Abstract
Rationale and Objective
National vascular access guidelines recommend placement of arteriovenous fistulas (AVF) over grafts (AVG) in hemodialysis patients, but have not been comprehensively assessed in the elderly. We evaluated clinically relevant vascular access outcomes in elderly patients receiving an AVF or AVG after hemodialysis initiation.
Study Design
Retrospective cohort study using national administrative data.
Settings and Partcipants
Claims data from United States Renal Data System of 9,458 U.S. patients age ≥67 years who initiated hemodialysis from 7/1/2010-6/30/2011 with a catheter and received an AVF (n=7,433) or AVG (n=2,025) within the ensuing six months.
Predictor
Arteriovenous access subtype, AVF or AVG.
Outcomes
Successful use of vascular access, interventions to make vascular access functional, duration of catheter-dependence prior to successful use of vascular access, frequency of interventions and abandonment after successful use of vascular access.
Analytical Approach
Multivariable logistic regression analysis was used to compare the need for intervention before successful use of AVFs and AVGs, and negative bionomial regression was used to calculate frequency of intervention after successful use of vascular access.
Results
Unsuccessful use of vascular access within 6 months of creation was higher for AVFs versus AVGs (51% vs 45%; adjusted hazard ratio (HR), 1.86; 95% confidence interval (CI), 1.73-1.99). Interventions to make vascular access functional were greater in AVFs versus AVGs (42% vs 23%; OR, 2.66; 95% CI, 2.26-3.12). AVFs had lower one-year abandonment rate after successful use compared to AVGs (OR, 0.71; 95% CI, 0.62-0.83), and required one-fourth fewer interventions after successful use (RR, 0.75; 95% CI, 0.69-0.81). Patients receiving an AVF had substantially longer catheter-dependence prior to successful use than those receiving an AVG (median time 3 vs 1 month, p<0.001).
Limitations
Residual confounding due to vascular access choice, restriction to an elderly population, and a one-year follow-up period.
Conclusions
In elderly hemodialysis patients initiating hemodialysis with a catheter, the optimal vascular access selection depends on trade-offs between shorter catheter-dependence and less frequent interventions to make the vascular access (AVG) functional vs longer access patency and fewer interventions after successful use of the vascular access (AVF).
Keywords: End-Stage Renal Disease (ESRD), Hemodialysis (HD), Vascular Access, Angioplasty Arteriovenous Fistula (AVF), Arteriovenous Graft (AVG), Central Venous Catheter (CVC), Fistula First, patency, interventions, catheter dependence, renal replacement therapy (RRT), elderly, dialysis initiation
Introduction
Vascular access is the “lifeline” for hemodialysis patients, providing a critical conduit for delivery of blood to the extracorporeal circuit. Over 80% of U.S. hemodialysis patients initiate dialysis with a central venous catheter1, with most subsequently undergoing placement of a permanent vascular access, either an arteriovenous fistula (AVF) or graft (AVG). Patients remain catheter-dependent until their AVF or AVG can be successfully used for dialysis, and longer duration of catheter dependence has been associated with increased risk for catheter-related bacteremia and death2-4. Surgical or endovascular interventions are frequently required to make vascular accesses functional for successful use on dialysis5-11. Even after successful use of a vascular access, a vascular access often requires additional interventions to maintain long-term patency for dialysis. Ultimately, many AVFs and AVGs are abandoned, usually due to irreversible thrombosis.
The Fistula First Initiative, launched by the Centers for Medicare & Medicaid Services (CMS) in 2002, urges providers to maximize AVF use in preference to an AVG12. The rationale is that AVFs have long-term survival superior to that of AVGs, and require fewer interventions to maintain such patency13. Implementation of the Fistula First Initiative recommendations has resulted in AVF placement in many elderly patients who would have previously received an AVG14. Concurrently, there has been a substantial increase of percutaneous interventions in AVFs, both to salvage AVFs that are unable to be successfully used for dialysis, as well as to maintain their long-term patency after maturation15. Such interventions delay successful AVF use, further prolonging catheter use. Two small observational studies reported that interventions to make the vascular access functional are also associated with a subsequent shortening of vascular access patency, and an increase in the frequency of interventions to maintain such patency16,17.
We previously compared clinical outcomes (deaths and hospitalizations) in a national cohort of elderly patients who initiated hemodialysis with a central venous catheter and subsequently had an AVF or AVG placed18. We found that placement of an AVF rather than an AVG is associated with greater patient survival, despite longer CVC-dependence18. The present study comprehensively compared several clinically relevant vascular access-related outcomes in the same cohort of elderly hemodialysis patients, to better understand the tradeoffs between AVF and AVG selection.
Methods
Data Sources and Study Population
We used standard analytic files derived from the United States Renal Data System (USRDS) for July 1, 2010 to December 31, 2013. Two-year pre-end stage renal disease (ESRD) Medicare data provided additional baseline information including co-morbidities, as previously published18,19. All incident hemodialysis patients ≥ 67 years of age who had their first ESRD service in the one-year period between 7/1/2010 and 6/30/2011 were identified as our baseline population. To ensure the catheter was the only vascular access present at the start of hemodialysis, patients were excluded from the study cohort if: (1) they were using an AVF or AVG, or had an AVF or AVG placed already but awaiting successful use at hemodialysis initiation, as reported in the 2728 Medical Evidence Form 20,21; or (2) they underwent AVF or AVG surgery in the two-year pre-ESRD period, as assessed by Current Procedural Terminology-4 procedure codes.
Since we used encrypted patient information and reported aggregate data, we did not require research ethics committee approval. Informed consent was also waived due to de-identified information.
Variables of Interest
The main study exposure was the vascular access type (AVG or AVF) inserted within 6 months of hemodialysis initiation, identified by using Current Procedural Terminology (CPT)-4 codes of 36818, 36819, 36820, 36821, and 36825 for AVF insertion and 36800, 36810, and 36830 for AVG insertion22,23.
Optimal vascular access management requires a complex set of consecutive processes of care, each of which must be overcome to achieve the goal of a successful and durable access. First, a vascular access must be surgically created in patients with a catheter. Second, it must reach the point of successful use and be used repeatedly to deliver dialysis. Third, once successful use of the vascular access has been achieved, it needs to remain patent for a prolonged period of time, often requiring subsequent interventions. Using this spectrum of care processes, we identified key study vascular access outcomes as indicated in the following.
Unsuccessful use of vascular access occurred if an AVF or AVG was not used for dialysis within 6 months of its creation. Effective July 2010, all dialysis units were required by CMS to report monthly vascular access use for all active hemodialysis patients using vascular access modifiers: V5 (catheter), V6 (AVG), or V7 (AVF). A patient with a concurrent CVC and a maturing AVF or AVG is reported as dialyzing with a CVC. These reports were used to ascertain when an AVF or AVG was successfully used for hemodialysis. Successful use of vascular access was deemed to have occurred during the first month in which the patient was reported as using it.
Interventions to make a vascular access functional were defined based on if an intervention(s) was required for the AVF or AVG prior to its successful use. Patients were considered to have an intervention to make the AVF or AVG functional for successful use on dialysis if they underwent a vascular access intervention prior to successful use of vascular access, and to have no intervention to make vascular access functional if they did not undergo such an intervention.
Catheter dependence was defined as the duration of catheter use from AVF/AVG placement to its successful use.
Loss of primary access patency at 1 year denoted a requirement for any access intervention after successful use of vascular access.
For access abandonment at 1 year, the duration of secondary AVF or AVG survival was calculated from its first successful use to abandonment, regardless of the need for interventions to maintain access patency. Abandonment was defined as three consecutive months of CVC use or new AVF or AVG placement.
Frequency of access interventions was calculated based on those interventions required to maintain AVF or AVG use in the one-year period after it matured.
Codes used to identify intervention procedures are listed in Table S1. Interventions to make a vascular access functional, duration of catheter dependency, access abandonment, and frequency of interventions after successful use of vascular access were examined among those who achieved successful use of vascular access.
The Medical Evidence Form provided patient demographics at hemodialysis initiation. Major co-morbidities were identified using one inpatient or two outpatient Medicare claims during the 2-year pre-ESRD period. Liu's comorbidity index was used24. We used the same baseline for all outcomes, defined as hemodialysis initiation. Start of follow-up was defined as the vascular access surgery date for both AVG and AVF patients.
Statistical Analysis
Baseline characteristics were summarized and compared between patients with AVF and AVG placement, respectively, using Pearson's chi-square tests for categorical variables, and nonparametric Wilcoxon rank-sum test for continuous variables. Duration of catheter dependency prior to successful use of vascular access was compared by using Kruskal-Wallis test among four groups: Intervention to make AVF functional, no intervention to make AVF functional, intervention to make AVG functional, and no intervention to make AVG functional. Since the USRDS data reports vascular access use for dialysis sessions on a monthly basis, we selected a discrete time-to-event framework and used complementary log-log models to estimate hazard ratios for successful use of vascular access, primary patency loss, and abandonment. Successful use of vascular access was determined within 6 months of access creation, and the primary patency loss and abandonment were determined at 1 year after successful use of vascular access. Death, a competing event, was treated as censoring in these analyses. We used logistic regression analysis to compare interventions to make vascular access functional between AVF and AVG placement. Frequency of access interventions after successful use was examined by negative binomial regression. All regression analyses were adjusted for age, gender, race, coronary artery disease (any history of myocardial infarction, coronary revascularization, or heart failure), history of stroke, and Liu's co-morbidity index25. Previous publications have associated these factors with successful AVF use 25,26. Statistical analyses were performed using SAS (version 9.3; SAS institute, Cary, NC). All statistical tests were two-sided and a p-value <0.05 considered statistically significant. Last, we used the STROBE guidelines to improve the reporting of our observational research study27.
Results
Baseline Characteristics of the Patients with Successful Use of Vascular Access
Our study population was derived from a cohort of 46,634 patients ≥67 years of age who initiated hemodialysis from 7/1/2010 to 6/30/2011, as previously described18. Of this cohort, 29,178 patients initiated hemodialysis with a CVC only, without an AVF or AVG placed in the pre-ESRD period, awaiting successful use. We then excluded (1) patients without pre-ESRD Medicare claims, (2) those with pre-ESRD AVF/AVG surgeries, (3) those who did not receive an AVF/AVG within 6 months of initiating hemodialysis, and (4) those who received a kidney transplant or switched to peritoneal dialysis. The final study cohort consisted of 9,458 elderly patients who initiated hemodialysis with a CVC and received an AVF (n=7,433) or an AVG (n=2,025) during the ensuing 6 months.
The baseline characteristics of the study cohort are summarized in Table 1. As compared to the group receiving an AVG, the group with an AVF creation was younger, had a greater proportion of males and whites, had a lower Liu co-morbidity index, and was less likely to have history of stroke, peripheral vascular disease, and chronic obstructive pulmonary disease. They also had fewer hospital days in the six months preceding initiation of dialysis. Duration of catheter-dependence from dialysis initiation to vascular access placement was similar (about 10 weeks) in both groups.
Table 1. Characteristics of elderly patients who initiated hemodialysis with only a catheter between July 1, 2010 and June 30, 2011 and had subsequent AVF or AVG placement within 6 months after dialysis initiation, by vascular access type.
| AVF SURGERIES | AVG SURGERIES | p-value | |
|---|---|---|---|
| Total Cohort | 7,433(78.6) | 2,025(21.4) | |
| Age at dialysis initiation, y | 76.7±6.4 | 77.8±6.8 | <0.001 |
| Age category | <0.001 | ||
| 67-<75 y | 3,127(42.1) | 735(36.3) | |
| 75-<85 y | 3,311(44.5) | 921(45.5) | |
| ≥85 y | 995(13.4) | 369(18.2) | |
| Sex | <0.001 | ||
| Male | 4,102(55.2) | 825(40.7) | |
| Female | 3,331(44.8) | 1,200(59.3) | |
| Race | <0.001 | ||
| White | 5,660(76.2) | 1,324(65.4) | |
| Black | 1,372(18.5) | 605(29.9) | |
| Other/unknown | 401(5.4) | 96(4.7) | |
| BMI | 28.5±7.3 | 28.2±7.3 | 0.02 |
| BMI category | 0.3 | ||
| <18.5 kg/m2 | 255(3.5) | 82(4.1) | |
| 18.5-<30 kg/m2 | 4,627(62.6) | 1,262(63.0) | |
| ≥30 kg/m2 | 2,509(34.0) | 658(32.9) | |
| Comorbid conditions | |||
| Liu co-morbidity index | 10.1±4.4 | 10.6±4.5 | <0.001 |
| Liu comorbidity index category | |||
| 0-7 | 1,619(21.8) | 394(19.5) | 0.01 |
| 7-13 | 3,236(43.5) | 861(42.6) | 0.009 |
| >13 | 2,578(34.7) | 768(38.0) | |
| Cardiovascular diseases | |||
| Congestive heart failure | 4,144(55.8) | 1,155(57) | 0.3 |
| Ischemic heart disease | 4,814(64.8) | 1,326(65.5) | 0.5 |
| Coronary revascularization | 837(11.3) | 187(9.2) | 0.009 |
| Acute myocardial infarction | 827(11.1) | 235(11.6) | 0.5 |
| Cerebrovascular disease | 2,365(31.8) | 724(35.8) | 0.001 |
| History of stroke | 1,362(18.3) | 467(23.1) | <0.001 |
| Peripheral vascular disease | 4,119(55.4) | 1,191(58.8) | 0.006 |
| Amputation | 211(2.8) | 67(3.3) | 0.3 |
| Diabetes | 5,123(68.9) | 1,406(69.4) | 0.7 |
| Cancer | 1,534(20.6) | 461(22.8) | 0.04 |
| COPD | 2,779(37.4) | 828(40.9) | 0.004 |
| Inability to ambulate | 1,758(23.7) | 674(33.3) | <0.001 |
| Depression | 975(13.1) | 356(17.6) | <0.001 |
| Dementia | 745(10.0) | 276(13.6) | <0.001 |
| Hospital days (6 mo before ESRD) | 17.1±16.9 | 20.9±20.6 | <0.001 |
| Catheter dependency (days to VA surgery) | 71.3±42.9 | 60.2±49.4 | <0.001 |
| Catheter dependency | <0.001 | ||
| At ≤60 d | 3,311(44.5) | 1,096(54.1) | |
| At >60 d | 4,122(55.5) | 929(45.9) |
Values for continuous data shown as mean +/- SD; for categorical data as count (percent). BMI, body mass index; COPD, Chronic obstructive pulmonary disease; AVF, _____; AVG, _____; ESRD, end-stage renal disease.
Overview of Clinically Relevant Vascular Access Outcomes
We determined the following clinically relevant vascular access outcomes in the patients receiving an AVF or AVG: unsuccessful use of vascular access, interventions to make a vascular access functional, duration of CVC-dependence from vascular access placement to successful use, primary access patency loss, and access abandonment. The numbers and proportions of patients attaining each vascular access outcome, except duration of CVC-dependence, are summarized in Figure 1. In subsequent analyses we calculated the adjusted odds ratios or relative risks of these outcomes for patients receiving an AVF vs an AVG.
Figure 1.
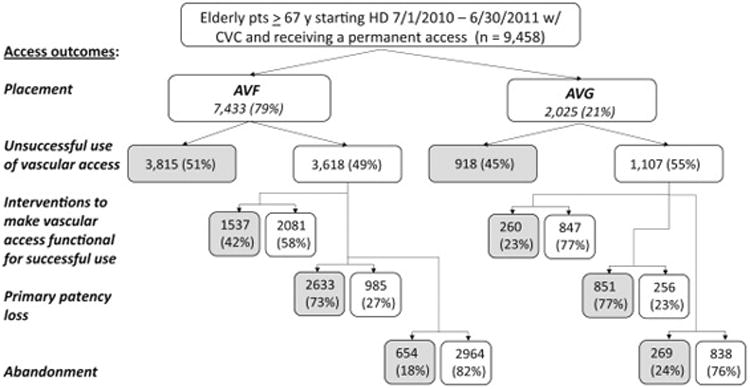
Flow diagram of study cohort. Our baseline population was derived from a cohort of 100,441 patients who initiated hemodialysis from 7/1/2010 to 6/30/2011, of whom 46,634 were >67 years old, and has been previously described. Of this cohort of elderly patients, 29,178 initiated hemodialysis with a catheter only, without a maturing AVF or AVG. We then excluded (1) patients without pre-ESRD Medicare claims, (2) those with pre-ESRD AVF/AVG surgeries, (3) those who did not receive an AVF/AVG within 6 months of initiating hemodialysis, and (4) those who received a kidney transplant or switched to peritoneal dialysis. The final study cohort consisted 9,458 elderly patients who initiated dialysis with a CVC and who received an AVF (n=7,433) or an AVG (n=2,025) during the 6 months after dialysis initiation. Note: Grey shading indicates access outcome occurred.
Unsuccessful Use of Vascular Access
A higher proportion of AVFs than AVGs failed to be used successfully for dialysis in the six-month period following their placement (51 vs 45%; adjusted HR, 1.86; 95% CI, 1.73-1.99)(Figure 2). A substantial proportion of vascular accesses required an intervention to make their vascular access functional. The proportion of patients requiring an intervention make their vascular access functional was higher in patients receiving an AVF vs an AVG (42 vs 23%; OR, 2.66; 95% CI, 2.26-3.12)(Figure 2).
Figure 2.
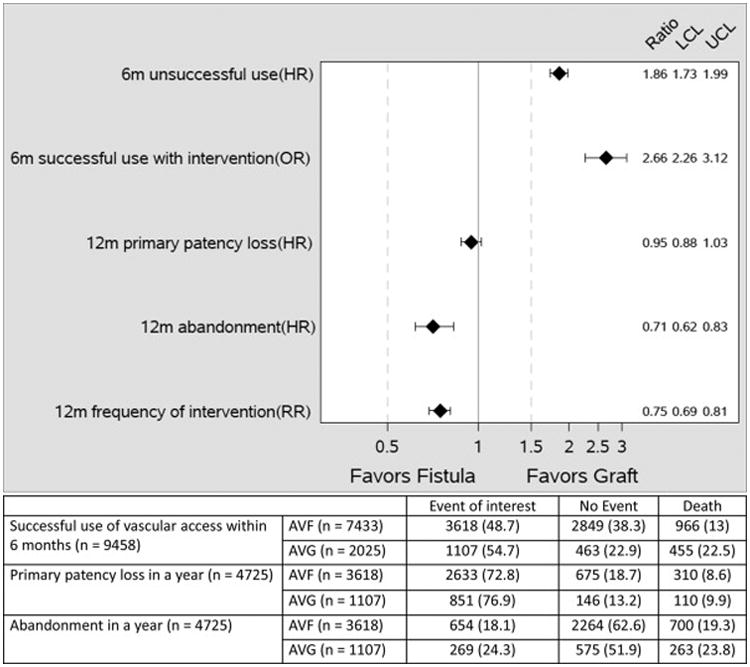
Adjusted hazard ratios (HR) and odds ratio (OR) and 95% confidence interval (CI) of unsuccessful use of vascular access unsuccessful use within 6 months of placement; Interventions to make vascular access functional for successful use on dialysis; primary patency loss (requirement for an intervention after successful use of vascular access; and access abandonment; and adjusted relative risk (RR) and 95% CI of frequency of access interventions in the first year after successful use of vascular access. All models were adjusted for age, gender, race, coronary artery disease (combine: history of myocardial infarction, coronary revascularization, heart failure), history of stroke, and Liu's co-morbidity index.
Duration of Catheter Dependence Before Successful Vascular Access Use
The median duration of catheter-dependence after vascular access creation and before its first use was greater in patients with requiring interventions vs no interventions to make AVFs functional for successful use on dialysis (4 (Q [quartile] 1-Q3, 3-5) vs. 3 (Q1-Q3, 2-4) months; p<0.001)(Figure 3). Likewise, it was greater in patients requiring interventions vs no interventions to make AVGs functional for successful use on dialysis (2 (IQR, 1.5-3) vs 1 (Q1-Q3, 1-2) months; p<0.001). Among patients with interventions to make the vascular functional for successful use on dialysis, catheter-dependence was greater in patients receiving an AVF vs AVG (4 (Q1-Q3, 3-5) vs 2 (Q1-Q3, 1.5-3), p<0.001). Similarly, among patients without interventions to make the vascular access functional for successful use on dialysis, catheter-dependence was greater in those undergoing AVF vs AVG placement (3 (Q1-Q3, 2-4) vs 1 (Q1-Q3, 1-2), p<0.001).
Figure 3.
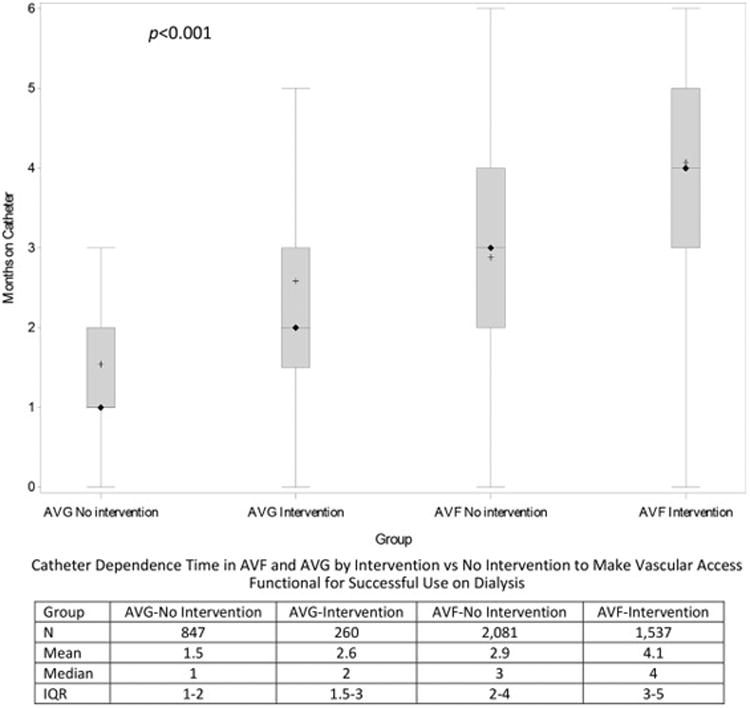
Catheter months before initial access use, calculated as months between the vascular access (AVF/AVG) placement date and its first use. Vascular access surgery must occur within 6 months of dialysis initiation and successful use of vascular access must occur within 6 months of creation surgery. Showing + Mean; ◊ Median; and 25th and 75th percentiles. Kruskal-Wallis test conducted to compare median values (p<0.0001 comparing catheter-dependence between the 4 groups). Pair-wise comparisons of mean duration of catheter dependence between AVFs requiring an intervention vs no intervention to make vascular access functional for successful use on dialysis, AVGs requiring an intervention vs no intervention to make vascular access functional for successful use on dialysis, AVF vs AVG requiring interventions to make vascular access functional for successful use on dialysis, AVF vs AVG not requiring interventions to make vascular access functional for successful use on dialysis had p<0.001.
Vascular Access Outcomes Following Successful Use of Access
In the one-year period after successful use of the vascular access, patients receiving an AVF and AVG were equally likely to lose primary access patency, i.e., require at least one intervention (HR, 0.95; 95% CI, 0.88-1.03)(Figure 2). The one-year access abandonment was lower in patients receiving an AVF vs AVG (HR, 0.71; 95% CI, 0.62-0.83)(Figure 2). The overall frequency of vascular access interventions in the year after successful use of vascular access was also lower among AVF patients vs AVG patients (2.35 vs 3.12; adjusted RR, 0.75; 95% CI, 0.69-0.81)(Figure 2).
Sensitivity Analyses
We had the precise date for each access intervention. In contrast, V codes reported the access in use at the end of each calendar month, without specifying the precise date during the month when the access was successfully used. In the subset of 1325 patients who had an access intervention during the same calendar month as a successfully used vascular access, there was uncertainty about whether the intervention occurred prior to successful use of vascular access (in which case the access was considered to have had an intervention to make the vascular access functional for successful use on dialysis) or whether it followed successful use of vascular access (in which case it was considered to be a intervention after successful use of vascular access). Our primary analysis assumed that all interventions occurring during that month of successful use of vascular access were considered interventions after successful use of vascular access. In a sensitivity analysis, we assumed the opposite, i.e, that all interventions during that month of successful use of vascular access represented interventions to make the vascular access functional. The new HR of interventions to make vascular access functional in AVFs vs AVGs was 1.82 (95% CI, 1.58-2.10) and the new HR for primary patency loss was 0.90 (95% CI, 0.83-0.98); both were lower but in the same direction as our primary study findings. The new RR for frequency of interventions after successful use of the vascular access was 0.75 (95% CI, 0.70-0.82), similar to that in the primary analysis.
Discussion
The current study evaluated several clinically relevant vascular access outcomes in a large national cohort of elderly patients who initiated hemodialysis with a catheter and subsequently received an AVF or AVG. Our main findings were: (1) AVFs have a higher likelihood than AVGs of successful use for dialysis within six months of their creation; (2) AVFs are significantly more likely than AVGs to require interventions to make the vascular access functional; (3) the duration of catheter-dependence following vascular access creation is substantially greater in patients receiving an AVF vs AVG; (4) vascular access abandonment at one year is less likely in patients with AVF placement, as compared to AVGs; and (5) AVFs require less frequent interventions after successful use, as compared to AVGs. In other words, vascular access events preceding successful use favor AVGs, whereas those occurring after successful use favor AVFs. These observations highlight important tradeoffs of AVF placement in elderly hemodialysis patients initiating hemodialysis with a catheter (Table 2). They suggest the need for a more nuanced approach to Fistula First recommendations that addresses the tradeoffs of earlier AVG access use versus longer AVF patency.
Table 2. Summary of parameters regarding vascular access choice for elderly ESRD patients who initiate dialysis with a catheter.
| Vascular Access Outcome | Fistula First Assumption | Current Study Findings vs Fistula First |
|---|---|---|
| Likelihood of access to be unsuccessful for dialysis use: AVF: 51% AVG: 45% |
Most AVFs will eventually be successfully used for dialysis | Contradictory—Among elderly HD patients, a higher proportion of new AVFs vs AVGs are not successfully used for dialysis within 6 months (adjusted OR, 1.86; 95% CI, 1.73-1.99) |
| Interventions to make vascular access functional: AVF: 42.5% AVG: 23.5% |
Most AVFs will be successfully used for dialysis without an intervention | Contradictory—An intervention to achieve successful use for dialysis was required more frequently in patients with an AVF vs those with an AVG (OR, 2.66; 95% CI 2.26-3.12) |
| Access abandonment in 1st year after initial use: AVF: 18% AVG: 24% |
Once they are successfully used for dialysis, AVFs have a lower failure rate than AVGs | Consistent—Among elderly HD patients, AVFs had a lower likelihood than AVGs of failing within one year of successful use (OR, 0.71; 95% CI, 0.62-0.83) |
| Frequency of access interventions required in 1st year after initial use: AVF: 2.35 AVG: 3.12 |
AVFs require fewer interventions than AVGs to maintain patency for HD | Consistent—AVFs required fewer interventions than AVGs (RR, 0.75; 95% CI, 0.69-0.81) |
| Median duration of catheter dependence: AVF: 3 mo AVG: 1 mo |
Placement of more AVFs will decrease catheter dependence | Contradictory—Patients receiving an AVF had a 2-month longer catheter dependence prior to successful use as compared to AVGs (p <0.001) |
AVF = Arteriovenous Fistula; AVG = Arteriovenous Graft; HD = Hemodialysis; OR = Odds Ratio; RR = Relative Risk; CI = Confidence Interval; ESRD, end-stage renal disease
There are currently over 450,000 dialysis patients in the U.S., of whom ∼90% utilize hemodialysis and 80% initiate hemodialysis with a catheter1. A functioning and durable vascular access is essential for the reliable delivery of hemodialysis. Catheters are widely regarded as an inferior access, due to the frequent occurrence of catheter-related bacteremia and dysfunction and their association with higher patient mortality28-31. For this reason, most patients initiating dialysis with a catheter subsequently receive an AVF or AVG. The Fistula First Initiative launched by CMS in 2002, building on the NKF-KDOQI (National Kidney Foundation–Kidney Disease Outcomes Quality Initiative) vascular access guidelines first released in 199732, strongly favors AVF placement in preference to an AVG 12. Recent multi-center vascular access studies have highlighted the high frequency of unsuccessfully used AVFs for dialysis and the requirement for interventions to makethe AVF functional. For example, the Dialysis Access Consortium Study (which enrolled patients from 2003 to 2007) reported that only 40% of new AVFs in U.S. centers were successfully used within six months of their creation33. Subsequently, the Hemodialysis Fistula Maturation Study (which enrolled patients from 2010 to 2013) observed that AVFs not requiring an intervention to make functional occurred in only 43.5% of U.S. patients34, suggesting an increased burden of procedures to achieve successful use. In the current study of elderly incident hemodialysis patients, 51% of AVFs were not successfully used for dialysis within 6 months of their creation, and even of those that were successfully used, 42% required an intervention to make the AVF functional. As a consequence, only 28% (ie, 2081/7433) of patients undergoing AVF creation achieved successful use without an intervention to make the AVF functional. In contrast, 42% (847/2025) of AVGs in this study achieved successful use without an intervention to make the AVG functional.
The Fistula First recommendations were proposed in 2002, at a time when only 24% of U.S. patients were dialyzing with an AVF35, unsuccessful use of AVFs for dialysis was relatively infrequent (20-30%)13, and AVFs requiring interventions to make the AVF functional was also relatively uncommon. As a consequence of patient selection, vascular access longevity after successful use was clearly superior for AVFs as compared to AVGs. Fifteen years later, now that AVFs are placed in most patients (78% of the current study cohort of elderly incident hemodialysis patients), their advantages over AVGs are not as evident in the elderly hemodialysis population. We previously compared clinical outcomes (deaths and hospitalizations) in the same national cohort of elderly dialysis patients who initiated hemodialysis with a central venous catheter and subsequently had an AVF or AVG placed18. That study reported that placement of an AVF rather than an AVG was associated with greater patient survival, despite longer CVC-dependence18, suggesting that the decision to place an AVF in a patient is a surrogate marker of better health. Our present study showed that placing AVFs rather than AVGs in this cohort was associated with several undesirable clinical vascular access consequences, including a higher likelihood of unsuccessful use of the AVF, much more frequent interventions to make the AVF functional, and prolonged catheter-dependence prior to successful use of the AVF. However, AVFs had superior vascular access patency after successful use as compared to AVGs, and required fewer interventions to maintain access patency. Following the Fistula First recommendations entails tradeoffs and entails accepting short term pain (longer catheter-dependence and a greater requirement for interventions to make the AVF functional) to realize long term gain (superior vascular access survival and fewer interventions after successful AVF use)(Table 2 and Fig 2). When the long term gain is not realized (i.e., unsuccessful AVF use), the original recommendations of the Fistula First Initiative may no longer be compelling, particularly in the elderly hemodialysis population. Thus, a clinician's selection of long-term vascular access type should factor both vascular access outcomes, as well as other clinical outcomes. If it were possible to accurately predict those elderly patients in whom the AVF is likely to have unsuccessful use for dialysis or require interventions to make the AVF functional, it would be possible to determine with greater confidence which ones may be better suited to undergoing AVG placement.
Our study has several important strengths. First, the large sample size is a census of incident Medicare-entitled United States elderly hemodialysis population in the current Fistula First era, and is thus broadly generalizable. Second, our vascular access data were captured using V codes reported monthly by every hemodialysis unit, analyzing information not previously available. Third, the current study has addressed a wide spectrum of clinically relevant vascular access outcomes, adding further support to recent publications questioning whether AVFs should be recommended as the vascular access of choice in elderly patients initiating hemodialysis with a catheter36-39.
Our study also has several limitations. First, it includes only elderly patients (≥67 years), and may not generalize to younger dialysis patients. However, the elderly are the fastest growing segment of the incident hemodialysis population1. They are also at greater risk of unsuccessful AVF use for dialysis40, and have shorter survival on hemodialysis, such that they frequently do not live long enough to realize the potential long-term benefits of AVFs. Second, two-stage basilic vein transpositions, a planned intervention prior to AVF use, differ from other interventions to make the AVF functional. Third, the recent introduction of V codes limited our analysis to only one-year followup after successful use of the vascular access. The relative differences in access events after interventions to make the vascular access functional (abandonment or frequency of interventions) between AVFs and AVGs may be more or less pronounced with longer follow-up. Finally, the observational nature of our study is unable to exclude residual confounding due to patient characteristics or local practice patterns (e.g. choice of placement of AVF or AVG, decision to intervene in an AVF or AVG) not available in the datasets used for this analysis. Ultimately, only a randomized clinical trial of elderly patients initiating hemodialysis with a catheter who are allocated to receive an AVF vs an AVG can definitively address the relative merits of the two vascular access types in this population.
In summary, our study demonstrates clear tradeoffs among elderly patients who initiate hemodialysis with a catheter and have a subsequent permanent vascular access placed. Compared to AVGs, AVFs are less likely to have successful use after creation, more likely to require interventions to make functional, and are associated with longer catheter dependence. In contrast, AVFs require fewer interventions to maintain patency after successful use and experience fewer abandoments in the first year after successful use. Ultimately, when considering selection and placement of the best vascular access in elderly patients initiating hemodialysis with a catheter, the clinician must balance the importance of removing the catheter and minimizing the need for interventions to make the vascular access functional (favoring AVG placement) versus a longer lasting vascular access with fewer maintenance interventions (favoring AVF placement).
Supplementary Material
Table S1: List of interventions and codes used to identify assistance for successful use of AVF and AVG and continued use for AVF and AVG using administrative data.
Supplementary Material Descriptive Text for Online Delivery
Supplementary Table S1 (PDF). List of interventions and codes used to identify assistance for successful use of AVF and AVG and continued use for AVF and AVG using administrative data.
Acknowledgments
Support: Dr. Lee is supported by grant 2R44DK109789-01 from National Institutes of Diabetes, Digestive and Kidney Diseases (NIDDK), grant 1R01HL139692-01 from the National Heart, Lung, and Blood Institutes, and grant 1I01BX003387-01A1 from a Veterans Affairs Merit Award. Dr. Allon is supported by grant 1R21DK104248-01A1 from the National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK). Dr. Thamer is supported by grant 1R21DK104248-01A1 from the National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK) and R01-HS-021229 from the Agency for Healthcare Research and Quality (AHRQ). Ms. Qian is supported by grant 1R21DK104248-01A1 from the National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK) and grant R03-HS-022931 from the Agency for Healthcare Research and Quality (AHRQ). These funding sources did not have a role in the study design, analysis, interpretation of data, writing the report, or the decision to submit the report for publication.
Footnotes
Authors' Contributions: Designed the study: TL, MT, MA; analyzed the data: MT, JQ, TL, MA. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Financial Disclosure: Dr. Lee is a consultant for Proteon Therapeutics, Merck, and Boston Scientific. Dr. Allon is a consultant for CorMedix. Dr. Thamer is a consultant for Proteon Therapeutics. The remaining authors declare that they have no relevant financial interests. Peer Review: Received _____. Evaluated by 3 external peer reviewers, with editorial input from a Statistics/Methods Editor and an Acting Editor-in-Chief (Associate Editor Sankar D. Navaneethan, MD, MPH). Accepted in revised form _____. The involvement of an Acting Editor-in-Chief to handle the peer-review and decision-making processes was to comply with AJKD's procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saran R, Li Y, Robinson B, et al. US Renal Data System 2015 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2016;67(3 Suppl 1):SVII–SVIII. doi: 10.1053/j.ajkd.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee T, Barker J, Allon M. Tunneled catheters in hemodialysis patients: reasons and subsequent outcomes. Am J Kidney Dis. 2005;46(3):501–508. doi: 10.1053/j.ajkd.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 3.Saad TF. Bacteremia associated with tunneled, cuffed hemodialysis catheters. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1999;34(6):1114–1124. doi: 10.1016/S0272-6386(99)70018-1. [DOI] [PubMed] [Google Scholar]
- 4.Lacson E, Jr, Wang W, Lazarus JM, Hakim RM. Change in vascular access and hospitalization risk in long-term hemodialysis patients. Clin J Am Soc Nephrol. 2010;5(11):1996–2003. doi: 10.2215/CJN.08961209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beathard GA. Fistula salvage by endovascular therapy. Adv Chronic Kidney Dis. 2009;16(5):339–351. doi: 10.1053/j.ackd.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Beathard GA, Settle SM, Shields MW. Salvage of the nonfunctioning arteriovenous fistula. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1999;33(5):910–916. doi: 10.1016/s0272-6386(99)70425-7. [DOI] [PubMed] [Google Scholar]
- 7.Beathard GA, Arnold P, Jackson J, Litchfield T. Aggressive treatment of early fistula failure. Kidney Int. 2003;64(4):1487–1494. doi: 10.1046/j.1523-1755.2003.00210.x. [DOI] [PubMed] [Google Scholar]
- 8.Nassar GM, Nguyen B, Rhee E, Achkar K. Endovascular Treatment of the “Failing to Mature” Arteriovenous Fistula. Clin J Am Soc Nephrol. 2006;1(2):275–280. doi: 10.2215/CJN.00360705. [DOI] [PubMed] [Google Scholar]
- 9.Tessitore N, Mansueto G, Lipari G, et al. Endovascular versus surgical preemptive repair of forearm arteriovenous fistula juxta-anastomotic stenosis: analysis of data collected prospectively from 1999 to 2004. Clin J Am Soc Nephrol. 2006;1(3):448–454. doi: 10.2215/CJN.01351005. [DOI] [PubMed] [Google Scholar]
- 10.Lipari G, Tessitore N, Poli A, et al. Outcomes of surgical revision of stenosed and thrombosed forearm arteriovenous fistulae for haemodialysis. Nephrol Dial Transplant. 2007;22(9):2605–2612. doi: 10.1093/ndt/gfm239. [DOI] [PubMed] [Google Scholar]
- 11.Tordoir JH, Bode AS, Peppelenbosch N, van der Sande FM, de Haan MW. Surgical or endovascular repair of thrombosed dialysis vascular access: is there any evidence? Journal of vascular surgery : official publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery, North American Chapter. 2009;50(4):953–956. doi: 10.1016/j.jvs.2009.06.058. [DOI] [PubMed] [Google Scholar]
- 12.Fistula First Catheter Last Initiative. [Accessed July 12, 2017]; Available at: http://esrdncc.org/ffcl/
- 13.Allon M, Robbin ML. Increasing arteriovenous fistulas in hemodialysis patients: problems and solutions. Kidney Int. 2002;62(4):1109–1124. doi: 10.1111/j.1523-1755.2002.kid551.x. [DOI] [PubMed] [Google Scholar]
- 14.Desilva RN, Patibandla BK, Vin Y, et al. Fistula first is not always the best strategy for the elderly. J Am Soc Nephrol. 2013;24(8):1297–1304. doi: 10.1681/ASN.2012060632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beathard GA, Urbanes A, Litchfield T. Changes in the Profile of Endovascular Procedures Performed in Freestanding Dialysis Access Centers over 15 Years. Clin J Am Soc Nephrol. 2017;12(5):779–786. doi: 10.2215/CJN.09730916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harms JC, Rangarajan S, Young CJ, Barker-Finkel J, Allon M. Outcomes of arteriovenous fistulas and grafts with or without intervention before successful use. J Vasc Surg. 2016;64(1):155–162. doi: 10.1016/j.jvs.2016.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee T, Ullah A, Allon M, et al. Decreased cumulative access survival in arteriovenous fistulas requiring interventions to promote maturation. Clinical journal of the American Society of Nephrology : CJASN. 2011;6(3):575–581. doi: 10.2215/CJN.06630810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee T, Thamer M, Zhang Q, Zhang Y, Allon M. Vascular Access Type and Clinical Outcomes among Elderly Patients on Hemodialysis. Clin J Am Soc Nephrol. 2017;12(11):1823–1830. doi: 10.2215/CJN.01410217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee T, Thamer M, Zhang Q, Zhang Y, Allon M. Reduced Cardiovascular Mortality Associated with Early Vascular Access Placement in Elderly Patients with Chronic Kidney Disease. Am J Nephrol. 2016;43(5):334–340. doi: 10.1159/000446159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.https://www.usrds.org/2012/pres/ASN2H/Ishani_Vascular_Access_Utilization_Dialysis_Population_final.pdf. Accessed November 1, 2017.
- 21.Solid CA, Collins AJ, Ebben JP, et al. Agreement of reported vascular access on the medical evidence report and on medicare claims at hemodialysis initiation. BMC Nephrol. 2014;15:30. doi: 10.1186/1471-2369-15-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solid CA, Carlin C. Timing of arteriovenous fistula placement and Medicare costs during dialysis initiation. Am J Nephrol. 2012;35(6):498–508. doi: 10.1159/000338518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hod T, Patibandla BK, Vin Y, Brown RS, Goldfarb-Rumyantzev AS. Arteriovenous fistula placement in the elderly: when is the optimal time? J Am Soc Nephrol. 2015;26(2):448–456. doi: 10.1681/ASN.2013070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Huang Z, Gilbertson DT, Foley RN, Collins AJ. An improved comorbidity index for outcome analyses among dialysis patients. Kidney Int. 2010;77(2):141–151. doi: 10.1038/ki.2009.413. [DOI] [PubMed] [Google Scholar]
- 25.Lok CE, Allon M, Moist L, Oliver MJ, Shah H, Zimmerman D. Risk equation determining unsuccessful cannulation events and failure to maturation in arteriovenous fistulas (REDUCE FTM I) J Am Soc Nephrol. 2006;17(11):3204–3212. doi: 10.1681/ASN.2006030190. [DOI] [PubMed] [Google Scholar]
- 26.Pisoni RL, Zepel L, Fluck R, et al. International Differences in the Location and Use of Arteriovenous Accesses Created for Hemodialysis: Results From the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2017 doi: 10.1053/j.ajkd.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 27.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):e296. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pastan S, Soucie JM, McClellan WM. Vascular access and increased risk of death among hemodialysis patients. Kidney Int. 2002;62(2):620–626. doi: 10.1046/j.1523-1755.2002.00460.x. [DOI] [PubMed] [Google Scholar]
- 29.Allon M, Depner TA, Radeva M, et al. Impact of dialysis dose and membrane on infection-related hospitalization and death: results of the HEMO Study. J Am Soc Nephrol. 2003;14(7):1863–1870. doi: 10.1097/01.asn.0000074237.78764.d1. [DOI] [PubMed] [Google Scholar]
- 30.Al-Solaiman Y, Estrada E, Allon M. The spectrum of infections in catheter-dependent hemodialysis patients. Clin J Am Soc Nephrol. 2011;6(9):2247–2252. doi: 10.2215/CJN.03900411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xue JL, Dahl D, Ebben JP, Collins AJ. The association of initial hemodialysis access type with mortality outcomes in elderly Medicare ESRD patients. Am J Kidney Dis. 2003;42(5):1013–1019. doi: 10.1016/j.ajkd.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 32.National Kidney Foundation-Dialysis Outcomes Quality Initiative. NKF-DOQI clinical practice guidelines for vascular access. Am J Kidney Dis. 1997;30(4 Suppl 3):S150–191. [PubMed] [Google Scholar]
- 33.Dember LM, Beck GJ, Allon M, et al. Effect of Clopidogrel on Early Failure of Arteriovenous Fistulas for Hemodialysis: A Randomized Controlled Trial. JAMA. 2008;299(18):2164–2171. doi: 10.1001/jama.299.18.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dember LM. Risk Factors for Access Failure: The Hemodialysis Fistula Maturation Study. Paper presented at: 2016 American Society of Nephrology Kidney Week Basic/Clinical Science Session, “Open for Business: New Insights to Hemodialysis Vascular Access”; November 19, 2016; Chicago, IL. Nov 19, 2016. [Google Scholar]
- 35.Pisoni RL, Young EW, Dykstra DM, et al. Vascular access use in Europe and the United States: results from the DOPPS. Kidney Int. 2002;61(1):305–316. doi: 10.1046/j.1523-1755.2002.00117.x. [DOI] [PubMed] [Google Scholar]
- 36.Allon M, Lok CE. Dialysis fistula or graft: the role for randomized clinical trials. Clin J Am Soc Nephrol. 2010;5(12):2348–2354. doi: 10.2215/CJN.06050710. [DOI] [PubMed] [Google Scholar]
- 37.Wish JB. Catheter last, fistula not-so-first. J Am Soc Nephrol. 2015;26(1):5–7. doi: 10.1681/ASN.2014060594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woo K, Ulloa J, Allon M, et al. Establishing patient-specific criteria for selecting the optimal upper extremity vascular access procedure. J Vasc Surg. 2017;65(4):1089–1103 e1081. doi: 10.1016/j.jvs.2016.10.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allon M. Arteriovenous Grafts: Much Maligned But in Need of Reconsideration? Semin Dial. 2017;30(2):125–133. doi: 10.1111/sdi.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richardson AI, 2nd, Leake A, Schmieder GC, et al. Should fistulas really be first in the elderly patient? The journal of vascular access. 2009;10(3):199–202. doi: 10.1177/112972980901000311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: List of interventions and codes used to identify assistance for successful use of AVF and AVG and continued use for AVF and AVG using administrative data.
Supplementary Material Descriptive Text for Online Delivery
Supplementary Table S1 (PDF). List of interventions and codes used to identify assistance for successful use of AVF and AVG and continued use for AVF and AVG using administrative data.


