Abstract
Atopic dermatitis (AD) patients are commonly colonized with Staphylococcus aureus (ADS.aureus+) but what differentiates this group from noncolonized AD subjects (ADS.aureus−) has not been well-studied. To evaluate whether these two groups have unique phenotypic or endotypic features we performed a multi-center, cross-sectional study enrolling ADS.aureus+ (N=51) and ADS.aureus− (N=45) subjects defined by the presence or absence of S. aureus by routine culture techniques and nonatopic, noncolonized controls NAS.aureus− (N=46). Filaggrin (FLG) genotypes were determined and disease severity (EASI, RJL, IGA, NRS and DLQI) was captured. Skin physiology was assessed (transepidermal water loss [TEWL], stratum corneum integrity, hydration and pH) and serum biomarkers were also measured. We found that ADS.aureus+ had more severe disease based on all scoring systems except itch (NRS). They had higher levels of type 2 biomarkers (eosinophil count, tIgE, CCL17, and periostin). Additionally, ADS.aureus+ had significantly greater allergen sensitization (Phadiatop and tIgE), barrier dysfunction (TEWL and SC integrity) and serum LDH than both ADS.aureus− and NAS.aureus− groups. FLG mutations did not associate with S.aureus+ colonization. In conclusion, adult AD participants who are colonized on their skin with S. aureus have more severe disease, greater type 2 immune deviation, allergen sensitization, barrier disruption, and LDH elevation than noncolonized AD subjects.
Keywords: Atopic dermatitis, eczema, Staphylococcus aureus, skin barrier, itch, type 2 immunity, disease severity
INTRODUCTION
Atopic dermatitis (AD) is a complex inflammatory skin disorder with highly variable clinical features and natural history. Many children follow a mild disease course, requiring only short-term anti-inflammatory topical therapy and experience near complete remission by late childhood. Other children endure a more protracted course, with more severe skin lesions and greater susceptibility to allergen sensitization and S. aureus or herpes viral skin infections. As many as 20% of AD cases are adult onset and these patients seem to have fewer concomitant atopic disorders but still experience complications from S. aureus or herpes simplex skin infections (Garmhausen et al., 2013). Significant efforts are underway to better identify the mechanistic explanations for these disparate clinical phenotypes in hopes that this may translate into more personalized treatment approaches with improved outcomes over what is achieved with current therapies (Muraro et al., 2016).
One AD phenotype under study by the Atopic Dermatitis Research Network (ADRN) funded by the National Institute of Allergy and Infectious Diseases is the population that is colonized with S. aureus on the skin surface. Although a number of acquired and/or genetic defects in epithelial or immune functions have been identified and are thought to explain the viral susceptibilities observed in AD patients (Gao et al., 2009, Gao et al., 2012, Howell et al., 2011, Shiohara et al., 2011, Leung et al., 2011, Bussman et al., 2008), the underlying mechanisms associated with S. aureus colonization and infection remain poorly understood. Both skin barrier dysfunction and impaired innate and adaptive immune responses have been implicated as causes of this S. aureus susceptibility (Nakatsuji et al., 2016, Leung 2013, Kuo et al., 2013). S. aureus, in turn, exacerbates AD via effects on innate immune pathways, T-cell activation and barrier integrity (Leung et al., 2017, Kobayashi et al., 2015, Brauweiler et al., 2014, Broccardo et al., 2011). The goal of this study was to more comprehensively evaluate the clinical features, biophysical characteristics of the skin barrier, and peripheral blood and serum biomarkers found in S. aureus− colonized versus non-S. aureus− colonized adult European American, non-Hispanic AD patients to test the hypothesis that there are unique phenotypic or endotypic features that associate with colonization. Understanding the endotypes and phenotypes of colonized patients may help identify patients at risk and generate the basis for rational approaches to modulating S. aureus colonization, preventing S. aureus infection and reducing disease severity in AD.
RESULTS
A total of 145 participants were enrolled, of which 142 met medication washout criteria and were able to complete all enrollment procedures (AD S. aureus+, n=51; AD S. aureus−, n=45; NA S. aureus−, n=46). Table 1 displays demographic, disease severity, and FLG status stratified by presence or absence of AD and S. aureus skin culture status. Compared to AD S. aureus− participants, AD S. aureus+ participants were more likely to be male. As expected, the distribution of FLG mutations differed between all AD participants and NA S. aureus− participants with 37% (29/78) of genotyped AD participants having any FLG mutation compared to only 4% (2/44) of NA S. aureus− participants (p<0.001). Importantly, among AD participants, FLG mutations were not associated with S. aureus colonization. Most AD S. aureus+ (96%) participants had a positive (≥0.35 PAU/L) Phadiatop or multi-aeroallergen screen and were noteworthy for their much higher values (median 51.9 PAU/L; IQR 5.3–301.5 PAU/L), compared to the 78% of AD S. aureus− and 39% of NA S. aureus− who were positive but with much lower values (median 6.1 PAU/L; IQR 0.76–23.1 PAU/L and median 0.18 PAU/L; IQR 0.12–1.2 PAU/L, respectively).
Table 1.
Demographics and Clinical Features
| Characteristic | AD S. aureus+ (N=51) | p-value | AD S. aureus− (N=45) | p-value | NA S. aureus− (N=46) | AD vs NA p-value |
|---|---|---|---|---|---|---|
| Gender, n (%) | ||||||
| Female | 27 (53%) | 0.06 | 33 (73%) | 0.37 | 29 (63%) | >0.99 |
| Male | 24 (47%) | 12 (27%) | 17 (37%) | |||
| Age (yrs), Mean (SD) | 39.1 (12.07) | 0.25 | 36.3 (12.14) | 0.42 | 34.3 (11.87) | 0.10 |
| Site, n (%) | ||||||
| NJH | 23 (45%) | 0.50 | 15 (33%) | >0.99 | 16 (35%) | 0.75 |
| OHSU | 16 (31%) | 16 (36%) | 15 (33%) | |||
| URMC | 12 (24%) | 14 (31%) | 15 (33%) | |||
| Aeroallergen Phadiatop (PAU/L) | ||||||
| Negative (<0.35) | 2 (4%) | 0.01 | 10 (22%) | <0.001 | 28 (61%) | <0.001 |
| Positive (≥0.35) | 49 (96%) | 35 (78%) | 18 (39%) | |||
| Filaggrin Status, n (%) | ||||||
| Compound Heterozygote | 4 (8%) | 0.46 | 1 (2%) | 0.002 | 0 (0%) | <0.001 |
| Heterozygote | 14 (27%) | 10 (22%) | 2 (4%) | |||
| Wild Type | 25 (49%) | 24 (53%) | 42 (91%) | |||
| Missing | 8 (16%) | 10 (22%) | 2 (4%) | |||
| EASI Severity Score, Mean (SD) | 23.8 (13.47) | <0.001 | 8.6 (5.73) | Not applicable | ||
| EASI Severity Category, n (%) | ||||||
| Mild (1.05–7) | 6 (12%) | <0.001 | 24 (53%) | Not applicable | ||
| Moderate (7.05–21) | 19 (37%) | 18 (40%) | ||||
| Severe (21.05–50) | 24 (47%) | 3 (7%) | ||||
| Very Severe (>50) | 2 (4%) | 0 (0%) | ||||
| Rajka-Langeland Severity Score, Mean (SD) | 8.1 (0.96) | <0.001 | 6.5 (1.17) | Not applicable | ||
| Rajka-Langeland Severity Category, n (%) | ||||||
| Mild (3–4) | 0 (0%) | <0.001 | 2 (4%) | Not applicable | ||
| Moderate (4.5–7.5) | 12 (24%) | 36 (80%) | ||||
| Severe (8–9) | 39 (76%) | 7 (16%) | ||||
| Investigator’s Global Assessment, n (%) | ||||||
| Almost Clear | 0 (0%) | <0.001 | 4 (9%) | Not applicable | ||
| Mild | 3 (6%) | 18 (40%) | ||||
| Moderate | 18 (35%) | 21 (47%) | ||||
| Severe | 25 (49%) | 2 (4%) | ||||
| Very Severe | 5 (10%) | 0 (0%) | ||||
| Itch NRS (1–10), Mean (SD) | 7.1 (2.19) | 0.12 | 6.4 (2.29) | Not applicable | ||
| Dermatology Quality of Life Index | ||||||
| Mild (0–5) | 13 (25%) | <0.001 | 17 (38%) | Not applicable | ||
| Moderate (6–10) | 9 (18%) | 19 (42%) | ||||
| Severe (11–30) | 29 (57%) | 9 (20%) |
Note: 11.8% (6/51) of AD S. aureus+ subjects had used systemic drugs (3 systemic steroids, 2 methotrexate and 1 cyclosporine). All had discontinued these ≥ 7 days prior to the enrollment visit. No subjects had been exposed to systemic or topical antibiotics within 7 days of enrollment.
Note: Pairwise comparisons of adjacent columns are done by the Wilcoxon two-sample test or Fisher’s exact test for continuous and discrete variables, respectively. Comparisons of filaggrin status exclude ‘Missing’ status.
All clinical severity scores that measure the signs of AD (EASI, Rajka-Langeland, and IGA scores) and quality of life impairment (DLQI) demonstrated that AD S. aureus+ participants had much more severe disease than AD S. aureus−. In fact, more than 50% of the AD S. aureus+ group had severe disease based on EASI, Rajka-Langeland, IGA and DLQI scores, whereas only 4–20% of AD S. aureus− were classified as severe using the same disease severity assessments. In contrast, AD S. aureus+ subjects did not experience greater itch intensity than AD S. aureus− participants (Table 1) (Rajka et al., 1989, Leshem et al., 2015).
AD S. aureus+ participants had significantly reduced barrier function as assessed by both a higher TEWL AUC and higher basal TEWL compared to AD S. aureus− and NA S. aureus− participants (Figures 1a, b). There was a trend toward reduced skin hydration in AD S. aureus+ compared to AD S. aureus− participants with both groups having significantly reduced hydration compared to NA participants (Figure 1c). Skin surface pH did not differ between the groups (Fig 1d). Serum LDH levels were more elevated in AD S. aureus+ compared to either AD S. aureus− or NA S. aureus− participants consistent with the suggestion that LDH may reflect epithelial damage and/or barrier disruption (Figure 2a) (Rusznak et al., 1999).
Figure 1.
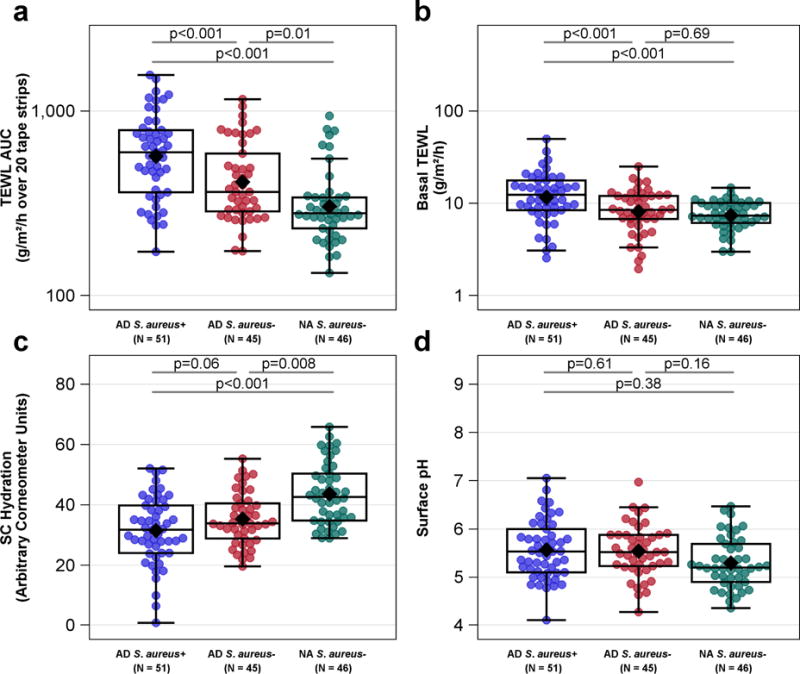
Skin Barrier Measurements by diagnostic group and the absence or presence of S. aureus skin colonization. (a) TEWL AUC was highest in ADS. aureus+ group and distinguished all three groups from each other. (b) Basal TEWL was highest in the ADS. aureus+ group compared to the ADS. aureus− and NAS. aureus− groups. AD S. aureus− was not different than NAS. aureus− group. (c) A borderline trend for lower SC hydration was observed in ADS. aureus+ group compared to the ADS. aureus− group and both AD groups were lower than the NAS. aureus− group. (d) Skin pH was not different between the three patient groups. Means (or geometric means for plots on the log scale) are displayed as black diamonds. Box plots are displayed. Comparisons of values (or log10 values for plots on the log scale) are computed using a generalized linear mixed model adjusting for gender, age, site and FLG mutation status.
Figure 2.
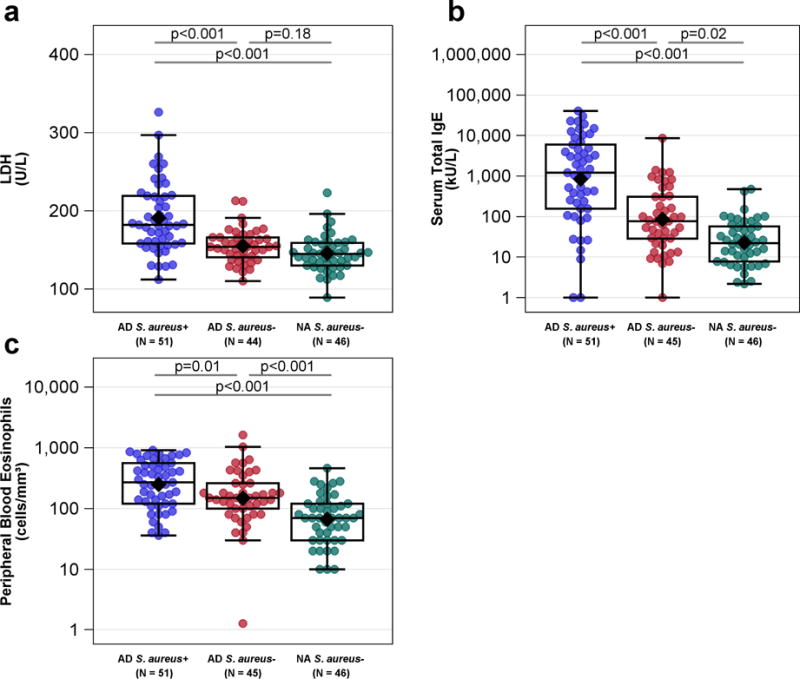
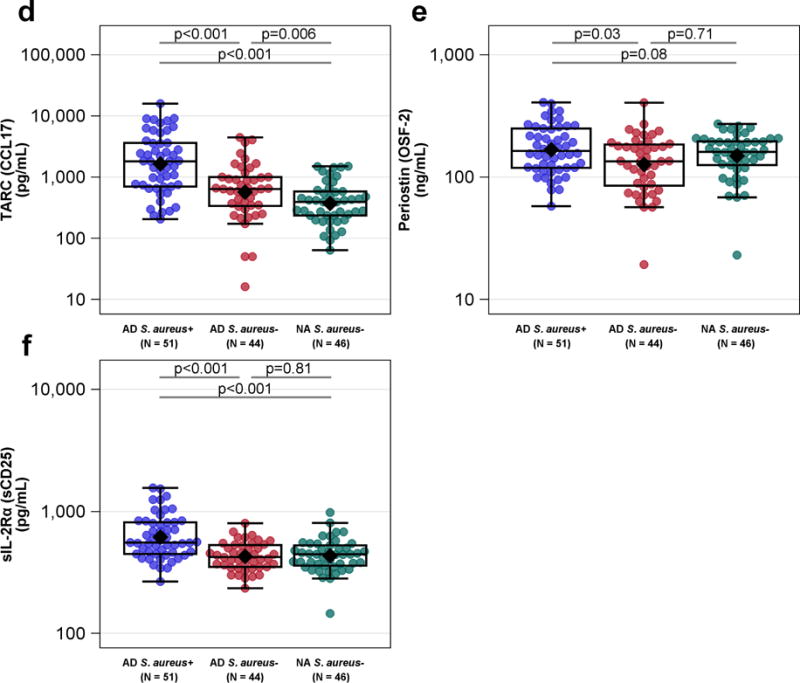
Peripheral Blood Biomarkers by diagnostic group and the absence or presence of S. aureus skin colonization. (b) Serum total IgE (c) peripheral eosinophil counts and (d) TARC (CCL17) were highest in the ADS. aureus+ group and distinguished all three groups from each other. (a) Serum LDH and (f) sIL-2R were highest in the ADS. aureus+ group compared to the ADS. aureus− and NAS. aureus− groups. ADS. aureus− and NAS. aureus− groups did not differ. (e) Periostin (OSF-2) was greater in the ADS. aureus+ than the ADS. aureus− group and had a borderline trend for greater values compared to the NAS. aureus− group. Means (or geometric means for plots on the log scale) are displayed as black diamonds. Box plots are displayed. Comparisons of values (or log10 values for plots on the log scale) are computed using a generalized linear mixed model adjusting for gender, age, site and FLG mutation status. For serum total IgE, 1 kAU/mL = 1000 kAU/L.
Serum total IgE and peripheral blood eosinophil counts were also significantly elevated in AD S. aureus+ participants compared to both AD S. aureus− and NA S. aureus− participants (Figures 2b, 2c). Consistent with these findings, several additional serum biomarkers indicative of type 2 immunity including serum CCL17/Thymus and activation-regulated chemokine (TARC), periostin and CCL26 (eotaxin-3) were able to discriminate between AD S. aureus+ and AD S. aureus− groups (Figures 2d, 2e, data not shown, respectively). Of these only CCL17 significantly discriminated between AD S. aureus− and NA S. aureus− groups. In contrast, serum CCL20 (MIP-3α), believed to reflect Th17 immunity, was not significantly altered as a function of either AD or S. aureus skin colonization (data not shown). Serum sIL-2Rα, a general immune activation marker, was higher in the AD S. aureus+ compared to AD S. aureus− and NA S. aureus− participants (Figure 2f). More than 50% of participant samples had values below the lower limit of detection for the cytokines IL-33, IL-36β and IL-6 and nothing could be inferred from this data (data not shown).
LDH has been shown to strongly correlate with AD disease severity (Thijs et al., 2015) and several groups have hypothesized that the elevations reflect epithelial barrier dysfunction or damage (Thijs et al., 2015). To address the clinical and biological relevance of serum LDH levels, we looked at associations of LDH with disease severity and skin barrier functions combining both AD subgroups. We also observed a strong association between LDH and EASI (β = 0.11, [95% CI] 0.04, 0.17; p<0.001). More importantly, we observed that LDH levels are associated with basal TEWL and inversely with SC hydration (Figures 3a, 3b). There was no significant association between TEWL AUC and LDH (Figure 3c).
Figure 3.
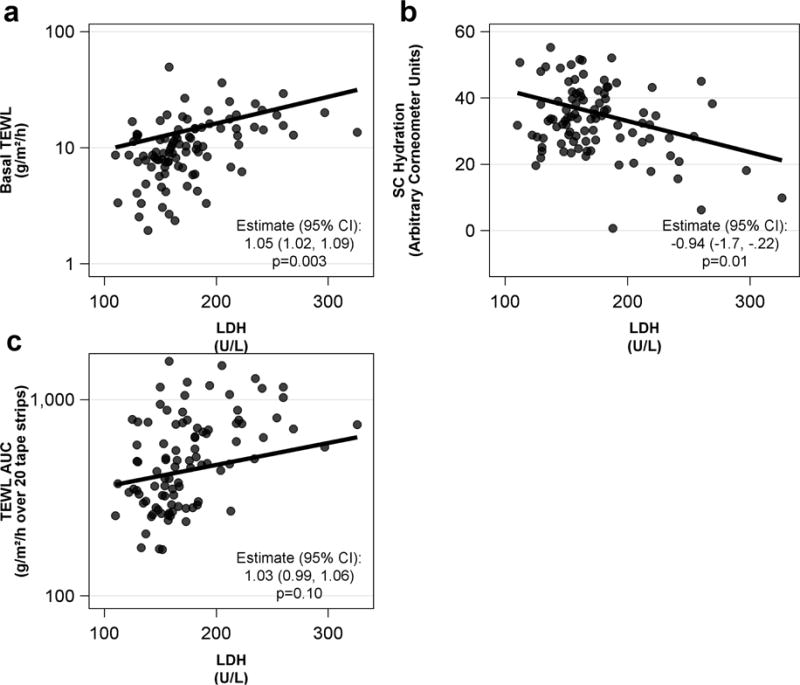
Relationship between serum LDH and skin barrier measurements in ADS. aureus+ and ADS. aureus− participants. LDH is strongly associated with (a) Basal TEWL, (b) SC hydration and (c) trends toward an association with TEWL AUC. The estimated geometric mean ratio (95% CI) reflects a percent change ([1-estimate] x 100) in the response variable for a 10 unit increase in LDH for Basal TEWL and TEWL AUC. The estimate for SC Hydration represents a .94 unit decrease in SC Hydration for every 10 unit increase in LDH. All estimates are computed using a generalized linear mixed model adjusting for diagnostic group, age, site, gender and FLG genotype. The regression line represents an ADS. aureus+ male of median age (38 years old) with wild-type filaggrin status from the URMC study site.
DISCUSSION
This comprehensive cross-sectional study of adult AD patients identified a distinct phenotype and endotype associated with the presence of culturable S. aureus. AD patients colonized with S. aureus have more severe disease and significantly greater impairments in key epidermal functions including the integrity of the stratum corneum, baseline permeability and skin hydration. It is important to emphasize that these epithelial abnormalities were observed in nonlesional skin and to minimize the effects of ultraviolet exposure and anatomical differences in biogeography all assessments were performed at the same location (nonflexural, non-sunexposed, upper extremity). Additionally, blood biomarkers clearly differentiated between subgroups, with S. aureus colonized patients displaying greater immune activation, type 2 immunity, allergen sensitization, and tissue damage compared to AD S. aureus− patients or NA S. aureus− healthy controls. These findings confirm a distinct AD phenotype and endotype in AD patients colonized with S. aureus and provide insight into the underlying mechanisms present in this more severe AD phenotype.
This study used four complementary and validated scoring systems (EASI, RJL, IGA and DLQI) to demonstrate that clinical severity was strongly correlated with S. aureus colonization as measured by routine culture techniques. Other studies using a single severity measure have observed the same association (Nilsson et al., 1992, Alsterholm et al., 2017, Gomes et al., 2011, Guzik et al., 2005, Lipnharski et al., 2013). Our observation was also consistent with the observation that S. aureus predominates in the skin microbiome during flares of AD and becomes less dominant (e.g. greater bacterial diversity) when the disease improves (Kong et al., 2012, Tauber et al., 2016). We objectively quantified itch in our AD participants and were surprised to see that there was no association between S. aureus colonization and itch as measured by NRS. Although it has been suggested that some S. aureus− derived toxins have protease activity and can activate itch-inducing protease-activated receptors-2 (Kong et al., 2012), our study would suggest that S. aureus is unlikely to be the key driver of pruritus in this disease (Kempkes et al., 2014).
Whether the link between S. aureus colonization and skin barrier function, AD severity, type 2 immunity or allergen sensitization is causal or may in fact be bidirectional has not been elucidated. On the one hand, type 2 cytokines may increase S. aureus susceptibility by enhancing epithelial expression of proteins such as fibronectin and fibrinogen that promote S. aureus adherence (Cho et al., 2001a, Cho et al., 2001b). Inflamed AD skin also displays an impaired ability to upregulate antimicrobial peptides (Ong et al., 2002) and reduced expression of a number of barrier proteins (FLG, loricrin, involucrin, long chain free fatty acids) due in large part to the actions of type 2 cytokines on keratinocytes (Howell et al., 2006, Kim et al., 2008, Danso et al., 2014). Additionally, type 2 cytokines have been shown to enhance S. aureus alpha toxin – mediated keratinocyte cell death which may provide an explanation for the elevated LDH and barrier disruption we observed in AD S. aureus+ participants (Brauweiler et al., 2014). Our serum biomarkers suggest that AD S. aureus+ subjects have a high Th2/Th17 ratio which may result in an insufficient production antimicrobial peptide and thereby perpetuating S. aureus colonization (Eyerich et al., 2009).
In contrast, S. aureus may drive disease severity by enhancing inflammation. S. aureus derived enterotoxins activate T cells, increase the expression of the skin T cell homing receptor called cutaneous lymphocyte antigen (CLA), activate Langerhans cells and dendritic cells and lead to IgE reactivity (Reginald et al., 2011, Bunikowski et al., 2000). Consistent with this, AD patients with more severe disease have higher levels of Staphylococcal enterotoxin (SE)-A and SEB-specific serum IgE levels (Ide et al., 2004, Bunikowski et al., 1999). Furthermore, murine studies have demonstrated that Staphylococcal enteroxins applied epicutaneously lead to a type 2 immune response (Laouini et al., 2003). Human clinical studies do not provide much clarity on whether S. aureus drives disease severity. Breuer, et al found intensive S. aureus decolonization alone reduces disese severity in a small uncontrolled study. In contrast, a systematic review of trials of antibiotics use in infected or uninfected AD did not find a significant benefit on disease severity. (Birnie et al., 2008, Breuer et al., 2000).
Our study found that S. aureus colonization was not only associated with clinical severity but also was associated with profound effects on skin barrier function of clinically unaffected or nonlesional skin. Our results confirm and expand upon other clinical studies showing a defective skin barrier in S. aureus colonized AD patients (Jinnestal et al., 2014, Tauber et al., 2016). One study in infants, did not find such an association, but they were looking at the relationship between the presence of S. aureus in the nares and skin barrier function (Berents et al., 2015). A feature of our study, that to our knowledge has not previously been reported, was the investigation of barrier integrity (e.g. TEWL AUC), in addition to simply basal permeability (e.g. TEWL). Barrier integrity is thought to reflect the function of the corneodesmosomes (Igawa et al., 2013, Jonca et al., 2011). We found S. aureus colonization negatively affected both basal permeability and skin integrity. Possible mechanisms underlying these findings include the ability of S. aureus to produce and/or activate serine proteases from keratinocytes ultimately leading to degradation of both desmosomal structures and lipid bilayers (Nakatsuji et al., 2016, Wang et al., 2017, Williams et al., 2017).
We found that FLG mutations were not associated with S. aureus colonization in this European American, non-Hispanic cohort. The role of FLG mutations in S. aureus colonization remains unclear. In vitro models predict filaggrin deficiency would promote S. aureus colonization as filaggrin breakdown products modestly inhibit S. aureus growth (Miajlovic et al., 2010). Consistent with these in vitro findings, Clausen et al. found an association of S. aureus colonization and FLG mutations (Clausen et al., 2017). In contrast, proteomic profiling of tape strips from AD participants colonized with S. aureus failed to show reduced filaggrin compared with noncolonized AD controls (Broccardo et al., 2011) consistent with our findings and the findings of a longitudinal cohort of infants (Berents et al., 2015). It is possible that the greater expression of type 2 cytokines found in our AD participants, may have sufficiently reduced FLG expression and thereby masked the effect of FLG null mutations (Howell et al., 2008). In other words, more severe AD patients would have a functional filaggrin deficiency that may blunt any associations with germline FLG mutations.
Analysis of peripheral blood biomarkers revealed that AD patients colonized with S. aureus have greater systemic immune activation, tissue damage, type 2 immune deviation and allergen sensitization than those without colonization. Consistent with our results, previous studies have correlated both CCL17/TARC and LDH (Thijs et al., 2015), with AD severity, but S. aureus colonization status was not addressed in these studies. LDH elevations are thought to reflect epidermal damage and in support of this theory our study demonstrated a strong association with basal TEWL measurements. Interestingly, LDH is also elevated in erythrodermic cutaneous T-cell lymphoma (Miyagaki et al., 2011), another type 2 immune disorder, and in contrast it is not a severity biomarker for the Th17-driven inflammatory skin disorder psoriasis. The high Phadiatop values observed in the AD S. aureus+ group suggest that colonization may somehow play a role in allergen sensitization. Interestingly, a similar association has been seen with food allergen sensitization and S. aureus skin colonization in children with AD (Jones et al., 2016). This suggests that reducing S. aureus colonization early in the course of AD may limit allergen sensitization and in so doing limit disease activity.
The limitations of this study include the cross-sectional design and inability to show causality for any of our associations. Other than FLG mutations, all biological markers measuring skin barrier function and type 2 inflammation can vary quite rapidly thus we were unable to determine whether these markers represented a state intrinsic to the patient or were in response to S. aureus colonization. This causality question could be addressed by an interventional trial where colonization is eradicated with antibacterial therapies, but such trials have not achieved a sustained reduction in S. aureus colonization (Breuer et al., 2002). An alternative approach would be to reduce S. aureus colonization using a skin microbiome transplant (Nakatsuji et al., 2017). To clarify the role that type 2 immunity plays in S. aureus colonization, one could treat patients with the anti-IL-4Rα targeted biologic, dupilumab, to determine if this affected S. aureus colonization and the kinetics of these affects on skin barrier restoration (Beck et al., 2014). Longitudinal studies of large cohorts may also begin to address causality. In fact two recent longitudinal studies of infants demonstrated two potentially compatible observations; namely, that the presence of S. aureus at three months or the absence of commensal staphylococci predicted AD development (Meylan et al., 2017, Kennedy et al., 2017).
In our study, we hypothesized that AD severity was a mediator of barrier dysfunction, consequently no adjustments were made for AD disease severity in our analyses. However, some may consider AD severity as a potential confounder and therefore the lack of adjustment may yield biased results. In that case, S. aureus colonization may not be the primary driver of our barrier or peripheral blood findings, and could possibly be explained by the underlying disease severity. Lastly, defining colonization by culture results may not provide a complete picture of the S. aureus burden as it captures only replicating bacteria and probably does not accurately mirror the in vivo situation where the host antimicrobial response and the actions of commensal bacteria would be relevant.
In conclusion, we have performed a comprehensive evaluation of adult AD participants who are colonized with S. aureus have found that they have a unique clinical phenotype and endotype, which does not show a clear association with FLG gentoype. Our most robust observations were the associations of cutaneous S. aureus with disease severity, Th2 biomarkers, allergen sensitization, skin barrier disruption and tissue damage. Notably, AD S. aureus+ patients were not troubled by more intense pruritus. Additionally, we have shown that barrier disruption and tissue damage may be intimately connected as evidenced by the strong association between barrier measures and serum LDH values. This highlights a potential opportunity to affect allergen sensitization by early interventions to normalize skin barrier defects and/or S. aureus colonization. Importantly, longitudinal studies are needed to address the causality of the associations we have observed.
MATERIALS & METHODS
Study Design
We report our observations made from a North American AD cross-sectional study which was performed at 3 academic centers: Oregon Health & Science University in Portland, Oregon; University of Rochester Medical Center in Rochester, New York; and National Jewish Medical Center in Denver, Colorado. All study documents and procedures were approved by university-based institutional review boards at each study site, and each participant provided written informed consent before study participation.
Population
We enrolled adult participants between 18 and 60 years of age with active AD diagnosed using published criteria (Eichenfield et al., 2003) and non-atopic (NA) controls defined as those without a personal or family history of atopic diseases. This study had the additional inclusion criterion of participants self-reporting as being of European descent and non-Hispanic because a focus of this study was to address the importance of filaggrin (FLG) mutations on S. aureus colonization and this is the only racial/ethnic group for which the major FLG mutations have been adequately identified (Irvine et al., 2011). Key exclusion criteria included: active systemic malignancy; skin disease other than AD that may affect skin barrier function or other severe immunological disease such as systemic lupus erythematosus; pregnant females; clinically active viral or bacterial skin infections; or life-threatening allergy to adhesives, lidocaine or latex. The full inclusion and exclusion criteria for this study are available in the online supplement.
Skin swabs for culture were taken from both lesional and non-lesional skin from the volar surface of the arms in all patients. Non-lesional skin was defined as skin free of inflammatory lesions consistent with AD-erythema, papules, lichenification, excoriation and approximately 2 cm from the edge of a lesion. Because xerosis may be present outside of inflamed skin (e.g. ichthyosis vulgaris) or from environmental conditions, xerotic skin was not considered lesional skin as long as no inflammatory signs were present. Participants were stratified into 3 groups based on their culture results and AD status: AD S. aureus+, AD S. aureus− and NA S. aureus− controls after the procedures listed below were performed. S. aureus positivity was defined by the growth of S. aureus from routine microbiological cultures obtained from either lesional (L) or non-lesional (Kong et al., 2012) skin swabs. The rationale behind including S. aureus positivity from either lesional or non-lesional was that we were interested in characterizing patients whose inflammatory skin disease was potentially driven or exacerbated by S. aureus in the absence of overt signs of infection. AD S. aureus− was defined as no growth from both skin sites. Only five NA controls (9.8%) were S. aureus positive and because of such low numbers, this group was not included in analyses.
Procedures
Skin Barrier Assessments
Barrier integrity was measured by transepidermal water loss (TEWL) before tape stripping (basal TEWL) and after 5, 10, 15 and 20 tape strips and evaluated as TEWL area under the curve (TEWL AUC). The D-SQUAME® standard skin sampling discs with diameter of 22.0 mm were pressed on the non-lesional, nonflexural skin of the upper extremity in patients with AD or NA participants and subsequently removed. The D-SQUAME pressure instrument D500 (http://store.cuderm.com/d100-d-squame-standard-sampling-discs/) was used to apply all tape strips with equivalent pressure (e.g. 225 g/cm2).
TEWL AUC was the primary endpoint for this study. TEWL AUC integrates the total TEWL change after tape stripping. Higher TEWL values reflect greater stratum corneum removal with each tape strip thus the AUC represents a measure of stratum corneum integrity. Basal TEWL reflects the baseline undisturbed permeability of the skin barrier. TEWL was assessed using the AquaFlux AF200 (Biox, London, UK). Skin hydration was assessed by measuring stratum corneum (SC) hydration using the Corneometer CM825 and surface pH using Skin-pH-Meter PH 905 (Courage and Khazaka Electronic GmbH, Kohn, GE). All skin barrier measurements were made on non-lesional, non-flexural, non-sun exposed skin from the upper extremity. All TEWL measurements were made in temperature and climate-controlled conditions with ambient temperatures between 21.3 and 28.7 degrees C (mean=23.6, SD=1.0) and ambient relative humidity between 9.2% and 63.4% (mean=35.0, SD=14.1). The observed variations in temperature and humidity seemed to reflect seasonal and geographic differences (data not shown). However, when adjusting for humidity and temperature in exploratory models, the TEWL results were not affected.
Disease Severity Assessments
Disease severity was determined using the Eczema Area and Severity Index (EASI) which evaluates the disease status at the time the procedures were performed, the Rajka-Langeland severity score (which provides a disease severity assessment that incorporates historical data and body surface area), the numerical rating scale (NRS) for pruritus (1–10), and an investigator’s global assessment (Igawa et al., 2013) asked on the question, “In the Investigator’s opinion, what is the overall severity of the participant’s atopic dermatitis at this visit?” To address the effect of S. aureus colonization has on quality of life metrics we had patients answer the Dermatology Life Quality (DLQ) questionnaire (Finlay, et al., 1994).
Routine Laboratory Assays
Blood samples were sent to Quest Diagnostics Laboratory for a complete blood count (CBC) with differential and to the Dermatology, Allergy, and Clinical Immunology (DACI) laboratory at Johns Hopkins Asthma and Allergy Center for serum total IgE (kilounits per liter) and a multiallergen, ImmunoCAP Phadiatop test (Phadia Arbitrary Units [PAU] per liter). A serum aliquot was also sent to UR Medicine Labs at University of Rochester Medical Center for lactate dehydrogenase (LDH) measurement (units per liter).
Serum Biomarker Measurements
The following serum biomarkers were quantified using a human premixed multi-analyte kit designed for human magnetic Luminex® screening assay (R&D Systems/Bio-Techne): Premixed 1: CXCL1, CXCL9, CXCL10, CXCL11, CXCL8, CCL2, CCL13; Premixed 2: periostin, CCL17, CCL20, CCL26 and sIL-2R alpha (sCD25); Individual: CCL18.
FLG Genotype
The four most common filaggrin (FLG) null mutations (2282del4, R501X, R2447X and S3247X) were identified by ABI Taqman genotyping with PCR primers for amplifying the polymorphic sequence of interest and two TaqMan MGB probes for distinguishing between alleles on 60 samples (19 AD S. aureus+, 21 AD S. aureus−, 20 NA S. aureus−) at National Jewish Health or Johns Hopkins School of Medicine. An additional 62 samples (24 AD S. aureus+, 14 AD S. aureus−, 24 NA S. aureus−) had their FLG mutations identified by whole genome sequencing using Illumina HiSeq at 30x depth of coverage. R501X, R2447X, and S3247X were derived from multi-sample SNPs.vcf files and 2282del4 was derived from multi-sample indels.vcf files.
Sample Size
A minimum of 45 participants per group (AD S. aureus+, AD S. aureus− and NA S. aureus−) was pre-specified in consideration of the available resources and time constraints for this pilot study. Using effect sizes from published data (Danby et al., 2011), an independent two-sample t-test and a two-sided significance level of 0.05, this sample size allowed us to detect a difference in TEWL AUC between the AD S. aureus+ and AD S. aureus− groups with at least 98% power.
Statistical Analyses
Demographics, AD clinical features and aeroallergen sensitization were compared between diagnostic groups using Fisher’s exact test for categorical measures and Wilcoxon 2-sample test for continuous measures; results are displayed as count (%) or mean (standard deviation) for discrete and continuous measures, respectively.
Comparisons between diagnostic groups in skin barrier measures (TEWL, SC hydration and surface pH), and serum and blood biomarkers were analyzed using a general linear regression model, adjusting for age, gender, study site and FLG genotype. Least squares means or geometric means (for non-normally distributed values) were estimated by diagnostic group. For normally distributed markers, estimates of the mean difference between diagnostic groups and 95% confidence interval were calculated. Non-normally distributed markers were log10 transformed prior to analysis, and results were back-transformed to estimate least squares geometric mean ratios and 95% confidence intervals between diagnostic groups. Except for the TEWL AUC comparison between the AD S. aureus+ and AD S. aureus− groups, all p-values reported were considered descriptive. No adjustments for multiple comparisons were made. SAS version 9.4 software (SAS Institute, Inc., Cary, NC) was used for all analyses.
Supplementary Material
Acknowledgments
The authors acknowledge Marshall Plaut, MD, NIAID project scientist and reviewer; Catherine Philpot at Rho, Inc. for centralized study coordination; and the following study coordinators for their hard work in recruiting human participants for this study: at University of Rochester Medical Center, Jean Sauvain, Nelissa Perez-Nazario, and Diane Wang, MD; at National Jewish Health, Patricia Taylor, FNP-C, Caroline Bronchick, RN, Amy Hoeft, RN and Anais Sanchez; and at Oregon Health & Science University, Emma Hill, Danielle McClanahan, Allison Wong, Susan Tofte, FNP, and Tamar Hajar, MD.
FUNDING SOURCES
This work was funded by the NIH/NIAID Atopic Dermatitis Research Network grants U19AI117673 and UM2AI117870.
Abbreviations
- AD
atopic dermatitis
- AUC
area under the curve
- CBC
complete blood count
- CCL
chemokine (C-C motif) ligand
- CFU
colony forming units
- CLA
cutaneous lymphocyte antigen
- CXCL
chemokine (C-X-C motif) ligand
- DACI
Dermatology, Allergy, and Clinical Immunology
- DLQI
Dermatology Life Quality Index
- DNA
deoxyribonucleic acid
- EASI
Eczema Area and Severity Index
- FLG
filaggrin
- IGA
Investigator’s Global Assessment Score
- LDH
Lactate Dehydrogenase
- NA
non-atopic
- NRS
Numerical Rating Scale
- PAU
Phadia Arbitrary Units
- qPCR
quantitative polymerase chain reaction
- RJL
Rajka and Langeland Severity Score
- S. aureus
Staphylococcus aureus
- SC
stratum corneum
- SE
Staphylococcal enterotoxin
- TARC
Thymus and activation-regulated chemokine
- TEWL
transepidermal water loss
- Th2
T helper type 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Alsterholm M, Strombeck L, Ljung A, Karami N, Widjestam J, Gillstedt M, et al. Variation in Staphylococcus aureus Colonization in Relation to Disease Severity in Adults with Atopic Dermatitis during a Five-month Follow-up. Acta Derm Venereol. 2017;97:802–7. doi: 10.2340/00015555-2667. [DOI] [PubMed] [Google Scholar]
- Beck LA, Thaci D, Hamilton JD, Graham NM, Bieber T, Rocklin R, et al. Dupilumab Treatment in Adults with Moderate-to-Severe Atopic Dermatitis. N Engl J Med. 2014;371(2):130–9. doi: 10.1056/NEJMoa1314768. [DOI] [PubMed] [Google Scholar]
- Berents TL, Carlsen KC, Mowinckel P, Skjerven HO, Kvenshagen B, Rolfsjord LB, et al. Skin Barrier Function and Staphylococcus aureus Colonization in Vestibulum Nasi and Fauces in Healthy Infants and Infants with Eczema: A Population-Based Cohort Study. PLoS One. 2015;10(6):e0130145. doi: 10.1371/journal.pone.0130145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnie AJ, Bath-Hextall FJ, Ravenscroft JC, Williams HC. Interventions to reduce Staphylococcus aureus in the management of atopic eczema. Cochrane Database Syst Rev. 2008:Cd003871. doi: 10.1002/14651858.CD003871.pub2. [DOI] [PubMed] [Google Scholar]
- Brauweiler AM, Goleva E, Leung DY. Th2 cytokines increase Staphylococcus aureus alpha toxin-induced keratinocyte death through the signal transducer and activator of transcription 6 (STAT6) J Invest Dermatol. 2014;134:2114–21. doi: 10.1038/jid.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer K, Haussler S, Kapp A, Werfel T. Staphylococcus aureus: colonizing features and influence of an antibacterial treatment in adults with atopic dermatitis. Br J Dermatol. 2002;147(1):55–61. doi: 10.1046/j.1365-2133.2002.04872.x. [DOI] [PubMed] [Google Scholar]
- Breuer K, Wittmann M, Bosche B, Kapp A, Werfel T. Severe atopic dermatitis is associated with sensitization to staphylococcal enterotoxin B (SEB) Allergy. 2000;55:551–5. doi: 10.1034/j.1398-9995.2000.00432.x. [DOI] [PubMed] [Google Scholar]
- Broccardo CJ, Mahaffey S, Schwarz J, Wruck L, David G, Schlievert PM, et al. Comparative proteomic profiling of patients with atopic dermatitis based on history of eczema herpeticum infection and Staphylococcus aureus colonization. J Allergy Clin Immunol. 2011;127(1):186–93. e1–11. doi: 10.1016/j.jaci.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunikowski R, Mielke M, Skarabis H, Herz U, Bergmann RL, Wahn U, et al. Prevalence and role of serum IgE antibodies to the Staphylococcus aureus-derived superantigens SEA and SEB in children with atopic dermatitis. J Allergy Clin Immunol. 1999;103:119–24. doi: 10.1016/s0091-6749(99)70535-x. [DOI] [PubMed] [Google Scholar]
- Bunikowski R, Mielke ME, Skarabis H, Worm M, Anagnostopoulos I, Kolde G, et al. Evidence for a disease-promoting effect of Staphylococcus aureus-derived exotoxins in atopic dermatitis. J Allergy Clin Immunol. 2000;105:814–9. doi: 10.1067/mai.2000.105528. [DOI] [PubMed] [Google Scholar]
- Bussmann C, Peng WM, Bieber T, Novak N. Molecular pathogenesis and clinical implications of eczema herpeticum. Expert Rev Mol Med. 2008;10:e21. doi: 10.1017/S1462399408000756. [DOI] [PubMed] [Google Scholar]
- Cho SH, Strickland I, Boguniewicz M, Leung DY. Fibronectin and fibrinogen contribute to the enhanced binding of Staphylococcus aureus to atopic skin. The Journal of allergy and clinical immunology. 2001;108:269–74. doi: 10.1067/mai.2001.117455. [DOI] [PubMed] [Google Scholar]
- Cho SH, Strickland I, Tomkinson A, Fehringer AP, Gelfand EW, Leung DY. Preferential binding of Staphylococcus aureus to skin sites of Th2-mediated inflammation in a murine model. J Invest Dermatol. 2001;116:658–63. doi: 10.1046/j.0022-202x.2001.01331.x. [DOI] [PubMed] [Google Scholar]
- Clausen ML, Edslev SM, Andersen PS, Clemmensen K, Krogfelt KA, Agner T. Staphylococcus aureus colonization in atopic eczema and its association with filaggrin gene mutations. Br J Dermatol. 2017 doi: 10.1111/bjd.15470. [DOI] [PubMed] [Google Scholar]
- Danby SG, Al-Enezi T, Sultan A, Chittock J, Kennedy K, Cork MJ. The effect of aqueous cream BP on the skin barrier in volunteers with a previous history of atopic dermatitis. Br J Dermatol. 2011;165:329–34. doi: 10.1111/j.1365-2133.2011.10395.x. [DOI] [PubMed] [Google Scholar]
- Danso MO, van Drongelen V, Mulder A, van Esch J, Scott H, van Smeden J, et al. TNF-alpha and Th2 cytokines induce atopic dermatitis-like features on epidermal differentiation proteins and stratum corneum lipids in human skin equivalents. J Invest Dermatol. 2014;134:1941–50. doi: 10.1038/jid.2014.83. [DOI] [PubMed] [Google Scholar]
- Eichenfield LF, Hanifin JM, Luger TA, Stevens SR, Pride HB. Consensus conference on pediatric atopic dermatitis. J Am Acad Dermatol. 2003;49:1088–95. doi: 10.1016/s0190-9622(03)02539-8. [DOI] [PubMed] [Google Scholar]
- Eyerich K, Pennino D, Scarponi D, Foerster S, Nasorri F, Behrendt H, et al. IL-17 in atopic ecezema: linking allergen-specific adaptive and mirobial-triggered innate immune response. J Allergy Clin Immunol. 2009;123(1):56–66.e4. doi: 10.1016/j.jaci.2008.10.031. [DOI] [PubMed] [Google Scholar]
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–6. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- Gao PS, Leung DY, Rafaels NM, Boguniewicz M, Hand T, Gao L, et al. Genetic variants in interferon regulatory factor 2 (IRF2) are associated with atopic dermatitis and eczema herpeticum. J Invest Dermatol. 2012;132:650–7. doi: 10.1038/jid.2011.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao PS, Rafaels NM, Hand T, Murray T, Boguniewicz M, Hata T, et al. Filaggrin mutations that confer risk of atopic dermatitis confer greater risk for eczema herpeticum. J Allergy Clin Immunol. 2009;124:507–13. e1–7. doi: 10.1016/j.jaci.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmhausen D, Hagemann T, Bieber T, Dimitriou I, Fimmers R, Diepgen T, et al. Characterization of different courses of atopic dermatitis in adolescent and adult patients. Allergy. 2013;68:498–506. doi: 10.1111/all.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes PL, Malavige GN, Fernando N, Mahendra MH, Kamaladasa SD, Seneviratne JK, et al. Characteristics of Staphylococcus aureus colonization in patients with atopic dermatitis in Sri Lanka. Clin Exp Dermatol. 2011;36:195–200. doi: 10.1111/j.1365-2230.2010.03962.x. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Bzowska M, Kasprowicz A, Czerniawska-Mysik G, Wójcik K, Szmyd D, et al. Persistent skin colonization with Staphylococcus aureus in atopic dermatitis: relationship to clinical and immunological parameters. Clin Exp Allergy. 2005;35:448–55. doi: 10.1111/j.1365-2222.2005.02210.x. [DOI] [PubMed] [Google Scholar]
- Howell MD, Fairchild HR, Kim BE, Bin L, Boguniewicz M, Redzic JS, et al. Th2 cytokines act on S100/A11 to downregulate keratinocyte differentiation. J Invest Dermatol. 2008;128(9):2248–58. doi: 10.1038/jid.2008.74. [DOI] [PubMed] [Google Scholar]
- Howell MD, Gallo RL, Boguniewicz M, Jones JF, Wong C, Streib JE, et al. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity. 2006;24:341–8. doi: 10.1016/j.immuni.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Howell MD, Gao P, Kim BE, Lesley LJ, Streib JE, Taylor PA, et al. The signal transducer and activator of transcription 6 gene (STAT6) increases the propensity of patients with atopic dermatitis toward disseminated viral skin infections. J Allergy Clin Immunol. 2011;128:1006–14. doi: 10.1016/j.jaci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide F, Matsubara T, Kaneko M, Ichiyama T, Mukouyama T, Furukawa S. Staphylococcal enterotoxin-specific IgE antibodies in atopic dermatitis. Pediatrics international: official journal of the Japan Pediatric Society. 2004;46:337–41. doi: 10.1111/j.1442-200x.2004.01880.x. [DOI] [PubMed] [Google Scholar]
- Igawa S, Kishibe M, Honma M, Marakami M, Mizuno Y, Suga Y, et al. Aberrant distribution patterns of corneodesmosomal components of tape-stripped corneocytes in atopic dermatitis and related skin conditions (ichthyosis vulgaris, Netherton syndrome and peeling skin syndrome type B) J Dermatol Sci. 2013;72:54–60. doi: 10.1016/j.jdermsci.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011;365:1315–27. doi: 10.1056/NEJMra1011040. [DOI] [PubMed] [Google Scholar]
- Jinnestal CL, Belfrage E, Back O, Schmidtchen A, Sonesson A. Skin barrier impairment correlates with cutaneous Staphylococcus aureus colonization and sensitization to skin-associated microbial antigens in adult patients with atopic dermatitis. Int J Dermatol. 2014;53:27–33. doi: 10.1111/ijd.12198. [DOI] [PubMed] [Google Scholar]
- Jonca N, Leclerc EA, Caubet C, Simon M, Guerrin M, Serre G. Corneodesmosomes and corneodesmosin: from the stratum corneum cohesion to the pathophysiology of genodermatoses. Eur J Dermatol. 2011;21(Suppl 2):35–42. doi: 10.1684/ejd.2011.1264. [DOI] [PubMed] [Google Scholar]
- Jones AL, Curran-Everett D, Leung DY. Food allergy is associated with Staphylococcus aureus colonization in children with atopic dermatitis. J Allergy Clin Immunol. 2016;137:1247–8. e1–3. doi: 10.1016/j.jaci.2016.01.010. [DOI] [PubMed] [Google Scholar]
- Kempkes C, Buddenkotte J, Cevikbas F, Buhl T, Steinhoff M. Role of PAR-2 in Neuroimmune Communication and Itch. In: Carstens E, Akiyama T, editors. Itch: Mechanisms and Treatment. Boca Raton (FL): CRC Press/Taylor & Francis; 2014. Chapter 11. Frontiers in Neuroscience. [PubMed] [Google Scholar]
- Kennedy EA, Connolly J, Hourihane JO, Fallon PG, McLean WH, Murray D, et al. Skin microbiome before development of atopic dermatitis: Early colonization with commensal staphylococci at 2 months is associated with a lower risk of atopic dermatitis at 1 year. J Allergy Clin Immunol. 2017;139(1):166–172. doi: 10.1016/j.jaci.2016.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BE, Leung DY, Boguniewicz M, Howell MD. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin Immunol. 2008;126:332–7. doi: 10.1016/j.clim.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Glatz M, Horiuchi K, Kawasaki H, Akiyama H, Kaplan DH, et al. Dysbiosis and Staphylococcus aureus Colonization Drives Inflammation in Atopic Dermatitis. Immunity. 2015;42:756–66. doi: 10.1016/j.immuni.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22:850–9. doi: 10.1101/gr.131029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo IH, Yoshida T, De Benedetto A, Beck LA. The cutaneous innate immune response in patients with atopic dermatitis. J Allergy Clin Immunol. 2013;131:266–78. doi: 10.1016/j.jaci.2012.12.1563. [DOI] [PubMed] [Google Scholar]
- Laouini D, Kawamoto S, Yalcindag A, Bryce P, Mizoguchi E, Oettgen H, et al. Epicutaneous sensitization with superantigen induces allergic skin inflammation. J Allergy Clin Immunol. 2003;112:981–7. doi: 10.1016/j.jaci.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Leshem YA, Hajar T, Hanifin JM, Simpson EL. What the Eczema Area and Severity Index score tells us about the severity of atopic dermatitis: an interpretability study. Br J Dermatol. 2015;172:1353–7. doi: 10.1111/bjd.13662. [DOI] [PubMed] [Google Scholar]
- Leung DY, Gao PS, Grigoryev DN, Rafaels NM, Streib JE, et al. Human atopic dermatitis complicated by eczema herpeticum is associated with abnormalities in IFN-gamma response. J Allergy Clin Immunol. 2011;127:965–73. e1–5. doi: 10.1016/j.jaci.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung DY. New insights into atopic dermatitis: role of skin barrier and immune dysregulation. Allergol Int. 2013;62:151–61. doi: 10.2332/allergolint.13-RAI-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung DYM, Jepson B, Beck LA, Hanifin JM, Schneider LC, et al. A clinical trial of intradermal and intramuscular seasonal influenza vaccination in patients with atopic dermatitis. J Allergy Clin Immunol. 2017;139:1575–82. doi: 10.1016/j.jaci.2016.12.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipnharski C, d’Azevedo PA, Quinto VP, Bessa G, Bonamigo RR. Colonization by S. aureus increases the EASI and the number of appointments by patients with atopic dermatitis: cohort with 93 patients. An Bras Dermatol. 2013;88:518–21. doi: 10.1590/abd1806-4841.20132046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meylan P, Lang C, Mermoud S, Johannsen A, Norrenberg S, Hohl D, et al. Skin Colonization by Staphylocossus aureus precedes the Clinical Diagnosis of Atopic Dermatitis in Infancy. J Invest Dermatol. 2017;137(12):2497–2504. doi: 10.1016/j.jid.2017.07.834. [DOI] [PubMed] [Google Scholar]
- Miajlovic H, Fallon PG, Irvine AD, Foster TJ. Effect of filaggrin breakdown products on growth of and protein expression by Staphylococcus aureus. J Allergy Clin Immunol. 2010;126:1184–90 e3. doi: 10.1016/j.jaci.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagaki T, Sugaya M. Erythrodermic cutaneous T-cell lymphoma: how to differentiate this rare disease from atopic dermatitis. J Dermatol Sci. 2011;64:1–6. doi: 10.1016/j.jdermsci.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Muraro A, Lemanske RF, Jr, Hellings PW, Akdis CA, Bieber T, Casale TB, et al. Precision medicine in patients with allergic diseases: Airway diseases and atopic dermatitis-PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2016;137:1347–58. doi: 10.1016/j.jaci.2016.03.010. [DOI] [PubMed] [Google Scholar]
- Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, Yun T, et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med. 2017;9(378) doi: 10.1126/scitranslmed.aah4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuji T, Chen TH, Two AM, Chun KA, Narala S, Geha RS, et al. Staphylococcus aureus Exploits Epidermal Barrier Defects in Atopic Dermatitis to Trigger Cytokine Expression. J Invest Dermatol. 2016;136:2192–200. doi: 10.1016/j.jid.2016.05.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson EJ, Henning CG, Magnusson J. Topical corticosteroids and Staphylococcus aureus in atopic dermatitis. JouJ Am Acad Dermatol. 1992;27:29–34. doi: 10.1016/0190-9622(92)70151-5. [DOI] [PubMed] [Google Scholar]
- Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–60. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- Rajka G, Langeland T. Grading of the severity of atopic dermatitis. Acta Derm Venereol Suppl (Stockh) 1989;144:13–4. doi: 10.2340/000155551441314. [DOI] [PubMed] [Google Scholar]
- Reginald K, Westritschnig K, Werfel T, Heratizadeh A, Novak N, Focke-Tejkl M, et al. Immunoglobulin E antibody reactivity to bacterial antigens in atopic dermatitis patients. Clin Exp Allergy. 2011;41:357–69. doi: 10.1111/j.1365-2222.2010.03655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusznak C, Sapsford RJ, Devalia JL, Justin JR, Hewitt EL, Lamont AG, et al. Cigarette smoke potentiates house dust mite allergen-induced increase in the permeability of human bronchial epithelial cells in vitro. Am J Respir Cell Mol Biol. 1999;20:1238–50. doi: 10.1165/ajrcmb.20.6.3226. [DOI] [PubMed] [Google Scholar]
- Shiohara T, Sato Y, Takahashi R, Kurata M, Mizukawa Y. Increased susceptibility to cutaneous viral infections in atopic dermatitis: the roles of regulatory T cells and innate immune defects. Curr Probl Dermatol. 2011;41:125–35. doi: 10.1159/000323306. [DOI] [PubMed] [Google Scholar]
- Tauber M, Balica S, Hsu CY, Jean-Decoster C, Lauze C, Redoules D, et al. Staphylococcus aureus density on lesional and nonlesional skin is strongly associated with disease severity in atopic dermatitis. J Allergy Clin Immunol. 2016;137(4):1272–1274. e1–3. doi: 10.1016/j.jaci.2015.07.052. [DOI] [PubMed] [Google Scholar]
- Thijs J, Krastev T, Weidinger S, Buckens CF, de Bruin-Weller M, Bruijnzeel-Koomen C, et al. Biomarkers for atopic dermatitis: a systematic review and meta-analysis. Curr Opin Allergy Clin Immunol. 2015;15:453–60. doi: 10.1097/ACI.0000000000000198. [DOI] [PubMed] [Google Scholar]
- Wang B, McHugh BJ, Qureshi A, Campopiano DJ, Clarke DJ, Fitzgerald JR, et al. IL-1beta-Induced Protection of Keratinocytes against Staphylococcus aureus-Secreted Proteases Is Mediated by Human beta-Defensin 2. J Invest Dermatol. 2017;137:95–105. doi: 10.1016/j.jid.2016.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MR, Nakatsuji T, Sanford JA, Vrbanac AF, Gallo RL. Staphylococcus aureus Induces Increased Serine Protease Activity in Keratinocytes. J Invest Dermatol. 2017;137:377–84. doi: 10.1016/j.jid.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


