Abstract
Neisseria gonorrhoeae is the causative agent of the sexually transmitted infection gonorrhea and is adapted to survive in humans, its only host. The N. gonorrhoeae cell wall is critical for maintaining envelope integrity, resisting immune cell killing, and production of cytotoxic peptidoglycan (PG) fragments. Deletion of the N. gonorrhoeae strain FA1090 genes encoding two predicted low-molecular-mass, penicillin-binding proteins (LMM PBPs), DacB and DacC, substantially altered the PG cross-linking. Loss of the DacB peptidase resulted in global alterations to the PG composition, while loss of the DacC protein affected a much narrower subset of PG peptide components. A double ΔdacB/ΔdacC mutant resembled the ΔdacB single mutant, but had an even greater level of cross-linked PG. While single ΔdacB or ΔdacC mutants did not show any major phenotypes, the ΔdacB/ΔdacC mutant displayed an altered cellular morphology, decreased resistance to antibiotics, and increased sensitivity to detergent-mediated death. Loss of the two proteins also drastically reduced the number of Type IV pili (Tfp), a critical virulence factor. The decreased piliation reduced transformation efficiency and correlated with increased growth rate. While these two LMM PBPs differentially alter the PG composition, their overlapping effects are essential to proper envelope function and expression of factors critical for pathogenesis.
Keywords: peptidoglycan, type IV pilus, endopeptidase, carboxypeptidase, gonorrhea
Graphical Abstract

Introduction
The Gram-negative, diplococcal bacterium Neisseria gonorrhoeae is the aetiologic agent of gonorrhea. This human-restricted pathogen causes an estimated 78 million infections annually worldwide (Francis Ndowa, 2012, Newman et al., 2015). N. gonorrhoeae typically colonizes the genitourinary tract resulting in non-complicated urethritis and cervicitis in men and women respectively. Presumably, due to the development of nucleic acid amplification tests, detection of rectal and pharyngeal gonorrhea, which often remains asymptomatic, has increased recently (Unemo & Shafer, 2014). Infection can, in rare cases, result in severe sequelae including pelvic inflammatory disease (PID), epididymitis, and bacteremia (Swasdio et al., 1996, O'Brien et al., 1983, Wasserheit, 1994). Resistance to all recommended antibiotics has been observed in N. gonorrhoeae, although not yet in a single strain (Goire et al., 2014). The observed resistance(s), coupled with the ability to undergo horizontal gene transfer, which can mediate spread of resistance, has lead the Centers for Disease Control to label N. gonorrhoeae an urgent threat (Prevention, 2013). A greater understanding of the basic biology and fundamental virulence factors of this pathogen is of critical public health importance.
As a human restricted pathogen, N. gonorrhoeae has no known environmental reservoir and has evolved under constant pressure from the human immune system (Virji, 2009). This restricted environment has led to exquisite adaptation to its niche with several virulence factors capable of undergoing antigenic variation to maintain function while avoiding immune surveillance (Zelewska et al., 2016). One such factor is the Type IV pilus (Tfp), an organelle widespread amongst Gram-negative bacteria and required for productive N. gonorrhoeae infection (Swanson et al., 1987). Tfp mediate an array of functions including microcolony formation, cellular adherence, modulation of host-cell signaling, and resistance to oxidative killing (Swanson, 1973, Dietrich et al., 2011, Bottcher, 2011, Stohl et al., 2013). The Tfp complex is also required for natural transformation, mediating at least the initial binding and uptake of DNA from the environment into the periplasmic space (Gangel et al., 2014, Obergfell & Seifert, 2015). In many Gram-negative bacteria, transformation efficiency is highly correlated with the expression of Tfp, and piliated strains of N. gonorrhoeae can undergo transformation more than a million times more frequently than a non-piliated strain (Long et al., 2003, Sparling, 1966, Craig et al., 2004).
The Tfp is a dynamic structure, undergoing cycles of extension and retraction that can result in pili extended several microns from the cell surface (Craig et al., 2004, Stephens et al., 1985). While these fibers are composed of thousands of polymerized pilin (PilE) subunits and are only six nm in width, they can exert significant force (Maier et al., 2002). Tfp are produced from an assembly complex that spans the bacterial envelope. The ATPases that drive extension and retraction (PilF and PilT, respectively) trade off occupying the inner membrane complex consisting of PilG, PilM, PilN, PilO (Berry & Pelicic, 2015, Chang et al., 2016). PilP spans the periplasmic space from the inner-membrane complex to the secretin PilQ. PilQ multimerizes to form a pore in the outer membrane through which the pilus extends. Both TsaP and PilQ mediate proper anchoring of the complex through interactions with the outer membrane and peptidoglycan (PG) layer (Siewering et al., 2014). Mpg, a zinc- dependent carboxy- and endopeptidase known to hydrolyze (PG) side chains, is required for stable pilus expression through an unknown mechanism (Stohl et al., 2013).
The characteristic coccal shape of N. gonorrhoeae requires the cell-wall PG sacculus (Zapun et al., 2008). In most bacteria, penicillin binding proteins (PBPs) polymerize and cross-link the cell-wall PG to form the large, macromolecular complex located in the periplasmic space (Typas et al., 2011). Transglycosylases polymerize the long glycan chains while transpeptidases establish the peptide cross-linking between the glycan strands. Along with hydrolase modification by carboxypeptidases and endopeptidases, these enzymes build a macromolecule, strong enough to withstand the osmotic pressure differential yet flexible enough to allow for cell growth and division. During N. gonorrhoeae cell-wall formation and maturation, PG fragments are released into the extracellular space that are cytotoxic and stimulate inflammation (Melly et al., 1984, Mavrogiorgos et al., 2014, Cloud-Hansen et al., 2008). The N. gonorrhoeae cell wall also helps to protect the organism from neutrophil-mediated killing (Ragland et al., 2017).
Similarly to other coccal bacteria, N. gonorrhoeae has a relatively small number of penicillin-binding proteins (PBPs) (Zapun et al., 2008, Barbour, 1981). Of the four identified PBPs in N. gonorrhoeae, only the two high molecular mass (HMM) proteins, PBP1 and PBP2 are essential for cell viability (Barbour, 1981). HMM PBPs are largely responsible for the synthesis of new PG. PBP1 is a class A HMM PBP, homologous to Escherichia coli PBP1a, which is likely responsible for transglycosylation and transpeptidation (Ropp & Nicholas, 1997). PBP2 is a class B HMM PBP, homologous to E. coli PBP3, which also catalyzes transpeptidation reactions (Spratt & Cromie, 1988, Zhang & Spratt, 1989, Dowson et al., 1989). Mutations in the penA gene encoding PBP2 account for the majority of the widespread resistance to β-lactams (Ameyama et al., 2002, Unemo & Nicholas, 2012).
Low molecular mass (LMM) PBPs tend to play non-essential roles in PG modification, PG recycling, and cell separation (Sauvage et al., 2008). The functional outcome of activity of LMM PBPs is typically harder to determine as LMM PBPs tend to be more numerous and often have overlapping functions; however, only two LMM PBPs have been identified and characterized in N. gonorrhoeae. PBP3 binds radiolabeled penicillin and is homologous to E. coli (DacB) (Stefanova et al., 2003, Barbour, 1981). DacB exhibits both carboxypeptidase activity and endopeptidase activity. N. gonorrhoeae PBP4 was identified through genetic analysis, is most similar to PBP7 of E. coli and does bind radiolabeled penicillin (Stefanova et al., 2003, Stefanova et al., 2004). PBP4 is a DD-carboxypeptidase with a preference for Nε-acylated substrates. While initial observation of single knockout strains of PBP3 or PBP4 of N. gonorrhoeae revealed no gross abnormalities, loss of PBP3 and PBP4 together was reported to reduce cell growth and alter the cellular morphology (Stefanova et al., 2003).
In this study, we mutated the N. gonorrhoeae genes encoding DacB (PBP3) and DacC, a third predicted LMM PBP not previously identified in radiolabeled penicillin binding assays in N. gonorrhoeae strain FA1090. Inactivation of dacB dramatically altered the PG cross-linking, while mutation of dacC had much subtler effects on the PG composition. Loss of the two proteins in tandem produced highly cross linked PG, and resulted in significant cellular abnormalities. As these abnormalities were largely absent in the single knockout strains, our data suggest these two proteins have overlapping effects on the N. gonorrhoeae cell wall despite uniquely altering the PG profile.
Results
ΔdacB, ΔdacC and ΔdacB/ΔdacC strains have altered cell wall PG profiles
While N. gonorrhoeae only has two identified LMM PBPs, a third predicted LMM PBP is expressed (Zielke et al., 2014). Here we investigated the role of DacB and the third predicted LMM PBP, encoded in locus NGO_0443 of N. gonorrhoeae FA1090. NGO_0443 protein sequence aligns fully to COG1686 (DacC) with an E-value of 4.36e-128 and hereafter will be referred to as dacC (Altschul et al., 1990, Marchler-Bauer et al., 2017). DacC homologs are DD-carboxypeptidases and members of the S11 peptidase family (Kanehisa et al., 2017). An alignment of N. gonorrhoeae DacC with DacC of E. coli shows that the three active sight motifs (SxxK, SxN, and KTG) are not conserved in N. gonorrhoeae (SFig. 1) (Chen et al., 2009). As DacB and DacC are confirmed and predicted LMM PBPs respectively, we constructed loss-of-function mutations in each gene to determine their role in bacterial physiology. The coding sequence of each gene was deleted and replaced with an antibiotic resistance cassette. A double mutant was also constructed. Both single mutants were viable as was the double mutant.
To determine whether the DacB and DacC modify the cell-wall PG, high performance liquid chromatography (HPLC) analysis of the PG was conducted. Cell-wall PG was extracted from the ΔdacB, ΔdacC, or ΔdacB/ΔdacC mutants, digested by mutanolysin to cleave glycan strands, and the resulting di-saccharide, peptide fragments were separated by HPLC (Fig. 1). Identities of the individual peaks were confirmed by mass spectrometry analysis and are consistent with those detailed by Antignac et al. (Antignac et al., 2003). Loss of DacB resulted in global changes to almost all identifiable peptide fragment peaks. The PG had fewer non-cross linked di, tri, and tetra peptides and increased penta peptides and cross-linked tetra-tri and tetra-penta peptides (Table 1). These results confirm previous biochemical analysis characterization of DacB as a carboxypeptidase and endopeptidase (Stefanova et al., 2003). Contrastingly, loss of DacC altered a smaller subset of PG peptides to a lesser degree. The mutant strain had fewer non-cross-linked peptides, an increase in cross-linked tetra-penta peptides, and a large increase in the larger cross-linked fragments with retention times longer than 100 min. The PG composition of the double mutant strain closely resembles that of the single ΔdacB mutant. Loss of the enzymes together results in PG that is much more heavily cross-linked than that of the parental strain. Loss of DacC in conjunction with DacB shifted the cross-linked fragments from tetra-tri towards tetra-penta and even larger fragments.
Figure 1. Peptidoglycan profiling of ΔdacB and ΔdacC mutants.
Purified sacculi were digested with mutanolysin and soluble fragments were separated by HPLC. A. Representative PG profiles of the parental, ΔdacB, ΔdacC, or ΔdacB/ΔdacC strains. The numbered peaks correspond to the identities listed in (Table 1) as determined by mass spectrometry. B. Representation of major peptidoglycan fragments identified. For simplicity, 1,6-anhydromuramic acid and O-acetylation of N-muramic acid species are not depicted. Dashed line indicates a possible minor DAP-DAP linked Tetra-tri fragment.
Table 1.
Percent area of peptidoglycan fragments separated by HPLC.
| Peak | Identity | Parental | ΔdacB | ΔdacC | ΔdacB/ΔdacC |
|---|---|---|---|---|---|
| 1 | Tri | 5.199 ± 0.718 | 0.799 ± 0.121 | 3.334 ± 0.552 | 0.584 ± 0.060 |
| 2 | Tetra (Gly) | 0.973 ± 0.106 | 0.510 ± 0.095 | 0.606 ± 0.080 | 0.211 ± 0.029 |
| 3 | Tetra | 9.279 ± 0.397 | 3.473 ± 0.281 | 7.209 ± 0.792 | 2.924 ± 0.204 |
| 4 | Penta (Gly) | - ± - | 1.283 ± 0.100 | - ± - | 1.390 ± 0.185 |
| 5 | Di | 2.768 ± 0.307 | 1.850 ± 0.300 | 1.162 ± 0.104 | 1.149 ± 0.178 |
| 6 | Tri (OAc) | 2.593 ± 0.132 | - ± - | 2.369 ± 0.466 | - ± - |
| 7 | Penta | 0.970 ± 0.212 | 5.108 ± 0.187 | 0.752 ± 0.117 | 4.730 ± 0.620 |
| 8 | Tetra (OAc) | 5.408 ± 0.326 | 2.110 ± 0.049 | 5.596 ± 0.722 | 1.501 ± 0.095 |
| 9 | Tri-tri | 1.301 ± 0.321 | 0.427 ± 0.063 | 1.065 ± 0.101 | 0.236 ± 0.094 |
| 10 | Tetra-tri | 2.480 ± 0.172 | 3.282 ± 0.440 | 1.340 ± 0.201 | 1.951 ± 0.112 |
| 11 | Penta (OAc) | 1.414 ± 0.036 | 3.282 ± 0.440 | 0.853 ± 0.088 | 2.072 ± 0.165 |
| 12 | Tetra-tetra | 6.395 ± 1.809 | 1.480 ± 0.174 | 3.936 ± 0.281 | 2.811 ± 1.118 |
| 13 | Tri (Anh) | 3.640 ± 0.808 | 1.265 ± 0.541 | 4.439 ± 0.544 | 1.189 ± 0.294 |
| 14 | Tetra-penta | 1.605 ± 0.459 | 3.873 ± 0.534 | 1.703 ± 0.227 | 5.926 ± 1.356 |
| 15 | Tetra-tetra (OAc) | 7.982 ± 0.778 | 1.815 ± 0.114 | 5.134 ± 0.776 | 2.391 ± 0.884 |
| 16 | Tetra (Anh) | 3.857 ± 1.226 | 1.429 ± 0.177 | 2.168 ± 0.128 | 1.816 ± 0.453 |
| 17 | Tetra-penta (OAc) | - ± - | 6.249 ± 0.543 | - ± - | 7.146 ± 1.408 |
Quantification of normalized percent area of peaks and standard deviation of peaks identified in (Fig. 1). Reported area is the mean of three independent experiments. Di: disaccharide dipeptide (disaccharide: N-acetylglucosamine-N-acetylmuramic acid); Tri: disaccharide tripeptide; Tetra: disaccharide tetrapeptide; Penta: disaccharide pentapeptide; Gly: indicates the replacement of an alanine with a glycine at the fourth or fifth position of the peptide stem; OAc: O-acetylation on N-acetylmuramic acid. Anh: 1, 6-anhydromuramic acid.
Mutation of dacB and dacC disrupts type IV pilus expression
The ΔdacB/ΔdacC double mutant strain, exhibited a non-piliated colony morphology similar to a pilE deletion strain (Fig. 2A). Neither individual mutant displayed an altered colony morphology, and complementation with dacB, and dacC under control of their native promoters at an ectopic locus restored the piliated colony morphology. To determine the extent to which piliation was disrupted in the double mutant, we assayed the transformation efficiency of the strains. While loss of either DacB or DacC individually did not alter transformation efficiency, the double knockout strain exhibited a 94% reduction in transformation efficiency (Fig. 2B). Although this represents a significant drop in transformation efficiency, the double mutant still exhibits relatively high levels of competence compared to the ΔpilE strain, which is non-transformable. The double complement strain fully restored transformation efficiency. As previously reported, N. gonorrhoeae strains with very low levels of pilin expression retain significant levels of transformation competence (Long et al., 2003, Long et al., 1998, Gibbs et al., 1989, Rudel et al., 1995). Loss of piliated colony morphology and transformation efficiency was not due to different expression levels of PilE as there as there is only a slight reduction in protein levels in the double mutant compared to the parental strain (Fig. 2C) (Haas et al., 1987). These data show that loss of DacB and DacC together, reduces piliation but does not completely abrogate pilus function.
Figure 2. Analysis of piliation in ΔdacB/ΔdacC N. gonorrhoeae.
A. Images of representative colonies grown on GCB solid media for 20hr and imaged using a stereo microscope. Non-piliated colonies are larger in diameter and are flatter rather than domed, resulting in different refraction of the light source. B. Transformation efficiencies of dacB and dacC mutant strains. Reported Transformation efficiencies are the mean ± standard deviation of at least four independent experiments. N.D. = transformants not detected. † represents statistically significant difference by one-way analysis of variance (ANOVA) followed by Tukey’s multiple-comparison test compared to the parental strain **p<0.001. [DC] = Double complement. C. PilE western blot of whole cell lysates of dacB and dacC mutant strains. Western blot analysis performed using the K36 anti-pilin peptide antibody.
To confirm the piliation status of the mutant strains, we imaged the strains using transmission electron microscopy (TEM). This technique allows qualitative analysis of the piliation state of the cell, but quantitative analysis is prevented by the inability to determine which cell a pilus originates from as well as the inability to differentiate individual pili form different sized pilus bundles. TEM analysis confirmed that both single mutants still expressed numerous pili, and the double mutant had few detectable pili per cell (Fig. 3).
Figure 3. Analysis of Piliation in ΔdacB and ΔdacC mutants.
Micrographs of N. gonorrhoeae strains grown for 20 hours on solid medium, negatively stained with uranyl acetate, and imaged on a TEM. The top panel has images of the detailed parental strains while the lower panel images are of the detailed strains harboring a pilT::erm allele.
To determine whether loss of both DacB and DacC disrupts pilus biogenesis or stability, we introduced a pilT loss-of-function mutation into the single and double mutant strains. Pilus expression can be restored in many mutants that decrease the number of surface exposed pili by inactivating pilT, encoding the ATPase that is required for pilus retraction (Wolfgang et al., 1998b, Wolfgang et al., 1998a). Deletion of pilT resulted in increased piliation in all strains (Fig. 3). Piliation of the ΔdacB/ΔdacC double mutant was restored to levels apparently similar to that of the parental strain, indicating that the loss of both DacB and DacC prevents stable pilus expression but that the pilus assembly process is not disrupted.
DacB and DacC alter cell fitness and morphology
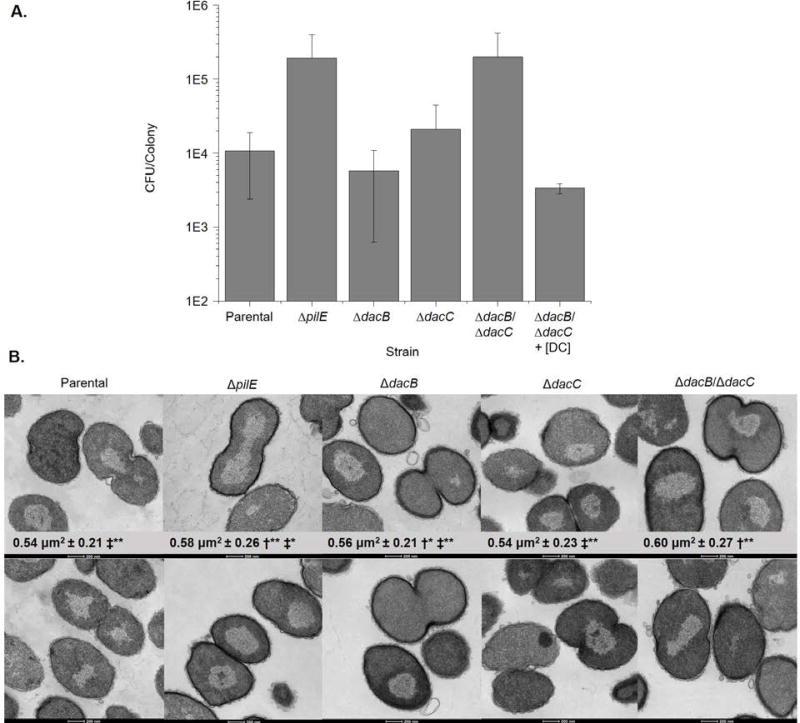
To determine whether DacB and DacC play a role in general cell fitness, we assayed the growth of the mutant strains on solid medium by determining the colony forming units (CFU) per colony after 16 hr (Fig. 4A). While loss of DacB or DacC individually did not alter the growth, loss of both gene products resulted in approximately a twentyfold increase in the number of CFU/colony compared to the wild type strain. This increase in growth is the same magnitude as that displayed by a ΔpilE strain, suggesting that rather than directly altering growth, the increased growth in the ΔdacB/ΔdacC strain is likely due to the loss of stable pilus expression.
Figure 4. Cell growth and morphology in ΔdacB and ΔdacC mutants.
A. Growth of N. gonorrhoeae strains grown on GCB solid media. At 16 hr post inoculation, six individual colonies per strain were picked from plates, diluted in liquid culture, and plated in triplicate to enumerate CFU/colony. Data points are the mean of at least three independent experiments. Error bars represent standard deviation. One-way analysis of variance (ANOVA) indicates statistically significant difference amongst the strains, but Tukey’s multiple-comparison test shows no statistical differences between paired strains. [DC] = Double complement. B. Representative electron micrographs of thin sections of N. gonorrhoeae strains. Mean cell cross sectional area ± standard deviation of at least 1300 individual cells is shown on each micrograph. †‡ symbols represent statistically significant difference by one-way analysis of variance (ANOVA) followed by Tukey’s multiple-comparison test compared to the parental strain (†) and ΔdacB/ΔdacC strain (‡). Asterix indicate degree of significance *p<0.05 **p<0.001.
As the murein sacculus is the scaffold on which the cell is shaped, we analyzed cellular morphology using TEM of thin-sections of the parental, ΔpilE, ΔdacB, ΔdacC, and ΔdacB/ΔdacC strains (Fig. 4B). While loss of either protein did not result in any gross morphologic abnormalities, the micrographs show increased cross-sectional area of the ΔdacB/ΔdacC cells. To analyze the change in cross-sectional area as a proxy for change in cell volume, we quantified the cross-sectional area of at least 1,300 cells per strain using ImageJ particle analysis. The analysis was gated on cells with a cross-sectional area of at least of 0.3µm2 to prevent analysis of slices that transited near the edge of cells. While all cross-sections do not necessarily transect the thickest part of the cell, analyzing over 1,300 cells allows for an appropriate comparison of the average cellular volume. The ΔdacB/ΔdacC strain exhibited a cross-sectional area 11% larger than the parental strain, which translates to a calculated 16% increase in total cell volume. While the ΔpilE strain also showed an increase in cross-sectional area, the magnitude of increase was less than that of the ΔdacB/ΔdacC strain. The ΔdacB strain also showed a very small but statistically significant increase in cell size. Together these data demonstrated that PG modification mediated by DacB and DacC constrains the cell size in FA1090 independent of their effect on piliation.
Antibiotic sensitivity is increased in the ΔdacB/ΔdacC strain
To determine the effect of inactivating DacB and DacC on antibiotic sensitivity, we determined the minimum inhibitory concentration of an array of antibiotics representing eight major classes (Table 2). Cell wall synthesis inhibitors included Ampicillin (Penicillins), Ceftazimide (Cephalosporins) and Imipenem (Carbapenems). Protein synthesis inhibitors included Spectinomycin (Aminoglycosides) and Erythromycin (Macrolides). Naldixic acid (Fluoroquinolones) inhibits DNA gyrase. RNA synthesis was inhibited by Rifampicin and cell membranes structure was disrupted with Polymyxin B. Loss of DacC did not alter antibiotic sensitivity of N. gonorrhoeae to any of the tested antibiotics. However, loss of DacB slightly increased the susceptibility to cell wall synthesis inhibitor, Ceftazidime. In contrast, the ΔdacB/ΔdacC double mutant strain was slightly more susceptible to all three classes of cell wall synthesis inhibitors tested. These data demonstrate that PG modification by either DacB or DacC serves to make N. gonorrhoeae more resistant to antibiotics that target PG. Moreover, the ΔdacB/ΔdacC double mutant strain was also slightly more sensitive to Erythromycin, Rifampicin, and Polymixin B. Complementation of the double mutant restored all antibiotic sensitivities to those displayed by the parental strain. Finally, the ΔdacB/ΔdacC double mutant shows increased detergent sensitivity suggesting that it is the changes to the cell wall that provides the alterations in sensitivity.
Table 2.
Antibiotic sensitivities of ΔdacB and ΔdacC mutant strains.
| Antibiotic | Parental | ΔpilE | ΔdacB | ΔdacC | ΔdacB/ΔdacC | ΔdacB/ΔdacC + [DC] |
|---|---|---|---|---|---|---|
| Ampicillin | 0.19–0.25 | 0.125–0.25 | 0.125–0.38 | 0.19–0.38 | 0.064–.125 | 0.19–0.25 |
| Ceftazidime | 0.064–0.125 | 0.047–0.094 | 0.047–0.064 | 0.064–0.125 | 0.016–0.032 | 0.064–0.094 |
| Imipenem | 0.064–0.094 | 0.064 | 0.064–0.094 | 0.064 | 0.023–0.032 | 0.064–0.094 |
| Spectinomycin | 12–16 | 12–16 | 12–16 | 12–24 | 12–16 | 16 |
| Erythromycin | 0.094–0.125 | 0.094 | 0.094–0.125 | 0.094–0.125 | .064–0.094 | N.D. |
| Naldixic Acid | 0.25–0.38 | 0.19–0.38 | 0.19–0.38 | 0.25–0.38 | 0.19–0.38 | 0.25–0.38 |
| Rifampicin | 0.023–0.032 | 0.023–0.032 | 0.023–0.032 | 0.023–0.032 | 0.012–0.023 | 0.023–0.032 |
| Polymixin B | 64–96 | 64–96 | 64 | 64–96 | 48–64 | 64–96 |
Minimum inhibitory concentrations (MIC) in µg/mL for N. gonorrhoeae strains grown on solid media and exposed to antibiotic E-test strips. MICs are represented as a range of at least three independent experiments. N.D. Not determined due to erythromycin resistance gene present in this strain.
When performing western blot analysis, we observed there was less total protein present in lysates from the ΔdacB/ΔdacC strain relative to the single mutants or the parental strain. This was despite normalizing the input cells in each sample. We hypothesized that the increased cross-linking observed in the ΔdacB/ΔdacC strain was interfering with SDS-mediated lysis in the bacterial cells. To test this hypothesis, lysozyme was added to the SDS lysis buffer to independently digest the PG cross-links. ΔdacB/ΔdacC cells lysed in the absence of lysozyme resulted in 15% less total protein as determined by densitometry of the coomassie stained gels as compared to cells lysed in the presence of lysozyme (Fig. 5A and B). No significant difference was observed in the parental and single mutant strains between the two conditions, and the ΔdacB/ΔdacC cells lysed in the presence of lysozyme resulted in the same total protein amounts as the other strains in both conditions.
Figure 5. Detergent sensitivity of ΔdacB and ΔdacC mutants.
A. Representative coomassie stained SDS-PAGE gel of protein samples of dacB and dacC mutants lysed by SDS sample buffer in the presence or absence of lysozyme. B. Ratio of total protein in samples lysed in SDS sample in the absence of lysozyme to samples lysed in the presence of lysozyme. Total protein was quantified by using Image Studio to analyze total protein run in a lane of a SDS-PAGE gel and stained by Coomassie Brilliant Blue. Ratio represents the mean of at four independent experiments. C. Relative survival of strains exposed to 0.001% SDS for 1hr. The reported ratio represents the number of CFU enumerated from cultures treated with SDS to the number of CFU enumerated from cultures treated with vehicle alone. Ratio represents the mean of at least three independent experiments. †‡ symbols represent statistically significant difference by one-way analysis of variance (ANOVA) followed by Tukey’s multiple-comparison test compared to the parental strain (†) and ΔdacB/ΔdacC strain (‡). A
To investigate if the observed resistance to SDS mediated lysis was biologically relevant, we assayed the sensitivity of the strains to SDS killing. When liquid cultures were exposed to 0.001% SDS for 1hr, the ΔdacB/ΔdacC strain was ten times more sensitive to SDS-mediated killing than the parental and ΔdacB strains (Fig. 5C). Contrastingly, the ΔpilE strain was not killed by SDS at this concentration, and the ΔdacC was killed less than the parental strain although all strains could be fully killed by 0.01% SDS. Complementation restored resistance to SDS killing to parental levels. Taken together, these results demonstrate that while increased cross-linking of the PG can hold the proteinaceous content of the cell together when exposed to detergent, the increased cross-linking observed in a ΔdacB/ΔdacC strain makes FA1090 more susceptible to killing by SDS.
Discussion
We have shown through loss-of-function mutations of dacB and dacC alone or in combination that mutants deficient for these proteins have distinct PG composition. Characterization of cell-wall PG from the single mutants revealed increased PG cross-linking. The decreased activity in the double mutant resulted in an even further cross-linked murein sacculus, confirming that these are not redundant activities. Deletion of each protein caused the loss of specific PG modification subsets. These results confirm that DacB acts as a carboxypeptidase and endopeptidase and show that even though DacC was not identified in radiolabeled penicillin binding assays, that it should be classified as a LMM PBP.
The analysis of the ΔdacC mutant PG showed that while there were obvious changes in the PG cross-linking, the PG structure was more similar to that found with the parental PG. In contrast, the ΔdacB mutant PG showed extensive increases in PG cross-links. These results suggest that DacB acts as a global modifier of PG peptides while the presence of DacC results in the alteration of a more specific subset of peptides. The PG profile observed in the DacB mutant closely resembles the profile observed when N. gonorrhoeae is treated with sub-minimum-inhibitory-concentration doses of beta-lactams (Garcia-Bustos & Dougherty, 1987). While loss of DacB drives the majority of the characteristic changes observed in the double mutant PG, concomitant loss of DacC is necessary for any large phenotypic changes. This finding is notable as the lack of conserved catalytic residues in N. gonorrhoeae DacC suggests the protein should be enzymatically inactive (SFig. 1). Our data clearly demonstrate, however, that expression of the dacC gene product results in an alteration of the PG. If the protein is indeed catalytically inactive, it may modify the PG profile through direct interaction with PG, PG precursers, or an additional PG modifying enzyme. Taken together these data demonstrate that DacB and DacC each have unique activities within the cell, and result in the modification of specific subsets of PG peptides. However, they have largely overlapping effects on cellular biology, as loss of both is required for phenotypes that demonstrate the importance of proper PG processing for normally cellular activities.
Because the study of cell division and PG synthesis has been mainly performed in rod-shaped bacteria, mechanistic insight into coccal growth and cell division is still relatively scarce (Pinho et al., 2013). Therefore, it is hard to predict how loss of DacB and DacC increases the overall cell volume, but it may involve regulation of the septal synthetic machinery at the division site (Zapun et al., 2008). Consistent with DacC playing a specific role in coccal growth, an alignment of DacC from different Neisseria species shows that the rod-shaped Neisseria have DacC orthologues that retain the catalytic residues while most coccal Neisseria species have at least one defective active site motif (SFig. 2). If the coccal forms were derived from rod-shaped precursors through the modification of PG processing factors, then the loss of DacC peptidase activity may have occurred during the transition from rod to coccus (Veyrier et al., 2015). We cannot determine whether the remaining DacC activity in the coccal Neisseria is shared with the DacC from the rod-shaped members of this genus, or whether there were other mutations that altered DacC function in the coccal organisms. The sequence conservation of the NGO_0443 gene product with prototypical members of the DacC family, support continued labeling of the gene product as DacC, but the lack of conserved catalytic residues show that it is not functional homolog. A detailed structure/function analysis will be required to determine exactly how the different DacC orthologues function in their representative cell types.
Alternatively, the increase in overall cell volume may be due to abnormal PG cross-linking interfering with tethering of the outer membrane to the PG. A recent study in Salmonella enterica demonstrated that outer-membrane lipoprotein tethering to the PG is important for proper periplasmic length, and loss of this tethering disrupted osmolalic balance between the cytoplasm and periplasm (Cohen et al., 2017). We did observe signs of outer membrane disruption and increased blebbing in thin section electron micrographs of the double mutant (Fig. 4B), although not to the extent observed in the lipoprotein mutant in S. enterica.
DacB and DacC expression is important for resistance to multiple classes of antibiotics. The increased susceptibility of the ΔdacB/ΔdacC strain to cell wall targeting antibiotics is likely due to the ΔdacB/ΔdacC induced cell wall perturbations making the cell less able to resist additional insults to the cell wall synthesis machinery. These data indicate that DacB and DacC play a functionally redundant role in protecting FA1090 (an possibly other gonococci) from beta-lactams. Of particular note is that the double mutant was also more sensitive to non-cell wall targeting antibiotics Erythromycin, Rifampicin, and Polymixin B. We presume that the altered cell wall in the ΔdacB/ΔdacC double mutant influences steady state levels of these compounds in the cell. The simplest explanation for an increase in cytoplasmic levels of antibiotics is that the barrier function of the PG sacculus is disrupted; However, if this explanation was correct, the sensitivity to most antibiotics should have increased. An alternative hypothesis is that expression or activity of outer membrane porins or efflux pumps is disrupted (Goire et al., 2014). It is also possible that the expression or availability of the targets of these antibiotics is altered in the double mutant, but we consider this option less likely.
Our data demonstrate that pilus functions critical for productive gonococcal infection are dependent on proper expression of either DacB or DacC. These findings contrast to the recent observation that Pseudomonas aeruginosa Tfp do not require PG-remodeling enzymes for proper pilus expression (Carter et al., 2017). Pseudomonas aeruginosa, which is rod shaped and only has polar pili, targets and preinstalls the pilus complexes into nascent poles. As N. gonorrhoeae is coccoid and has peritrichous pili, a different strategy appears to be necessary for pilus elaboration. Rather than insertion of the complex into nascent poles prior to PG formation, our data suggest that the peritrichous nature of N. gonorrhoeae pili requires specific PG remodeling for proper pilus elaboration.
The ΔdacB/ΔdacC strain exhibited both an increased cell volume and increased growth relative to the parental strain or either individual mutant. We presume these phenotypes are partially dependent on the loss of stable pilus expression since the ΔpilE strain also shows similar changes, but we do not presently have a direct link of the change in cell volume to the loss of pilus elaboration. One possibility to explain these phenotypes is that there is an increase in ATP availability when pili are lost. Assembly and retraction of the pilus requires the sequential insertion or removal of pilin subunits and each subunit step is predicted to require hydrolysis of two ATP molecules (Chang et al., 2016, McCallum et al., 2017). As pili consist of thousands of subunits per fiber, extension and retraction of large numbers of pili per cell would be energetically expensive. Hence, cells not having to waste energy on pilus dynamics could put more energy towards cell growth and division.
Since the single mutants have no phenotype with respect to pilus expression, either DacB or DacC activity is sufficient to module the cell wall to allow stable pilus expression. An additional N. gonorrhoeae carboxypeptidase/endopeptidase, Mpg (Stohl et al., 2013), acts on PG cross-links and is also required for stable Tfp expression,. This Zinc metalloprotease also acts on the PG side chains, but there is no change in bulk PG, suggesting the Mpg activity is localized to the pilus complex. As with the ΔdacB/ΔdacC mutant, the piliation defect observed in the mpg mutant strain can also be rescued by mutation of the pilT gene. Pilus expression can be restored in a subset of pilus complex mutations that decrease the number of surface exposed pili by inactivating pilT (Wolfgang et al., 1998b). As pili are undergoing frequent cycles of extension and retraction, deletion of the ATPase responsible for retraction can increase the number of pili per cell by locking all elaborated pili in the extended position. Non-stable pili are also retracted through the action of PilT, and thus, deletion of the ATPase prevents retraction of non-stable pilus fibers and results in an increased number of surface exposed pili. Taken together the data from these studies as well as the Mpg studies clearly demonstrate a critical role for PG modification in pilus biogenesis in N. gonorrhoeae.
One possible explanation for the requirement for PG modification is that it is necessary to create holes in the PG layer through which the macromolecular Tfp complex can assemble. This would be consistent with the lack of PG-modifying enzymes required in P. aeruginosa, as the complex can form prior to cell wall formation (Carter et al., 2017). However, as neither loss of Mpg cleavage nor loss of DacB and DacC activity abrogated all piliation and a large number of pili could be restored in ΔpilT strains, it is unlikely that the sole function of PG modification in pilus biogenesis by these peptidases is to cut holes in the cell wall to allow proper pilus complex assembly. While limiting the amount of cross-linking probably plays a role in allowing for type IV complexes to assemble through the cell wall, disruption of PG remodeling by loss of DacB and DacC as well as Mpg may alter the PG structure in a manner that disrupts anchoring to the cell wall. This loss of stability would result in fewer pili per cell due to increased PilT-mediated retraction and help account for the restoration of piliation in the pilT mutant. PilQ levels were not reduced in either single mutant or the double mutant; therefore PilQ assembly does not explain this phenotype (SFig. 3) (Helm et al., 2007). Regardless of the mechanism, it is clear that PG modification is necessary for proper type IV complex expression. Taken together these data demonstrate the critical role DacB and DacC mediated PG modification plays in normal Neisseria physiology. The fact that loss of both activities is necessary to produce an observable phenotype shows that a major shift in the PG cross-linking is required to alter the role(s) of the cell wall in cellular processes and underscores the importance of PG remodeling for maintaining cell wall architecture to allow for proper elaboration of fundamental cellular processes.
Experimental Procedures
Bacterial Strains and Growth
All experiments were performed using derivatives of strain FA1090 with the 1–81-S2 pilE sequence and an IPTG inducible recA6 allele (Seifert, 1997, Seifert et al., 1994). The ΔpilE strain contains a deletion from the upstream silent copy through the coding region of pilE including the promoter and ribosome binding site (Chen et al., 2004). The ΔpilT strains contain a pilT::erm allele described previously (Wolfgang et al., 1998a). For N. gonorrhoeae growth, solid media was GC Medium Base (Difco) plus Kellogg supplements I and II (GCB) at 37°C with 5% CO2, and liquid medium was GCB Liquid Broth (GCBL: 1.5% peptone protease no. 3 [Difco], 0.4% K2HPO4 [Fisher], 0.1% KH2PO4 [Fisher], 0.1% NaCl [Fisher]) plus Kellogg supplements I and II and 0.042% sodium bicarbonate (GCBL+). Antibiotic resistant N. gonorrhoeae were selected on the following antibiotics and concentrations: Chloramphenicol (Cm) 1 ug/ml, Kanamycin (Kan) 50 ug/ml, Naldixic Acid (Nal) 0.75 ug/ml, and Erythromycin (Erm) 2 ug/ml. E. coli were grown on Luria-Bertani (LB) agar or broth at 37°C. Plasmids were propagated in E. cloni 10G ELITE electrocompetent E. coli (Lucigen) or BH10C containing a mutated pcnB gene for low plasmid copy number (Howell-Adams & Seifert, 2000). Antibiotic resistant E. coli were selected on the following concentrations: Kanamycin (Kan) 50 ug/ml, Ampicillin (Amp) 100 ug/ml, Chloramphenicol (Cm) 20 ug/ml.
Construction of ΔdacB, ΔdacC, ΔdacB/ΔdacC
PCR was used to amplify the upstream and downstream region of NGO_0443 (dacC) and NGO_0107(dacB) using primers KP264-267 and KP272-275 respectively. The 3’ primer of the upstream region and 5’ primer of the downstream region contained complementary sequence including a KpnI restriction site. This region of complementarity was used in a splicing overlap extension PCR (SOE-PCR) to combine the upstream and downstream regions with a KpnI restriction site in-between. This construct was cloned into pSMART LCAmp (Lucigen) following manufacturer’s instructions and introduced in E. coli by electroporation. Following confirmation by sequencing, the resulting plasmids (pKP152, 154) were isolated using the QIAprep Spin Miniprep Kit (Qiagen), digested with KpnI (NEB), and gel purified using the Wizard SV Gel and PCR Clean-Up System (Promega). The Kan resistant nptII gene was digested from plasmid pBSL86 using KpnI and gel purified. The nptII allele was cloned into the KpnI digested plasmids using T4 DNA ligase (NEB), electroporated into E. coli and selected for on Amp and Kan. For use in the double mutant, a Cm resistance cassette was digested from plasmid (pKP133) using KpnI and cloned into the NGO_0443 upstream and downstream containing plasmid (pKP152) resulting in plasmid (pKP157). Sequencing confirmed the plasmid sequences and the nptII containing plasmids were used to transform the parental strain with selection on Kanamycin. Deletion was confirmed by PCR using primers KP293–294 (ΔdacC) and KP297–298(ΔdacB). The double mutant was made by transforming the Kan resistant ΔdacB with the dacC::cat containing plasmid (pKP157).
Construction of ΔdacB/ΔdacC double complement
NGO_0443(dacC) and the flanking region was PCR amplified using primers KP309 and KP310 containing an AatII and a SpeI restriction site, respectively. NGO_0107(dacB) and the flanking region was PCR amplified using primers KP311 and KP312 containing a SpeI and a PacI restriction site, respectively. Each amplicon was cloned separately into pSMART LCKan and electroporated into E. coli. The sequence confirmed dacC containing plasmid (pKP178) was digested with AatII and SpeI, the dacB containing plasmid (pKP179) was digested with SpeI and PacI. The dacC and dacB containing fragments were gel purified and cloned into AatII and PacI cut pGCC2 in a single ligation reaction (Mehr et al., 2000). The ligation reaction was electroporated into DH5α pcnB. The resulting plasmid pKP180 was confirmed by PCR amplification and sequencing and and was transformed into the ΔdacB/ΔdacC strain with selection on Erm for correct chromosomal insertion between lctP and aspC. Transformants were verified by PCR amplification and sequencing.
Spot transformation of N. gonorrhoeae
Constructs were transformed into N. gonorrhoeae through coculture on solid media (spot transformation). Strains to be transformed were plated from frozen stocks on GCB solid media and grown for 18 hr. Several isolated colonies were streaked as lawns onto GCB solid media supplemented with 1mM IPTG, 10µL of DNA containing solution was mixed with 10µL GCBL containing supplements I and II and 5 mM MgSO4, and was immediately spotted onto the lawn. Following incubation for 20hr at 37°C, the bacteria from the area where the DNA solution was spotted were suspended in GCBL and dilutions were plated on GCB solid media containing the proper selective antibiotics.
Cell wall PG characterization by HPLC and mass spectrometry
Triplicate cultures of each strain were grown to exponential phase in amended GCBL. PG was isolated as described previously (Dillard & Hackett, 2005). Briefly, cells were spun down at 3,800g for 10min at 4°C and washed twice with cold 25mM sodium phosphate buffer pH6. Following resuspension in cold, 10mL phosphate buffer, the cells were added drop wise to an equal volume of boiling 8% SDS solution, boiled for 1hr, and spun down for at 30,000g for 30min at 15°C. Boiling and centrifugation were repeated. The pellet was washed 5 times in 10mL of phosphate buffer following centrifugation at 30,000g for 30min. PG was collected through ultracentrifugation for 30min at 162,000g with resuspension in 3mL phosphate buffer. Cell wall PG was digested for 48 hours in 20ug/mL mutanolysin (Sigma) and Amico-Ultra 10kDA filters were used to eliminate insoluble fragments. Soluble PG was reduced with 10 mg/mL sodium borohydride in 0.5 M borate buffer pH=8 for 20 minutes with the reaction stopped by lowering sample pH to 2 using o-phosphoric acid. HPLC separation of soluble PG fragments was performed as previously described (Dougherty, 1985). Fractions of individual peaks were collected and desalted by rp-HPLC using an acetonitrile gradient. Fractions of interest were analyzed by positive ion electrospray ionization time-of-flight mass spectrometry at the University of Wisconsin Biotechnology Center.
Transformation assays
Strains were plated for lawn growth on GCB solid media and grown for 20 hr. Lawns were swabbed into 1mL transformation media (GCBL, 1mM IPTG, 5mM MgSO4 and Kellogg supplements I and II, pH7.2) using a sterile polyester swab (Puritan) to an optical density (OD) at 550nm of approximately 1.5. 20uL of cell suspension was added to 200uL of transformation media containing 150ng of pSY6, a plasmid containing the gyrB1 allele conferring resistance to Nal (Stein et al., 1991). The cell and DNA suspensions were incubated at 37°C for 20 min prior to dilution into 2mL of 37°C transformation media. Cells were incubated for 4hr at 37°C, 5% CO2 before serial dilution. 10uL serial dilutions were plated in triplicate on GCB solid media and GCB solid media containing Nal. Reported transformation efficiencies are the ratio of antibiotic resistant CFUs to total CFUs and are the mean of at least three replicate experiments.
Imaging of colony morphology
Strains were grown on GCB solid media for 20 hr and imaged using a Nikon DS-Fi1 camera attached to a Nikon SMZ-10A stereo microscope.
Measuring growth on solid media
Cells were plated on GCB from frozen stocks and grown for 20 hr. Several isolated colonies were suspended in GCBL and dilutions were plated on GCB for isolated colony growth. At 16hr post inoculation, 6 single colonies per strain were picked using a sterile 6 mm filter disk (GE Healthcare) and dispersed in 500uL GCBL by vortexing. 10uL of serial dilutions were spot plated in triplicate and the resulting colonies enumerated.
Transmission Electron Microscopy
Whole cell TEMs were taken from preparations as previously described (Obergfell & Seifert, 2016). Briefly, strains were grown on solid media and adhered to 300-mesh nickel grids. Cells were fixed with PFA and glutaraldehyde and negatively stained with uranyl acetate. Thin-section micrographs were taken from samples prepared by Northwestern’s Center for Advanced Microscopy. Strains were plated for lawn growth on GCB solid media and grown for 20hr. Two plates per strain were swabbed into 1mL phosphate buffered saline pH7.4 (PBS) and pelleted at 14,000g for 1min. The pellet was suspended in 1% paraformaldehyde in PBS and kept at room temperature (RT) for 1hr. Cell culture samples were fixed in 0.1 M sodium cacodylate buffer (pH 7.3) containing 2% paraformaldehyde and 2.5% glutaraldehyde and were postfixed with 2% osmium tetroxide in 0.1 M sodium cacodylate buffer. They were then rinsed with distilled water, en bloc stained with 3% uranyl acetate, rinsed a second time with distilled water, dehydrated in ascending grades of ethanol, transitioned with propylene oxide, embedded in the resin mixture of the EMbed 812 kit, and cured in a 60°C oven. Samples were sectioned on a Leica Ultracut UC6 ultramicrotome. 70 nm thin sections were collected on 200 mesh copper grids, post stained with 3% uranyl acetate and Reynolds lead citrate. All imaging was performed on a FEI Tecnai Spirit G2 120-KV with digital images captured on FEI Eagle camera at Northwestern’s Center for Advanced Microscopy.
Quantification of average N. gonorrhoeae cross-sectional area
Thin-section TEMs were imaged at 1900–2900X magnification. Images were imported into ImageJ, converted to binary format, inverted, and particle size was analyzed with exclusion on the edges and gating on a cross-sectional area between 0.3 and 2.0um2 to eliminate sections through the edges of cells and cell clusters. Reported cell cross-sectional area is the mean ± standard deviation of at least 1300 cells imaged.
Western blots
Protein was isolated from cell lysates of N. gonorrhoeae strains grown as lawns on GCB solid media for 20hr. Bacteria was swabbed into 1mL PBS, spun down at 4,000g for 5 min and washed with 0.5mL PBS. Cells were suspended in 550uL PBS and 50uL of the cell suspension was reserved for BCA analysis. SDS lysis buffer (60mM Tris-HCl, 25% glycerol, 2% SDS, 14.4mM 2-mercaptoethanol, 0.1% bromophenol blue, pH6.8) and 1mg/mL chicken egg white lysozyme was added to the cell suspension and rotated for 2hr at 4°C. Samples were passed through a needle to sheer DNA and boiled for 5 min. Samples were loaded and run on SDS-Page gels and blotted as described previously (Obergfell & Seifert, 2016). K36 α-PilE peptide antibody (Forest et al., 1996) was used at 1:50,000 dilution as the primary antibody and Peroxidase-conjugated AffinPure Gt α-Rabbit IgG (Jackson ImmunoResearch) was used as the secondary antibody at 1:10,000 dilution.
For the PilQ blot, protein samples were normalized by coomassie quantification and were run on a Mini-PROTEAN TGX 4–15% gel (BioRad). PilQ antibody was used at a dilution of 1:10,000 (graciously provided by C. E. Wilde, Indiana University School of Medicine) and Peroxidase-conjugated AffinPure Gt α-Rabbit IgG was again used as the secondary antibody. Quantification of blots was done using ImageJ.
Antibiotic sensitivity testing
Minimum inhibitory concentrations (MIC) were assayed using Etest strips (Biomerieux). N. gonorrhoeae was grown in lawn cultures for 20hr and suspended in GCBL to an OD550 of approximately 1.0. Cell suspensions were diluted 1:4 into 48°C GCB-Top agar (23.2g GCB in a 1:5 dilution of GCBL). 4mL of GCB-Top-agar, cell suspensions were spread onto 37°C GCB agar plates and allowed to solidify at RT for 15min. A single E-test strip was placed in the center of each plate. Following incubation at 37°C, 5% CO2 for 20hr the zone of clearing was used to determine the MIC for each strain and each antibiotic. Reported MICs are the mean of at least three independent experiments.
Assaying detergent mediated lysis
Lawn growths of strains were grown for 20hr on GCB solid media and suspended in 1mL PBS. Cells were centrifuged at 14,000g and suspended in 1mL PBS two times. A 50µL aliquot was taken for each strain and used to determine the total protein concentration using a bicinchoninic acid assay (Peirce BCA Assay). Cells were then centrifuged at 14,000g and resuspended to a normalized concentration based upon the BCA results. Cells were then lysed in SDS lysis buffer ± 1mg/mL chicken egg white lysozyme for 2hr rotating at 4°C. Samples were then passed through a small bore needle to shear genomic DNA, boiled for 5min and equal volumes were loaded and electrophoresed on 10% SDS-PAGE gels. Total protein was visualized using Coomassie Brilliant Blue and quantified using Image Studio (LiCor).
SDS sensitivity testing
Strains were grown for 22hr on GCB solid media and 20 colonies were picked and struck for lawn growth on fresh GCB solid media. Lawns were grown for 8hr, suspended in 6mL amended GCBL, and incubated at 30°C overnight in a rotating drum. Liquid cultures were back diluted 1:2 in fresh amended GCBL and grown for 2.5hr at 37°C in a rotating drum. Cultures were back diluted to an OD550 of 0.07 and grown for 4hr at 37°C in a rotating drum. 900µL of culture from each strain was incubated with either 100µL PBS or 0.001% SDS in PBS for 1hr at 37°C in a rotating drum. Cultures were serially diluted in GCBL and 10µL of serial dilutions were spotted on GCB solid media in triplicate. CFUs were enumerated and sensitivity was reported as the ratio of CFUs of SDS treated culture to CFUs of PBS treated cultures. The reported ratio represents the mean of at least three independent experiments.
Supplementary Material
Summary.
The composition and architecture of the bacterial cell wall is an essential factor in proper growth and communication of the bacterium with the outside world. This work shows that inactivation of two proteins that modify the cell wall of Neisseria gonorrhoeae, the bacterium that causes gonorrhea, produces viable bacterial cells that actually grow faster but are more sensitive to antibiotics and no longer express a major virulence factor.
Acknowledgments
Electron microscopy was performed at Northwestern’s Center for Advanced Microscopy. DNA sequencing was completed by The Genomics Core at the Northwestern Center for Genetic Medicine. Mass spectrometry was completed at the University of Wisconsin Biotechnology Center. This work was supported by National Institutes of Health grants R37 Al033493 to HSS and R01 AI097157 JPD.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Ameyama S, Onodera S, Takahata M, Minami S, Maki N, Endo K, Goto H, Suzuki H, Oishi Y. Mosaic-Like Structure of Penicillin-Binding Protein 2 Gene (penA) in Clinical Isolates of Neisseria gonorrhoeae with Reduced Susceptibility to Cefixime. Antimicrob. Agents Chemother. 2002;46:3744–3749. doi: 10.1128/AAC.46.12.3744-3749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antignac A, Rousselle JC, Namane A, Labigne A, Taha MK, Boneca IG. Detailed structural analysis of the peptidoglycan of the human pathogen Neisseria meningitidis. J. Biol. Chem. 2003;278:31521–31528. doi: 10.1074/jbc.M304749200. [DOI] [PubMed] [Google Scholar]
- Barbour AG. Properties of penicillin-binding proteins in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 1981;19:316–322. doi: 10.1128/aac.19.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry JL, Pelicic V. Exceptionally widespread nanomachines composed of type IV pilins: the prokaryotic Swiss Army knives. FEMS Microbiol. Rev. 2015;39:134–154. doi: 10.1093/femsre/fuu001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottcher JP. Mathematics and Natural Sciences. Humboldt University of Berlin; 2011. Piliated Neisseria gonorrhoeae induce host cell signaling to stabilize extracellular colonization and microcolony formation; p. 144. [Google Scholar]
- Carter T, Buensuceso RN, Tammam S, Lamers RP, Harvey H, Howell PL, Burrows LL. The Type IVa Pilus Machinery Is Recruited to Sites of Future Cell Division. mBio. 2017:8. doi: 10.1128/mBio.02103-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YW, Rettberg LA, Treuner-Lange A, Iwasa J, Sogaard-Andersen L, Jensen GJ. Architecture of the type IVa pilus machine. Science. 2016;351:aad2001. doi: 10.1126/science.aad2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, Tobiason DM, Thomas CE, Shafer WM, Seifert HS, Sparling PF. A mutant form of the Neisseria gonorrhoeae pilus secretin protein PilQ allows increased entry of heme and antimicrobial compounds. J. Bacteriol. 2004;186:730–739. doi: 10.1128/JB.186.3.730-739.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhang W, Shi Q, Hesek D, Lee M, Mobashery S, Shoichet BK. Crystal structures of penicillin-binding protein 6 from Escherichia coli. J. Am. Chem. Soc. 2009;131:14345–14354. doi: 10.1021/ja903773f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloud-Hansen KA, Hackett KT, Garcia DL, Dillard JP. Neisseria gonorrhoeae uses two lytic transglycosylases to produce cytotoxic peptidoglycan monomers. J. Bacteriol. 2008;190:5989–5994. doi: 10.1128/JB.00506-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen EJ, Ferreira JL, Ladinsky MS, Beeby M, Hughes KT. Nanoscale-length control of the flagellar driveshaft requires hitting the tethered outer membrane. Science. 2017;356:197–200. doi: 10.1126/science.aam6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig L, Pique ME, Tainer JA. Type IV pilus structure and bacterial pathogenicity. Nature reviews. Microbiology. 2004;2:363–378. doi: 10.1038/nrmicro885. [DOI] [PubMed] [Google Scholar]
- Dietrich M, Bartfeld S, Munke R, Lange C, Ogilvie LA, Friedrich A, Meyer TF. Activation of NF-kappaB by Neisseria gonorrhoeae is associated with microcolony formation and type IV pilus retraction. Cell Microbiol. 2011;13:1168–1182. doi: 10.1111/j.1462-5822.2011.01607.x. [DOI] [PubMed] [Google Scholar]
- Dillard JP, Hackett KT. Mutations affecting peptidoglycan acetylation in Neisseria gonorrhoeae and Neisseria meningitidis. Infect. Immun. 2005;73:5697–5705. doi: 10.1128/IAI.73.9.5697-5705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty TJ. Analysis of Neisseria gonorrhoeae peptidoglycan by reverse-phase, high-pressure liquid chromatography. J. Bacteriol. 1985;163:69–74. doi: 10.1128/jb.163.1.69-74.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowson CG, Jephcott AE, Gough KR, Spratt BG. Penicillin-binding protein 2 genes of non-beta-lactamase- producing, penicillin-resistant strains of Neisseria gonorrhoeae. Mol. Microbiol. 1989;3:35–41. doi: 10.1111/j.1365-2958.1989.tb00101.x. [DOI] [PubMed] [Google Scholar]
- Forest KT, Bernstein SL, Getzoff ED, So M, Tribbick G, Geysen HM, Deal CD, Tainer JA. Assembly and antigenicity of the Neisseria gonorrhoeae pilus mapped with antibodies. Infect. Immun. 1996;64:644–652. doi: 10.1128/iai.64.2.644-652.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis Ndowa ML-N. Global action plan to control the spread and impact of antimicrobial resistance in Neisseria gonorrhoeae. World Health Organization; 2012. [Google Scholar]
- Gangel H, Hepp C, Müller S, Oldewurtel ER, Aas FE, Koomey M, Maier B. Concerted Spatio-Temporal Dynamics of Imported DNA and ComE DNA Uptake Protein during Gonococcal Transformation. PLOS Pathogens. 2014;10:e1004043. doi: 10.1371/journal.ppat.1004043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bustos JF, Dougherty TJ. Alterations in peptidoglycan of Neisseria gonorrhoeae induced by sub-MICs of beta-lactam antibiotics. Antimicrob. Agents Chemother. 1987;31:178–182. doi: 10.1128/aac.31.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs CP, Reimann BY, Schultz E, Kaufmann A, Haas R, Meyer TF. Reassortment of pilin genes in Neisseria gonorrhoeae occurs by two distinct mechanisms. Nature. 1989;338:651–652. doi: 10.1038/338651a0. [DOI] [PubMed] [Google Scholar]
- Goire N, Lahra MM, Chen M, Donovan B, Fairley CK, Guy R, Kaldor J, Regan D, Ward J, Nissen MD, Sloots TP, Whiley DM. Molecular approaches to enhance surveillance of gonococcal antimicrobial resistance. Nature reviews. Microbiology. 2014;12:223–229. doi: 10.1038/nrmicro3217. [DOI] [PubMed] [Google Scholar]
- Haas R, Schwarz H, Meyer TF. Release of soluble pilin antigen coupled with gene conversion in Neisseria gonorrhoeae. Proc. Natl. Acad. Sci. U. S. A. 1987;84:9079–9083. doi: 10.1073/pnas.84.24.9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm RA, Barnhart MM, Seifert HS. pilQ Missense mutations have diverse effects on PilQ multimer formation, piliation, and pilus function in Neisseria gonorrhoeae. J. Bacteriol. 2007;189:3198–3207. doi: 10.1128/JB.01833-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell-Adams B, Seifert HS. Molecular models accounting for the gene conversion reactions mediating gonococcal pilin antigenic variation. Mol. Microbiol. 2000;37:1146–1158. doi: 10.1046/j.1365-2958.2000.02067.x. [DOI] [PubMed] [Google Scholar]
- Kanehisa M, Furumichi M, Tanabe M, Sato Y, Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45:D353–d361. doi: 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long CD, Madraswala RN, Seifert HS. Comparisons between colony phase variation of Neisseria gonorrhoeae FA1090 and pilus, pilin, and S-pilin expression. Infect. Immun. 1998;66:1918–1927. doi: 10.1128/iai.66.5.1918-1927.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long CD, Tobiason DM, Lazio MP, Kline KA, Seifert HS. Low-level pilin expression allows for substantial DNA transformation competence in Neisseria gonorrhoeae. Infect. Immun. 2003;71:6279–6291. doi: 10.1128/IAI.71.11.6279-6291.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier B, Potter L, So M, Long CD, Seifert HS, Sheetz MP. Single pilus motor forces exceed 100 pN. Proc. Natl. Acad. Sci. U. S. A. 2002;99:16012–16017. doi: 10.1073/pnas.242523299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler-Bauer A, Bo Y, Han L, He J, Lanczycki CJ, Lu S, Chitsaz F, Derbyshire MK, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Lu F, Marchler GH, Song JS, Thanki N, Wang Z, Yamashita RA, Zhang D, Zheng C, Geer LY, Bryant SH. CDD/SPARCLE: functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 2017;45:D200–d203. doi: 10.1093/nar/gkw1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrogiorgos N, Mekasha S, Yang Y, Kelliher MA, Ingalls RR. Activation of NOD receptors by Neisseria gonorrhoeae modulates the innate immune response. Innate Immun. 2014;20:377–389. doi: 10.1177/1753425913493453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum M, Tammam S, Khan A, Burrows LL, Howell PL. The molecular mechanism of the type IVa pilus motors. Nature communications. 2017;8:15091. doi: 10.1038/ncomms15091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehr IJ, Long CD, Serkin CD, Seifert HS. A homologue of the recombination-dependent growth gene, rdgC, is involved in gonococcal pilin antigenic variation. Genetics. 2000;154:523–532. doi: 10.1093/genetics/154.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melly MA, McGee ZA, Rosenthal RS. Ability of monomeric peptidoglycan fragments from Neisseria gonorrhoeae to damage human fallopian-tube mucosa. J. Infect. Dis. 1984;149:378–386. doi: 10.1093/infdis/149.3.378. [DOI] [PubMed] [Google Scholar]
- Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, Stevens G, Gottlieb S, Kiarie J, Temmerman M. Global Estimates of the Prevalence and Incidence of Four Curable Sexually Transmitted Infections in 2012 Based on Systematic Review and Global Reporting. PloS one. 2015;10:e0143304. doi: 10.1371/journal.pone.0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien JP, Goldenberg DL, Rice PA. Disseminated gonococcal infection: a prospective analysis of 49 patients and a review of pathophysiology and immune mechanisms. Medicine (Baltimore) 1983;62:395–406. [PubMed] [Google Scholar]
- Obergfell KP, Seifert HS. Mobile DNA in the pathogenic Neisseria. Microbiol Spectr. 2015;3:0015–2014. doi: 10.1128/microbiolspec.MDNA3-0015-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obergfell KP, Seifert HS. The Pilin N-terminal Domain Maintains Neisseria gonorrhoeae Transformation Competence during Pilus Phase Variation. PLoS Genet. 2016;12:e1006069. doi: 10.1371/journal.pgen.1006069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho MG, Kjos M, Veening JW. How to get (a)round: mechanisms controlling growth and division of coccoid bacteria. Nature reviews. Microbiology. 2013;11:601–614. doi: 10.1038/nrmicro3088. [DOI] [PubMed] [Google Scholar]
- Prevention, C.f.D.C.a. Antibiotic resistance threats in the United States, 2013. Atlanta: CDC; 2013. [Google Scholar]
- Ragland SA, Schaub RE, Hackett KT, Dillard JP, Criss AK. Two lytic transglycosylases in Neisseria gonorrhoeae impart resistance to killing by lysozyme and human neutrophils. Cell Microbiol. 2017:19. doi: 10.1111/cmi.12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropp PA, Nicholas RA. Cloning and characterization of the ponA gene encoding penicillin-binding protein 1 from Neisseria gonorrhoeae and Neisseria meningitidis. J. Bacteriol. 1997;179:2783–2787. doi: 10.1128/jb.179.8.2783-2787.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel T, Facius D, Barten R, Scheuerpflug I, Nonnenmacher E, Meyer TF. Role of pili and the phase-variable PilC protein in natural competence for transformation of Neisseria gonorrhoeae. Proc. Natl. Acad. Sci. U. S. A. 1995;92:7986–7990. doi: 10.1073/pnas.92.17.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvage E, Kerff F, Terrak M, Ayala JA, Charlier P. The penicillin-binding proteins: structure and role in peptidoglycan biosynthesis. FEMS Microbiol. Rev. 2008;32:234–258. doi: 10.1111/j.1574-6976.2008.00105.x. [DOI] [PubMed] [Google Scholar]
- Seifert HS. Insertionally inactivated and inducible recA alleles for use in Neisseria. Gene. 1997;188:215–220. doi: 10.1016/s0378-1119(96)00810-4. [DOI] [PubMed] [Google Scholar]
- Seifert HS, Wright CJ, Jerse AE, Cohen MS, Cannon JG. Multiple gonococcal pilin antigenic variants are produced during experimental human infections. J. Clin. Invest. 1994;93:2744–2749. doi: 10.1172/JCI117290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siewering K, Jain S, Friedrich C, Webber-Birungi MT, Semchonok DA, Binzen I, Wagner A, Huntley S, Kahnt J, Klingl A, Boekema EJ, Sogaard-Andersen L, van der Does C. Peptidoglycan-binding protein TsaP functions in surface assembly of type IV pili. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E953–961. doi: 10.1073/pnas.1322889111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling PF. Genetic transformation of Neisseria gonorrhoeae to streptomycin resistance. J. Bacteriol. 1966;92:1364–1371. doi: 10.1128/jb.92.5.1364-1371.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt BG, Cromie KD. Penicillin-binding proteins of gram-negative bacteria. Rev. Infect. Dis. 1988;10:699–711. doi: 10.1093/clinids/10.4.699. [DOI] [PubMed] [Google Scholar]
- Stefanova ME, Tomberg J, Davies C, Nicholas RA, Gutheil WG. Overexpression and enzymatic characterization of Neisseria gonorrhoeae penicillin-binding protein 4. Eur. J. Biochem. 2004;271:23–32. doi: 10.1046/j.1432-1033.2003.03886.x. [DOI] [PubMed] [Google Scholar]
- Stefanova ME, Tomberg J, Olesky M, Holtje JV, Gutheil WG, Nicholas RA. Neisseria gonorrhoeae penicillin-binding protein 3 exhibits exceptionally high carboxypeptidase and beta-lactam binding activities. Biochemistry (Mosc) 2003;42:14614–14625. doi: 10.1021/bi0350607. [DOI] [PubMed] [Google Scholar]
- Stein DC, Danaher RJ, Cook TM. Characterization of a gyrB mutation responsible for low-level nalidixic acid resistance in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 1991;35:622–626. doi: 10.1128/aac.35.4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DS, Whitney AM, Rothbard J, Schoolnik GK. Pili of Neisseria meningitidis. Analysis of structure and investigation of structural and antigenic relationships to gonococcal pili. J. Exp. Med. 1985;161:1539–1553. doi: 10.1084/jem.161.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohl EA, Dale EM, Criss AK, Seifert HS. Neisseria gonorrhoeae metalloprotease NGO1686 is required for full piliation, and piliation is required for resistance to H2O2- and neutrophil-mediated killing. mBio. 2013:4. doi: 10.1128/mBio.00399-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J. Exp. Med. 1973;137:571–589. doi: 10.1084/jem.137.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J, Robbins K, Barrera O, Corwin D, Boslego J, Ciak J, Blake M, Koomey JM. Gonococcal pilin variants in experimental gonorrhea. J. Exp. Med. 1987;165:1344–1357. doi: 10.1084/jem.165.5.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swasdio K, Rugpao S, Tansathit T, Uttavichai C, Jongusuk P, Vutayavanich T, Oranratanachai A, Pruthitada N, Peerakom S, Ittipunkul W, Rowe PJ, Ward ME. The association of Chlamydia trachomatis/gonococcal infection and tubal factor infertility. J. Obstet. Gynaecol. Res. 1996;22:331–340. doi: 10.1111/j.1447-0756.1996.tb00985.x. [DOI] [PubMed] [Google Scholar]
- Typas A, Banzhaf M, Gross CA, Vollmer W. From the regulation of peptidoglycan synthesis to bacterial growth and morphology. Nature reviews. Microbiology. 2011;10:123–136. doi: 10.1038/nrmicro2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unemo M, Nicholas RA. Emergence of multidrug-resistant, extensively drug-resistant and untreatable gonorrhea. Future microbiology. 2012;7:1401–1422. doi: 10.2217/fmb.12.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unemo M, Shafer WM. Antimicrobial Resistance in Neisseria gonorrhoeae in the 21st Century: Past, Evolution, and Future. Clin. Microbiol. Rev. 2014;27:587–613. doi: 10.1128/CMR.00010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veyrier FJ, Biais N, Morales P, Belkacem N, Guilhen C, Ranjeva S, Sismeiro O, Pehau-Arnaudet G, Rocha EP, Werts C, Taha MK, Boneca IG. Common Cell Shape Evolution of Two Nasopharyngeal Pathogens. PLoS Genet. 2015;11:e1005338. doi: 10.1371/journal.pgen.1005338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virji M. Pathogenic Neisseriae: surface modulation, pathogenesis and infection control. Nature reviews. Microbiology. 2009;7:274–286. doi: 10.1038/nrmicro2097. [DOI] [PubMed] [Google Scholar]
- Wasserheit JN. Effect of changes in human ecology and behavior on patterns of sexually transmitted diseases, including human immunodeficiency virus infection. Proc. Natl. Acad. Sci. U. S. A. 1994;91:2430–2435. doi: 10.1073/pnas.91.7.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfgang M, Lauer P, Park HS, Brossay L, Hebert J, Koomey M. pilT mutations lead to simultaneous defects in competence for natural transformation and twitching motility in piliated Neisseria gonorrhoeae. Mol. Microbiol. 1998a;29:321–330. doi: 10.1046/j.1365-2958.1998.00935.x. [DOI] [PubMed] [Google Scholar]
- Wolfgang M, Park HS, Hayes SF, van Putten JP, Koomey M. Suppression of an absolute defect in type IV pilus biogenesis by loss-of-function mutations in pilT, a twitching motility gene in Neisseria gonorrhoeae. Proc. Natl. Acad. Sci. U. S. A. 1998b;95:14973–14978. doi: 10.1073/pnas.95.25.14973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapun A, Vernet T, Pinho MG. The different shapes of cocci. FEMS Microbiol. Rev. 2008;32:345–360. doi: 10.1111/j.1574-6976.2007.00098.x. [DOI] [PubMed] [Google Scholar]
- Zelewska MA, Pulijala M, Spencer-Smith R, Mahmood HA, Norman B, Churchward CP, Calder A, Snyder LA. Phase variable DNA repeats in Neisseria gonorrhoeae influence transcription, translation, and protein sequence variation. Microbial genomics. 2016;2:e000078. doi: 10.1099/mgen.0.000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QY, Spratt BG. Nucleotide sequence of the penicillin-binding protein 2 gene of Neisseria meningitidis. Nucleic Acids Res. 1989;17:5383. doi: 10.1093/nar/17.13.5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielke RA, Wierzbicki IH, Weber JV, Gafken PR, Sikora AE. Quantitative proteomics of the Neisseria gonorrhoeae cell envelope and membrane vesicles for the discovery of potential therapeutic targets. Molecular & cellular proteomics : MCP. 2014;13:1299–1317. doi: 10.1074/mcp.M113.029538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.