Abstract
Uterine leiomyomas are common and life-altering for many women. Despite a wide range of symptoms, varying characteristics of the uterus and the leiomyomas themselves, and many alternatives, hysterectomy accounts for almost three quarters of all surgical therapy. Yet tThere is increasing evidence for a variety of procedural therapies for symptomatic leiomyomas and a new generation of medical therapies under development. With increasing evidence of long-term risk to hysterectomy and new data regarding leiomyoma biology, individualized medical approaches to leiomyomas are likely in the near future. Key biologic attributes that influence this disease process and potential treatments are common driver mutations and the new appreciation of the interaction of smooth muscle cells and fibroblasts. Additionally, the interaction between cell types and steroid hormone responsiveness likely plays a role in pathogenesis that can be leveraged in individualized therapy. However, given the independent clonal nature of leiomyomas within the same uterus, moving in the direction of biopsies for individual leiomyomas to understand the biology is unlikely to be fruitful. Use of advanced imaging will likely continue to evolve not only to accurately predict malignant disease, including sarcomas, but to predict leiomyoma subtypes and/or response to therapy. We predict the continued evolution of therapy from excisional or interventional therapies to medical therapies and ultimately prediction of at-risk individuals. Ideally, individualized therapies will offer primary prevention for women at high risk of leiomyomas and secondary prevention after initial treatment.
Introduction
Uterine leiomyomas are benign monoclonal neoplasms that have a heterogeneous array of sizes, symptoms and locations.(1) They are common: occurring in up to 80% of women, with symptoms affecting up to 50%. (2) The most common leiomyoma symptom is heavy or prolonged menstrual bleeding (HMB), also termed abnormal uterine bleeding related to leiomyomas or AUB-L within the International Federation of Gynecology and Obstetrics (FIGO) classification system. Other common leiomyomas symptoms are anemia secondary to HMB, pelvic pressure, and urinary frequency. Symptomatic leiomyomas decrease quality of life, lead to lost time at work, limit work potential and represent a significant health disparity given their impact on women of African descent. (3) The already high healthcare costs associated with leiomyomas are predicted to rise 20–30% in the next few decades in large part to the increasing racial and ethnic diversity in the US with more than 400,000 leiomyoma-related predicted hospitalizations per year by 2050. (4)
Epidemiology
Risk factors for the development of leiomyomas have been well-studied and include African ancestry, increasing age until age of menopause, family history of uterine leiomyomas, time since last birth, hypertension and consumption of food additives and soybean milk. (5, 6) Factors which have been found to be associated with decreased leiomyoma risk include increasing parity, the use of oral contraceptives or depot medroxyprogesterone acetate, and smoking in women with low BMI.(6) Modifiable risk factors, such as physical activity, diet, and body mass index are less consistent across studies, but likely influence leiomyoma development. Risk factor profiles differ based on whether studies depend on imaging-diagnosed leiomyomas or study only symptomatic women. Ultrasound-screening studies can help find associations with the presence of leiomyomas, while studies that require clinical symptoms may be more informative on the risk factors that lead to treatment or predict leiomyoma growth. (5)
Even within the same uterus there is substantial biologic heterogeneity. Leiomyomata grow and shrink at varying rates and there are often leiomyomas from different genetic subgroups.(7) There is evidence to suggest that leiomyomas closer to the endometrial cavity (submucosal) contribute more to HMB than other leiomyomas(8), but ascribing other symptoms to specific leiomyomas or predicting symptoms based on uterine volume is problematic. Despite, or because of this heterogeneity, leiomyomata have traditionally been treated as a single disease with the primary therapy being hysterectomy.
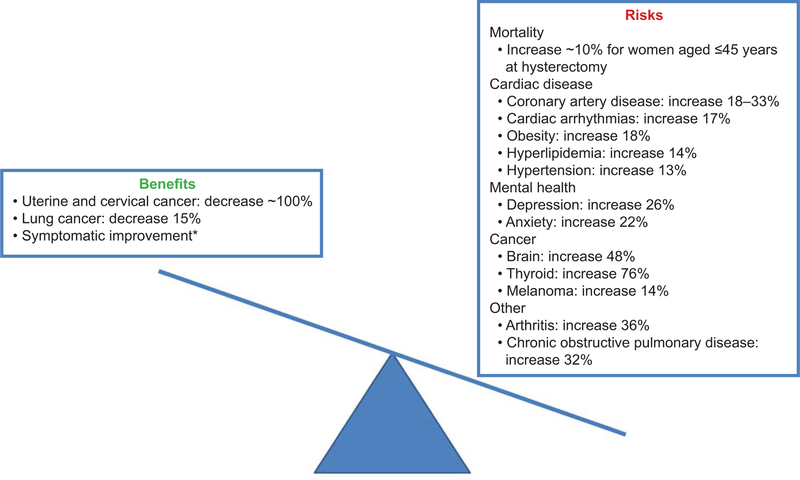
Innovative approaches to treatment are paramount for several reasons. First, increasing data show that hysterectomy, even with ovarian conservation, has long-term consequences (Figure 1). (9–11) Hysterectomy can shorten the time to menopause by 2–4 years and increase the long-term risks of cardiovascular disease, in particular in women who undergo hysterectomy at a young age, likely through the detrimental effects on ovarian function. (11, 12) If bilateral oophorectomy is performed with hysterectomy, risks are even higher including an 8% risk of excess risk of mortality.(13) Secondly, women seek alternatives to hysterectomy for a variety of reasons: to maintain fertility, to preserve ovarian function, to limit time away from work, family and other activities, and to maintain body integrity. Guidelines from the American College of Obstetricians and Gynecologists (ACOG) support women pursuing alternatives to hysterectomy whether or not they desire future childbearing. (14)
Figure 1.
Risks and benefits associated with hysterectomy with bilateral ovarian conservation at any age.9-11,58-60*Although symptoms may be alleviated with other less invasive treatment options.
Many women also seek alternatives to any type of surgery specifically medical therapies that treat leiomyoma symptoms and decrease size with fewer side effects than the currently available options. New classes of medication, some currently in clinical trials, offer women a non-surgical treatment option that may be used earlier in the disease process and potentially for a longer period of time. If proven safe, future use could include secondary prevention of leiomyoma growth in women following interventional therapy and even primary prevention in young women who wish to delay child-bearing without putting future fertility at risk.
Understanding of leiomyoma biology coupled with increased interest in alternative treatment options will lead to unique opportunities for women with leiomyomas. In this review, we explore the innovative diagnostic and treatment options currentlyavailable and those becoming available to individualize leiomyoma treatment.
New Insights into Pathophysiology
New research is challenging some classical teaching regarding leiomyoma biology. First, the somatic mutations that are a key part of leiomyoma pathogenesis, where normal myometrial cells are transformed into leiomyoma progenitors, have been well characterized and have some correlation with phenotype (Table 1). (15) Studies in multiple populations now confirm mutations in the mediator complex subunit 12 (MED12) gene to be the most common leiomyoma driver mutation, representing roughly 70% of all leiomyomas. MED12 mutations produce a phenotype of smaller tumors with more extracellular matrix (ECM) than other genotype groups. (16) However, examination of downstream signaling pathways shows overlap between the MED12 group and two other key groups, those showing mutations in high mobility group AT-hook 2 (HMGA2) or collagen type IV alpha 5 or alpha 6 chain (COL4A5 and COL4A6) genes. Thus, simply knowing the genotype may not get us directly to individualized therapy in the near term.
Table 1.
Common Leiomyoma Mutations and Associated Phenotypes.
| Common leiomyoma mutations | Phenotypes | Mutation information | |
|---|---|---|---|
| Somatic mutations |
MED12 |
Small tumors made of SMC and fibroblasts |
Most common mutation |
|
HMGA2 |
90% SMC, second most common mutation | Gene disrupted in fibroids with t(12;14) | |
| COL4A5, COL4A6 | Rarely associated with diffuse leiomyomatosis/Alport syndrome which has X-linked inheritance | OMIM # 301050 | |
| Autosomally -dominant inherited or sporadic somatic mutations | Fumarate hydratase (FH) | Inherited mutations associated with HLRCC syndrome: cutaneous leiomyomas and renal cell cancer also atypical and cellular fibroids and increased risk of uterine sarcoma. Also gene responsible for syndrome previously described as Reed’s Syndrome |
OMIM # 150800 Immunohistochemistry suggests alteration in FH in both inherited and somatic mutations. |
However, one genotypic group, the fumarate hydratase (FH) mutation group does appear to have distinct characteristics that are important clinically. (17) Most FH leiomyomas have autosomally-dominant inherited mutations and not somatic mutations. Families with this dominant mutation often have cutaneous leiomyomas and an increased risk of uterine and renal cancers and thus Hereditary Leiomyomatosis and Renal Cell Cancer (HLRCC) syndrome. Increasing use of immunohistochemistry to detect products of the anaerobic metabolism of these FH leiomyomas during pathologic exam of excised leiomyoma tissue may increase recognition of HLRCC syndrome and those at risk of malignancy. (18) Because of both the familial risk and the risk of malignant disease, women with evidence of FH uterine leiomyomas should be referred for genetic counseling. Finally, based on downstream signaling, FH leiomyoma appear to have non-redundant pathways with other types of leiomyomas suggesting distinct treatment options can be developed for this rare but important genetic subgroup.
Secondly, there is new appreciation of the role of both fibroblasts and smooth muscle cells (SMC) in leiomyoma biology. (19, 20) There is significant heterogeneity in cellular composition associated with genotype: MED12 leiomyomas are comprised equally of SMC and fibroblasts while HMGA2 leiomyomas, the second most common group, are 90% SMC. (20) Although steroid-responsiveness has been a key descriptor for leiomyoma clinical behavior, there has been substantial heterogeneity of response. Key findings are that stem cells appear to be more steroid-responsive than mature leiomyoma cells, progesterone is the key regulator of SMC, and fibroblasts are estrogen-dependent. (20, 21) Finally, the active role of ECM and even mechanical signaling appears to play a key role in this disease.
Diagnosis
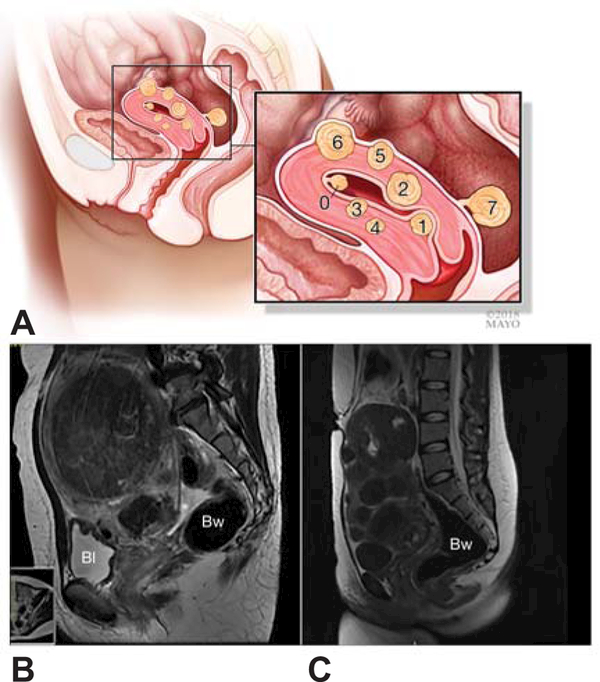
Traditionally, ultrasound is used to confirm the diagnosis of leiomyomata after indicated by symptoms or physical examination. Ultrasound reports are sometimes detailed as to leiomyoma number, size and location, but usually just report leiomyomas are present with a measurement of the largest diameter. In addition to their classification of abnormal uterine bleeding, FIGO has proposed a classification system for leiomyoma location with reference to the endometrium and serosa (Figure 2) (22), which has not been fully implemented. Categorizing using ultrasound, or even magnetic resonance imaging (MRI), is more difficult with larger or multiple leiomyomas where the uterine landmarks are distorted. (23)
Figure 2.
FIGO classification system labels leiomyoma types 0 through 7 (A). FIGO classification can be more difficult to implement in patients with large (B) or multiple leiomyomas (C). BI, bladder; BW, bowel. Figure 2A used with permission of Mayo Foundation for Medical Education and Research. All rights reserved.
Although cost effective and minimally invasive, ultrasound is effective at mapping up to 4 leiomyomata or to a uterine volume of 375mL (24), but above those limits, magnetic resonance imaging generally performs better. The relationship of the leiomyoma with the uterine cavity is important for understanding symptoms and treatment options; intracavitary involvement is better outlined with hysteroscopy or saline -infusionsonogram. (25)
With the increasing use of radiologic and minimally invasive procedures focusing on tissue destruction, rather than removal, as well as the emerging concerns about unsuspected malignancy, MRI is more frequently used. For the former concern, MRI provides an excellent pre-procedural evaluation of leiomyoma location with reference to surrounding structures, number, contrast enhancement, and heterogeneity. (26) These details allow physicians to determine the likely benefits and effectiveness of each procedure. (27) Signal intensity on MRI for both T2- and T1-weighted images have been studied for predicting outcomes of uterine artery embolization (UAE) and magnetic resonance focused ultrasound (MRgFUS), but no consensus has been found. (27, 28) These discrepancies may be due to the interpretation of a high T2-weighted signal which may indicate either high vascularity (good for UAE outcomes) or high cellularity (poor for UAE outcomes). (28)
The Key Issue of Differentiating Leiomyomas from Malignancy
The Food and Drug Administration focused attention of the importance and difficulty of differentiating ordinary leiomyomas from leiomyosarcomas and other uterine malignancies in 2014. Any new treatment paradigm will need to address this significant issue and to identify markers of malignancy and potential prognostic indicators.
Distinguishing between leiomyomata and leiomyosarcoma has been difficult. Some risk factors for leiomyosarcoma differ from those for leiomyomas including higher risk for malignancy with postmenopausal status, older age, tamoxifen use, history of retinoblastoma, pelvic irradiation, and HLRCC syndrome. However, premenopausal women and women of African descent are at risk for both leiomyomas and leiomyosarcoma. Many new imaging techniques are emerging, some of which increase the sensitivity and specificity of the tests. However, the low prevalence of leiomyosarcoma, ranging from 0.023% to 0.2%, limits the positive predictive value of any test. (29) For instance, with a prior probability of sarcoma of less than 0.2%, the positive predictive value of a test that is 90% sensitive and 99% specific would be 3% (personal communication, Evan R. Myers MD MPH). Tests that reliably rule out leiomyosarcoma fare better; in this scenario, the negative predictive value is 99.98% and may be helpful in avoiding unnecessary hysterectomy. MRI that shows a dark, homogenous tumor on T2-weighted imaging is highly predictive of a benign leiomyoma. (30)
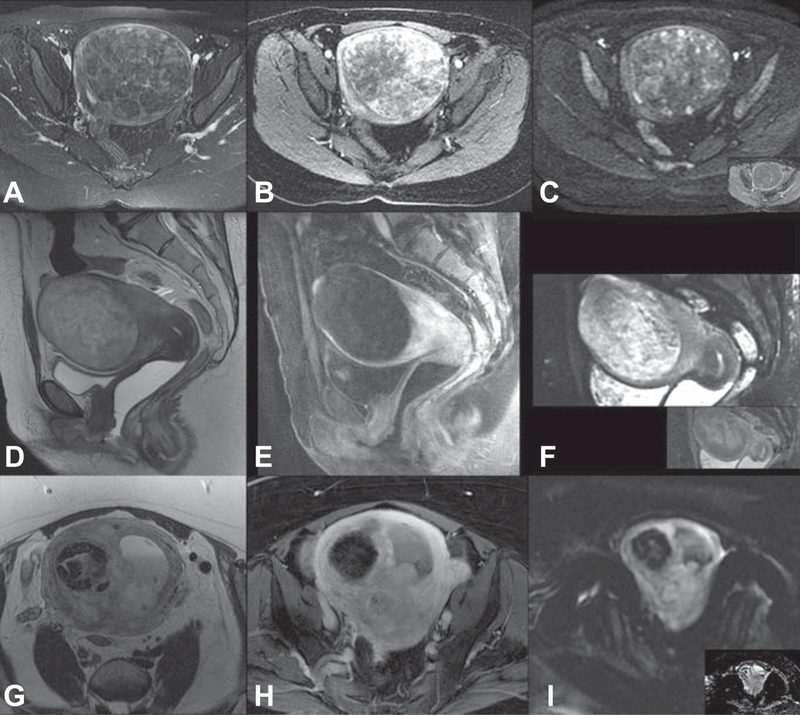
Dynamic or functional MRI scans improve diagnosis over T2-weighted imaging alone. The addition of gadolinium contrast can highlight the increased vascularity of normal leiomyoma relative to myometrium; however, this is not universal as lack of enhancement may indicate complete degeneration of a benign leiomyoma (Figure 3). Enhancement of lesions at 60 seconds using gadolinium contrast is highly predictive of normal leiomyomata. (31)
Figure 3.
Use of T2-weighted images, dynamic magnetic resonance imaging with contrast enhancement, and diffusion weighted imaging (with inset apparent diffusion coefficient map) for leiomyoma or leiomyosarcoma diagnosis. Benign leiomyoma: mildly heterogeneous low T2 signal (A), early and heterogeneous avid enhancement (B) no significant restricted diffusion (C). Degenerated leiomyoma: heterogeneous high T2-weighted signal (D) T1-weighted imaging with fat saturation shows homogeneous hypoenhancement (E) heterogeneous diffusion restriction (F). Leiomyosarcoma: infiltrative, ill-defined mass with intermediate T2 signal (G) enhancement of the viable tumoral tissue (H) and hyperintense on high B-value imaging with subsequent restricted dark signal on the apparent diffusion coefficient map (I).
An emerging MRI technique that is widely available and can potentially help distinguish tumors with malignant potential from benign leiomyomas is diffusion weighted imaging (DWI). DWI relies on diffusion motion of water molecules in the extracellular space that is restricted in tumors with high cellularity, such as tumors with malignant potential. (32, 33) DWI can be quantified using an apparent diffusion coefficient (ADC) and used as an adjunct to traditional MRI, but T2- and T1-weighted imaging is still needed to characterize leiomyoma biology. For instance, leiomyosarcomas tend to have a low ADC, but ADC values overlap with “ordinary” leiomyomas because some ordinary leiomyomas have high ECM content of leiomyomas limiting diffusion. (32) However, a low signal intensity on T2- and T1-weighted imaging and a smooth border would indicate a typical homogeneous, highly fibrotic leiomyoma, even if ADC was low. High signal intensity on T2- and/or T1-weighted images could indicate a leiomyosarcoma, cellular leiomyoma, or a degenerated leiomyoma; here, DWI with high ADC may be reassuring that it is more likely a degenerated leiomyoma (Figure 3) while low ADC would be more worrisome, but not confirmatory, for malignancy. When ADC and T2-weighted imaging were used together, accuracy for the classification of benign and malignant tumors was 92.4%.(30) On-going studies continue to try to improve positive predictive value for malignancy.
Other preoperative tests that could serve as biomarkers for sarcomas such as endometrial biopsy and lactate dehydrogenase (LDH) blood markers also suffer from low positive predictive values for the diagnosis of leiomyosarcoma and are not routinely used. The sensitivity of endometrial biopsy for stromal malignancies, such as leiomyosarcoma and endometrial stromal sarcoma are between 38% and 86%. (34, 35) However, ACOG recommends that women 45 years and older with abnormal uterine bleeding have an endometrial biopsy to rule out endometrial cancer or hyperplasia (36); this important test should not be overlooked even if bleeding is presumed to be due to leiomyomata (AUB-L).
Combining pre-operative testing into scoring systems to improve the diagnosis of leiomyosarcoma is increasingly popular.(33) The revised Preoperative Sarcoma Score (rPRESS) found that patient age, blood levels of lactate dehydrogenase (LDH) markers, and endometrial cytology were sufficient for a high predictive value (92.3%) and did not include MRI features in the final system. (37, 38) Negative predictive value for rPRESS model was 94.0% and accuracy 93.7%.
The future of diagnosis
Future diagnostic techniques ideally would not only be able to differentiate a benign leiomyoma from a malignancy, but would be able to predict the clinical course or the appropriate treatment. It is not yet clear if genotype or phenotype will be the key to individualized therapy given the redundancies in signaling pathways especially as multiple genotypes and phenotypes of leiomyomata can occur in a single uterus. The Fibroid Growth Study demonstrated a wide range of growth rates for different tumors in the same woman. Both rapidly growing and spontaneously regressing leiomyomata occurred in 7 women, and the overall within-woman variation was greater than between-woman. (7) Leiomyoma biopsies have been proposed, but which leiomyomata would be biopsied with this great variation? Due to the heterogeneity of leiomyomas and the difficulty diagnosing leiomyosarcoma on small samples, core biopsies are thought overall to be unreliable as preoperative diagnostic testing. Finally, not all leiomyomas can be easily biopsied in an ambulatory setting due to position within the uterus.
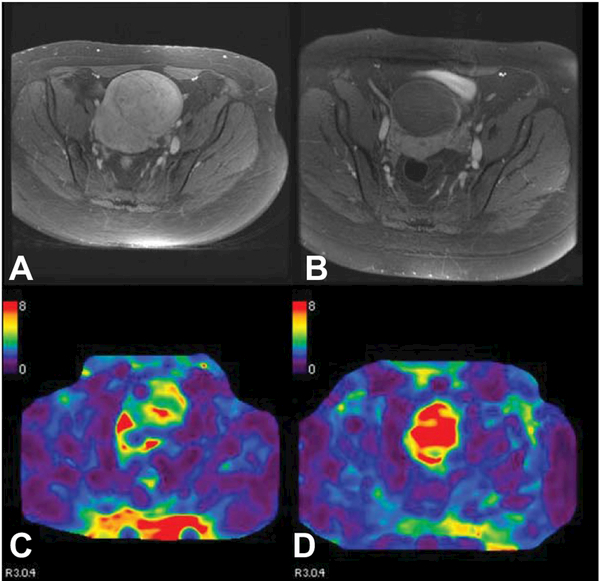
One promising phenotypic marker of a typical leiomyomata, and target of therapy, is the large amount of ECM, compared with leiomyoma variants, which have some facet of malignant behavior but in general are regarded as benign, or malignancies which tend to be more cellular or degenerated. Fibrotic ECM may be evaluated by ultrasound or magnetic resonance elastography (MRE), a test of the stiffness of these tumors, with the potential for predicting response to treatments including Focused Ultrasound Ablation Surgery (FUS). MRE induces harmonic vibrations and images these propagations to generate a stiffness map, quantifying the mechanical properties of the tissue (Figure 4). (39) Already proven useful in liver tumors, feasibility trials are ongoing in leiomyomata. (39) Typical leiomyomata demonstrate higher stiffness than cellular or degenerated tumors.
Figure 4.
Typical leiomyoma demonstrates contrast enhancement on T1-weighted imaging (A) and has average stiffness (C). After uterine artery embolization, leiomyoma no longer enhances (B) and has increased stiffness (D).
A second innovative idea is being able to image the pseudocapsule that encompasses a leiomyoma to better determine leiomyoma location, which would help surgical planning for myomectomy and may have potential to distinguish benign from malignant tumors. Intramural leiomyomata that have an intracavitary component increase the risk of abdominal or laparoscopic myomectomy (40); this knowledge is paramount for preoperative counseling. Under electron microscope, the pseudocapsule appears to be comprised of smooth muscle cells whose cytoplasm is packed with thin filaments and that have a surrounding basal membrane. (41) This structure lacks the stiffer ECM of the typical leiomyoma allowing it to be more elastic and stretch for growth. It is also described as a neurovascular bundle which should be preserved during myomectomy. (40) The pseudocapsule can be imaged on ultrasound and appears as a “ring of fire”. (40)
Lastly, being able to define biomarkers to diagnose and predict the clinical course of leiomyomata is vital. Early detection of colon and endometrial cancers has been vastly advanced by the detection of methylated DNA shed by the tumors into the stool and menstrual samples, respectively. (42, 43) Given AUB-L, it is likely that DNA could be collected in a similar fashion during menses. (42) The limitations are that leiomyomata are not generally necrotic, where tumor shedding may be increased, and the tumor itself may not have direct contact with the endometrium. However, studies have demonstrated endometrial changes even distant from the leiomyoma. (44, 45)
With leiomyomata, we would want to move beyond differentiating between benign and malignant tumors. Finding molecular markers for each of the genetic subtypes that are shed in menstrual blood could offer a window into the predicted phenotype of the leiomyomata in the uterus, allowing for individualized treatment options.
Current Treatment Options
The first step in determining the best treatment option currently is determining whether the patient is symptomatic. Asymptomatic leiomyomata do not require treatment and can be managed expectantly. There is little risk to waiting for symptoms before re-imaging or treating an asymptomatic leiomyoma; available short-term studies of expectant management demonstrate that leiomyoma have minimal growth and bleeding changes within one year (46). One exception is for women with submucosal leiomyomata who desire fertility, where hysteroscopic resection is indicated even if asymptomatic according to some guidelines. (47)
Second, treatment options should be directed at the symptoms, whether that is HMB, bulk symptoms, or both. Leiomyomata that grow into the uterine cavity (Type 0, 1, or 2) can cause HMB even at a small size and can be addressed hysteroscopically.(1) HMB can also be addressed medically with tranexamic acid, NSAIDs, or hormonal therapy including combined estrogen-progestin pills or progestin-only intrauterine devices (IUD).(1) Studies on progestin-releasing IUDs demonstrate excellent control of HMB with little or no change in the size of leiomyomas; treatment failure for the IUD, including a higher risk of expulsion, was lower than women who used oral contraceptive pills in one randomized study.(48) For the woman who has completed childbearing, and has a fairly normal endometrial cavity, endometrial ablation can be an option to address HMB. However, contraception will still be necessary for the sexually-active woman since the cavity is not necessarily obliterated and fertilization can occur.
Bulk symptoms are caused by larger leiomyomata that cause pressure on surrounding organs such as the bladder (urinary frequency), bowel (constipation), or distort the contour of the abdominal wall. These leiomyomata are amenable to destructive or excisional alternatives to hysterectomy. Myomectomy has long been the gold standard uterine-sparing option, especially to optimize fertility or for very large uteri. Myomectomy can be done hysteroscopically, laparoscopically, or with an open laparotomy depending on the size and location of the leiomyomata. Because this is an excisional procedure, it allows for complete removal of the leiomyoma,or 100% volume reduction, and pathological evaluation. Comparatively, nonexcisional procedures leave leiomyomata behind and typically result in 30–60 % volume reduction. The FDA guidance about morcellation of unsuspected leiomyosarcoma which state that the use of power morecellators are contraindicated for removal of uterine tissue in women who are peri- or postmenopausal have decreased the utilization of myomectomy in perimenopausal women. (49)
Current procedures that destroy the leiomyoma without excising it include UAE, MRgFUS, and radiofrequency ablation; all are effective in reducing leiomyoma size and treating symptoms with less complications than myomectomy or hysterectomy. However, recurrent symptoms or regowth of leiomyomas can be an issue. In most studies, women treated closer to the time of menopause will have lower risk of re-intervention than younger women. (27)(46) In a randomized controlled trial comparing UAE to MRgFUS, symptoms were well-controlled by both treatments, but more leiomyoma re-interventions were more common in the MRgFUS arm. (50)
Optimizing fertility is important for many women with leiomyomas and in fact the sole indication for treatment in some women. The conclusions of most small studies suggest that leiomyomas that distort the uterine cavity affect fertility and removal of these leiomyomas improves fertility. However, a recent large study that was powered to examine other confounders investigated the relationship between leiomyomas and miscarriage. (51) This study showed that leiomyomas were associated with miscarriage in univariable analysis. However, when other confounders were examined, this relationship disappeared and increased age appeared to be the key association. While miscarriage risk and fertility risk have commonalities, they are not the same. Nonetheless the findings from this well-designed study with large numbers of participants raises questions about the smaller studies of infertility and whether the same confounding effect of age may be present for infertility. Unfortunately, in the United States (U.S.), where many women have insurance coverage for surgical procedures, but not for infertility care, the bias toward surgical therapy for leiomyomas is likely to persist.
Innovative Treatment Options
Medical therapies to treat symptoms and decrease leiomyoma size have been long sought by women. In a national survey regarding current treatment options, 81% of women were concerned about the invasiveness of the procedure and 72% were worried about permanence. Nearly ¾ of women were concerned about missing days from work. (3) Not only is this important for personal preference, but the annual healthcare costs for the leiomyoma treatment in the U.S. is about $2 bllion dollars, which was mainly due to hysterectomies. (52)
New medical therapies offer decreases in bleeding and leiomyoma size with fewer side effects than the currently available long-acting GnRH agonists. Leuprolide acetate is currently FDA-approved for preoperative treatment of anemia secondary to leiomyomata; because it mimics menopause, women have significant vasomotor side effects and long-term use is associated with loss of bone mineral density. (53)
Selective progesterone receptor modulators (PRMs) work by decreasing progesterone’s effect on leiomyoma growth. Several PRMs are undergoing trials in the U.S. (Table 2). Ulipristal acetate (UA) is currently available for the treatment of leiomyomata outside the U.S.; however, there have been three reports of liver failure that are being investigated by the European Medicines Agency. (54) PRMs have been shown to control HMB and reduce uterine and leiomyoma volumes, with a side effect profile that is improved over GnRH agonists. (53) Trials in the U.S. with a more diverse and obese population have shown lower amenorrhea rates. (55)
Table 2.
Investigational status of new medical therapies for uterine leiomyomata
| Medication | Status | U.S. Clinical Trial Phase | Clinicaltrials.gov |
|---|---|---|---|
|
Selective progesterone receptor medications | |||
| Ulipristal Acetate | Approved in Europe, Asia, Canada for repeated intermittent use | VENUS I & II, complete NDA reported accepted 10/10/2017 |
NCT02147197 NCT02147158 |
| Viliprisan | Investigational | ASTEROID Phase III Study launched 8/7/2017 |
NCT03240523 NCT03194646 |
| Proellex | Investigational | Completed Phase II studies for vaginal and oral administration |
NCT00737282 NCT00683917 |
| GnRH antagonists | |||
| Elagolix | Investigational | Phase IIIb study completed, IIIb enrolling |
NCT02654054 NCT02691494 NCT03271489 |
| Relugolix | Investigational | Phase III LIBERTY 1 and 2 enrolling |
NCT03049735 NCT03103087 |
| OBE2109 | Investigational | Phase III PRIMROSE 1 and 2 enrolling |
NCT03070899 NCT03070951 |
A second long-term medical option in clinical trials are oral GnRH antagonists (Table 2). Compared with placebo, these agents demonstrate greater reductions in menstrual blood loss and leiomyoma volume. (56) Add-back therapy attenuated results, but also decreased side effects such as hot flushes.
Medical therapy to date has focused on hormone receptors. The accumulation of ECM contributes to the large size, bulk symptoms, and rapid growth of leiomyomata. Many growth factors, cytokines, and microRNAs in addition to hormones affect the ECM and are potential targets for medical intervention. (57) Antiproliferative drugs such as epigallocatechin gallate (found in green tea) have shown some promising results in early trials. All-trans retinoic acid has been tested in cell lines and found to down regulate cell proliferation and ECM formation. Vitamin D insufficiency has been associated with an increased risk of leiomyomata; in animal models, vitamin D has been shown to reduce leiomyoma size likely via a TGF-β pathway. (57)
Conclusion
Leiomyomata are a heterogeneous condition that display some molecularly distinct pathways associated with the known genetic subtypes and some common molecular pathways. We believe treatment focus will undergo a paradigm shift analogous to that of treating malignancies, where location of the tumor is secondary to understanding its molecular alterations. Knowing the molecular pathways and their effect on leiomyoma growth can create innovative treatment options that focus on hormone receptors, ECM, or disordered collagen that can be specified to a tumor’s genotype. These non-surgical options are appealing to women who wish to preserve their uterus for fertility and long-term health benefits. Additionally, because of the high prevalence, the burden of healthcare costs for leiomyoma treatment will continue to grow. To have viable medical therapies, though, the diagnostic tools to identify these genotype-phenotype subgroups are needed. Future research should seek non-surgical options with the potential for long-term treatment or even secondary prevention. Developing a risk algorithm for high risk women and aiming for primary prevention will be the long term goal for this disease.
Supplementary Material
Précis.
25 words (present tense, stating conclusion)
Newly understood leiomyoma biology raises the possibility for innovative individualized leiomyoma care; innovative diagnostic techniques will be required to correlate leiomyoma subtype with therapeutic response.
Acknowledgments
Supported by grant number P50HS023418 from the Agency for Healthcare Research and Quality.
Financial Disclosure
Shannon K. Laughlin-Tommaso received personal fees from Allergan and grants from Halt Medical outside the submitted work. Elizabeth A. Stewart received personal fees from AbbVie, Allergan, Astellas, Bayer, GlaxoSmithKline, Gynesonics, Myovant, and Welltwigs from outside the submitted work.
References
- 1.Stewart EA, Laughlin-Tommaso SK, Catherino WH, Lalitkumar S, Gupta D, Vollenhoven B. Uterine fibroids. Nat Rev Dis Primers 2016. June 23;2:16043. [DOI] [PubMed] [Google Scholar]
- 2.Day Baird D, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 2003. January;188(1):100-7. [DOI] [PubMed] [Google Scholar]
- 3.Stewart EA, Nicholson WK, Bradley L, Borah BJ. The burden of uterine fibroids for African-American women: results of a national survey. J Womens Health (Larchmt) 2013. October;22(10):807-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wechter ME, Stewart EA, Myers ER, Kho RM, Wu JM. Leiomyoma-related hospitalization and surgery: prevalence and predicted growth based on population trends. Am J Obstet Gynecol 2011. November;205(5):492 e1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wise LA, Laughlin-Tommaso SK. Epidemiology of Uterine Fibroids: From Menarche to Menopause. Clin Obstet Gynecol 2016. March;59(1):2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart EA, Cookson CL, Gandolfo RA, Schulze-Rath R. Epidemiology of uterine fibroids: a systematic review. BJOG 2017. September;124(10):1501-12. [DOI] [PubMed] [Google Scholar]
- 7.Peddada SD, Laughlin SK, Miner K, Guyon JP, Haneke K, Vahdat HL, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci U S A 2008. December 16;105(50):19887-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puri K, Famuyide AO, Erwin PJ, Stewart EA, Laughlin-Tommaso SK. Submucosal fibroids and the relation to heavy menstrual bleeding and anemia. Am J Obstet Gynecol 2014. January;210(1):38 e1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeh JS, Cheng HM, Hsu PF, Sung SH, Liu WL, Fang HL, et al. Hysterectomy in young women associates with higher risk of stroke: A nationwide cohort study. Int J Cardiol 2013. October 3;168(3):2616-21. [DOI] [PubMed] [Google Scholar]
- 10.Ingelsson E, Lundholm C, Johansson AL, Altman D. Hysterectomy and risk of cardiovascular disease: a population-based cohort study. Eur Heart J 2011. March;32(6):745-50. [DOI] [PubMed] [Google Scholar]
- 11.Laughlin-Tommaso SK, Khan Z, Weaver AL, Smith CY, Rocca WA, Stewart EA. Cardiovascular and metabolic morbidity after hysterectomy with ovarian conservation: a cohort study. Menopause 2017. December 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farquhar CM, Sadler L, Harvey SA, Stewart AW. The association of hysterectomy and menopause: a prospective cohort study. Bjog 2005. July;112(7):956-62. [DOI] [PubMed] [Google Scholar]
- 13.Parker WH, Broder MS, Liu Z, Shoupe D, Farquhar C, Berek JS. Ovarian conservation at the time of hysterectomy for benign disease. Obstetrics and gynecology 2005. August;106(2):219-26. [DOI] [PubMed] [Google Scholar]
- 14.Alternatives to hysterectomy in the management of leiomyomas. ACOG Practice Bulletin No. 96. Obstet Gynecol 2008;112:387-400. [DOI] [PubMed] [Google Scholar]
- 15.Mehine M, Kaasinen E, Makinen N, Katainen R, Kampjarvi K, Pitkanen E, et al. Characterization of uterine leiomyomas by whole-genome sequencing. N Engl J Med 2013. July 4;369(1):43-53. [DOI] [PubMed] [Google Scholar]
- 16.Makinen N, Mehine M, Tolvanen J, Kaasinen E, Li Y, Lehtonen HJ, et al. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science 2011. October 14;334(6053):252-5. [DOI] [PubMed] [Google Scholar]
- 17.Mehine M, Kaasinen E, Heinonen HR, Makinen N, Kampjarvi K, Sarvilinna N, et al. Integrated data analysis reveals uterine leiomyoma subtypes with distinct driver pathways and biomarkers. Proc Natl Acad Sci U S A 2016. February 02;113(5):1315-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alsolami S, El-Bahrawy M, Kalloger SE, AlDaoud N, Pathak TB, Chung CT, et al. Current morphologic criteria perform poorly in identifying hereditary leiomyomatosis and renal cell carcinoma syndrome-associated uterine leiomyomas. Int J Gynecol Pathol 2014. November;33(6):560-7. [DOI] [PubMed] [Google Scholar]
- 19.Holdsworth-Carson SJ, Zaitseva M, Vollenhoven BJ, Rogers PA. Clonality of smooth muscle and fibroblast cell populations isolated from human fibroid and myometrial tissues. Molecular Hum Reprod 2014. March;20(3):250-9. [DOI] [PubMed] [Google Scholar]
- 20.Wu X, Serna VA, Thomas J, Qiang W, Blumenfeld ML, Kurita T. Subtype-specific tumor-associated fibroblasts contribute to the pathogenesis of uterine leiomyoma. Cancer Res 2017. October 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishikawa H, Ishi K, Serna VA, Kakazu R, Bulun SE, Kurita T. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology 2010. June;151(6):2433-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munro MG, Critchley HO, Fraser IS. The FIGO classification of causes of abnormal uterine bleeding in the reproductive years. Fertility and Sterility 2011. June;95(7):2204-8, 8 e1–3. [DOI] [PubMed] [Google Scholar]
- 23.Laughlin-Tommaso SK, Hesley GK, Hopkins MR, Brandt KR, Zhu Y, Stewart EA. Clinical limitations of the International Federation of Gynecology and Obstetrics (FIGO) classification of uterine fibroids. Int J Gynaecol Obstet 2017. November;139(2):143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Accuracy of magnetic resonance imaging and transvaginal ultrasonography in the diagnosis, mapping, and measurement of uterine myomas. Am J Obstet Gynecol 2002. March;186(3):409-15. [DOI] [PubMed] [Google Scholar]
- 25.Soguktas S, Cogendez E, Kayatas SE, Asoglu MR, Selcuk S, Ertekin A. Comparison of saline infusion sonohysterography and hysteroscopy in diagnosis of premenopausal women with abnormal uterine bleeding. Eur J Obstet Gynecol Reprod Biol 2012. March;161(1):66-70. [DOI] [PubMed] [Google Scholar]
- 26.Gorny KR, Borah BJ, Brown DL, Woodrum DA, Stewart EA, Hesley GK. Incidence of additional treatments in women treated with MR-guided focused US for symptomatic uterine fibroids: review of 138 patients with an average follow-up of 2.8 years. J Vasc Interv Radiol 2014. October;25(10):1506-12. [DOI] [PubMed] [Google Scholar]
- 27.Gorny KR, Borah BJ, Weaver AL, Brown D, Woodrum DA, Stewart EA, et al. Clinical predictors of successful magnetic resonance-guided focused ultrasound (MRgFUS) for uterine leiomyoma. J Ther Ultrasound 2013;1:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee MS, Kim MD, Jung DC, Lee M, Won JY, Park SI, et al. Apparent diffusion coefficient of uterine leiomyoma as a predictor of the potential response to uterine artery embolization. J Vasc Interv Radiol 2013. September;24(9):1361-5. [DOI] [PubMed] [Google Scholar]
- 29.Parker WH, Kaunitz AM, Pritts EA, Olive DL, Chalas E, Clarke-Pearson DL, et al. U.S. Food and Drug Administration's Guidance Regarding Morcellation of Leiomyomas: Well-Intentioned, But Is It Harmful for Women? Obstetrics and gynecology 2016. January;127(1):18-22. [DOI] [PubMed] [Google Scholar]
- 30.Thomassin-Naggara I, Dechoux S, Bonneau C, Morel A, Rouzier R, Carette MF, et al. How to differentiate benign from malignant myometrial tumours using MR imaging. Eur Radiol 2013. August;23(8):2306-14. [DOI] [PubMed] [Google Scholar]
- 31.Goto A, Takeuchi S, Sugimura K, Maruo T. Usefulness of Gd-DTPA contrast-enhanced dynamic MRI and serum determination of LDH and its isozymes in the differential diagnosis of leiomyosarcoma from degenerated leiomyoma of the uterus. Int J Gynecol Cancer 2002. Jul-Aug;12(4):354-61. [DOI] [PubMed] [Google Scholar]
- 32.Tamai K, Koyama T, Saga T, Morisawa N, Fujimoto K, Mikami Y, et al. The utility of diffusion-weighted MR imaging for differentiating uterine sarcomas from benign leiomyomas. Eur Radiol 2008. April;18(4):723-30. [DOI] [PubMed] [Google Scholar]
- 33.Barral M, Place V, Dautry R, Bendavid S, Cornelis F, Foucher R, et al. Magnetic resonance imaging features of uterine sarcoma and mimickers. Abdom Radiol (NY) 2017. June;42(6):1762-72. [DOI] [PubMed] [Google Scholar]
- 34.Bansal N, Herzog TJ, Burke W, Cohen CJ, Wright JD. The utility of preoperative endometrial sampling for the detection of uterine sarcomas. Gynecol Oncol 2008. July;110(1):43-8. [DOI] [PubMed] [Google Scholar]
- 35.Leibsohn S, d'Ablaing G, Mishell DR Jr.,Schlaerth JB. Leiomyosarcoma in a series of hysterectomies performed for presumed uterine leiomyomas. Am J Obstet Gynecol 1990. April;162(4):968-74; discussion 74–6. [DOI] [PubMed] [Google Scholar]
- 36.Management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. ACOG Committee Opinion No. 557. American College of Obstetricians and Gynecologists. Obstet Gynecol 2013;121:891-6. [DOI] [PubMed] [Google Scholar]
- 37.Nagai T, Takai Y, Akahori T, Ishida H, Hanaoka T, Uotani T, et al. Novel uterine sarcoma preoperative diagnosis score predicts the need for surgery in patients presenting with a uterine mass. Springerplus 2014;3:678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagai T, Takai Y, Akahori T, Ishida H, Hanaoka T, Uotani T, et al. Highly improved accuracy of the revised PREoperative sarcoma score (rPRESS) in the decision of performing surgery for patients presenting with a uterine mass. Springerplus 2015;4:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jondal DE, Wang J, Chen J, Gorny KR, Felmlee J, Hesly G, et al. Uterine fibroids: correlations between MRI appearance and stiffness via magnetic resonance elastography. Abdom Radiol (NY) 2017. September 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tinelli A, Hurst BS, Hudelist G, Tsin DA, Stark M, Mettler L, et al. Laparoscopic myomectomy focusing on the myoma pseudocapsule: technical and outcome reports. Hum Reprod 2012. February;27(2):427-35. [DOI] [PubMed] [Google Scholar]
- 41.Malvasi A, Cavallotti C, Morroni M, Lorenzi T, Dell'Edera D, Nicolardi G, et al. Uterine fibroid pseudocapsule studied by transmission electron microscopy. Eur J Obstet Gynecol Reprod Biol 2012. June;162(2):187-91. [DOI] [PubMed] [Google Scholar]
- 42.Bakkum-Gamez JN, Wentzensen N, Maurer MJ, Hawthorne KM, Voss JS, Kroneman TN, et al. Detection of endometrial cancer via molecular analysis of DNA collected with vaginal tampons. Gynecol Oncol 2015. April;137(1):14-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imperiale TF, Ransohoff DF, Itzkowitz SH, Levin TR, Lavin P, Lidgard GP, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med 2014. April 3;370(14):1287-97. [DOI] [PubMed] [Google Scholar]
- 44.Deligdish L, Loewenthal M. Endometrial changes associated with myomata of the uterus. J Clin Pathol 1970. November;23(8):676-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang X, Lu B, Huang X, Xu H, Zhou C, Lin J. Endometrial nerve fibers in women with endometriosis, adenomyosis, and uterine fibroids. Fertil Steril 2009. November;92(5):1799-801. [DOI] [PubMed] [Google Scholar]
- 46.Hartmann KE, Fonnesbeck C, Surawicz T, Krishnaswami S, Andrews JC, Wilson JE, Velez-Edwards D, Kugley S, Sathe NA. Management of Uterine Fibroids. Comparative Effectiveness Review No. 195. (Prepared by the Vanderbilt Evidence-based Practice Center under Contract No. 290–2015-00003-I.) AHRQ Publication No. 17(18)-EHC028-EF. Rockville, MD: Agency for Healthcare Research and Quality; December 2017. www.effectivehealthcare.ahrq.gov/reports/final.cfm. doi: https://doi.org/10.23970/AHRQEPCCER195. [PubMed]
- 47.Marret H, Fritel X, Ouldamer L, Bendifallah S, Brun JL, De Jesus I, et al. Therapeutic management of uterine fibroid tumors: updated French guidelines. Eur J Obstet Gynecol Reprod Biol 2012. December;165(2):156-64. [DOI] [PubMed] [Google Scholar]
- 48.Sayed GH, Zakherah MS, El-Nashar SA, Shaaban MM. A randomized clinical trial of a levonorgestrel-releasing intrauterine system and a low-dose combined oral contraceptive for fibroid-related menorrhagia. Int J Gynaecol Obstet 2011. February;112(2):126-30. [DOI] [PubMed] [Google Scholar]
- 49.Barron KI, Richard T, Robinson PS, Lamvu G. Association of the U.S. Food and Drug Administration Morcellation Warning With Rates of Minimally Invasive Hysterectomy and Myomectomy. Obstet Gynecol 2015. December;126(6):1174-80. [DOI] [PubMed] [Google Scholar]
- 50.Laughlin-Tommaso SK, Weaver AL, Vaughan LE, Jacoby V, Stewart EA. Long Term Outcomes in a Randomized Controlled Trial of Uterine Artery Embolization and Mr-Guided Focused Ultrasound: The Firstt Study. Fertility and Sterility 2017. September;108(3):E26-E. [Google Scholar]
- 51.Hartmann KE, Velez Edwards DR, Savitz DA, Jonsson-Funk ML, Wu P, Sundermann AC, et al. Prospective Cohort Study of Uterine Fibroids and Miscarriage Risk. Am J Epidemiol 2017. June 07:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flynn M, Jamison M, Datta S, Myers E. Health care resource use for uterine fibroid tumors in the United States. Am J Obstet Gynecol 2006. October;195(4):955-64. [DOI] [PubMed] [Google Scholar]
- 53.Donnez J, Tatarchuk TF, Bouchard P, Puscasiu L, Zakharenko NF, Ivanova T, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med 2012. February 2;366(5):409-20. [DOI] [PubMed] [Google Scholar]
- 54.http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Esmya/human_referral_prac_000070.jsp&mid=WC0b01ac05805c516f
- 55.Liu J, Soper D, Lukes AS, Gee P, Kimble T, Kroll R, et al. Venus Ii: The Second Us-Based Phase 3 Study of Ulipristal Acetate (Upa) for Treatment of Symptomatic Uterine Fibroids (Uf). Fertility and Sterility 2017. September;108(3):E27-E8. [Google Scholar]
- 56.Archer DF, Stewart EA, Jain RI, Feldman RA, Lukes AS, North JD, et al. Elagolix for the management of heavy menstrual bleeding associated with uterine fibroids: results from a phase 2a proof-of-concept study. Fertil Steril 2017. June 01. [DOI] [PubMed] [Google Scholar]
- 57.Islam MS, Protic O, Giannubilo SR, Toti P, Tranquilli AL, Petraglia F, et al. Uterine leiomyoma: available medical treatments and new possible therapeutic options. J Clin Endocrinol Metab 2013. March;98(3):921-34. [DOI] [PubMed] [Google Scholar]
- 58. Temkin SM, Minasian L, Noone AM. The End of the Hysterectomy Epidemic and Endometrial Cancer Incidence: What Are the Unintended Consequences of Declining Hysterectomy Rates? Front Oncol 2016;6:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Altman D, Yin L, Falconer H. Long-term cancer risk after hysterectomy on benign indications: Population-based cohort study. Int J Cancer 2016. June 1;138(11):2631-8. [DOI] [PubMed] [Google Scholar]
- 60. Gierach GL, Pfeiffer RM, Patel DA, Black A, Schairer C, Gill A, et al. Long-term overall and disease-specific mortality associated with benign gynecologic surgery performed at different ages. Menopause 2014. June;21(6):592-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.