Abstract
Objectives
To assess the cost‐effectiveness of Embrace, an integrated primary care service for older adults.
Data Sources
Care and support claims from health care insurers, long‐term care administration, and municipalities for enrolled older adults between 2011 and 2013.
Study Design
A total of 1,456 older adults, listed with 15 general practitioners practices in the Netherlands, were stratified into risk profiles (“Robust,” “Frail,” and “Complex care needs”) and randomized to Embrace or care‐as‐usual groups. Incremental costs were calculated per quality‐adjusted life year, per day able to age in place, and per percentage point risk profile improvement.
Principal Findings
Total average costs were higher for Embrace compared to care‐as‐usual. Differences in health‐associated outcomes were small and not statistically significant. Probabilities that Embrace is cost‐effective were below 80 percent, except for “risk profile improvements” within risk profile “Complex care needs.” Complete case analysis resulted in smaller differences in total average costs across conditions and differences in health‐associated outcomes remained small.
Conclusions
According to current standards, Embrace is not considered cost effective after 12 months. However, it could be considered worthwhile in terms of “risk profile improvements” for older adults with “Complex care needs,” if society is willing to invest substantially.
Keywords: Cost‐effectiveness analysis, primary health care, older adults, integrated care
The unprecedented aging of the population is having a major impact on modern societies and requires transformations within the health care system and community (Bloom et al. 2015; World Health Organization 2015b). It is assumed that preventive, person‐centered, and integrated primary care services for older adults will lead to better clinical outcomes, along with reduced service use and cost (Beswick et al. 2008; Boult and Wieland 2010; Milani and Lavie 2015). However, evidence for this remains scarce (Eklund and Wilhelmson 2009; Low, Yap, and Brodaty 2011; World Health Organization 2015a). Moreover, most of the studies on integrated care services focus on older adults already in need of care (Eng et al. 1997; Hebert et al. 2003; Boyd et al. 2007), without taking into account that the health status of older adults may suddenly change and take a turn for the worse (Boult and Wieland 2010). Opportunities for proactive and preventive care and support to postpone a decline in health are therefore not exploited (Fries 1980; Stuck et al. 1999).
We developed Embrace (in Dutch “SamenOud”) (Spoorenberg et al. 2013) as an integrated care service for all older adults living in the community. Embrace combines the Chronic Care Model (CCM)(Wagner et al. 2001)—a well‐known generic framework for improvements in health care—with a Population Health Management model (PHM), the Kaiser Permanente Triangle (Singh and Ham 2006). One of the main goals of Embrace is to improve the health outcomes of older adults and to modify the factors that may influence these health outcomes (Kindig 2007). Embrace uses the self‐reported levels of “case complexity”(Peters et al. 2013) and “frailty”(Peters et al. 2012) in order to stratify older adults into nondisease and nonservice‐specific risk profiles (Lynn et al. 2007; Lafortune et al. 2009), with the intensity of care and support offered depending on the risk profile.
Whether integrated primary care services for older adults, as Embrace is, fulfill its aims are still undecided (Spoorenberg et al. 2015b, 2018; Uittenbroek et al. 2017), and endpoints such as quality‐adjusted life years and Embrace's impact on costs have not been evaluated. Furthermore, it is not clear whether or not older adults, who received Embrace care and support, were able to age in place longer, or whether their risk profiles improved. In this study, we have therefore assessed the cost‐effectiveness of the integrated primary care service, Embrace, using various outcomes.
Methods
We performed a cost‐utility analysis and cost‐effectiveness analysis from a societal perspective alongside a randomized controlled trial that compared Embrace with care‐as‐usual. A detailed description of the study protocol has been published previously (Spoorenberg et al. 2013). The Medical Ethics Committee of the University Medical Center Groningen assessed the Embrace study proposal and concluded that their approval was not required (Reference METc2011.108). Study performance met the Helsinki Regulations (World Medical Association 2013).
Setting, Participants, and Procedure
Fifteen general practitioner (GP) practices (also referred to as family physicians in the United States) participated; they were evenly distributed over the three participating municipalities in the northern parts of the Netherlands. All adults aged 75 and older, listed with these GP practices, were invited to participate. Exclusion criteria at baseline were long‐term admission to a nursing home, involvement in a comparable integrated care service, or participating in another scientific study. After written consent was provided, including permission to obtain data on service use and cost, the participants completed self‐report questionnaires at baseline and after 12‐month follow‐up.
Randomization and Blinding
Participating older adults were stratified into three risk profiles, based on self‐reported case complexity (assessed with the INTERMED for the Elderly, self‐assessment, INTERMED‐E‐SA) (Peters et al. 2013) and frailty (assessed with the Groningen Frailty Indicator, GFI) (Peters et al. 2012). The risk profiles were as follows: “Robust” (INTERMED‐E‐SA score <16 and GFI score <5), “Frail” (INTERMED‐E‐SA score <16 and GFI score ≥5), and “Complex care needs” (INTERMED‐E‐SA score ≥16). After stratification, a concealed and computerized balanced allocation procedure was performed to achieve equal distributions between the intervention (Embrace) and control groups for those characteristics that potentially affect intervention outcomes. The balancing criteria were age, sex, complexity of care needs, frailty, living situation, number of chronic conditions, whether or not receiving home care, and whether or not receiving help with filling out the questionnaires.
Due to the nature of the study, Elderly Care Team members knew which participants were assigned to Embrace; however, members of the Elderly Care Teams did not know the risk profiles of the participants in the control group. The participants were informed in writing as to whether they were assigned to an intervention or a control group. The data manager was not blinded; researchers were blinded until the analyses started.
Intervention
Within Embrace, a GP‐led Elderly Care Team was assembled for each participating GP‐practice, which also consisted of an elderly care physician (also known as a nursing home physician), a community nurse (case manager for older adults with risk profile “Complex care needs”), and a social worker (case manager for older adults with risk profile “Frail”). The Elderly Care Team provided older adults with comprehensive, person‐centered, proactive, and preventive care and support. Participants within the profiles “Frail” and “Complex care needs” received individual care and support from a case manager, who visited the older adults at home and focused on the older adults’ self‐defined problems such as mobility of joint functions, emotional well‐being, and exercise tolerance (Spoorenberg et al. 2015a). Older adults within the profile “Robust” were monitored by the Elderly Care Team, which reviewed their medical files and medications at least once a year. All participating older adults were offered a self‐management support and prevention program that emphasized preventive measures and endorsed a healthy lifestyle, while maintaining self‐management abilities. See Supplementary Table S1 for more details.
Care‐as‐usual
The control group received care‐as‐usual as provided by their GP, and local health care and social care organizations. Patients enter the health care system via primary health care, in which the GP also acts as a gatekeeper for specialized (secondary) medical care. The mean number of GP visits in the Netherlands increases with age from four visits per year at age 45–64 to 10 visits per year for people aged 75 years and older (Statistics Netherlands 2013).
Resource Use and Valuation
Data on costs of Dutch health care and social care for all older adults were obtained from the three sources of reimbursement, based on Dutch health care and social care legislation (Schafer et al. 2010). These data come closest to reality, as these are actually reimbursed costs. However, copayments or out‐of‐pocket costs are not included (e.g., a compulsory copayment for curative care of 220 EUR per person per year). Costs of medical (curative) care—for example, primary care, hospital care, medications, or paramedical care—are covered by the Health Insurance Act and are reimbursed by various competing health insurers. In the Netherlands, this insurance is compulsory for all Dutch residents. In this study, we obtained data from two of the major health care insurers active throughout the Netherlands (Menzis and Zilveren Kruis), covering 77 percent of the study population. Missing data on medical (curative) care costs for the remaining 23 percent were handled as missing data and imputed by multiple imputation (see also statistical analysis paragraph). Long‐term care—for example, institutional care and home care—for all older adults is covered by the Chronic Care Act, tax funded with additional patient copayment and reimbursed by the dominant health insurer's long‐term care administration office (Menzis). We were able to obtain data on all participating older adults for this. Finally, social care—for example, home help and home adjustment—is covered by the Social Support Act, tax funded with additional patient copayment, and reimbursed by the municipalities, and we obtained data from three municipalities, covering all participating older adults. Informal care was assessed using self‐report questionnaire, the minimal dataset‐economic evaluation (MDS‐e), older adults filled out as well. Hours of care provided (per week) were multiplied by cost prices, according to Dutch guidelines for economic evaluations (EUR 13.27 per hour) (Hakkaart‐van Roijen, Tan, and Bouwmans 2011) and converted to annual costs. Intervention costs were available on an individual basis, from the records of funding, as granted by the Dutch Healthcare Authority and reimbursed by the long‐term care administration office (see Table S1).
The total sum of care and support expenditures was calculated for the year prior to the intervention (baseline) and for the year after the start of the intervention. Data on costs that could not be retrieved were treated as missing values. All costs are presented in Euros (EUR) for the intervention period of 12 months and based on unit prices for the reference year (2012). If needed, prices were indexed to the reference year using a consumer price index of 1.02 per year (Hakkaart‐van Roijen, Tan, and Bouwmans 2011).
Health‐Associated Main Outcome Measurements
For the cost‐utility analysis, the outcome was health‐related quality of life expressed as quality‐adjusted life years (QALYs). QALYs were calculated by multiplying the utility score of a state of health, assessed by means of the EuroQol (EQ‐5D‐3L) (Brooks 1996), by the amount of time an older adult spent in that particular state of health. Linear interpolation was used for transitions between states of health. For those who died during the intervention period, we multiplied baseline utility scores by total survival time. Dutch tariffs were used to estimate the utility score (0–1) for each participating older adult (Lamers et al. 2005).
For the cost‐effectiveness analyses, the first outcome was the number of days an older adult was “able to age in place,” that is, to have no long‐term stay in a nursing home. Data on these stays were obtained from the long‐term care administration office. Number of days “able to age in place” was then computed as 365 days—number of days in a nursing home. Second outcome for the cost‐effectiveness analysis was the proportion of older adults, whose risk profile remained “Robust” or was improved at 12‐month follow‐up. The latter involved being assigned a profile with a lower risk.
Statistical Analysis
First, we assessed baseline characteristics, overall, and per risk profile. Next, differences in main outcomes between Embrace and care‐as‐usual groups were assessed using t‐statistics for the QALYs, and “days able to age in place” and logistic regression for the binary outcome “risk profile improvement.” Costs were presented as arithmetic means, and differences between groups were compared using t‐test and nonparametric bootstraps (Thompson and Barber 2000). To assess consistency and homogeneity of the effect over all Elderly Care Teams and to account for potential skewness, we used multilevel analyses with older adults as lower level and GP practices as higher level, adjusted for age, gender, and baseline values. In addition, we calculated the differences in costs within conditions during the year before the intervention and during the 1‐year intervention period, and applied multilevel analyses to assess differences in change between conditions.
Missing items regarding patient‐reported outcomes, costs, and informal care hours were assumed to be missing at random and imputed at item level by multiple imputation using Bayesian techniques (van Buuren 2007), generating 20 imputed datasets. We used condition, risk profile, GP, sex, marital status, living situation, educational level, income, available data on health care and social care cost, and receipt of help with filling in the questionnaire as covariates of the missing predictor models. Measurement instrument scale scores of patient‐reported outcomes, which were missing due to loss to follow‐up, were imputed by the mean change of deterioration of completed cases, since we assumed that older adults deteriorate over time (Eklund and Wilhelmson 2009).
Incremental cost‐effectiveness ratios (ICERs) were calculated for all outcomes by dividing the difference in costs between the intervention and care‐as‐usual groups by the difference in effectiveness between both. For the bootstrap simulations, we randomly resampled cost‐effectiveness pairs from the imputed trial datasets, equally many as the number of participants per group. We calculated averages of outcomes and costs per bootstrap simulation. Given the structure of the dataset, being an imputed dataset, we replicated these calculations 10,000 times to estimate uncertainty intervals (Briggs, Wonderling, and Mooney 1997). Results of the bootstraps are presented in cost‐effectiveness planes and cost‐effectiveness acceptability curves (CEACs) (Briggs and Fenn 1998). Cost‐effectiveness planes show differences in costs on the vertical axis and differences in effect on the horizontal axis. For example, bootstrapped cost‐effectiveness pairs located in the southeast quadrant show Embrace to be more effective and less costly than care‐as‐usual. Then again, the preference for an intervention depends on the threshold value, that is, what society is willing to pay for an effectiveness gain. The CEACs show the probability that the intervention is cost effective in comparison with care‐as‐usual for a range of ceiling ratios, which are defined as willingness to pay to gain one unit of effect (Fenwick and Byford 2005).
Although a formal threshold for willingness to pay has not been defined within the Netherlands, a ceiling ratio between EUR 20,000 and EUR 80,000 for a QALY gained is most often assumed (Boersma, Broere, and Postma 2010). Regarding an additional “day able to age in place” and a percentage point of “risk profile improvement,” no thresholds are available, as these outcomes have never been used in a cost‐effectiveness analysis before. Nevertheless, as an approximation, we used EUR 200 to EUR 250, the cost of a day in a nursing home (Hakkaart‐van Roijen, Tan, and Bouwmans 2011; United States Department of Health & Human Services 2015), as it is most likely that a societal willingness to pay for a “day able to age in place” would be of that order of magnitude. For percent of “risk profile improvement,” we presented the probability of the intervention being cost effective for a range of ceiling ratios: EUR 0 to EUR 10,000. Peters et al. (2015) found that a point increase in “frailty” or “complexity of care needs”—instruments we used for assignment to risk profiles—was associated with a 15 percent or 6 percent increase in cost the next year, respectively.
Finally, we performed sensitivity analyses. We performed CEA and cost‐utility analysis using complete cases only. Cases were considered complete if data on medical costs were available from the registries and an older adult had completed the 12‐month intervention period. All analyses were performed on the level of the total sample and per risk profile. We used SPSS/PASW 23 (IBM Corp. Released 2015. IBM SPSS Statistics for Windows. IBM Corp., Armonk, NY) for statistical analysis. For bootstrapping, we used Microsoft Excel 2010.
Results
Participant and Baseline Characteristics
Figure 1 shows the flow of older adults through the study, and in Table 1, the baseline characteristics are presented. Of the 1,456 older adults who started the intervention, 1,131 (78 percent) completed it. Most older adults were lost to the researcher's data acquisition (see Figure 1). Loss to follow‐up and number of older adults with missing data on medical care costs, and older adults who were lost to follow‐up and had also missing data on medical care costs, were similar for the intervention and control groups, overall, and per risk profile. Older adults lost to follow‐up were significantly (p < .01) older, more frail, had a greater complexity of care needs, had a lower health‐related quality of life, and had higher costs during the intervention period than other older adults who completed the 12‐month intervention period. Furthermore, some statically significant differences were found between older adults with incomplete data versus older adults with complete data, and between older adults that completed the intervention period and had complete data, versus those that did not (see Table S2).
Figure 1.
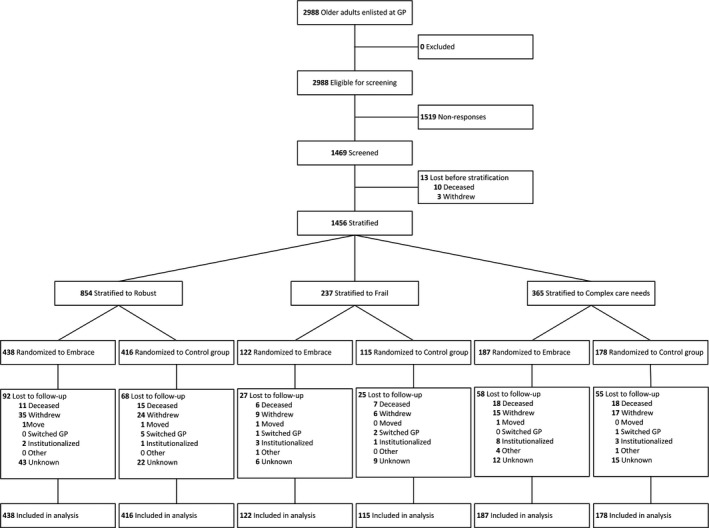
Flow of the Participants
Table 1.
Characteristics of Participating Older Adults at Baseline (n = 1,456), Overall, and Per Risk Profile
| Baseline Characteristics | Total | Complex Care Needs | Frail | Robust | ||||
|---|---|---|---|---|---|---|---|---|
| (n = 1,456) | (n = 365) | (n = 237) | (n = 854) | |||||
| Embrace | Care‐as‐Usual | Embrace | Care‐as‐Usual | Embrace | Care‐as‐Usual | Embrace | Care‐as‐Usual | |
| Number of participants | 747 | 709 | 187 | 178 | 122 | 115 | 438 | 416 |
| Age, mean (SD) | 80.7 (4.5) | 80.8 (4.7) | 81.8 (4.6) | 81.5 (4.9) | 81.6 (5.1) | 82.8 (5.5) | 79.9 (4.0) | 79.9 (4.1) |
| Female, n (%) | 405 (54.2) | 394 (55.6) | 121 (64.7) | 115 (64.6) | 82 (67.2) | 80 (69.6) | 202 (46.1) | 199 (47.8) |
| Widow(er), single, or divorced, n (%) | 320 (42.8) | 290 (41.0) | 87 (46.5) | 79 (44.4) | 77 (63.1) | 72 (63.2) | 156 (35.6) | 139 (33.5) |
| Low educational level, n (%) | 370 (49.9) | 374 (53.4) | 106 (57.0) | 116 (66.3) | 66 (54.1) | 69 (60.0) | 198 (45.7) | 189 (46.0) |
| Low household income, n (%) | 261 (35.7) | 231 (33.6) | 80 (43.5) | 77 (43.8) | 53 (44.5) | 51 (45.1) | 128 (29.9) | 103 (25.9) |
| Complexity of care needs | ||||||||
| IM‐E‐SA, median (IQR) | 10 (6–15) | 10 (6–15) | 19 (17–22) | 20 (17–24) | 12 (10–14) | 12 (9–13) | 7 (5–10) | 8 (5–10) |
| Frailty | ||||||||
| GFI, median (IQR) | 3 (2–6) | 3 (2–6) | 7 (5–8) | 7 (5–9) | 6 (5–7) | 6 (5–7) | 2 (1–3) | 2 (1–3) |
| Health‐related quality of life | ||||||||
| EQ‐5D‐3L, means (SD) | 0.79 (0.15) | 0.78 (0.16) | 0.65 (0.16) | 0.64 (0.17) | 0.74 (0.11) | 0.74 (0.13) | 0.86 (0.10) | 0.86 (0.09) |
| EQ‐5D‐VAS, means (SD) | 70.7 (17.5) | 69.8 (18.3) | 56.7 (16.7) | 53.8 (19.4) | 67.2 (15.6) | 70.0 (13.5) | 77.7 (14.1) | 76.5 (14.5) |
Low‐education means primary school, low‐vocational training, or less.
Low household income means less than EUR 1351 per month.
EQ‐5D, EuroQol Health‐related quality of life; GFI, Groningen Frailty Indicator; IM‐E‐SA, INTERMED Elderly Self‐Assessment; IQR, interquartile range; SD, standard deviation; VAS, Visual Analogue Scale.
Care and Support Costs
In Table 2 health care utilization in the year before the intervention period (baseline), the mean costs per participating older adult during the 1‐year intervention period, and the difference between these years are presented. We found baseline total health care utilization within the Embrace groups to be higher than care as usual (EUR 9203 vs. EUR 8346), although differences were not statically significant, with the exception for informal care within the Robust profile.
Table 2.
Mean Costs (Standard Deviation) and Differences in Change in Costs within and between Embrace and Care‐as‐Usual during the Year Prior Intervention (Baseline) and during the 1‐Year Intervention Period (in Euros)
| Embrace | Care‐as‐Usual | Differences between Intervention vs. Baseline Period | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline Mean (SD) | Intervention Period Mean (SD) | Change Mean (SD) | Baseline Mean (SD) | Intervention Period Mean (SD) | Change Mean (SD) | B (95% CI) | p‐Value | |
| Total sample | n = 747 | n = 709 | ||||||
| Total costs | 9,203 (15,702) | 13,073a (18,104) | 3,870 (15,635) | 8,346 (12,854) | 10,677a (14,476) | 2,331 (11,514) | 1,555 (−27 to 3,137) | .05 |
| Intervention | 684 (777) | |||||||
| Subtotal service use | 9,203 (15,702) | 12,389 (17,851) | 3,186 (15,581) | 8,346 (12,854) | 10,677 (14,476) | 2,331 (11,514) | 866 (−713 to 2,446) | .28 |
| Medical care | 4,901 (9,343) | 6,458 (10,738) | 1,557 (10,580) | 4,345 (6,546) | 5,689 (8,076) | 1,344 (7,912) | 198 (−965 to 1,361) | .74 |
| Long‐term care | 2,225 (7,489) | 2,667 (7,038) | 1,312 (7,585) | 1,605 (5,574) | 1,933 (5,729) | 877 (6,258) | 462 (−251 to 1,175) | .20 |
| Social care | 697 (1,413) | 667 (1,288) | −30 (1,115) | 649 (1,411) | 625 (1,347) | −24 (1,059) | −13 (−123 to 97) | .82 |
| Informal care | 747 (3,045) | 1,094 (4,355) | 348 (4,289) | 587 (1,763) | 721 (2,653) | 135 (2,644) | 217 (−200 to 634) | .31 |
| Complex care needs | n = 187 | n = 178 | ||||||
| Total costs | 19,268 (24,822) | 24,622 (24,376) | 5,354 (24,737) | 16,363 (17,291) | 19,959 (19,294) | 3,596 (16,539) | 1,747 (−2,961 to 6,456) | .47 |
| Intervention | 1,675 (392) | |||||||
| Subtotal service use | 19,268 (24,822) | 22,947 (24,430) | 3,679 (24,728) | 16,363 (17,291) | 19,959 (19,294) | 3,596 (16,539) | 70 (−4,638 to 4,778) | .98 |
| Medical care | 9,408 (16,119) | 10,301 (15,097) | 893 (16,416) | 7,032 (8,684) | 8,565 (10,104) | 1,532 (9,841) | −634 (−3,881 to 2,613) | .70 |
| Long‐term care | 6,887 (13,688) | 9,325 (14,430) | 2,437 (11,879) | 6,545 (12,133) | 8,416 (13,573) | 1,871 (9,689) | 531 (−1,697 to 2,759) | .64 |
| Social care | 1,345 (1,789) | 1,316 (1,567) | −29 (1,563) | 1,265 (1,862) | 1,134 (1,806) | −130 (1,723) | 102 (−234 to 438) | .55 |
| Informal care | 1,627 (5,020) | 2,005 (6,053) | 377 (5,447) | 1,521 (2,842) | 1,843 (4,450) | 322 (4,527) | 47 (−1,124 to 1,218) | .94 |
| Frail | n = 122 | n = 115 | ||||||
| Total costs | 9,948 (13,024) | 16,413 (19,628) | 6,465 (15,126) | 10,374 (16,578) | 12,261 (14,428) | 1,887 (11,625) | 4,629 (898 to 8,359) | .02b |
| Intervention | 1,399 (233) | |||||||
| Subtotal service use | 9,948 (13,024) | 15,014 (19,699) | 5,066 (15,167) | 10,374 (16,578) | 12,261 (14,428) | 1,887 (11,625) | 3,235 (−501 to 6,971) | .09 |
| Medical care | 4,347 (5,298) | 6,672 (12,099) | 2,325 (10,117) | 4,282 (7,553) | 5,532 (7,135) | 1,250 (8,472) | 899 (−1,693 to 3,349) | .50 |
| Long‐term care | 3,956 (9,117) | 5,736 (11,563) | 1,780 (8,517) | 4,515 (11,206) | 4,929 (10,515) | 415 (6,207) | 1,610 (−293 to 3,513) | .10 |
| Social care | 957 (1,569) | 866 (1,488) | −91 (1,384) | 945 (1,573) | 910 (1,476) | −35 (1,032) | −83 (−383 to 217) | .59 |
| Informal care | 688 (1,604) | 1,740 (6,279) | 1,052 (6,199) | 633 (1,701) | 889 (2,324) | 256 (2,588) | 784 (−644 to 2,211) | .28 |
| Robust | n = 438 | n = 416 | ||||||
| Total costs | 4,698 (6,823) | 7,213 (10,184) | 2,515 (9,345) | 4,355 (5,759) | 6,267 (9,132) | 1,912 (8,406) | 606 (−757 to 1,970) | .38 |
| Intervention | 62 (5) | |||||||
| Subtotal service use | 4,698 (6,823) | 7,150 (10,184) | 2,452 (9,345) | 4,355 (5,759) | 6,267 (9,132) | 1,912 (8,406) | 544 (−819 to 1,908) | .43 |
| Medical care | 3,132 (4,272) | 4,757 (7,027) | 1,626 (6,858) | 3,213 (4,541) | 4,501 (6,962) | 1,289 (6,738) | 328 (−778 to 1,434) | .56 |
| Long‐term care | 832 (3,866) | 1,533 (5,322) | 701 (4,086) | 664 (2,810) | 1,243 (4,324) | 579 (3,920) | 131 (−402 to 664) | .64 |
| Social care | 348 (1,017) | 334 (929) | −13 (737) | 304 (959) | 328 (934) | 24 (585) | −37 (−126 to 52) | .41 |
| Informal care | 387c (1,970) | 526a (2,224) | 139 (2,798) | 174c (707) | 195a (1,099) | 21 (1,123) | 122 (−201 to 444) | .46 |
Significant differences between conditions after the 1‐year intervention period.
Significant differences in change between conditions after the 1‐year intervention period.
Significant differences between conditions at baseline.
B, unstandardized regression coefficient; CI, confidence interval.
Mean total costs during the 1‐year intervention period (including intervention costs) were significantly higher for older adults in the Embrace group, compared to older adults in the care‐as‐usual group (EUR 13,073 including EUR 684 intervention costs vs. EUR 10,677, MD: 2397, CI: 547; 427, p = .01). For the risk profiles separately, the mean total costs during the 1‐year intervention period did not differ significantly between conditions. Regarding subsets, costs of “informal care” in the risk profile “Robust” were statistically significantly higher for the intervention group (EUR 526 vs. EUR 195, MD: 331, CI: 61;600, p = .02).
Finally, increase or decrease in costs in the 1‐year intervention period compared with the year before intervention did not differ with statistical significance between conditions. For the risk profiles separately, the total costs (including intervention cost) differed significantly between conditions within the risk profile ‘Frail’ (EUR 6465 vs. EUR 1887, MD: 4629, CI: 898;8359, p = .02). Same trends were found in MDS‐e data (not presented), and no differences were found between GP practices.
Effects on Health‐associated Main Outcomes
Differences between conditions were small for all health‐associated outcomes (see Table 3) and were consistent and homogeneous over all Elderly Care Teams. Differences in QALYs ranged from 0.00 for the risk profile “Frail” to a maximum of 0.02 for the risk profile “Robust” with small confidence intervals ranging from −0.01 to 0.05. Differences in days “able to age in place” were small as well: 358 in the Embrace group and 357 in the control group (MD: 0.84, CI: −3.76;5.46). The proportion of older adults in the total sample, whose risk profile remained stable or improved, was 61.3 percent for the intervention group and 60.2 percent for the control group (MD: 1.17, CI: −3.86;6.18). Details on changes between the risk profiles at baseline and follow‐up are presented in Table S3.
Table 3.
Results of Cost‐utility and Cost‐effectiveness Analyses Based on Data Completed by Imputation (n = 1,456)
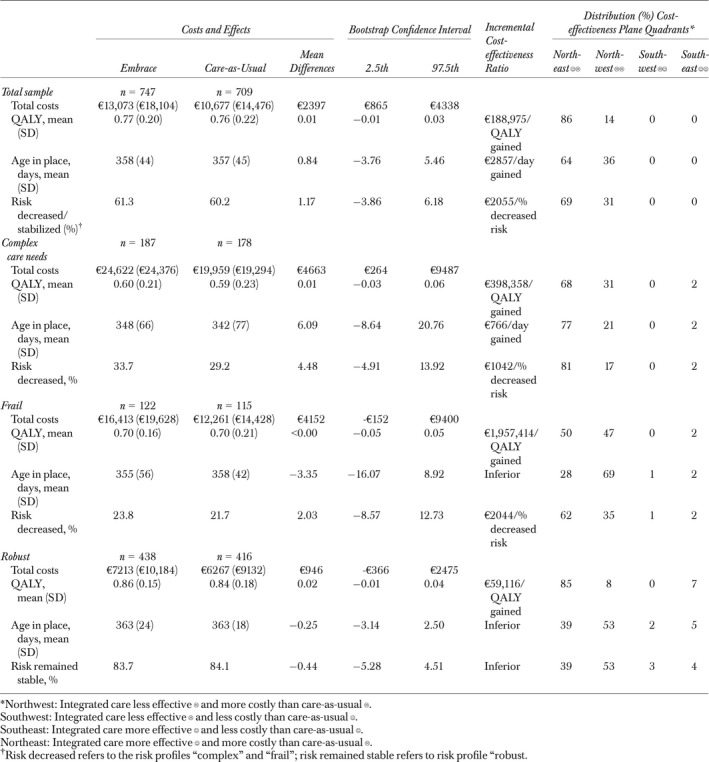
Cost‐utility Analyses
Overall, the ICER was EUR 188,975 for an additional QALY gained in the intervention group. Of the bootstrapped cost‐effect pairs, 86 percent were located in the northeast quadrant, indicating that Embrace was more effective and more expensive than care‐as‐usual (see Table 3 and Figure 2). The CEACs indicated that, for a willingness to pay EUR 20,000 for a QALY gained, the probability that Embrace is cost effective was 1 percent. For a willingness to pay EUR 80,000, the probability was 14 percent. Comparable results were found for the three risk profiles (see Table 3 and Figure 2).
Figure 2.
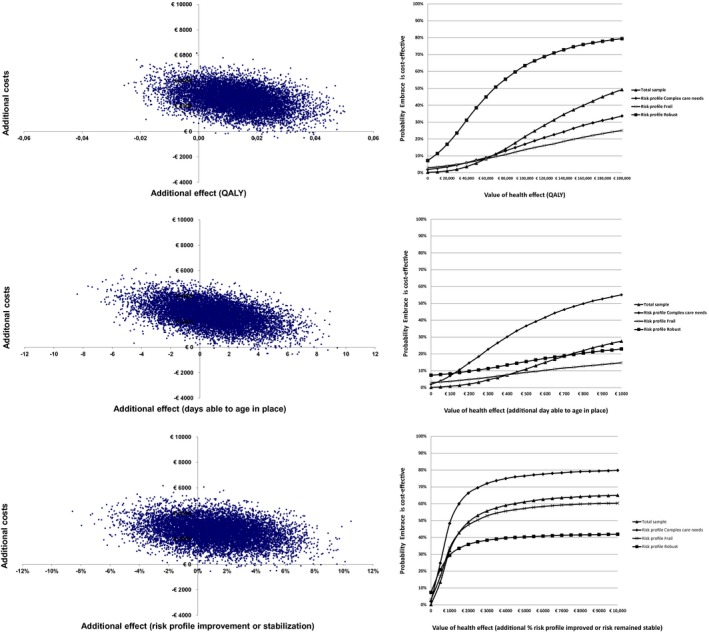
Cost‐effectiveness Planes for Total Sample and Probabilities of Embrace Being Cost Effective [Color figure can be viewed at http://wileyonlinelibrary.com] [Correction added on 11 April 2018, after first online publication: the three graphs in Figure 2 depicting the probabilities of Embrace being cost effective have been corrected for typographical and graphical errors.]
Cost‐effectiveness Analyses
Cost‐effectiveness analyses showed that the ICER for the total sample was EUR 2857 for an additional day to age in place (see Table 3). For the risk profile “Complex care needs,” the ICER was EUR 766 and, for the risk profiles “Robust” and “Frail,” Embrace was less effective and more costly, and therefore inferior to care‐as‐usual. The CEACs (see Figure 2) indicated that the probability that Embrace is cost effective were <80 percent for a willingness to pay EUR 250 for an additional “day able to age in place.”
Concerning “risk profile improvement or stabilization,” the ICER for the total sample was EUR 2055. For the risk profiles “Complex care needs” and “Frail,” these ICERs were EUR 1042 and EUR 2044, respectively. For the risk profile “Robust,” Embrace was inferior to care‐as‐usual. Within the risk profile “Complex care needs,” the probability that Embrace would be cost effective was ≥80 percent in case of a willingness to pay EUR 10,000 for an additional percentage point of “risk profile improvement” (see Figure 2).
Sensitivity Analyses
Of the 1456 older adults, we included 904 (62 percent) older adults in the complete case analysis. The results showed that differences in total costs decreased, while differences in outcomes between conditions remained small (see Table S4). In contrast to the ITT analysis, the probability that Embrace is cost effective was <80 percent at a willingness to pay 10,000 for a percentage point of “risk profile improvement” for the risk profile “Complex care needs.” Furthermore, the probability that Embrace would be cost effective was >80 percent at a willingness to pay EUR 250 for an additional “day to age in place” within the risk profile “Complex care needs” (see Figure S1).
Discussion
Our study results show that, within a time horizon of 12 months, Embrace, a comprehensive person‐centered integrated care service for older adults living in the community, was not cost effective. Embrace might be considered worthwhile in terms of “risk profile improvements” for older adults with “Complex care needs,” if society is willing to invest substantially.
Our finding of a lack of overall advantages for Embrace might be explained in several ways. First, the EQ‐5D as used might have been too insensitive vis‐à‐vis the benefits of the intervention. Although the EQ‐5D is widely used as a measurement of health status, it focuses primarily on physical functioning; integrated care services, however, target psychological functioning, and social well‐being as well (van Leeuwen et al. 2015b; Makai et al. 2015). Second, the proactive approach by the Elderly Care Team may have increased the awareness of a need of care leading to more use of services and informal care early in the care trajectory, and thus leading to higher costs in the intervention groups. In similar studies, it was found that this “investment” may need more time than 12 months to have an effect on patient outcomes (Toseland et al. 1997; Boult et al. 2011; You et al. 2012). We found that Embrace improved quality of care (Uittenbroek et al. 2017). Results of a qualitative study indicated that Embrace reinforced the participants’ ability to stay in control, and feel safe and secured, in contrast to the experienced fears, decreasing social contacts, and loss of control before Embrace (Spoorenberg et al. 2015b). These outcomes could potentially be an indication of positive outcomes in the longer run. Third, Embrace might not have been sufficiently integrated into the health care system to have effects on service use and costs. Older adults also use a wide range of services outside the domain of the integrated care as offered, such as hospital and paramedical care. These services were not included in Embrace and may have diluted the contrast between intervention and control groups. Finally, although not statically significant, differences in utilization between both groups were already present at baseline to the detriment of the intervention group, despite the randomization with balanced allocation. However, adjustment for these differences at baseline did not show differences in costs between conditions, with the exception of the risk profile “Frail” in which total costs (intervention costs included) differed between conditions.
We introduced two new outcomes so as to be able to capture the specific effects of integrated care and found that Embrace could be cost effective in terms of “risk profile improvements” for older adults with “Complex care needs.” However, one should proceed cautiously, as society's willingness to pay for these novel outcomes has not yet been established (Gafni and Birch 2006). Given the dissimilarities in average costs between risk profiles, one percentage point of “risk profile improvement” might be equivalent to a EUR 10,000 cost reduction in the following year. For willingness to pay for a “day able to age in place,” we suggested a threshold based on residential care costs. However, we might have double counted the benefit of the intervention, as we included a nursing home day as both numerator and denominator in the incremental cost‐effectiveness ratio. Nevertheless, the additional gain from not having to stay in a nursing home might be valued even higher than the reallocation of costs, and it may also have nonfinancial benefits, such as increased dignity, independence, social contacts, and even physical health. (Wiles et al. 2012; Young et al. 2015). Further research on society's willingness to pay for these outcomes is needed.
The lack of effects found in our study—and in other recent studies (van Leeuwen et al. 2015a; Makai et al. 2015; Metzelthin et al. 2015; Blom et al. 2016)—may also indicate that new payment models and accountability agreements are essential to overcome fragmentation in health care provision and financing (Enthoven 2009; Schneider, Hussey, and Schnyer 2011; Song et al. 2014). An example of a cost‐effective integrated care service for older adults is the “Program of All‐inclusive Care for the Elderly (PACE)” (Eng et al. 1997). This service is provided to nursing home‐eligible older adults with the aim of maximizing their autonomy and continued residence in the community, in addition to providing quality care at a lower cost. It is provided by highly integrated and accountable care organizations and leads to both improved health outcomes and reduced costs (Hirth, Baskins, and Dever‐Bumba 2009; Wieland et al. 2010; Meret‐Hanke 2011). Unlike Embrace, however, this service focuses on older adults already in need of care. When targeting all older adults living in the community, revolutionary changes in health care and in long‐term and social care payment models will be needed (Humphries 2015).
Strengths of this study are its design, that is, a randomized controlled trial with balanced allocation (Zielhuis et al. 1990), in addition to a large community‐based sample. Moreover, we used health care registries as primary data sources, resulting in highly reliable data that are also used for financial reimbursement (Smeets, de Wit, and Hoes 2011). Some (potential) limitations need to be addressed as well. We randomized participating older adults within the GP practices, which may have caused some contamination of the control group and thus an underestimation of the advantages of Embrace. We found no significant differences between conditions in the intention to treat and complete case analysis. However, the differences between older adults lost to follow‐up and older adults who completed the 12‐month intervention period might indicate that there is a selective dropout and generalization of our findings requires further investigation. Furthermore, we were not able to retrieve data from all health insurers. This is unlikely to have caused bias, as rates of older adults without complete data on costs did not differ vis‐à‐vis conditions, although this incompleteness might have reduced accuracy. In addition, we found comparable trends in data that were based on insurance data and self‐report questionnaires (MDS‐e) between groups and profiles. Finally, for the complete case analyses, we included participants who completed the 12‐month intervention period and had complete data on medical care cost. This restriction of the subsample may have affected our findings.
In conclusion, our study shows that it is feasible to provide care and support that correlates with intensity levels that depend on risk profiles. According to current standards, Embrace is not considered cost effective; however, it might be considered worthwhile in terms of “risk profile improvements” for those older adults with “Complex care needs.” Given the short time horizon of the study, the effects may be shown to increase in the longer run (Toseland et al. 1997). Other results regarding Embrace, such as improved quality of care and reinforced ability of the participant to stay in control, may lead to more positive outcomes in a longer follow‐up. Research on its long‐term cost‐effectiveness is therefore recommended. Furthermore, our findings need confirmation in other populations of community‐living elderly. Much can be gained in that regard not only in terms of costs but also most importantly in terms of health, well‐being, and quality of care.
Supporting information
Appendix SA1: Author Matrix.
Table S1: Embrace Care and Support, Per Risk Profile.
Table S2: Mean Costs (Standard Deviation) for Older Adults That (Not) Completed the Intervention and with (in)Complete Data, Overall and Per Risk Profile.
Table S3: Changes in Risk Profiles between Baseline and Twelve Months Later (n =1,456).
Table S4: Results of Cost‐utility and Cost‐effectiveness Analysis Based on Complete Cases (n =904).
Figure S1: Probability of Embrace Being Cost‐effective; Cost‐effectiveness Acceptability Curves (Complete Cases).
Acknowledgments
Joint Acknowledgment/Disclosure Statement: We would like to thank the participating older adults and health care professionals from the 15 GP practices, the health care organization “Zorggroep Meander,” and the welfare organization “Tinten welzijnsgroep,” especially Coen Ronde, without whom this study could not have been carried out. Furthermore, for their help providing data on service use and costs, we would like to thank the health care insurance company Menzis, especially Frits Plat, and health care insurance company Zilveren Kruis (Zilveren Kruis Health Database), especially Anne Hollinga, Paul van der Bel, and Hugo Smeets; the long‐term care administration office, Zorgkantoor Menzis: Alex Tiehuis and Richard Trigg; and the municipalities of Stadskanaal, Pekela, and Veendam: Floor Aukema, Bouwiena Huls, Jannie Drent, and Tjaart Burema. Finally, we would like to thank J. Almansa Ortiz, PhD, for his statistical support.
Disclosures: None.
Disclaimers: None.
References
- Beswick, A. D. , Rees K., Dieppe P., Ayis S., Gooberman‐Hill R., Horwood J., and Ebrahim S.. 2008. “Complex Interventions to Improve Physical Function and Maintain Independent Living in Elderly People: A Systematic Review and Meta‐analysis.” Lancet 371 (9614): 725–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom, J. , den Elzen W., van Houwelingen A. H., Heijmans M., Stijnen T., Van den Hout W., and Gussekloo J.. 2016. “Effectiveness and Cost‐effectiveness of a Proactive, Goal‐oriented, Integrated Care Model in General Practice for Older People. A Cluster Randomised Controlled Trial: Integrated Systematic Care for older People‐the ISCOPE study.” Age and Ageing 45 (1): 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom, D. E. , Chatterji S., Kowal P., Lloyd‐Sherlock P., McKee M., Rechel B., Rosenberg L., and Smith J. P.. 2015. “Macroeconomic Implications of Population Ageing and Selected Policy Responses.” Lancet 385 (9968): 649–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma, C. , Broere A., and Postma M. J.. 2010. “Quantification of the Potential Impact of Cost‐Effectiveness Thresholds on Dutch Drug Expenditures Using Retrospective Analysis.” Value in Health: The Journal of the International Society for Pharmacoeconomics and Outcomes Research 13 (6): 853–6. [DOI] [PubMed] [Google Scholar]
- Boult, C. , and Wieland G. D.. 2010. “Comprehensive Primary Care for Older Patients with Multiple Chronic Conditions: ‘Nobody Rushes You Through.’” Journal of the American Medical Association 304 (17): 1936–43. [DOI] [PubMed] [Google Scholar]
- Boult, C. , Reider L., Leff B., Frick K. D., Boyd C. M., Wolff J. L., Frey K., Karm L., Wegener S. T., Mroz T., and Scharfstein D. O.. 2011. “The Effect of Guided Care Teams on the Use of Health Services: Results from a Cluster‐randomized Controlled Trial.” Archives of Internal Medicine 171 (5): 460–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd, C. M. , Boult C., Shadmi E., Leff B., Brager R., Dunbar L., Wolff J. L., and Wegener S.. 2007. “Guided Care for Multimorbid Older Adults.” Gerontologist 47 (5): 697–704. [DOI] [PubMed] [Google Scholar]
- Briggs, A. , and Fenn P.. 1998. “Confidence Intervals or Surfaces? Uncertainty on the Cost‐effectiveness Plane.” Health Economics 7 (8): 723–40. [DOI] [PubMed] [Google Scholar]
- Briggs, A. H. , Wonderling D. E., and Mooney C. Z.. 1997. “Pulling Cost‐effectiveness Analysis up by Its Bootstraps: A Non‐parametric Approach to Confidence Interval Estimation.” Health Economics 6 (4): 327–40. [DOI] [PubMed] [Google Scholar]
- Brooks, R. 1996. “EuroQol: The Current State of Play.” Health Policy (Amsterdam, Netherlands) 37 (1): 53–72. [DOI] [PubMed] [Google Scholar]
- van Buuren, S. 2007. “Multiple Imputation of Discrete and Continuous Data by Fully Conditional Specification.” Statistical Methods in Medical Research 16 (3): 219–42. [DOI] [PubMed] [Google Scholar]
- Eklund, K. , and Wilhelmson K.. 2009. “Outcomes of Coordinated and Integrated Interventions Targeting Frail Elderly People: A Systematic Review of Randomised Controlled Trials.” Health & Social Care in the Community 17 (5): 447–58. [DOI] [PubMed] [Google Scholar]
- Eng, C. , Pedulla J., Eleazer G. P., McCann R., and Fox N.. 1997. “Program of All‐inclusive Care for the Elderly (PACE): An Innovative Model of Integrated Geriatric Care and Financing.” Journal of the American Geriatrics Society 45 (2): 223–32. [DOI] [PubMed] [Google Scholar]
- Enthoven, A. C. 2009. “Integrated Delivery Systems: The Cure for Fragmentation.” American Journal of Managed Care 15 (10 Suppl): S284–90. [PubMed] [Google Scholar]
- Fenwick, E. , and Byford S.. 2005. “A Guide to Cost‐effectiveness Acceptability Curves.” British Journal of Psychiatry: The Journal of Mental Science 187: 106–8. [DOI] [PubMed] [Google Scholar]
- Fries, J. F. 1980. “Aging, Natural Death, and the Compression of Morbidity.” New England Journal of Medicine 303 (3): 130–5. [DOI] [PubMed] [Google Scholar]
- Gafni, A. , and Birch S.. 2006. “Incremental Cost‐effectiveness Ratios (ICERs): The Silence of the Lambda.” Social Science & Medicine (1982) 62 (9): 2091–100. [DOI] [PubMed] [Google Scholar]
- Hakkaart‐van Roijen, L. , Tan S., and Bouwmans C. A. M.. 2011. Handleiding voor kostenonderzoek, methoden en richtlijnprijzen voor economische evaluaties in de gezondheidszorg (geactualiseerde versie 2010). Diemen, the Netherlands: College voor zorgverzekeringen. [Google Scholar]
- Hebert, R. , Durand P. J., Dubuc N., Tourigny A., and PRISMA Group . 2003. “Frail Elderly Patients. New Model for Integrated Service Delivery.” Canadian Family Physician Medecin de Famille Canadien 49: 992–7. [PMC free article] [PubMed] [Google Scholar]
- Hirth, V. , Baskins J., and Dever‐Bumba M.. 2009. “Program of All‐inclusive Care (PACE): Past, Present, and Future.” Journal of the American Medical Directors Association 10 (3): 155–60. [DOI] [PubMed] [Google Scholar]
- Humphries, R. 2015. “Integrated Health and Social Care in England–Progress and Prospects.” Health Policy (Amsterdam, Netherlands) 119 (7): 856–9. [DOI] [PubMed] [Google Scholar]
- Kindig, D. A. 2007. “Understanding Population Health Terminology.” Milbank Quarterly 85 (1): 139–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafortune, L. , Beland F., Bergman H., and Ankri J.. 2009. “Health State Profiles and Service Utilization in Community‐living Elderly.” Medical Care 47 (3): 286–94. [DOI] [PubMed] [Google Scholar]
- Lamers, L. M. , Stalmeier P. F., McDonnell J., Krabbe P. F., and van Busschbach J. J.. 2005. “Measuring the Quality of Life in Economic Evaluations: The Dutch EQ‐5D tariff.” Nederlands Tijdschrift Voor Geneeskunde 149 (28): 1574–8. [PubMed] [Google Scholar]
- van Leeuwen, K. M. , Bosmans J. E., Jansen A. P., Hoogendijk E. O., Muntinga M. E., van Hout H. P., Nijpels G., van der Horst H. E., and van Tulder M. W.. 2015a. “Cost‐Effectiveness of a Chronic Care Model for Frail Older Adults in Primary Care: Economic Evaluation Alongside a Stepped‐Wedge Cluster‐Randomized Trial.” Journal of the American Geriatrics Society 63 (12): 2494–504. [DOI] [PubMed] [Google Scholar]
- van Leeuwen, K. M. , Jansen A. P., Muntinga M. E., Bosmans J. E., Westerman M. J., van Tulder M. W., and van der Horst H. E.. 2015b. “Exploration of the Content Validity and Feasibility of the EQ‐5D‐3L, ICECAP‐O and ASCOT in Older Adults.” BMC Health Services Research 15: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low, L. F. , Yap M., and Brodaty H.. 2011. “A Systematic Review of Different Models of Home and Community Care Services for Older Persons.” BMC Health Services Research 11: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn, J. , Straube B. M., Bell K. M., Jencks S. F., and Kambic R. T.. 2007. “Using Population Segmentation to Provide Better Health Care for All: The ‘Bridges to Health’ Model.” Milbank Quarterly 85 (2): 185–208; discussion 209‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makai, P. , Looman W., Adang E., Melis R., Stolk E., and Fabbricotti I.. 2015. “Cost‐effectiveness of Integrated Care in Frail Elderly Using the ICECAP‐O and EQ‐5D: Does Choice of Instrument Matter?” European Journal of Health Economics: HEPAC: Health Economics in Prevention and Care 16 (4): 437–50. [DOI] [PubMed] [Google Scholar]
- Meret‐Hanke, L. A. 2011. “Effects of the Program of All‐inclusive Care for the Elderly on Hospital Use.” Gerontologist 51 (6): 774–85. [DOI] [PubMed] [Google Scholar]
- Metzelthin, S. F. , van Rossum E., Hendriks M. R., De Witte L. P., Hobma S. O., Sipers W., and Kempen G. I.. 2015. “Reducing Disability in Community‐dwelling Frail Older People: Cost‐effectiveness Study Alongside a Cluster Randomised Controlled Trial.” Age and Ageing 44 (3): 390–6. [DOI] [PubMed] [Google Scholar]
- Milani, R. V. , and Lavie C. J.. 2015. “Health Care 2020: Reengineering Health Care Delivery to Combat Chronic Disease.” American Journal of Medicine 128 (4): 337–43. [DOI] [PubMed] [Google Scholar]
- Peters, L. L. , Boter H., Buskens E., and Slaets J. P.. 2012. “Measurement Properties of the Groningen Frailty Indicator in Home‐Dwelling and Institutionalized Elderly People.” Journal of the American Medical Directors Association 13 (6): 546. [DOI] [PubMed] [Google Scholar]
- Peters, L. L. , Boter H., Slaets J. P., and Buskens E.. 2013. “Development and Measurement Properties of the Self Assessment Version of the INTERMED for the Elderly to Assess Case Complexity.” Journal of Psychosomatic Research 74 (6): 518–22. [DOI] [PubMed] [Google Scholar]
- Peters, L. L. , Burgerhof J. G., Boter H., Wild B., Buskens E., and Slaets J. P.. 2015. “Predictive Validity of a Frailty Measure (GFI) and a Case Complexity Measure (IM‐E‐SA) on Healthcare Costs in an Elderly Population.” Journal of Psychosomatic Research 79 (5): 404–11. [DOI] [PubMed] [Google Scholar]
- Schafer, W. , Kroneman M., Boerma W., van den Berg M., Westert G., Deville W., and van Ginneken E.. 2010. “The Netherlands: Health System Review.” Health Systems in Transition 12 (1): v–xxvii, 1‐228. [PubMed] [Google Scholar]
- Schneider, E. C. , Hussey P. S., and Schnyer C.. 2011. Payment Reform: Analysis of Models and Performance Measurement Implications. Santa Monica, CA: RAND Corporation. [PMC free article] [PubMed] [Google Scholar]
- Singh, D. , and Ham C.. 2006. Improving Care for People with Long‐term Conditions: A Review of UK and International Frameworks. Birmingham: University of Birmingham, Health Services Management Centre. [Google Scholar]
- Smeets, H. M. , de Wit N. J., and Hoes A. W.. 2011. “Routine Health Insurance Data for Scientific Research: Potential and Limitations of the Agis Health Database.” Journal of Clinical Epidemiology 64 (4): 424–30. [DOI] [PubMed] [Google Scholar]
- Song, Z. , Rose S., Safran D. G., Landon B. E., Day M. P., and Chernew M. E.. 2014. “Changes in Health Care Spending and Quality 4 Years into Global Payment.” New England Journal of Medicine 371 (18): 1704–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoorenberg, S. L. , Uittenbroek R. J., Middel B., Kremer B. P., Reijneveld S. A., and Wynia K.. 2013. “Embrace, a Model for Integrated Elderly Care: Study Protocol of a Randomized Controlled Trial on the Effectiveness Regarding Patient Outcomes, Service Use, Costs, and Quality of Care.” BMC Geriatrics 13 (1): 62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoorenberg, S. L. , Reijneveld S. A., Middel B., Uittenbroek R. J., Kremer H. P., and Wynia K.. 2015a. “The Geriatric ICF Core Set Reflecting Health‐related Problems in Community‐living Older Adults Aged 75 Years and Older without Dementia: Development and Validation.” Disability and Rehabilitation 37 (25): 2337–43. [DOI] [PubMed] [Google Scholar]
- Spoorenberg, S. L. , Wynia K., Fokkens A. S., Slotman K., Kremer H. P., and Reijneveld S. A.. 2015b. “Experiences of Community‐Living Older Adults Receiving Integrated Care Based on the Chronic Care Model: A Qualitative Study.” PLoS ONE 10 (10): e0137803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoorenberg, S. L. W. , Wynia K., Uittenbroek R. J., Kremer H. P. H., and Reijneveld S. A.. 2018. “Effects of a Population‐based, Person‐centered, and Integrated Care Service on Health, Wellbeing, and Self‐Management of Community‐living Older Adults: Results of a Randomized Controlled Trial on Embrace.” PLoS ONE 13 (1): e0190751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistics Netherlands . 2013. “Data on Contacts Registered by the GP (age and sex)” [accessed on September 10, 2015]. Available at http://statline.cbs.nl/Statweb/publication/?DM=SLNL&PA=80191ned&D1=4&D2=a&D3=21-26&D4=0&D5=a&HDR=G3,G1,G4&STB=T,G2&VW=T.
- Stuck, A. E. , Walthert J. M., Nikolaus T., Bula C. J., Hohmann C., and Beck J. C.. 1999. “Risk Factors for Functional Status Decline in Community‐living Elderly People: A Systematic Literature Review.” Social Science & Medicine (1982) 48 (4): 445–69. [DOI] [PubMed] [Google Scholar]
- Thompson, S. G. , and Barber J. A.. 2000. “How Should Cost Data in Pragmatic Randomised Trials Be Analysed?” BMJ (Clinical Research ed.) 320 (7243): 1197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toseland, R. W. , O'Donnell J. C., Engelhardt J. B., Richie J., Jue D., and Banks S. M.. 1997. “Outpatient Geriatric Evaluation and Management: Is There an Investment Effect?” Gerontologist 37 (3): 324–32. [DOI] [PubMed] [Google Scholar]
- Uittenbroek, R. J. , Kremer H. P. H., Spoorenberg S. L. W., Reijneveld S. A., and Wynia K.. 2017. “Integrated Care for Older Adults Leads to Better Quality of Care: Results of a Randomized Controlled Trial of Embrace.” Journal of General Internal Medicine 32 (5): 516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Department of Health & Human Services . 2015. “Costs of Care” [accessed on December 9, 2015]. Available at http://longtermcare.gov/costs-how-to-pay/costs-of-care/
- Wagner, E. H. , Austin B. T., Davis C., Hindmarsh M., Schaefer J., and Bonomi A.. 2001. “Improving Chronic Illness Care: Translating Evidence into Action.” Health Affairs 20 (6): 64–78. [DOI] [PubMed] [Google Scholar]
- Wieland, D. , Boland R., Baskins J., and Kinosian B.. 2010. “Five‐year Survival in a Program of All‐inclusive Care for Elderly Compared with Alternative Institutional and Home‐ and Community‐based Care.” Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 65 (7): 721–6. [DOI] [PubMed] [Google Scholar]
- Wiles, J. L. , Leibing A., Guberman N., Reeve J., and Allen R. E.. 2012. “The Meaning of ‘Aging in Place’ to Older People.” Gerontologist 52 (3): 357–66. [DOI] [PubMed] [Google Scholar]
- World Health Organization . 2015a. People‐centred and Integrated Health Services: An Overview of the Evidence. Geneva: World Health Organization. [Google Scholar]
- World Health Organization . 2015b. WHO Global Strategy on People‐centred and Integrated Health Services. Geneva: World Health Organization. [Google Scholar]
- World Medical Association . 2013. “World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects.” Journal of the American Medical Association 310 (20): 2191–4. [DOI] [PubMed] [Google Scholar]
- You, E. C. , Dunt D., Doyle C., and Hsueh A.. 2012. “Effects of Case Management in Community Aged Care on Client and Carer Outcomes: A Systematic Review of Randomized Trials and Comparative Observational Studies.” BMC Health Services Research 12: 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, Y. , Kalamaras J., Kelly L., Hornick D., and Yucel R.. 2015. “Is Aging in Place Delaying Nursing Home Admission?” Journal of the American Medical Directors Association 16 (10): 900.e1–6. [DOI] [PubMed] [Google Scholar]
- Zielhuis, G. A. , Straatman H., van ‘t Hof‐Grootenboer A. E., van Lier H. J., Rach G. H., and van den Broek P.. 1990. “The Choice of a Balanced Allocation Method for a Clinical Trial in Otitis Media with Effusion.” Statistics in Medicine 9 (3): 237–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.
Table S1: Embrace Care and Support, Per Risk Profile.
Table S2: Mean Costs (Standard Deviation) for Older Adults That (Not) Completed the Intervention and with (in)Complete Data, Overall and Per Risk Profile.
Table S3: Changes in Risk Profiles between Baseline and Twelve Months Later (n =1,456).
Table S4: Results of Cost‐utility and Cost‐effectiveness Analysis Based on Complete Cases (n =904).
Figure S1: Probability of Embrace Being Cost‐effective; Cost‐effectiveness Acceptability Curves (Complete Cases).


