Abstract
Background
Atrial fibrillation (AF) is an important public health problem across race/ethnic groups. Data from US cohort studies initiated in the 1980s suggest a higher prevalence of AF risk factors among African‐Americans (AAs) than whites, but lower AF incidence. The Jackson Heart Study (JHS) is a community‐based study of 5306 AAs recruited starting in 2000.
Hypothesis
Demographic, anthropometric, cardiovascular, and/or electrocardiographic factors are associated with AF incidence in JHS.
Methods
Using baseline participant characteristics and incident AF identified through hospital surveillance, study electrocardiogram, and Medicare claims, we estimated age‐ and sex‐specific AF incidence rates, compared them with rates in AA participants in the Multi‐Ethnic Study of Atherosclerosis (MESA) and Cardiovascular Health Study (CHS), and examined associations of cardiovascular risk factors with AF.
Results
A total of 66 participants had prevalent AF at baseline. Over an average follow‐up of 8.5 years, 242 cases of incident AF were identified. Age‐ and sex‐specific AF incidence rates in JHS were similar to those among AAs in MESA and appeared slightly lower than those among AAs in CHS. In an age‐ and sex‐adjusted model, associations with incident AF were observed for modifiable risk factors: high body weight (HR = 1.23 per 15 kg, 95%CI 1.13‐1.35), systolic blood pressure (HR = 1.29 per 20 mmHg, 95%CI 1.13‐1.47), and current smoking (HR = 1.80, 95%CI 1.27‐2.55). Risk estimates associated with these risk factors were only slightly attenuated after multivariable adjustments.
Conclusions
These findings underscore the potential additional benefits of interventions for weight management, control of hypertension, and smoking cessation for the prevention of AF among AAs.
Keywords: arrhythmia, atrial fibrillation, Jackson Heart Study
1. INTRODUCTION
Although the burden of atrial fibrillation (AF) is well‐characterized among white Americans, less is understood about the burden of AF in African‐Americans (AA). In an early publication from the Multi‐Ethnic Study of Atherosclerosis (MESA), AA participants had a 49% lower risk of incident AF than whites, although the number of AF cases among AAs in that analysis was small (n = 64).1 The Atherosclerosis Risk in Communities (ARIC) study found a 41% lower risk of incident AF among AAs than whites.2 However, the ARIC cohort was recruited over 25 years ago, 1987 to 1989, at a time when the prevalence of AF risk factors among AAs likely differed compared with more recent times. Obesity and diabetes are more prevalent in recent years, while current smoking is less common now than in the 1980s.
To provide additional information about incident AF in a large contemporary cohort of AAs, we examined data from the Jackson Heart Study (JHS). Our aims were (1) to identify incident AF and describe the methods of AF event ascertainment; (2) to compare age‐ and sex‐specific AF incidence in JHS with that of AA participants in MESA and the Cardiovascular Health Study (CHS); and (3) to describe associations of demographic, anthropometric, cardiovascular, and electrocardiographic risk factors with AF incidence in JHS participants.
2. METHODS
2.1. Study population
JHS is a community‐based cohort study of cardiovascular disease in AAs that recruited 5306 residents from the Jackson, Mississippi (MS), metropolitan area who completed a baseline examination between 2000 and 2004. Participants were examined at two additional in‐person follow‐up study exams (2005‐2008 and 2009‐2013). JHS participants were recruited from community volunteers (25%), a random sample of the community (17%), the families of JHS participants (22%), living ARIC participants (31%), and the families of ARIC participants (5%). Details of the JHS design, methods, and recruitment are published elsewhere.3, 4
2.2. Baseline examination
At the baseline examination, JHS participants reported their age, smoking status, health insurance status, educational achievement, current medication use, and history of hypertension and myocardial infarction (MI).4 A history of heart failure was self‐reported at exam 2 in 2005 to 2008. The baseline examination included a blood draw in the fasting state and measurement of height and weight, forced expiratory volume in 1 s (FEV1), and sitting blood pressure. Cardiac rhythm, PR interval, and evidence of past MI were assessed through 12‐lead resting electrocardiograms (ECGs).5 Fasting plasma glucose was measured, and glycemic status was categorized as normal (fasting glucose<100 mg/dL and HbA1c < 5.7% with no use of diabetic medications), impaired (fasting glucose ≥100 and < 126 mg/dL or HbA1c ≥ 5.7, and < 6.5% with no use of diabetic medications), or diabetes (use of diabetes medication, fasting glucose ≥ 126 mg/dL, or HbA1c ≥ 6.5%). Cystatin C was measured at baseline exam and used to determine the estimated glomerular filtration rate (eGFR) by applying the Chronic Kidney Disease Epidemiology Collaboration Equation.6
2.3. AF ascertainment
AF cases in JHS were identified from three sources: 12‐lead ECGs at baseline and exam 3, surveillance of hospital discharge diagnosis codes from 2000 to 2012 (and from 1987 to 2000 for participants also enrolled in ARIC), and from Medicare claims data from 1991 to 2012 for participants enrolled in fee‐for‐service Medicare. Atrial flutter was included with AF in the present analysis. Prevalent AF was defined by an AF diagnosis from before JHS enrollment or AF on the baseline exam ECG. Hospitals in the Jackson, MS, area reported hospital discharge diagnosis International Classification of Diseases, Ninth Revision (ICD‐9) codes for all JHS participants over the course of follow‐up. Fee‐for‐service Medicare claims data included inpatient services, institutional outpatient services, carrier claims for physician services, and home health claims. ICD‐9 diagnosis codes of 427.31 or 427.32 in any position were considered evidence of AF. AF diagnoses associated with open cardiac surgery were ignored in the definition. We also examined the effect on AF ascertainment of requiring two or more, rather than a single, outpatient diagnosis code for evidence of AF.
Updated data on incident AF among AAs in MESA and CHS was obtained for comparison with JHS. MESA is a multicenter observational study of subclinical cardiovascular disease in 6814 participants (1891 AA) recruited in 2000 to 2002 from six US sites. CHS is a multicenter study of cardiovascular disease in 5888 participants (911 AA) who were 65 years of age or older at recruitment in 1989 to 1990 and 1992 to 1993. Incident AF was assessed in CHS and MESA through 2012 using ascertainment methods very similar to those in JHS, involving study ECGs, hospital discharge diagnosis code surveillance, and Medicare claims.7, 8 All participants provided written informed consent, and institutional review board approval was obtained. The JHS, MESA, and CHS investigations were conducted in accordance with the Declaration of Helsinki.
2.4. Statistical analysis
Continuous data are presented as mean (standard deviation) and categorical data as frequencies (percentages). Data were missing for less than 5% of participants for all variables except FEV1, which was missing for 6.2% of participants. For participants with missing values in any covariate of interest, values were imputed using multiple imputation by chained equations (Stata release 14.2, StataCorp LP, College Station, Texas).9 Of the participants without prevalent AF at baseline, person‐years at risk for incident AF were calculated from baseline until the first diagnosis of AF, death, loss to follow up, or the end of follow up (December 31, 2012).
We identified 15 potential risk factors for AF from the existing literature,10, 11, 12, 13, 14, 15, 16, 17 including sex, age, height, weight, glycemic status, use of antihypertensive medication, systolic (SBP) and diastolic blood pressure, history of MI, FEV1, eGFR, PR interval, smoking status, health insurance status, and educational attainment. We examined individual models for each risk factor adjusted for age and sex, and additionally fit a multivariable Cox proportional hazards model. We used splines to investigate nonlinear associations between suspected risk factors and AF log hazard. Based on changes in the hazard ratio (HR) of interest, we found no evidence of nonlinearity for the risk factors examined. Because information on incident heart failure was only collected following the JHS exam 2 (2005 to 2008), we conducted a secondary multivariable analysis adding heart failure as a risk factor, in which participants began accruing time at risk for incident AF at the date of exam 2. For this analysis, values from exam 2 were used when possible. When these data were unavailable, baseline measurements were used.
3. RESULTS
Among the 5306 JHS participants, 5240 were free of prevalent AF at baseline. In total, 3330 women and 1910 men were included in our analysis, with an average age of 55 years at enrollment. Participants had a high average weight at baseline (Table 1). Cohort‐wide prevalence was high for diabetes but relatively low for current smoking (Table 1). Compared with participants who did not develop AF during follow up, participants who developed AF were, on average, older and had higher SBP, lower FEV1, and longer electrocardiographic PR interval. A larger proportion of those who developed AF were current smokers, had diabetes (this difference was particularly prominent among women), had a history of MI, and used antihypertensive medication.
Table 1.
Characteristics of 5240 Jackson Heart Study participants free of prevalent atrial fibrillation at baseline (2000‐2004) overall and among men and women without and with a subsequent diagnosis of incident atrial fibrillation
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| All participants | All | Without incident AF | With incident AF | All | Without incident AF | With incident AF | ||
| N | 5240 | 1910 | 1814 | 96 | 3330 | 3184 | 146 | |
| Age, years, mean (SD) | 55.2 (12.8) | 54.4 (12.9) | 53.9 (12.8) | 63.5 (11.2) | 55.6 (12.7) | 55.1 (12.6) | 66.9 (9.5) | |
| Height, cm, mean (SD) | 168.9 (9.3) | 177.5 (7.0) | 177.5 (7.0) | 177.1 (7.3) | 164.0 (6.4) | 164.0 (6.4) | 164.2 (6.0) | |
| Weight, km, mean (SD) | 90.1 (21.4) | 94.3 (21.2) | 94.1 (20.8) | 97.3 (27.5) | 88.4 (21.2) | 88.3 (21.2) | 90.2 (22.1) | |
| Glycemic Status, (%) | ||||||||
| Normal | 44.2 | 43.3 | 43.7 | 34.8 | 44.8 | 45.6 | 27.2 | |
| Impaired | 34.1 | 36.6 | 36.3 | 41.0 | 32.6 | 32.6 | 32.1 | |
| Diabetes | 21.7 | 20.2 | 19.9 | 24.2 | 22.6 | 21.8 | 40.7 | |
| Antihypertensive medication (%) | 50.8 | 43.3 | 41.9 | 68.8 | 55.1 | 54.0 | 81.0 | |
| Systolic BP (mm Hg) | 127.4 (16.9) | 128.4 (16.5) | 128.0 (16.1) | 136.4 (21.2) | 126.9 (17.1) | 126.5 (17.0) | 135.3 (17.1) | |
| Diastolic BP (mm Hg) | 75.8 (8.8) | 78.0 (8.8) | 78.0 (8.8) | 77.0 (9.7) | 74.5 (8.5) | 74.5 (8.5) | 73.9 (8.5) | |
| History of MI (%) | 7.3 | 9.4 | 8.4 | 27.1 | 6.1 | 5.7 | 15.8 | |
| FEV1, L, mean (SD) | 2.39 (0.71) | 2.91 (0.71) | 2.94 (0.70) | 2.45 (0.68) | 2.09 (0.51) | 2.10 (0.51) | 1.71 (0.41) | |
| eGFR (mL/min per m2) | 94.4 (21.8) | 93.1 (20.5) | 93.5 (20.1) | 84.7 (25.3) | 95.2 (22.5) | 95.8 (22.3) | 81.7 (22.6) | |
| ECG PR interval, (ms), mean (SD) | 165.9 (26.9) | 170.0 (27.8) | 169.5 (27.7) | 179.8 (27.8) | 163.5 (26.1) | 163.3 (25.8) | 169.2 (31.6) | |
| Current smoker, (%) | 13.2 | 18.2 | 18.0 | 20.1 | 10.3 | 10.2 | 13.9 | |
| Insured, (%) | 86.4 | 86.0 | 85.7 | 92.7 | 86.6 | 86.2 | 94.5 | |
| High school graduate (%) | 79.9 | 79.1 | 79.5 | 71.1 | 80.4 | 81.5 | 54.8 | |
Abbreviations: AF, atrial fibrillation; BP, blood pressure; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; FEV1, forced expiratory volume in 1 s; MI, myocardial infarction; SD, standard deviation.
We compared two methods of AF ascertainment in the JHS cohort. We first used study ECGs and hospital surveillance data, hereafter referred to as “Method 1.” Using Method 1, we identified 43 participants with prevalent AF at baseline and 239 cases of incident AF during an average of 8.5 years of follow up. In Method 2, we added Medicare claims data to Method 1 sources and identified 66 cases of prevalent AF and 242 incident AF cases during follow‐up (Table 2). A total of 90% of the incident AF cases identified by Medicare claims data were also identified by hospital surveillance. Compared with Method 1, Method 2 identified an additional 23 prevalent and 15 incident AF cases. Of the additional 23 prevalent cases, 12 had been identified as incident cases by Method 1. In addition, of the AF cases identified by both Medicare claims data and hospital surveillance, the inclusion of Medicare claims data in Method 2 identified AF cases an average of 37 days earlier than Method 1. All subsequent analyses used Method 2. In Method 2, a requirement for at least two outpatient claims for AF rather than only one resulted in the identification of 62, rather than 66, prevalent AF cases at baseline but did not change the number of incident AF cases identified.
Table 2.
Incident and prevalent atrial fibrillation among 5306 Jackson Heart Study participants by method of ascertainment
| Method 2b | |||||
|---|---|---|---|---|---|
| No AF | Prevalent AF | Incident AF | Total | ||
| Method 1 a | No AF | 4998 | 11 | 15 | 5024 |
| Prevalent AF | 0 | 43 | 0 | 43 | |
| Incident AF | 0 | 12 | 227 | 239 | |
| Total | 4998 | 66 | 242 | 5306 | |
Abbreviation: AF, atrial fibrillation.
Study ECGs and hospital surveillance were used to ascertain prevalent and incident AF.
Method 1 plus medicare claims data were used to ascertain prevalent and incident AF.
Of the 66 prevalent and 242 incident AF cases, 46 (70%) prevalent and 119 (49%) incident AF cases occurred among JHS participants who were also enrolled in ARIC, from which JHS drew 31% of its participants. Participants enrolled in both studies were considerably older at the JHS baseline exam than the JHS cohort as a whole (67 vs 55 years).
Age‐ and sex‐specific AF incidence rates in JHS were similar to those of AAs in MESA (Figure 1), with similar point estimates and overlapping confidence intervals, and to published rates in ARIC (Table S1, Supporting information). Incidence rates in CHS were higher than in JHS and MESA, particularly among participants aged 80 to 89 years (Figure 1). In all three cohorts, AF incidence was substantially higher in older age groups. JHS and MESA showed similar age‐specific incidence in men and women across the age range, while in the age ranges represented in CHS, AF incidence in men tended to be higher than in women.
Figure 1.
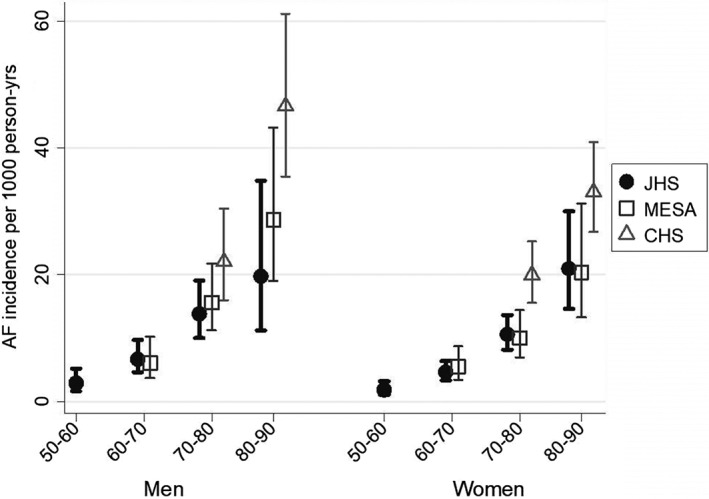
Atrial fibrillation incidence among African‐Americans in the Jackson Heart Study (JHS), Cardiovascular Health Study (CHS), and Multi‐Ethnic Study of Atherosclerosis (MESA), by age group and sex
Table 3 summarizes hazard ratios for potential AF risk factors from individual age‐ and sex‐adjusted models and from a single multivariable model. In the age‐ and sex‐adjusted models, higher mean age and weight, antihypertensive medication use, higher SBP, history of MI, lower FEV1 and eGFR (<60 mL/min per m2), and current smoking were associated with incident AF. In the multivariable model, point estimates associated with SBP and current smoking were only slightly attenuated, and age, weight, MI, and lower FEV1 remained strongly associated with incident AF.
Table 3.
Age‐ and sex‐adjusted and multivariable‐adjusted hazard ratios of incident atrial fibrillation in Jackson Heart Study participants (N = 5240) according to baseline characteristics, 2000 to 2004
| Age‐ & sex adjusted onlya | Multivariable modelb | |||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Male | 1.35 | 1.04, 1.75 | 1.34 | 0.90, 1.98 |
| Age (per 5 years) | 1.47 | 1.39, 1.56 | 1.31 | 1.20, 1.43 |
| Height (per 10 cm) | 1.24 | 1.02, 1.50 | 1.29 | 1.04, 1.60 |
| Weight (per 15 kg) | 1.23 | 1.13, 1.35 | 1.18 | 1.07, 1.30 |
| Glycemic status | ||||
| Normal | Ref. | N/A | Ref. | N/A |
| Impaired | 1.04 | 0.76, 1.43 | 0.89 | 0.64, 1.22 |
| Diabetes | 1.45 | 1.05, 1.98 | 0.96 | 0.68, 1.36 |
| Antihypertensive medication | 1.87 | 1.36, 2.59 | 1.52 | 1.08, 2.15 |
| Systolic BP (per 20 mm Hg) | 1.29 | 1.13, 1.47 | 1.23 | 1.05, 1.45 |
| Diastolic BP (per 10 mm Hg) | 1.09 | 0.74, 1.26 | 0.99 | 0.83, 1.18 |
| History of MI | 2.30 | 1.67, 3.16 | 1.77 | 1.28, 2.45 |
| FEV1 (per 0.5 L) | 0.72 | 0.63, 0.82 | 0.74 | 0.63, 0.86 |
| eGFR <60 mL/min per m2 vs ≥60 | 1.79 | 1.24, 2.59 | 1.46 | 1.01, 2.11 |
| ECG PR interval, ms | ||||
| 120‐199 | Ref. | N/A | Ref. | N/A |
| <120 | 1.36 | 0.56, 3.32 | 1.54 | 0.63, 3.77 |
| ≥ 200 | 1.10 | 0.76, 1.59 | 1.00 | 0.69, 1.44 |
| Current smoking | 1.80 | 1.27, 2.55 | 1.65 | 1.14, 2.39 |
| Insured | 1.39 | 0.82, 2.36 | 1.39 | 0.81, 2.38 |
| High school graduate | 0.79 | 0.59, 1.04 | 0.90 | 0.67, 1.20 |
Abbreviations: BP, blood pressure; CI, confidence interval; ECG, electrocardiographic; eGFR, estimated glomerular filtration rate; FEV1, forced expiratory volume in 1 second; Hx, history; HR, hazard ratio; MI, myocardial infarction.
Separate models for each characteristic.
One model with all characteristics included.
Our secondary analysis including heart failure as a covariate showed similar magnitudes of association with AF for all variables (Table S2) except history of MI, for which the association was attenuated by adjustment for heart failure. Heart failure was strongly associated with incident AF, with a hazard ratio of 3.20 in the multivariable model (95%CI: 1.64‐6.23).
4. DISCUSSION
In JHS, a contemporary cohort of AA, we found higher rates of incident AF with increasing age and similar age‐specific AF incidence in men and women. AF incidence rates in JHS were similar to those in MESA and to published rates in ARIC (with the caveat that almost half of JHS incident AF cases occurred in people who were also ARIC participants) but lower than those in CHS in the oldest age ranges. We found that AF ascertainment was increased with the inclusion of Medicare claims data. Modifiable risk factors, including weight, smoking, and SBP, were strongly associated with the risk of incident AF in age‐ and sex‐adjusted models. In a fully adjusted multivariable model, risk estimates for these factors were only slightly attenuated. We also identified associations of higher age and weight, lower FEV1, and history of MI and heart failure with a risk of incident AF.
Strengths of this study include the JHS cohort, which offers a large sample size and a contemporary population of AA participants. With access to Medicare claims data, in addition to study ECGs and hospital surveillance, the ascertainment of AF in JHS is likely more complete than in cohort studies without such data. We demonstrated the utility of adding the Medicare claims data for defining and dating the clinical recognition of AF. However, several limitations should be recognized. Because AF can be transitory and/or asymptomatic, it may not have come to medical attention and would not be identified using our ascertainment methods. While we included data from fee‐for‐service Medicare claims and hospital diagnosis codes between 1987 and 2000 for ARIC participants, not all participants were age 65 or older and eligible for Medicare and not all were in ARIC. In addition, for those 65 years or older, not all were enrolled in fee‐for‐service Medicare.
A previous analysis in ARIC found that the inclusion of Medicare claims data for AF ascertainment offered a more complete picture of AF incidence.18 Our current study builds on those findings, showing that including Medicare claims data can help identify, as prevalent cases, participants who, through study surveillance, had otherwise been categorized as incident cases. In addition, better dating of AF diagnosis through the inclusion of Medicare claims data improves precision when calculating person‐time and AF incidence rates. Finally, in our study, the inclusion of Medicare claims data yielded an additional 15 incident AF events, or 6% of total cases, a small but important increase over ascertainment limited to study ECG and ICD9 hospital surveillance data. Hospital discharge diagnosis codes for AF have been shown to have a positive predictive value of 89%,19 but the positive predictive value of Medicare outpatient, carrier, and home health claims for AF have not been determined. Additional studies are needed of the validity of these types of Medicare claims data.
Our results show lower estimates for AF incidence in AA men and women than have been observed for white men and women in other cohorts.1, 2, 10 Published findings from large cohort studies of mostly white participants, including CHS and the Framingham Heart Study, demonstrate higher rates of AF among men than women in all age groups, related at least in part to differences in height and weight.11 However, among AA participants in JHS and MESA, there was little evidence of higher AF incidence rates in men than in women in any age stratum. By contrast, in CHS, older AA men aged 80 to 89 years appeared to be at greater risk of AF than women of the same age. However, confidence intervals in all three cohorts were wide, and estimated AF incidence, particularly in the older age strata, is imprecise.
In age‐ and sex‐adjusted models, modifiable risk factors, including smoking status, weight, and SBP, were among those most strongly associated with AF risk. In the multivariable Cox proportional hazards model, FEV1, weight, and MI and/or heart failure remained strongly associated with AF risk, consistent with results from predominantly white cohorts.10, 11 In both age‐ and sex‐adjusted and multivariable models, higher AF risk was observed among participants with greater weight and height, consistent with existing analyses of body mass index and AF incidence.10, 20, 21 In contrast to previous studies of biethnic populations,10, 11, 22 diabetes was not associated with AF risk in our multivariable model, which included adjustment for weight and height. The relative contributions of higher weight and diabetes to AF risk in AA require further study.23
5. CONCLUSION
Findings from JHS indicate that Medicare claims data are useful for AF ascertainment in cohort studies. In this contemporary AA cohort with a high mean weight, high prevalence of diabetes, and a relatively low prevalence of current smoking, age‐ and sex‐specific AF incidence was similar to that reported in AA from the MESA cohort and slightly lower than in the CHS cohort. Associations with established AF risk factors were similar to results in other cohorts and underscore the importance of smoking cessation and efforts to control blood pressure and body weight for the prevention of AF.
Supporting information
TABLE S1. Person‐time (PT) at risk, atrial fibrillation (AF) events, and incidence rates of AF among African Americans in the Jackson Heart Study (JHS), Multi‐Ethnic Study of Atherosclerosis (MESA), Cardiovascular Health Study (CHS), and Atherosclerosis Risk in Communities (ARIC) study by gender and age group
TABLE S2. Age and gender‐adjusted and multivariable‐adjusted hazard ratios of incident atrial fibrillation in Jackson Heart Study participants (N = 4205) according to baseline and Exam 2 characteristics, including a history of heart failure
ACKNOWLEDGMENT
The authors thank the participants and data collection staff of the Jackson Heart Study, the Multi‐Ethnic Study of Atherosclerosis, and the Cardiovascular Health Study for their valuable contributions. The Jackson Heart Study is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, and HHSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities. Additional support is from R01 HL121348 and R01 HL127659 from the National Heart, Lung, and Blood Institute. This research in the Cardiovascular Health Study was supported by contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, and N01HC85086 and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). This research in the Multi‐Ethnic Study of Atherosclerosis was supported by contracts HHSN268201500003I, N01‐HC‐95159, N01‐HC‐95160, N01‐HC‐95161, N01‐HC‐95162, N01‐HC‐95163, N01‐HC‐95164, N01‐HC‐95165, N01‐HC‐95166, N01‐HC‐95167, N01‐HC‐95168 and N01‐HC‐95169 from the National Heart, Lung, and Blood Institute and by grants UL1‐TR‐000040, UL1‐TR‐001079, and UL1‐TR‐001420 from NCATS.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; the National Institute for Occupational Safety and Health; or the U.S. Department of Health and Human Services.
Conflict of interest
The authors declare no potential conflict of interests
Austin TR, Wiggins KL, Blackshear C, et al. Atrial fibrillation in an African‐American cohort: The Jackson Heart Study. Clin Cardiol. 2018;41:1049–1054. 10.1002/clc.23020
Funding information National Center for Advancing Translational Sciences (NCATS); National Heart, Lung, and Blood Institute (NHLBI); National Institute on Aging (NIA); National Institute of Neurological Disorders and Stroke (NINDS); National Heart, Lung, and Blood Institute (NHLBI); National Heart, Lung, and Blood Institute (NHLBI); National Institute on Minority Health and Health Disparities (NIMHD); National Heart, Lung, and Blood Institute (NHLBI)
REFERENCES
- 1. Rodriguez CJ, Soliman EZ, Alonso A, et al. Atrial fibrillation incidence and risk factors in relation to race‐ethnicity and the population attributable fraction of atrial fibrillation risk factors: the Multi‐Ethnic Study of Atherosclerosis. Ann Epidemiol. 2015;25(2):71‐76e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alonso A, Agarwal SK, Soliman EZ, et al. Incidence of atrial fibrillation in whites and African‐Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158(1):111‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fuqua SR, Wyatt SB, Andrew ME, et al. Recruiting African‐American research participation in the Jackson Heart Study: methods, response rates, and sample description. Ethn Dis. 2005;15(4 Suppl (6)):S6‐18‐S6‐29. [PubMed] [Google Scholar]
- 4. Taylor HA Jr, Wilson JG, Jones DW, et al. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;15(4 Suppl 6):S6–4‐S6–17. [PubMed] [Google Scholar]
- 5. Carpenter MA, Crow R, Steffes M, et al. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci. 2004;328(3):131‐144. [DOI] [PubMed] [Google Scholar]
- 6. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wallace ER, Siscovick DS, Sitlani CM, et al. Incident atrial fibrillation and disability‐free survival in the cardiovascular health study. J Am Geriatr Soc. 2016;64(4):838‐843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kwon Y, Gharib SA, Biggs ML, et al. Association of sleep characteristics with atrial fibrillation: the Multi‐Ethnic Study of Atherosclerosis. Thorax. 2015;70(9):873‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carlin JB, Galati JC, Royston P. A new framework for managing and analyzing multiply imputed data in Stata. Stata J. 2008;8(1):49‐67. [Google Scholar]
- 10. Jensen PN, Thacker EL, Dublin S, Psaty BM, Heckbert SR. Racial differences in the incidence of and risk factors for atrial fibrillation in older adults: the cardiovascular health study. J Am Geriatr Soc. 2013;61(2):276‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alonso A, Krijthe BP, Aspelund T, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE‐AF consortium. J Am Heart Assoc. 2013;2(2):e000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population‐based cohort. The Framingham Heart Study. JAMA. 1994;271(11):840‐844. [PubMed] [Google Scholar]
- 13. Psaty BM, Manolio TA, Kuller LH, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96(7):2455‐2461. [DOI] [PubMed] [Google Scholar]
- 14. Chamberlain AM, Agarwal SK, Folsom AR, et al. Smoking and incidence of atrial fibrillation: results from the Atherosclerosis Risk in Communities (ARIC) study. Heart Rhythm. 2011;8(8):1160‐1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Misialek JR, Rose KM, Everson‐Rose SA, et al. Socioeconomic status and the incidence of atrial fibrillation in whites and blacks: the Atherosclerosis Risk in Communities (ARIC) study. J Am Heart Assoc. 2014;3(4):e001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bansal N, Zelnick LR, Alonso A, et al. eGFR and albuminuria in relation to risk of incident atrial fibrillation: a meta‐analysis of the Jackson Heart Study, the multi‐ethnic study of atherosclerosis, and the cardiovascular health study. Clin J Am Soc Nephrol. 2017;12(9):1386‐1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alonso A, Lopez FL, Matsushita K, et al. Chronic kidney disease is associated with the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123(25):2946‐2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bengtson LG, Kucharska‐Newton A, Wruck LM, et al. Comparable ascertainment of newly‐diagnosed atrial fibrillation using active cohort follow‐up versus surveillance of centers for medicare and medicaid services in the atherosclerosis risk in communities study. PLoS One. 2014;9(4):e94321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):141‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Frost L, Hune LJ, Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Med. 2005;118(5):489‐495. [DOI] [PubMed] [Google Scholar]
- 21. Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new‐onset atrial fibrillation. JAMA. 2004;292(20):2471‐2477. [DOI] [PubMed] [Google Scholar]
- 22. Huxley RR, Alonso A, Lopez FL, et al. Type 2 diabetes, glucose homeostasis and incident atrial fibrillation: the Atherosclerosis Risk in Communities study. Heart. 2012;98(2):133‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heckbert SR, Wiggins KL, Blackshear C, et al. Pericardial fat volume and incident atrial fibrillation in the Multi‐Ethnic Study of Atherosclerosis and Jackson Heart Study. Obesity (Silver Spring). 2017;25(6):1115‐1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1. Person‐time (PT) at risk, atrial fibrillation (AF) events, and incidence rates of AF among African Americans in the Jackson Heart Study (JHS), Multi‐Ethnic Study of Atherosclerosis (MESA), Cardiovascular Health Study (CHS), and Atherosclerosis Risk in Communities (ARIC) study by gender and age group
TABLE S2. Age and gender‐adjusted and multivariable‐adjusted hazard ratios of incident atrial fibrillation in Jackson Heart Study participants (N = 4205) according to baseline and Exam 2 characteristics, including a history of heart failure


