Abstract
Objective
To determine characteristics and trends in opioid use, questionable use, and prescribing in Medicare.
Study Setting
Opioid prescriptions filled through Medicare Part D for beneficiaries with full‐year, fee‐for‐service Medicare coverage during 2006 to 2012.
Study Design
Retrospective analysis of a 20 percent sample of Medicare claims data. Estimates are adjusted using multivariable regression analysis.
Data Collection
Opioid use, opioid abuse, questionable opioid use, and opioid prescribing by specialty.
Principal Findings
Opioid use in Medicare was stable from 2006 to 2012 on average. More than 1 in 3 beneficiaries filled an opioid prescription annually; about 1 in 10 were chronic opioid users. The distribution of opioid users shifted in favor of diagnoses often associated with chronic pain. Opioid users were increasingly likely to abuse opioids or display patterns of questionable use from 2006 to 2010, with a slowdown in later years. Average outcomes mask significant variation as the distribution of opioid use widened over the analysis period. Prescribing quantity and intensity varied by specialty. The largest quantity increases were among nurse practitioners and physician assistants.
Conclusions
Opioid utilization and prescribing are increasingly heterogeneous from 2006 to 2012. Future research should focus on explaining differential trends in utilization and prescribing.
Keywords: Opioids, Medicare Part D, prescribing behavior
Use and abuse of prescription opioids are a growing problem in the United States. As the rates of opioid prescribing have quadrupled over the past few decades, the rates of opioid‐related overdose deaths have grown steadily (Paulozzi et al. 2011). In 2015, there were 33,091 overdose deaths in the United States involving opioids, a more than 200 percent increase over the level in 2000 (Rudd 2016; Rudd et al. 2016). While the bulk of these deaths occur in the under‐65 population, there are high and rising rates of opioid‐related overdose among seniors who comprise most of the Medicare population (Rudd et al. 2016). In addition to opioid‐related deaths, studies have shown that seniors are vulnerable to increased rates of falls and fractures and a higher risk of hospitalizations as a result of opioid use (Saunders, Dunn et al. 2010; Solomon et al. 2010).
Within Medicare, studies have shown that the disabled, under‐65 population are chronic, high‐intensity users of opioids (Morden et al. 2014). In addition, single‐year studies of opioid use in Medicare found that 15 percent of hospitalizations result in opioid prescriptions for beneficiaries, 41 percent of opioid users utilize multiple physicians to obtain opioids, and opioid prescribing is more uniformly distributed than in other populations (Jena et al. 2014; Chen et al. 2016; Jena, Goldman, and Karaca‐Mandic 2016). An analysis of Medicare Advantage beneficiaries in a single insurance plan found rising rates of diagnosed opioid abuse (Dufour et al. 2014).
Despite the evidence that opioid use among Medicare beneficiaries could lead to negative outcomes, and the evidence from single‐year or limited population studies of opioid use and prescribing, there is no comprehensive study of the trajectory or characteristics of opioid use in the broader Medicare population. These single‐year studies or studies examining a subset of the Medicare population provide important insights, but they cannot explain how opioid use, abuse, or prescribing has evolved. Furthermore, as policy changes within Medicare are applied broadly, it is important to understand Medicare utilization as a whole not just within specific subpopulations. This study provides a comprehensive analysis of opioid use and prescribing and in doing so fills important gaps in understanding of trends and characteristics of opioid utilization, questionable use, and prescribing among Medicare beneficiaries.
Methods
This study was exempted by the Institutional Review Board at the University of Southern California.
Data and Study Sample
The sample of beneficiaries and prescribers was drawn from a 20 percent random sample of administrative claims data for all Medicare beneficiaries. The administrative claims include information on all Medicare Part A (inpatient), Medicare Part B (outpatient and physician care), and Medicare Part D (prescription drug) claims. They also contain information on the timing and cost of these claims, demographic and eligibility information about beneficiaries, prescriber and pharmacy identifiers, and details on prescriber training and specialty. All prescription drug claims include a national drug code (NDC), which is used to identify opioids.
The analysis is limited to those beneficiaries with full‐year, fee‐for‐service Medicare enrollment from 2006 to 2012. Beneficiaries are not necessarily enrolled for all years from 2006 to 2012. The full‐year enrollment restriction ensures that the claims cover all inpatient, outpatient, and prescription drug claims for the beneficiary‐year. For the analysis of beneficiary outcomes, the unit of analysis is the beneficiary‐year, and for the prescribing results, the unit of analysis is the prescriber‐year.
Within the beneficiary analyses, the prescription opioid‐using population is divided into two subpopulations to facilitate comparisons across different types of users. The full population of prescription opioid users is defined as beneficiaries with any prescription opioid use. This population fills one or more opioid prescriptions in a year. The first subpopulation is beneficiaries filling one or more opioid prescriptions in a year following a year with no prescription opioid use. This prescription opioid‐initiating population represents the opioid naïve patients who may be less frequent or less intense users of opioids. The other subpopulation is chronic opioid users. This population is defined in two ways. Either these beneficiaries fill six or more opioid prescriptions in a year or they have an episode of 90 or more days of consecutive opioid use in a year. Both subpopulations are defined to be comparable with existing literature on opioid use (Von Korff et al. 2008; Morden et al. 2014). These subpopulations are not mutually exclusive as a patient could both initiate opioid use and develop into a chronic user over the course of a year.
Statistical Analysis
Across categories of prescription opioid users, prescription opioid utilization, questionable use, and prescribing outcomes are operationalized in a variety of ways. To describe prescription opioid utilization, this paper examines the probability of use, the quantity of utilization, and the potency of opioid utilization. Quantity is measured through the total fills in a year as well as the total days supply. Potency is measured by the daily milligrams of morphine‐equivalent opioids prescribed (MME per day) and the probability of receiving a prescription with a potency higher than 100 mg of morphine equivalence per day (>100 MED). Quantity outcomes come directly from the claims data, and morphine equivalence was calculated as the product of the prescription's active ingredient MME conversion factor, dosage in milligrams, and quantity. The active ingredient conversion factors are available in Table S2. MME per day is calculated as the total MME prescribed divided by the total days supply prescribed over the year.
Opioid abuse is defined as a diagnosis of opioid abuse in an inpatient, outpatient, physician, or emergency department setting, excluding ICD‐9 diagnosis codes that relate to heroin. Opioid overdose is defined similarly as a diagnosis of opioid‐related overdose that is not attributed to heroin. The data do not include information on whether the overdose attempt was fatal, so this outcome measures overdose attempts rather than death due to overdose. Specific ICD‐9 codes are available in Table S1.
Questionable opioid use or opioid misuse is operationalized through estimates of doctor shopping and pharmacy shopping. These measures are associated with an increase in negative outcomes like opioid overdoses. A recent study found that the use of four or more unique pharmacies and an early opioid fill within a 90‐day period was significantly associated with elevated opioid overdose risk (Yang et al. 2015). Thus, pharmacy shopping is defined as the use of four or more unique pharmacies to fill opioids prescriptions and an overlapping opioid fill from distinct providers within a 90‐day observation period, and extrapolating from that study, doctor shopping is defined as the use of four or more unique prescribers for opioid prescriptions and an overlapping opioid fill from distinct prescribers in a 90‐day observation period. While these estimates of questionable opioid use or misuse are imperfect—and may instead be signals of fragmented patient care—they serve as useful starting points for identifying patients whose opioid use may need to be observed or managed.
Opioid prescribing by specialty is measured in four ways to illuminate both the quantity and intensity of prescribing by specialty. The main quantity outcome examines the share of all prescriptions filled, by prescriber specialty. The main intensity outcome examines the average number of prescriptions filled per prescriber, by specialty. This study also reports the distribution of prescribers in the top decile of total fills prescribed by specialty. Because such aggregate measures are difficult to translate to clinical practice, this paper includes a more episode‐based measure of prescribing. Following literature showing that many patients receive opioid prescriptions after an overdose attempt and that such behavior increases the risk of future overdoses, the study estimates a measure of the share of prescribers by specialty who prescribe opioids within 60 days of a documented overdose event (Larochelle et al. 2016). Results reflect trends in prescribing for the top 15 specialties by quantity in 2012.
All results reported are regression‐adjusted estimates of these use, questionable use, or prescribing outcomes. Regression adjustment controls for differences in patient demographics, eligibility status, health status, year, and indicators for various diagnoses. Health status is measured by calculating a patient's RxHCC score, which is used by CMS for risk‐adjusting payments to Medicare Advantage (Robst, Levy, and Ingber 2007). Where the outcomes of interest are counts, I employ log‐linear regressions and report predicted outcomes using the smearing methodology from Duan (1983) to account for differences in the underlying distribution functions when transforming the results from log scale back to counts (Duan 1983). Such methods have shown to be both appropriate and efficient for dealing with the challenges of the restricted range and skewness of utilization data (Buntin and Zaslavsky 2004). Where outcomes are binary, I employ logit regressions and report predicted probabilities. Analyses were conducted using Stata version 14 (StataCorp LP, College Station, TX, USA).
Results
Baseline Characteristics
More than 1 in 3 Medicare beneficiaries fill at least one opioid prescription in a year. About 1 in 10 beneficiaries initiate opioid use after not filling opioids for a year, and about 1 in 10 beneficiaries can be classified as chronic opioid users. As shown in Table 1, individuals with any use of opioids tend to be somewhat sicker (health status score of 1.26 vs. 1.11 and higher rates of many selected diagnoses), younger (67.6 vs. 69.9 years), more likely to be low income (as measured by dual eligibility for Medicare and Medicare or receipt of a low‐income subsidy for Part D), and more likely to be eligible for Medicare due to disability (42 percent vs. 31 percent) than the general Medicare population. Within the opioid‐using population, those divergences are attenuated or reversed among the opioid‐initiating population and magnified among the chronic use populations.
Table 1.
Description of Population and Unadjusted Outcome Variables
| Beneficiary‐Years | Full Population | Any Opioid Use (>0 Opioid Fills) | Initiate Opioid Usea | Chronic Use (≥6 Fills Per Year) | Chronic Use (≥90 Consecutive Days) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 21,120,682 | 7,764,111 | 2,071,056 | 2,724,496 | 2,095,858 | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Share of beneficiary‐years | 1.000 | 0.000 | 0.368 | 0.482 | 0.098 | 0.297 | 0.129 | 0.335 | 0.099 | 0.299 |
| Opioid utilization outcomes | ||||||||||
| Mean total fills | 2.283 | 5.562 | 6.201 | 7.727 | 2.183 | 2.577 | 14.093 | 8.470 | 14.892 | 9.180 |
| Mean total days supply | 43.760 | 121.742 | 118.853 | 177.001 | 22.258 | 44.546 | 295.756 | 197.040 | 356.075 | 184.620 |
| Share with prescription >100 MED | 0.059 | 0.236 | 0.161 | 0.367 | 0.107 | 0.309 | 0.251 | 0.433 | 0.250 | 0.433 |
| Mean total MME per day | 17.487 | 48.109 | 47.495 | 69.721 | 44.350 | 49.698 | 51.459 | 64.940 | 52.137 | 69.465 |
| Opioid abuse outcomes | ||||||||||
| Opioid abuse diagnosisb | 0.006 | 0.080 | 0.015 | 0.123 | 0.003 | 0.056 | 0.035 | 0.185 | 0.043 | 0.202 |
| Opioid overdose diagnosis | 0.001 | 0.035 | 0.003 | 0.056 | 0.001 | 0.027 | 0.007 | 0.083 | 0.008 | 0.090 |
| Questionable opioid use outcomes | ||||||||||
| Opioid pharmacy shopping | 0.001 | 0.071 | 0.003 | 0.057 | 0.000 | 0.019 | 0.009 | 0.095 | 0.010 | 0.101 |
| Opioid doctor shopping | 0.005 | 0.035 | 0.014 | 0.117 | 0.004 | 0.063 | 0.037 | 0.190 | 0.036 | 0.187 |
| Beneficiary characteristics | ||||||||||
| Share white | 0.765 | 0.424 | 0.765 | 0.424 | 0.774 | 0.419 | 0.781 | 0.414 | 0.791 | 0.407 |
| Share male | 0.384 | 0.486 | 0.366 | 0.482 | 0.386 | 0.487 | 0.350 | 0.477 | 0.363 | 0.481 |
| Mean health status score (RxHCC score) | 1.107 | 0.440 | 1.258 | 0.476 | 1.199 | 0.439 | 1.363 | 0.508 | 1.357 | 0.510 |
| Mean age | 69.931 | 14.358 | 67.584 | 15.033 | 70.943 | 13.914 | 64.295 | 15.385 | 63.532 | 15.290 |
| Share dually eligible for Medicare and Medicaid | 0.387 | 0.487 | 0.462 | 0.499 | 0.371 | 0.483 | 0.578 | 0.494 | 0.587 | 0.492 |
| Share receiving low‐income subsidy (LIS) | 0.057 | 0.231 | 0.065 | 0.247 | 0.053 | 0.225 | 0.079 | 0.269 | 0.082 | 0.274 |
| Share who died | 0.050 | 0.218 | 0.063 | 0.243 | 0.060 | 0.238 | 0.050 | 0.218 | 0.057 | 0.232 |
| Share eligible due to disability | 0.310 | 0.463 | 0.420 | 0.494 | 0.281 | 0.450 | 0.589 | 0.492 | 0.623 | 0.485 |
| Share with end‐stage renal disease (ESRD) | 0.016 | 0.124 | 0.026 | 0.159 | 0.019 | 0.137 | 0.025 | 0.157 | 0.021 | 0.142 |
| Share of beneficiary‐years with selected diagnoses | ||||||||||
| Myalgia | 0.317 | 0.465 | 0.439 | 0.496 | 0.401 | 0.490 | 0.518 | 0.500 | 0.513 | 0.500 |
| Cancer | 0.162 | 0.368 | 0.187 | 0.390 | 0.217 | 0.412 | 0.155 | 0.362 | 0.148 | 0.355 |
| Non‐opioid drug abuse | 0.089 | 0.285 | 0.153 | 0.360 | 0.101 | 0.301 | 0.235 | 0.424 | 0.248 | 0.432 |
| Alcohol abuse | 0.016 | 0.124 | 0.023 | 0.151 | 0.017 | 0.130 | 0.031 | 0.174 | 0.031 | 0.173 |
| Back pain | 0.157 | 0.364 | 0.267 | 0.442 | 0.208 | 0.406 | 0.366 | 0.482 | 0.386 | 0.487 |
| Headache | 0.106 | 0.307 | 0.161 | 0.368 | 0.125 | 0.331 | 0.205 | 0.403 | 0.202 | 0.402 |
| Fracture | 0.073 | 0.261 | 0.128 | 0.335 | 0.139 | 0.346 | 0.137 | 0.344 | 0.125 | 0.330 |
| Sprain or strain | 0.105 | 0.307 | 0.169 | 0.375 | 0.165 | 0.371 | 0.183 | 0.387 | 0.171 | 0.376 |
| Mental health disorder | 0.225 | 0.417 | 0.294 | 0.456 | 0.235 | 0.424 | 0.395 | 0.489 | 0.406 | 0.491 |
| Hepatitis C | 0.012 | 0.111 | 0.022 | 0.146 | 0.012 | 0.108 | 0.035 | 0.184 | 0.038 | 0.191 |
| Chronic pain | 0.045 | 0.208 | 0.107 | 0.309 | 0.037 | 0.188 | 0.226 | 0.418 | 0.260 | 0.439 |
| Arthritis | 0.305 | 0.460 | 0.423 | 0.494 | 0.382 | 0.486 | 0.490 | 0.500 | 0.484 | 0.500 |
Doctor shopping are beneficiaries who fill opioid prescriptions from four or more unique providers and have at least one overlapping opioid prescription in a 90‐day period.
Pharmacy shopping are beneficiaries who fill opioid prescriptions at four or more unique pharmacies and have at least one overlapping opioid prescription in a 90‐day period.
Specific ICD‐9 codes used to generate estimates of various diagnoses are available in Table S1.
Opioid abuse and overdoes diagnoses are defined as in Table S1.
Beneficiaries who initiate opioid use are individuals who fill an opioid in a calendar year who did not fill an opioid in the previous calendar year.
Due to data redaction of substance abuse claims, there are no claims for opioid abuse in 2012.
Opioid users are more likely to have been diagnosed with the comorbidities noted in Table 1 than the average Medicare beneficiary, and the distribution of opioid users by those comorbidities has shifted from 2007 to 2012. As shown in Figure 1, the share of opioid users with a diagnosis commonly associated with acute pain like a fracture (13 percent) as well as those with a cancer diagnosis (19 percent) remained flat from 2007 to 2012. Meanwhile, the share of opioid users with comorbidities often associated with chronic pain like low back pain grew by 4 percentage points and the share with an explicit diagnosis of chronic pain grew 11 percentage points. These growth rates exceed those in the full population. The share of opioid users with diagnoses of abuse of other drugs, abuse of opioids, and major mental health diagnoses also grew over the period. These growth rates are more dramatic in the population of beneficiaries with chronic opioid use, where fracture diagnoses decreased by 1 percentage point and lower back pain diagnoses increased by 6 percentage points.
Figure 1.
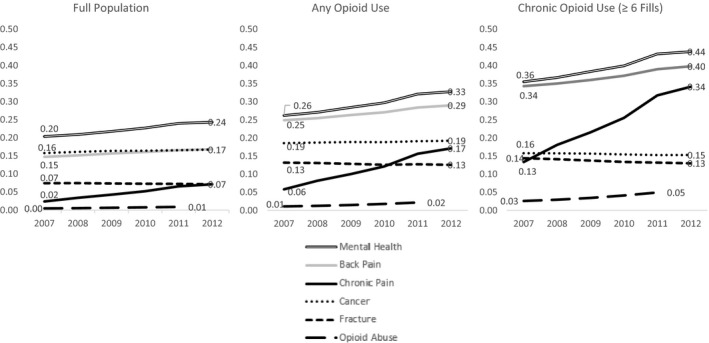
Distribution of Selected Patient Comorbidities by Opioid Utilization Category, 2007–2012 Notes: Specific ICD‐9 codes for these diagnoses are available in Table S1.
In addition to observing a substantial shift in the distribution of comorbidities of opioid users over this period, diagnoses of chronic pain and low back pain are also associated with stronger, lengthier prescriptions (see Table S3).
Opioid Utilization
Use of opioids by Medicare beneficiaries is high and stable. From 2006 to 2012, about 5 percent of all prescriptions filled each year through Medicare Part D were for opioids. Controlling for differences by patient characteristics, as shown in Table 2, the share of beneficiaries with any opioid use rose slightly from 36.8 percent in 2007 to 37.8 percent in 2010 when it peaked and then fell slightly in 2011 and 2012 to 35.3 percent. Initiation of opioid use and chronic opioid use by Medicare beneficiaries followed a similar pattern of small or no increase from 2007 to 2010 and then slight decreases in 2011 and 2012. Patterns of utilization—total fills and total days supply—increased until 2011 and then dropped in 2012.
Table 2.
Regression‐Adjusted Opioid Utilization Results by Opioid Use Subpopulation, 2006–2012
| 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | |
|---|---|---|---|---|---|---|---|
| Beneficiaries with any opioid use | |||||||
| Share of beneficiaries (%) | 37.59 | 36.83 | 37.26 | 37.57 | 37.75 | 36.01 | 35.29 |
| Mean total fillsa | 6.04 | 6.03 | 6.26 | 6.45 | 6.62 | 6.72 | 6.55 |
| Mean total days supplya | 120.41 | 129.28 | 143.53 | 153.92 | 166.19 | 174.75 | 168.37 |
| Mean total MME per daya | 52.04 | 50.17 | 48.41 | 51.29 | 49.52 | 41.71 | 41.77 |
| Share with prescription >100MED (%) | 21.98 | 21.02 | 19.70 | 18.91 | 16.65 | 9.02 | 8.70 |
| Beneficiaries initiating opioid useb | |||||||
| Share of beneficiaries (%) | 7.46 | 11.79 | 11.87 | 11.75 | 10.76 | 10.79 | |
| Mean total fillsa | 2.23 | 2.17 | 2.16 | 2.15 | 2.11 | 2.10 | |
| Mean total days supplya | 22.53 | 22.16 | 21.84 | 22.15 | 21.55 | 21.40 | |
| Mean total MME per daya | 47.57 | 47.36 | 49.34 | 46.83 | 38.01 | 38.23 | |
| Share with prescription >100MED (%) | 17.50 | 16.46 | 15.02 | 12.12 | 2.97 | 2.87 | |
| Beneficiaries with chronic opioid use (measured by ≥6 fills) | |||||||
| Share of beneficiaries (%) | 13.12 | 12.33 | 12.77 | 13.16 | 13.46 | 12.93 | 12.61 |
| Mean total fillsa | 14.12 | 14.02 | 14.16 | 14.17 | 14.17 | 14.09 | 13.79 |
| Mean total days supplya | 267.47 | 275.74 | 288.87 | 296.89 | 308.35 | 317.13 | 315.76 |
| Mean total MME per daya | 52.88 | 51.12 | 50.82 | 54.35 | 54.12 | 49.06 | 48.45 |
| Share with prescription >100MED (%) | 30.48 | 29.23 | 27.86 | 27.36 | 25.73 | 19.62 | 18.72 |
| Beneficiaries with chronic opioid use (measured by ≥90 consecutive days of use) | |||||||
| Share of beneficiaries (%) | 9.38 | 8.89 | 9.48 | 10.01 | 10.55 | 10.44 | 10.33 |
| Mean total fillsa | 15.45 | 15.26 | 15.26 | 15.10 | 14.96 | 14.72 | 14.22 |
| Mean total days supplya | 345.32 | 351.13 | 357.93 | 359.28 | 361.43 | 359.24 | 350.97 |
| Mean total MME per daya | 51.82 | 50.28 | 50.83 | 55.18 | 55.85 | 51.87 | 50.79 |
| Share with prescription >100MED (%) | 28.91 | 27.60 | 26.75 | 26.50 | 25.64 | 21.95 | 20.69 |
Findings control for differences in age, sex, race, dual eligibility, low‐income status, disability status, ESRD status, end‐of‐life status, various diagnoses, and health status.
All results are on a per‐year basis.
Additional estimates and complete regression results are available in the Tables S3–S4.
These estimates are predicted using the smearing estimator in Duan (1983).
Beneficiaries initiating opioid use are individuals who fill an opioid in a given calendar year who did not fill an opioid in the previous calendar year.
Given the skewed nature of these utilization measures, mean outcomes mask substantial variation in the distribution of these outcomes. Table S4 provides a detailed distribution of the total fills and total days supply outcomes. In general, utilization at the top end of the distribution is intensifying, while use at the bottom end of the distribution is falling. Among beneficiaries with any opioid use, the 25th percentile of total fills fell from 4.1 fills per year in 2006 to 3.6 in 2012 while the 90th percentile of total fills increased from 9.3 fills per year in 2006 to 12.8 in 2012. Growth at the 95th percentile is even more exaggerated, rising from 11.1 fills per year to 17.3 in 2012. A similar pattern holds for the distribution of total days supply where the bottom half of the distribution decreased from 2006 to 2012 while the top 25 percent increased.
Among the opioid‐initiating subpopulation, only the top 5 percent of the total fills and total days supply distributions increased, while the rest of the distribution is stable or decreasing. Among the chronic use subpopulations, the top decile of total fills and the top 25 percent of the total days supply distributions increased, while the rest of the distributions are stable or decreasing.
Utilization measures that adjust for the potency of opioids do not follow the same patterns. As shown in Table 2, total MME per day fell in most years after 2006. These decreases were observed across all opioid subpopulations and at all levels of the distribution of total MME per days (see Table S4). The share of patients receiving a prescription with a potency >100 MED decreased across all years from 2006 to 2012. These decreases accelerated markedly in 2011 and 2012, but varied in their magnitude by opioid subpopulation. Among beneficiaries with any opioid use, the share with prescriptions exceeding 100 MED more than halved from 22 percent to 9 percent. Among chronic users, that share fell from 30 percent to 19 percent or 29 percent to 21 percent, depending on the definition of chronic use (see Table 2).
Questionable Opioid Use
Opioid abuse or questionable opioid use is common in Medicare. As shown in Table 3, the share of beneficiaries with any opioid use who are diagnosed as opioid abusers increased from 1.2 to 2.2 percent, the share of chronic users (as measured by fills) who abuse opioids grew from 2.6 to 4.9 percent, and the share of chronic users (as measured by consecutive use) who abuse opioids grew from 3.2 to 5.6 percent. While opioid overdose attempts are a rare outcome, they grew to as high as 1 percent of chronic opioid users (as measured by consecutive use) in 2011 before slowing in 2012.
Table 3.
Regression‐Adjusted Questionable Opioid Use and Abuse Estimates by Opioid Use Subpopulation, 2006–2012
| 2006 (%) | 2007 (%) | 2008 (%) | 2009 (%) | 2010 (%) | 2011 (%) | 2012 (%) | |
|---|---|---|---|---|---|---|---|
| Beneficiaries with any opioid use | |||||||
| Opioid abuse diagnosisa | 1.17 | 1.10 | 1.26 | 1.49 | 1.78 | 2.17 | – |
| Opioid overdose diagnosis | 0.23 | 0.22 | 0.24 | 0.29 | 0.37 | 0.41 | 0.36 |
| Doctor shopping | 1.35 | 1.27 | 1.37 | 1.45 | 1.47 | 1.42 | 1.34 |
| Pharmacy shopping | 0.29 | 0.31 | 0.33 | 0.36 | 0.35 | 0.33 | 0.29 |
| Beneficiaries with chronic opioid use (measured by ≥6 fills) | |||||||
| Opioid abuse diagnosisa | 2.56 | 2.60 | 2.97 | 3.46 | 4.13 | 4.94 | – |
| Opioid overdose diagnosis | 0.54 | 0.53 | 0.56 | 0.66 | 0.81 | 0.89 | 0.78 |
| Doctor shopping | 3.68 | 3.60 | 3.80 | 3.94 | 3.89 | 3.75 | 3.55 |
| Pharmacy shopping | 0.81 | 0.90 | 0.95 | 1.00 | 0.98 | 0.91 | 0.81 |
| Beneficiaries with chronic opioid use (measured by ≥90 consecutive days of use) | |||||||
| Opioid abuse diagnosisa | 3.16 | 3.22 | 3.63 | 4.16 | 4.83 | 5.63 | – |
| Opioid overdose diagnosis | 0.68 | 0.66 | 0.68 | 0.78 | 0.92 | 1.01 | 0.86 |
| Doctor shopping | 3.78 | 3.69 | 3.83 | 3.87 | 3.73 | 3.48 | 3.20 |
| Pharmacy shopping | 0.97 | 1.10 | 1.12 | 1.16 | 1.11 | 0.99 | 0.86 |
Findings control for differences in age, sex, race, dual eligibility, low‐income status, disability status, ESRD status, end‐of‐life status, various diagnoses, and health status.
Opioid abuse and overdoes diagnoses are defined as in Table S1.
Doctor shopping are beneficiaries who fill opioid prescriptions from four or more unique providers and have at least one overlapping opioid prescription in a 90‐day period.
Pharmacy shopping are beneficiaries who fill opioid prescriptions at four or more unique pharmacies and have at least one overlapping opioid prescription in a 90‐day period.
Due to data redaction of substance abuse claims, there are no claims for opioid abuse in 2012.
Estimates of questionable opioid use that are associated with an increased risk of opioid overdose, like doctor shopping and pharmacy shopping, had similar rates of prevalence to opioid abuse and overdose outcomes in the full population of opioid users. Like measures of opioid abuse or overdose, both doctor and pharmacy shopping rates were somewhat higher in the chronic use population. Among chronic users (as measured by fills), rates of doctor shopping peaked at 3.9 percent in 2009, while rates of pharmacy shopping peaked at 1.2 percent in 2009 (as measured by consecutive use). These outcomes grew less quickly and steadily over the analysis period than did diagnoses of abuse or overdose—across all opioid‐using populations. The share of patients who have both medical and nonmedical indicators of questionable opioid use in a year is small (see Table 3).
While these are not perfect measures of questionable opioid use and could instead be indicative of fragmented or poorly managed care, they indicate that the scope of questionable opioid use is somewhat different than captured by medical diagnoses of abuse or overdose.
Opioid Prescribing by Specialty
Total prescribing of opioids across all specialties grew slightly from 2006 to 2012. That growth peaked in 2010 and slowed in 2011 and 2012. As shown in Table 4, the bulk of opioid prescriptions (50.4 percent in 2006) were written by family practice and internal medicine physicians, but their relative share of opioid prescriptions fell by 2.1 and 0.85 percentage points, respectively, from 2006 to 2012. The largest increases in the quantity of prescribing came among nurse practitioners and physician assistants whose share of total filled opioid prescriptions grew by 3.0 and 2.5 percentage points, respectively, from 2006 to 2012. Notably, prescribing by nurse practitioners and physician assistants increased linearly in every year from 2006 to 2012 with no peak in 2010. Dentists and pain medicine physicians also experienced relatively high increases of 0.8 and 0.6 percentage points, respectively (see Table 4).
Table 4.
Regression‐Adjusted Prescribing Measures by Prescriber Specialty, 2006–2012
| Share of Total Filled Prescriptions (%) | Mean Number of Fills per Prescriber | Share Prescribing <60 Days after an Overdose (%) | Distribution of Prescribers in the Top 10% of Fills (%) | |||||
|---|---|---|---|---|---|---|---|---|
| 2006 | 2012 | 2006 | 2012 | 2006 | 2012 | 2006 | 2012 | |
| All specialties | 100 | 100 | 13.07 | 14.02 | 0.61 | 0.85 | 100 | 100 |
| Family medicine | 29.20 | 27.09 | 24.03 | 26.28 | 0.83 | 1.23 | 66.06 | 63.88 |
| Internal medicine | 21.23 | 20.37 | 15.91 | 17.16 | 0.73 | 1.07 | 5.91 | 6.31 |
| Surgery | 8.82 | 8.63 | 11.89 | 12.55 | 0.33 | 0.45 | 0.19 | 0.21 |
| Nurse practitionera | 2.55 | 5.55 | 10.03 | 11.08 | 0.60 | 0.77 | – | 0.06 |
| Emergency medicine | 5.09 | 4.93 | 9.52 | 10.52 | 0.69 | 0.90 | – | – |
| Physician assistanta | 2.21 | 4.69 | 8.91 | 9.67 | 0.58 | 0.73 | – | – |
| Dentist | 2.95 | 3.77 | 4.90 | 5.13 | 0.12 | 0.14 | – | – |
| Pain medicine | 1.94 | 2.50 | 103.20 | 120.16 | 6.12 | 9.12 | 2.46 | 2.92 |
| Physical medicine | 1.91 | 2.14 | 26.25 | 29.80 | 2.07 | 3.18 | 5.51 | 6.57 |
| Oncology | 2.02 | 2.10 | 12.59 | 13.42 | 0.40 | 0.52 | 0.15 | 0.23 |
| Anesthesiology | 1.61 | 2.01 | 28.41 | 32.45 | 3.18 | 4.65 | 5.27 | 6.91 |
| Geriatric medicine | 1.88 | 1.69 | 35.26 | 38.20 | 0.95 | 1.59 | 6.43 | 5.71 |
| Rheumatology | 1.85 | 1.62 | 33.77 | 36.39 | 0.75 | 0.97 | 6.41 | 5.63 |
| Psychiatry/neurology | 1.21 | 1.35 | 9.12 | 10.37 | 1.45 | 1.63 | – | – |
| General medicine | 1.19 | 1.05 | 21.15 | 22.88 | 0.94 | 1.51 | 1.57 | 1.56 |
All findings are adjusted at the prescriber level and control for differences by patient age, sex, race, dual eligibility, low‐income status, disability status, ESRD status, end‐of‐life status, various diagnoses, and health status.
The top 15 specialties (as defined by their total share of fills in 2012) are presented in this table. All specialty definitions are defined following the Medicare health care provider taxonomy available here: https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/MedicareProviderSupEnroll/Downloads/TaxonomyCrosswalk.pdf. Physicians with both a classification and a specialization (e.g., Internal Medicine & Emergency Medicine) are reported by their specialization.
– Indicates that there were either no observations for that specialty or too few to report.
Nurse practitioners and physicians assistants were grouped together by type, regardless of specialization.
Conditional on prescribing any opioids, all of the top 15 specialties experienced at least a small increase in the average intensity of prescribing as measured by the mean number of prescriptions per prescriber‐year. Both the levels of and increases in prescribing were highest among pain medicine physicians (mean of 120.2 prescriptions in 2012; 17 more prescriptions written in 2012 than in 2006 on average). Other high‐intensity specialties included geriatric medicine, rheumatology, anesthesiology, and physical medicine and rehabilitation. Again, the growth followed a pattern of small increases until 2010, when intensity peaked and then fell in 2011 and 2012.
An alternate measure of intensity focusing on individual prescribers—the share of prescribers by specialty in the top 10 percent of the total fills distribution—is dominated by family practice physicians. While these physicians account for 64 percent of the top decile of prescribers in 2012, their share decreased by 2.2 percentage points from 2006. Specialties whose prescribers are more likely to be in the top decile in 2012 compared to 2006 include anesthesiology (1.6 percentage point increase), physical medicine and rehabilitation (1.1 percentage point increase), and pain medicine (0.5 percentage point increase). In addition, nurse practitioners entered the top decile of prescribing in 2009, and their share grew to 0.06 percent of all top decile prescribers in 2012.
In addition to variation by volume and intensity of prescribing, prescribing by specialty varies with respect to the patient population. Controlling for average patient characteristics, all specialties were more likely to have ever prescribed an opioid to a patient within 60 days of an overdose event between 2006 and 2012 (0.2 percentage point increase). The probability and change were highest among high‐intensity specialties like pain medicine (3.0 percentage points) and anesthesiology (1.5 percentage points), but difference in probability from 2006 to 2012 was also elevated among geriatric medicine physicians (0.6 percentage point increase), general practice physicians (0.6 percentage point increase), and physical medicine and rehabilitation physicians (1.1 percentage point increase).
Discussion
Utilization of prescription of opioids in Medicare from 2006 to 2012 generally mirrored trends in the non‐Medicare population. Namely, average measures of utilization in Medicare were stable until 2010, but after 2010 there was a reversal in trend and average utilization is decreasing. Similarly, total sales of opioids—across all populations—climbed until 2011 when total sales decreased through 2013 (Dart et al. 2015). Furthermore, according to CDC data, prescription opioid‐related overdoses grew until 2011, when the trend reversed and prescription opioid overdose‐related deaths fell slightly (Dowell, Noonan, and Houry 2017). While a single year of data is not a trend, opioid‐related overdose attempts in Medicare rose until 2011 and then fell in 2012. Additionally, the estimate of opioid overdose diagnosis in these data is not identical to opioid overdose deaths in CDC data and could be subject to some measurement error as claims related to substance abuse were redacted in 2012, but other indicators of questionable opioid use or misuse follow similar trajectories.
While these average trends are encouraging—utilization and questionable use decreased in the latter part of the analysis period—a key insight of these results is that the observed trends are not uniform across the distribution of opioid utilization. Of most interest is a general tendency for the lower end of the distribution of utilization measures to decrease over time, while the higher end increases—resulting in stable or decreasing average outcomes. These results imply that for opioid users at the high end of the utilization distribution, use may be escalating. This phenomenon has been described in commercial and Medicaid populations where increases in opioid use are attributable to disproportionate increases in the high end of the utilization distribution (Sullivan et al. 2008; Edlund et al. 2010). Thus, there is a universal need to routinely and reliably identify patients at the high end of the utilization distribution to try to reduce any inappropriate or excessive utilization.
One of the more striking findings is that the share of patients—across all user types—receiving high‐dose prescriptions fell precipitously in 2011 and 2012. However, the magnitude of the decrease is substantially lower among the chronic use populations than it is in the opioid‐initiating population or among all users. Furthermore, given the elevated risk of opioid abuse, overdose, or questionable use among chronic opioid users, it is likely that these opioid users with high and rising utilization are also those at an increased risk of negative opioid‐related outcomes. Among all opioid users, there was a 1.0 percentage point increase in the share of users diagnosed with abuse from 2006 to 2011, but a 2.4 percentage point increase among the chronic use subpopulations. These high‐intensity and high‐risk beneficiaries are precisely the population whose utilization should be closely monitored to reduce the risk of negative opioid‐related outcomes, but they are the beneficiaries who received high and rising quantities of opioids. Again, prior research has identified this phenomenon of “adverse selection” in non‐Medicare settings (Sullivan and Howe 2013).
In addition to utilization varying within the distribution of opioid users, prescription opioid users also vary by diagnosis. Over the study period, patients using opioids were increasingly likely to have diagnoses associated with chronic pain rather than those associated with acute or malignant pain. While limitations of the data make it impossible to confirm which prescriptions are directly tied to these diagnoses, the shift in comorbidities among opioid users is striking, particularly among chronic opioid users. With increasing evidence that opioids may not be appropriate for the treatment of chronic pain or other musculoskeletal diagnoses like lower back pain, it is troubling that a greater share of Medicare opioid users is being diagnosed with such ailments (Deshpande et al. 2007; Martell et al. 2007).
Finally, the analysis of prescribing by specialty highlights the growth in the quantity of prescribing in nonphysician specialties and in the intensity of prescribing among specialties like physical medicine and rehabilitation who focus on the treatment of chronic pain. While nonphysician prescribers account for a small share of total prescriptions, their growth is anomalous. Given the reductions in prescribing by family, internal medicine, and general medicine physicians, it is possible that they are simply replacing prior prescribing, but it is also possible that they are treating unobservably sicker patients or are looser prescribers (as indicated by nurse practitioners’ entry into the top decile of prescribing in 2009). The growth in opioid prescribing intensity among physical medicine and rehabilitation physicians is also notable given their treatment of patients whose chronic pain may not be amenable to treatment with opioids. Again, their patient population may be unobservably sicker, or they may be relatively looser prescribers than other specialties. Future research should determine which mechanism is responsible for these trends.
Together these findings show that, much like opioid use in the broader population from 2006 to 2012, utilization in Medicare was ripe for stricter regulation and policy intervention. Since the start of the study period, there has been an increase in the level of regulation of prescription opioids. For example, many states have adopted a wide variety of policies like prescription drug monitoring programs (PDMPs) aimed at stemming opioid misuse; the federal government reclassified hydrocodone to control access more tightly; and CMS has specifically focused on identifying and intervening with Medicare beneficiaries whose opioid utilization appears anomalous. While there is some reason to believe such policies could have a large impact on Medicare beneficiaries’ utilization of opioids, a recent study showed that among disabled Medicare beneficiaries, there was no impact of the implementation of PDMPs or any other state‐level interventions on a range of measures of opioid use or overdose (Meara et al. 2016).
While these results cannot inform whether the change in regulatory environment related to opioids has had an impact on utilization in Medicare, they do point to a few avenues for further study. For example, the marked decrease in the share of patients receiving high‐dose prescriptions followed closely on the finding and publication of results showing that similar doses carry an increased risk of overdose, perhaps signaling the amenability of Medicare utilization or prescribers to information‐based policy change (Dunn, Saunders et al. 2010). If that is the case, the prescribing results highlight the need to ensure that any such campaigns related to opioid prescribing are reaching nurse practitioners and physician assistants as they appear to be increasingly important to the volume of opioid prescriptions. Additionally, given the heterogeneity in outcomes and trends by intensity of use, patient diagnoses, and prescriber specialty, successful interventions may need to be targeted to individual patients or prescribers rather than implemented broadly as in the state polices noted above.
What these results show most clearly is that there is significant heterogeneity in prescription opioid use within Medicare. Future research should focus on identifying explanatory factors like patient diagnoses, geography, prescriber specialty, and others that account for these differential patterns of utilization and trends. By better explaining these divergent trends, perhaps Medicare will be able to help target interventions that treat pain while not exacerbating opioid misuse.
Limitations
While these results reflect a broad, representative sample of Medicare beneficiaries, they are subject to the limitations of administrative claims data. For example, the data do not indicate whether prescriptions were consumed, consumed as prescribed, or consumed by the beneficiaries in question. These findings reflect the share of prescription opioids that are filled through Medicare Part D—rather than true consumption patterns of Medicare beneficiaries. These prescriptions do not capture any opioids that are paid for out‐of‐pocket or are otherwise not filled through Part D. All prescription‐based outcomes measures are a conservative estimate of a beneficiary's total consumption and could exclude the most at‐risk beneficiaries. Finally, because prescriptions are not tied directly to a medical encounter in the claims data, measures of diagnoses reflect the comorbidities of patients receiving opioids rather than the direct diagnosis for which the opioid was prescribed. Future research should focus on explaining the relationship between pain‐related diagnoses and resulting trajectories of opioid utilization or misuse.
Conclusions
On average, opioid use in Medicare was stable from 2006 to 2012. More than 1 in 3 beneficiaries filled an opioid prescription annually; about 1 in 10 were chronic opioid users. These average outcomes mask significant variation in the distribution of opioid use. Opioid users at the high end of the utilization distribution intensified their utilization from 2006 to 2012, while those at the low end stabilized or reduced their opioid prescriptions. Chronic opioid users were increasingly likely to abuse opioids or display patterns of questionable use as compared to all prescription opioid users. But, over the same time period, high‐dose prescriptions (>100 MME) were reduced substantially for all prescription opioid use subpopulations. In addition, patients were increasingly likely to have comorbidities associated with chronic rather than acute pain and were more likely to receive opioids from nonphysician prescribers. While these results do not account for many of the changes to the regulatory landscape regarding opioids, they point to the need for more research to understand and explain these differential outcomes within the distribution of opioid utilization.
Supporting information
Table S1. ICD‐9 Codes for Diagnosis Definitions.
Table S2. Morphine Equivalence by Active Ingredient.
Table S3. Utilization Regression Results for Population with Any Opioid Use.
Table S4. Detailed Distribution of Count‐Based Opioid Utilization Measures by Opioid Subpopulation, 2006–2012.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: This research was supported by a National Institute on Aging grant (5P01AH033559) and a grant from the Agency for Healthcare Research and Quality (1R36HS024251‐01). The funders had no role in the design or conduct of the study. I want to thank Dana Goldman and Geoffrey Joyce for their thoughtful comments on earlier drafts and their support of this project. No other disclosures.
Disclosures: None.
Disclaimer: None.
References
- Buntin, M. B. , and Zaslavsky A. M.. 2004. “Too Much Ado about Two‐part Models and Transformation?: Comparing Methods of Modeling Medicare Expenditures.” Journal of Health Economics 23 (3): 525–42. [DOI] [PubMed] [Google Scholar]
- Chen, J. H. , Humphreys K., Shah N. H., and Lembke A.. 2016. “DIstribution of Opioids by Different Types of Medicare Prescribers.” JAMA Internal Medicine 176 (2): 259–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dart, R. C. , Surratt H. L., Cicero T. J., Parrino M. W., Severtson S. G., Bucher‐Bartelson B., and Green J. L.. 2015. “Trends in Opioid Analgesic Abuse and Mortality in the United States.” New England Journal of Medicine 372 (3): 241–8. [DOI] [PubMed] [Google Scholar]
- Deshpande, A. , Furlan A. D., Mailis‐Gagnon A., Atlas S., and Turk D.. 2007. Opioids for Chronic Low‐back Pain. Cochrane Database of Systematic Reviews. 3 (3): CD004959. [DOI] [PubMed] [Google Scholar]
- Dowell, D. , Noonan R. K., and Houry D.. 2017. “Underlying Factors in Drug Overdose Deaths.” Journal of the American Medical Association 318: 2295–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan, N. 1983. “Smearing Estimate: A Nonparametric Retransformation Method.” Journal of the American Statistical Association 78 (383): 605–10. [Google Scholar]
- Dufour, R. , Joshi A. V., Pasquale M. K., Schaaf D., Mardekian J., Andrews G. A., and Patel N. C.. 2014. “The Prevalence of Diagnosed Opioid Abuse in Commercial and Medicare Managed Care Populations.” Pain Practice 14 (3): E106–15. [DOI] [PubMed] [Google Scholar]
- Dunn, K. M. , Saunders K. W., Rutter C. M., Banta‐Green C. J., Merrill J. O., Sullivan M. D., Weisner C. M., Silverberg M. J., Campbell C. I., Psaty B. M., and Von Korff M.. 2010. “Opioid Prescriptions for Chronic Pain and Overdose: A Cohort Study.” Annals of Internal Medicine 152 (2): 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund, M. J. , Martin B. C., Fan M.‐Y., Braden J. B., Devries A., and Sullivan M. D.. 2010. “An Analysis of Heavy Utilizers of Opioids for Chronic Noncancer Pain in the TROUP Study.” Journal of Pain and Symptom Management 40 (2): 279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jena, A. B. , Goldman D., and Karaca‐Mandic P.. 2016. “Hospital Prescribing of Opioids to Medicare Beneficiaries.” JAMA Internal Medicine 176 (7): 990–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jena, A. B. , Goldman D., Weaver L., and Karaca‐Mandic P.. 2014. “Opioid Prescribing by Multiple Providers in Medicare: Retrospective Observational Study of Insurance Claims.” BMJ: British Medical Journal 348: g1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle, M. R. , Liebschutz J. M., Zhang F., Ross‐Degnan D., and Wharam J. F.. 2016. “Opioid Prescribing After Nonfatal Overdose and Association with Repeated Overdose: A Cohort Study of Opioid Prescribing after Nonfatal Overdose.” Annals of Internal Medicine 164 (1): 1–9. [DOI] [PubMed] [Google Scholar]
- Martell, B. A. , O'Connor P. G., Kerns R. D., Becker W. C., Morales K. H., Kosten T. R., and Fiellin D. A.. 2007. “Systematic Review: Opioid Treatment for Chronic Back Pain: Prevalence, Efficacy, and Association with Addiction.” Annals of Internal Medicine 146 (2): 116–27. [DOI] [PubMed] [Google Scholar]
- Meara, E. , Horwitz J. R., Powell W., McClelland L., Zhou W., O'Malley A. J., and Morden N. E.. 2016. “State Legal Restrictions and Prescription‐opioid Use among Disabled Adults.” New England Journal of Medicine 375 (1): 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morden, N. E. , Munson J. C., Colla C. H., Skinner J. S., Bynum J. P., Zhou W., and Meara E. R.. 2014. “Prescription Opioid Use among Disabled Medicare Beneficiaries: Intensity, Trends and Regional Variation.” Medical Care 52 (9): 852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulozzi, L. J. , Jones C. M., Mack K. A., and Rudd R. A.. 2011. “Vital Signs: Overdoses of Prescription Opioid Pain Relievers – United States, 1999–2008.” MMWR. Morbidity and Mortality Weekly Report 40 (43): 1487–92. [PubMed] [Google Scholar]
- Robst, J. , Levy J. M., and Ingber M. J.. 2007. “Diagnosis‐Based Risk Adjustment for Medicare Prescription Drug Plan Payments.” Health Care Financing Review 28 (4): 15–30. [PMC free article] [PubMed] [Google Scholar]
- Rudd, R. A. , Seth P., David F., and Scholl L.. 2016. “Increases in Drug and Opioid‐Involved Overdose Deaths—United States, 2010–2015.” MMWR. Morbidity and Mortality Weekly Report 65 (50–51): 1445–52. [DOI] [PubMed] [Google Scholar]
- Rudd, R. A. , Aleshire N., Zibbell J. E., and Matthew Gladden R.. 2016. “Increases in Drug and Opioid Overdose Deaths—United States, 2000–2014.” American Journal of Transplantation 16 (4): 1323–7. [DOI] [PubMed] [Google Scholar]
- Saunders, K. W. , Dunn K. M., Merrill J. O., Sullivan M., Weisner C., Braden J. B., Psaty B. M., and Von Korff M.. 2010. “Relationship of Opioid Use and Dosage Levels to Fractures in Older Chronic Pain Patients.” Journal of General Internal Medicine 25 (4): 310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon, D. H. , Rassen J. A., Glynn R. J., Garneau K., Levin R., Lee J., and Schneeweiss S.. 2010. “The Comparative Safety of Opioids for Nonmalignant Pain in Older Adults.” Archives of Internal Medicine 170 (22): 1979–86. [DOI] [PubMed] [Google Scholar]
- Sullivan, M. D. , and Howe C. Q.. 2013. “Opioid Therapy for Chronic Pain in the United States: Promises and Perils.” PAIN 154 (Supplement 1): S94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan, M. D. , Edlund M. J., Fan M.‐Y., DeVries A., Braden J. B., and Martin B. C.. 2008. “Trends in Use of Opioids for Non‐cancer Pain Conditions 2000–2005 in Commercial and Medicaid Insurance Plans: The TROUP Study.” Pain 138 (2): 440–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Korff, M. , Saunders K., Ray G. T., Boudreau D., Campbell C., Merrill J., Sullivan M. D., Rutter C., Silverberg M., and Banta‐Green C.. 2008. “Defacto Long‐term Opioid Therapy for Non‐cancer Pain.” Clinical Journal of Pain 24 (6): 521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z. , Wilsey B., Bohm M., Weyrich M., Roy K., Ritley D., Jones C., and Melnikow J.. 2015. “Defining Risk of Prescription Opioid Overdose: Pharmacy Shopping and Overlapping Prescriptions among Long‐term Opioid Users in Medicaid.” Journal of Pain 16 (5): 445–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. ICD‐9 Codes for Diagnosis Definitions.
Table S2. Morphine Equivalence by Active Ingredient.
Table S3. Utilization Regression Results for Population with Any Opioid Use.
Table S4. Detailed Distribution of Count‐Based Opioid Utilization Measures by Opioid Subpopulation, 2006–2012.


