Abstract
Objective
To examine the impact of the 340B drug discount program on the site of cancer drug administration and cancer care spending in Medicare.
Data Sources/Study Setting
2010–2013 Medicare claims data for a random sample of Medicare Fee‐for‐Service beneficiaries with cancer.
Study Design
We identified the 340B effect using variation in the availability of 340B hospitals across markets. We considered beneficiaries from markets that newly gained a 340B hospital during the study period (new 340B markets) as the treatment group. Beneficiaries in markets with no 340B hospital were the control group. We used a difference‐in‐differences approach with market fixed effects.
Data Collection
Secondary data analysis.
Principal Findings
The probability of a patient receiving cancer drug administration in hospital outpatient departments (HOPDs) versus physician offices increased 7.8 percentage points more in new 340B markets than in markets with no 340B hospital. Per‐patient spending on other cancer care increased $1,162 more in new 340B markets than in markets with no 340B hospital.
Conclusions
The 340B program shifted the site of cancer drug administration to HOPDs and increased spending on other cancer care. As the program expands, continuing assessment of its impact on service utilization and spending would be needed.
Keywords: 340B program, site of cancer drug administration, cancer care spending, Medicare Part B drugs
Background
A federal drug pricing program called “340B” allows covered entities—certain types of hospitals and federal grantees—to obtain most outpatient prescription drugs except vaccines at substantially low prices, up to 25–50 percent savings in drug costs (Health Policy Brief 2014). Its original intent was to give financial support to providers serving low‐income uninsured people. When implemented in 1992, the program covered only a small number of hospitals and other providers (e.g., federally qualified health centers) that offer care to the poor. The program has grown substantially over the past decade: 340B drug sales increased from $2.4 billion in 2005 to $12 billion in 2015 (Fein 2016).
The expansion of the program occurred through two channels: First, the number of 340B‐covered hospitals increased due to the eligibility expansion by the 2010 Affordable Care Act (ACA). Prior to 2010, certain disproportionate share hospitals (DSHs) and children's hospitals were covered by 340B, but the ACA expanded the 340B eligibility to other types of hospitals, including critical access hospitals (CAHs), sole community hospitals, rural referral centers, and free‐standing cancer hospitals. The number of 340B DSHs increased from 583 in 2005 to 1,001 in 2010 and remained stable since then; the number of 340B CAHs reached 940 in 2014; and 234 other types of hospitals were covered by 340B in 2014 (MedPAC 2015). Second, a significant number of community‐based clinics joined the program through 340B hospitals. Individual clinics cannot be an independent 340B entity but can join the program by being affiliated/consolidated with a 340B hospital and billing 340B drug use through the hospital. The number of clinics affiliated with a 340B DSH increased from 1,847 in 2010 to 6,529 in 2013, and the number of 340B CAH affiliated clinics was 903 in 2013 (Figure 1).
Figure 1.
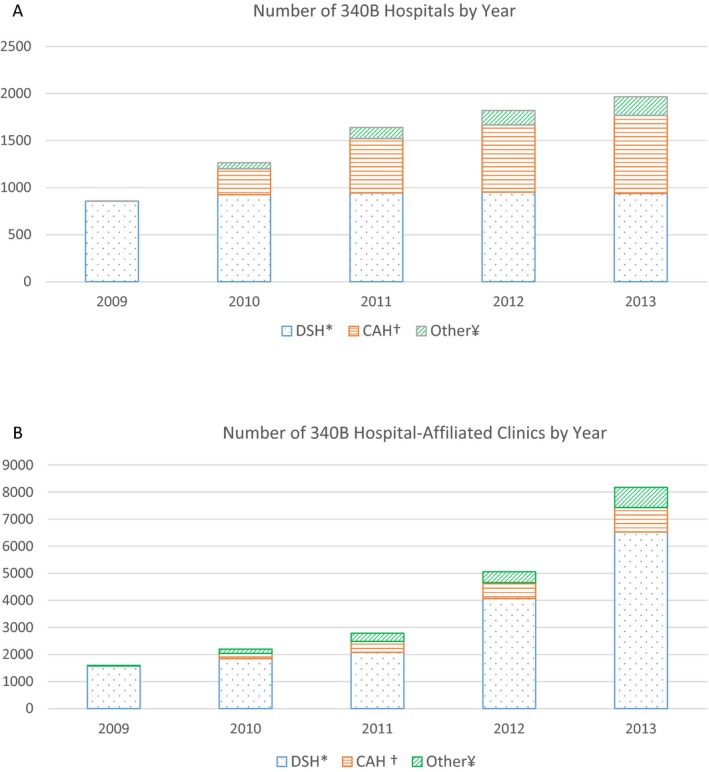
- Notes: This figure counts the number of 340B hospitals and affiliated clinics that maintained 340B coverage until the end of each year. *DSH, disproportionate share hospital; † CAH, critical access hospitals; ¥Other, includes children's hospitals, rural referral centers, sole community hospitals, and free‐standing cancer hospitals.
A concern about the growth of the 340B program is that the program creates incentives for 340B hospitals and affiliated clinics to change their practice patterns without necessarily benefiting poor patients. 340B hospitals generate revenues when they treat insured patients with 340B drugs because payers’ reimbursements for drugs do not depend on drug acquisition prices. In Medicare, Part B pays the same rate for provider‐administered drugs regardless of care setting or 340B status. However, the program does not limit use of the revenue created by 340B to poor patients only. Thus, 340B hospitals have incentives to increase 340B drug administration in their outpatient departments and/or shift drug administration to hospital outpatient departments (HOPDs) from physicians’ offices (Offices) by developing affiliation/consolidation with community‐based practices. The trend in Medicare Part B drug use during the period of 340B growth supported this possibility: HOPD‐administered cancer drugs accounted for 15 percent of Part B cancer drug claims in 2005, but that number increased to 33 percent in 2012 (Vandervelde, Miller, and Younts 2014). This attracted attention of policy makers, leading them to call for a scrutiny of the 340B program (Government Accountability Office [GAO] 2015). Researchers expressed a concern that this shift can increase care spending because it may be more costly to provide care in HOPDs than in Offices (Conti and Bach 2013).
However, empirical evidence on the 340B impacts is limited, and no study provides estimates on the 340B effects on changes in the site of care and care spending. A GAO analysis (2015) documented that per‐patient Part B cancer drug spending was higher in 304B DSHs than in non‐340B DSHs ($4,779 vs. $3,632 in 2008; and $7,801 vs. $5,432 in 2012). Conti and Bach (2014) reported that clinics newly affiliated with 340B‐DSHs tend to serve areas with the well‐off, which deviates from the original intent of the program. Alpert, Hsi, and Jacobson (2017) examined the impact of 340B on consolidation between hospitals and community oncologists. They reported that consolidation was greater in markets with newly eligible hospitals under the ACA (e.g., CAHs) than in markets without such hospitals by 4.5 percentage points (a 13 percent increase) among midsize practices. They discussed that consolidation had been a national trend since 2010 and the contribution of 340B to consolidation was limited. However, focusing on 340B DSHs, Desai and McWilliams (2017) found a large effect of 340B on consolidation: 340B led each participating DSH to have 2.5 more consolidated oncologists (a 208 percent increase). They also showed that 340B DSHs had higher per‐patient spending on Medicare Part B drugs than non‐304B DSHs (by $1,067) but that 340B DSHs did not increase care for poor patients.
These studies support that 340B changes provider practice patterns without enhancing care for poor patients. However, no estimates exist on the extent to which the program changes the site of care and increases care spending, although these are primary concerns over the program (Conti and Bach 2013). Both GAO (2015) and Desai and McWilliams (2017) analyzed drug spending aggregated at the hospital level. Thus, they could not estimate the degree of shift in the site of drug administration to HOPDs. Also, both studies focused only on spending on outpatient drugs, for which Medicare pays the same between HOPDs and Offices. For most services other than drugs, Medicare pays more in HOPDs than in Offices (Avalere Health, 2016). Patients often use other services (e.g., lab tests and radiology services) while visiting providers to receive drug administration. Thus, shifts in the site of drug administration to HOPDs may increase spending on other cancer‐related services. Yet no evidence exists on this issue.
Our study provides the first estimates on 340B impacts on the site of provider‐administered cancer drugs and care spending in Medicare. We focus on cancer care because drugs are a main modality to treat cancer, and cancer drugs make up the largest share of Part B drugs (Vandervelde, Miller, and Younts 2014). We identified the 340B effect using across‐market variation in the availability of 340B hospitals. During our study period, many markets newly gained 340B hospitals—mainly CAHs due to the ACA eligibility expansion. We considered beneficiaries in those markets as the treatment group and compared their cancer drug use/spending and care spending with those from beneficiaries in markets that remained having no 340B hospital. We used a difference‐in‐differences model with market fixed effects to addresses potential selection related to 340B (e.g., providers with sick patients may acquire 340B status) by controlling for all time‐invariant differences in patient risk and provider practice patterns across markets.
Study Sample and Data
The study population is a random sample of Medicare beneficiaries with cancer between 2010 and 2013. The random sample was created in two steps. First, the Centers for Medicare and Medicaid Services (CMS) identified all patients with cancer from 100 percent of Medicare claims based on the standard algorithm it uses to create cancer indicators in the Chronic Condition Warehouse files: having ≥1 inpatient or skilled nursing facility claim with a cancer diagnosis or ≥2 outpatient claims of cancer in a given year. The condition of two outpatient claims is to select a confirmed diagnosis through a follow‐up visit. Second, CMS randomly selected about a half million unique beneficiaries out of those cancer patients, and we received the data from that random sample. We estimated that our data contained a 10 percent random sample of patients with cancers. While the sample was not stratified to be random by cancer type, the sample size of each cancer reflected the prevalence of that cancer type. We restricted the analysis to cancer patients that had both Part A and Part B coverage for the full year. We excluded enrollees in Medicare Advantage Plans because their claims data were not available to researchers.
The primary data were Medicare Outpatient files, which contain records on services in HOPDs, and Carrier files, which have claims on services by noninstitutional providers. Both Outpatient and Carrier claims include information on diagnosis, service date, service type, and payments. Master Beneficiary Summary Files provided beneficiaries’ demographic characteristics and disease indicators, and American Community Survey supplied ZIP‐level income, education, and unemployment rates.
We identified 340B hospitals from the database maintained by the Health Resources and Services Administration (HRSA) Office of Pharmacy Affairs. This database is updated daily and provides information on entities that have ever been covered by the 340B program, including their name, city, state, ZIP, and the date of 340B qualification.
Methods
Outcomes
We constructed one outcome measure for the entire sample: an indicator of a patient receiving any provider‐administered cancer drug in either HOPDs or Offices in a given year. This examines whether 340B providers increase overall drug use by treating more patients with cancer drugs, which is a potential provider response to 340B. We then limited the sample to patients who received any provider‐administered cancer drugs (“users”). We measured five annual outcomes for each user: (1) receipt of provider‐administered cancer drugs in HOPDs versus Offices; (2) the frequency of cancer drug use (Part B cancer drug claims); (3) spending on cancer drugs; (4) spending on cancer care other than drugs; and (5) spending on any care.
We identified provider‐administered cancer drugs using the Healthcare Common Procedure Coding System (HCPCS) Level II (J‐codes) in Carrier and Outpatient files. Appendix reports J‐codes used for the study. We selected claims with both a cancer diagnosis and a cancer drug J‐code to exclude cases using cancer drugs for other conditions. Carrier claims with the service place code of hospital outpatient departments were considered as HOPD claims. A claim of chemotherapy (the same J‐code on the same day) can be reported in both Carrier and Outpatient files. We removed such duplicates from Carrier to avoid double counting.
We measured all spending variables by the allowed payments, which include both Medicare reimbursements and patient out‐of‐pocket spending. For spending on cancer drugs, we summed up the payments across all cancer drug claims of the patient. Medicare pays the same for a drug regardless of care site or 340B status: 106 percent1 of the Average Sales Price (ASP), which is the average net price given to the manufacturer for the drug after any rebates/discounts except 340B discounts and is higher than the 340B price. Thus, shifting drug administration to HOPDs without changes in quantity (or substitution for costlier drugs) would not lead to differences in drug spending between the two settings. For spending on other cancer care, we added up the payments across all claims with a cancer diagnosis except claims of cancer drugs. These claims include cancer drug administration, evaluation and management visits, lab tests, and diagnostic or radiologic procedures. For these services, Medicare payments are usually higher in HOPDs than in Offices (The Moran Company 2013; Avalere Health 2016). Because patients use those services when visiting providers to receive drug administration, a shift in the site of care to HOPDs can increase spending on other cancer care. Finally, we obtained spending on any care as the sum of the payments across all claims of the patient. We adjusted all spending measures to 2013 dollars based on the Consumer Price Index for medical services.
Market Definition and Analytic Approaches
We defined the market by Hospital Referral Region (HRR), which represents a regional health care market for tertiary medical care (Dartmouth Atlas 2015). HRRs align a patient's residence with the likely area (s)he receives health care, and they have been used in the prior work on chemotherapy utilization (Polsky et al. 2006). In addition, use of HRRs allows us to identify 340B impacts by an eligible hospital, which is a unit of organization serving as an independent 340B entity, and the hospital's affiliated clinics.
We identified the 340B effect by exploiting across‐market variation in the availability of new 340B hospitals. We selected markets that had no 340B hospital before the study period and classified them into two groups: markets that newly gained a 340B hospital during the study period (“new 340B markets”) and markets that remained having no 340B hospital. Patients from new 340B markets were the treatment group. Patients in markets with no 340B hospitals were the control group. We then used a difference‐in‐differences approach with market fixed effects to address possible selection (e.g., providers with sick patients may acquire 340B status) by controlling for all time‐invariant market factors, such as patient risk and provider practice patterns. This market‐level identification strategy enables us to examine the effect of 340B in shifts in the site of care. It also allows us to avoid potential selection that a hospital's having 340B status itself changes the hospital's patient risk: When 340B hospitals develop affiliation with clinics, their patient risk changes because patients from affiliated clinics are moved to the hospitals’ outpatient departments. Accounting for this issue is important because patient risk differs between HOPDs and Offices: for example, the distribution of cancer types differs between the two settings (Avalere Health, 2012) and patterns of cancer drug use/spending differ by cancer type (Jung, Feldman, and McBean 2017). However, changes in patient risk in 340B hospitals were not properly addressed in prior work based on a hospital‐level identification approach (Government Accountability Office 2015; Desai and McWilliams 2017).
To indicate new 340B markets, we obtained a list of new 340B hospitals each year based on the 340B qualification date from the HRSA database2 and identified HRRs of those new 340B hospitals based on the hospitals’ ZIP codes. We then linked the new 340B market information by ZIP‐year to Medicare claims, which include each patient's ZIP in a given year. We considered a new 340B market as treated in the initial year when the market newly gained a 340B hospital regardless of the (gaining) date. We thus identified partial‐year effects for the first year of the 340B qualification while capturing full‐year effects for subsequent years. New 340B markets in 2013 (the last year of the data) contributed to the analysis only through the partial‐year effects.
Estimation
Our model is written as
| (1) |
where Y imt is an outcome for beneficiary i in market m in year t. NEW340B is an indicator of a new 340B market in a year when a 340B hospital was available. Its coefficient (β) represents the 340B effect as changes in outcomes between pre‐ and postgaining 340B hospitals in new 340B markets relative to those in markets with no 340B hospital. MARKET m and YEAR t are market and year fixed effects, respectively. X imt is a vector of patient and time‐varying market characteristics. ε imt is an error term.
For control variables (X imt), we included age, gender, race, state buy‐in status (an indicator of Medicaid paying the patient's Part B premium), indicators of several chronic conditions, the number of chronic conditions, and the numbers of cancer‐related hospitalizations and physician visits in the prior year. Market factors were average income, percent college educated, and unemployment rates.
We estimated equation (1) using linear regressions, where inclusion of market fixed effects is straightforward. Standard errors were clustered within markets. We analyzed the model for the entire sample to examine the probability of a patient using any cancer drug. We then estimated the model among users to assess the five outcomes we constructed for each user as described earlier.
Additional Analyses
First, we analyzed the model including markets that had had a 340B hospital since the years before the study period (“existing 340B markets”). We excluded these markets from the primary analysis because those markets had been exposed to the “treatment”—340B hospitals—and those existing 340B hospitals continuously contributed to the 340B expansion by affiliating with clinics during the study period. However, the primary analysis was limited to a small sample because a large share of HRRs already had a 340B hospital before/in 2010. We thus included those existing 340B markets and identified the 340B effect using variation in the availability of new types of 340B hospitals due to the ACA expansion. We considered, as the treatment group, beneficiaries in any markets that gained a new type of 340B hospitals because of the ACA expansion (e.g., CAHs) regardless of whether the market had a 340B hospital before the study period. The control group included beneficiaries in markets that did not add a new 340B hospital due to the ACA expansion. By including a much larger number of markets, this analysis helps confirm that our results reflect a general picture of the 340B impacts.
Second, we performed the analysis defining the market by Primary Care Service Area (PCSA), which corresponds to Medicare patients’ travel to primary care based on the definition by the Dartmouth Atlas. The analysis based on HRRs identifies the 340B effect by newly covered 340B hospitals (mostly CAHs). However, as described earlier, the 340B growth also came through (existing) 340B hospitals’ affiliation with community‐based clinics because many clinics became affiliated with a 340B DSH, which joined the program in early years of the program. More than 4,500 clinics became affiliated with a 340B DSH between 2010 and 2013 (Figure 1). We thus estimated how a clinic becoming affiliated with a 340B hospital changed the site of care and cancer care spending within small areas (PCSAs).
Finally, we conducted a falsification test to check whether the results in the primary analysis were driven by differential time trends between the treatment and control groups. We limited the data of the treatment group to the period before gaining a 340B hospital and created a false NEW340B dummy (1 for 1/2 years prior to the 340‐hospital availability, and 0 for 2/3 years prior to that availability). We used 2010–2012 data for the control group. Under this counterfactual, we expected no significant 340B effect.
Results
Table 1 shows descriptive data for the study sample. Most characteristics were similar between the treatment and control groups. However, the treatment group had a slightly smaller share of whites, a larger share of college‐educated, higher unemployment rates, and higher income than the control group.
Table 1.
Descriptive Data for the Study Sample*
| Variable | Mean (SD) or % | |
|---|---|---|
| Treatment Group† (N = 51,258) | Control Group ‡ (N = 26,818) | |
| Patient characteristics | ||
| Age group | ||
| ≤65 (%) | 5.53 | 4.88 |
| 66–75 (%) | 41.92 | 43.03 |
| 76–85 (%) | 37.46 | 37.72 |
| ≥86 (%) | 15.09 | 14.37 |
| Female (%) | 49.66 | 46.34 |
| White (%) | 93.67 | 95.49 |
| Having diabetes (%) | 27.54 | 30.05 |
| Having hypertension (%) | 67.57 | 72.16 |
| Having ischemic heart disease (%) | 35.61 | 43.73 |
| Having hyperlipidemia (%) | 58.55 | 67.55 |
| Having depression (%) | 15.15 | 15.55 |
| Having congestive heart failure (%) | 17.73 | 18.29 |
| Having cataract (%) | 25.41 | 26.99 |
| Having chronic obstructive pulmonary disease (%) | 15.21 | 18.14 |
| Number of chronic conditions | 4.37 (2.61) | 4.84 (2.66) |
| Number of cancer‐related hospitalizations in the previous year | 0.18 (0.56) | 0.19 (0.56) |
| Number of cancer‐related physician visits in the previous year | 3.17 (4.80) | 3.25 (4.80) |
| Market characteristics | ||
| Percent unemployed | 7.66 (2.28) | 10.56 (3.02) |
| Median household income ($) | 67,752 (22,079) | 53,119 (12,733) |
| Percent college educated | 22.28 (10.37) | 18.15 (6.29) |
*Sample includes beneficiaries from markets (Hospital Referral Region) that had no 340B hospital before the study period.
†Treatment group includes beneficiaries from markets that newly gained a 340B hospital during the study period.
‡Control group includes beneficiaries in markets with no 340B hospital.
Descriptive trends in outcome measures are shown in Figure 2. The rate of cancer drug administration in either HOPDs or Offices was almost the same between the two groups in each year. Among users, the share of HOPD‐administered drugs out of the total provider‐administered cancer drugs increased from 22.2 percent in 2010 to 39.9 percent in 2013 in the treatment group, while it changed from 16.8 percent to 20.1 percent in the control group. Each user in the treatment group had an average of 8.1 cancer drug claims per year, while the corresponding number in the control group was 9.8. Reflecting this pattern, the average cancer drug spending per user was lower in the treatment group than in the control group ($12,673 vs. $15,323). Spending on other cancer care increased from $13,321 in 2010 to $15,435 in 2015 in the treatment group, but from $15,773 to $16,742 in the control group. Higher other cancer care spending in the control group appears to be due to greater use of services: Our data indicated that the numbers of radiology services, evaluation/management visits, and lab tests were all higher in the control group than in the treatment group (data not shown). The average total care spending per user was also lower in the treatment group than in the control group ($33,838 vs. $38,792).
Figure 2.
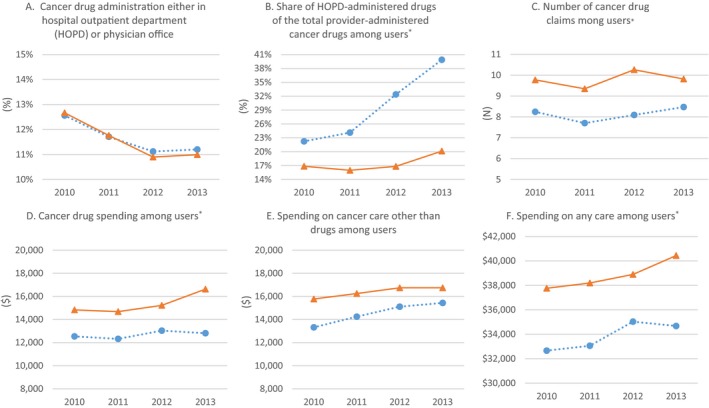
- Notes: Data are from beneficiaries in markets (Hospital Referral Region) that had no 340B hospital before the study period; *Among patients who received any provider‐administered cancer drug;
 treatment group includes beneficiaries from markets that newly gained a 340B hospital during the study period;
treatment group includes beneficiaries from markets that newly gained a 340B hospital during the study period;  control group includes beneficiaries in markets with no 340B hospital.
control group includes beneficiaries in markets with no 340B hospital.
Table 2 presents the regression results. We found no significant 340B effect on the probability of a patient receiving provider‐administered cancer drugs in any setting. However, the program had a significant effect on the change in the site of cancer drug administration: the probability of a patient receiving cancer drug administration in HOPDs versus Offices increased 7.8 percentage points more in new 340B markets than in markets with no 340B hospital (a 34.8 percent increase). The program had no significant effect on spending on cancer drugs. However, it increased spending on other cancer care: the Medicare allowed payments for other cancer care for each patient receiving cancer drug administration was $1,162 higher in markets newly gaining a 340B hospital than in markets with no 340B hospital (a 8.4 percent increase). The impact of 340B on total care spending was positive but statistically insignificant.
Table 2.
Regression Results
| Variable | All Study Sample† (N = 78,076) | Among Users† (N = 9,062) | ||||
|---|---|---|---|---|---|---|
| Marginal Effect (Robust SE) | Marginal Effect (Robust SE) | |||||
| Use of Cancer Drug in HOPD or Office | Use of Cancer Drug in HOPD | Number of Cancer Drug Claims | Cancer Drug Spending | Spending on Cancer Care Other Than Drugs | Spending on Any Care | |
| NEW340B indicator‡ | 0.49 (0.40) | 7.76 (2.60)*** | 0.04 (0.36) | −611 (909) | 1,162 (570)** | 424 (1,235) |
| Age ≤65§ | −0.58 (0.82) | 24.30 (3.95)*** | 5.75 (0.64)*** | 13,608 (1,562)*** | 12,900 (1,026)*** | 30,110 (2,548)*** |
| Age 66–75 | −0.78 (0.48) | 10.80 (1.70)*** | 4.64 (0.32)*** | 8,506 (685)*** | 11,179 (501)*** | 21,749 (1,010)*** |
| Age 76–85 | −0.86 (0.36)** | 5.22 (1.51)*** | 2.74 (0.25)*** | 3,399 (603)*** | 6,375 (490)*** | 11,290 (1,006)*** |
| Female | −7.03 (0.45)*** | 5.50 (2.71)* | 5.18 (0.29)*** | 5,959 (630)*** | 5,219 (477)*** | 11,511 (877)*** |
| White | −1.55 (0.48)*** | −5.75 (2.86)* | 0.27 (0.51) | 435 (1,259) | 1,275 (645)* | 2,981 (1,454)** |
| Having diabetes | 0.58 (0.36) | −4.31 (1.15)*** | −0.24 (0.40) | −604 (866) | −1,729 (412)*** | −3,849 (1,088)*** |
| Having hypertension | −0.64 (0.29)** | 0.57 (1.37) | 0.11 (0.23) | 50 (515) | 816 (478)* | 30 (775) |
| Having ischemic heart disease | −0.84 (0.32)** | −1.84 (1.27) | 0.10 (0.24) | 180 (719) | 74 (386) | 166 (795) |
| Having hyperlipidemia | −2.79 (0.32)*** | −1.77 (0.99)* | −1.14 (0.33)*** | −1,571 (701)** | −1,104 (393)*** | −4,329 (1,023)*** |
| Having depression | 0.29 (0.43) | 1.79 (1.19) | −1.17 (0.29)*** | −3,019 (704)*** | −426 (399) | −3,775 (794)*** |
| Having congestive heart failure | −0.18 (0.48) | −1.85 (1.02)* | −1.11 (0.29)*** | −911 (688) | −2,701 (476)*** | −4,412 (934)*** |
| Having cataract | −1.92 (0.28)*** | −2.50 (1.14)** | −1.22 (0.27)*** | −378 (758) | −1,467 (476)*** | −2,670 (993)** |
| Having COPD¶ | 1.63 (0.37)*** | −0.64 (1.57) | 0.37 (0.33) | −1,217 (717)* | 943 (642) | −446 (1,033) |
| Number of chronic conditions | 0.44 (0.12)*** | 1.05 (0.32)*** | 0.29 (0.07)*** | 335 (213) | 926 (128)*** | 2,854 (276)*** |
| Number of prior year hospitalizations | −0.44 (0.26)* | 0.03 (0.96) | −0.27 (0.16) | −660 (399) | −216 (245) | −741 (531) |
| Number of prior‐year cancer‐related physician visits | 1.67 (0.07)*** | 0.38 (0.11)*** | 0.27 (0.04)*** | 654 (58)*** | 241 (27)*** | 838 (61)*** |
| Unemployment | 0.00 (0.14) | −0.77 (1.95) | −0.44 (0.23)* | −546 (468) | −749 (341)** | −1,433 (774)* |
| Median household income | 0.00 (0.00)*** | 0.00 (0.00)* | −0.00 (0.00) | 0 (0) | 0 (0)** | 0 (0) |
| College educated | 0.10 (0.10) | −2.49 (1.10)** | 0.03 (0.16) | 96 (375) | −116 (170) | −54 (387) |
| 2011 | −1.23 (0.32)*** | −2.77 (1.69) | −0.62 (0.26)** | −87 (682) | 39 (329) | −160 (854) |
| 2012 | −2.27 (0.41)*** | 4.42 (2.84) | 0.11 (0.43) | 598 (1,009) | 1,181 (633)* | 1,832 (1,625) |
| 2013 | −2.44 (0.44)*** | 11.30 (4.03)*** | 0.24 (0.48) | 780 (1,226) | 1,427 (744)* | 2,131 (1,832) |
| Constant | −4.84 (7.39) | 193.30 (87.80)** | 12.59 (11.02) | 11,007 (22,651) | 33,991 (15,639)** | 40,592 (28,032) |
†Sample includes beneficiaries from markets (Hospital Referral Region) that had no 340B hospital before the study period; users refer to patients who received any provider‐administered cancer drug.
‡Dummy that identifies observations from a market that newly gained a 340B hospital in a year when a 340B hospital was available.
§Age >85 years is reference group.
¶Chronic obstructive pulmonary disease.
*p < 0.10, **p < 0.05, ***p < 0.01. Market fixed effects are included in all models; standard errors are accounted for clustering within a market; and spending measures are adjusted to 2013 dollars.
HOPD, hospital outpatient department; SE, standard errors.
Table 3 reports the results from the additional analyses, which all supported the findings from the primary analysis. First, the analysis including existing 340B markets indicated that the program increased the probability of a patient using cancer drugs in HOPDs versus Offices by 2.7 percentage points and the program increased each user's spending on other cancer care by $523. These estimates are smaller than those from the primary analysis. This may be because 340B hospitals and affiliated clinics had been present in existing 340B markets and thus the impact of a newly covered 340B hospital in those markets may have been small. Second, the analysis based on PCSAs also confirmed that 340B shifted the site of care to HOPDs. However, it found no significant impact on other cancer care spending. This is probably because the analysis using PCSA as the market estimated the 340B effect by a 340B clinic(s) within small areas, which may not capture all services patients in that area received. Finally, we found no significant coefficient on the 340B indicator from the falsification tests. This confirms that our results were not driven by different time trends in cancer drug use/spending and care spending between the treatment and control groups.
Table 3.
Results from Additional Analyses
| Variable | All Sample | Among Users† | ||||
|---|---|---|---|---|---|---|
| Marginal Effect (Robust SE) | Marginal Effect (Robust SE) | |||||
| Use of Cancer Drugs in HOPD or Office | Use of Cancer Drug in HOPD | Number of Cancer Drug Claims | Cancer Drug Spending | Spending on Cancer Care Other Than Drugs | Spending on Any Care | |
| Analysis including markets that had had a 340B hospital since years before the study period | ||||||
| (N = 611,948) | (N = 71,135) | |||||
| NEW340B indicator‡ | −0.04 (0.18) | 2.68** (1.04) | −0.10 (0.13) | 112 (322) | 523* (283) | 592 (501) |
| Analysis using Primary Care Service Area (PCSA) as the market | ||||||
| (N = 797,585) | (N = 95,169) | |||||
| NEW340B indicator§ | −0.26 (0.16) | 2.51*** (0.76) | −0.22 (0.14) | −315 (310) | 99 (236) | −154 (455) |
| Using false indicator for presence of 340B hospital | ||||||
| (N = 40,451) | (N = 4,809) | |||||
| False NEW340B¶ | 0.12 (0.71) | 3.12 (2.07) | −0.07 (0.79) | 513 (1,226) | 425 (766) | 1,192 (1,752) |
†Patients who received any provider‐administered cancer drug.
‡Dummy indicating observations from a market (hospital referral region) that gained a new 340B hospital in a year when the 340B hospital was available.
§Dummy indicating observations from a market that newly gained a new 340B hospital/clinic in a year when the 340B hospital/clinic was available.
¶False indicator of the presence of a 340B hospital in the market (hospital referral region).
* p < 0.10, **p < 0.05, ***p < 0.01. All regressions control for market and year fixed effects, and time‐varying patient and market characteristics; standard errors are accounted for clustering within a market; and spending measures are adjusted to 2013 dollars.
HOPD, hospital outpatient department; SE, standard errors.
Discussion
The 340B program allows covered entities to earn revenue from outpatient drug prescriptions. 340B entities are expected to use such revenue for low‐income populations, but no evidence indicates increases in care for poor patients by 340B hospitals. As the program expands, a policy concern has been raised that the program may merely increase care spending by changing providers’ practice patterns. Our study offers empirical estimates on this concern in Medicare. We report three major findings.
First, we found that the 340B program shifted the place of cancer drug administration to HOPDs: the probability of a patient receiving cancer drug administration in HOPDs versus Offices increased 7.8 percentage points more in markets that newly gained a 340B hospital than in markets with no 340B hospitals. This confirms the prior reports of the increasing trend in HOPD‐administered drugs during the period of the 340B growth (Vandervelde, Miller, and Younts 2014).
Second, we found no significant effect of 340B on use or spending on provider‐administered cancer drugs. The program did not increase the probability of a patient receiving cancer drug administration and did not change the frequency of cancer drug claims among users. No change in use/quantity led to the insignificant effect of 340B on cancer drug spending because Medicare pays the same for outpatient drugs regardless of setting. Our finding differs from the prior work, which reported 340B DSHs had higher cancer drug spending than non‐340B DSHs (Government Accountability Office 2015; Desai and McWilliams 2017). This difference is likely because (1) we identified the 340B effect mainly by newly covered 340B hospitals under the ACA (e.g., CAHs), while prior work examined 340B DSHs (early participants); (2) prior work examined cancer drug spending aggregated at the hospital level and did not control for a change in patient risk at 340B hospitals. As discussed earlier, acquiring 340B status changes the hospital's patient risk because of a shift of patients from affiliated clinics; and (3) our market‐level identification estimated the impact of patients having access to 340B hospitals, while prior work analyzed the impact of patients using 340B hospitals.
Finally, the 340B program increased spending on other cancer care: per‐patient other cancer care spending increased $1,162 more in markets that newly gained a 340B hospital than in markets with no 340B hospitals. This is consistent with the concern that the program increases care spending by moving patients to HOPDs (Conti and Bach 2013). Patients visiting HOPDs for cancer drug administration may receive additional services that might not be offered if they visited physicians’ offices. Also, Medicare payments for those services are higher in HOPDs than in Offices. Our finding thus confirms the discussion that the impact of 340B reaches beyond drug use/spending because the program can change service utilization and spending down the road by shifting the site of care to HOPDs.
The implications of the 340B program for the delivery of care can be even broader. For example, the 340B impact on the shift in care site indicates that 340B hospitals developed/expanded affiliation with individual clinics. We did not examine the contribution of 340B to the hospital‐clinic integration, but prior work showed a positive role of 340B in promoting the integration (Alpert, Hsi, and Jacobson 2017; Desai and McWilliams 2017). Hospital‐physician affiliation could also influence quality of care, such as clinics’ referring patients only to their affiliated hospital, not just affecting quantity of services. Further, it can affect service prices in the commercial sector. Evidence suggests that integration between physicians and hospitals led to disruption in patients’ referral patterns, high prices, but mixed quality results (Carlin, Dowd, and Feldman 2015; Neprash et al. 2015; Carlin, Feldman, and Dowd 2016). These are important potential consequences of the 340B expansion. Our study was limited to analyzing the 340B impact on cancer drug use and cancer care spending. Assessing broader implications of the program should be pursued in future research.
Our finding of the 340B impact on increases in HOPD‐administered cancer drugs implies increased revenue of 340B hospitals. Yet prior studies reported that 340B hospitals did not expand their services to poor patients (Desai and McWilliams 2017), while newly 340B hospital‐affiliated clinics tend to serve well‐off communities (Conti and Bach 2014). This evidence from prior work and our finding suggest that the 340B program changed providers’ practice patterns without necessarily benefiting poor patients. This raises a question over what the 340B program accomplishes against its original intent.
Some suggested that modifications of the 340B program are needed for the program to better serve its original intent (Conti and Bach 2013; Health Policy Brief, 2014). For example, HRSA could limit the use of 340B drugs to poor patients or restrict the use of 340B‐generated revenues to the care for the poor (e.g., extending services to the needy or paying off charity or uncompensated care). Recently, CMS proposed that Medicare lower reimbursements for 340B drugs. This change would decrease hospitals’ 340B revenue, which could in turn slow down a shift in the site of care to hospital outpatient departments and help save Medicare spending on Part B drugs. This approach would limit financial support for 340B hospitals. However, if savings from such changes would be used to provide direct subsidies to the poor, it would better serve poor patients, which is an ultimate goal of the program. The first step toward these efforts could be to increase transparency over how hospitals use revenues generated by 340B. The 340B program is expected to continue to expand in the coming years, and debates will likely continue over how to address the 340B growth (Fein 2016; Vandervelde and Blalock 2016). Continuing assessment of how 340B changes patterns of health care use and spending will help advance the debates and develop policy options over the program.
We note several limitations of the study. First, we did not examine pharmacy‐dispensed cancer drugs, which are covered by Part D. 340B hospitals can contract with retail or specialty pharmacies to dispense discounted drugs to patients. Future analysis including pharmacy‐dispensed drugs would provide a more comprehensive evaluation of the program. Second, our analysis identified the 340B effect by the change in the availability of new 340B hospitals during the study period. Its results may not be generalizable to the effects by 340B DSHs—early participants. Moreover, our results do not represent long‐term impacts of the 340B program. Third, some factors affecting cancer care use and spending, such as stage of cancer, might remain unobserved, particularly in the analysis of the entire sample to examine the probability of a patient using any provider‐administered cancer drug. However, those factors are limited to time‐varying elements, such as differential changes in patients’ health risk over time across markets, because all time‐invariant factors were controlled for by market fixed effects. The impact of unobserved time‐varying factors is likely to be small given that the falsification test showed no differential trends in cancer drug use/spending and care spending during the period prior to gaining a new 340B hospital. Finally, our analysis is limited to cancer care in Medicare. Its results may not be generalizable to other conditions or to the commercial sector.
Our analysis provided the first evidence on the contribution of 340B to the shift in the site of provider‐administered cancer drugs to HOPDs. We also showed that the 340 program increased spending on cancer care other than drugs. As the program further expands, its impacts on patterns of service use and spending should be continuously evaluated.
Supporting information
Appendix SA1: Author Matrix.
Appendix SA2: Chemotherapy J‐codes (HCPCS Level II Codes) Used for the Study: J9000‐J9999, J8521, J8560, J8520, J8530, and J2180.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: This work is supported by NIH/NIA grant number 1R01AG047934‐01 and NIH grant number R24 HD041025.
Disclosures: None.
Disclaimer: None.
Notes
Between 2010 and 2012, Medicare paid 104–105 percent of ASP in HOPDs while paying 106 percent of ASP in Offices.
We did not consider other types of 340B entities, such as federally qualified community centers or Ryan White clinics. Most markets already had at least one of these entities in 2010. Thus, their effects are absorbed by market fixed effects.
References
- Alpert, A. , Hsi H., and Jacobson M.. 2017. “Evaluating the Role of Payment Policy in Driving Vertical Integration in the Oncology Market.” Health Affairs 36 (4): 680–8. 10.1377/hlthaff.2016.0830. [DOI] [PubMed] [Google Scholar]
- Avalere Health . 2012. “Total Cost of Cancer Care by Site of Service: Physician Office vs Outpatient Hospital,” Washington, DC [accessed on January 1, 2018]. Available at http://www.communityoncology.org/pdfs/avalere-cost-of-cancer-care-study.pdf
- Avalere Health . 2016. “Medicare Payment Differentials across Outpatient Settings of Care,” Washington, DC [accessed on January 1, 2018]. Available at http://www.physiciansadvocacyinstitute.org/Portals/0/assets/docs/Payment-Differentials-Across-Settings.pdf
- Carlin, C. S. , Dowd B., and Feldman R.. 2015. “Changes in Quality of Health Care Delivery after Vertical Integration.” Health Services Research 50 (4): 1043–68. 10.1111/1475-6773.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin, C. S. , Feldman R., and Dowd B.. 2016. “The Impact of Hospital Acquisition of Physician Practices on Referral Patterns: Vertical Integration and Referral Patterns.” Health Economics 25 (4): 439–54. 10.1002/hec.3160. [DOI] [PubMed] [Google Scholar]
- Conti, R. M. , and Bach P. B.. 2013. “Cost Consequences of the 340B Drug Discount Program.” Journal of American Medical Association 309 (19): 1995–6. 10.1001/jama.2013.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti, R. , and Bach P.. 2014. “The 340B Drug Discount Program: Hospitals Generate Profits by Expanding to Reach More Affluent Communities.” Health Affairs 33 (10): 1786–92. 10.1377/hlthaff.2014.0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartmouth Atlas of Health Care . 2015. “Research Methods” [accessed on January 1, 2018]. Available at http://www.dartmouthatlas.org/downloads/methods/research_methods.pdf
- Desai, S. , and McWilliams J. M.. 2017. “Subsidizing Consolidation? Unintended Consequences of a Federal Drug Discount Program” [accessed on September 13, 2017]. Available at http://scholar.harvard.edu/files/desai/files/desai_jmp.pdf
- Fein, A. 2016. “340B Purchases Hit $12 Billion in 2015—And Almost Half of the Hospital Market.” Drug Channels [accessed on January 1, 2018]. Available at http://www.drugchannels.net/2016/02/340b-purchases-hit-12-billion-in.html
- Government Accountability Office . 2015. “Medicare Part B Drugs: Action Needed to Reduce Financial Incentives to Prescribe 340B Drugs at Participating Hospitals.” GAO‐15‐442. Washington, DC [accessed on January 1, 2018]. Available at https://www.gao.gov/products/GAO-15-442
- Health Policy Brief . 2014. “The 340B Drug Discount Program.” Health Affairs [accessed on January 1, 2018]. Available at https://www.healthaffairs.org/do/10.1377/hpb20141117.14335/full/healthpolicybrief_130.pdf [Google Scholar]
- Jung, J. , Feldman R., and McBean M.. 2017. “The Price Elasticity of Specialty Drug Use: Evidence from Medicare Part D Enrollees with Cancer.” Forum for Health Economics and Policy. doi: 10.1515/fhep-2016-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicare Payment Advisory Committee . 2015. Report to the Congress: Overview of the 340B Drug Pricing Program. Washington, DC: MedPAC. [Google Scholar]
- The Moran Company . 2013. “Cost Differences in Cancer Care across Settings” [accessed on January 1, 2018]. Available at https://www.communityoncology.org/UserFiles/Moran_Cost_Site_Differences_Study_P2.pdf
- Neprash, H. T. , Chernew M. E., Hicks A. L., Gibson T., and McWilliams J. M.. 2015. “Association of Financial Integration between Physicians and Hospitals with Commercial Health Care Prices.” JAMA Internal Medicine 175 (12): 1932–9. 10.1001/jamainternmed.2015.4610. [DOI] [PubMed] [Google Scholar]
- Polsky, D. , Armstrong K. A., Randall T. C., Ross R. N., Even‐SHoshan O., Rosenbaum P. R., and Silver J. H.. 2006. “Variation in Chemotherapy Utilization in Ovarian Cancer: The Relative Contribution of Geography.” Health Services Research 41 (6): 2201–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandervelde, A. , and Blalock E.. 2016. “340B Program Sales Forecast: 2016–2021,” Berkeley Research Group White Paper. Washington, DC.
- Vandervelde, A. , Miller H., and Younts J.. 2014. “Impact on Medicare Payments of Shift in Site of Care for Chemotherapy Administration.” Berkeley Research Group White Paper [accessed on January 1, 2018]. Available at https://www.communityoncology.org/UserFiles/BRG_340B_SiteofCare_ReportF_6-9-14.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.
Appendix SA2: Chemotherapy J‐codes (HCPCS Level II Codes) Used for the Study: J9000‐J9999, J8521, J8560, J8520, J8530, and J2180.


