Abstract
Objective.
Previous studies suggest that the brain-derived neurotrophic factor (BDNF) Val66Met (rs6265) polymorphism may influence symptom onset in Alzheimer’s disease (AD). Our recent cross-sectional findings suggest that Met66 may influence clinical expression in dominantly inherited AD (DIAD) through its effects on tau. However, it remains unclear whether carriage of Met66 in DIAD results in faster increases in CSF tau and ptau181, and whether these increases are associated with accelerated brain volume loss and memory decline.
Methods.
A total of 211 subjects (101 mutation non-carriers, 110 mutation carriers), who were cognitively normal, as defined by a Clinical Dementia Rating (CDR) global score of 0, completed assessments of cognitive function, neuroimaging and CSF sampling over 3.5 years as part of the Dominantly Inherited Alzheimer’s Network (DIAN).
Results.
In mutation carriers, Met66 carriers showed faster memory decline (4x), hippocampal volume loss (16x), and CSF tau and ptau181 increases (6x) than Val66 homozygotes. BDNF did not influence rates of cortical β-amyloid accumulation or change in CSF Aβ42 levels in mutation carriers. In mutation non-carriers, BDNF genotype had no effect on change in cognition, brain volume, cortical β-amyloid accumulation or change in any CSF measures of tau, ptau181, and CSF Aβ42.
Interpretation.
As in sporadic AD, the deleterious effects of β-amyloid on cognitive function, brain volume loss and CSF tau in DIAD mutation carriers are less in Val66 homozygotes. The BDNF Val66Met polymorphism should be considered as a potential moderator of clinical trial outcomes in current treatment and prevention trials in DIAD and sporadic AD.
Introduction
Dominantly inherited Alzheimer’s disease (DIAD) is caused by mutations in the amyloid precursor protein (APP), presenilin-1 (PSEN1) and presenilin-2 (PSEN2) which lead to a rapid accumulation of beta-amyloid (Aβ), which in turn gives rise to increased cerebrospinal fluid (CSF) tau, neurofibrillary tangles, loss of brain volume, cognitive decline and ultimately dementia.1, 2 The temporal sequence of biomarker changes and their clinical manifestation in DIAD is acknowledged widely as being similar to that in sporadic AD.1–4 Hence, understanding disease progression in DIAD is important for understanding AD pathophysiological process in general. As DIAD mutations have almost complete penetrance, we have developed predictive models for the timing of symptom onset in DIAD mutation carriers.1, 5 However, these account for only ~50% of the variance in symptom onset in mutation carriers,5 suggesting other factors may modify relationships between DIAD mutations and their phenotypic expression.
Converging evidence from sporadic AD and DIAD studies suggest that the brain-derived neurotrophic factor (BDNF) Val66Met (rs6265) polymorphism may influence symptom onset.6–9 In sporadic AD, Met66 carriers show faster cognitive decline and hippocampal volume loss in both preclinical and prodromal stages.6–10 However, the Met66 allele does not increase Aβ accumulation, and in β-amyloid-negative individuals, does not influence cognitive decline or neurodegeneration.6, 7 This led us to propose that in β-amyloid-positive non-demented older adults, Met66 reduces resilience to β-amyloid associated neurotoxicity,11, 12 possibly through modifying CNS BDNF levels as a response to inflammatory processes that occur early in the course of AD.13
In DIAD, BDNF Val66Met can also influence symptom onset. Cognitively normal young DIAD mutation carriers who were Met66 carriers showed greater cognitive impairment and lower hippocampal glucose metabolism than matched Val66 homozygotes.10 This was despite both Met66 carriers and Val66 homozygotes having elevated and equivalent levels of cortical Aβ compared to matched controls.10 These cross-sectional findings also suggested that Met66 influenced clinical expression in DIAD by increasing tau because DIAD mutation carriers who carried the Met66 allele showed CSF tau and phosphorylated tau (ptau181) levels that were 25% greater than DIAD mutation carriers who were Val66 homozygotes.10
The hypothesis that BDNF influences tau levels requires challenge in a prospective study as it is not known whether carriage of Met66 in DIAD is associated with faster increases in CSF tau and ptau181, and whether such increases accompany greater brain volume loss and memory decline. Further, to confirm the role of Met66 in AD, it is necessary to show that it does not influence memory decline, brain volume loss and tau levels in β-amyloid-negative mutation non-carriers.
Methods
Subjects
Individuals at risk for carrying a DIAD mutation (i.e., presenilin 1 [PSEN1], presenilin 2 [PSEN2], or amyloid precursor protein [APP] mutations) were enrolled in the Dominantly Inherited Alzheimer Network (DIAN) study and followed over an average of 3 years. Subjects from families with known pathogenic DIAD mutations were recruited from 197 families at sites in the United States (6), United Kingdom (1) and Australia (3).1 Recruitment and enrolment processes have been detailed previously.1 Subjects were included for analysis in this study if they had more than one cognitive and biomarker assessment. Prospective data from 211 cognitively normal subjects (101 mutation non-carriers, 110 preclinical mutation carriers), defined by a Clinical Dementia Rating (CDR) of 0,14 and who had completed assessments of cognition, neuroimaging, CSF sampling and BDNF Val66Met genotyping from Jan 2009 to June 2017 were included (DataFreeze 12).
On average, preclinical mutation carriers were followed over a period of 3.56 (SD 1.51) years. Similarly, mutation non-carriers were followed over a period of 3.75 (SD 1.53) years. Table 1a shows the demographic characteristics of the sample. Table 1b shows the demographic characteristics of the sub-sample who underwent lumbar punctures.
Table 1a.
Baseline demographic and clinical characteristics.
| NCVal66/Val66 | NC Met66 | p | MCVal66/Val66 | MC Met66 | p | |
|---|---|---|---|---|---|---|
| N | 72 | 29 | 77 | 33 | ||
| N (%) Female | 46 (63.9%) | 18 (62.1%) | .864 | 49 (63.6%) | 19 (57.6%) | .549 |
| N(%)AP0E ε4 | 18 (25.0%) | 8 (27.6%) | .788 | 21 (27.3%) | 10 (30.3%) | .746 |
| N (%) Caucasian | 67 (93.1%) | 27 (93.1%) | .993 | 68 (88.3%) | 25 (75.8%) | .119 |
| NAPP/PS1/PS2 | - | - | - | 20/50/7 | 4/28/1 | .106 |
| Age | 38.87 (10.47) | 38.69 (10.54) | .937 | 35.84 (9.81) | 33.54 (9.06) | .252 |
| EYO | −7.67 (11.09) | −7.41 (12.16) | .920 | −11.76 (8.99) | −12.49 (9.21) | .699 |
| Education | 14.86 (2.41) | 15.17 (3.21) | .596 | 14.38 (2.63) | 15.06 (3.14) | .242 |
| GDS | 1.15 (1.61) | 2.14 (2.40) | .019 | 1.77 (2.13) | 1.36 (1.71) | .339 |
| CDRsum of boxes | 0.00 (0.00) | 0.00 (0.00) | .999 | 0.03 (0.11) | 0.06 (0.17) | .203 |
| MMSE | 29.22 (1.21) | 29.32 (1.02) | .703 | 28.93 (1.36) | 29.24 (0.97) | .242 |
| Years Follow-Up | 3.75 (1.53) | 3.56 (1.51) | .569 | 3.64 (1.60) | 3.22 (1.26) | .184 |
| No. Assessments | 2.61 (0.86) | 2.38 (0.73) | .206 | 2.66 (1.01) | 2.30 (0.64) | .062 |
Note: NC = Non-mutation Carriers; MC Val66/Val66 = CDR 0 DIAD Mutation Carriers (Val66 homozygotes); MC Met66 = CDR 0 DIAD Mutation Carriers (Met66 Carriers); EYO = Estimated Years to Symptom Onset; GDS = Geriatric Depression Scale; CDR = Clinical Dementia Rating Scale; MMSE = Mini Mental State Examination
Table 1b.
Demographic and clinical characteristics of CSF sample
| NC Val66/Val66 | NC Met66 | p | MC Val66/Val66 | MC Met66 | p | |
|---|---|---|---|---|---|---|
| N | 31 | 9 | 34 | 10 | ||
| N (%) Female | 21 (67.7%) | 3 (33.3%) | .064 | 21 (61.8%) | 6 (60.0%) | .835 |
| N(%)AP0E ε4 | 11 (35.5%) | 3 (33.3%) | .905 | 9 (26.5%) | 3 (30.0%) | .715 |
| N (%) Caucasian | 28 (90.3%) | 8 (88.9%) | .900 | 32 (94.1%) | 7 (70.0%) | .106 |
| NAPP/PS1/PS2 | - | - | - | 9/23/2 | 0/9/1 | .176 |
| Age | 42.14(10.31) | 43.42 (11.68) | .751 | 38.09 (10.00) | 38.93 (5.12) | .922 |
| EYO | −4.70 (8.32) | −0.58 (14.56) | .281 | −8.08 (8.73) | −7.27 (6.02) | .852 |
| Education | 15.23 (2.20) | 14.78 (2.99) | .623 | 14.24 (2.77) | 15.40 (3.63) | .267 |
| GDS | 0.84 (1.10) | 1.00 (1.00) | .695 | 1.68 (2.00) | 1.40 (1.26) | .665 |
| CDRsum of boxes | 0.00 (0.00) | 0.00 (0.00) | .999 | 0.01 (0.09) | 0.00 (0.00) | .588 |
| MMSE | 28.87 (1.48) | 29.00 (1.32) | .815 | 28.59 (1.54) | 29.30 (0.95) | .153 |
| Years Follow-Up | 3.92 (1.56) | 3.17 (1.55) | .217 | 2.21 (1.35) | 2.74 (0.99) | .303 |
| No. Assessments | 2.97(0.87) | 2.22 (0.44) | .019 | 2.50 (0.86) | 2.50 (0.97) | .878 |
Note: NC = Non-mutation Carriers; MC Val66/Val66 = CDR 0 DIAD Mutation Carriers (Val66 homozygotes); MC Met66 = CDR 0 DIAD Mutation Carriers (Met66 Carriers); EYO = Estimated Years to Symptom Onset; GDS = Geriatric Depression Scale; CDR = Clinical Dementia Rating Scale; MMSE = Mini Mental State Examination
All participants provided written informed consent. All study procedures were approved by the Washington University Human Research Protection Office and the local institutional review boards of each participating site.
Clinical Assessment
Without reference to performance on the neuropsychological tests, a clinician assessed each subject for the presence and severity of clinical symptoms of dementia at baseline (using the CDR scale, for which a CDR total score of 0 indicates cognitive normality).14 Subjects also completed the Mini Mental State Examination (MMSE) and the Geriatric Depression Scale (GDS) at baseline.
Neuropsychological Assessment
The DIAN neuropsychological test battery included the Wechsler Memory Scale–Revised Logical Memory (Story A only, immediate and delayed recall); Digit Symbol from the Wechsler Adult Intelligence Scale–Revised (WAIS–R); and immediate and delayed recall of a single presentation of a 16-item word list.15 These tasks, their standardisation and quality control, have been detailed previously and were administered according to standard protocols by trained research assistants.15
Outcome measures for each neuropsychological test were standardized against the baseline mean and standard deviation of non-mutation carriers. Standardized scores were then averaged to form a composite score for episodic memory (Logical Memory delayed recall, word list learning delayed recall) and for global cognition (Logical Memory delayed recall, word list learning delayed recall, Digit Symbol, MMSE).10
Genotyping
DNA sequencing to identify pathogenic mutations in APP, PSEN1, and PSEN2 was performed on DNA extracted from peripheral blood samples using methods described previously.16 Samples were also genotyped with the Infinium HumanExomeCore V1.0 Beadchip (Illumina, Inc). Genotyping was performed at The Genome Technology Access Center (GTAC; https://gtac.wustl.edu/) at Washington University. All samples and genotypes underwent stringent quality control (QC). Genotype data were cleaned by applying a minimum call rate for SNPs and individuals (98%). SNPs not in Hardy-Weinberg equilibrium (P< 1×10−6) were excluded. No SNPs were removed for low MAF. Gender identification was verified by analysis of X-chromosome SNPs. Unanticipated duplicates were identified using pairwise genome-wide estimates of proportion identity-by-descent using PLINK v1.9. Genotype data for the BDNF Val66Met (rs6265) polymorphism were extracted using PLINK. Clinicians were blinded to all genetic information and genetic polymorphisms were not used diagnostically.
Neuroimaging
DIAN neuroimaging protocols have been described previously.17, 18 Briefly, images from positron emission tomography (PET) using Pittsburgh compound B (PiB) (PiB-PET) were co-registered with individual MRI images for region-of-interest (ROI) determination. 3 Tesla volumetric T1-weighted MRI scans from DIAN subjects were acquired and processed through FreeSurfer 5.3 (Martinos Center, Boston, MA).17 Whole brain and hippocampal regions were automatically segmented. Volumetric measures were corrected for total intracranial volume. β-amyloid imaging was performed with a bolus injection of approximately 15 mCi of [11C] PiB. Dynamic imaging acquisition started either at injection for 70 minutes or 40 minutes post-injection for 30 minutes. For analysis, PiB-PET data between 40 to 70 minutes were used. For PiB-PET, total neocortical standardized uptake value ratio (SUVR) was used to determine levels of cortical Aβ deposition, using cerebellar grey matter as the reference region and applying partial volume correction using a regional point spread function.18
Biochemical Analysis
Fasted CSF was collected in the morning via lumbar puncture. Samples were shipped on dry ice to the DIAN Biomarker Core laboratory. CSF concentrations of Aβ42, total tau, and tau phosphorylated at threonine 181 (ptau181) were measured by immunoassay (AlzBio3, Fujirebio [formerly Innogenetics, Ghent, Belgium]). All values met quality-control standards, including a coefficient of variation of 25% or less, kit “controls” within the expected range as defined by the manufacturer, and measurement consistency between plates of a common sample that was included in each run. All samples were run on a single assay lot number, with serial samples from a given individual run on the same assay plate.
Estimated year to symptom onset
The estimated year to expected symptom onset (EYO) was calculated as the age of the participant at the time of the baseline assessment minus the mean age of symptom onset of all other individuals with the same mutation type.5 Analyses were also repeated using parental EYO, defined as an individual’s age minus the age of symptom onset of that individual’s parent.5
Data Analysis
All analyses were conducted in the statistical program R v3.5.0, using the following packages: “ggplot2”, “psych”, “lme4” and “lmerTest”. Data was analysed separately for preclinical mutation carriers and non-mutation carriers.
First, a series of t-tests were conducted to determine whether there were differences on any demographic or mood variables between Met66 carriers and Val66 homozygotes at baseline (Table 1). Any demographic or mood variable that were different between groups were then included as a covariate in subsequent analyses. While the number of subjects who identified as Caucasian or non-Caucasian were not different between Met66 carriers and Val66 homozygotes (in either preclinical mutation carriers or non-mutation carriers), we included race as a as a covariate in all analyses as there is a wide variation in the population prevalence of the Met66 allele in different races.
In preclinical mutation carriers, we conducted a series of linear mixed models with an unstructured covariance matrix, with each outcome measure (cognition, brain volume, tau, Aβ) as the dependent variable, and interactions between BDNF group (Met66 carriers vs Val66 homozygotes) x EYO (defined as continuous time-dependent variable) specified as fixed factors. EYO and subjects were included as random factors. Besides race, no other covariates were included in the model as no demographic characteristics differed between groups (Table 1). Further, as we have shown that APOE ε4 does not influence disease progression in DIAD5 and does not interact with BDNF to influence cognitive or biomarker outcomes in DIAD,10 APOE ε4 status was not included as a term in these statistical models.
In Aβ negative non-mutation carriers, we conducted a series of linear mixed models with an unstructured covariance matrix, with each outcome measure (cognition, brain volume, tau, Aβ) as the dependent variable, and interactions between BDNF group (Met66 carriers vs. Val66 homozygotes) x subjects’ age (defined as continuous time-dependent variable) specified as fixed factors. EYO and subjects were included as random factors. As Met66 carriers showed higher levels of depressive symptoms than Val66 homozygotes (Table 1), depressive symptom scores were included in the model as a covariate. We also included APOE ε4 status as a covariate in these analyses as it presents an increased risk to age-related cognitive decline.
For all analyses, the statistical significance for comparisons was set at p < .05. Analyses were not adjusted for multiple comparisons because this is a novel area of experimental investigation that provides an important hypothesis to be tested, and the outcome measures are highly correlated. Furthermore, for each comparison, measures of effect sizes were used to quantify the magnitude of difference in rates of change between groups, where effect sizes <0.2 classified as trivial and not interpreted regardless of their statistical significance, thus reducing the likelihood of Type I error. Within the preclinical mutation carrier group, the reference group for effect size calculation were Val66 homozygotes; similarly, within non-mutation carriers, the reference group for effect size calculation were Val66 homozygotes. We extracted slope estimate and standard error for each group through the mixed effects model. Cohen’s d was determined by calculating the mean difference between groups and dividing the result by the pooled standard deviation.
Results
Demographic and clinical characteristics
In preclinical mutation carriers, EYO did not differ significantly between Val66 homozygotes and Met66 carriers. In non-mutation carriers, age did not differ significantly between Val66 homozygotes and Met66 carriers. Preclinical mutation carriers and mutation non-carriers did not differ on any other demographic characteristic.
Effect of BDNF Val66Met on rates of cognitive decline and brain volume loss in preclinical mutation carriers
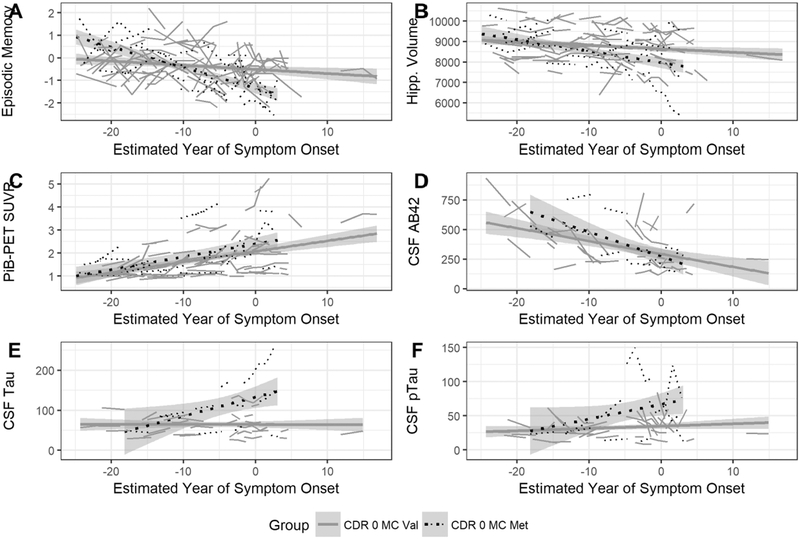
In preclinical mutation carriers, decline in episodic memory (Figure 1A) and global cognition were significantly greater in Met66 carriers compared to Val66 homozygotes (Table 2a), with the magnitude of difference, by convention, large (d>0.8; Table 2b). Similarly, when compared to Val66 homozygotes, Met66 carriers also showed significantly faster rates of hippocampal volume loss (Figure 1B), but not greater reduction in precuneus thickness (Table 2a). These results were unchanged with parental EYO treated as the continuous time-dependent variable.
Figure 1.
Rate of episodic memory decline (A), hippocampal volume loss (B), cortical Aβ accumulation (C), decreases in CSF Aβ42 (D), increases in CSF tau (E) and increases in CSF ptau181 (F), with increasing estimated years to symptom onset in preclinical DIAD mutation carriers who are Val66 homozygotes and Met66 carriers.
Table 2a.
Effect of BDNF Val66Met on each cognitive and biomarker outcome measure over time.
| BDNFVal66Met | EYO | BDNFVal66Met × EYO | ||||
|---|---|---|---|---|---|---|
| Estimate (SE) | p | Estimate (SE) | p | Estimate (SE) | p | |
| Episodic Memory | −0.579 (0.218) | .009 | −0.020 (0.008) | .019 | −0.055 (0.016) | .0006 |
| PACC | −0.710 (0.226) | .002 | −0.022 (0.009) | .012 | −0.070 (0.016) |
3.14×10−5 |
| Hippocampal volume | −531.664 (237.430) | .027 | −22.780 (6.021) | .005 | −31.520 (15.818) | .037 |
| Precuneus Thickness | −0.062 (0.082) | .451 | −0.019 (0.003) | 9.65×10−10 | −0.002 (0.006) | .718 |
| CSFtau | 65.085 (15.294) | .0001 | 0.527 (0.577) | .363 | 5.453 (1.345) | .0001 |
| CSFptau181 | 39.626 (12.023) | .002 | 0.262 (0.383) | .497 | 2.136 (0.990) | .021 |
| PiB-PET SUVR | 0.263 (0.260) | .313 | 0.041 (0.007) | 1.91×10−7 | 0.008 (0.013) | .549 |
| CSF Aβ42 | −9.992 (81.416) | .903 | −11.787 (3.599) | .003 | −1.957 (8.139) | .811 |
Note: All models have been adjusted for race; CSF = Cerebrospinal fluid; EYO = Estimated Years to Symptom Onset; PACC = Preclinical Alzheimer Cognitive Composite; PT = Precuneus Thickness; PiB-PET SUVR = Positron Emission Tomography (using Pittsburgh Compound B) Standardized Uptake Value Ratio
Table 2b.
Mean estimate (standard error) rate of change of each cognitive and biomarker outcome measure for preclinical DIAD mutation carriers who are Val66 homozygotes and preclinical DIAD mutation carriers who are Met66 carriers.
| Preclinical MC Val66 homozygotes | Preclinical MC Met66 carriers | Cohens’ d | |||||
|---|---|---|---|---|---|---|---|
| Estimate (SE) | p | N | Estimate (SE) | p | N | ||
| Episodic Memory | −0.020 (0.008) | .019 | 77 | −0.076 (0.012) | 9.82×10−8 | 34 | 0.80 (0.38,1.21) |
| PACC | −0.022 (0.009) | .012 | 77 | −0.092 (0.014) | 4.05×10−10 | 34 | 0.88 (0.45,1.29) |
| Hippocampal Volume | −22.780 (6.021) | .005 | 75 | −54.300 (13.570) | 8.69×10−5 | 33 | 0.52 (0.10,0.93) |
| Precuneus Thickness | −0.019 (0.003) | 9.65×10−10 | 75 | −0.021 (0.004) | 3.21×10−5 | 33 | 0.08 (−0.33, 0.49) |
| CSF tau | 0.527 (0.577) | .363 | 34 | 5.980 (1.222) | 4.03×10−6 | 10 | 1.57 (0.76,2.31) |
| CSF ptau181 | 0.262 (0.383) | .497 | 34 | 2.398 (0.912) | .011 | 10 | 0.89 (0.15,1.61) |
| PiB-PET SUVR | 0.041 (0.007) | 1.91×10−7 | 73 | 0.049 (0.011) | 3.35×10−5 | 30 | 0.13 (−0.29, 0.56) |
| CSF Aβ42 | −11.787 (3.599) | .003 | 34 | −13.745 (7.353) | .070 | 10 | 0.09 (−0.79, 0.62) |
Note: All estimates have been adjusted for race; CSF = Cerebrospinal fluid; MC = Mutation Carrier; PACC = Preclinical Alzheimer Cognitive Composite; SE = Standard Error; PiB-PET SUVR = Positron Emission Tomography (using Pittsburgh Compound B) Standardized Uptake Value Ratio
Effect of BDNF Val66Met on rate of change in Aβ and tau in preclinical mutation carriers
In preclinical mutation carriers, Met66 carriers and Val66 homozygotes showed equivalent rates of cortical Aβ accumulation and decreases in CSF Aβ42 (Table 2a; Figure 1C, 1D), with the difference in rates of change for both Aβ biomarkers non-significant and small in magnitude (d=0.1; Table 2b). However, compared to Val66 homozygotes, Met66 carriers showed significantly greater increases over time in CSF tau and ptau181 (Table 2a; Figure 1E, 1F), with these differences large in magnitude (d’s~1; Table 2b). These results were unchanged when parental EYO was used.
As some have reported sex-specific effects of BDNF Val66Met previously,19 we repeated our analyses on the main outcome measures (episodic memory, hippocampal volume, CSF tau and SUVR) accounting for the effects of sex. Our results remained unchanged, and participants’ sex was not related to any aspect of memory, neuroimaging or biomarker change, nor did it interact with BDNF to influence change in any of these clinical disease markers (Supplementary Table 1).
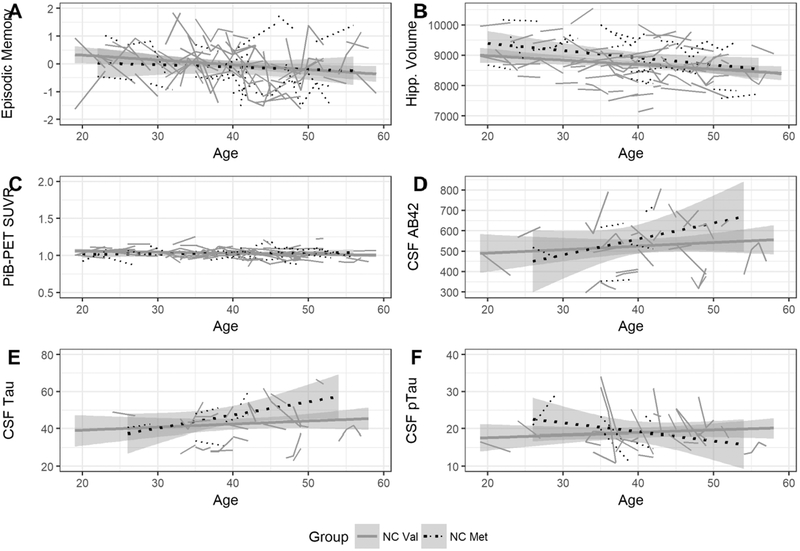
No effect of BDNF Val66Met on rates of change in cognition, brain volume, tau and Aβ in mutation non-carriers who do not have amyloidosis
In β-amyloid-negative non-mutation carriers, there was no effect of BDNF Val66Met on rates of change in cognition, brain volume, CSF tau (and CSF ptau181) and Aβ (cortical Aβ and CSF Aβ42) as a function of increasing age (Table 3a, Figure 2). Mean estimates (and standard error) of change for Val66 homozygotes and Met66 carriers are provided in Table 3b.
Table 3a.
Effect of BDNF Val66Met on each cognitive and biomarker outcome measure over time.
| BDNF | Age | BDNF × Age | ||||
|---|---|---|---|---|---|---|
| Estimate (SE) | p | Estimate (SE) | p | Estimate (SE) | p | |
| Episodic Memory | −0.344(0.673) | .610 | −0.015 (0.008) | .069 | 0.010(0.016) | .560 |
| PACC | −0.132(0.527) | .803 | −0.014(0.006) | .037 | 0.002(0.013) | .855 |
| Hippocampal volume | −8.747 (503.814) | .986 | −18.949 (6.155) | .002 | 4.439 (12.437) | .722 |
| Precuneus thickness | 0.126(0.187) | .503 | −0.009 (0.002) | 9.42 ×10−5 | −0.005 (0.005) | .256 |
| CSF tau | −6.534 (21.802) | .765 | 0.352(0.236) | .141 | 0.156(0.500) | .755 |
| CSF ptau181 | 10.109(10.064) | .321 | 0.152(0.106) | .162 | −0.241 (0.234) | .308 |
| PiB-PET SUVR | −0.036(0.070) | .604 | −0.001 (0.001) | .330 | 0.001(0.002) | .710 |
| CSF Aβ42 | −166.661 (257.338) | .408 | −0.454 (2.716) | .868 | 5.244(6.285) | .408 |
Note: All models have been adjusted for APOE ε4, depressive levels and race; CSF = Cerebrospinal fluid; EYO = Estimated Years to Symptom Onset; PACC = Preclinical Alzheimer Cognitive Composite; PiB-PET SUVR = Positron Emission Tomography (using Pittsburgh Compound B) Standardized Uptake Value Ratio
Figure 2.
Rate of episodic memory decline (A), hippocampal volume loss (B), cortical Aβ accumulation (C), decreases in CSF Aβ42 (D), increases in CSF tau (E) and increases in CSF ptau181 (F), with increasing age in healthy Aβ negative non-mutation carriers, who are Val66 homozygotes and Met66 carriers.
Table 3b.
Mean estimate (standard error) rate of change of each cognitive and biomarker outcome measure for non-mutation carriers who are Val66 homozygotes and non-mutation carriers who are Met66 carriers.
| NC Val66 homozygotes | NC Met66 carriers | Cohens’ d | |||||
|---|---|---|---|---|---|---|---|
| Estimate (SE) | p | N | Estimate (SE) | p | N | ||
| Episodic Memory | −0.015 (0.008) | .069 | 72 | −0.009 (0.016) | .594 | 29 | 0.08 (−0.35, 0.51) |
| PACC | −0.014(0.006) | .037 | 72 | −0.011 (0.012) | .374 | 29 | 0.05 (−0.38, 0.49) |
| Hippocampal Volume | −18.949 (6.155) | .002 | 71 | −15.332 (11.340) | .178 | 26 | 0.07 (−0.38, 0.52) |
| Precuneus Thickness | −0.009 (0.002) | 9.42 ×10−5 | 71 | −0.015 (0.004) | .0004 | 26 | 0.34 (−0.12, 0.79) |
| CSF tau | 0.352(0.236) | .141 | 31 | 0.614 (0.460) | .187 | 9 | 0.20 (−0.55, 0.94) |
| CSF ptau181 | 0.152(0.106) | .162 | 31 | −0.185 (0.194) | .344 | 9 | 0.57 (−0.19,1.31) |
| PiB-PET SUVR | −0.001 (0.001) | .330 | 71 | −0.0003(0.0015) | .858 | 23 | 0.09 (−0.39, 0.56) |
| CSF Aβ42 | −0.454 (2.716) | .868 | 31 | 4.790 (5.638) | .399 | 9 | 0.34 (−0.41,1.08) |
Note: All estimates have been adjusted for APOE ε4, depressive levels and race; CSF = Cerebrospinal fluid; NC = Non-mutation Carrier; PACC = Preclinical Alzheimer Cognitive Composite; SE = Standard Error; PiBPET SUVR = Positron Emission Tomography (using Pittsburgh Compound B) Standardized Uptake Value Ratio
Among β-amyloid-negative mutation non-carriers, Val66 homozygotes showed no significant deterioration in cognition or hippocampal volume loss compared to Met66 carriers (Table 3a; Figure 2A–B), with all group differences small (d=0.20; Table 3b). Similarly, in β-amyloid-negative mutation non-carriers, there were no significant differences between Val66 homozygotes and Met66 carriers on the rate of change in CSF tau, CSF ptau181, and Aβ (cortical Aβ and CSF Aβ42) (Table 3a; Figure 2C–F).
Discussion
The results of this study show that the phenotypic expression of DIAD mutations is influenced by the BDNF Val66Met polymorphism in that carriage of a Met66 allele hastens the onset of AD symptoms through the acceleration of Aβ+ related tau accumulation and neurodegeneration. Preclinical mutation carriers who carried the Met66 allele showed faster decline in episodic memory, accompanied by greater loss of hippocampal volume compared to Val66 homozygotes (Figure 1A–B). This finding is consistent with, and extends, our previous cross-sectional observation that in preclinical DIAD mutation carriers, Met66 carriers show worse memory performance and lower hippocampal glucose metabolic activity than Val66 homozygotes.10 These results also accord with observations in preclinical and prodromal sporadic AD, where β-amyloid-positive Met66 carriers also showed faster cognitive decline and hippocampal volume loss when compared to β-amyloid-positive Val66 homozygotes.6–9
Our data also show for the first time that in preclinical mutation carriers, Met66 carriers have a faster (i.e., 5 and 7 times) rate of increase in CSF tau and ptau181 than Val66 homozygotes. (Figure 1E–F, Table 2b). This is consistent with, and extends our previous cross-sectional observation that in preclinical DIAD mutation carriers, CSF tau and ptau181 levels were 25% higher in Met66 carriers than in Val66 homozygotes.10 In preclinical mutation carriers who were Val66 homozygotes, levels of CSF tau and ptau181 were higher than those in non-mutation carriers, but interestingly, no increase in CSF tau or ptau181 levels were observed over the mean follow-up period of 3 years (Table 2b). In contrast, cortical Aβ accumulation, as measured by PiB-PET and decreases in CSF Aβ42 levels, in preclinical mutation carriers were unrelated to allelic variation in the BDNF Val66Met polymorphism (Figure 1C–D, Table 2b).
This dissociation between the rate of increase in CSF tau and the rate of cortical Aβ accumulation, and the development of clinical symptoms in DIAD, raises the hypothesis that in preclinical mutation carriers, increased rate of cognitive decline and hippocampal volume loss observed in Met66 carriers is a consequence of biological processes associated with increasing CSF tau and ptau181, most likely reflecting neuronal injury and presence of neurofibrillary tangles.
Finally, we confirmed that β-amyloid positivity is necessary for the cognitive decline, neurodegeneration and tau accumulation associated with Met66 carriers. In age-matched mutation non-carriers who showed no evidence of amyloidosis, rates of cognitive decline, hippocampal volume loss, increases in CSF tau and ptau181, and cortical Aβ accumulation did not change with increasing age for either Met66 carriers or Val66 homozygotes (Figure 2). This is consistent with observations by us,6–8 and others9 that BDNF Val66Met has no effect on cognitive decline or hippocampal volume loss in β-amyloid-negative adults. The necessity of β-amyloid positivity for cognitive decline in Met66 carriers led us to propose that equivocal findings on the effect of BDNF Val66Met on cognitive decline observed in previous studies,20 is likely due to their not accounting for the presence of β-amyloid positivity in their samples. Recently, in a large group of middle-aged adults, Met66 carriers showed faster cognitive decline than Val66 homozygotes.9 However, when Aβ levels were considered, only β-amyloid-positive Met66 carriers showed increased cognitive decline when compared to matched β-amyloid-negative controls.9 Thus, our current results, and that of previous studies,6–10 indicate that while Met66 is unrelated to accumulation of Aβ itself, β-amyloid positivity is necessary for cognitive decline and hippocampal volume loss in Met66 carriers.
The finding that in preclinical DIAD mutation carriers, the presence of at least one copy of the BDNF Met66 allele increased the rate of cognitive decline, hippocampal volume loss, as well as the rate of increase in CSF tau and ptau181 indicates that Met66 acts to accelerate symptom onset early in DIAD. This is consistent with and extends our previous cross-sectional findings that the Met66 allele is important for the timing of cognitive decline in DIAD.10 It also accords with studies of preclinical sporadic AD in different samples, where the Met66 allele is associated with increased cognitive decline and brain volume loss.6–9 The effect of the Met66 allele on the clinical expression of DIAD is important as the rate of cognitive decline in Met66 carriers was ~4 times faster, and the rate of hippocampal volume loss was ~16 times faster than in Val66 homozygotes. Additionally, Met66 carriers showed increases in levels of CSF tau and ptau181 that were ~6 times greater than those observed in Val66 homozygotes.
The effects of the BDNF Met66 allele on AD clinical markers and biomarkers in this study also illustrate an important dissociation between the effects of tau and Aβ on the clinical manifestation of AD pathophysiology. While it is generally agreed that both Aβ and tau accumulation are necessary for the development of AD dementia,21 the processes by which these aggregating proteins interact to influence clinical disease progression remain unclear.22 It is well-established that a DIAD mutation results in a rapid increase in Aβ accumulation, neurodegeneration, and cognitive decline.1, 5 However, the finding that the Met66 allele is associated with faster cognitive decline and hippocampal volume loss, and with increases in CSF tau, but is not associated with cortical Aβ accumulation, suggests strongly that the deleterious effects of tau on neurons occurs downstream of Aβ positivity. Furthermore, our data suggest that the effect of Aβ positivity on neurodegeneration and cognitive decline is crucially linked to increasing CSF tau and ptau181.
While the current findings are consistent with the proposed temporal sequence of AD biomarker changes and their clinical manifestation,23 it remains unclear how Met66 intersects these models to increase the rate of change in CSF tau and ptau181. For example, Met66 may be associated with lower levels of CNS BDNF, which may facilitate increases in tau, which in turn accelerate neurodegeneration. However, it is also possible that lower levels of CNS BDNF allow faster Aβ positive related neurodegeneration, of which increased tau is a consequence. It is suggested commonly that the clinical manifestation of AD is related more strongly to tau rather than to Aβ.24 The current data suggests this statement is too simple. Rather, while early clinical presentation of AD is related strongly to increasing CSF tau and ptau181, this is only in the presence of β-amyloid positivity.
Current in vitro models show the expression of human tau and BDNF levels are inversely related, even prior to the formation of neurofibrillary tangles. However, these models propose that it is tau that down-regulates BDNF expression.25 If the faster cognitive decline and neurodegeneration in Met66 carriers observed here were a consequence of greater down-regulation of BDNF by tau, then increases in CSF tau should be equivalent in preclinical mutation carriers who are Met66 carriers and Val66 homozygotes. The specific association between Met66 and increasing CSF tau observed here suggests that increases in CSF tau were a consequence, rather than a cause, of reduced CNS BDNF in humans. This suggestion is supported by our observation that in the absence of β-amyloid positivity (i.e., in non-mutation carriers), carriage of the Met66 allele did not influence tau levels (Figure 2). It is also consistent with findings that reduced BDNF in cells slows the de-phosphorylation of tau through slowing TrkB activation,26 and that in AD post-mortem samples, BDNF loss is specific to tangle-bearing neurons.27 As the Met66 allele is associated with lower expression of CNS BDNF, the large effect of the BDNF Met66 allele on CSF tau, cognitive decline and neurodegeneration in the preclinical DIAD mutation carriers also accords with the importance of CNS BDNF in synaptic excitation, long-term potentiation and neuronal plasticity.28, 29 It is also consistent with studies using AD rodent models which show that BDNF mRNA is reduced substantially in the hippocampus and temporal lobe,28–30 with the extent of BDNF loss associated with the magnitude of cognitive impairment.28, 30, 31 Further, post-mortem analysis of BDNF RNA in AD patients suggest that low CNS BDNF expression is associated with increased cognitive decline before death.31
Another pathway through which BDNF may potentially operate to influence rates of neurodegeneration and cognitive decline is by responding to inflammatory processes early in the AD course.13, 32 For example, BDNF production by astrocytes increases to counteract the deleterious effects of Aβ,32 and this is posited to represent an attempt to rescue or buffer neurons against AD pathogenesis.13 Additionally, administration of pioglitazone significantly restored BDNF levels and attenuated inflammatory markers in mice treated with intracerebroventricular Aβ.33 Thus, it is possible that lower levels of CNS BDNF in Met66 carriers may increase susceptibility to Aβ and tau toxicity by moderating inflammation. Future studies could test this hypothesis in humans by determining whether inflammatory markers are related to allelic variation in BDNF Val66Met in a manner similar to that observed for CSF tau, brain volume loss and cognitive decline.
Generalizations of the results from this study should take into account the relatively small sample size, and the short follow-up period (~3.5 years). It will be important for these findings to be replicated in future studies of DIAD mutation carriers over longer periods of follow-up. Given the complete penetrance of DIAD, we expect that if followed over longer periods, MC Val66 homozygotes will start to show cognitive decline arising from neurodegeneration and this will also be associated with increasing levels of CSF tau and ptau181, albeit at an older age. Nonetheless, this study demonstrates that the deleterious effects of Aβ in DIAD were increased in preclinical mutation carriers who carried the BDNF Met66 allele. Another avenue of future investigation will be the relationship between peripheral markers of BDNF and increases in tau, brain volume loss and cognitive decline. While some studies observe CSF and plasma levels of BDNF to be reduced in sporadic AD, this finding is not consistent and the extent to which such changes relate to clinical disease markers has not been established.34, 35 Taken together, the results of this study provide more evidence that common biological factors influence the development of dementia in DIAD and sporadic AD. The strength and consistency of our results with those in sporadic AD also suggest that increasing CNS BDNF levels may be a viable therapeutic strategy for reducing the effects of amyloidosis on disease progression. Our results also suggest strongly that the BDNF Val66Met polymorphism should be considered as a potential moderator of clinical trial outcomes in current treatment and prevention trials in DIAD and sporadic AD.
Supplementary Tables
Acknowledgements
Data collection and sharing for this project was supported by The Dominantly Inherited Alzheimer’s Network (DIAN, U19AG032438) funded by the National Institute on Aging (NIA), the German Center for Neurodegenerative Diseases (DZNE), Raul Carrea Institute for Neurological Research (FLENI), Partial support by the Research and Development Grants for Dementia from Japan Agency for Medical Research and Development, AMED, and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI). This manuscript has been reviewed by DIAN Study investigators for scientific content and consistency of data interpretation with previous DIAN Study publications.
YYL is supported by the National Health and Medical Research Council (GNT1111603, GNT 1147465).
We acknowledge the altruism of the participants and their families and contributions of the DIAN research and support staff at each of the participating sites for their contributions to this study.
Footnotes
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1002/ana.25299
Potential Conflicts of Interest
Nothing to report.
References
- 1.Bateman RJ, Xiong C, Benzinger TLS, et al. Clinical and biomarker changes in Dominantly Inherited Alzheimer’s disease. The New England Journal of Medicine 2012;367:795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleisher AS, Chen K, Quiroz YT, et al. Florbetapir PET analysis of amyloid-β deposition in the presenilin 1 E280A autosomal dominant Alzheimer’s disease kindred: a cross-sectional study. Lancet Neurology 2012;11:1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bateman RJ, Aisen PS, De Strooper B, et al. Autosomal-dominant Alzheimer’s disease: A review and proposal for the prevention of Alzheimer’s disease. Alzheimer’s Research & Therapy 2011;3:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang F, Gordon BA, Ryman DC, et al. Cerebral amyloidosis associated with cognitive decline in autosomal dominant Alzheimer disease. Neurology 2015;85:790–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryman DC, Acosta-Baena N, Aisen PS, et al. Symptom onset in autosomal dominant Alzheimer disease: A systematic review and meta-analysis. Neurology 2014;85:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim YY, Villemagne VL, Laws SM, et al. BDNF Val66Met, Aβ amyloid and cognitive decline in preclinical Alzheimer’s disease. Neurobiology of Aging 2013;34:2457–2464. [DOI] [PubMed] [Google Scholar]
- 7.Lim YY, Villemagne VL, Laws SM, et al. BDNF Val66Met moderates Aβ-related memory decline and hippocampal atrophy in prodromal Alzheimer’s disease: A preliminary study. PLoS One 2014;9:e86498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim YY, Villemagne VL, Laws SM, et al. APOE and BDNF polymorphisms moderate amyloid β-related cognitive decline in preclinical Alzheimer’s disease. Molecular Psychiatry 2015;20:1322–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boots EA, Schultz SA, Clark LR, et al. BDNF Val66Met predicts cognitive decline in the Wisconsin Registry for Alzheimer’s Prevention. Neurology 2017;88:2098–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim YY, Hassenstab J, Cruchaga C, et al. BDNF Val66Met moderates memory impairment, hippocampal function and tau in preclinical autosomal dominant Alzheimer’s disease. Brain 2016;139:2766–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizui T, Ishikawa Y, Kumanogoh H, et al. BDNF pro-peptide actions facilitate hippocampal LTD and are altered by the common BDNF polymorphism Val66Met. Proceedings of the National Academy of Sciences of the United States of America 2015;112:E3067–3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ieraci A, A.I. M, Mallei A, Lee FS, Popoli M. Brain-derived neurotrophic factor Val66Met human polymorphism impairs the beneficial exercise-induced neurobiological changes in mice. Neuropsychopharmacology 2016;41:3070–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faria M, Gonçalves G, Rocha N, et al. Increased plasma levels of BDNF and inflammatory markers in Alzheimer’s disease. Journal of Psychiatric Research 2014;53:166–172. [DOI] [PubMed] [Google Scholar]
- 14.Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 1983;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 15.Storandt M, Balota DA, Aschenbrenner AJ, Morris JC. Clinical and psychological characteristics of the initial cohort of the Dominantly Inherited Alzheimer Network (DIAN). Neuropsychology 2014;28:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talbot C, Lendon C, Craddock N, Shears S, Morris JC, Goate A. Protection against Alzheimer’s disease with apoE epsilon 2. Lancet 1994;343:1432–1433. [DOI] [PubMed] [Google Scholar]
- 17.Benzinger TL, Blazey T, Jack CR, et al. Regional variability of imaging biomarkers in autosomal dominant Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America 2013;110:E4502–E4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su Y, Blazey TM, Snyder AZ, et al. Partial volume correction in quantitative amyloid imaging. NeuroImage 2015;107:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watts A, Andrews SJ, Anstey KJ. Sex Differences in the Impact of BDNF Genotype on the Longitudinal Relationship between Physical Activity and Cognitive Performance. Gerontology 2018;doi: 10.1159/000486369. [DOI] [PubMed] [Google Scholar]
- 20.Mandelman SD, Grigorenko EL. BDNF Val66Met and cognition: All, none or some? A meta-analysis of the genetic association. Genes, Brain and Behavior 2012;11:127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han SD, Gruhl J, Beckett L, et al. Beta Amyloid, Tau, Neuroimaging, and Cognition: Sequence Modeling of Biomarkers for Alzheimer’s Disease. Brain Imaging and Behavior 2012;6:610–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tosun D, Landau S, Aisen PS, et al. Association between tau deposition and antecedent amyloid-B accumulation rates in normal and early symptomatic individuals. Brain 2017;140:1499–1512. [DOI] [PubMed] [Google Scholar]
- 23.Jack CR, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurology 2010;9:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ittner LM, Götz J. Amyloid-β and tau - a toxic pas de deux in Alzheimer’s disease. Nature Reviews, Neuroscience 2011;12:65–72. [DOI] [PubMed] [Google Scholar]
- 25.Rosa E, Mahendram S, Ke YD, Ittner LM, Ginsberg SD, Fahnestock M. Tau downregulates BDNF expression in animal and cellular models of Alzheimer’s disease. Neurobiology of Aging 2016;48:135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elliott E, Atlas R, Lange A, Ginzburg I. Brain-derived neurotrophic factor induces a rapid dephosphorylation of tau protein through a PI-3 kinase signalling mechanism. European Journal of Neuroscience 2005;22:1081–1089. [DOI] [PubMed] [Google Scholar]
- 27.Ferrer I, Marín C, Rey MJ, et al. BDNF and full-length and truncated TrkB expression in Alzheimer disease: Implications in therapeutic strategies. Journal of Neuropathology and Experimental Neurology 1999;58:729–739. [DOI] [PubMed] [Google Scholar]
- 28.Garzon DJ, Fahnestock M. Oligomeric amyloid decreases basal levels of brain-derived neurotrophic factor (BDNF) mRNA via specific downregulation of BDNF transcripts IV and V in differentiated human neuroblastoma cells. The Journal of Neuroscience 2007;27:2628–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee ST, Chu K, Jung KH, et al. miR-206 regulates brain-derived neurotrophic factor in Alzheimer disease model. Annals of Neurology 2012;72:269–277. [DOI] [PubMed] [Google Scholar]
- 30.Peng S, Wuu J, Mufson EJ, Fahnestock M. Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the preclinical stages of Alzheimer’s disease. Journal of Neurochemistry 2005;93:1412–1421. [DOI] [PubMed] [Google Scholar]
- 31.Buchman AS, Yu L, Boyle PA, Schneider JA, De Jager PL, Bennett DA. Higher brain BDNF gene expression is associated with slower cognitive decline in older adults. Neurology 2016;86:735–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kimura N, Takahashi M, Tashiro T, Terao K. Amyloid beta up-regulates brain-derived neurotrophic factor production from astrocytes: rescue from amyloid beta-related neuritic degeneration. Journal of Neuroscience Research 2006;84:782–789. [DOI] [PubMed] [Google Scholar]
- 33.Prakash A, Kumar A. Role of nuclear receptor on regulation of BDNF and neuroinflammation in hippocampus of β-amyloid animal model of Alzheimer’s disease. Neurotoxicity Research 2014;25:335–347. [DOI] [PubMed] [Google Scholar]
- 34.Qin X, Cao C, Cawley N, et al. Decreased peripheral brain-derived neurotrophic factor levels in Alzheimer’s disease: A meta-analysis study (N=7277). Molecular Psychiatry 2017;22:312–320. [DOI] [PubMed] [Google Scholar]
- 35.Lim YY, Rainey-Smith S, Lim Y, et al. BDNF Val66Met in preclinical Alzheimer’s disease is associated with short-term changes in episodic memory and hippocampal volume but not serum mBDNF. Int Psychogeriatr 2017;29:1825–1834. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.