Abstract
Background
Sex-based disparities in liver transplantation (LT) are incompletely understood. We assessed the role of height, MELD, MELD-Na and exception points in the disparate access to LT.
Methods
Adults waitlisted for LT at Organ Procurement and Transplantation Network (OPTN) between 2002 and 2013 were included. Covariates associated with likelihood of LT were analyzed by Cox proportional model. In a separate cohort of waitlisted adults with glomerular filtration rate (GFR) measurement by iothalamate clearance (n=611), we determined the number of creatinine-derived MELD points in men vs women, across all ranges of GFR. The impact of correcting the MELD score deficit in women on LT was modeled.
Results
Among 90,720 OPTN registrants, women had higher mortality than men (4 years after listing: 22% vs 18%, p<0.0001), and lower likelihood of LT (49% vs 58%, p<0.0001); women were 20% less likely to be transplanted (HR= 0.80, 95% CI= 0.78–0.81). Differences in height and MELD exception scores accounted for most of the LT deficit in women (HR=0.91, 95% CI 0.89–0.94). Women received between 1 and 2.4 fewer creatinine-derived MELD points than men with similar renal dysfunction. MELD-Na worsened the gender disparity. Addition of 1 or 2 MELD points to women significantly impacted LT access.
Conclusion
Differences in height and MELD exception points explained most of the sex-based disparity in LT. Additionally, MELD score underestimated disease severity in women by up to 2.4 points and MELD Na exacerbated this disparity. The degree of underestimation based on MELD had significant impact on allocation.
Sex-based disparities in liver transplantation (LT) have been recognized but not well understood. Women are 30% less likely to undergo LT and the disparity has increased after the introduction of MELD-based allocation system 1. Furthermore, in the MELD era women are more likely than men to die while waiting for a donor organ 1,2,3. Population-based studies showed that women are at a disadvantage through all stages in the process of transplant evaluation, from diagnosis of liver disease to enrollment on the waiting list 4.
The disparity in liver transplantation rates between men and women have been examined in various analyses, and several contributing factors have been speculated. One proposed factor was a systematic bias in MELD score, which disadvantages women given their lower muscle mass and, consequently, their serum creatinine 5,6,7. However, no study was able to show that sex-adjustment of MELD score would eliminate this inequity 8. Women have smaller body size, which may limit the acceptability of a potential liver allograft if the available organ comes from a larger individual 9, 10. Height contributes, but does not entirely explain the disparities in wait list mortality and access to LT between men and women 3.
The disparities in LT between sexes are likely multifactorial and extend beyond height and listing MELD score 11. Differences in liver disease etiology and progression (predominantly hepatitis C and alcoholic liver disease in men; primary biliary cholangitis predominance in women) 12,13, as well as the incidence of hepatocellular carcinoma (HCC) 14 are factors that have not been accounted for in previous studies. Patients who develop HCC are given extra MELD score points (‘exception points’) in order to facilitate access to LT for patients with HCC in whom their biological MELD score alone does not represent the urgency in the need for LT. More importantly, it is unclear if women’s higher risk of death while waiting for a liver graft is related to lower access to transplantation, or to biological reasons associated with female sex, inaccurately captured by the MELD score. A more detailed examination of the factors contributing to the disparities is required to guide strategies for an impartial allocation of this very limited lifesaving resource.
In this study we explored i) differences and determinants of waitlist mortality and liver transplantation rates between men and women; ii) the impact of renal function underestimation based on serum creatinine use in MELD score in disparities to liver transplantation; and iii) whether correction of deficient MELD points improves women’s deficit in liver transplantation.
METHODS
Study population
To determine the waitlist mortality and liver transplantation rates in men and women, we studied all adult patients listed for liver transplantation in the Organ Procurement and Transplantation Network (OPTN) database between March 2002 and December 2013. Subjects listed as Status 1 were excluded, because their liver allocation is urgency-based and they do not compete through the usual MELD-based pathway. Candidates were followed from the time of waitlist registration to the earlier of 1) liver transplantation, 2) removal from the waitlist or 3) death. Reasons for removal included being too sick to transplant, improved, medically unsuitable or refusal of LT. Subjects who were not alive 30 days after waitlist removal were included in the death group. Candidates listed at more than 1 LT center but removed from 1 center’s list, were considered active (not removed from OPTN) if they remained active at any other center.
Exploring factors that impact LT disparities between men and women
Competing risk analysis based on cause-specific hazards (methods of Putter et al)15,16 was used to assess the rates of 3 outcomes: death, removal and LT. To illustrate the rates of outcomes, we used Aalen-Johansen curves (Figure 1A), which are an extension of Kaplan-Meier curves when more than 2 outcomes are possible. The curves are stratified by the following covariates at the time of listing: calculated MELD score, age, UNOS region, blood type and height. Additionally, the Putter method allows to examine how a certain predictor of interest (ie, sex) impacts each of the competing risks and thus, is able to analyze how disparities in one outcome (ie, LT) influence the other (ie, death). For example, it can examine the question if women are less likely to undergo LT because a) female sex is an independent risk factor for LT, or b) women are more likely to die or be removed from the list, thus their lower likelihood of LT is an indirect consequence of higher waitlist mortality or removal. The results of this regression model are shown in the Table included under the graph in Figure 1A. Alternatively, their mortality may be affected by factors inherently associated to the female sex (ie, lower incidence of HCC, different MELD score progression after listing). Akin to Kaplan-Meier, the Aalen-Johansen curves give an accurate view of the outcomes over time, however they can only be fit using baseline covariates, thus the impact of MELD fluctuations on the waitlist cannot be assessed.
Figure 1.
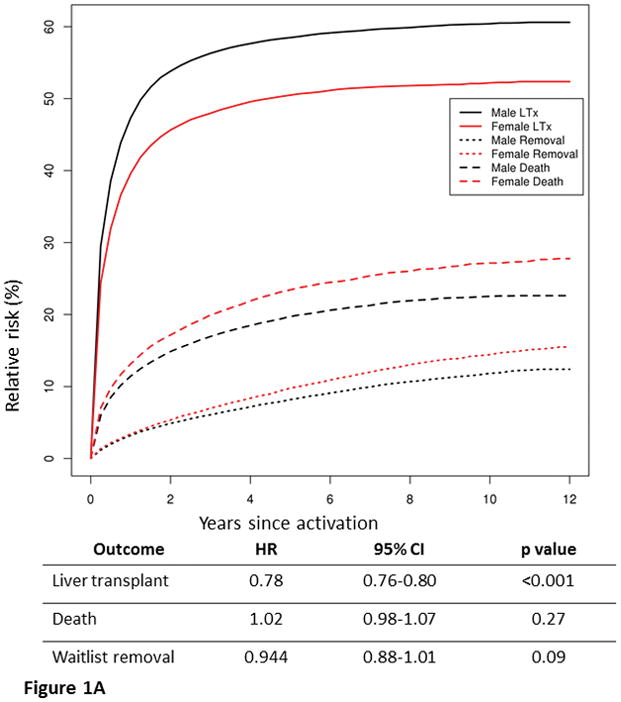
Figure 1A. Risk of liver transplantation, waitlist mortality and removal in men and women among 90,720 OPTN registrants between 2002–2013. The graph represents Aalen-Johansen curves, which are an extension of Kaplan-Meier curves when more than 2 outcomes are possible. The curves are stratified for the following covariates at the time of listing: calculated MELD score, age, UNOS region, blood type and height. Women were less likely than men to undergo LT, more likely to die on the waitlist and to be removed from the list.
The Table shows results of regression analysis of the impact of female sex on the 3 outcomes, when stratified by calculated MELD score at listing, age, UNOS region, blood type and height. Female sex is independent risk factor for LT risk but not for waitlist mortality or removal.
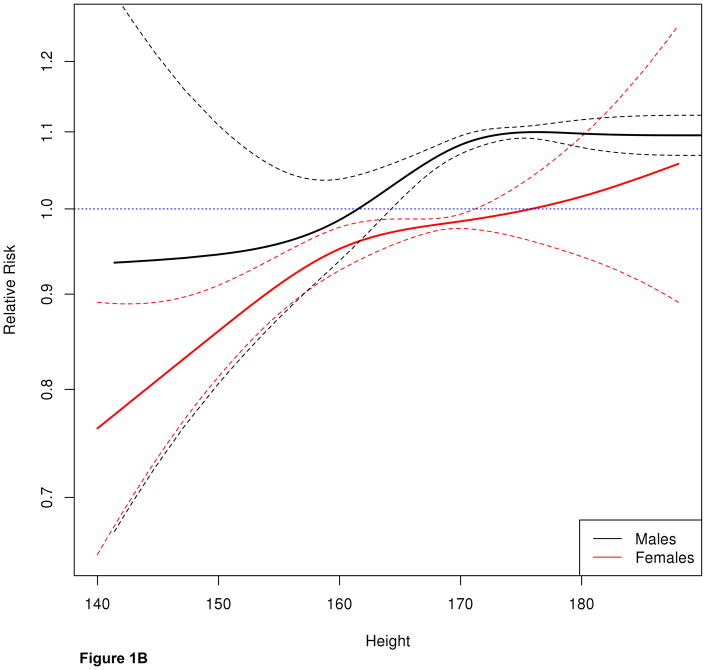
Figure 1B. Spline curve illustrating the effect of height on liver transplantation stratified by calculated MELD, blood type, age and region. In men and women, the likelihood of LT increased with height in a linear fashion. While the overall pattern was similar between men and women, even the tallest (170 cm) women were approximately 10% less likely than men with similar covariates to receive LT.
To address this limitation and further explore the differences in access to LT as they relate not only to sex, but also to sex-dependent differences in disease progression over time, we used Cox regression analysis. We modeled variables well-known to impact allocation (age, blood type, UNOS region), and then tabulated how much they affect the LT deficit in women. Next, keeping the “stable” covariates constant (age, blood type and region), we added MELD score in a series of different models: calculated MELD at listing (cMELD), to reflect the impact of disease severity at baseline; allocated MELD (aMELD) at listing, to explore the impact of receipt of MELD exception scores; and time-dependent aMELD (aMELD recorded at multiple consecutive time-points between listing and LT), to assess the role of disease progression or exception score upgrade after the initial listing. Lastly, the additional role of height in LT was explored in the model that included the parameters that authors considered the most rational: age, blood type, region and time-dependent allocated MELD. The effect of height on LT in men versus women is further illustrated in a spline curve derived from the adjusted Cox model last described above, to show the impact on LT across all height ranges.
Creatinine-derived MELD points in men and women with similar renal function
In the second part of the study we explored differences in renal function estimation by serum creatinine between men and women and the impact these differences have in the number of MELD points acquired by men vs women with similar renal function. We included all adult patients listed for LT at Mayo Clinic, Rochester MN between 2002 and 2014. The transplant evaluation protocol included measurement of GFR by iothalamate clearance, the gold standard method of renal function assessment. For each GFR range, we determined the corresponding serum creatinine level (obtained within 30 days of iothalamate clearance measurement) in men and women and calculated the number of MELD points derived from creatinine, using the established MELD equation: MELD = 9.57 × ln(Cr) + 3.78 × ln(bilirubin) + 11.20 × ln(INR) + 6.43. As the addition of Na increases the predictive ability of MELD and is not influenced by muscle mass or body size, we then analyzed if MELD Na corrects the disparity between sexes. Similar to the analysis described above, we determined the number of MELD Na points derived from serum creatinine and serum sodium for each GFR range, based on the established formula: MELD-Na = MELD + 1.32 x (137-Na) – [0.033 x MELD*(137-Na)].
Impact of creatinine-based MELD correction on LT disparities
In the last part of the analysis we returned to the OPTN cohort used in the first aim and modeled the impact of the deficient number of creatinine-derived MELD points in female LT candidates to their access to LT. We determined the probability of receiving a transplant stratified by the current recipient allocated MELD, height, blood type and region and then used the model to predict the probability of receiving a transplant after adding 1 or 2 points to the calculated MELD of women with serum creatinine between 1 and 4 mg/dl. This range was chosen because creatinine is capped at a minimum of 1 and a maximum of 4 in the MELD formula. The expected number of transplants for each MELD range was obtained from the Cox model residuals: the residual for each subject is (observed - expected), where “observed” is 1 if LT was received or 0 if LT was not received; the “expected” number of transplants take into account different selection probabilities stratified on MELD, region, blood type, and height. While holding the coefficients fixed, we repeated the predictions after adding 1 or 2 MELD points to each female subject with creatinine 1–4 mg/dL 17. In this stratified model, the potential competitors for the organ are all adult men and women who were active on the list in the same region, with similar blood type, similar or higher MELD score and height within ±20 cm than that of the actual recipient. A similar analysis was performed after replacing MELD with MELDNa.
Statistical analyses were performed in SAS v9.4 (SAS Institute; Cary, NC) and R statistical software, version 3.2.0 (R Foundation for Statistical Computer, Vienna). The study was approved by the Mayo Clinic Institutional Review Board.
RESULTS
Disparities in LT and waitlist mortality between men and women
A total of 90,720 waitlist registrants, consisting of 59,899 men and 30,821 women (34%), were included in the analysis (Table 1). Female candidates were slightly older (56 vs 55 years, p<0.0001), shorter (163 vs 178 cm, p<0.0001), more likely to have cholestatic or fatty liver disease (39% vs 18%, p<0.0001) and less likely to have HCC (11% vs 20%, p<0.0001). Compared to men, women had similar calculated MELD and MELD Na scores at listing but were less likely to receive MELD exception points (21% vs 29%, p<0.0001).
Table 1.
Characteristics of waitlisted adults in the Organ Procurement and Transplantation Network between 2002 and 2013.
| Recipient characteristics | Men N= 59,899 |
Women N= 30,821 |
P value |
|---|---|---|---|
| Age, median (IQR) | 55 (49–60) | 56 (49–61) | <0.0001 |
| Race | <0.0001 | ||
| White | 72% | 69% | |
| Black | 8% | 9% | |
| Asian/Pacific Islander | 5% | 4% | |
| Other | 15% | 18% | |
| Height, cm - median (IQR) | 178 (170–183) | 163 (157–168) | <0.0001 |
| Body mass index, kg/m2 – median (IQR) | 27.7 (24.5–31.5) | 27.5 (23.6–32.4) | <0.0001 |
| Liver disease etiology, % | <0.0001 | ||
| Alcoholic | 21% | 14% | |
| Viral | 58% | 44% | |
| Cholestatic | 5% | 14% | |
| NAFLD/cryptogenic | 13% | 25% | |
| Other | 3% | 3% | |
| HCC Diagnosis | 20% | 11% | <0.0001 |
| MELD at listing, median (IQR) | 15 (11–20) | 15 (11–20) | 0.0002 |
| MELD- Na at listing, median (IQR) | 16 (12–22) | 16 (11–22) | <0.0001 |
| MELD at transplant, median (IQR) | 19 (13–28) | 21 (14–30) | <0.0001 |
| MELD-Na at transplant, median (IQR) | 21 (14–29) | 22 (15–30) | <0.0001 |
| Received exception points, % | 29% | 21% | 0.0005 |
| OPTN region % | <0.0001 | ||
| 1 | 5% | 4% | |
| 2 | 13% | 12% | |
| 3 | 12% | 12% | |
| 4 | 10% | 11% | |
| 5 | 18% | 18% | |
| 6 | 3% | 3% | |
| 7 | 8% | 9% | |
| 8 | 6% | 7% | |
| 9 | 9% | 8% | |
| 10 | 7% | 8% | |
| 11 | 9% | 8% |
Figure 1A illustrates the results of the competing risks analysis of waitlist outcomes in men and women followed up to 12 years since listing. Compared to men with similar covariates (age, blood group, UNOS region and calculated MELD at listing), women were less likely to undergo LT, more likely to die on the waitlist and to be removed from the list. For example, at 4 years since listing 49% of women versus 58% of men (p<0.0001) underwent LT, whereas 22% of women versus 18% of men died while waiting for LT (p<0.0001). The magnitude of disparity in LT and waitlist mortality varied slightly between the United Network for Organ Sharing (UNOS) regions (Figure S1). The Cox regression model in Figure 1A shows that female sex is associated with lower LT risk independently and not indirectly by increasing the risk of other competing risks such as mortality or waitlist removal.
Factors that impact LT disparities between men and women
Table 2 summarizes a series of Cox regression models in which the association of sex, height and other plausible variables with the likelihood of LT was analyzed. In model 1, adjusted for age, blood group and region, women were 20% less likely than men to undergo LT (HR =0.80, 95% CI= 0.78–0.81). Additional adjustment for calculated MELD at listing (model #2) minimally increased the disparity (HR= 0.78, 95% CI 0.76–0.79). However, adjustment for baseline allocated MELD (which replaced calculated MELD) in model #3 attenuated the differences in LT access (HR=0.82, 95% CI 0.80–0.84), which suggests that the MELD exception scores accounted for part of the men’s access to LT. Further adjustment for allocated MELD score in a time dependent fashion (model #4) increased the HR to 0.85 (95% CI= 0.83–0.87), suggesting that men’s access to LT was partly related to faster progression of MELD score while waiting for a donor. Finally, including recipient height in model #5 brought the HR of LT associated with female sex closer towards unity (HR= 0.91, 95% CI 0.89–0.94). Other factors such as race and functional status did not play a role in the disparities (data not shown).
Table 2.
Covariates associated with disparities in liver transplantation between men and women. Each individual Cox regression model was stratified by age, blood group, UNOS region, whereas MELD scores were sequentially substituted. In the last model, height was added to model 4.
| Model | Covariates | HR of liver transplantation (F/M) | 95% CI |
|---|---|---|---|
| 1 | Age, blood group, region | 0.80 | 0.78–0.81 |
| 2 | Age, blood group, region, lab MELD at listing | 0.78 | 0.76–0.79 |
| 3 | Age, blood group, region, allocated MELD at listing | 0.82 | 0.80–0.84 |
| 4 | Age, blood group, region, time-dependent allocated MELD | 0.85 | 0.83–0.87 |
| 5 | Age, blood group, region, time-dependent allocated MELD, height | 0.91 | 0.89–0.94 |
Figure 1B further demonstrates that recipient height was an important determinant of liver transplantation. In both men and women, the likelihood of LT increased with height in a linear fashion. While the overall pattern was similar between men and women, even the tallest (170 cm) women were approximately 10% less likely than men of the same height, calculated MELD and from the same region, to receive LT. For female candidates with height between 160 and 170 cm, the likelihood of LT did not markedly change, whereas it decreased rapidly in men. Finally, in women < 160cm, the decrease was as rapid as in men who are 10 cm taller. The slope of the 2 curves in those segments suggests that a 10cm height difference translated to roughly a 10% change in the likelihood of LT.
Creatinine-derived MELD points in men and women with similar renal function
To explore the disparity in renal function estimation by serum creatinine in MELD score, we used a cohort of 611 subjects listed for liver transplantation at Mayo Clinic, Rochester, MN, where renal function measurement by iothalamate clearance was part of the routine evaluation for LT candidacy. As seen in Table 3, women had a lower median mGFR than men: 68 (45–97) vs 80 (55–103) ml/min/BSA) at the time of listing. Nevertheless, women’s serum creatinine level was lower: 0.8 (0.7–1.2 mg/dl) vs 1.0 (0.8–1.2) mg/dl in men. As a reflection of lower muscle mass, women’s creatinine was consistently lower than that of men at all ranges of measured GFR (Table 4). Accordingly, the number of MELD points derived from serum creatinine in women was between 1.15 and 2.38 points lower than that of men with similar mGFR. The deficit appeared widest at a mGFR between 40–49 ml/min/BSA. Additionally, women started accruing creatinine-derived MELD points at a later stage of renal dysfunction than men (50 ml/min/BSA vs 60 ml/min/BSA) (Figure 2-solid lines). When MELD Na formula was used, the number of MELD points derived from creatinine and serum sodium remained higher in men than women across all mGFR ranges (Table 4). Compared to MELD, the disparity in the number of acquired points using MELD Na started at an even higher GFR (69 vs 59 ml/min/BSA) and worsened through nearly all GFR ranges. Due to higher serum creatinine and lower serum sodium despite similar degrees of renal dysfunction, men acquired between 0.47 and 4.86 more MELD-Na points than women (Figure 2-dotted lines).
Table 3.
Characteristics of the Mayo Clinic patient cohort.
| Characteristics at listing Median (IQR) |
Women N=262 |
Men N=349 |
Total N=611 |
p-value* |
|---|---|---|---|---|
| Age | 55 (47–61) | 54 (44–59) | 54 (45–60) | 0.104 |
| Measured GFR (ml/min/BSA) | 68 (45–97) | 80 (55–103) | 75 (50–101) | 0.001 |
| Creatinine (mg/dl) | 0.8 (0.7–1.2) | 1.0 (0.8–1.2) | 0.9 (0.7–1.2) | <0.001 |
| Bilirubin (mg/dl) | 2.5 (1.3–5.0) | 2.6 (1.3–4.5) | 2.5 (1.3–4.7) | 0.850 |
| INR | 1.2 (1.1–1.4) | 1.2 (1.1–1.5) | 1.2 (1.1–1.5) | 0.097 |
| MELD | 13 (9–18) | 14 (10–19) | 14 (10–18) | 0.046 |
| MELD-Na | 16 (11–20) | 16 (12–21) | 16 (11–21) | 0.114 |
P-values are from the Kruskal-Wallis test
Table 4.
The number of MELD points derived from serum creatinine and serum sodium in men (gray) and women (white), stratified by ranges of renal function.
| Measured GFR (ml/min/BSA) and number of M and F | Serum creatinine (mg/dl) (median, IQR) | MELD points derived from Cr (median, IQR) | Δ MELD points M vs W | Serum sodium (mmol/L) (median, IQR) | MELD Na points derived from Cr and Na (median, IQR) | Δ MELD-Na points M vs W |
|---|---|---|---|---|---|---|
|
| ||||||
| ≥70 M: 232 F: 138 |
0.80 0.70–0.96 |
0.00 0.00–0.00 |
0.00 | 139 136–140 |
0.00 0.00–0.97 |
0.00 |
|
|
|
|||||
| 0.66 0.56–0.76 |
0.00 0.00–0.00 |
138 137–140 |
0.00 0.00–0.00 |
|||
|
| ||||||
| 69–60 M: 34 F: 28 |
1.00 0.90–1.16 |
0.00 0.00–1.42 |
0.00 | 136 132–138 |
2.21 0.91–4.72 |
2.21 |
|
|
|
|||||
| 0.66 0.76–0.95 |
0.00 0.00–0.00 |
138 133.5–142 |
0.00 0.00–2.63 |
|||
|
| ||||||
| 59–50 M: 36 F: 49 |
1.20 1.03–1.40 |
1.74 0.28–3.22 |
1.74 | 137.5 134.5–140 |
2.98 1.422–4.53 |
1.56 |
|
|
|
|||||
| 1.00 0.9–1.16 |
0.00 0.00–1.42 |
138 135–141 |
1.42 0.56–2.94 |
|||
|
| ||||||
| 49–40 M: 29 F: 38 |
1.36 1.15–1.66 |
2.94 1.43–4.85 |
2.38 | 137 133–139 |
4.62 2.21–6.44 |
2.54 |
|
|
|
|||||
| 1.06 0.90–1.20 |
0.56 0.00–1.74 |
138 134–141 |
2.08 0.00–3.81 |
|||
|
| ||||||
| 39–30 M: 36 F: 26 |
1.50 1.33–1.91 |
3.88 2.73–6.19 |
1.15 | 137.5 134.5–139.5 |
5.02 3.77–7.61 |
1.94 |
|
|
|
|||||
| 1.33 1.06–1.40 |
2.73 0.56–3.22 |
137.5 132–141 |
3.08 1.45–6.36 |
|||
|
| ||||||
| 29–20 M: 21 F: 24 |
1.76 1.60–2.26 |
5.41 4.50–7.80 |
1.34 | 136 135–140 |
6.51 4.74–9.81 |
0.47 |
|
|
|
|||||
| 1.53 1.36–1.88 |
4.07 2.94–6.04 |
136.5 134–141 |
6.04 3.90–7.36 |
|||
|
| ||||||
| <20 M: 10 F: 12 |
2.56 2.10–3.5 |
9.00 7.10–11.99 |
1.54 | 134 128–137 |
12.50 9.00–14.55 |
4.86 |
|
|
|
|||||
| 2.18 1.71–2.93 |
7.46 5.13–10.28 |
138 134–140 |
7.64 6.5–11.74 |
|||
Figure 2.
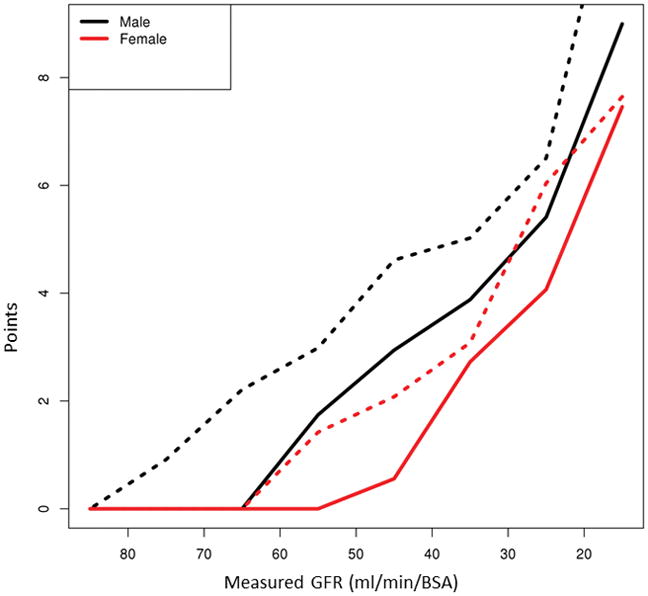
The number of creatinine-derived MELD points (solid lines) and creatinine+sodium derived MELD Na points (dashed lines) in men versus women by range of glomerular filtration rate. Women accrue fewer MELD points derived from serum creatinine and starting at a later stage of renal dysfunction than men with similar measured GFR. Using MELD Na formula, the number of MELD points derived from creatinine plus serum sodium remained higher in men than women across all measured GFR ranges.
Impact of creatinine-based MELD correction on LT disparities
To explore whether a difference of 1 MELD point would have an impact on the disparities in LT rates in women, we modeled the number of LT in women as follows: observed (white bars), expected (light grey bars), estimated after adding 1 point to cMELD (dark grey bars) and estimated after adding 2 points to cMELD (black bars) (Figure 3). In calculating the expected transplants, the contenders were all men and women with the same allocated MELD (calculated or exception MELD) and with a height within 20 cm of the recipient of the actual LT recipient. This method accounted for the inherent differences related to MELD exception points and height between men and women and estimated the effect of MELD points addition independently from these factors.
Figure 3.
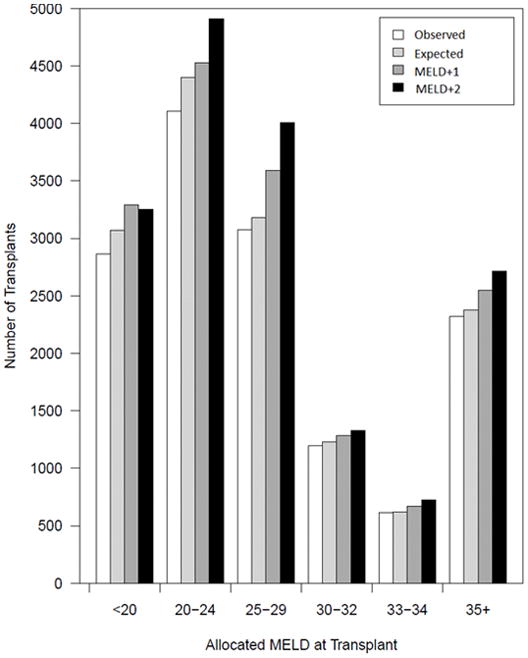
Estimated impact of 1 or 2 MELD points addition on the expected number of liver transplants in women. The bars show the actual LT rates (observed), the probability of receiving a transplant stratified by the current recipient allocated MELD, height, blood type and region (expected) and the predicted probability of receiving a transplant after adding 1 (MELD+1) or 2 (MELD+2) points to the calculated MELD of women with abnormal serum creatinine. The model c-statistic was 0.58.
The observed number of LTs in women was lower than that of expected LTs across all ranges of MELD at transplant, except 30–34. The estimated total deficit (deficit= expected – observed) was 698 transplants. Addition of 1 MELD point resulted in an increase in the expected LT numbers in women across all MELD ranges. The largest effect was seen at MELD 20–29 (estimated 486 more LTs), a range where most of the liver transplants occurred. Of 1,761 women with MELD score of 34, addition of 1 point to those with creatinine between 1 and 4 mg/dl increased their MELD to 35 (the threshold for regional sharing) in 948 women (54%) (Table S1). A 2 point addition to women with MELD 33 brought 1,090 women (48%) to the MELD 35 threshold (Table S2).
Lastly, we explored the observed and expected LTs if allocated MELD Na would have hypothetically been used instead of MELD, understanding that the MELD Na policy was not in place during the study timeframe. Figure S2 shows that women’s LT deficit would have worsened for women listed with a MELD Na <25 and ≥35, and “overcorrected” for the intermediate MELD Na ranges.
DISCUSSION
Despite the efforts of policy makers to achieve equitable organ allocation, women continue to have less access to liver transplantation and experience higher death rates on the waitlist than men. While most of the LT deficit in women is explained by their shorter stature, differences in disease characteristics between sexes reflected in the rate of disease progression and receipt of MELD exception points also play a role. However, even after accounting for these anatomical and biological differences, women remain less likely than men to be selected for LT. Due to lower muscle mass, serum creatinine underestimates renal dysfunction in women, resulting in 1–2 fewer MELD points than men. MELD-Na exacerbates this disparity, which can reach up to 4 MELD points. One MELD point deficit appears to have considerable impact on women’s access to available livers, but the exact extent should be further assessed with complex simulation models.
Since the introduction of MELD score in liver allocation, women became less likely than men to undergo transplantation; LT deficit in women increased from 9% to 14% in the MELD era 2. In our study, extending the analysis to 11 years since MELD score implementation, the unadjusted LT deficit in women seems to have become even larger, at 20%. The “sickest first” principle is designed to identify subjects who are most likely to die without receipt of a liver allograft. However, women are more likely to remain waiting for a liver, and consequently die.
We found that differences in height play the biggest role in the sex-based disparity in liver transplantation rates. When referenced to subjects of 175 cm in height, short candidates (165 cm or less) are approximately 10–15% less likely to undergo LT. More than half of the women and only a minority of men listed for LT fall into this category. Nevertheless, the small percentage of tall women, whose height range should not constitute a surgical limitation, remains disadvantaged, with LT rates that are much lower than those of men with similar height. The reasons remain obscure and require further study.
We explored several other potential factors contributing to the LT deficit in women. An important contributor is that more men are listed with MELD exception scores. Several previous studies have shown that patients who are listed with MELD exception points are transplanted at higher rates than those with equivalent biologic MELD scores and have lower rates of waitlist dropouts 18,19,20. Of patients who receive exception points for HCC, 77.5% are male 18, and the rate of exception scores allocation is increasing over time. Akin to previous published data, HCC was more prevalent in men in the OPTN cohort studied in this work. If exception patients are favored disproportionally to their predicted risk, this represents an added disadvantage for women, for whom exception points are less often granted.
Other factors have been speculated to account for lower LT rates in women. A systematic bias within MELD score has been described in various studies, which demonstrate that for the same GFR, female candidates for LT have lower MELD scores than male candidates5. Women have lower muscle mass, hence lower serum creatinine levels relative to their true renal function. Since creatinine is a component of MELD score, their mortality risk may not be fully captured by the calculated MELD. Nevertheless, correcting MELD score by sex does not improve mortality prediction6.
In our cohort, differences in renal function estimation based on serum creatinine translate to 1–2.4 fewer MELD points in women compared to men with similar renal dysfunction. For reasons that are not entirely clear (but probably related to higher volume of total body water), men have lower serum sodium; thus, the recent implementation of MELD-Na could potentially widen the disparity in liver disease estimation and prioritization for LT. Perhaps somewhat unexpected, addition of 1–2 points to the MELD of women with renal dysfunction had a substantial impact in LT rates in our model. In the current era of Share 35, addition of 1 or 2 MELD points brings a significant number of women to the threshold of 35 that opens the door to regional donors. The purpose of our model is not to propose the addition of MELD points to women with renal dysfunction as a definitive solution to LT disparities, but to highlight that a seemingly insignificant deficit in creatinine-derived points has a considerable impact on women’s LT access, the magnitude of which that had not been clearly demonstrated in previous studies.
This study improves the understanding of sex-based inequities in liver allocation and explains most of the transplant deficit in women. In addition to candidate body size, we took into account differences in liver disease between men and women by modeling MELD score in a time-dependent fashion, to account for its dynamic nature and different progression rates; we have analyzed MELD exception points received either at listing or accrued over time while on the waitlist, to account for differences in liver disease complications, including but not limited to HCC. After controlling for region and all biologically plausible differences between sexes, the liver transplant deficit in women decreases from 20% to 9%. Thus, a part of the inequity remains unexplained. It remains to be explored if disparate access to liver transplantation is also attributed to concealed provider biases that unintentionally keep women longer on the waitlist, or other arbitrary boundaries.
This work sheds light into potential steps the transplant community should take, beyond recognizing the unequal access to LT for women as an unfortunate reality. When waitlist mortality (as opposed to equal access to transplantation) is the criterion for organ allocation, some inequity between men and women in the LT rates may be unavoidable. Further amendments of the allocation rules for MELD exception scores for HCC may partially attenuate the unequal access to donor organs. However, women’s major disadvantage is unrelated to unique features of liver disease. Body size poses limitations on both the feasibility of a surgical operation (due to height and related differences in abdominal cavity size) and the accurate estimation of their disease severity (due to lower muscle mass). Increased awareness of this barrier should motivate providers to encourage women to seek living donation. The impact of 1 or 2 MELD point addition to women, especially in the MELD Na era, should be carefully considered in future allocation policies aimed at reducing disparity in access to liver transplantation. The quest for fairness and equitable allocation of organs to all liver transplant candidates must also address sex-based disparities.
Supplementary Material
Acknowledgments
Grant support: National Institute of Diabetes and Digestive and Kidney Diseases DK-34238, DK-92336 (WRK)
Abbreviations
- GFR
glomerular filtration rate
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- LT
liver transplantation
- MELD
model for end stage liver disease
- OPTN
Organ Procurement and Transplantation Network
- UNOS
United Network for Organ Sharing
Footnotes
Conflict of interest: The authors have nothing to disclose.
Author contributions: study concept and design: AMA, WRK, PSK; acquisition of data: JJL; statistical analysis: JJL, KCM, TMT; analysis and interpretation of data: AMA, JKH, WRK, PSK, TMT; drafting of the manuscript: AMA; critical revision of the manuscript for important intellectual content: JKH, WRK, PSK, TMT.
References
- 1.Moylan CA, Brady CW, Johnson JL, et al. Disparities in liver transplantation before and after introduction of the MELD score. JAMA. 2008;300:2371–8. doi: 10.1001/jama.2008.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathur AK, Schaubel DE, Gong Q, et al. Sex-based disparities in liver transplant rates in the United States. Am J Transplant. 2011;11:1435–43. doi: 10.1111/j.1600-6143.2011.03498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai JC, Terrault NA, Vittinghoff E, et al. Height contributes to the gender difference in wait-list mortality under the MELD-based liver allocation system. Am J Transplant. 2010;10:2658–64. doi: 10.1111/j.1600-6143.2010.03326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryce CL, Angus DC, Arnold RM, et al. Sociodemographic differences in early access to liver transplantation services. Am J Transplant. 2009;9:2092–101. doi: 10.1111/j.1600-6143.2009.02737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cholongitas E, Marelli L, Kerry A, et al. Female liver transplant recipients with the same GFR as male recipients have lower MELD scores--a systematic bias. Am J Transplant. 2007;7:685–92. doi: 10.1111/j.1600-6143.2007.01666.x. [DOI] [PubMed] [Google Scholar]
- 6.Huo SC, Huo TI, Lin HC, et al. Is the corrected-creatinine model for end-stage liver disease a feasible strategy to adjust gender difference in organ allocation for liver transplantation? Transplantation. 2007;84:1406–12. doi: 10.1097/01.tp.0000282867.92367.d0. [DOI] [PubMed] [Google Scholar]
- 7.Mindikoglu AL, Regev A, Seliger SL, et al. Gender disparity in liver transplant waiting-list mortality: the importance of kidney function. Liver Transpl. 2010;16:1147–57. doi: 10.1002/lt.22121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myers RP, Shaheen AA, Aspinall AI, et al. Gender, renal function, and outcomes on the liver transplant waiting list: assessment of revised MELD including estimated glomerular filtration rate. J Hepatol. 2011;54:462–70. doi: 10.1016/j.jhep.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Mindikoglu AL, Emre SH, Magder LS. Impact of estimated liver volume and liver weight on gender disparity in liver transplantation. Liver Transpl. 2013;19:89–95. doi: 10.1002/lt.23553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowring MG, Ruck JM, Haugen CE, et al. Deceased-Donor Liver Size and the Sex-Based Disparity in Liver Transplantation. Transplantation. 2017;101:e329. doi: 10.1097/TP.0000000000001898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Axelrod DA, Pomfret EA. Race and sex disparities in liver transplantation: progress toward achieving equal access? JAMA. 2008;300:2425–6. doi: 10.1001/jama.2008.732. [DOI] [PubMed] [Google Scholar]
- 12.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825–32. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 13.Parikh-Patel A, Gold EB, Worman H, et al. Risk factors for primary biliary cirrhosis in a cohort of patients from the united states. Hepatol. 2001;33:16–21. doi: 10.1053/jhep.2001.21165. [DOI] [PubMed] [Google Scholar]
- 14.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterol. 2007;132:2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 15.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26:2389–430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 16.Kim WR, Therneau TM, Benson JT, et al. Deaths on the liver transplant waiting list: an analysis of competing risks. Hepatol. 2006;43:345–51. doi: 10.1002/hep.21025. [DOI] [PubMed] [Google Scholar]
- 17.Therneau TM, Grambsch, Patricia M. Modeling Survival Data: Extending the Cox Model. New York: Springer-Verlag; 2000. [Google Scholar]
- 18.Massie AB, Caffo B, Gentry SE, et al. MELD Exceptions and Rates of Waiting List Outcomes. Am J Transplant. 2011;11:2362–71. doi: 10.1111/j.1600-6143.2011.03735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Washburn K, Edwards E, Harper A, et al. Hepatocellular carcinoma patients are advantaged in the current liver transplant allocation system. Am J Transplant. 2010;10:1643–8. doi: 10.1111/j.1600-6143.2010.03127.x. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg D, French B, Abt P, et al. Increasing disparity in waitlist mortality rates with increased model for end-stage liver disease scores for candidates with hepatocellular carcinoma versus candidates without hepatocellular carcinoma. Liver Transpl. 2012;18:434–43. doi: 10.1002/lt.23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.