Abstract
Background
Prosthesis rehabilitation after dysvascular transtibial amputation (TTA) is focused on optimizing functional capacity with limited emphasis on promoting health self-efficacy. Self-efficacy interventions decrease disability for people living with chronic disease, but the influence of self-efficacy on disability is unknown for people with dysvascular TTA.
Objectives
To identify if self-efficacy mediates the relationship between self-reported functional capacity and disability after dysvascular TTA.
Design
Cross-sectional, secondary data analysis.
Setting
Outpatient rehabilitation facilities.
Participants
Thirty-eight men (63.6 ± 9.1 years old) with dysvascular TTA.
Methods
Participants had been living with an amputation for less than 6 months and using walking as their primary form of locomotion using a prosthesis. The independent variable, functional capacity, was measured using the Prosthesis Evaluation Questionnaire-Mobility Scale (PEQ-MS). The proposed mediator, self-efficacy, was measured with the Self-Efficacy of Managing Chronic Disease questionnaire (SEMCD).
Main outcome measure
Disability was measured using the World Health Organization Disability Assessment Schedule 2.0 (WHODAS 2.0) questionnaire.
Results
The relationship between self-reported functional capacity and disability is partially mediated by self-efficacy. Relationships between WHODAS 2.0 and PEQ-MS (r = −0.61), WHODAS 2.0 and SEMCD (r = −0.51), and PEQ-MS and SEMCD (r = 0.44) were significant (P < .01). Controlling for SEMCD (P = .04), the relationship between PEQ-MS and WHODAS 2.0 remained significant (P < .01). Statistically significant mediation was determined by a bootstrap method for the product of coefficients (95% CI: −2.23, −7.39).
Conclusions
This study provides initial evidence that the relationship between self-reported functional capacity and disability is partially mediated by self-efficacy after dysvascular TTA. The longitudinal effect of self-efficacy should be further examined to identify causal pathways of disability after dysvascular amputation. Furthermore, additional factors contributing to the relationship between self-reported functional capacity and disability need to be identified.
Keywords: Dysvascular Amputation, Self-efficacy, Physical Function, Complex Conditions, Disability
Introduction
The majority of lower limb amputations are due to dysvascular etiologies including peripheral artery disease (PAD) and diabetes mellitus (DM).1 Rehabilitation outcomes following dysvascular amputation are poor, with people experiencing greater disability than 95% of the general population.2 Severe disability may be related to the etiology of amputation and presence of high comorbidity burden.1,3 Current prosthesis rehabilitation, regardless of etiology, is focused on maximizing functional capacity in the first year after amputation.4 Despite improvements in performance and self-reported functional capacity after prosthesis rehabilitation,5 people with dysvascular transtibial amputation (TTA) continue to report dissatisfaction with mobility,6 have significant limitations in their ability to ambulate in the community,7 and achieve 3 times fewer than the recommended 7,100 steps/day for people with disabilities.8,9
Self-efficacy, the belief in one’s ability to execute a given behavior, may account for differences in disability status following dysvascular TTA and has been studied in various chronic conditions.10,11 Effective behavior-based interventions use strategies (e.g., patient-directed goal setting, problem solving, self-monitoring during exercise) to specifically target improved self-efficacy.12–15 For example, a group-mediated behavior-based intervention focused on enhancing self-efficacy among people with PAD increased participation in everyday physical activity, distance of walking in six minutes, and pain-free walking distance at six months.16 Furthermore, self-efficacy mediates the effects of chronic pain perception and morbidity on disability outcomes for older adults.17,18
The extent to which self-efficacy attenuates disability outcomes after dysvascular TTA has not been established. Understanding the relationships between perceived functional capacity, patient self-efficacy, and disability is needed to inform the development of more effective rehabilitation interventions aimed at reducing long-term disability in an understudied, medically complex population with low odds of rehabilitation success. Therefore, the purpose of this study was twofold: 1) describe the relationships between perceived functional capacity, self-efficacy, and disability and 2) identify if self-efficacy mediates the relationship between self-reported functional capacity and disability after dysvascular TTA.
Materials/Methods
Participants
Baseline data from a randomized clinical trial aimed at improving rehabilitation outcomes after dysvascular transtibial amputation (TTA) were analyzed for the purposes of this study. Data for this analysis were collected by a physical therapist during a baseline test, before clinical trial randomization and intervention. Briefly, participants were recruited from outpatient rehabilitation clinics throughout the front range of Colorado and the Veterans Affairs Eastern Colorado Health Care System. Individuals between 50 and 85 years of age were included in the study if they had a dysvascular TTA less than 6 months prior to enrollment and were walking independently using a prosthesis with or without an assistive device. Individuals were excluded if they used a wheelchair as a primary form of locomotion, their amputation was due to cancer or trauma, or they had unstable cardiac, neurologic, or orthopedic conditions. The study protocol was approved by the Colorado Multiple Institutional Review Board and Veterans Affairs Research and Development office.
Variables
Variables were selected due to hypothesized relationships among self-reported functional capacity, disability, and self-efficacy based on the International Classification of Functioning and Social Cognitive theory (Figure 1). More specifically, we hypothesized that greater self-reported functional capacity would be associated with greater self-efficacy, leading to reduced disability. Descriptive variables including age, body mass index, number of comorbid conditions, and weeks since amputation were also collected.
Figure 1.
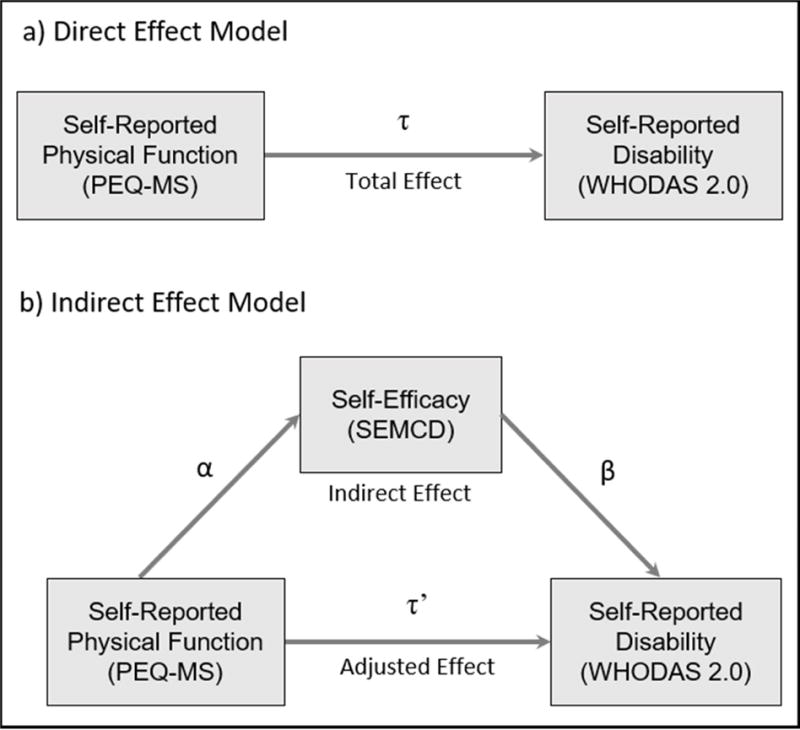
Proposed relationships among Self-Reported Physical Function, Self-Reported Disability, and Chronic Disease Management Self-Efficacy
Independent Variable
Self-reported functional capacity was assessed with the 12-item Prosthesis Evaluation Questionnaire – Mobility Scale (PEQ-MS). The PEQ-MS is a well-established, commonly used outcome measure with demonstrated reliability (ICC=0.73–0.90) and internal consistency (Cronbach’s alpha=0.96) for use with people who have dysvascular TTA.19,20 Participants were asked to rate the amount of difficulty they perceive completing a range of ambulation and transfer tasks (e.g., walk in confined spaces, walk up a steep hill, sit down and get up from a chair); with scores ranging from being unable or hardly able (0) to having no problems (4). An average score across the 12-item questionnaire was used in the analysis (score range: 0–4).
Dependent Variable
Self-reported disability was assessed with the World Health Organization Disability Assessment Schedule 2.0 (WHODAS 2.0) 12-item questionnaire.21,22 The WHODAS 2.0 was developed to measure disability due to any health condition across domains of cognition, mobility, self-care, getting along, life activities, and participation.22 The WHODAS 2.0 has strong internal consistency (Cronbach’s alpha=0.89) and has demonstrated severe disability with people who have dysvascular TTA.2,23 Participants in this study were asked to rate the amount of difficulty completing 12 daily activities (e.g., learning a new task, taking care of household responsibilities, joining community activities) over the past 30 days, with scores ranging from 1 (no difficulty) to 5 (extreme difficulty/cannot do).22 Overall disability was calculated by summing the scores for the 12 items; higher scores indicated greater disability (score range: 12–60).
Mediating Variable
The Self-Efficacy of Managing Chronic Disease (SEMCD) scale was used to assess participant confidence in using strategies to minimize the negative effects of chronic disease on everyday living. The SEMCD consists of 6 questions in domains related to symptom control, emotional functioning, and communicating with physicians and others. The questionnaire is internally consistent (Cronbach’s alpha=0.91) and valid for use with people living with a variety of chronic conditions, including DM and PAD.24 Participants indicated their confidence on a scale of 1 (not confident) to 10 (totally confident). An average score was obtained over the 6 items and used in the analysis (score range: 1–10).
Statistical Analysis/Analysis Plan
Sample size was determined by the parent randomized control trial (NCT01929018). Univariate linear regression and Pearson correlation coefficients (r) were used to identify statistically significant relationships between self-reported functional capacity, self-efficacy, and disability. In the presence of significant relationships (P < .05), mediation analysis was used to assess the hypothesized relationship among self-reported disability, self-reported functional capacity, and self-efficacy for chronic disease management (Figure 1). The intent was to test if the independent variable (I; PEQ-MS) was related to the dependent variable (Y; WHODAS 2.0) through the mediating variable (M; SEMCD). Testing mediation requires analysis of regression coefficients from 3 equations: (1) The total effect of the independent variable (τ) on the dependent variable (Y = β0(1) + τI + ε(1)), (2) the effect of the independent variable (α) on the mediating variable (M = β0(2) + αI + ε(2)), and (3) the effect of the independent variable on the dependent variable (τ′) when the mediating variable (β) is added to regression one (Y = β0(3) + βM + τ′I + ε(3)). The null hypothesis is then HO: τ-τ′ = 0 or HO: αβ = 0. The nature of cross-sectional data precludes the assessment of causal directionality of variables in our analysis. Additionally, sample size limitations prevented inclusion of covariates in regression models.
MacKinnon et al compared 14 methods for testing the null hypothesis, generally differing in the way the variance of τ-τ′ or αβ is computed and whether the test statistic is normally distributed25. The most powerful test when the null hypothesis was false and yielding the most accurate type I error rate was the product of coefficients test, αβ ± CL√(α2σ2β + β2σ2α) where σαβ = √(α2σ2β + β2σ2α). Confidence intervals for αβSEMCD were obtained using the bootstrap method. We also report the effect of adding the mediator to the model (ie, τ and τ′). Full mediation is indicated if τ′ is no longer significant when controlling for the mediating variable (SEMCD). Partial mediation is indicated if τ′ is less than τ but remains statistically significant when controlling for the mediating variable (SEMCD).
Results
One hundred three people were screened and full data were collected from 38 men enrolled in the study. People did not enroll because they declined participation (n=12), did not meet inclusion criteria (n=27), or were unable to be contacted (n=26). Participants with dysvascular TTA had a mean age of 63.6 years (SD: ± 9.1), body mass index of 30.6 kg/m2 (±8.6), 6.6 comorbid conditions (±2.5), and 17.9 weeks (±6.6) since amputation. The average WHODAS 2.0 score was 38.5 (±8.8), PEQ-MS score 2.5 (±0.8), and SEMCD score of 7.7 (±1.7). There were no significant differences in descriptive characteristics between Veteran (n=20) and non-Veteran (n=18) participants (Table 1).
Table 1.
Participant characteristics by Veteran/non-Veteran status*
| Non-Veteran | Veteran | p | |
|---|---|---|---|
| (n = 18) | (n = 20) | ||
| Age (years) | 61.00 ± 9.39 | 65.85±8.39 | .63 |
| Body mass index (kg/m2) | 30.83 ±7.75 | 30.44±9.45 | .41 |
| Comorbid conditions (number) | 6.00±2.33 | 7.10±2.61 | .63 |
| Time since amputation (weeks) | 21.50±4.34 | 14.70±6.74 | .07 |
| WHODAS 2.0 score | 38.22±8.02 | 38.80±9.698 | .44 |
| PEQ-MS score | 2.53±0.79 | 2.53±0.78 | .97 |
| SEMCD score | 7.35±1.87 | 8.025±1.61 | .53 |
Values are mean ± SD
WHODAS 2.0 = World Health Organization Disability Schedule 2.0; PEQ-MS = Prosthesis Evaluation Questionnaire Mobility Scale; SEMCD = Self Efficacy of Managing Chronic Disease Questionnaire
Relationships between WHODAS 2.0 and PEQ-MS (r = −0.61), WHODAS 2.0 and SEMCD (r = −0.51), and PEQ-MS and SEMCD (r = 0.44) were significant (Table 2). The point estimate for the total effect of PEQ-MS on WHODAS 2.0 (τ) was −6.93 and PEQ-MS on SEMCD (α) was 0.99 (Table 3). When adding SEMCD to model 1, the effect of PEQ-MS on WHODAS 2.0 was reduced and remained significant (τ′= −5.44). Bootstrap 95% confidence intervals of αβSEMCD do not include zero (−7.39, −2.23). The non-inclusion of zero in the bootstrap confidence intervals demonstrate significant mediation effect of PEQ-MS on WHODAS 2.0 by SEMCD. The effect of PEQ-MS maintaining significance when SEMCD was added to the model, indicates the significant mediation is partial. Since the effect of PEQ-MS on WHODAS 2.0 remained significant after adding SEMCD to the model, there was not full mediation.
Table 2.
Relationships between variables of interest
| PEQ-MS | SEMCD | |||
|---|---|---|---|---|
|
|
||||
| r | R2 | r | R2 | |
| WHODAS 2.0 | −0.61 | 0.37 | −0.51 | 0.26 |
| (p<.001) | (p=.001) | |||
|
| ||||
| PEQ-MS | 0.44 | 0.19 | ||
| (p=.006) | ||||
Table 3.
Parameter estimates of regression equations
| Tau (p) | Tau‘ (p) | Alpha (p) | Beta (p) | R2 | |
|---|---|---|---|---|---|
| Eq 1: PEQ-MS | −6.93 (p<.001) |
0.37 | |||
| Eq 2: SEMCD | 0.99 (p=006) |
0.19 | |||
| Eq 3: PEQ-MS & SEMCD | −5.44 (p=.002) |
−1.50 (p=042) |
0.44 |
Discussion
The results of this study demonstrate that self-efficacy partially mediates the relationship between self-reported functional capacity and disability among individuals with dysvascular TTA. Specifically, higher self-efficacy for chronic disease management results in lower levels of disability. Partial mediation suggests other factors, such as performance-based functional capacity, may also account for disability outcomes after dysvascular TTA.
Our findings build on prior evidence indicating that greater self-efficacy results in lesser disability. The effect of self-efficacy in health behavior for people with chronic disease has been well established by Bandura and others; 10,11,26 yet, to the best of our knowledge, this is the first study to explore the mediation effect of self-efficacy with people who have dysvascular TTA. Schulz et al.17 demonstrated self-efficacy partially mediated the relationship between pain intensity and pain-related disability for older adults with more than three comorbid conditions, yet the focus on pain in this population prevents generalization of these findings to people with dysvascular TTA. McAuley et al.27 demonstrated partial mediation effect of self-efficacy and functional performance on the relationship between physical activity and disability in older women. Although not specifically related to individuals with dysvascular TTA, the evidence agrees with our findings of partial mediation effect of self-efficacy within the larger context of self-reported physical function and disability with patients who have complex medical conditions.
Veteran and non-Veteran participants in this sample were similar, having an average of six or more co-morbid conditions in the presence of severe disability as measured by the WHODAS 2.0. Our findings suggest self-efficacy of chronic disease management may be a target of intervention to improve chronic disability with Veterans and non-Veterans. In addition to complex medical histories, people with dysvascular TTA commonly have poor health behaviors including history of smoking,28 non-adherence with diabetes management recommendations,29 and low levels of physical activity.8 Behavior-based interventions targeting improved self-efficacy have been developed to increase physical activity behavior with people who have PAD or DM16,30 and improve health outcomes for those specifically with DM.31 Unfortunately, integration of behavior-based rehabilitation strategies targeting improved self-efficacy after dysvascular TTA are not common.
Developing interventions to improve self-efficacy for chronic disease management within rehabilitation may improve long-term disability after dysvascular TTA. Although not specifically addressed with this study, functional performance may account for the remaining portion of the relationship between self-reported physical function and disability.27 Current prosthesis rehabilitation targeting range of motion, strength, and balance effectively improves functional performance, regardless of etiology, health behaviors, or comorbidity burden.4 Persistence of severe disability outcomes following rehabilitation suggest the presence of rehabilitation targets that have gone unaddressed, such as self-efficacy. Integration of behavior-based strategies to improve self-efficacy, including patient-directed goal setting, problem solving, and self-monitoring during exercise may enable participants to seek greater participation in life roles and decrease chronic disability.
There are several study limitations to consider. Cross-sectional data from 38 men were used for analysis may bias the findings of this analysis and limit generalizability. Larger sample size studies that include women are needed to provide greater statistical power for making robust inferences about the effect of self-efficacy on disability.25 In addition, our partial mediation findings suggest that other factors beyond self-efficacy may also contribute to the relationship between self-reported functional capacity and disability. Although similarities between Veterans and non-Veterans were identified in this study, future work should clarify potential differences between groups (e.g., diagnosis of PTSD). Furthermore, the cross-sectional nature of the data limits our ability to assess for causal directionality of self-efficacy and self-reported functional status on disability. Longitudinal studies will be required to determine causal directionality and how the effects of self-efficacy may change over time. Finally, the results of this study are based on self-reported functional capacity and further study is needed to identify how performance-based measures (e.g., timed up and go, two-minute walk, gait speed) of functional capacity may influence disability outcomes with relation to self-efficacy.
Conclusion
This exploratory data analysis identified significant relationships between self-reported functional capacity, self-efficacy, and disability for people with dysvascular TTA. Although causality is not definitive, this mediation analysis of cross-sectional data provided initial evidence that the relationship between perceived functional capacity and self-reported disability is partially mediated by self-efficacy after dysvascular TTA. Additionally, the absence of full mediation suggests that other factors, beyond self-efficacy, may also partially account for the relationship between self-reported functional capacity and disability. Further research is needed to identify additional targets of rehabilitation intervention and determine if interventions specifically targeting self-efficacy in this population reduce disability after dysvascular TTA.
Acknowledgments
Funding: This work was supported by the National Institutes of health [NIH K12 HD055931, NIH/NCATS UL1-TR001082] and the Foundation for Physical Therapy [Promotion of Doctoral Studies Scholarship]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethical Approval: Study protocol was approved by Colorado Multiple Institutional Review Board and Veterans Affairs Research and Development office (COMIRB #13-0179).
Conflict of Interest: The authors report no relevant conflicts of interest for conducting this research
References
- 1.Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89(3):422–429. doi: 10.1016/j.apmr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Coffey L, Gallagher P, Desmond D. Goal pursuit and goal adjustment as predictors of disability and quality of life among individuals with a lower limb amputation: a prospective study. Arch Phys Med Rehabil. 2014;95(2):244–252. doi: 10.1016/j.apmr.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Dillingham TR, Pezzin LE, MacKenzie EJ. Limb amputation and limb deficiency: epidemiology and recent trends in the United States. South Med J. 2002;95(8):875–883. doi: 10.1097/00007611-200208000-00018. [DOI] [PubMed] [Google Scholar]
- 4.VA/DoD Clinical Practice Guideline for Rehabilitation of Lower Limb Amputation. Department of Veterans Affairs & Department of Defense; 2008. [Google Scholar]
- 5.Christiansen CL, Fields T, Lev G, Stephenson RO, Stevens-Lapsley JE. Functional Outcomes After the Prosthetic Training Phase of Rehabilitation After Dysvascular Lower Extremity Amputation. PM R. 2015;7(11):1118–1126. doi: 10.1016/j.pmrj.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norvell DC, Williams RM, Turner AP, Czerniecki JM. The development and validation of a novel outcome measure to quantify mobility in the dysvascular lower extremity amputee: the amputee single item mobility measure. Clinical rehabilitation. 2016;30(9):878–889. doi: 10.1177/0269215516644308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies B, Datta D. Mobility outcome following unilateral lower limb amputation. Prosthet Orthot Int. 2003;27(3):186–190. doi: 10.1080/03093640308726681. [DOI] [PubMed] [Google Scholar]
- 8.Paxton RJ, Murray AM, Stevens-Lapsley JE, Sherk KA, Christiansen CL. Physical activity, ambulation, and comorbidities in people with diabetes and lower-limb amputation. J Rehabil Res Dev. 2016;53(6):1069–1078. doi: 10.1682/JRRD.2015.08.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tudor-Locke C, Craig CL, Aoyagi Y, et al. How many steps/day are enough? For older adults and special populations. Int J Behav Nutr Phys Act. 2011;8:80. doi: 10.1186/1479-5868-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bandura A. Health promotion by social cognitive means. Health education & behavior : the official publication of the Society for Public Health Education. 2004;31(2):143–164. doi: 10.1177/1090198104263660. [DOI] [PubMed] [Google Scholar]
- 11.Rimal RN. Closing the knowledge-behavior gap in health promotion: the mediating role of self-efficacy. Health communication. 2000;12(3):219–237. doi: 10.1207/S15327027HC1203_01. [DOI] [PubMed] [Google Scholar]
- 12.Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. Jama. 2002;288(19):2469–2475. doi: 10.1001/jama.288.19.2469. [DOI] [PubMed] [Google Scholar]
- 13.McDermott MM, Domanchuk K, Liu K, et al. The Group Oriented Arterial Leg Study (GOALS) to improve walking performance in patients with peripheral arterial disease. Contemporary clinical trials. 2012;33(6):1311–1320. doi: 10.1016/j.cct.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDermott MM, Guralnik JM, Criqui MH, et al. Home-based walking exercise in peripheral artery disease: 12-month follow-up of the GOALS randomized trial. J Am Heart Assoc. 2014;3(3):e000711. doi: 10.1161/JAHA.113.000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lorig KR, Ritter P, Stewart AL, et al. Chronic disease self-management program: 2-year health status and health care utilization outcomes. Med Care. 2001;39(11):1217–1223. doi: 10.1097/00005650-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 16.McDermott MM, Liu K, Guralnik JM, et al. Home-based walking exercise intervention in peripheral artery disease: a randomized clinical trial. JAMA. 2013;310(1):57–65. doi: 10.1001/jama.2013.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulz S, Brenk-Franz K, Kratz A, et al. Self-efficacy in multimorbid elderly patients with osteoarthritis in primary care–influence on pain-related disability. Clinical rheumatology. 2015;34(10):1761–1767. doi: 10.1007/s10067-014-2766-0. [DOI] [PubMed] [Google Scholar]
- 18.McAuley E, Morris KS, Doerksen SE, et al. Effects of change in physical activity on physical function limitations in older women: mediating roles of physical function performance and self-efficacy. J Am Geriatr Soc. 2007;55(12):1967–1973. doi: 10.1111/j.1532-5415.2007.01469.x. [DOI] [PubMed] [Google Scholar]
- 19.Legro MW, Reiber GD, Smith DG, del Aguila M, Larsen J, Boone D. Prosthesis evaluation questionnaire for persons with lower limb amputations: assessing prosthesis-related quality of life. Arch Phys Med Rehabil. 1998;79(8):931–938. doi: 10.1016/s0003-9993(98)90090-9. [DOI] [PubMed] [Google Scholar]
- 20.Franchignoni F, Giordano A, Ferriero G, Orlandini D, Amoresano A, Perucca L. Measuring mobility in people with lower limb amputation: Rasch analysis of the mobility section of the prosthesis evaluation questionnaire. Journal of rehabilitation medicine. 2007;39(2):138–144. doi: 10.2340/16501977-0033. [DOI] [PubMed] [Google Scholar]
- 21.Ustun TB, Chatterji S, Kostanjsek N, et al. Developing the World Health Organization Disability Assessment Schedule 2.0. Bull World Health Organ. 2010;88(11):815–823. doi: 10.2471/BLT.09.067231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ustun T, Kostanjsek N, Chatterji S, Rehm J, editors. Measuring health and disability: manual for WHO disability assessment schedule (WHODAS 2.0) Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 23.Luciano JV, Ayuso-Mateos JL, Aguado J, et al. The 12-item World Health Organization Disability Assessment Schedule II (WHO-DAS II): a nonparametric item response analysis. BMC medical research methodology. 2010;10:45. doi: 10.1186/1471-2288-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorig KR, Sobel DS, Ritter PL, Laurent D, Hobbs M. Effect of a self-management program on patients with chronic disease. Eff Clin Pract. 2001;4(6):256–262. [PubMed] [Google Scholar]
- 25.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7(1):83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marks R, Allegrante JP, Lorig K. A review and synthesis of research evidence for self-efficacy-enhancing interventions for reducing chronic disability: implications for health education practice (part I) Health promotion practice. 2005;6(1):37–43. doi: 10.1177/1524839904266790. [DOI] [PubMed] [Google Scholar]
- 27.McAuley E, Konopack JF, Morris KS, et al. Physical activity and functional limitations in older women: influence of self-efficacy. The journals of gerontology Series B, Psychological sciences and social sciences. 2006;61(5):P270–277. doi: 10.1093/geronb/61.5.p270. [DOI] [PubMed] [Google Scholar]
- 28.Kulkarni J, Pande S, Morris J. Survival rates in dysvascular lower limb amputees. International journal of surgery (London, England) 2006;4(4):217–221. doi: 10.1016/j.ijsu.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 29.Quilici MT, Del Fiol Fde S, Vieira AE, Toledo MI. Risk Factors for Foot Amputation in Patients Hospitalized for Diabetic Foot Infection. Journal of diabetes research. 2016;2016:8931508. doi: 10.1155/2016/8931508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collins TC, Lunos S, Carlson T, et al. Effects of a home-based walking intervention on mobility and quality of life in people with diabetes and peripheral arterial disease: a randomized controlled trial. Diabetes Care. 2011;34(10):2174–2179. doi: 10.2337/dc10-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGowan P. The relative effectiveness of self-management programs for type 2 diabetes. Can J Diabetes. 2015;39(5):411–419. doi: 10.1016/j.jcjd.2015.04.005. [DOI] [PubMed] [Google Scholar]


