Abstract
Background
Gastric bypass surgery for weight reduction often corrects dysglycemia in diabetic patients, but a full understanding of the underlying biochemical pathways continues to be investigated. We explored the effects of weight loss by surgical and dietary interventions on plasma metabolites using both targeted and discovery-oriented metabolomics platforms.
Setting
An academic medical center in the United States.
Methods
Improvement in HOMA-IR, as an index of insulin resistance, was compared at six months in eleven patients that underwent Roux-en-Y gastric bypass (RYGB) against eleven patients that were matched for weight loss in the Weight Loss Maintenance (WLM) program. Metabolites in plasma were evaluated by non-targeted gas chromatography/mass spectrometry (GC/MS) for the potential detection of more than 1100 biochemical markers.
Results
Among multiple metabolites detected, 2-hydroxybutyric acid (2-HBA) declined most significantly after six months in comparing patients that underwent RYGB versus those in WLM (P < 0.001), corresponding with declines in HOMA-IR (P = 0.025). Baseline levels of 2-HBA for all patients were correlated with pre-intervention levels of HOMA-IR (R2 = 0.565, P < 0.001). Moreover, the changes in 2-HBA after six months were correlated with changes in HOMA-IR (R2 = 0.399, P = 0.0016).
Conclusions
Correlation between insulin resistance and 2-hydroxybutyric acid suggests the utility of the latter as an excellent biomarker for tracking glycemic improvement, and offers further insight into the pathways that control diabetes. This is the first report of a decline in 2-HBA in response to bariatric surgery.
Keywords: Insulin resistance, Diabetes, Weight loss, Roux-en-Y gastric bypass, Gastric bypass surgery, Bariatric surgery, Metabolomics, 2-Hydroxybutyric acid
INTRODUCTION
Restoration of normoglycemia in diabetic patients who have undergone Roux-en Y gastric bypass (RYGB) often occurs prior to and independently of weight loss (1). Previous studies have sought to understand the underlying mechanisms controlling this effect through the analysis of metabolic markers (2). In a study by Laferrère et al(3), patients who underwent RYGB were matched against patients who achieved similar weight loss by dietary restriction, and their plasma metabolomes profiled by targeted tandem mass spectrometry. Amino acids, most notably the branched-chain amino acids (BCAA) valine, leucine, and isoleucine, and their related catabolites declined significantly after surgery, but not with diet. Associations of plasma BCAA with insulin resistance and other measures of obesity-related metabolic syndrome appear to correlate with metabolic changes in the muscle, liver, and adipose, as well as the gut microbiome(4). Metabolomics may help predict the metabolic response to weight loss treatments (2).
In the present study, we aimed to gain a broader view of the metabolic changes of RYGB-associated diabetic remission by application of a non-targeted metabolomics approach. Using gas chromatography/mass spectrometry (GC/MS), assisted by application of a spectral library of over 1100 metabolites, we explored a larger cross-section of the human metabolome, including amino acids, fatty acids, organic acids, ketone bodies, sterols, and carbohydrates. Among the metabolites examined was 2-hydroxybutyric acid (2-HBA), of recent interest based on its emergence as an indicator for dysglycemia and a potential early marker of diabetes (5, 6). Through our investigation, we further establish 2-HBA as a key component in the overall metabolome by demonstrating its decrease with the improvement in insulin resistance, as effectively accomplished by surgical intervention.
MATERIALS AND METHODS
Eleven patients that had undergone RYGB at the Metabolomics and Weight loss Surgery Center, Duke University Medical Center(7), were matched for weight loss against eleven participants in the Weight Loss Maintenance (WLM) program that included a low-fat diet and support counseling(8). The RYGB cohort included morbidly obese individuals with type 2 diabetes (T2DM) who were taking oral diabetes medications, glucagon-like peptide-1 receptor agonists (GLP-1 RA) or insulin, while WLM enrolled obese subjects who were taking medications for hypertension and/or dyslipidemia. The weight of each participant was measured at the start of intervention and after six months, at which times blood was drawn and immediately processed for EDTA-plasma. Participant heights were also measured for subsequent calculation of body mass index (BMI) in kg/m2. Studies involving all participants were registered in clinicaltrial.gov (NCT00054925 and NCT00787670) and were approved by the Institutional Review Board (IRB) at our institute.
Plasma glucose and other metabolites were measured by enzymatic assays on a DxC600 clinical analyzer (Beckman-Coulter, Brea, CA); insulin by immunoassay from Meso Scale Discovery (Rockville, MD), standardized against WHO 66/304; and a panel of fifteen amino acids quantitatively measured by targeted MS/MS using stable-isotope dilution, as previously described(9). Homeostasis model assessment was used as an index of insulin resistance (HOMA-IR)(10), calculated as the product of fasting glucose (mg/dL) and insulin (μU/mL) divided by 405. For non-targeted GC/MS, plasmas were extracted and derivatized by methoximation and trimethylsilylation (TMS) according to a procedure previously described(11), and run using instrumentation from Agilent Technologies (Santa Clara, CA), which included a 6890N GC and a 5975B MS using electron ionization. GC features were deconvoluted using AMDIS(12), and annotated by matching against known analytes in a spectral library based on both retention time and mass fragmentation pattern. The library we used was initially created by Fiehn and co-workers(13), and since supplemented by our laboratory to include more than 1100 metabolites. Integrated peak areas of chromatographic features were log2-transformed and grouped across samples based on their annotation.
Statistical analysis
Continuous data were presented as mean (± standard deviation). Comparisons between groups were performed using independent sample t-tests, and comparisons of change between baseline and 6 months done by dependent sample t-tests. Pearson correlation coefficients were calculated to evaluate the relationship between the continuous variables. Statistics including t-tests and Pearson correlations were performed using Microsoft Excel, and multiple regressions by SAS, version 9.4 (Cary, NC).
RESULTS
Characteristics of the participants in the RYGB and WLM groups are outlined in Table 1. Pre-intervention weight and BMI were not statistically different between groups. Participant age tended slightly higher for WLM, which had more males, whereas the RYGB group was mostly females. Baseline plasma glucose, insulin and HOMA-IR were significantly higher in the RYGB group. This was expected for the RYGB cohort, which included only patients with T2DM, while the WLM cohort enrolled obese subjects with hypertension and dyslipidemia. Other targeted metabolites that were increased in the RYGB cohort include NEFA, valine, branched-chain amino acids (BCAA, combined leucine/isoleucine and valine), and glutamate/glutamine, whereas glycine was decreased, as have previously been associated with dysglycemia(4). Each metabolite is shown here to have a significant correlation with HOMA-IR.
TABLE 1.
Baseline characteristics and six-month changes for patients that underwent Roux-en-Y gastric bypass (RYGB) surgery and dietary weight loss (WLM).
| Baseline Characteristics | 6-month Changes | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Pre-RYGB (n-11) | Pre-WLM (n-11) | Pgroup | Pcorr | Δ RYGB | Δ WLM | Pgroup | Pcorr | |
|
| ||||||||
| Age | 50.5 ± 7.8 | 56.5 ± 11.4 | 0.160 | |||||
|
| ||||||||
| Male (%) | 3 (27.3) | 7 (63.6) | 0.095 | |||||
|
| ||||||||
| Race | 1.000 | |||||||
| White (%) | 9 (81.8) | 9 (81.8) | ||||||
| Black (%) | 2 (18.2) | 2 (18.2) | ||||||
|
| ||||||||
| Weight (kg) | 112.3± 15.5 | 119.9±15.5 | 0.262 | 0.77 | −24.0 ± 4.3 | −23.6 ± 4.6 | 0.84 | 0.720 |
|
| ||||||||
| BMI (kg/m2) | 41.3 ± 3.0 | 38.2 ± 4.6 | 0.078 | 0.14 | −8.9 ± 1.9 | −7.5 ± 1.2 | 0.051 | 0.820 |
|
| ||||||||
| HOMA-IR | 17.1 ± 18.3 | 2.5 ± 2.0 | 0.025 | −15.3 ± 17.2 | −1.6 ± 1.8 | 0.025 | ||
|
| ||||||||
| Targeted metabolites | ||||||||
|
| ||||||||
| Insulin (μU/mL) | 39.9 ± 41.9 | 9.9 ± 6.9 | 0.040 | −33.1 ± 38.1 | −5.9 ± 5.8 | 0.040 | ||
|
| ||||||||
| Glucose (mg/dL) | 161.9 ± 35.9 | 100.4 ± 10.8 | <0.001 | −56.4 ± 42.4 | −8.1 ± 7.1 | 0.004 | ||
|
| ||||||||
| NEFA (mmol/L) | 0.90 ± 0.20 | 0.43 ± 0.11 | <0.001 | 0.012 | −0.20 ± 0.26 | 0.04 ± 0.12 | 0.015 | 0.14 |
|
| ||||||||
| Valine (μmol/L) | 311.1±56.4 | 262.3 ± 27.4 | 0.021 | 0.002 | −89.3 ± 48.0 | −24.7 ± 33.3 | 0.002 | 0.002 |
|
| ||||||||
| BCAA (μmol/L) | 515.0 ± 90.5 | 433.6 ± 39.9 | 0.017 | 0.003 | −140.2 ± 80.9 | −49.9 ± 45.1 | 0.005 | 0.009 |
|
| ||||||||
| Glutamate/Glutamine (μmol/L) | 160.6 ± 43.7 | 77.2 ± 17.8 | <0.001 | <0.001 | −23.8 ± 36.1 | −9.4 ± 46.8 | 0.43 | 0.76 |
|
| ||||||||
| Glycine (μmol/L) | 260.6 ± 69.1 | 325.8 ± 43.9 | 0.017 | 0.023 | 88.7 ± 70.2 | 58.6 ± 41.0 | 0.24 | 0.99 |
|
| ||||||||
| Non-targeted metabolites (log2) | ||||||||
|
| ||||||||
| 2-Hydroxbutyric acid | 20.69 ± 0.79 | 19.35 ± 0.47 | <0.001 | <0.001 | −1.63 ± 0.76 | 0.15 ± 0.6 | <0.001 | 0.002 |
|
| ||||||||
| 2-Hydroxyvaleric acid | 17.28 ± 0.83 | 16.10 ± 1.06 | 0.009 | 0.007 | −1.33 ± 1.07 | −0.55 ± 0.88 | 0.077 | 0.019 |
|
| ||||||||
| 2-Aminobutyric acid | 16.76 ± 0.68 | 16.72 ± 0.68 | 0.89 | 0.33 | −0.92 ± 0.90 | 0.17 ± 0.93 | 0.011 | 0.033 |
|
| ||||||||
| 3-Hydroxybutyric acid | 21.47 ± 1.16 | 19.81 ± 0.73 | 0.001 | 0.096 | −0.61 ± 1.38 | 1.21 ± 0.87 | 0.002 | 0.033 |
|
| ||||||||
| Acetoacetic acid | 16.53 ± 1.23 | 15.18 ± 0.40 | 0.012 | 0.23 | −1.35 ± 1.05 | 1.19 ± 0.68 | 0.001 | 0.22 |
|
| ||||||||
| CMPF | 17.24 ± 1.83 | 14.78 ± 1.75 | 0.040 | 0.80 | −2.75 ± 1.55 | 0.10 ± 1.28 | 0.010 | 0.27 |
|
| ||||||||
| gamma-Tocopherol | 16.62 ± 1.03 | 15.52 ± 0.91 | 0.019 | 0.072 | −0.83 ± 1.04 | −0.91 ± 1.11 | 0.86 | 0.70 |
|
| ||||||||
| Hypoxanthine | 14.85 ± 0.83 | 13.29 ± 0.54 | <0.001 | 0.19 | −1.61 ± 0.41 | 1.74 ± 0.26 | 0.002 | 0.40 |
|
| ||||||||
| Norepinephrine | 14.60 ± 0.85 | 13.69 ± 0.66 | 0.016 | 0.20 | −0.23 ± 1.25 | 0.07 ± 0.86 | 0.61 | 0.37 |
|
| ||||||||
| Oleic acid | 23.42 ± 0.62 | 22.13 ± 0.47 | <0.001 | 0.052 | −0.25 ± 0.72 | 0.15 ± 0.60 | 0.17 | 0.55 |
|
| ||||||||
| Linoleic acid | 22.63 ± 0.60 | 21.50 ± 0.27 | <0.001 | 0.050 | −0.28 ± 0.70 | −0.10 ±0.47 | 0.49 | 0.64 |
|
| ||||||||
| Cysteine | 18.87 ± 0.56 | 17.98 ± 1.06 | 0.028 | 0.017 | −0.56 ± 0.71 | −0.39 ± 0.90 | 0.43 | 0.66 |
BMI = body mass index. HOMA-IR = Homeostatic Model Assessment for Insulin Resistance. NEFA = non-esterified fatty acids. BCAA = Branched-chain amino acids, BCAA, include valine, leucine and isoleucine. CMPF = 3-carboxy-4-methyl-5-propyl-2-furanpropionic acid.
RYGB = Roux-en-Y gastric bypass. WLM = Weight loss maintenance.
Data are presented as mean ± standard deviation and number (%).
P group is the probability for the differences between groups for both baseline and 6-month changes. P corr is the probability of correlation versus HOMA-IR for all patients.
At six months, subjects in both groups lost significant weight (RYGB −23.98 ± 4.34 kg versus WLM −23.60 ± 4.63 kg, P = 0.845) and at an equivalent level, as was intended. Combined with the loss in weight, patients in the RYGB group showed significant decreases in fasting insulin and glucose, and thereby HOMA-IR, achieving post-intervention levels that were more in line with normoglycemia. This resulted in levels becoming more similar to those for WLM, in which patients were already normoglycemic. Moreover, 10/11 (90.9%) patients in the RYGB cohort no longer needed diabetes medications and insulin at 6 months after surgery. Significant differences in six-month changes between RYGB and WLM therefore mostly reflect changes for RYGB (with WLM mostly unchanged) and since weight loss was equivalent between groups, suggest an association with glycemic improvement. In addition to insulin, glucose, and HOMA-IR, six-month changes for NEFA, valine, and BCAA were significantly decreased for RYGB relative to WLM (P = 0.015, 0.002, and 0.005, respectively). The association is consistent with our earlier study(3), in which branched-chain amino acids declined more for RYGB relative to a dietary intervention. We further show here a significant correlation between changes of HOMA-IR and changes for either valine (P = 0.002) or BCAA (P = 0.009), respectively), when measured by targeted MS/MS.
We used non-targeted GC/MS to explore a much broader array of relevant metabolites. A total of 118 non-targeted metabolites were detected, of which Table 1 lists twelve that displayed significant differences and that were not in the targeted panels. Not included are amino acids (except cysteine), fatty acids, and glucose, which were measured with greater sensitivity in the targeted assays, and are instead displayed with all non-targeted metabolites in Table S1.
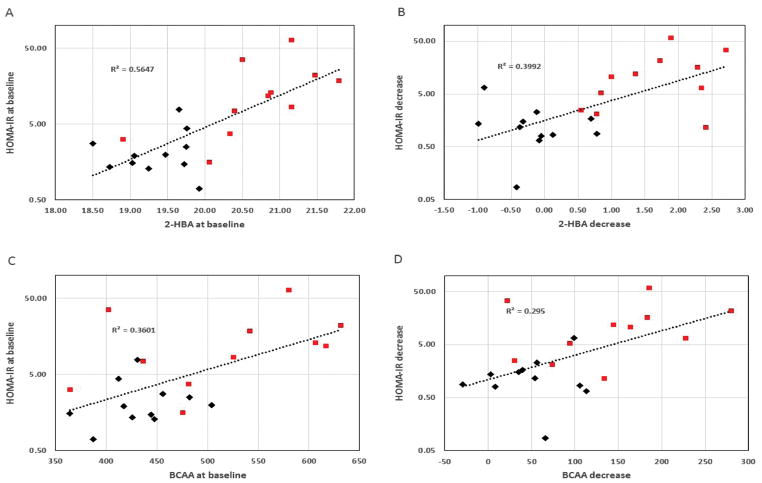
Prominent among the non-targeted metabolites is 2-HBA, which showed a significant decline for the RYGB intervention relative to WLM (P < 0.001). The effect was again due to higher pre-intervention levels of 2-HBA for RYGB (P < 0.001), which became reduced after surgery to the levels seen with WLM. 2-HBA levels for WLM, by contrast, did not substantially change. These observations are consistent with the apparent association between 2-HBA and insulin resistance, wherein patients electing RYGB were more dysglycemic at baseline. Thus, Figure 1A shows HOMA-IR for all patients covering a large range, but being generally higher for those in the RYGB group. A clear correlation between HOMA-IR, representing insulin resistance, and 2-HBA (r2 = 0.565, P < 0.001) is observed. Moreover, as Figure 1B displays, the declines in HOMA-IR with intervention, as more strongly produced by RYGB, were highly correlated with the corresponding declines in 2- HBA (r2 = 0.399, P = 0.016). These correlations between the glycemic index HOMA-IR and 2-HBA were stronger than for any other measured metabolite, including the BCAAs, as indicated in Figures 1C–D.
FIGURE 1.
Correlation plots relating A) pre-intervention levels of HOMA-IR to 2-hydroxybutyric acid (2-HBA), B) decrease in HOMA-IR to decrease in 2-HBA, C) pre-intervention HOMA-IR to combined branched-chain amino acids (BCAA), and D) decrease in HOMA-IR to decrease in BCAA. ◆ Patients from WLM; ■ patients from RYGB. 2-HBA was measured by non-targeted gas chromatography/mass spectrometry (GC/MS) with levels reported as log2 transforms of integrated peak areas. BCAA are combined valine and leucine/isoleucine measured by targeted MS/MS.
Other metabolites detected by non-targeted GC/MS showed significant differences when comparing RYGB against WLM. Of the twelve selected for Table 1, eleven showed significantly higher levels for RYGB at baseline relative to WLM. Of these, five also showed significant differences in six-month changes between RYGB and WLM, including 2-HBA, 3-hydroxybutyric acid (3-HBA), acetoacetic acid, CMPF, and hypoxanthine. These changes generally appeared as a decrease for RYGB, and an increase for WLM. Considering the baseline correlations with HOMA-IR, only those for 2-HBA, 2-hydroxyvaleric acid (2-HVA), and cysteine were significant. Of these, only 2-HBA and 2-HVA also showed significant correlations with six-month changes of HOMA-IR. 2-Aminobutyric acid (2-ABA) and 3-HBA were also significant for the six-month correlations, but not for correlations with HOMA-IR at baseline.
As our study was exploratory in nature, aimed at discovery rather than validation, we did not correct for multiple comparisons. Applying a Bonferroni correction(14) for the 118 non-targeted metabolites nevertheless did not alter our statistical conclusions for 2-HBA, including that for differences between the two interventions in levels of change (P corrected <0.001). The effect for changes in 2-HBA also remained significant when adjusted for patient baseline levels, including those for HOMA-IR, BMI, and 2-HBA (P = 0.0083). In addition, the correlation between changes in 2-HBA and HOMA-IR was strongly significant (P = 0.0016), whereas that between changes in 2-HBA and BMI was not (P = 0.067), indicating the association is with glycemic improvement and not weight loss. In contrast, correlations between changes in HOMA-IR and BMI were not significant (P = 0.77).
DISCUSSION
Studying plasma in patients that underwent RYGB allowed us to observe large improvements in glycemic status and thereby detect any significant metabolic correlations using both targeted and non-targeted metabolomics. Our preliminary study found evidence that supports 2-HBA as a potential new marker for dysglycemia and insulin resistance, not only confirming its previously shown association at baseline(5, 6), but now further showing that 2-HBA declines in a manner strongly correlated to the improvement of insulin resistance after treatment. Previous work by others had shown no apparent change in 2-HBA with surgery(15), however those patients were first exposed to a low-caloric liquid diet before surgical intervention, such that the effect was possibly obscured, and also observed after only 28 days. Here, we measured changes at six-month post-surgery, when the full effects of the intervention became more apparent. In subsequent work by Ferrannini and coworkers, improvement in insulin resistance by pharmaceutical means to increase levels of the incretin, glucagon like peptide (GLP)-1, also showed 2-HBA to decrease(16). Other factors like circulating pigment epithelium-derived factor levels(17) or circulating lipopolysaccharide-binding protein (LBP)(18) have also been shown to associate with insulin resistance and decrease after weight loss, possibly involved in the observed metabolic changes and improvement of insulin resistance after RYGB. There may also be additional unidentified factors involved in insulin sensitivity and energy balance regulation(19).
A mechanism previously proposed to link insulin resistance with 2-HBA suggests channeling through the precursor 2-ketobutyric acid (2-KBA)(5, 6), which increases as a consequence of oxidative stress-related glutathione synthesis, thus driving increased glutamate and cysteine, and decreased glycine. Through a separate pathway, BCAA and non-esterified fatty acids (NEFA) are also expected to increase 2-KBA. Our observations for the baseline levels of amino acids and NEFA, comparing RYGB versus WLM, are consistent with these mechanisms, as are the correlations with the individual baseline levels of HOMA-IR (Table 1). Correlations for six-month changes for these metabolites are less compelling however, the exception being BCAA, driven predominantly by valine. The association of BCAA with insulin resistance and other metabolic syndrome effects have been noted in many previous studies(4), for which the operative pathways may be manifold, and possibly overlapping those connected with 2-HBA.
Finding markers like 2-HBA that are associated with glycemic status are important for revealing pathways in dysglycemic conditions such as diabetes, and can offer unique perspectives for tracking efficacy of therapeutic interventions. The criteria for such a marker are that 1) it shows a significant distinction pre-intervention between a population of dysglycemic individuals and a population with normoglycemia, 2) pre-intervention levels are significantly correlated with an index for glycemia, like HOMA-IR, 3) changes following intervention between the dysglycemic and normoglycemic population are significantly different, and 4) these changes are significantly correlated with the changes in the index. In the present study, the dysglycemic population are those with T2DM who undergo RYGB, and the normoglycemic were those in WLM. The index used was HOMA-IR. Table 1 clearly demonstrates that 2-HBA meets all four of the above criteria, with highly significant levels observed for all statistical probabilities that are listed. The BCAA, as strongly influenced by valine, also meet the criteria, although to a much lower level of significance.
There is currently no clear consensus on what constitutes a success in bariatric surgery. A common definition of successful postoperative outcome is a loss of 50% of excess weight at 1–2 years postoperatively (20). However, the success of bariatric surgery cannot be defined in terms of absolute weight loss without describing the health and quality of life improvements associated with weight loss(21). Preoperative levels of metabolites or their changes after bariatric surgery, particularly for 2-HBA, may be used as predictors or new criteria to define success of bariatric/metabolic surgery.
Other metabolites detected by non-targeted GC/MS appear closely related to 2-HBA, and share some similarities in their relationship to insulin resistance. Among these are 2-aminobutyric acid (2-ABA), formed by the transamination of 2-KBA, the precursor to 2-HBA; and 2-hydroxyvaleric acid (2-HVA), the five-carbon analog of 2-HBA. Both decline significantly with RYGB only (Table S1), and also show correlation of changes with changes in HOMA-IR, although clearly not as strongly as 2-HBA (Table 1). Neither meet all four criteria outlined above for consideration as a marker of glycemic status. We also observed significant differences in baseline and six-month changes between groups for the ketone bodies, 3-hydroxybutyric acid (3-HBA) and acetoacetic acid. Neither ketone body marker however meets the criteria for correlation with HOMA-IR at baseline (and acetoacetic acid also fails for the changes). Changes at six months, unlike with 2-HBA, are mostly influenced by increasing levels for WLM, and comprise smaller declines for RYGB (Table S1). We suspect these changes are more likely a reflection of the weight loss in both groups, with WLM patients exhibiting a larger relative shift towards reliance on fatty acid oxidation. Ketone bodies in the diabetic RYGB group were high at baseline (as were also targeted NEFA and oleic and linoleic acids) due to compensatory mechanisms for failure to adequately utilize glucose. A catabolic influence to increase levels following surgery occurred as metabolic fuels became less supplied, but were offset by the opposing influence of improving insulin sensitivity. We also observe a decline in RYGB patients only for 3-carboxy-4-methyl-5-propyl-2-furanpropionic acid (CMPF), a furan recently implicated in diabetes development(22), although here showing no particular correlation with HOMA-IR.
We acknowledge some limitations in this preliminary study, including its small sample size, and its comparison of two different populations, one with obese normoglycemic individuals and the other with diabetic obese subjects that underwent RYGB. A larger study is needed to further explore the extent to which metabolomics changes, particularly for 2-HBA, in diabetic obese individuals are the result of weight loss per se or bariatric surgery. Despite these limitations, the present study findings are important and highlight the complexity of mechanisms of weight loss and comorbidities resolution after bariatric surgery.
CONCLUSION
We provide further evidence to confirm 2-HBA as a reliable marker for insulin resistance and incipient diabetes, and indicate its additional utility in tracking therapeutic improvement. We believe that metabolomics will continue to serve a valuable discovery role in rapidly revealing key metabolites that illuminate the pathways giving rise to diabetes.
Supplementary Material
Highlight.
This is the first report of a decline in 2-hydroxybutyric acid in response to bariatric surgery. Correlation between insulin resistance and 2-hydroxybutyric acid suggests the utility of the latter as an excellent biomarker for tracking glycemic improvement, and offers further insight into the pathways that control diabetes.
Acknowledgments
Alessandro Mor, M.D., provided helpful discussion. Haijing Song and Huaxia Cui helped with targeted and non-targeted metabolomic assays. This study was supported by grants from the US National Institutes of Health (P01-DK058398 and 5U01HL068734).
Footnotes
Conflict of Interest
The authors declare no conflicts of interests.
Author Contributions
All authors contributed to the study design and analysis of the data. M.M., P.S., J.B., and O.I. performed the measurements. M.M. and P.S. co-wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ribaric G, Buchwald JN, McGlennon TW. Diabetes and weight in comparative studies of bariatric surgery vs conventional medical therapy: a systematic review and meta-analysis. Obes Surg. 2014;24:437–55. doi: 10.1007/s11695-013-1160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tulipani S, Griffin J, Palau-Rodriguez M, et al. Metabolomics-guided insights on bariatric surgery versus behavioral interventions for weight loss. Obesity (Silver Spring, Md) 2016;24:2451–66. doi: 10.1002/oby.21686. [DOI] [PubMed] [Google Scholar]
- 3.Laferrere B, Reilly D, Arias S, et al. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci Transl Med. 2011;3:80re2. doi: 10.1126/scitranslmed.3002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newgard CB. Metabolomics and Metabolic Diseases: Where Do We Stand? Cell Metab. 2017;25:43–56. doi: 10.1016/j.cmet.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gall WE, Beebe K, Lawton KA, et al. alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS One. 2010;5:e10883. doi: 10.1371/journal.pone.0010883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrannini E, Natali A, Camastra S, et al. Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance. Diabetes. 2013;62:1730–7. doi: 10.2337/db12-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khoo CM, Chen J, Pamuklar Z, Torquati A. Effects of Roux-en-Y gastric bypass or diabetes support and education on insulin sensitivity and insulin secretion in morbidly obese patients with type 2 diabetes. Ann Surg. 2014;259:494–501. doi: 10.1097/SLA.0b013e318294d19c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brantley P, Appel L, Hollis J, et al. Design considerations and rationale of a multi-center trial to sustain weight loss: the Weight Loss Maintenance Trial. Clin Trials. 2008;5:546–56. doi: 10.1177/1740774508096315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tai ES, Tan ML, Stevens RD, et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia. 2010;53:757–67. doi: 10.1007/s00125-009-1637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–95. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 11.Banerjee R, He J, Spaniel C, et al. Non-targeted metabolomics analysis of cardiac Muscle Ring Finger-1 (MuRF1), MuRF2, and MuRF3 in vivo reveals novel and redundant metabolic changes. Metabolomics : Official journal of the Metabolomic Society. 2015;11:312–22. doi: 10.1007/s11306-014-0695-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mallard WE, Reed J. Automated Mass Spectral Deconvolution and Identification System: AMDIS User Guide. National Institute of Standards and Technology, US Department of Commerce; 1997. [Google Scholar]
- 13.Kind T, Wohlgemuth G, Lee DY, et al. FiehnLib: mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal Chem. 2009;81:10038–48. doi: 10.1021/ac9019522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunn OJ. Multiple comparisons among means. J Am Stat Assoc. 1961;56:52–64. [Google Scholar]
- 15.Sarosiek K, Pappan KL, Gandhi AV, et al. Conserved Metabolic Changes in Nondiabetic and Type 2 Diabetic Bariatric Surgery Patients: Global Metabolomic Pilot Study. Journal of diabetes research. 2016;2016:3467403. doi: 10.1155/2016/3467403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muscelli E, Frascerra S, Casolaro A, et al. The amino acid response to a mixed meal in patients with type 2 diabetes: effect of sitagliptin treatment. Diabetes Obes Metab. 2014;16:1140–7. doi: 10.1111/dom.12350. [DOI] [PubMed] [Google Scholar]
- 17.Sabater M, Moreno-Navarrete JM, Ortega FJ, et al. Circulating pigment epithelium-derived factor levels are associated with insulin resistance and decrease after weight loss. J Clin Endocrinol Metab. 2010;95:4720–8. doi: 10.1210/jc.2010-0630. [DOI] [PubMed] [Google Scholar]
- 18.Moreno-Navarrete JM, Ortega F, Serino M, et al. Circulating lipopolysaccharide-binding protein (LBP) as a marker of obesity-related insulin resistance. Int J Obes (Lond) 2012;36:1442–9. doi: 10.1038/ijo.2011.256. [DOI] [PubMed] [Google Scholar]
- 19.Fruhbeck G, Gomez-Ambrosi J. Rationale for the existence of additional adipostatic hormones. FASEB J. 2001;15:1996–2006. doi: 10.1096/fj.00-0829hyp. [DOI] [PubMed] [Google Scholar]
- 20.Shantavasinkul PC, Omotosho P, Corsino L, Portenier D, Torquati A. Predictors of weight regain in patients who underwent Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis. 2016;12:1640–5. doi: 10.1016/j.soard.2016.08.028. [DOI] [PubMed] [Google Scholar]
- 21.Fruhbeck G. Bariatric and metabolic surgery: a shift in eligibility and success criteria. Nat Rev Endocrinol. 2015;11:465–77. doi: 10.1038/nrendo.2015.84. [DOI] [PubMed] [Google Scholar]
- 22.Prentice KJ, Luu L, Allister EM, et al. The furan fatty acid metabolite CMPF is elevated in diabetes and induces beta cell dysfunction. Cell Metab. 2014;19:653–66. doi: 10.1016/j.cmet.2014.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.