Abstract
Objective
To assess trends in use of long-acting opioids during delivery hospitalizations.
Methods
The Perspective database, an administrative inpatient database that includes medication receipt, was analyzed to evaluate patterns of long-acting opioids use during delivery hospitalizations from January 2006 through March 2015. Medications evaluated included methadone, formulations including buprenorphine and extended-release formulations of oxycodone, morphine, fentanyl and other opioids. Temporal trends in use of these medications were determined. Unadjusted and adjusted models evaluating the role of demographic and hospital factors were created evaluating both use of these medications and risk for severe morbidity. Risk for severe morbidity was determined based on Centers for Disease Control and Prevention criteria.
Results
Our analysis included 2,994,630 delivery hospitalizations meeting study criteria. Over the entire study period, use of long-acting opioids increased significantly from 457 to 844 per 100,000 deliveries. While buprenorphine and methadone use increased, use of other long-acting opioids decreased. In 2006, methadone and buprenorphine accounted for less than a third of all long-acting opioids used during delivery hospitalizations. By 2015 buprenorphine and methadone represented 73.5% of long-acting opioids used. In adjusted and unadjusted models, risk for severe morbidity was significantly lower with buprenorphine or methadone compared to other long-acting opioids. Restricting the cohort to only women with drug abuse or dependence, risk for severe morbidity was lower with methadone and buprenorphine than without any long-acting opioids.
Conclusion
Increased use of methadone and buprenorphine in this study supports the feasibility of use of these medications during pregnancy and uptake of clinical recommendations for women with opioid use disorder. Use of methadone and buprenorphine are associated with decreased maternal morbidity although causation cannot be presumed from this study model.
INTRODUCTION
Use of prescription opioid medications and opioid abuse are both common in the United States with opioid prescriptions and overdose deaths becoming more frequent in recent decades.1–4 Compared to short acting opioid prescriptions, patients treated with long-acting opioid formulations such as controlled-release oxycodone, sustained-release morphine, and fentanyl patches are at greater risk from overdose.5 The opioid epidemic has affected the obstetric population with up to 22% of women prescribed opioids during their pregnancy.2,6 Additionally, maternal opioid abuse and dependence, also known as opioid use disorder (OUD), have risen dramatically.7 For patients with OUD, including pregnant women, opioid agonist pharmacotherapy with methadone or buprenorphine is recommended compared to abstinence due to lower rates of relapse and attendant risks.8
While use of both methadone and buprenorphine are supported by the American College of Obstetricians and Gynecologists (ACOG) for women with OUD it is unclear to what degree these medications are being used in clinical practice.8 Additionally, it is unclear to what degree women are receiving long-acting opioids not specifically for treatment of OUD such as controlled-release oxycodone, sustained-release morphine, and fentanyl patches. Little is known about obstetric outcomes among pregnant women taking buprenorphine and methadone versus other long-acting opioids, and how use of these may differ during delivery hospitalizations. Deliveries complicated by maternal OUD are associated with increased risk for maternal mortality and severe morbidity,7 and optimal management of this condition may be associated with decreased risk. While there is increasing data for outcomes for infants exposed to these agents during pregnancy,9 maternal data is limited.
Given the knowledge gap regarding trends and outcomes related to use of long-acting opioids including methadone and buprenorphine during delivery hospitalizations, the objectives of this study were (i) to describe temporal trends in long-acting opioid medication use, and (ii) evaluate maternal outcomes for women taking these medications during delivery hospitalizations.
MATERIALS AND METHODS
The Perspective database was used for this analysis. The Premier Perspective data base includes a sample of more than 500 hospitals and reports 100% of hospitalizations for each of these hospitals. Hospitals in Perspective are diverse geographically within the United States and include non-teaching and teaching hospitals. About 15% of hospitalizations are nationally are included in this database. The Premier Perspective database includes information on patient demographics, hospital characteristics, and discharge codes similar to other commonly used administrative datasets. Additionally, Perspective includes data on medications and devices received during hospitalizations and includes other information including costs estimated by internal hospital accounting systems. Perspective is commercially available and is commonly used across medical specialties for research that evaluates inpatient medication use.10–15 The Perspective database is maintained by Premier Incorporated (Charlotte, NC) and undergoes 95 quality assurance and validation checks prior to being used for research.16 The Columbia University Institutional Review Board deemed the study exempt given the data was de-identified.
Inclusion criteria for this analysis included women 15 to 49 years of age who underwent a delivery hospitalization from January 2006 through March 2015. Delivery hospitalizations were identified based on International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) billing codes using an approach that ascertains more than 95% of deliveries (ICD-9-CM codes 650 and V27.x).17 Women with a long-acting opioid prescription during their delivery hospitalization were identified. These mediations were presumed to be prescribed for an indication other than short term pain control after delivery for which short-acting opioids or non-opioid medications are used primarily. Based on the type of long-acting opioid, cases were classified in one of three groups: (i) oral methadone, (ii) oral buprenorphine, and (iii) other long-acting opioids or opiates including fentanyl patches and sustained release, timed release, extended release, controlled delivery, controlled release, or long-acting oral formulations of oxycodone, morphine, tramadol, hydromorphone, oxymorphone, and tapentadol. For reporting demographics we classified women who simultaneously received both methadone and buprenorphine in the methadone category given that methadone may be administered to women for whom buprenorphine is inadequate.
We evaluated (i) temporal trends in any long-acting opioid use, and (ii) temporal trends in methadone, buprenorphine, and other long-acting opioids use individually. Hospitals are included in the Perspective database for varying durations of time. To account for confounding secondary to this changing sampling frame we restricted the analysis to only hospitals that contributed data throughout the entire January 2006 to March 2015 study period. In addition to evaluating overall temporal trends, we evaluated hospital level rates of opioid prescription for obstetric patients; in evaluating hospital-level rates of opioid prescription we restricted the analysis to hospitals contributing ≥1000 deliveries over the entire study period.
To determine which factors were associated with methadone or buprenorphine use among women using long-acting opioids, adjusted log-linear models including demographic and hospital factors were applied to the cohort of women using long-acting opioids. Results were reported as adjusted risk ratios (aRR) with 95% confidence intervals (95% CI) as measures of effect. Hospital characteristics included location (urban versus rural), teaching status (teaching versus nonteaching), geographic region (Midwest, Northeast, South, West), and hospital size (small (<400 beds), medium (400 to 600 beds), large (>600 beds)). Demographic characteristics included maternal age at delivery (<20, 20–24, 25–29, 30–34, or ≥35 years), maternal race (white, black, Hispanic, other), marital status (married, single, unknown), year of delivery (2006 to 2015), and insurance status (commercial, Medicare, Medicaid, uninsured, and unknown).
Risk for severe maternal morbidity was evaluated using criteria from the Centers for Disease Control and Prevention (CDC). The CDC definition of severe maternal morbidity includes 21 diagnoses including shock, stroke, heart failure, transfusion, and other conditions all identified using ICD-9-CM codes (Box 1).18 We evaluated risk for severe maternal morbidity among women receiving (i) no long-acting opioids, (ii) buprenorphine, (iii) methadone, and (iv) other long-acting opioids. To determine adjusted risk associated with long-acting opioid use, we created multivariable log linear regression models for risk for severe morbidity including hospital and demographic factors. Additionally, we controlled for comorbid risk as measured by an obstetric comorbidity index.19 While comorbidity indices are commonly used in analyses of administrative data to adjust for risk, risk factors for morbidity and death for the general medical and surgical population likely differ significantly from obstetric patients.20 This comorbidity index provides weighted scores for comorbidity for individual patients based on the presence of specific diagnosis codes and demographic factors present in administrative data. Factors in the comorbidity index include hypertensive diseases of pregnancy, multiple gestation, cardiac conditions, abnormal placentation, substance abuse, pre-existing diabetes, and previous cesarean delivery. Higher scores are associated with increased risk for severe morbidity. In the initial study validating the comorbidity index, patients with the lowest score of 0 had a 0.68% risk of severe morbidity whereas a score of >10 was associated with a risk of severe morbidity of 10.9%.19 This comorbidity index was subsequently validated in an external population.21 Because the comorbidity index includes drug abuse, we modified this scoring system excluding this diagnosis for the present analysis. Additionally, because the comorbidity index includes maternal age as a factor, we did not include maternal age in our models adjusted for comorbidity. Finally, we performed an unadjusted analysis for severe morbidity risk restricted to women with opioid drug abuse and dependence diagnoses (ICD-9-CM 304.0x, 304.7x, 305.5x); for this group of women we compared risk for severe morbidity (i) with buprenorphine or methadone use, (ii) other long-acting opioids, or (iii) no long-acting opioids. To protect patient confidentiality cell counts <10 for demographic factors are not demonstrated. All analyses were performed with SAS 9.4 (SAS Institute, Cary, NC).
Box 1. Centers for Disease Control and Prevention Severe Maternal Morbidity Indicators.
| Conditions |
|---|
| Acute myocardial infarction |
| Aneurysm |
| Acute renal failure |
| Adult respiratory distress syndrome |
| Amniotic fluid embolism |
| Cardiac arrest/ventricular fibrillation |
| Conversion of cardiac rhythm |
| Disseminated intravascular coagulation |
| Eclampsia |
| Heart failure/arrest during surgery or procedure |
| Puerperal cerebrovascular disorders |
| Pulmonary edema/acute heart failure |
| Severe anesthesia complications |
| Sepsis |
| Shock |
| Sickle cell disease with crisis |
| Air and thrombotic embolism |
| Blood transfusion |
| Hysterectomy |
| Temporary tracheostomy |
| Ventilation |
Modified from Centers for Disease Control and Prevention. Severe maternal morbidity indicators and corresponding ICD codes during delivery hospitalizations. Available at: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/smm/severe-morbidity-ICD.htm. Retrieved July 2, 2018.
RESULTS
A total of 2,994,630 delivery hospitalizations from 2006 through the first quarter of 2015 were included in the analysis. Demographics of the 2,932 women taking buprenorphine, the 7,034 women taking methadone, and 8,589 women taking other long-acting opioids are in Table 1. Of all medications, long-acting oxycodone was most frequently used, (n=7,813) followed by methadone and buprenorphine (Table 2). Transdermal fentanyl patches were fourth most common (n=608) while long-acting oral morphine was fifth most common (n=296). Use of other long-acting opioids was uncommon and no women were noted to have received long-acting formulations of tapentadol. Women receiving long-acting opioids were more likely to be single ((59.8% (11,099/18,555)) than women not receiving opioids ((40.5% (1,206,441/2,976,075)). Women receiving long-acting opioids were also more likely to have Medicaid insurance (65.0% (12,068/18,555) than women not receiving long-acting opioids 42.8% (1,272,499/2,976,075) (p<0.01 for both). Buprenorphine, methadone, and other long-acting opioids use was most common in the Northeast compared to other regions (1.7% of patients in the Northeast (8,801/530,390) compared to 0.3% in the Midwest (1,559/534,591), 0.3% in the South (4,099/1,429,317), and 0.2% in the West (1,096/477,332), p<0.01). The highest rates of comorbidity were noted among women taking long-acting opioids other than buprenorphine and methadone. For example, 9.5% (95% CI 8.5–10.7%) of women receiving buprenorphine had a comorbidity index score >1 compared to 12.1% (95% CI 11.4–12.9%)of women receiving methadone, and 19.3% of women receiving other opioids (95% CI 18.4–20.1%) (p<0.01).
Table 1.
Demographic characteristics of women using long-acting opioids
| Buprenorphine, n (%) | Methadone | Other opioids | No opioids | |
|---|---|---|---|---|
| All | 2,932 | 7,034 | 8,589 | 2,976,075 |
|
| ||||
| Age, years | ||||
| 15–17 | 30 (1.0%) | 58 (0.8%) | 163 (1.9%) | 79,388 (2.7%) |
| 18–24 | 1,028 (35.1%) | 2,015 (28.7%) | 2,357 (27.4%) | 898,110 (30.2%) |
| 25–29 | 1,052 (35.9%) | 2,546 (36.2%) | 2,470 (28.8%) | 848,908 (28.5%) |
| 30–34 | 597 (20.4%) | 1,611 (22.9%) | 2,134 (24.9%) | 723,769 (24.3%) |
| >34 | 225 (7.7%) | 804 (11.4%) | 1,465 (17.1%) | 425,900 (14.3%) |
| Year | ||||
| 2006 | <10 (na) | 452 (6.4%) | 979 (11.4%) | 328,122 (11.0%) |
| 2007 | <10 (na) | 559 (8.0%) | 1,050 (12.2%) | 336,994 (11.3%) |
| 2008 | 20 (0.7%) | 578 (8.2%) | 989 (11.5%) | 330,228 (11.1%) |
| 2009 | 28 (1.0%) | 627 (8.9%) | 1,001 (11.7%) | 325,823 (11.0%) |
| 2010 | 119 (4.1%) | 836 (11.9%) | 952 (11.1%)4,784,3807 (111 | 318,312 (10.7%) |
| 2011 | 247 (8.4%) | 1190 (16.9%) | 898 (10.5%) | 316,798 (10.6%) |
| 2012 | 635 (21.7%) | 769 (10.9%) | 876 (10.2%) | 314,494 (10.6%) |
| 2013 | 682 (23.3%) | 889 (12.6%) | 831 (9.7%) | 313,051 (10.5%) |
| 2014 | 947 (32.3%) | 915 (13.0%) | 846 (9.9%) | 317,301 (10.7%) |
| 2015 (1Q) | 243 (8.3%) | 219 (3.1%) | 167 (1.9%) | 74,952 (2.5%) |
| Marital status | ||||
| Married | 530 (18.1%) | 1,515 (21.5%) | 3,306 (38.5%) | 1,413,699 (47.5%) |
| Single | 2,162 (73.7%) | 4,916 (69.9%) | 4,021 (46.8%) | 1,206,441 (40.5%) |
| Unknown | 240 (8.2%) | 603 (8.6%) | 1,262 (14.7%) | 355,935 (12.0%) |
| Hospital bed size | ||||
| Small | 782 (26.7%) | 3,328 (47.3%) | 1,336 (15.6%) | 1,614,382 (54.3%) |
| Medium | 567 (19.3%) | 1,881 (26.7%) | 1,193 (13.9%) | 825,145 (27.7%) |
| Large | 1,583 (54.0%) | 1,825 (26.0%) | 6,060 (70.6%) | 536,548 (18.0%) |
| Insurance status | ||||
| Private | 477 (16.3%) | 1,084 (15.4%) | 3,846 (44.9%) | 1,499,553 (50.4%) |
| Medicare | 115 (3.9%) | 178 (2.5%) | 175 (2.0%) | 18,104 (0.6%) |
| Medicaid | 2,258 (77.0%) | 5,465 (77.7%) | 4,345 (50.6%) | 1,272,499 (42.8%) |
| Uninsured | 33 (1.1%) | 216 (3.1%) | 77 (0.9%) | 78,421 (2.6%) |
| Other/unknown | 49 (1.7%) | 91 (1.3%) | 136 (1.6%) | 107,498 (3.6%) |
| Hospital Location | ||||
| Rural | 184 (6.3%) | 444 (6.3%) | 128 (1.5%) | 240,577 (8.1%) |
| Urban | 2,748 (93.7%) | 6,590 (93.7%) | 8,461 (98.5%) | 2,735,498 (91.9%) |
| Hospital Region | ||||
| Northeast | 1,439 (49.1%) | 2,119 (30.1%) | 5,243 (61.0%) | 521,589 (17.5%) |
| Midwest | 181 (6.2%) | 1,118 (15.9%) | 260 (3.0%) | 553,032 (18.6%) |
| South | 1,218 (41.5%) | 3,345 (47.6%) | 2,536 (29.5%) | 1,425,218 (47.9%) |
| West | 94 (3.2%) | 452 (6.4%) | 550 (6.4%) | 476,236 (16.0%) |
| Teaching | ||||
| Non-teaching | 780 (26.6%) | 3,458 (49.2%) | 7,139 (83.1%) | 1,655,134 (55.6%) |
| Teaching | 2,152 (73.4%) | 3,576 (50.8%) | 1,450 (16.9%) | 1,320,941 (44.4%) |
| Comorbidity score | ||||
| 0 | 1,959 (66.8%) | 4,382 (62.3%) | 3,580 (41.7%) | 1,903,197 (64.0%) |
| 1 | 693 (23.6%) | 1,800 (25.6%) | 3,357 (39.1%) | 726,983 (24.4%) |
| 2 | 203 (6.9%) | 571 (8.1%) | 1,166 (13.6%) | 256,547 (8.6%) |
| 3 | 56 (1.9%) | 199 (2.8%) | 349 (4.1%) | 68,304 (2.3%) |
| 4 | 10 (0.3%) | 52 (0.7%) | 106 (1.2%) | 16,162 (0.5%) |
| >4 | 11 (0.4%) | 30 (0.4%) | 31 (0.4%) | 4,882 (0.2%) |
| Race | ||||
| Non-Hispanic white | 2,297 (78.3%) | 5,413 (77.0%) | 5,623 (65.5%) | 1,616,881 (54.3%) |
| Non-Hispanic black | 175 (6.0%) | 602 (8.6%) | 856 (10.0%) | 468,433 (15.7%) |
| Hispanic | <10 (na) | 173 (2.5%) | 617 (7.2%) | 241,503 (8.1%) |
| Other | 451 (15.4%) | 841 (12.0%) | 1,491 (17.4%) | 647,655 (21.8%) |
| Unknown | <10 (na) | <10 (na) | <10 (na) | 1,603 (0.1%) |
Patients that received methadone along with other opioids were categorized as receiving methadone. All comparisons across demographic categories differed significantly (p<0.01).
Table 2.
Patterns of opioid use among the 18,555 women taking prescription opioids
| n | |
|---|---|
| All Patients | 2,994,630 |
| Long–acting opioid * | 18,803 (0.63%) |
| Methadone | 7,034 |
| Buprenorphine | 3,026 |
| Oxycodone | 7,813 |
| Morphine | 296 |
| Transdermal fentanyl | 608 |
| Other long-acting opioids | 26 |
Other long-acting opioids include oxymorphone, tramadol, and hydromorphone. 248 women used >1 opioid.
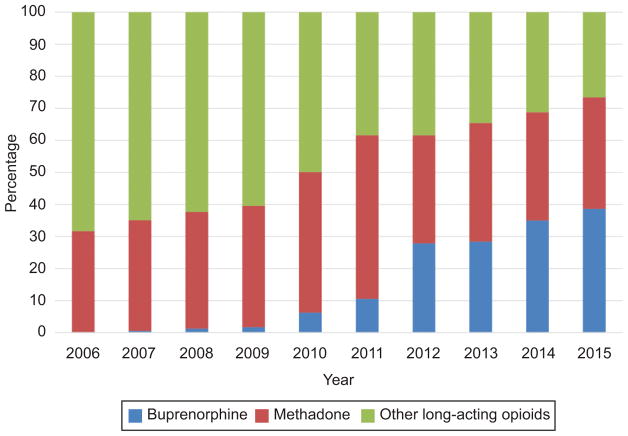
Evaluating temporal trends, use of both methadone and buprenorphine increased more than 2-fold as a proportion of overall long-acting opioid administration over the study period (Figure 1). In 2006, methadone accounted for 31.5% of all long-acting opioids, buprenorphine <0.1%, with other long-acting opioids representing the remaining 68.5%. By 2015 buprenorphine and methadone represented 73.5% of long-acting opioids administered. Over the entire study period, long-acting opioids use increased significantly from 457 to 844 per 100,000 deliveries (Figure 2) (p<0.01). While use of both methadone and buprenorphine increased from the beginning compared to the end of the study period, use of other long-acting opioids decreased from 304 to 256 per 100,000 deliveries. Hospital level rates of long-acting opioid use are demonstrated in Figure 3. Of the 185 hospitals that demonstrated at least 1000 deliveries over the study period 164 (88.6%) had a rate of long-acting opioid use of <1%.
Figure 1.
Proportion of women taking long-acting opioids using methadone and buprenorphine over the study period. The figure demonstrates the proportion of women taking buprenorphine, methadone, and other long-acting opioids among the cohort using long-acting opioids between 2006 through the first quarter of 2015. The proportion of women using either methadone or buprenorphine increased for each period while the proportion of other long acting opioids decreased significantly (P<.01).
Figure 2.
Number of women using methadone, buprenorphine, and other long-acting opioids per 100,000 deliveries. The figure demonstrates methadone, buprenorphine, other long acting opioids or any long acting opioid use per 100,000 deliveries over the study period. Over the study period, methadone and buprenorphine use increased more than twofold (P<0.01), while use of other long-acting opioids decreased.
Figure 3.
Hospital-level rates of long-acting opioids receipt during delivery hospitalizations. Hospitals were ranked from lowest rates of long-acting opioid receipt during delivery hospitalizations to the highest. Only hospitals (n=185) that contributed ≥1000 or more deliveries over the course of the study period were included. To facilitate interpretation given that majority of hospitals had opioid receipt of <1% (n=164), a logarithmic scale was used for the y-axis. Thirteen hospitals had no patients who received long acting opioids, thus they are not represented in the figure because of the logarithmic scale.
The unadjusted and adjusted models evaluating buprenorphine or methadone use among the cohort of women using long-acting opioids (N=18,555) are demonstrated in Table 3. In the unadjusted model factors associated with increased use of buprenorphine or methadone included later study year with higher use in 2015 compared to 2006 (RR 2.32 95% CI 2.04–2.64), being single compared to married (RR 1.67 95% CI 1.59–1.75), delivering at a teaching compared to non-teaching hospital (RR 2.14 95% CI 2.06–2.23) and Medicaid compared to private insurance (RR 2.22 95% CI 2.10–2.34). Factors associated with decreased likelihood of methadone or buprenorphine use included being Hispanic compared to white (RR 0.39, 95% CI 0.33–0.45), delivering at a large compared to small hospital (RR 0.48, 95% CI 0.46–0.50), and being in a urban compared to rural setting (RR 0.63, 95% CI 0.58–0.68). In the adjusted model, many of these factors retained significant relationships with factors associated with increased likelihood of methadone or buprenorphine use including delivering in 2015 compared to 2006 (aRR 1.59, 95% CI 1.39–1.81), being single compared to being married (aRR 1.30, 95% CI 1.23–1.37), receiving Medicaid compared to private insurance (aRR 1.70, 95% CI 1.61–1.80), and delivering in a teaching compared to nonteaching hospital (aRR 2.01, 95% CI 1.92–2.10). Factors associated with decreased buprenorphine or methadone use in the adjusted analysis included delivering in a large hospital compared to small hospital (aRR 0.47, 95% CI 0.45–0.50) and Hispanic compared to non-Hispanic white race (aRR 0.60, 95% CI 0.52–0.70).
Table 3.
Likelihood of receiving buprenorphine or methadone among all women using opioids
| RR | 95% CI | aRR | 95% CI | |
|---|---|---|---|---|
| Year | ||||
| 2006 | 1.00 | Reference | 1.00 | Reference |
| 2007 | 1.11 | 0.98–1.25 | 1.05 | 0.93–1.19 |
| 2008 | 1.19 | 1.05–1.34 | 1.11 | 0.98–1.26 |
| 2009 | 1.25 | 1.11–1.41 | 1.09 | 0.97–1.23 |
| 2010 | 1.58 | 1.41–1.77 | 1.26 | 1.13–1.41 |
| 2011 | 1.94 | 1.75–2.16 | 1.47 | 1.32–1.64 |
| 2012 | 1.94 | 1.75–2.16 | 1.50 | 1.34–1.67 |
| 2013 | 2.06 | 1.86–2.29 | 1.53 | 1.38–1.70 |
| 2014 | 2.17 | 1.96–2.40 | 1.54 | 1.39–1.71 |
| 2015 | 2.32 | 2.04–2.64 | 1.59 | 1.39–1.81 |
| Marital status | ||||
| Single | 1.67 | 1.59–1.75 | 1.30 | 1.23–1.37 |
| Married | 1.00 | Reference | 1.00 | Reference |
| Unknown | 1.05 | 0.97–1.14 | 1.11 | 1.02–1.21 |
| Hospital bed size | ||||
| Small | 1.00 | Reference | 1.00 | Reference |
| Medium | 0.89 | 0.85–0.94 | 0.69 | 0.65–0.74 |
| Large | 0.48 | 0.46–0.50 | 0.47 | 0.45–0.50 |
| Insurance status | ||||
| Medicare | 2.17 | 1.92–2.46 | 1.79 | 1.57–2.03 |
| Medicaid | 2.22 | 2.10–2.34 | 1.70 | 1.61–1.80 |
| Private | 1.00 | Reference | 1.00 | Reference |
| Uninsured | 2.65 | 2.32–3.03 | 1.82 | 1.59–2.08 |
| Other | 1.76 | 1.48–2.09 | 1.42 | 1.19–1.69 |
| Hospital Location | ||||
| Rural | 1.00 | Reference | 1.00 | Reference |
| Urban | 0.63 | 0.58–0.68 | 0.94 | 0.86–1.02 |
| Hospital Region | ||||
| Northeast | 1.00 | Reference | 1.00 | Reference |
| Midwest | 2.06 | 1.93–2.20 | 1.20 | 1.11–1.30 |
| South | 1.59 | 1.52–1.66 | 1.28 | 1.22–1.34 |
| West | 1.23 | 1.13–1.35 | 1.02 | 0.93–1.12 |
| Teaching | ||||
| Non-teaching | 1.00 | Reference | 1.00 | Reference |
| Teaching | 2.14 | 2.06–2.23 | 2.01 | 1.92–2.10 |
| Comorbidity score | ||||
| 0 | 1.00 | Reference | 1.00 | Reference |
| 1 | 0.67 | 0.64–0.70 | 0.79 | 0.75–0.82 |
| 2 | 0.62 | 0.58–0.67 | 0.79 | 0.73–0.85 |
| 3 | 0.66 | 0.58–0.75 | 0.77 | 0.68–0.87 |
| 4 | 0.58 | 0.45–0.74 | * | * |
| >4 | 0.89 | 0.66–1.21 | * | * |
| Race | ||||
| Non-Hispanic black | 0.82 | 0.76–0.89 | 0.73 | 0.68–0.79 |
| Non-Hispanic white | 1.00 | Reference | 1.00 | Reference |
| Hispanic | 0.39 | 0.33–0.45 | 0.60 | 0.52–0.70 |
| Unknown | 1.23 | 0.51–2.97 | * | * |
| Other | 0.80 | 0.76–0.85 | 0.81 | 0.77–0.86 |
Adjusted model included all factors in this table (year, marital status, bed size, insurance status, hospital location, hospital region, hospital teaching status, comorbidity index score, race). RR, risk ratio. aRR, adjusted risk ratio.
these risk estimates were omitted given the of the small number of cases in the model.
Adjusted and unadjusted models for severe morbidity are demonstrated in Table 4. Severe morbidity was most common among women receiving long-acting opioids other than buprenorphine and methadone (4.7%, 400 of 4589 women), followed by women receiving methadone (2.6% 182 of 7034 women), buprenorphine (2.5%, 72 of 2932), and women receiving no opioids (1.6%, 47,876 of 2,976076 women). Unadjusted and adjusted risk for severe morbidity was highest for women using long-acting opioids other than methadone and buprenorphine (RR 2.90 95%, CI 2.62–3.19 and aRR 2.59, 95% 1.77–3.79, respectively). In the unadjusted model risks for severe morbidity were higher with methadone (RR 1.61, 95% CI 1.39–1.86) and buprenorphine (RR 1.53 95% CI 1.21–1.92) compared with no opioid use, but significantly lower than risk associated with other long-acting opioids. In the adjusted model, buprenorphine was not significantly associated with severe morbidity compared with no opioid use (aRR 1.22 95% CI 0.95–1.56), while methadone was associated with 35% increased risk (aRR 1.35 95% CI 1.14–1.61) which was significantly less than that associated with other long-acting opioids use. Other factors associated with increased risk for severe morbidity in both adjusted and unadjusted analyses included later study year, increasing comorbidity index score, Medicare compared to private insurance, and black compared to white race. In both adjusted and unadjusted comparisons severe morbidity risk did not differ significantly between methadone and buprenorphine. Because long-acting oxycodone was the most commonly used opioid we evaluated the unadjusted risk ratio for severe morbidity associated with receipt of this medication compared to women not receiving long-acting opioids and found the risk to be significantly elevated (RR 2.06 95% CI 1.82–2.33). When the cohort was restricted to women with a diagnosis of drug abuse or dependence, risk for severe morbidity was significantly lower among women receiving buprenorphine or methadone compared to women not receiving opioids (2.3% versus 3.0%, p<0.01).
Table 4.
Risk for severe morbidity (including transfusion) in unadjusted and adjusted models
| RR | 95% CI | aRR | 95% CI | |
|---|---|---|---|---|
| Long acting opioids | ||||
| None | 1.00 | Reference | 1.00 | Reference |
| Methadone | 1.61 | 1.39–1.86 | 1.35 | 1.14–1.61 |
| Buprenorphine | 1.53 | 1.21–1.92 | 1.22 | 0.95–1.56 |
| Other long-acting opioids | 2.90 | 2.62–3.19 | 2.59 | 1.77–3.79 |
| Year | ||||
| 2006 | 1.00 | Reference | 1.00 | Reference |
| 2007 | 1.05 | 1.00–1.09 | 1.04 | 0.98–1.09 |
| 2008 | 1.15 | 1.10–1.20 | 1.14 | 1.07–1.21 |
| 2009 | 1.28 | 1.23–1.34 | 1.26 | 1.17–1.36 |
| 2010 | 1.37 | 1.31–1.42 | 1.35 | 1.24–1.46 |
| 2011 | 1.37 | 1.32–1.43 | 1.35 | 1.24–1.46 |
| 2012 | 1.39 | 1.34–1.45 | 1.36 | 1.27–1.49 |
| 2013 | 1.41 | 1.36–1.47 | 1.37 | 1.27–1.49 |
| 2014 | 1.47 | 1.41–1.53 | 1.42 | 1.32–1.54 |
| 2015 | 1.49 | 1.40–1.58 | 1.43 | 1.28–1.60 |
| Marital status | ||||
| Single | 1.27 | 1.25–1.30 | 1.16 | 1.13–1.19 |
| Married | 1.00 | Reference | 1.00 | Reference |
| Unknown | 1.13 | 1.10–1.16 | 1.07 | 0.98–1.16 |
| Hospital bed size | ||||
| Small | 1.00 | Reference | 1.00 | Reference |
| Medium | 1.16 | 1.13–1.18 | 1.08 | 0.90–1.30 |
| Large | 1.33 | 1.30–1.36 | 1.21 | 0.96–1.53 |
| Insurance status | ||||
| Medicare | 2.15 | 1.98–2.33 | 1.46 | 1.33–1.62 |
| Medicaid | 1.21 | 1.19–1.23 | 1.11 | 1.07–1.15 |
| Private | 1.00 | Reference | 1.00 | Reference |
| Uninsured | 1.18 | 1.12–1.25 | 1.14 | 1.05–1.24 |
| Other | 1.16 | 1.11–1.22 | 1.17 | 1.11–1.23 |
| Hospital Location | ||||
| Rural | 1.00 | Reference | 1.00 | Reference |
| Urban | 1.22 | 1.17–1.26 | 1.01 | 0.83–1.22 |
| Hospital Region | ||||
| Northeast | 1.00 | Reference | 1.00 | Reference |
| Midwest | 0.85 | 0.83–0.88 | 1.00 | 0.77–1.30 |
| South | 0.97 | 0.95–0.99 | 1.08 | 0.86–1.36 |
| West | 0.73 | 0.71–0.75 | 0.91 | 0.68–1.20 |
| Teaching | ||||
| Non-teaching | 1.00 | Reference | 1.00 | Reference |
| Teaching | 1.28 | 1.26–1.31 | 1.06 | 0.88–1.29 |
| Comorbidity score | ||||
| 0 | 1.00 | Reference | 1.00 | Reference |
| 1 | 1.57 | 1.54–1.60 | 1.55 | 1.52–1.60 |
| 2 | 2.33 | 2.27–2.39 | 2.33 | 2.25–2.42 |
| 3 | 4.31 | 4.16–4.47 | 4.18 | 3.96–4.41 |
| 4 | 6.54 | 6.17–6.92 | * | * |
| >4 | 11.9 | 11.0–12.8 | * | * |
| Race | ||||
| Non-Hispanic black | 1.77 | 1.73–1.81 | 1.44 | 1.37–1.51 |
| Non-Hispanic white | 1.00 | Reference | 1.00 | Reference |
| Hispanic | 1.05 | 1.02–1.09 | 1.16 | 1.08–1.23 |
| Unknown | 1.56 | 1.12–2.17 | * | * |
| Other | 1.17 | 1.14–1.20 | 1.14 | 1.09–1.20 |
Adjusted model included all factors in this table (opioid receipt, year, marital status, bed size, insurance status, hospital location, hospital region, hospital teaching status, comorbidity index score, and race). RR, risk ratio. aRR, adjusted risk ratio.
these risk estimates were omitted because given the of the small number of cases in the model.
DISCUSSION
In this large cohort of women, use of long-acting opioids increased over the study period. In particular, the proportion of women receiving buprenorphine and methadone increased while the proportion of women receiving other long-acting opioids decreased. The change represents both an uptake in clinical recommendations supporting use of these interventions for pregnant women with OUD as well as an increase in the proportion of women with this diagnosis. Increased use of methadone and buprenorphine likely reflects increased maternal opioid abuse and dependence among obstetric patients that has paralleled the opioid epidemic in the United States. While causation cannot be inferred from this study model, use of buprenorphine and methadone during the delivery hospitalization was associated with decreased risk for severe maternal morbidity. Over the study period, use of long-acting opioids besides methadone and buprenorphine decreased significantly. These findings support that uptake into practice is occurring for recommendations for opioid agonist pharmacotherapy as first-line treatment for pregnant women with opioid use disorder.8 That use of long-acting opioids besides buprenorphine and methadone was found to decrease may be representative of providers using more caution in prescribing these medications as well as greater access to medically-assisted treatment of OUD in general and buprenorphine in particular.
The increasing choice of buprenorphine over methadone may point to advantages of this medication in the treatment of opioid use disorder for some patients. Outpatient treatment may be more convenient and readily available for patients receiving buprenorphine from approved providers in comparison to regular attendance at a registered opioid treatment center. For other patients methadone may offer benefits including higher rates of retention in some populations,26 and currently there is insufficient evidence to recommend one medication over the other in pregnancy. 27 That use of methadone and buprenorphine compared to other long-acting opioids was more likely in teaching than non-teaching hospitals in adjusted analyses, but less likely among larger hospitals, suggests that certain administrative and organizational resources may be important factors in offering maintenance to pregnant women.
In evaluating outcomes for this study, women who received methadone or buprenorphine were at significantly decreased risk for severe morbidity compared to women receiving other long-acting opioids. This difference in morbidity may be due to a benefit from appropriate treatment. However, unmeasured confounding is also a possibility and women using long-acting opioids other than buprenorphine or methadone may be at a priori increased risk secondary to medical or social factors not accounted for in our model. When the analysis was restricted to the women with opioid abuse or dependence (“comparing apples to apples”) morbidity was significantly lower for women receiving treatment with methadone or buprenorphine; this finding was reassuring and supports the benefit of treatment. Future comparative research is needed to clarify best approaches for treatment of opioid use disorder that optimize maternal and neonatal outcomes.
This analysis has a number of limitations. First, we presume that long-acting opioid medications taken in the hospital during delivery hospitalizations are continuations from outpatient treatment regimens. Because Perspective only includes inpatient information we are not able to model for actual outpatient pharmacological management. It is possible that long-acting opioid treatment could be changed acutely during delivery hospitalizations in which case medication intake during the hospitalization may not have been representative of care predating the hospitalization. A second major limitation is that demographic and social data are limited and we are not able to determine to what degree other outpatient support services, factors, and comorbidities may predispose women to better or worse outcomes. Optimal outcomes research on opioid use disorder would incorporate antenatal, inpatient, and postpartum management data. A third major limitation of this study is that maternal delivery hospitalizations are not linked to neonatal outcomes, and we are not able to make comparisons regarding neonatal morbidity and outcomes. Fourth, estimates in observational studies should be interpreted cautiously given the potential for unmeasured confounding. Comparisons demonstrating risk ratios of <2 may be less likely to be representative of true differences than those of larger magnitude.18 Fifth, the granularity of this study is limited in that we are not able to determine dose and number of administrations of medications. Sixth, because of our study construct we cannot determine that the relationship between severe maternal morbidity and long-acting opioid use necessarily represents a causal relationship. Women with OUD who seek treatment with buprenorphine or methadone may be at a priori lower risk for adverse outcomes than women with OUD disorder who do not seek treatment with these medications or who receive other long-acting opioids for another indication. Furthermore, the differences in patient and hospital characteristics associated with use of buprenorphine and methadone compared to other long-acting opioids may represent either underlying differences in populations at risk of opioid use disorder or differences in access to opioid agonist therapy and practice patterns. Strengths of this study include a large sample size, a long study period during a historically relevant time during the United States opioid crisis, and the use of clinically meaningful maternal outcome measures. The database provided information on a wide range of both patient and hospital-level characteristics and represents a geographically and clinically diverse sample.
In summary, this study found increasing buprenorphine and methadone use among pregnant women admitted for delivery while other long-acting opioid medications decreased. Use of methadone and buprenorphine are associated with decreased maternal morbidity although causation cannot be presumed from this study model. Increased use of methadone and buprenorphine in this sample indicates the feasibility of use of these medications and uptake of clinical recommendations.
Acknowledgments
Dr. Friedman is supported by a career development award (K08HD082287) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Footnotes
Presented at the Annual Meeting for the Society for Maternal Fetal Medicine, February 1, 2018, Dallas, Texas
Financial Disclosure
Dr. Wright has served as a consultant for Tesaro and Clovis Oncology. The other authors did not report any potential conflicts of interest.
Each author has indicated that he or she has met the journal’s requirements for authorship.
References
- 1.Volkow ND, McLellan TA, Cotto JH, Karithanom M, Weiss SR. Characteristics of opioid prescriptions in 2009. JAMA. 2011;305:1299–301. doi: 10.1001/jama.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desai RJ, Hernandez-Diaz S, Bateman BT, Huybrechts KF. Increase in prescription opioid use during pregnancy among Medicaid-enrolled women. Obstet Gynecol. 2014;123:997–1002. doi: 10.1097/AOG.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuehn BM. Opioid prescriptions soar: increase in legitimate use as well as abuse. JAMA. 2007;297:249–51. doi: 10.1001/jama.297.3.249. [DOI] [PubMed] [Google Scholar]
- 4.Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in Drug and Opioid Overdose Deaths--United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2016;64:1378–82. doi: 10.15585/mmwr.mm6450a3. [DOI] [PubMed] [Google Scholar]
- 5.Miller M, Barber CW, Leatherman S, et al. Prescription opioid duration of action and the risk of unintentional overdose among patients receiving opioid therapy. JAMA Intern Med. 2015;175:608–15. doi: 10.1001/jamainternmed.2014.8071. [DOI] [PubMed] [Google Scholar]
- 6.Bateman BT, Hernandez-Diaz S, Rathmell JP, et al. Patterns of opioid utilization in pregnancy in a large cohort of commercial insurance beneficiaries in the United States. Anesthesiology. 2014;120:1216–24. doi: 10.1097/ALN.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maeda A, Bateman BT, Clancy CR, Creanga AA, Leffert LR. Opioid abuse and dependence during pregnancy: temporal trends and obstetrical outcomes. Anesthesiology. 2014;121:1158–65. doi: 10.1097/ALN.0000000000000472. [DOI] [PubMed] [Google Scholar]
- 8.Opioid use and opioid use disorder in pregnancy. Committee Opinion No. 711. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2017;130:e81–e94. doi: 10.1097/AOG.0000000000002235. [DOI] [PubMed] [Google Scholar]
- 9.Jones HE, Kaltenbach K, Heil SH, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. The New England journal of medicine. 2010;363:2320–31. doi: 10.1056/NEJMoa1005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang MC, Maselli J, Lurie JD, Lindenauer PK, Pekow PS, Auerbach AD. Use and outcomes of venous thromboembolism prophylaxis after spinal fusion surgery. Journal of thrombosis and haemostasis: JTH. 2011;9:1318–25. doi: 10.1111/j.1538-7836.2011.04326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kulik A, Rassen JA, Myers J, et al. Comparative effectiveness of preventative therapy for venous thromboembolism after coronary artery bypass graft surgery. Circulation Cardiovascular interventions. 2012;5:590–6. doi: 10.1161/CIRCINTERVENTIONS.112.968313. [DOI] [PubMed] [Google Scholar]
- 12.Prabhakaran S, Herbers P, Khoury J, et al. Is prophylactic anticoagulation for deep venous thrombosis common practice after intracerebral hemorrhage? Stroke. 2015;46:369–75. doi: 10.1161/STROKEAHA.114.008006. [DOI] [PubMed] [Google Scholar]
- 13.Ritch JM, Kim JH, Lewin SN, et al. Venous thromboembolism and use of prophylaxis among women undergoing laparoscopic hysterectomy. Obstet Gynecol. 2011;117:1367–74. doi: 10.1097/AOG.0b013e31821bdd16. [DOI] [PubMed] [Google Scholar]
- 14.Wright JD, Lewin SN, Shah M, et al. Quality of venous thromboembolism prophylaxis in patients undergoing oncologic surgery. Annals of surgery. 2011;253:1140–6. doi: 10.1097/SLA.0b013e31821287ac. [DOI] [PubMed] [Google Scholar]
- 15.Zacharia BE, Youngerman BE, Bruce SS, et al. Quality of Postoperative Venous Thromboembolism Prophylaxis in Neuro-oncologic Surgery. Neurosurgery. 2017;80:73–81. doi: 10.1227/NEU.0000000000001270. [DOI] [PubMed] [Google Scholar]
- 16.Stulberg JJ, Delaney CP, Neuhauser DV, Aron DC, Fu P, Koroukian SM. Adherence to surgical care improvement project measures and the association with postoperative infections. Jama. 2010;303:2479–85. doi: 10.1001/jama.2010.841. [DOI] [PubMed] [Google Scholar]
- 17.Kuklina EV, Whiteman MK, Hillis SD, et al. An enhanced method for identifying obstetric deliveries: implications for estimating maternal morbidity. Maternal and child health journal. 2008;12:469–77. doi: 10.1007/s10995-007-0256-6. [DOI] [PubMed] [Google Scholar]
- 18.Grimes DA, Schulz KF. False alarms and pseudo-epidemics: the limitations of observational epidemiology. Obstet Gynecol. 2012;120:920–7. doi: 10.1097/AOG.0b013e31826af61a. [DOI] [PubMed] [Google Scholar]
- 19.Bateman BT, Mhyre JM, Hernandez-Diaz S, et al. Development of a comorbidity index for use in obstetric patients. Obstet Gynecol. 2013;122:957–65. doi: 10.1097/AOG.0b013e3182a603bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Metcalfe A, Lix LM, Johnson JA, et al. Validation of an obstetric comorbidity index in an external population. BJOG: an international journal of obstetrics and gynaecology. 2015;122:1748–55. doi: 10.1111/1471-0528.13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemon LS, Caritis SN, Venkataramanan R, Platt RW, Bodnar LM. Methadone Versus Buprenorphine for Opioid Use Dependence and Risk of Neonatal Abstinence Syndrome. Epidemiology. 2018;29:261–8. doi: 10.1097/EDE.0000000000000780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tolia VN, Murthy K, Bennett MM, et al. Antenatal methadone vs buprenorphine exposure and length of hospital stay in infants admitted to the intensive care unit with neonatal abstinence syndrome. Journal of perinatology: official journal of the California Perinatal Association. 2018;38:75–9. doi: 10.1038/jp.2017.157. [DOI] [PubMed] [Google Scholar]
- 24.Zedler BK, Mann AL, Kim MM, et al. Buprenorphine compared with methadone to treat pregnant women with opioid use disorder: a systematic review and meta-analysis of safety in the mother, fetus and child. Addiction. 2016;111:2115–28. doi: 10.1111/add.13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker AM. Confounding by indication. Epidemiology. 1996;7:335–6. [PubMed] [Google Scholar]
- 26.Jones HE, Heil SH, Baewert A, et al. Buprenorphine treatment of opioid-dependent pregnant women: a comprehensive review. Addiction. 2012;107(Suppl 1):5–27. doi: 10.1111/j.1360-0443.2012.04035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Minozzi S, Amato L, Bellisario C, Ferri M, Davoli M. Maintenance agonist treatments for opiate-dependent pregnant women. Cochrane Database Syst Rev. 2013:CD006318. doi: 10.1002/14651858.CD006318.pub3. [DOI] [PubMed] [Google Scholar]