOI] [PubMed] [Google Scholar]
- 46.MacKinnon AC, Liu X, Hadoke PWF, Miller MR, Newby DE, Sethi T. Inhibition of galectin-3 reduces atherosclerosis in apolipoprotein E-deficient mice. Glycobiology. 2013;23:654–663. doi: 10.1093/glycob/cwt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rebholz CM, Selvin E, Liang M, et al. Plasma galectin-3 levels are associated with the risk of incident chronic kidney disease. Kidney. Int. 2018;93:252–259. doi: 10.1016/j.kint.2017.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gopal DM, Kommineni M, Ayalon N, et al. Relationship of Plasma Galectin-3 to Renal Function in Patients With Heart Failure: Effects of Clinical Status, Pathophysiology of Heart Failure, and Presence or Absence of Heart Failure. Journal of the American Heart Association. 2012;1 doi: 10.1161/JAHA.112.000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meijers WC, van der Velde AR, Ruifrok WP, et al. Renal handling of galectin-3 in the general population, chronic heart failure, and hemodialysis. Journal of the American Heart Association. 2014;3:e000962. doi: 10.1161/JAHA.114.000962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ji F, Zhang S, Jiang X, et al. Diagnostic and prognostic value of galectin-3, serum creatinine, and cystatin C in chronic kidney diseases. Journal of clinical laboratory analysis. 2017;31 doi: 10.1002/jcla.22074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zamora E, Lupon J, de Antonio M, et al. Renal function largely influences Galectin-3 prognostic value in heart failure. Int J Cardiol. 2014;177:171–177. doi: 10.1016/j.ijcard.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 52.Daniels LB, Clopton P, Iqbal N, Tran K, Maisel AS. Association of ST2 levels with cardiac structure and function and mortality in outpatients. American heart journal. 2010;160:721–728. doi: 10.1016/j.ahj.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 53.Januzzi JL, Mebazaa A, Di Somma S. ST2 and prognosis in acutely decompensated heart failure: the International ST2 Consensus Panel. Am J Cardiol. 2015;115:26b–31b. doi: 10.1016/j.amjcard.2015.01.037. [DOI] [PubMed] [Google Scholar]
- 54.Pascual-Figal DA, Lax A, Perez-Martinez MT, del Carmen Asensio-Lopez M, Sanchez-Mas J. Clinical relevance of sST2 in cardiac diseases. Clinical chemistry and laboratory medicine. 2016;54:29–35. doi: 10.1515/cclm-2015-0074. [DOI] [PubMed] [Google Scholar]
- 55.Januzzi JL., Jr ST2 as a cardiovascular risk biomarker: from the bench to the bedside. Journal of cardiovascular translational research. 2013;6:493–500. doi: 10.1007/s12265-013-9459-y. [DOI] [PubMed] [Google Scholar]
- 56.Ky B, French B, McCloskey K, et al. <span hwp:id="article-title-1" class="article-title">High-Sensitivity ST2 for Prediction of Adverse Outcomes in Chronic Heart Failure</span><span hwp:id="article-title-32" class="sub-articletitle">Clinical Perspective</span>. Circulation: Heart Failure. 2011;4:180–187. doi: 10.1161/CIRCHEARTFAILURE.110.958223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weir RA, Miller AM, Petrie CJ, et al. Interleukin-21--a biomarker of importance in predicting myocardial function following acute infarction? Cytokine. 2012;60:220–225. doi: 10.1016/j.cyto.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 58.Weir RA, Miller AM, Murphy GE, et al. Serum soluble ST2: a potential novel mediator in left ventricular and infarct remodeling after acute myocardial infarction. J Am Coll Cardiol. 2010;55:243–250. doi: 10.1016/j.jacc.2009.08.047. [DOI] [PubMed] [Google Scholar]
- 59.Demissei BG, Valente MA, Cleland JG, et al. Optimizing clinical use of biomarkers in high-risk acute heart failure patients. Eur J Heart Fail. 2016;18:269–280. doi: 10.1002/ejhf.443. [DOI] [PubMed] [Google Scholar]
- 60.Bayes-Genis A, Zamora E, de Antonio M, et al. Soluble ST2 Serum Concentration and Renal Function in Heart Failure. Journal of Cardiac Failure. 2013;19:768–775. doi: 10.1016/j.cardfail.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 61.Seki K, Sanada S, Kudinova AY, et al. Interleukin-33 prevents apoptosis and improves survival after experimental myocardial infarction through ST2 signaling. Circulation. Heart failure. 2009;2:684–691. doi: 10.1161/CIRCHEARTFAILURE.109.873240. [DOI] [PubMed] [Google Scholar]
- 62.Pascual-Figal DA, Januzzi JL. The Biology of ST2: The International ST2 Consensus Panel. The American Journal of Cardiology. 2015;115:3B–7B. doi: 10.1016/j.amjcard.2015.01.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Scatterplots of eGFR and UACR with GDF-15, Gal-3, and sST2.
Figure S2. Incidence rates of study outcomes based on GDF-15, Gal-3, and sST-2 quartiles.
Table S1. Baseline characteristics of study population across quartiles of GDF-15, Gal-3, and sST2.
Table S2. Multivariable linear regression analysis of patient baseline characteristics and GDF-15, Gal-3, sST2 levels.