Highlights
-
•
In cases of NOMI, it is difficult to determine the area of bowel resection.
-
•
Using ICG fluorescence imaging, we can evaluate ischemia of the intestine.
-
•
We adeptly resected the ischemic intestine in NOMI using ICG fluorescence imaging.
Keywords: Case reports, Non-occlusive mesenteric ischemia, Indocyanine green fluorescence imaging, Case report
Abstract
Introduction
Non-occlusive mesenteric ischemia (NOMI) is a type of acute intestinal ischemia, and its associated mortality is very high. In laparotomy of NOMI, we often have difficulty determining the area of bowel resection. We herein describe a case in which we detected the area of bowel resection using indocyanine green (ICG) fluorescence imaging.
Presentation of the case
An 89-year-old man diagnosed as having advanced gastric cancer underwent distal gastrectomy. On the night of postoperative day 4, he strongly complained of distention of the abdomen. The laboratory data indicated severe metabolic acidosis and dehydration. The abdominal computed tomography scan showed a dilated small bowel, but there were no specific signs suggestive of bowel necrosis. We suspected NOMI and decided to perform emergency laparotomy because we could not exclude the possibility of bowel necrosis. During the operation, we could not detect the necrotic bowel macroscopically. After injecting 2.5 mg of ICG, the ischemic area of the bowel became visible as a region with poor fluorescence emission using the Photodynamic Eye™ (Hamamatsu Photonics K.K.). We resected the ischemic bowel and performed anastomosis. We confirmed that he was alive at 4 months after the operation of NOMI.
Conclusion
Intraoperative ICG fluorescence imaging makes it possible to detect necrotic intestine that cannot be found with the naked eye. By using this method, planned reoperation to find any newly developed necrotic intestine might be unnecessary. Intraoperative ICG fluorescence imaging is useful for defining the area of ischemic bowel in a patient with NOMI.
1. Introduction
Non-occlusive mesenteric ischemia (NOMI) can be critical owing to its challenging diagnosis [1]. In the case that bowel resection is necessary, we often have difficulty determining the area of bowel resection because the ischemic bowel area in a patient with NOMI is unclear and not sectionalized in contrast to superior mesenteric artery occlusion [2]. We herein describe a survival case of NOMI in which we determined the ischemic bowel area using indocyanine green (ICG) fluorescence imaging, and the patient underwent resection and anastomosis during one surgery. This case report has been written in line with the SCARE criteria [3].
2. Presentation of case
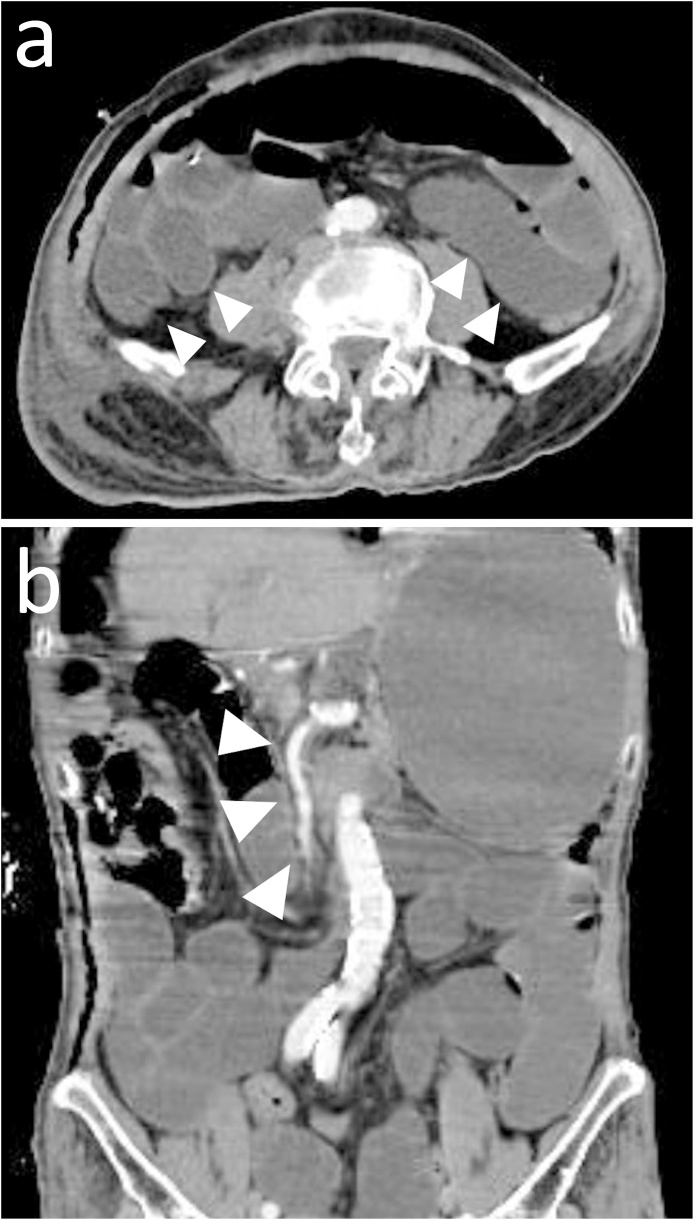
An 89-year-old man was diagnosed as having progressive gastric cancer. He underwent distal gastrectomy, lymph node dissection, gastrojejunal anastomosis (Billoth II), and jejunostomy for nutrition. On postoperative day 3, he started eating meals. On the night of postoperative day 4, he complained of distention of the abdomen and gradually became in a state of agitation. Based on the laboratory data, dehydration was suggested because of the elevated levels of creatinine (1.96 mg/dL [reference range, 0.5–1 mg/dL]) and blood urea nitrogen (34.7 mg/dL [8–19.7 mg/dL]). An electrolyte abnormality was also revealed (sodium level, 120 mEq/L [140–146 mEq/L]; potassium level, 5.6 mEq/L [3.5–4.8 mEq/L]). Moreover, severe metabolic acidosis (pH, 7.185 [7.35–7.45]; bicarbonate level, 6.9 mmol/L [21–27 mmol/L]; base excess level, −19.5 mmol/L [−2 to 2 mmol/L]) was indicated in the arterial blood gas analysis. The contrast-enhanced computed tomography (CT) scan showed dilatation of the small bowel. Obvious superior mesenteric artery occlusion or bowel strangulation was not observed. There were also no findings of bowel necrosis, such as pneumatosis intestinalis or venous gas (Fig. 1a, b). On the basis of nonspecific ileus-like findings detected with CT and metabolic acidosis, we suspected NOMI. Although there seemed to be no CT findings that indicated bowel necrosis, we could not rule out bowel necrosis because of severe acidosis. Therefore, we decided to perform laparotomy.
Fig. 1.
Contrast-enhanced computed tomography (CT) scan. a The dilated small bowel is seen (arrowhead) in the axial postcontrasted CT image, but pneumatosis intestinalis or venous gas is not demonstrated. b Coronal reconstructed CT image shows no thrombus in the superior mesenteric artery (arrowhead).
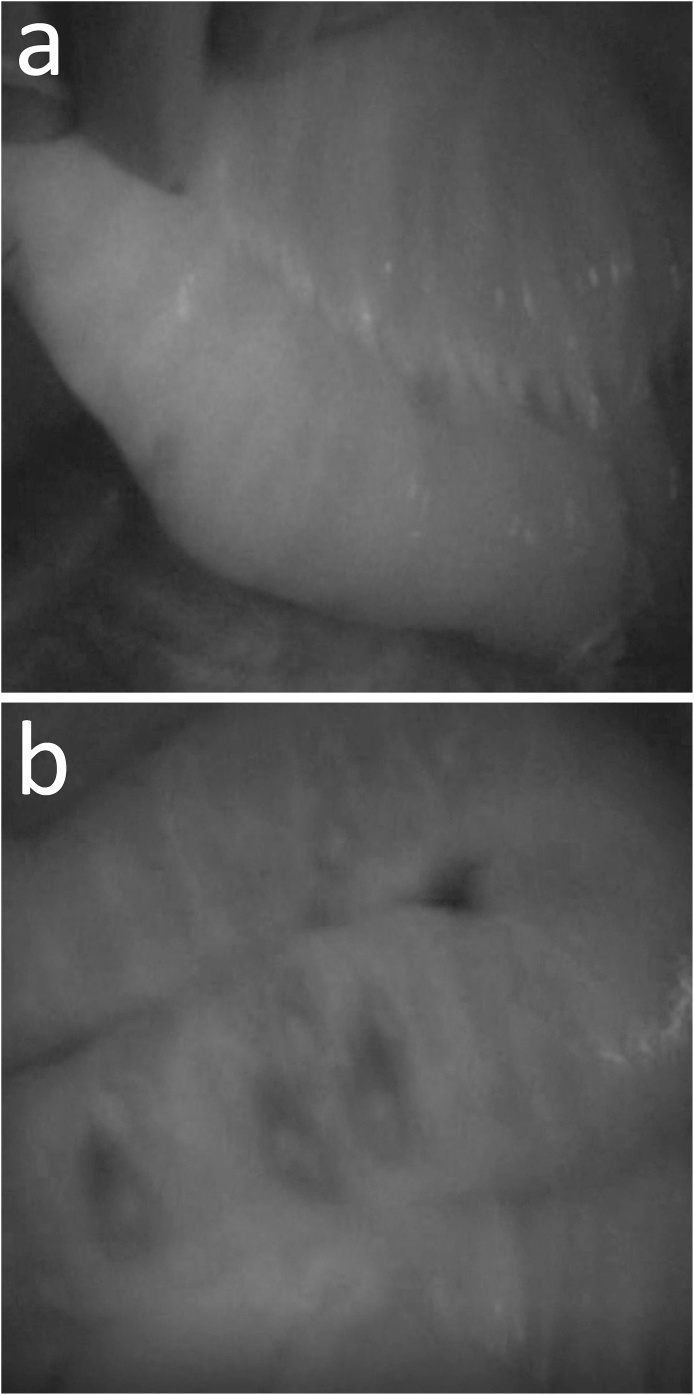
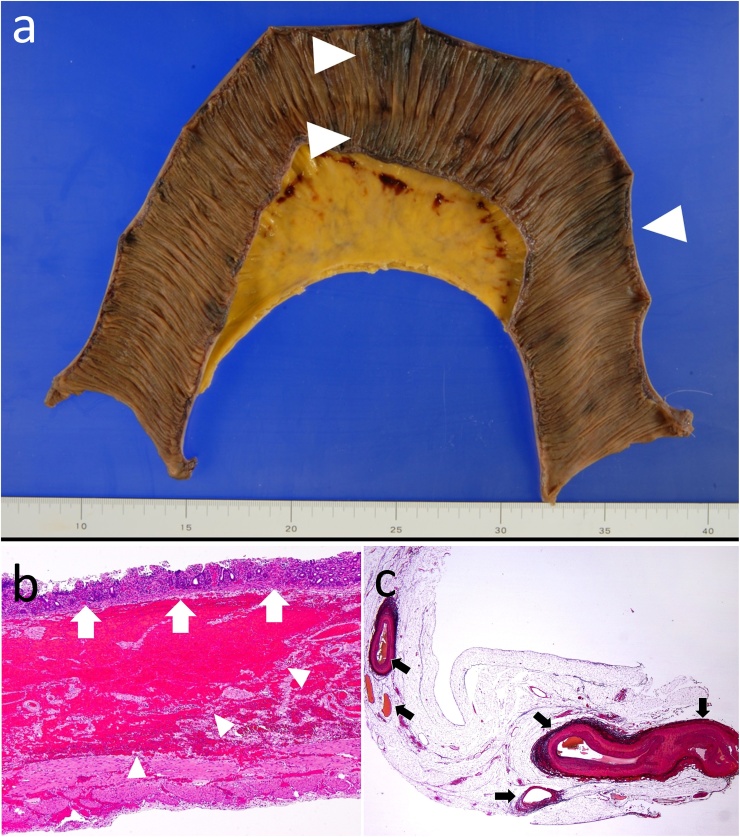
The operation revealed the dilated small bowel, but there seemed to be no necrotic bowel macroscopically, although a small area of the bowel was slightly ischemic. After injecting 0.25 mg of ICG, we observed the bowel using the Photodynamic Eye™ (PDE, Hamamatsu Photonics K.K.). The small bowel wall, measuring 40 cm in length, showed poor fluorescence emission, indicating ischemia (Fig. 2a, b). We decided to resect the ischemic lesion and to confirm that there was no other ischemic intestine. The resected intestine was anastomosed in a functional end-to-end manner. The histopathological examination showed various degrees of ischemic change in the bowel wall and mucosal necrosis (Fig. 3a–c).
Fig. 2.
Intraoperative indocyanine green fluorescence imaging. a Fluorescence is observed in the bowel, in which blood flow is normal. b The jejunum, measuring 40 cm in length, reveals poor fluorescence visualized as skipped dark spots.
Fig. 3.
Resected specimen. a Macroscopically, various degrees of ischemic changes (arrowhead) are observed in the mucosa. b Mucosal atrophy (white arrow) with a high degree of hemorrhage (arrowhead) in the submucosa is shown, but transmural necrosis is not observed histopathologically. c A thrombus is not observed in the mesenteric vessels (black arrow).
After intestinal resection, the patient developed congestive heart failure owing to a large venous injection; however, he improved with the administration of a diuretic agent. On postoperative day 26, he was transferred to another hospital for rehabilitation. We confirmed that he is alive at 7 months after operative treatment of NOMI.
3. Discussion
NOMI is very rare clinical condition, but it has a very high mortality rate of 30–68.5% even now [[4], [5], [6], [7]]. The pathogenesis of NOMI is vasospasm of mesenteric arteries, and the blood flow is not completely interrupted in contrast to superior mesenteric artery occlusion [8]. It has been reported that angiography of the mesenteric artery is useful for detecting spastic mesenteric arteries [9]. Additionally, the usefulness of arterial infusion therapy for vasodilators following angiography has been recognized. However, angiography takes time, and in the case when bowel necrosis is suspected, it is most urgent to perform intestinal resection.
ICG fluorescence imaging captures the fluorescence of ICG injected into the body using a digital video camera. ICG becomes excited by infrared light (wavelength, 750–810 nm) and emits infrared fluorescence (peak wavelength, 830 nm) of different wavelengths. All wavelengths are light in the wavelength range that can easily transmit through about 10 mm of human soft tissue [10]. Although they cannot be seen directly with one’s naked eye, PDE can excite ICG injected into the body and simultaneously capture fluorescence emission. Intravenously injected ICG is transported to peripheral vessels within a few seconds, and it becomes visible when observed with PDE. In a tissue or an organ where blood flow is inhibited, the fluorescence emission of ICG weakens.
The evaluation of blood flow using ICG fluorescence imaging is applied to breast reconstruction [11], coronary artery bypass grafting [12], and colorectal resection [13]. Using this technique, blood flow can be evaluated in real time, and it can be easily introduced to those aforementioned operations, except in patients who are allergic to ICG. The usefulness of ICG fluorescence imaging in patients with NOMI has already been reported. Ishizuka et al. used this method in 6 patients with NOMI [2] who underwent bowel resection and anastomosis in one operation. The authors reported that 5 of 6 patients were discharged uneventfully.
In the surgical management of NOMI, planned reoperation within 24–48 h after the initial operation is often necessary, with the aim to find any newly developed necrotic lesions. Ward et al. reported that 50% of patients with NOMI who underwent second-look laparotomy underwent further bowel resection [14]. The arteries in the intestinal wall perforate the longitudinal and circular muscles from the serosal side to the mucosal side. Therefore, even if the intestinal serosa is normal during initial laparotomy of NOMI, it is possible that the mucosa becomes ischemic or sometimes necrotic. In addition, the ischemic lesion of the intestine is missed because the mesenteric blood flow is not completely interrupted in contrast to superior mesenteric artery thrombosis. These are the reasons why planned reoperation is often performed in patients with NOMI.
In our case, using PDE, we could identify mucosal necrosis, which is not visible macroscopically. This was proven by the pathological findings. In the case that the intestine to be resected is unclear in NOMI, like in our case, ICG fluorescence imaging using PDE is thought to be very useful. We also suggest that planned reoperation might not be necessary. The limitation of ICG fluorescence imaging is that this method largely depends on the subjectivity of the operator, and the quantity of fluorescence emitted on the screen has not been quantified. Therefore, this method should be used as an auxiliary method for determining the resection area.
On the other hand, this method has the risk of over-resection of the intestine. The short bowel syndrome following massive intestinal resection results in a poor clinical course. Therefore, if there is concern about short bowel syndrome after intestinal resection, it might be effective to remove only macroscopically necrotic intestine and to perform the planned reoperation.
4. Conclusions
ICG fluorescence imaging was useful for evaluating mesenteric blood flow in a patient with NOMI. By using this method, planned reoperation might not be necessary.
Conflicts of interest
None.
Funding source
None.
Ethical approval
Ethical approval was not required by our institution for this case study.
Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Author’s contribution
Yutaka Nakagawa performed the operation, collected the clinical data, and drafted this manuscript. Kazuaki Kobayashi participated in the clinical management of the patient. Shirou Kuwabara supervised the clinical management. Hiroyuki Shibuya contributed to the acquisition and interpretation of the pathological data. Tadashi Nishimaki supervised the writing of this manuscript. All authors read and approved final manuscript.
Registration of research studies
This case report was not registered in a publicly accessible database.
Guarantor
Yutaka Nakagawa.
Provenance and peer review
Not commissioned, externally peer-reviewed.
References
- 1.Bourcier S., Oudjit A., Goudard G., Charpentier J., Leblanc S., Coriat R. Diagnosis of non-occlusive acute mesenteric ischemia in the intensive care unit. Ann. Intensive Care. 2016;6:112. doi: 10.1186/s13613-016-0213-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishizuka M., Nagata H., Takagi K., Iwasaki Y., Yamagishi H., Tanaka G. Usefulness of intraoperative observation using a fluorescence imaging instrument for patients with nonocclusive mesenteric ischemia. Int. Surg. 2015;100:593–599. doi: 10.9738/INTSURG-D-14-00038.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agha R.A., Fowler A.J., Saeta A., Barai I., Rajmohan S., Orgill D.P. The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;34:180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Cocorullo G., Mirabella A., Falco A., Fontana T., Tutino R., Licari L. An investigation of bedside laparoscopy in the ICU for cases of non-occlusive mesenteric ischemia. World J. Emerg. Surg. 2017;12:4. doi: 10.1186/s13017-017-0118-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazzei M.A., Guerrini S., Squitieri C.N., Vindigni C., Grassi R., Volterrani L. Reperfusion in non-occlusive mesenteric ischaemia (NOMI): effectiveness of CT in an emergency setting. Br. J. Radiol. 2016;89:20150956. doi: 10.1259/bjr.20150956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adaba F., Askari A., Dastur J., Patel A., Gabe S.M., Vaizey C.J. Mortality after acute primary mesenteric infarction: a systematic review and meta-analysis of observational studies. Colorectal Dis. 2015;17:556–577. doi: 10.1111/codi.12938. [DOI] [PubMed] [Google Scholar]
- 7.Yukaya T., Saeki H., Taketani K., Ando K., Ida S., Kimura Y. Clinical outcomes and prognostic factors after surgery for non-occlusive mesenteric ischemia: a multicenter study. J. Gastrointest. Surg. 2014;18:1642–1647. doi: 10.1007/s11605-014-2579-0. [DOI] [PubMed] [Google Scholar]
- 8.Bassiouny H.S. Nonocclusive mesenteric ischemia. Surg. Clin. North Am. 1997;77:319–326. doi: 10.1016/s0039-6109(05)70551-x. [DOI] [PubMed] [Google Scholar]
- 9.Trompeter M., Brazda T., Remy C.T., Vestring T., Reimer P. Non-occlusive mesenteric ischemia: etiology, diagnosis, and interventional therapy. Eur. Radiol. 2002;12:1179–1187. doi: 10.1007/s00330-001-1220-2. [DOI] [PubMed] [Google Scholar]
- 10.Inoue K., Fukuhara H., Shuin T., Kurabayashi A., Furihata M., Tanimura M. Endoscopic surgery using fluorescence navigation. Jpn. J. Endourol. 2013;26:158–162. [Google Scholar]
- 11.Griffiths M., Chae M.P., Rozen W.M. Indocyanine green-based fluorescent angiography in breast reconstruction. Gland Surg. 2016;5:133–149. doi: 10.3978/j.issn.2227-684X.2016.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Handa T., Katare R.G., Nishimori H., Wariishi S., Fukutomi T., Yamamoto M. New device for intraoperative graft assessment: HyperEye charge-coupled device camera system. Gen. Thorac. Cardiovasc. Surg. 2010;58:68–77. doi: 10.1007/s11748-009-0536-8. [DOI] [PubMed] [Google Scholar]
- 13.Boni L., David G., Mangano A., Dionigi G., Rausei S., Spampatti S. Clinical applications of indocyanine green (ICG) enhanced fluorescence in laparoscopic surgery. Surg. Endosc. 2015;29:2046–2055. doi: 10.1007/s00464-014-3895-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward D., Vernava A.M., Kaminski D.L., Ure T., Peterson G., Garvin P. Improved outcome by identification of high-risk nonocclusive mesenteric ischemia, aggressive reexploration, and delayed anastomosis. Am. J. Surg. 1995;170:577–581. doi: 10.1016/s0002-9610(99)80019-1. [DOI] [PubMed] [Google Scholar]