Abstract
Background
Advanced chronic kidney disease (CKD) is associated with an elevated risk of cognitive impairment. However, it is not known if and how cognitive impairment is associated with planning and preparation for end stage renal disease.
Study Design
Retrospective observational study.
Setting & Participants
630 adults participating in the CRIC (Chronic Renal Insufficiency Cohort) Study who had cognitive assessments in late stage CKD, defined as an estimated glomerular filtration rate (eGFR) ≤ 20 ml/min/1.73m2, and subsequently initiated maintenance dialysis.
Predictor
Pre-dialysis cognitive impairment, defined as a score on the Modified Mini-Mental State Examination below previously-derived age-based threshold scores. Covariates included age, race/ethnicity, educational attainment, comorbid conditions, and health literacy.
Outcomes
Peritoneal dialysis (PD) as first dialysis modality, pre-emptive permanent access placement, venous catheter avoidance at dialysis initiation, and pre-emptive wait-listing for kidney transplantation.
Measurements
Multivariable-adjusted logistic regression.
Results
Pre-dialysis cognitive impairment was present in 117 participants (19%). PD was the first dialysis modality among 16% of participants (n=100), 75% had pre-emptive access placed (n=473), 45% avoided using a venous catheter at dialysis initiation (n=279), and 20% were pre-emptively wait-listed (n=126). Pre-dialysis cognitive impairment was independently associated with an 78% lower odds of PD as the first dialysis modality (adjusted Odds Ratio [aOR], 0.22; 95% Confidence Interval [CI], 0.06–0.74; p=0.02) and a 42% lower odds of venous catheter avoidance at dialysis initiation (aOR, 0.58; 95% CI, 0.34–0.98; p=0.04). Pre-dialysis cognitive impairment was not independently associated with pre-emptive permanent access placement or wait-listing.
Limitations
Potential unmeasured confounders; single measure of cognitive function.
Conclusions
Pre-dialysis cognitive impairment is associated with a lower likelihood of PD as a first dialysis modality and of venous catheter avoidance at dialysis initiation. Future studies may consider addressing cognitive function when testing strategies to improve patient transitions to dialysis.
Index words: chronic kidney diseases (CKD), end-stage renal disease (ESRD), CKD to ESRD transition, cognitive impairment, dialysis modality, dialysis access, peritoneal dialysis (PD), central venous catheter (CVC), executive function, memory, incident ESRD, dementia, transplant waitlisting
Introduction
Numerous consensus guidelines promote strategies to improve patients’ transitions from chronic kidney disease (CKD) to end stage renal disease (ESRD), including individualized decision-making on a preferred dialysis modality, pre-emptive placement of appropriate dialysis access, and early assessment of eligibility for the kidney transplant waiting list.1–3 However, underutilization of home dialysis therapies such as peritoneal dialysis (PD),4 widespread use of venous catheters,1 and the low prevalence of pre-emptive wait-listing5 indicate that the transition to dialysis remains suboptimal for many patients. While prior studies have observed variation across different patient subgroups in achieving these important outcomes,5–9 whether cognitive impairment contributes to the likelihood of optimal transition to ESRD remains unknown.
Despite recognition that CKD is associated with increased risks of cognitive impairment, cognitive deficits are frequently underdiagnosed in clinical settings.10–13 CKD-associated cognitive impairment commonly manifests as diminished executive function and delayed memory,14 and patients with these deficits may have diminished abilities to focus, plan, retain new knowledge, and complete tasks that are crucial to achieving optimal preparation for ESRD.15,16
Therefore, the goal of this study was to evaluate whether differences in pre-dialysis cognitive function may contribute to variation in ESRD transition outcomes. Among participants enrolled in the Chronic Renal Insufficiency Cohort (CRIC) study who reached late-stage CKD and initiated maintenance dialysis, we assessed the independent association of predialysis cognitive impairment with the likelihood of four outcomes: peritoneal dialysis (PD) as a first dialysis modality, pre-emptive permanent access placement, avoidance of venous catheter use at dialysis initiation, and pre-emptive wait-listing for kidney transplantation.
Methods
Study Population
The CRIC Study is an ongoing multicenter prospective study of risk factors for CKD progression and cardiovascular disease. The design and methods of the study and inclusion criteria for study participants have been described previously.17,18 Briefly, the CRIC Study recruited 3,939 participants aged 21–74 years with an estimated glomerular filtration rate (eGFR) in the range 20–70 mL/min/1.73m2 from 2003 through 2008. All participants provided informed consent. Participants completed questionnaires at enrollment about sociodemographic information and medical history, and returned for yearly visits during which time this information was updated. All questionnaires remained confidential and for research purposes, and were offered in English and Spanish, corresponding to participants’ native language and preference. The study protocol was approved by the institutional review boards of all participating centers (University of Pennsylvania [coordinating center] IRB protocol #807882), and all research practices are in accordance with the Declaration of Helsinki.
Given evidence that lower levels of kidney function are associated with increased risks of cognitive impairment,19–21 in the current analyses, we focused on the association of cognitive impairment and ESRD transition outcomes among those CRIC participants who initiated dialysis after progression to late-stage CKD. Therefore, we restricted our study to CRIC participants who had a CRIC visit with an eGFR that was equal to or less than 20 ml/min/1.73m2 by the Modification of Diet in Renal Disease (MDRD) Study equation.22 This eGFR threshold was chosen because it is also the cut-off for kidney transplant waiting list eligibility in the United States (US) (see Figure 1, Participant Flow Diagram).23 We defined the visit with the qualifying eGFR as the index visit.
Figure 1.
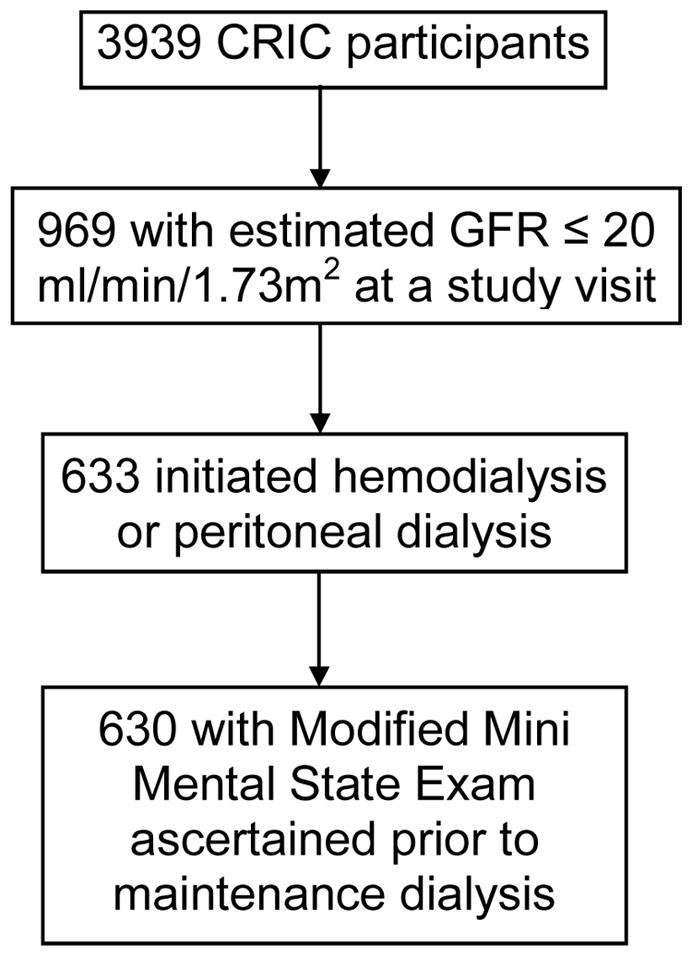
Participant Inclusion Flow Diagram. Abbreviations: GFR, glomerular filtration rate; CRIC, Chronic Renal Insufficiency Cohort
Primary Exposure: Pre-Dialysis Cognitive Impairment
We ascertained pre-dialysis cognitive function by performance on the Modified Mini-Mental State (3MS) exam. The 3MS, a test of global cognitive function including components that test memory, orientation, concentration, language, and praxis,24 was administered at baseline and during annual or bi-annual CRIC visits. 3MS scores range from 0 to 100, with higher scores indicative of better cognitive function. We included the 3MS score for each participant that was closest in time to the index visit and before dialysis onset. We then considered two previously-recognized strategies to define pre-dialysis cognitive impairment among individuals with CKD based on 3MS performance.25,26 In our primary analysis, we defined cognitive impairment as 3MS scores below previously-derived age-based threshold scores:25,27 <85 for participants <65 years of age, <80 for participants aged 65 to 79 years, and <75 for those 80 years of age or older. In secondary analyses, we examined the association of scores >1 standard deviation below the cohort mean score (3MS<80)26,28 on ESRD transition outcomes.
Covariates
As our goal was to assess the independent relationship of cognitive impairment with ESRD transition outcomes, we selected covariates a priori for our multivariable models that are commonly utilized in clinical practice for risk assessment. From the CRIC enrollment visit, we used participant self-reported sex, race/ethnicity, annual household income, educational attainment, and marital status (as a metric of social support). We also included information on the following self-reported medical conditions: diabetes, cardiovascular disease, peripheral vascular disease, congestive heart failure, stroke, and non-skin cancer. At the index visit, we ascertained age (in years), systolic and diastolic blood pressure (in mm Hg), body mass index (in kg/m2), tobacco use, and functional status measured by the Kidney Disease Quality of Life Survey Short Form Physical Component Scale. 29 We used laboratory measures from the enrollment and index visit to calculate slope of eGFR decline prior to the cognitive assessment visit (ml/min/1.73m2 per year), and included serum albumin and serum hemoglobin (both in g/dL) collected at the index visit.
Outcomes
We evaluated four ESRD transition outcomes: 1) PD as the first dialysis modality, 2) pre-emptive permanent access placement, 3) venous catheter avoidance at dialysis initiation, and 4) pre-emptive placement on the transplant waiting list. We defined permanent access as an arteriovenous fistula, graft, or peritoneal dialysis catheter. We verified the date and modality of dialysis initiation, presence of a maturing permanent access at dialysis initiation, type of first access used for dialysis, and date of wait-listing by linkage of CRIC data to the United States Renal Data Service (USRDS) database, including the USRDS Medical Evidence Form 2728, unless this information was not available. As the latest data linkage between CRIC and the USRDS provided outcome information up to April 2015, we used CRIC self-reported data on dates of dialysis initiation and permanent access placement for participants who initiated dialysis after this date. In cases where CRIC participants endorsed wait-listing events after April 2015, we used the date of the first CRIC study visit at which the participant reported being wait-listed. CRIC data on dialysis initiation dates, permanent access placement, and waiting list outcomes were available on participants through February 2016.
Statistical Analysis
All analyses were performed using Stata, version 14.1. All hypothesis tests were 2-sided, with a significance level of 0.05. Descriptive statistics were summarized as means (standard deviations) or medians (interquartile ranges) for continuous variables, and as frequencies (proportions) for categorical variables. Continuous and categorical variables were compared using Wilcoxon rank sum or chi-square tests, respectively.
First, we examined the association between pre-dialysis cognitive impairment and ESRD transition outcomes using unadjusted logistic regression models. Next, we fit the following sequentially adjusted models: Model 1 (participant age (continuous), race/Ethnicity (Non-Hispanic White, Non-Hispanic Black, Hispanic), and sex); Model 2 (educational attainment (categorized as < high school, high school graduate, or > high school), highest quartile of eGFR decline (>-4.4 ml/min/1.73m2/year), history of diabetes, cardiovascular disease, peripheral vascular disease, stroke, congestive heart failure, non-skin cancer, obesity (body mass index≥30 kg/m2), systolic blood pressure (categorized as < or ≥ 140 mmHg), diastolic blood pressure (categorized as < or ≥ 90 mmHg), hemoglobin level (categorized as < or ≥ 10.0 g/dL), serum albumin level (categorized as < or ≥ 4.0 g/dL), current smoker, marital status (currently married/formerly married/never married), functional status score (continuous), timing of cognitive assessment relative to dialysis onset (< or ≥3 years)); and the final, fully adjusted Model 3 (low income status (categorized as <$20,000 vs. other), and CRIC Center (Center 5 vs. other)).
Missing Data
With the exception of eGFR slope (missing=65), all covariates were missing among <3% of participants. The primary analysis included only participants with non-missing data for all key exposures and covariates. In sensitivity analyses, we imputed data for missing observations of eGFR slope using multiple imputation with 10 iterations. Odds ratios were calculated by taking the exponentiation of the estimates from the averaged estimates.30
Sensitivity Analyses
Given knowledge of the potential importance of health literacy in achieving optimal transitions to dialysis,31 we fit multivariable models with health literacy as a covariate among participants with non-missing data. As health literacy was only measured, by protocol, in a sub-cohort of CRIC participants, these models included 71% (n=449) of the participants in the current study cohort. Further, given the distinct steps required in access planning based on dialysis modality, we also performed sensitivity analyses for the outcome of venous catheter avoidance at dialysis initiation in which we excluded participants who initiated PD. Additionally, among participants with preemptive access placement, to examine whether adjustment for health literacy or the timing of pre-emptive vascular access placement strengthened or attenuated the association of cognitive impairment and venous catheter avoidance for hemodialysis initiation, we first imputed the date of pre-emptive permanent vascular access placement as the mid-point date between the date of the first yearly CRIC questionnaire when participants indicated that a permanent access had been placed and the date of the prior questionnaire, or as the date between the last pre-dialysis questionnaire when participants indicated no permanent access was placed and the dialysis date. Then, among participants who initiated hemodialysis after pre-emptive access placement, we examined multivariable logistic regression models for venous catheter avoidance that were sequentially adjusted for all original covariates, health literacy, and whether participants had pre-emptive access placement < or ≥180 before hemodialysis initiation.
Results
Baseline Characteristics
Of 630 eligible CRIC participants, pre-dialysis cognitive impairment (defined by age-based 3MS score) was present in 19% (n=117) of the cohort. Table 1 demonstrates participant characteristics stratified by the presence of pre-dialysis cognitive impairment. Cognitive impairment was not associated with age (mean 60 vs. 61 years, p=0.4). Compared to participants without cognitive impairment, those with cognitive impairment were more likely to be Hispanic (44% vs 20%, p<0.001), report an annual income <$20,000 (62% vs 37%, p<0.001), and have less than a high school education (63% vs 21%, p<0.001).
Table 1.
Demographic and Clinical Characteristics of CRIC Participants Who Initiated Dialysis, by Pre-Dialysis Cognitive Function
| Participant Characteristics | No Cognitive Impairment n=513 | Cognitive Impairment n=117 | P Value |
|---|---|---|---|
| Age at Cognitive Assessment, y | 60 (51–67) | 61 (54–67) | 0.4 |
| Female sex | 229 (45%) | 55 (47%) | 0.6 |
| Race/Ethnicity | <0.001 | ||
| Non-Hispanic White | 150 (29%) | 5 ( 4%) | |
| Non-Hispanic Black | 259 (50%) | 60 (51%) | |
| Hispanic | 104 (20%) | 52 (44%) | |
| Annual income <=$20,000 | 191 (37%) | 73 (62%) | <0.001 |
| Educational Attainment | <0.001 | ||
| Less than High School | 109 (21%) | 74 (63%) | |
| High School Graduate | 104 (20%) | 25 (21%) | |
| Some College/College Graduate | 300 (58%) | 18 (15%) | |
| Marital Status | 0.1 | ||
| Currently Married | 267 (52%) | 63 (54%) | |
| Never Married | 92 (18%) | 12 (10%) | |
| Formerly Married | 154 (30%) | 42 (36%) | |
| Diabetes mellitus | 341 (66%) | 88 (75%) | 0.07 |
| Cardiovascular Disease | 251 (49%) | 53 (45%) | 0.5 |
| Congestive Heart Failure | 88 (17%) | 18 (15%) | 0.6 |
| Stroke | 66 (13%) | 14 (12%) | 0.8 |
| Peripheral Vascular Disease | 55 (11%) | 14 (12%) | 0.7 |
| Cancer (Non-Skin) | 43 ( 8%) | 7 ( 6%) | 0.4 |
| Change in eGFR prior to cognitive assessment, ml/min/1.73 m2 per year | −3.75 (−4.40—3.34) | −3.78 (−4.48—3.34) | 0.6 |
| Body Mass Index ≥30 kg/m2 | 321 (63%) | 60 (51%) | 0.02 |
| Systolic Blood Pressure ≥140mmHg | 250 (49%) | 71 (61%) | 0.02 |
| Diastolic Blood Pressure ≥ 90mmHg) | 47 (9%) | 10 (9%) | 0.8 |
| Current Smoker | 69 (13%) | 15 (13%) | 0.9 |
| KDQOL SF12 PCS Score | 37 (28–48) | 36 (29–44) | 0.2 |
| Serum Albumin <4.0 g/dL | 355 (69%) | 87 (74%) | 0.3 |
| Serum Hemoglobin <10.0 g/dL | 116 (23%) | 27 (23%) | 0.9 |
| eGFR at Index Visit | 17.0 (15.0–19.0) | 17.0 (14.0–18.0) | 0.4 |
| Days from Index visit to 3MS Assessment | 0 (−365− 0) | 0 (−370− 0) | 0.9 |
| Days from 3MS Assessment to Dialysis Initiation | 628 (376–1046) | 547(229–859) | 0.08 |
Values for continuous variables expressed as mediann (interquartile range); for categorical variables as count (percentage).
Abbreviations: eGFR—estimated glomerular filtration rate; KDQOL SF12 PCS—Kidney Disease Quality of Life Short Form 12 Physical Component Score; k—kilograms; m—meters; mmHg – millimeters mercury; 3MS—Modified Mini Mental State Examination; g—grams, dL--deciliter
Association of Pre-Dialysis Cognitive Function with ESRD Transition Outcomes
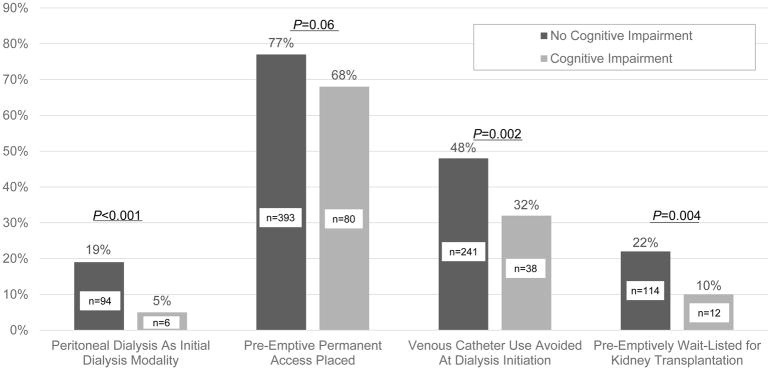
PD was the first dialysis modality among 16% of the cohort (n=100), 75% of participants (n=473) had pre-emptive permanent access placed, 45% of participants (n=279) avoided venous catheter use at dialysis initiation, and 20% (n=126) were preemptively waitlisted. Figure 2 displays proportions of CRIC participants with each ESRD transition outcome, stratified by pre-dialysis cognitive function.
Figure 2.
Proportion of CRIC Participants with ESRD outcomes, Stratified by Pre-dialysis Cognitive Function. Figure displays p-values for the unadjusted associations between pre-dialysis cognitive impairment, defined by age-based cut-off scores on the Modified Mini Mental State Examination, and ESRD transition outcomes.
Outcome 1: Pre-Dialysis Cognitive Impairment and PD as Initial Dialysis Modality
Table 2 demonstrates the results of our iterative model building strategy. Pre-dialysis cognitive impairment retained a statistically significant and inverse association with the odds of dialysis initiation with PD after adjustment for demographics (Model 1), educational attainment, marital status, and clinical covariates (Model 2), and income and CRIC Center 5 (Model 3). In the final, fully adjusted model (Model 3), participants with pre-dialysis cognitive impairment had 78% lower odds of utilizing PD as an initial dialysis modality (aOR for cognitive impairment, 0.22; 95% CI, 0.06–0.74; p=0.02).
Table 2.
Results of Multivariable Logistic Regression Models of the Association of Pre-Dialysis Cognitive Impairment, Defined Using Age-based 3MS Cut-off Scores, with ESRD Transition Outcomes.
| ESRD Transition | Frequency | Unadjusted | Adjusted OR (95% CI) | ||
|---|---|---|---|---|---|
| Outcome | N(%) | OR(95%CI) | Model 1* | Model 2** | Model 3:,Fully-Adjusted Model*** |
| PD as First Dialysis Modality | 100 (16%) | 0.24 (0.10,0.55) | 0.29 (0.12,0.70) | 0.20 (0.06,0.69) | 0.22 (0.06,0.74) |
| Permanent Access Placed before Dialysis Initiation | 473 (75%) | 0.66 (0.43,1.03) | 0.83 (0.53,1.33) | 0.75 (0.42,1.35) | 0.76 (0.44,1.33) |
| CVC Use Avoided at Dialysis Initiation | 279 (45%) | 0.51 (0.34,0.79) | 0.63 (0.41, 0.98) | 0.55 (0.32, 0.92) | 0.58 (0.34, 0.98) |
| Pre-emptively Waitlisted | 126 (20%) | 0.40 (0.21,0.75) | 0.49 (0.25,0.94) | 0.61 (0.28,1.33) | 0.68 (0.31,1.50) |
3MS, Modified Mini Mental State Examination; ESRD, end stage renal disease; OR, odds ratio; 95% CI, 95% Confidence Interval, CVC, central venous catheter; PD, peritoneal dialysis Underlined: p<0.05
Model 1: Adjusted for Age (years), Sex, Race/Ethnicity
Model 2: Model 1+ Educational Attainment, Diabetes, Systolic Blood Pressure ≥140mmHg, Diastolic Blood Pressure ≥90 mmHg, Stroke, Cardiovascular Disease, Congestive Heart Failure, Peripheral Vascular Disease, Tobacco Use, Marital Status, Physical Function Score, Body Mass Index ≥30 kg/m2, Non-skin cancer, Serum Albumin <4.0 g/dL, Serum Hemoglobin<10.0 g/dL, Slope of eGFR decline >4.4ml/min/1.73 m2 per year, cognitive assessment ≤3 years before dialysis.
Model 3: Model 2+ Annual Income, CRIC Center 5
Outcome 2: Pre-Dialysis Cognitive Impairment and Pre-Emptive Permanent Access Placement
Compared to participants without cognitive impairment, there was a nominally lower frequency of pre-emptive permanent access placement among CRIC participants with pre-dialysis cognitive impairment, but this difference did not meet criteria for statistical significance (unadjusted OR for cognitive impairment, 0.66; 95% CI, 0.43–1.03; p=0.06).
Outcome 3: Pre-Dialysis Cognitive Impairment and Venous Catheter Avoidance at Dialysis Initiation
In unadjusted analyses and in Models 1, 2, and the fully adjusted model (Model 3), pre-dialysis cognitive impairment was independently associated with a lower odds of venous catheter avoidance at dialysis initiation. In the fully adjusted model, pre-dialysis cognitive impairment was associated with a 42% lower odds of venous catheter avoidance at dialysis initiation (aOR for cognitive impairment, 0.58; 95% CI, 0.34–0.98; p=0.04).
Outcome 4: Pre-Dialysis Cognitive Impairment and Pre-emptive Transplant Wait-listing
After adjustment for age, race, and sex (Model 1), pre-dialysis cognitive impairment was associated with a 51% lower odds of pre-emptive wait-listing (aOR, 0.49; 95% CI, 0.25–0.94; p=0.03). However, this association was no longer significant after further covariate adjustment (Models 2 and 3). Effect estimates for all variables in the multivariable models are available in Table S1.
Secondary Analysis: Alternative Definition of Pre-Dialysis Cognitive Impairment
In analyses in which we utilized a single threshold 3MS score (<80) to indicate pre-dialysis cognitive impairment, 14% of participants (n=89) met criteria for cognitive impairment. Cognitive impairment was associated with all four ESRD transition outcomes in unadjusted analyses. These associations were attenuated and no longer significant for any of the outcomes in fully-adjusted models (Table 3).
Table 3.
Results of Multivariable Logistic Regression Models of the Association of Pre-Dialysis Cognitive Impairment, Defined as 3MS<80, with ESRD Transition Outcomes
| ESRD Transition | Frequency | Unadjusted | Adjusted OR (95% CI) | ||
|---|---|---|---|---|---|
| Outcome | N(%) | OR(95%CI) | Model 1* | Model 2** | Model 3:,Fully-Adjusted Model*** |
| PD as First Dialysis Modality | 100 (16%) | 0.22 (0.08,0.60) | 0.27 (0.10,0.78) | 0.24 (0.05,1.03) | 0.25 (0.06,1.09) |
| Permanent Access Placed before Dialysis Initiation | 473 (75%) | 0.57 (0.35,0.92) | 0.71 (0.43,1.18) | 0.58 (0.31,1.06) | 0.61 (0.33,1.12) |
| CVC Use Avoided at Dialysis Initiation | 279 (45%) | 0.57 (0.35,0.91) | 0.71 (0.43,1.16) | 0.67 (0.37, 1.20) | 0.72 (0.39,1.32) |
| Pre-emptively Wait- listed | 126 (20%) | 0.30 (0.14,0.67) | 0.38 (0.17,0.87) | 0.51 (0.20,1.34) | 0.54 (0.20,1.44) |
3MS, Modified Mini Mental State Examination; ESRD, end stage renal disease; OR, odds ratio; 95% CI, 95% Confidence Interval, CVC, central venous catheter; PD, peritoneal dialysis
Underlined: p<0.05
Model 1: Adjusted for Age, Sex, Race/Ethnicity
Model 2: Model 1+ Educational Attainment, Diabetes, Systolic Blood Pressure ≥140mmHg, Diastolic Blood Pressure ≥90 mmHg, Stroke, Cardiovascular Disease, Congestive Heart Failure, Peripheral Vascular Disease, Tobacco Use, Marital Status, Physical Function Score, Body Mass Index ≥30 kg/m2, Non-skin cancer, Serum Albumin < 4.0 g/dL, Serum Hemoglobin < 10.0 g/dL, Slope of eGFR decline >4.4ml/min/year, cognitive assessment ≤3 years before dialysis
Model 3: Model 2+ Low Income, CRIC Center 5
Sensitivity Analyses
We observed similar associations between cognitive impairment and ESRD transition outcomes in analyses in which we imputed missing data on eGFR slope (Table S2). Among participants with information on health literacy, pre-dialysis cognitive impairment was associated with an 88% lower odds of PD utilization (aOR for cognitive impairment, 0.12; 95% CI, 0.02–0.99; p=0.05), and a 67% lower odds of venous catheter avoidance at dialysis initiation (aOR, 0.33; 95% CI, 0.14–0.76; p=0.009) in fully-adjusted models that included health literacy. When we restricted analyses of venous catheter avoidance to participants who initiated hemodialysis (n=526), pre-dialysis cognitive impairment was associated with lower odds of venous catheter avoidance at dialysis initiation in the fully adjusted model (Model 3), but this did not reach statistical significance (Table S3). Among participants with pre-emptive permanent vascular access placement, the median days from access placement to hemodialysis was similar between those with and without cognitive impairment (69 versus 61 days, p=0.5). Access was placed ≥180 days before hemodialysis onset among 61 participants (16%). Among participants with information on health literacy who initiated hemodialysis with preemptive vascular access placement (n=222), independent of the timing of access placement and health literacy, cognitive impairment was associated with a 71% lower odds of venous catheter avoidance at hemodialysis initiation (aOR for cognitive impairment, 0.29; 95% CI, 0.11–0.79; p=0.02).
Discussion
In this study of CRIC participants with advanced CKD who progressed to dialysis, we found that pre-dialysis cognitive impairment was strongly associated with lower utilization of PD and higher utilization of venous catheters at dialysis initiation. These findings were robust to adjustment for age, race, comorbidities, and functional status. Our findings have implications for future efforts to improve ESRD preparation and for quality monitoring of dialysis providers, who are evaluated in part based on their utilization of venous catheters. Our study suggests that some of the center-to-center variation observed in ESRD transition outcomes32 could be associated with commonly unmeasured patient factors, such as cognitive function.
Although there is greater awareness of the burden of cognitive impairment among patients with advanced CKD in the literature,13,33,34 the clinical implications are still being defined. Our findings suggest that cognitive impairment may have important impacts on utilization of PD. In-center hemodialysis remains the most common scenario for patients who initiate dialysis in the US,35 and PD is especially infrequently utilized in settings of urgent dialysis initiation.3,36 Some CKD patients with cognitive impairment may have limited ability to perform self-care, which may lead nephrologists to prefer hemodialysis for these patients. However, functional limitations may not be a universal barrier to PD, particularly in the presence of strong social support.37,38 Further, PD may represent an important dialysis modality for patients with limited vascular access options, and may result in lower risks for progressive cerebral damage and cognitive decline than hemodialysis.39,40 Therefore, future studies are needed to define potentially modifiable factors that result in low PD utilization among patients with pre-dialysis cognitive impairment.
In our study, we observed that despite the fact that 75% of CRIC participants had pre-emptive placement of a permanent access, over half of the cohort initiated dialysis using a venous catheter. Pre-dialysis cognitive impairment was associated with a lower likelihood of venous catheter avoidance at dialysis initiation, a finding that could be explained by numerous mechanisms. Patients with pre-dialysis cognitive impairment may have diminished capacity to understand clinical instructions that facilitate successful vascular access placement and maturation.41 Lower utilization of PD among cognitively impaired participants may have contributed substantially to our findings on venous catheter avoidance, given the distinct steps in access planning and placement required for PD. Finally, cognitive impairment may be a marker for vascular disease,42,43 which is a risk factor for vascular access maturation failure.44 Future studies are needed to determine whether alternative strategies for permanent access placement (e.g., earlier placement, closer clinical monitoring) may reduce the need for venous catheters among cognitively impaired CKD patients.
We also explored the relationship of pre-dialysis cognitive impairment and preemptive placement on the kidney transplant waiting list. Although there was no statistically significant association between pre-dialysis cognitive impairment and the likelihood of pre-emptive listing at the conventional p-value cut off of 5%, the 95% confidence interval was wide, and should not be interpreted as concluding that cognitive impairment has no impact on the outcome, or that cognitive function is not a relevant factor in determining waiting list eligibility. Carefully selected patients with mild cognitive impairment may derive benefits from transplantation, and numerous studies have suggested that some patients may experience improvements in cognition post-transplantation.45–47 On the other hand, among older recipients, high rates of incident dementia have been observed post-transplantation, associated with poorer survival.48 Therefore, future studies are needed to identify CKD patients with cognitive impairment who are most likely to benefit from kidney transplantation, and determine barriers to transplant access among these patients.
Our finding that nearly 20% of CRIC participants with late-stage CKD displayed evidence of cognitive impairment also points to a larger challenge than improving any single ESRD transition outcome - ensuring that patients with cognitive impairment are supported to make timely and informed decisions about their care through the course of their illness. Prior educational interventions to improve ESRD preparation have highlighted the importance of patient knowledge and decision-making ability as key factors impacting these outcomes.49–52 However, no strategies to date have considered differential effects of such interventions based on patients’ levels of cognitive function. The 3MS is a validated measure of global cognitive function but includes limited assessments of other cognitive domains, such as executive function and memory.11,14,53 Therefore, studies with more granular assessments of cognitive deficits are needed to determine whether some CKD patients may benefit from interventions validated in non-CKD populations, such as repetitive instruction to boost memory54 or use of cognitive training exercises,55 when receiving modality education and access planning prior to ESRD.
Our study has several strengths. To our knowledge, this is the first study to assess cognitive impairment as a potential barrier to ESRD transition outcomes. The CRIC cohort is large, racially and geographically diverse, and collected information on several known health barriers and other important confounders, including physical function, health literacy, and health behaviors. Given the CRIC linkage to the USRDS, we were also able to confirm important patient outcomes including dialysis access and wait-listing.
However, our study was subject to certain limitations. Our study design did not provide insight on differences in ESRD transition outcomes among CRIC participants with different severity of cognitive impairment, or on the reasons why many participants with pre-emptively placed vascular access did not use it to initiate dialysis. We did not focus on the potentially important role of cognitive decline prior to dialysis as a risk factor for poor ESRD transition outcomes, given a recent prospective, longitudinal study of CRIC participant cognitive function that found that global cognitive function remained relatively stable for the majority of participants prior to initiating dialysis.12 Further, despite the fact that many of our patient characteristics mirror similar studies of national dialysis cohorts,56 our observed rates of pre-emptive permanent access placement were considerably higher.1,57 CRIC participants may have had greater awareness of their CKD and experienced different patterns in access referral and placement compared to patients in the community.58 However, our cohort’s low rate of pre-emptive wait-listing (20%) is comparable to national rates,5 illustrating that knowledge of CKD is only one of the determinants of ESRD preparedness. Further, by conditioning inclusion in our cohort on reaching late-stage CKD and on initiating dialysis, our retrospective study design may be subject to selection bias, and should be reproduced in other settings with prospective study designs.
In summary, we found that in a large, diverse cohort of patients with late-stage CKD who initiated dialysis, pre-dialysis cognitive impairment was associated with lower utilization of PD and higher utilization of venous catheters at dialysis initiation. Typical approaches to ESRD preparation, which rely heavily on patients to drive the process forward, may not be equally effective for patients with cognitive impairment. Additional studies are needed to determine whether interventions directed at mitigating the effects of poor cognitive function can improve preparation for ESRD.
Supplementary Material
Fully adjusted multivariable logistic regression models for ESRD transition outcomes.
Results of sensitivity analysis with multiple imputation for missing eGFR slope.
Association of predialysis cognitive impairment, defined using age-based 3MS cut-off scores, and avoidance of venous catheter use at hemodialysis initiation, with adjustment for health literacy and timing of pre-emptive access placement.
Acknowledgments
Support: Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases P20 GM109036, Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131. Dr. Harhay is funded by NIDDK K23 DK105207. Dr. Lash is funded by the NIDDK K24DK092290 and R01-DK072231-91 Awards. The CRIC COG study is supported by DK069406-01 from NIDDK. None of the funders of this study had any role in the current study design, collection, analysis, and interpretation of data, writing the report, or the decision to submit the report for publication.
Footnotes
Authors’ Contributions: Research Idea and Study Design: MNH, KY, PPR, MKT; Data Analysis/Interpretations: MNH, DX, XZ, CH, EV, ASG, SMS, JB, SS, JC, RD, MD, SA, PPR, JPL, KY, MKT; Supervision or Mentorship: PPR, JPL, KY, MKT. Each author contributed important intellectual content during manuscript drafting or revision, and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy of integrity of any portion of the work are appropriately investigated and resolved.
Financial Disclosure: Dr. Reese serves as a consultant for Collaborative Healthcare Research & Data Analytics (COHRDATA). The remaining authors declare that they have no relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Foley RN, Chen SC, Collins AJ. Hemodialysis access at initiation in the United States, 2005 to 2007: still “catheter first”. Hemodialysis international International Symposium on Home Hemodialysis. 2009;13(4):533–542. doi: 10.1111/j.1542-4758.2009.00396.x. [DOI] [PubMed] [Google Scholar]
- 2.Friedewald JJ, Reese PP. The kidney-first initiative: what is the current preemptive transplantation? Advances in chronic kidney disease. 2012;19(4):252–256. doi: 10.1053/j.ackd.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schreiber MJ., Jr Changing Landscape for Peritoneal Dialysis: Optimizing Utilization. Seminars in dialysis. 2017;30(2):149–157. doi: 10.1111/sdi.12576. [DOI] [PubMed] [Google Scholar]
- 4.Mehrotra R, Soohoo M, Rivara MB, et al. Racial and Ethnic Disparities in Use of and Outcomes with Home Dialysis in the United States. Journal of the American Society of Nephrology : JASN. 2016;27(7):2123–2134. doi: 10.1681/ASN.2015050472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keith D, Ashby VB, Port FK, Leichtman AB. Insurance type and minority status associated with large disparities in prelisting dialysis among candidates for kidney transplantation. Clinical journal of the American Society of Nephrology : CJASN. 2008;3(2):463–470. doi: 10.2215/CJN.02220507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schold JD, Gregg JA, Harman JS, Hall AG, Patton PR, Meier-Kriesche HU. Barriers to evaluation and wait listing for kidney transplantation. Clin J Am Soc Nephrol. 2011;6(7):1760–1767. doi: 10.2215/CJN.08620910. [DOI] [PubMed] [Google Scholar]
- 7.Patzer RE, Paul S, Plantinga L, et al. A Randomized Trial to Reduce Disparities in Referral for Transplant Evaluation. Journal of the American Society of Nephrology : JASN. 2017;28(3):935–942. doi: 10.1681/ASN.2016030320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldfarb-Rumyantzev AS, Syed W, Patibandla BK, et al. Geographic disparities in arteriovenous fistula placement in patients approaching hemodialysis in the United States. Hemodialysis international International Symposium on Home Hemodialysis. 2014;18(3):686–694. doi: 10.1111/hdi.12141. [DOI] [PubMed] [Google Scholar]
- 9.Patibandla BK, Narra A, Desilva R, et al. Disparities in arteriovenous fistula placement in older hemodialysis patients. Hemodialysis international International Symposium on Home Hemodialysis. 2014;18(1):118–126. doi: 10.1111/hdi.12099. [DOI] [PubMed] [Google Scholar]
- 10.Anand S, Johansen KL, Kurella Tamura M. Aging and chronic kidney disease: the impact on physical function and cognition. The journals of gerontology Series A, Biological sciences and medical sciences. 2014;69(3):315–322. doi: 10.1093/gerona/glt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurella M, Chertow GM, Luan J, Yaffe K. Cognitive impairment in chronic kidney disease. Journal of the American Geriatrics Society. 2004;52(11):1863–1869. doi: 10.1111/j.1532-5415.2004.52508.x. [DOI] [PubMed] [Google Scholar]
- 12.Kurella Tamura M, Vittinghoff E, Hsu CY, et al. Loss of executive function after dialysis initiation in adults with chronic kidney disease. Kidney international. 2017;91(4):948–953. doi: 10.1016/j.kint.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray AM, Knopman DS. Cognitive impairment in CKD: no longer an occult burden. Am J Kidney Dis. 2010;56(4):615–618. doi: 10.1053/j.ajkd.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yaffe K, Ackerson L, Kurella Tamura M, et al. Chronic kidney disease and cognitive function in older adults: findings from the chronic renal insufficiency cohort cognitive study. Journal of the American Geriatrics Society. 2010;58(2):338–345. doi: 10.1111/j.1532-5415.2009.02670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sachdev PS, Blacker D, Blazer DG, et al. Classifying neurocognitive disorders: the DSM-5 approach. Nat Rev Neurol. 2014;10(11):634–642. doi: 10.1038/nrneurol.2014.181. [DOI] [PubMed] [Google Scholar]
- 16.Moist LM, Lok CE. Incident Dialysis Access in Patients With End-Stage Kidney Disease: What Needs to Be Improved. Semin Nephrol. 2017;37(2):151–158. doi: 10.1016/j.semnephrol.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. Journal of the American Society of Nephrology : JASN. 2003;14(7 Suppl 2):S148–153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 18.Lash JP, Go AS, Appel LJ, et al. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4(8):1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurella Tamura M, Muntner P, Wadley V, et al. Albuminuria, kidney function, and the incidence of cognitive impairment among adults in the United States. Am J Kidney Dis. 2011;58(5):756–763. doi: 10.1053/j.ajkd.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurella Tamura M, Wadley V, Yaffe K, et al. Kidney function and cognitive impairment in US adults: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am J Kidney Dis. 2008;52(2):227–234. doi: 10.1053/j.ajkd.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray AM, Bell EJ, Tupper DE, et al. The Brain in Kidney Disease (BRINK) Cohort Study: Design and Baseline Cognitive Function. Am J Kidney Dis. 2016;67(4):593–600. doi: 10.1053/j.ajkd.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 23.Israni AK, Salkowski N, Gustafson S, et al. New national allocation policy for deceased donor kidneys in the United States and possible effect on patient outcomes. Journal of the American Society of Nephrology : JASN. 2014;25(8):1842–1848. doi: 10.1681/ASN.2013070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 25.Kurella Tamura M, Yaffe K, Hsu CY, et al. Cognitive Impairment and Progression of CKD. Am J Kidney Dis. 2016;68(1):77–83. doi: 10.1053/j.ajkd.2016.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torres RV, Elias MF, Seliger S, Davey A, Robbins MA. Risk for cognitive impairment across 22 measures of cognitive ability in early-stage chronic kidney disease. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2017;32(2):299–306. doi: 10.1093/ndt/gfw005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones TG, Schinka JA, Vanderploeg RD, Small BJ, Graves AB, Mortimer JA. 3MS normative data for the elderly. Arch Clin Neuropsychol. 2002;17(2):171–177. [PubMed] [Google Scholar]
- 28.Kurella Tamura M, Larive B, Unruh ML, et al. Prevalence and correlates of cognitive impairment in hemodialysis patients: the Frequent Hemodialysis Network trials. Clinical journal of the American Society of Nephrology : CJASN. 2010;5(8):1429–1438. doi: 10.2215/CJN.01090210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1.0. Health Econ. 1993;2(3):217–227. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 30.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley & Sons; 1987. [Google Scholar]
- 31.Taylor DM, Bradley JA, Bradley C, et al. Limited health literacy in advanced kidney disease. Kidney international. 2016;90(3):685–695. doi: 10.1016/j.kint.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 32.Fuller DS, Robinson BM. Facility Practice Variation to Help Understand the Effects of Public Policy: Insights from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Clinical journal of the American Society of Nephrology : CJASN. 2017;12(1):190–199. doi: 10.2215/CJN.03930416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murray AM, Tupper DE, Knopman DS, et al. Cognitive impairment in hemodialysis patients is common. Neurology. 2006;67(2):216–223. doi: 10.1212/01.wnl.0000225182.15532.40. [DOI] [PubMed] [Google Scholar]
- 34.Berger I, Wu S, Masson P, et al. Cognition in chronic kidney disease: a systematic review and meta-analysis. BMC Med. 2016;14(1):206. doi: 10.1186/s12916-016-0745-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2016 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2017;69(3S1):A7–A8. doi: 10.1053/j.ajkd.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arramreddy R, Zheng S, Saxena AB, Liebman SE, Wong L. Urgent-start peritoneal dialysis: a chance for a new beginning. Am J Kidney Dis. 2014;63(3):390–395. doi: 10.1053/j.ajkd.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oliver MJ, Quinn RR. Selecting Peritoneal Dialysis in the Older Dialysis Population. Perit Dial Int. 2015;35(6):618–621. doi: 10.3747/pdi.2014.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oliver MJ, Garg AX, Blake PG, et al. Impact of contraindications, barriers to self-care and support on incident peritoneal dialysis utilization. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2010;25(8):2737–2744. doi: 10.1093/ndt/gfq085. [DOI] [PubMed] [Google Scholar]
- 39.Madero M, Sarnak MJ. Does hemodialysis hurt the brain? Seminars in dialysis. 2011;24(3):266–268. doi: 10.1111/j.1525-139X.2011.00857.x. [DOI] [PubMed] [Google Scholar]
- 40.Wolfgram DF, Szabo A, Murray AM, Whittle J. Risk of dementia in peritoneal dialysis patients compared with hemodialysis patients. Perit Dial Int. 2015;35(2):189–198. doi: 10.3747/pdi.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moist LM, Lee TC, Lok CE, et al. Education in vascular access. Seminars in dialysis. 2013;26(2):148–153. doi: 10.1111/sdi.12055. [DOI] [PubMed] [Google Scholar]
- 42.Weiner DE, Scott TM, Giang LM, et al. Cardiovascular disease and cognitive function in maintenance hemodialysis patients. Am J Kidney Dis. 2011;58(5):773–781. doi: 10.1053/j.ajkd.2011.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yaffe K, Ackerson L, Hoang TD, et al. Retinopathy and cognitive impairment in adults with CKD. Am J Kidney Dis. 2013;61(2):219–227. doi: 10.1053/j.ajkd.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allon M, Greene T, Dember LM, et al. Association between Preoperative Vascular Function and Postoperative Arteriovenous Fistula Development. Journal of the American Society of Nephrology : JASN. 2016;27(12):3788–3795. doi: 10.1681/ASN.2015020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harciarek M, Biedunkiewicz B, Lichodziejewska-Niemierko M, Debska-Slizien A, Rutkowski B. Continuous cognitive improvement 1 year following successful kidney transplant. Kidney international. 2011;79(12):1353–1360. doi: 10.1038/ki.2011.40. [DOI] [PubMed] [Google Scholar]
- 46.Griva K, Thompson D, Jayasena D, Davenport A, Harrison M, Newman SP. Cognitive functioning pre- to post-kidney transplantation--a prospective study. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2006;21(11):3275–3282. doi: 10.1093/ndt/gfl385. [DOI] [PubMed] [Google Scholar]
- 47.Kramer L, Madl C, Stockenhuber F, et al. Beneficial effect of renal transplantation on cognitive brain function. Kidney international. 1996;49(3):833–838. doi: 10.1038/ki.1996.115. [DOI] [PubMed] [Google Scholar]
- 48.McAdams-DeMarco MA, Bae S, Chu N, et al. Dementia and Alzheimer's Disease among Older Kidney Transplant Recipients. Journal of the American Society of Nephrology : JASN. 2017;28(5):1575–1583. doi: 10.1681/ASN.2016080816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanko J, Romann A, Taylor P, Copland M, Beaulieu M. Optimizing AVF creation prior to dialysis start: the role of predialysis renal replacement therapy choices. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2012;27(11):4205–4210. doi: 10.1093/ndt/gfs378. [DOI] [PubMed] [Google Scholar]
- 50.Patzer RE, Basu M, Mohan S, et al. A Randomized Controlled Trial of a Mobile Clinical Decision Aid to Improve Access to Kidney Transplantation: iChoose Kidney. Kidney Int Rep. 2016;1(1):34–42. doi: 10.1016/j.ekir.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lopez-Vargas PA, Craig JC, Gallagher MP, et al. Barriers to timely arteriovenous fistula creation: a study of providers and patients. Am J Kidney Dis. 2011;57(6):873–882. doi: 10.1053/j.ajkd.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 52.Lacson E, Jr, Wang W, DeVries C, et al. Effects of a nationwide predialysis educational program on modality choice, vascular access, and patient outcomes. Am J Kidney Dis. 2011;58(2):235–242. doi: 10.1053/j.ajkd.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 53.Davey A, Elias MF, Robbins MA, Seliger SL, Dore GA. Decline in renal functioning is associated with longitudinal decline in global cognitive functioning, abstract reasoning and verbal memory. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2013;28(7):1810–1819. doi: 10.1093/ndt/gfs470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McDougall GJ., Jr Cognitive interventions among older adults. Annu Rev Nurs Res. 1999;17:219–240. [PMC free article] [PubMed] [Google Scholar]
- 55.Kelly ME, Loughrey D, Lawlor BA, Robertson IH, Walsh C, Brennan S. The impact of cognitive training and mental stimulation on cognitive and everyday functioning of healthy older adults: a systematic review and meta-analysis. Ageing Res Rev. 2014;15:28–43. doi: 10.1016/j.arr.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 56.Szeifert L, Bragg-Gresham JL, Thumma J, et al. Psychosocial variables are associated with being wait-listed, but not with receiving a kidney transplant in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2012;27(5):2107–2113. doi: 10.1093/ndt/gfr568. [DOI] [PubMed] [Google Scholar]
- 57.Malas MB, Canner JK, Hicks CW, et al. Trends in incident hemodialysis access and mortality. JAMA surgery. 2015;150(5):441–448. doi: 10.1001/jamasurg.2014.3484. [DOI] [PubMed] [Google Scholar]
- 58.Tuot DS, Plantinga LC, Hsu CY, et al. Chronic kidney disease awareness among individuals with clinical markers of kidney dysfunction. Clinical journal of the American Society of Nephrology : CJASN. 2011;6(8):1838–1844. doi: 10.2215/CJN.00730111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fully adjusted multivariable logistic regression models for ESRD transition outcomes.
Results of sensitivity analysis with multiple imputation for missing eGFR slope.
Association of predialysis cognitive impairment, defined using age-based 3MS cut-off scores, and avoidance of venous catheter use at hemodialysis initiation, with adjustment for health literacy and timing of pre-emptive access placement.