Abstract
Background
Gastrointestinal stromal tumor (GIST) is the most common mesenchymal neoplasm of the gastrointestinal tract. The major risk factors of recurrence and metastasis are mitotic index and tumor size. This study investigates the risk of recurrence and metastasis in solely gastric GIST. The primary outcome is to evaluate risk of recurrence and metastasis. The secondary outcome is to analyse survival rates of patients who have recurrence and metastasis after curative resection.
Method
A cohort of patients who underwent curative resection of gastric GIST between January 2006 to December 2016 was reviewed. The diagnosis was confirmed with positive CD34, DOG1 or KIT (CD117) immunohistochemistry. Risk factors of recurrence and metastasis were analyzed.
Results
Sixty-eight patients who received curative resection and diagnosed as gastric GIST were included in this study. Twenty (29.41%) had recurrence or metastasis. The median follow up time was 31.95 months. The mostcommon type of surgery was partial gastric resection. There were statistically-significant differences between mitotic index 6 HPF or 6 HPF in tumor size 0-5 cm, 5-10 cm and 10 cm and the risk of recurrence or metastasis (p-value 0.036). In tumors sized 6-10 cm, patients with mitotic index 6 HPF had longer survival than patients with mitotic index 6 HPF (p-value 0.0147).
Conclusion
The factor that determines the outcome of recurrence or metastasis in solely gastric GIST is high mitotic index count. Patients who have abdominal pain may be suspected as advanced disease. The type of operation and tumor size are not associated with recurrence or metastasis.
Keywords: Gastric GIST, Mitotic index, Survival, Prognosis, Recurrence
Highlights
-
•
The factor that determines the outcome of recurrence or metastasis in gastric GIST is high mitotic index count more than 6.
-
•
Patients who have abdominal pain may be suspected as advanced disease.
-
•
The tumor size are not associated with recurrence or metastasis.
-
•
The overall survival did not depend on the tumor size, in contrast, the overall survival depends on the mitotic index.
1. Introduction
Gastrointestinal stromal tumor (GIST) is a relatively rare tumor [1]. GISTs are the most common mesenchymal tumors of the digestive tract. They account for the majority of intramural tumors and can vary widely in appearance, from small intraluminal lesions to exophytic masses that protrude into the peritoneal cavity, commonly with areas of hemorrhage or necrosis. GISTs originate within the smooth muscle layer in the wall of the tubular gastrointestinal tract and grow mostly toward the serosa, far less often toward the mucosa.
The cell origin of GIST arises from interstitial cell of Cajal and is characterized by mutation of KIT and platelet derived growth factor receptor (PDGFR) [2]. The standard curative treatment of GIST is complete surgical resection with negative margin from the tumor. The evaluation risk s of recurrence and metastasis depend on various factors including tumor location, mitotic rate, tumor size, and tumor rupture. Fletcher et al. classified the risk of recurrence or metastasis of surgically resected primary GIST as very low, low, intermediate, and high risk by using the tumor size and mitotic count [3]. Miettinen and Lasota developed a method to predic t the risk of recurrence and metastasis which included the tumor location i.e. stomach, jejunum and ileum, duodenum, and rectum together with the tumor size and mitotic rate [4]. The other characteristic s for high -risk of recurrence are high cellularity, inva sion of adjacent organs, and tumor rupture.
Gastrointestinal stromal tumors (GISTs) are rare life threatening forms of cancer representing 0.1–3% of all the GI malignancies. When one compares between gastric GIST and GIST from other locations, i.e. esophagus, small intestine, colon, rectum or extra-gastrointestinal GIST (EGIST), gastric GIST have a better prognosis. The recurrence free survival of gastric GIST is longer than that of GIST from other organs with the same size or mitotic rate [2,5,6]. Because GIST are heterogeneous of clinical and morphology which are difficult to predict the prognosis of the disease [7]. Few studies have focused on the specific risks of solely gastric GIST and some reports have shown the size not correlated the risk of recurrence [8]. Therefore, the primary aim of this study was to evaluate the risk of recurrence and metastasis of solely gastric GIST receiving curative resection. The secondary aim was to analyse the survival rate of solely gastric GIST patients who have recurrence and metastasis.
2. Materials and methods
A retrospective cohort of all patients aged 18 years or older who presented with gastric GIST and underwent curative surgical resection from January 2006 to December 2016 was reviewed. Ethical issues were approved by Faculty of Medicine Ramathibodi Hospial Ethics Committee and registered in Research Registry. The patients who had involvement of other organs or metastasis before curative surgery were.
excluded. Curative surgical resection was defined as total resection of all tissue s invaded by the tumor with a free margin, including lymphatic resection if nessesary. The tumor specimen s were diagnosed by a pathologist and confirmed by immunohistochemistry with one of the followings: CD34, DOG1 or KIT (CD117). The patients who had recurrence or metastasis at the time of follow up were identified. The diagnosis tools for detection of recurrence or metastasis were esophagogastroduodenoscopy (EGD) or imaging studies include ultrasonography, computer tomography (CT), or magnetic resonance imaging (MRI). Age, gender, chief complaint s, EGD finding s, imaging finding s, tissue dianosis before surgery, intraoperative finding s, tissue immunohistochemistry after complete surg ical resection, adjuvant treatment, and the time of recurrence or metastasis were analysed.
2.1. Statistical analysis
The data were analysed by using STATA version 14. The logistic regression was used to evaluate the risk of recurrence or metastasis and reported 95% CI (confidence interval) of OR (odds ratio). The Pearson Chi Square was used to compare and categorize between size and mitotic index for risk of recurrence and metastasis. The p-value < 0.05 was considered statistically significant.
3. Results
Between January 1, 2006 and December 31, 2016, 68 patients were diagnosed as solely gastric GIST and received curative resection. Forty-eight patients had no recurrence or metastases, whereas 20 patients (29.41%) had recurrence or metastasis. The median follow up time was 31.95 months (IQR 14.6–52.7). The patients' characteristics are shown in Table 1. Diabetes mellitus was significantly associated with increased risk of recurrence or metastasis (p = 0.025). However, after multivariated analysis, no underlying disease was found to be associated with increased risk of recurrence or metastasis. Like diabetes mellitus, abdominal pain was the only presenting symptom which was significantly associated with recurrence or metastasis (p = 0.022); however, the result was not different in multivariated analysis.
Table 1.
The patient's characteristic in patient with or without recurrent or metastasis gastric GIST.
| Factors | Patients without recurrence or metastasis group nb (%) | Patients with recurrence or metastasis group nb (%) | Univariate P - value | Multivariate p- value |
|---|---|---|---|---|
| Sex (male) | 21 (43.75) | 9 (45) | 0.925 | 0.996 |
| Age (±SD) | 59.25 ± 15.28 | 63.25 ± 12.29 | 0.303 | – |
| Underlying disease | ||||
| DM | 9 (18.75) | 9 (45) | 0.025 | 0.996 |
| HT | 21 (43.75) | 12 (60) | 0.222 | – |
| DLP | 14 (29.17) | 5 (25) | 0.727 | 0.989 |
| CVA | 2 (4.17) | – | 0.354 | – |
| CAD | 2 (4.17) | 2 (10) | 0.354 | – |
| Cirrhosis | – | 1 (5) | 0.119 | – |
| Chief complain | ||||
| UGIH | 3 (6.25) | 3 (15) | 0.246 | – |
| Melena | 15 (31.25) | 2 (10) | 0.065 | – |
| Weight loss | 1 (1.08) | – | 0.516 | – |
| Abdominal mass | 13 (27.08) | 4 (20) | 0.539 | – |
| Abdominal pain | 4 (8.33) | 7 (35) | 0.022 | 0.986 |
| Anemia | 1 (1.08) | 2 (10) | 0.147 | – |
| Vomit | 8 (16.67) | 2 (10) | 0.479 | – |
| Tumor location | ||||
| Upper part | 15 (46.88) | 9 (60) | 0.666 | 0.994 |
| Middle part | 13 (40.3) | 5 (33.33) | ||
| Lower part | 4 (12.50) | 1 (6.67) | ||
| Type of operation | ||||
| Total gastrectomy | 2 (4.55) | 1 (5.56) | 0.965 | 0.996 |
| Subtotal gastrectomy | 4 (9.09) | 2 (11.11) | ||
| Partial gastrectomy | 32 (72.73) | 11 (61.11) | ||
| Distal gastrectomy | 1 (2.27) | – | ||
| Proximal gastrectomy | 5 (11.36) | 3 (16.66) | ||
| Othera | – | 1 (5.56) | ||
DM: diabetes mellitus, HT: hypertension, DPL: dyslipidemia, CVA: cerebrovascular accident, CAD: coronary artery disease, UGIH: upper gastrointestinal hemorrhage.
Other: en bloc resection of tumor with splenectomy and distal pancreatectomy.
Due to retrospective studies, some data was not recorded completely thus the number of patients in some data is not equal to others.
The location of tumors was classified as proximal, middle, and distal part of the stomach according to Japanese classification of Gastric Carcinoma [9]. The most-common tumor location in all patients was proximal, followed by middle, and lower part, respectively. No association between location of tumor and recurrence or metastasis was found (p = 0.666). There was no statistical significance between the types of the operation and recurrence or metastasis (p = 0.965). Rupture of tumor during operation was found in 3 cases in the group of patients without recurrence or metastasis and one case in.
3.1. Group of patients with recurrence or metastasis. `
The tumor size, mitotic index and the correlation of recurrence and metastasis are shown in Table 2, Table 3, Table 4. There is a statistically significant association between mitotic index >6 HPF (95% CI 1.802–24.653, p = 0.004) and risk of recurrence or metastasis (Table 3). There is a statistically significant difference between mitotic index ≤6 HPF or >6 HPF in tumor size 0–5 cm, > 5–10 cm and >10 cm and the risk of recurrence or metastasis (p-value 0.036) (Table 4).
Table 2.
Compare categories between size and mitotic index for risk of recurrence and metastasis in patients with or without recurrent and metastasis.
| Factors | patients without recurrence or metastasis group | patients with recurrence or metastasis group | P - value |
|---|---|---|---|
| Tumor size mean ± SD (cm) | 8.26 ± 4.28 | 8.99 ± 4.43 | 0.612 |
| Mitotic index mean ± SD (HPF) | 5.81 ± 8.03 | 24.31 ± 22.66 | 0.000 |
Table 3.
Tumor size and mitotic index in risk of recurrence and metastasis in patients with recurrent and metastasis.
| Factors | Odd ratio | 95% C.I. | P-value (<0.05) | |
|---|---|---|---|---|
| Tumor size | 0–5 cm. | 1 | – | – |
| >5–10 cm. | 1.2 | 0.239–6.024 | 0.825 | |
| >10 cm. | 3 | 0.495–18.168 | 0.232 | |
| Mitotic index | ≤6 HPF | 1 | – | – |
| >6 HPF | 6.66 | 1.802–24.653 | 0.004 | |
Table 4.
Compare category between size and mitotic index for risk of recurrence and metastasis in patients with recurrent and metastasis.
| Size (cm.) | Mitotic index |
P-value (<0.05) | |
|---|---|---|---|
| ≤6 HPF (n%) | >6 HPF (n%) | ||
| 0–5 | 11 (40.74) | 1 (7.69) | 0.036 |
| >5-10 | 12 (44.44) | 6 (46.15) | |
| >10 | 4 (14.81) | 6 (46.15) | |
In patients who had recurrence or metastasis (20 patients), 7 (35%) had liver metastasis, 1 (5%) had peritoneal metastasis, 1 (5%) had lung and liver metastasis and 11 (55%) had tumor recurrence. Most patients received systemic treatment following the standard protocol, except for some patients who did not receive systemic treatment due to personal financial constraints. Seventeen out of twenty patients in the recurrence or metastasis group received Imatinib. In survival analysis, the size of tumor did not affect overall survival in either group of mitotic indexes less or more than 6. However, the mitotic index less than or equal to 6/HPF and more than 6/HPF had a statistically significant difference in each size of tumor (range 0–5 cm, > 5–10 cm and >10 cm).
In the tumor size 0–5 cm, patients with mitotic index ≤6 HPF had longer survival than patients with mitotic index >6 HPF, but does not show statistical significance (p-value 0.0827). In the tumor size 6–10 cm, the patients with mitotic index ≤6 HPF had longer survival than patients with mitotic index >6 HPF (p-value 0.0147). For tumor size >10 cm, there is no statistical difference between mitotic index ≤6 HPF and >6 HPF (p-value 0.4254).
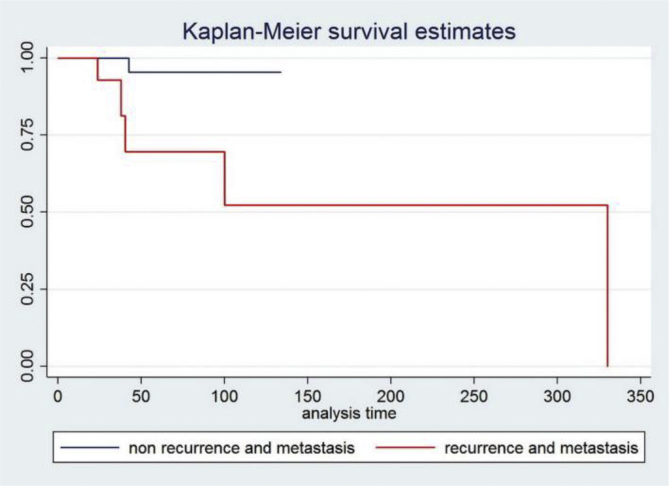
The median follow up time was 31.5 months (range 0.4–330 months). The median follow up time for patients with liver metastasis, liver and lung metastasis, and peritoneal metastasis were 24.19, 11.53 and 10.52 months, respectively. The survival analysis was performed for patients with recurrence or metastasis (Fig. 1). The mortality rate for patients who had recurrence or metastasis and non-recurrence or non-metastasis patients were 0.444/100/month (5.33 patients/year) and 0.051/100/month (0.61 patients/year), respectively.
Fig. 1.
Kaplan-Meier survival graph shows survival analysis in patients with non-recurrence or metastasis and patients with recurrence or metastasis.
4. Discussion
In this study, we included 68 patients who were diagnosed with gastric GIST. At the initial presentation, all patients had no metastasis and underwent curative resection surgery, and some patients.
received systemic treatment following standard protocol. We found that tumor size between patients with and without recurrent or metastasis group does not show a statistical significance (p-value 0.612). Only high mitotic index shows a statistical significance (p-value 0.004) that could predict the high risk of recurrence or metastasis in these patients.
For patients in the recurrence or metastasis group in this study, the correlation between size and mitotic index in all patients did not affect the recurrence or metastasis. Independent to mitotic count, the data do not show a statistical significance in all ranges of tumor. Regarding the mitotic index, a statistical significance is found in mitotic index >6 HPF (95% CI. 1.802–24.653, p-value 0.004). The correlation between tumor size and mitotic index shows a statistical significance (p-value 0.036) in mitotic count more than 6 HPF by independent to size of tumor which like some report has shown the size not correlated with the risk of recurrence [8]. Finally, these data were interpreted that only mitotic index more than 6 HPF affected recurrence or metastasis and does not depend on tumor size.
After follow up time, (median follow up time was 31.5 months), we found 20 patients had recurrence or metastasis. In the patients with recurrence and metastasis, the total mean age was 63.25 years and 59.25 years in patients with and without recurrent or metastasis group, respectively. This is similar to several other studies which found the mean age more than 50 years [10].
In these data, we found that the most-common underlying disease which affected the recurrent or metastasis in gastric GIST was diabetes mellitus (p-value 0.025). However, multivariate analysis dose not show statistical significance. This result was probably due to confounding from many factors of patients and unbalance of each group. The most presenting symptom in this report was found to be abdominal pain in the recurrent or metastasis group that correlates with the size of tumor (p-value 0.022). Other symptoms do not show a statistical significance in either group of patients. Previous reports showed that the most-frequent location was the stomach (50%) and small bowel (25%) [10,11]. In this study, we included only gastric GIST and presented the gastric location of tumors which were evaluated by EGD and CT abdomen, and we found that the most-frequent location was at the fundus and greater curvature followed by lesser curvature, antrum and cardia respectively. From these data, the location of tumor does not affect the risk of recurrence or metastasis of gastric GIST. The standard treatment of gastric GIST was curative surgical resection [12]. The type of operation and reconstruction depends on the tumor location. In this study, the most-frequent tumor location was found at greater curvature. Therefore, most patients could undergo partial or wedge resection of the stomach. However, some patients underwent proximal.
gastrectomy due to the location of tumor located at the cardia, and some underwent subtotal gastrectomy or total gastrectomy because of large tumors entering the stomach. However, the difference of operation did not affect the outcome of patients in term of recurrence and metastasis. In 2010, Hohenberger et al. reported risk of recurrence at nearly 100% if the tumor had ruptured [13]. Only 1 patient had a ruptured tumor during the operation and recurrence had occurred. Several studies show the factors which increase the risk of recurrence or metastasis including tumor size, mitotic index or ruptured tumor. Zhao WY et al. reported that the serosal invasion may be an adverse predictive factor in high-risk patients. But this study found that tumors invading adjacent organs increased the risk of recurrence and metastasis and shows a statistical significance (p-value 0.009) [5,[14], [15], [16], [17]].
In overall survival of patients by tumor size and mitotic index, the overall survival of patients did not depend on the size of tumor. The data have previously shown that correlation with the size of tumor in patients with or without recurrence or metastasis showed no difference in both groups. In contrast, the overall survival of patients depends on the mitotic index. Thus, the large tumor size together with high mitotic index affects the survival of patients.
The median follow-up time was 31.5 months (range 0.4–298 months). We found tumor recurrence (55%) and the most-common metastasis at liver (35%), followed by peritoneal metastasis (5%). Only 1 case had lung and liver metastasis. All patients received systemic treatment following the standard protocol. Most patients received systemic treatment for approximately 12–24 months (median time 16.67 months). We found that the follow-up time in patients who had single liver metastasis was 24.19 months, which was better than patients who had peritoneal metastasis whose follow up time was 10.52 months. For the survival analysis, due to one patient dying more than 330 months after the follow up time, the Kaplan-Meier survival graph at the time of 330 month had no survival but the median time survival of patients with recurrence or metastasis was 40.3 months. Patients who had recurrence or metastasis had a higher mortality rate when compared with the patients who did not have recurrence or metastasis. Although recent studies advocate the use of Imatinib after surgery in cases in which there is a high risk of recurrence, in developing countries this is often impractical. These other studies refer to ‘high risk’ cases of tumor diameter >10 cm, mitotic count >10/50 HPFs, tumor diameter >5 cm and mitotic count >5/50 HPFs, or tumor rupture before or at surgery [16,18,19]. Because this study is a retrospective analysis, the data for some patients were not completely recorded and the sample size was small. In the future, we hope our study can lead to randomized control trials to reduce our limitation. However, the data show the mitotic index count could affect to recurrence and metastasis.
5. Conclusion
The affecting factors determining the outcome of recurrence or metastasis in gastric GIST were patients with underlying diabetes mellitus, or tumor invasion and high mitotic index count, especially mitotic index count more than 6 HPF. Although the tumor size does not show a statistical significance in this study, the large tumor size increases the risk of recurrence or metastasis. Extensive research work including large clinical trials is needed to confirm our findings.
Ethical approval
Committee on Human Rights Related to Research Involving Human Subjects, Faculty of Medicine, Ramathibodi Hospital, Mahidol University.
Protocol Number: ID 03-59-52.
Sources of funding
No sources of funding for our research.
Author contribution
Chumpon Wilasrusmee and Chairat Supsamutchai who performed the study design. Teerawut Rakchob and Pattawia Choikrua who performed data collection and data analysis. Teerawut Rakchob who writing the article and re-checked by Chumpon Wilasrusmee. Pitichote Hiranyatheb and Jakrapan Jirasiritham were contributors.
Conflicts of interest
No conflicts of interest and source of funding in this article.
Research registration number
Chairat Supsamutchai.
researchregistry3614.
Guarantor
Chumpon Wilasrusmee and Chairat Supsamutchai.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Acknowledgement
We thank Professor Amnuay Thithapandha for his help with the English editing of this manuscript.
References
- 1.Nilsson B., Bumming P., Meis-Kindblom J.M., Oden A., Dortok A., Gustavsson B. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer. 2005;103(4):821–829. doi: 10.1002/cncr.20862. [DOI] [PubMed] [Google Scholar]
- 2.Miettinen M., Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch. Pathol. Lab Med. 2006;130(10):1466–1478. doi: 10.5858/2006-130-1466-GSTROM. [DOI] [PubMed] [Google Scholar]
- 3.Fletcher C.D., Berman J.J., Corless C., Gorstein F., Lasota J., Longley B.J. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum. Pathol. 2002;33(5):459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 4.Lasota J., Miettinen M. KIT and PDGFRA mutations in gastrointestinal stromal tumors (GISTs) Semin. Diagn. Pathol. 2006;23(2):91–102. doi: 10.1053/j.semdp.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Joensuu H., Vehtari A., Riihimaki J., Nishida T., Steigen S.E., Brabec P. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13(3):265–274. doi: 10.1016/S1470-2045(11)70299-6. [DOI] [PubMed] [Google Scholar]
- 6.Jones R.L. Practical aspects of risk assessment in gastrointestinal stromal tumors. J. Gastrointest. Canc. 2014;45(3):262–267. doi: 10.1007/s12029-014-9615-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rutkowski P., Nowecki Z.I., Michej W., Debiec-Rychter M., Wozniak A., Limon J. Risk criteria and prognostic factors for predicting recurrences after resection of primary gastrointestinal stromal tumor. Ann. Surg Oncol. 2007;14(7):2018–2027. doi: 10.1245/s10434-007-9377-9. [DOI] [PubMed] [Google Scholar]
- 8.Abdel-Ghaffar M. Large gastrointestinal stromal tumor size does not imply early recurrence. Int. Med. Case Rep. J. 2010;3:13–17. doi: 10.2147/imcrj.s9729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Japanese Gastric Cancer A. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14(2):101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 10.DeMatteo R.P., Lewis J.J., Leung D., Mudan S.S., Woodruff J.M., Brennan M.F. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann. Surg. 2000;231(1):51–58. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubin B.P., Heinrich M.C., Corless C.L. Gastrointestinal stromal tumour. Lancet. 2007;369(9574):1731–1741. doi: 10.1016/S0140-6736(07)60780-6. [DOI] [PubMed] [Google Scholar]
- 12.Group E.S.E.S.N.W. Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014;25(Suppl 3):iii21–i26. doi: 10.1093/annonc/mdu255. [DOI] [PubMed] [Google Scholar]
- 13.Hohenberger P., Ronellenfitsch U., Oladeji O., Pink D., Strobel P., Wardelmann E. Pattern of recurrence in patients with ruptured primary gastrointestinal stromal tumour. Br. J. Surg. 2010;97(12):1854–1859. doi: 10.1002/bjs.7222. [DOI] [PubMed] [Google Scholar]
- 14.Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum. Pathol. 2008;39(10):1411–1419. doi: 10.1016/j.humpath.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 15.Yanagimoto Y., Takahashi T., Muguruma K., Toyokawa T., Kusanagi H., Omori T. Re- appraisal of risk classifications for primary gastrointestinal stromal tumors (GISTs) after complete resection: indications for adjuvant therapy. Gastric Cancer. 2015;18(2):426–433. doi: 10.1007/s10120-014-0386-7. [DOI] [PubMed] [Google Scholar]
- 16.Gold J.S., Gonen M., Gutierrez A., Broto J.M., Garcia-del-Muro X., Smyrk T.C. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol. 2009;10(11):1045–1052. doi: 10.1016/S1470-2045(09)70242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao W.Y., Xu J., Wang M., Zhang Z.Z., Tu L., Wang C.J. Evaluation of high-risk clinicopathological indicators in gastrointestinal stromal tumors for prognosis and imatinib treatment outcome. BMC Gastroenterol. 2014;14:105. doi: 10.1186/1471-230X-14-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corless C.L., Ballman K.V., Antonescu C.R., Kolesnikova V., Maki R.G., Pisters P.W. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J. Clin. Oncol. 2014;32(15):1563–1570. doi: 10.1200/JCO.2013.51.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joensuu H., Eriksson M., Sundby Hall K., Hartmann J.T., Pink D., Schutte J. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. J. Am. Med. Assoc. 2012;307(12):1265–1272. doi: 10.1001/jama.2012.347. [DOI] [PubMed] [Google Scholar]