Abstract
cGMP-dependent protein kinase 1 (PKG1) plays an important role in nitric oxide (NO)/cGMP–mediated maintenance of vascular smooth muscle cell (VSMC) phenotype and vasorelaxation. Inflammatory cytokines, including tumor necrosis factor-α (TNFα), have long been understood to mediate several inflammatory vascular diseases. However, the underlying mechanism of TNFα-dependent inflammatory vascular disease is unclear. Here, we found that TNFα treatment decreased PKG1 expression in cultured VSMCs, which correlated with NF-κB–dependent biogenesis of miR-155-5p that targeted the 3′-UTR of PKG1 mRNA. TNFα induced VSMC phenotypic switching from a contractile to a synthetic state through the down-regulation of VSMC marker genes, suppression of actin polymerization, alteration of cell morphology, and elevation of cell proliferation and migration. All of these events were blocked by treatment with an inhibitor of miR-155-5p or PKG1, whereas transfection with miR-155-5p mimic or PKG1 siRNA promoted phenotypic modulation, similar to the response to TNFα. In addition, TNFα-induced miR-155-5p inhibited the vasorelaxant response of de-endothelialized mouse aortic vessels to 8-Br-cGMP by suppressing phosphorylation of myosin phosphatase and myosin light chain, both of which are downstream signal modulators of PKG1. Moreover, TNFα-induced VSMC phenotypic alteration and vasodilatory dysfunction were blocked by NF-κB inhibition. These results suggest that TNFα impairs NO/cGMP-mediated maintenance of the VSMC contractile phenotype and vascular relaxation by down-regulating PKG1 through NF-κB–dependent biogenesis of miR-155-5p. Thus, the NF-κB/miR-155-5p/PKG1 axis may be crucial in the pathogenesis of inflammatory vascular diseases, such as atherosclerotic intimal hyperplasia and preeclamptic hypertension.
Keywords: tumor necrosis factor (TNF), protein kinase G (PKG), microRNA (miRNA), NF-kappa B (NF-κB), vascular smooth muscle cells, cGMP-dependent protein kinase 1
Introduction
Endothelial nitric-oxide synthase (eNOS) 2-derived nitric oxide (NO) in the endothelium diffuses into the smooth muscle, where it modulates vascular tone and VSMC proliferation by soluble guanylyl cyclase–dependent cGMP synthesis with subsequent activation of PKG1 (1). Activated PKG1, a serine/threonine-specific protein kinase, phosphorylates a number of important targets and is implicated in the maintenance of vasorelaxation and contractile phenotype, which are characteristic of healthy VSMCs. In physiological states, the sequential activation of the NO/cGMP/PKG1 pathway by communication between endothelial cells and VSMCs plays a key role in vascular homeostasis and remodeling. However, impairment of this pathway is directly associated with various vascular disorders, such as atherosclerosis, hypertension, and preeclampsia (2–4).
In addition to endothelial dysfunction with decreased eNOS/NO activity, impaired VSMC function also contributes to human vascular diseases, such as atherosclerotic intima formation, hypertension, and vascular aneurysm (5). VSMCs generally exist in a differentiated, nonproliferative, and contractile phenotype; however, they exhibit an extraordinary capacity or plasticity to undergo transition from the contractile to the synthetic state in response to stimulation by oxidative stress and inflammatory cytokines, which are known as pathogenic factors in a variety of vascular disorders through activation of NF-κB (6). The inflammatory cytokines TNFα and interleukin-1β (IL-1β) are known as major risk factors for inflammatory cardiovascular diseases. Both cytokines inhibit PKG1 expression in primary cultured VSMCs, leading to phenotypic switching from the contractile to the synthetic state (7). This evidence suggests that NF-κB activation may dysregulate VSMC function by down-regulating PKG1 expression in the pathological environment associated with vascular inflammation, subsequently causing inflammatory cardiovascular disorders. However, the underlying pathophysiological mechanisms have not been clearly elucidated.
Increasing evidence has demonstrated that miRNAs (miRs) are involved in the pathogenesis of various cardiovascular diseases, such as atherosclerosis, hypertension, and stroke, through the post-transcriptional suppression of gene expression by targeting the 3′-UTR of specific mRNAs (8). Although several miRNAs, such as miR-22, miR-31, miR-133, and miR-145 (9–12), have been shown to be implicated in VSMC proliferation and phenotypic modulation, leading to the development of vascular dysfunction and cardiovascular disorders, the molecular mechanism associated with miRNAs in phenotypic modulation of VSMCs by inflammatory cytokines has yet to be fully understood. Our recent studies demonstrated that TNFα causes vascular dysfunction through down-regulation of eNOS expression due to increased NF-κB–dependent biogenesis of miR-155-5p (13). However, little is known about the functional role of miR-155-5p in NF-κB-dependent PKG1 expression and VSMC phenotypic modulation in inflammatory conditions.
In this study, we found that TNFα-induced biogenesis of NF-κB–responsive miR-155-5p promotes VSMC phenotypic switching and vasodilatory dysfunction by inhibiting PKG1 expression in VSMCs. These findings suggest that miR-155-5p plays an important role in VSMC dysfunction associated with intima formation and hypertension and thus may be a potential therapeutic target for inflammatory vascular diseases.
Results
TNFα down-regulates PKG1 expression
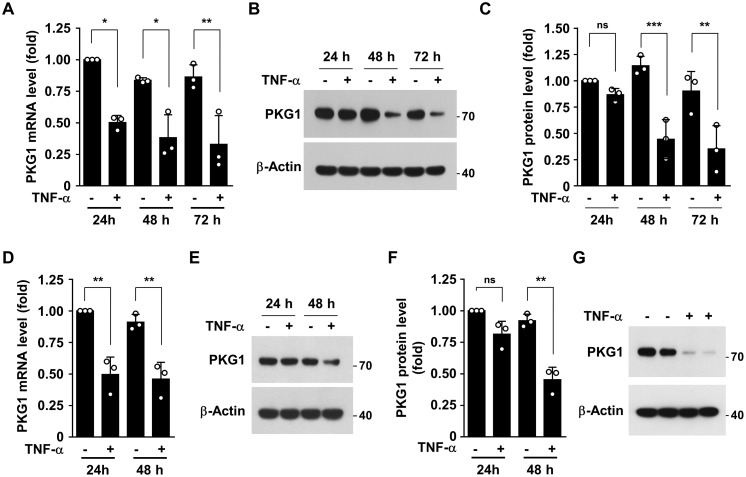
As pro-inflammatory cytokines, including TNFα, are crucially involved in the pathogenesis of various inflammatory vascular diseases (14), we investigated whether TNFα regulates PKG1 expression in human aortic smooth muscle cells (HASMCs) and isolated primary mouse aortic smooth muscle cells (MASMCs). Treatment of HASMCs with TNFα resulted in a significant decrease in PKG1 mRNA levels at 24 h, with a further reduction until 72 h (Fig. 1A). Consistent with this, TNFα treatment dramatically suppressed PKG1 protein levels at 48 h (Fig. 1, B and C). Similar suppressive effects on PKG1 expression were observed in HASMCs stimulated with other inflammatory stimulants, such as IL-1β, IL-6, and lipopolysaccharide (Fig. S1, A and B). In addition, TNFα treatment also decreased mouse PKG1 mRNA and protein levels in cultured MASMCs (Fig. 1, D–F). Furthermore, ex vivo TNFα stimulation of de-endothelialized mouse aortic vessels down-regulated mouse PKG1 expression (Fig. 1G). These results suggest that TNFα down-regulates PKG1 expression in cultured primary human and mouse VSMC and isolated mouse aortic vessels.
Figure 1.
TNFα regulates PKG1 expression in HASMCs and MASMCs. A–C, HASMCs were stimulated with TNFα (10 ng/ml) for the indicated time periods. A, PKG1 mRNA levels were measured by qRT-PCR. B and C, PKG1 protein levels were determined by Western blotting and quantified using ImageJ software. D–F, MASMCs were stimulated with TNFα (10 ng/ml) for the indicated time periods. D, mouse PKG1 mRNA levels were measured by qRT-PCR. E and F, mouse PKG1 protein levels were determined by Western blotting and quantified using ImageJ software. G, de-endothelialized mouse aortic vessels were stimulated with TNFα (10 ng/ml) for 48 h. Mouse PKG1 protein levels were determined by Western blotting. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, not significant. Error bars, S.D.
TNFα-mediated PKG1 down-regulation depends on NF-κB–responsive miR-155-5p
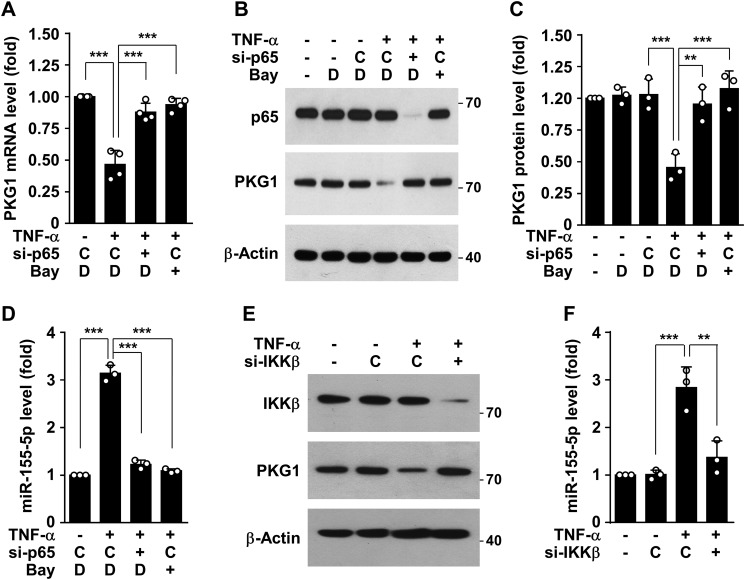
Because NF-κB plays a crucial role in regulating the immune response and stimulating inflammation-associated gene expression, we next examined whether NF-κB could be involved in TNFα-mediated negative regulation of PKG1 expression. Pretreatment with the NF-κB inhibitor Bay 11-7082 or transfection with siRNAs for NF-κB subunit p65 siRNA rescued down-regulation of PKG1 mRNA and protein levels in HASMCs stimulated with TNFα (Fig. 2, A–C), suggesting that NF-κB is an important player in TNFα-induced negative regulation of PKG1 expression. It has recently been shown that NF-κB–responsive miR-155-5p contributes to the inhibition of expression of some genes, including eNOS, in the vasculature (14). We analyzed whether PKG1 is a possible target for miR-155-5p using TargetScan (www.targetscan.org). 3 We found that miR-155-5p could negatively regulate PKG1 expression by complementary binding to the 3′-UTR of its mRNA, and its target site was highly conserved in several species, including humans, nonhuman primates, and rodents (Fig. S2A). As expected, TNFα elevated miR-155-5p biogenesis in HASMCs, and this effect was blocked by treatment with Bay 11-7082 or knockdown of the NF-κB subunit p65 (Fig. 2D). Similar regulatory effects on PKG1 expression and miR-155-5p biogenesis were observed in HASMCs transfected with siRNA specific for IκB kinase β (IKKβ) (Fig. 2, E and F), further confirming the involvement of NF-κB in biogenesis of miR-155-5p. As expected, several activators NF-κB, such as IL-1β, IL-6, and lipopolysaccharide, stimulated miR-155-5p biogenesis (Fig. 1C) and subsequently down-regulated PKG1 protein levels (Fig. 1, A and B). These results suggest that NF-κB–responsive miR-155-5p could negatively regulate PKG1 expression in HASMC stimulated with TNFα.
Figure 2.
TNFα inhibits PKG1 expression in a NF-κB–dependent manner. HASMCs were transfected with control siRNA (C), NF-κB p65 siRNA (si-p65), or IKKβ siRNA (si-IKKβ), followed by stimulation with TNFα (10 ng/ml) in the presence or absence of Bay 11-7082 (5 μm) or DMSO (D) as a vehicle for 48 h. A, PKG1 mRNA levels were determined by qRT-PCR. B and C, PKG1 and NF-κB p65 protein levels were determined by Western blotting and quantified using ImageJ software. D, miR-155-5p levels were determined by qRT-PCR. E, PKG1 and IKKβ protein levels were determined by Western blotting. F, miR-155-5p levels were determined by qRT-PCR. **, p < 0.01; ***, p < 0.001. Error bars, S.D.
PKG1 is a bona fide target of NF-κB–responsive miR-155-5p
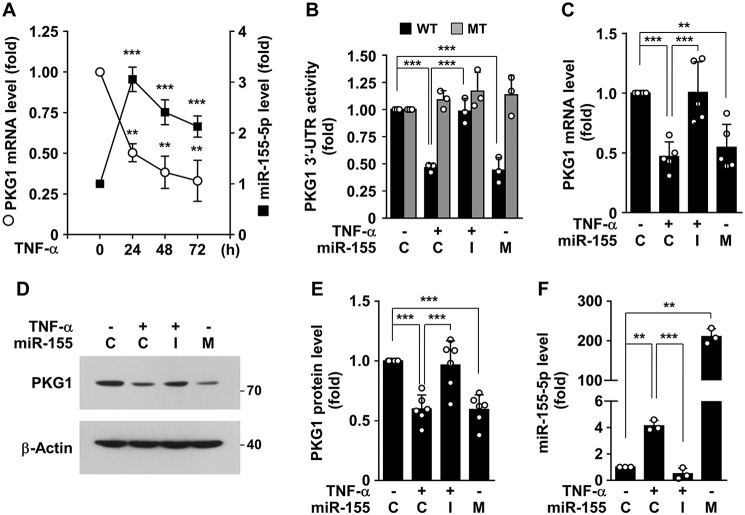
To examine the possibility that miR-155-5p down-regulates PKG1 expression, we first assessed the correlation between miR-155-5p and PKG1 mRNA levels in HASMCs stimulated with TNFα. Following stimulation with TNFα, PKG1 mRNA levels were markedly decreased in a time-dependent manner, whereas miR-155-5p levels were rapidly increased 24 h after stimulation and thereafter slightly decreased (Fig. 3A), indicating that PKG1 mRNA and miR-155-5p levels are inversely correlated. This suggests that NF-κB–responsive miR-155-5p is a negative regulator of PKG1 expression. We further examined whether miR-155-5p regulates the expression of human PKG1 by complementary binding to its 3′-UTR. Treatment with TNFα suppressed the activity of a PKG1 mRNA 3′-UTR–based reporter, but not the activity of its mutant reporter, and the decreased WT reporter activity was rescued by a miR-155-5p inhibitor (Fig. 3B). In addition, transient transfection of miR-155-5p mimic inhibited the 3′-UTR activity, but not its mutant activity, as did TNFα (Fig. 3B). Consistent with this, TNFα or miR-155-5p mimic attenuated PKG1 mRNA and protein levels, and the inhibitory effect of TNFα was reversed by a miR-155-5p inhibitor (Fig. 3, C–E). We next determined comparative levels of miR-155-5p in HASMCs treated with either TNFα or miR-155-5p mimic or inhibitor. As expected, transfection with miR-155-5p mimic highly increased cellular miR-155-5p levels, whereas the miR-155-5p inhibitor suppressed TNFα-induced elevation of miR-155-5p (Fig. 3F). Collectively, these findings suggest that TNFα negatively regulates PKG1 expression by NF-κB–responsive miR-155-5p biogenesis.
Figure 3.
TNFα-induced miR-155-5p inhibits PKG1 expression by targeting the PKG1 mRNA 3′-UTR. A, HASMCs were stimulated with TNFα (10 ng/ml) for the indicated time periods. PKG1 mRNA and miR-155-5p levels were determined by qRT-PCR (n = 3). B–E, HASMCs were transfected with control miRNA (C), miR-155-5p mimic (M), or miR-155-5p inhibitor (I) or in combination with psiCHECK-2-PKG1 3′-UTR-reporter constructs (WT and mutant (MT)), followed by stimulation with TNFα (10 ng/ml) for 48 h. B, luciferase activity was determined in cell lysates. C–E, PKG1 mRNA and protein levels were determined by qRT-PCR and Western blotting, and protein levels were quantified using ImageJ software. F, cellular levels of miR-155-5p were determined by qRT-PCR. **, p < 0.01; ***, p < 0.001. Error bars, S.D.
TNFα induces VSMC phenotypic switching by miR-155-5p–mediated down-regulation of PKG1
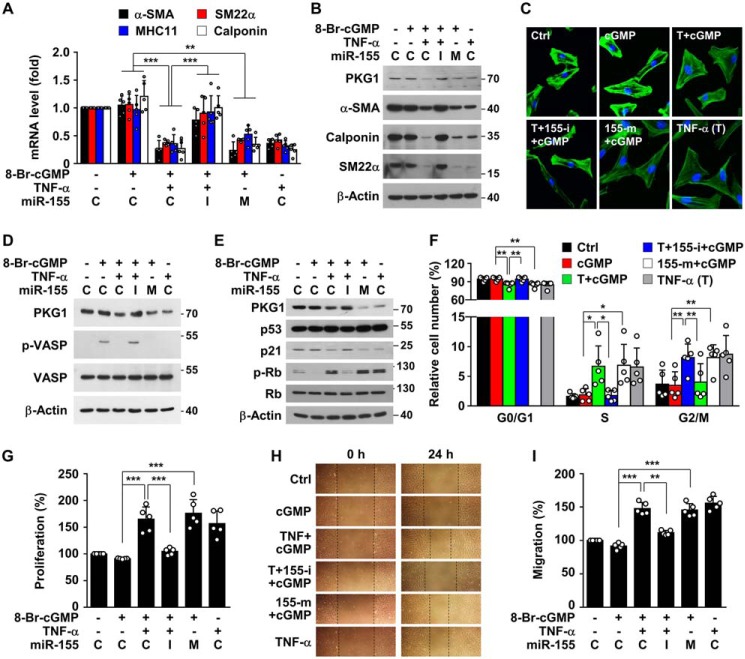
To examine the role of TNFα-induced miR-155-5p in the regulation of VSMC-specific contractile gene expression, HASMCs were incubated with the membrane-permeable 8-Br-cGMP, an endogenous mimic product of the eNOS/soluble guanylyl cyclase pathway, following treatment with TNFα, TNFα plus miR-155 inhibitor, or miR-155-5p mimic. TNFα treatment suppressed mRNA and protein levels of contractile genes in HASMCs, such as α-smooth muscle actin (α-SMA), smooth muscle 22α (SM22α), smooth muscle myosin heavy chain 11 (SM-MHC11), and calponin, and this suppression was reversed by transfection with a miR-155-5p inhibitor (Fig. 4, A and B). As expected, transfection with miR-155-5p mimic inhibited expression of the contractile genes, as was seen with TNFα treatment (Fig. 4, A and B). We further examined the effect of TNFα-induced miR-155-5p on cell morphology and actin cytoskeleton rearrangement in HASMCs. Cells treated with TNFα or miR-155-5p mimic underwent morphological changes from spindle-shaped to a spread-out or rhomboid/polygonal shape with reduced cytoskeletal rearrangement, and the TNFα-induced morphological changes mostly disappeared by an miR-155-5p inhibitor (Fig. 4C). Moreover, we examined whether TNFα-induced miR-155-5p regulates cell proliferation and migration, characterized by VSMC phenotypic switching. TNFα and miR-155-5p mimic strongly inhibited 8-Br-cGMP–mediated phosphorylation of vasodilator-stimulated phosphoprotein (VASP), known as a cGMP/PKG1-dependent downstream mediator for inhibiting VSMC proliferation (15), and the inhibitory effect of TNFα was blocked by a miR-155-5p inhibitor (Fig. 4D). As expected, TNFα and miR-155-5p decreased p21 protein levels and promoted phosphorylation of retinoblastoma protein (Rb), without affecting p53 expression, and the regulatory effects of TNFα were mitigated by an miR-155-5p inhibitor (Fig. 4E). As a result, treatment with TNFα or miR-155-5p mimic resulted in a decrease in G0/G1 phase and a subsequent increase in S and G2/M phases, and TNFα-induced cell cycle progression was blocked by a miR-155-5p inhibitor (Fig. 4F). Similar results were also observed in terms of VSMC proliferation (Fig. 4G). In addition, TNFα and miR-155-5p mimic increased VSMC migration, and TNFα-induced VSMC migration was also attenuated by a miR-155-5p inhibitor (Fig. 4, H and I). Taken together, these findings suggest that TNFα stimulates VSMC phenotypic switching from the contractile to the adverse proliferative or synthetic state by miR-155-5p-mediated down-regulation of PKG1.
Figure 4.
TNFα-induced miR-155-5p stimulates VSMC phenotypic switching. HASMCs were transfected with control miRNA (C), miR-155-5p mimic (M or 155-m), or miR-155-5p inhibitor (I or 155-i) and treated with TNFα (T; 10 ng/ml) for 48 h, followed by further stimulation with 8-Br-cGMP (100 μm) for 24 h, except for cell cycle analysis, which was performed at 12 h. A and B, target gene mRNA and protein levels were determined by qRT-PCR and Western blotting, respectively. C, cells were stained with Alexa Fluor 488 phalloidin and DAPI. Representative images show morphological alternation of VSMCs with actin cytoskeleton rearrangement. D and E, target protein levels were determined by Western blotting. F, cells were stained with PI, and cell cycle distribution was analyzed by flow cytometry. G, cell proliferation was determined by a [3H]thymidine incorporation assay. H and I, cell migration was assessed using a scratch wound-healing assay and quantified by measuring relative distance of cell migration into the cell-free area. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Error bars, S.D.
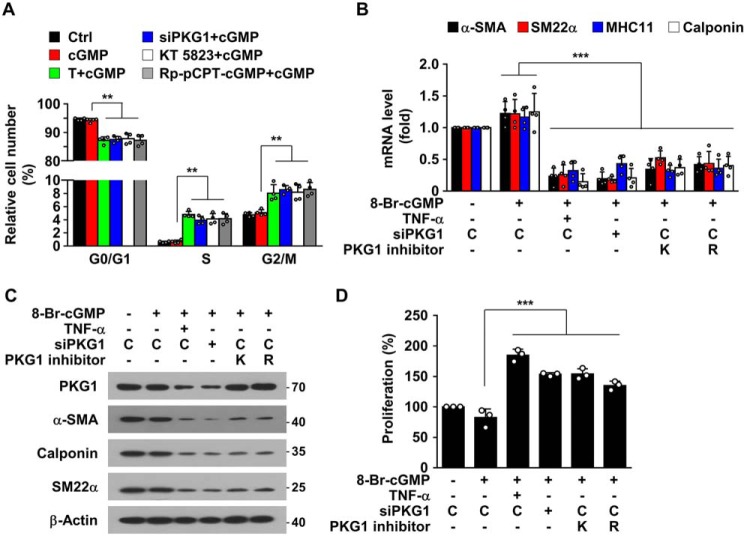
PKG1 inhibitors mimic TNFα-mediated VSMC phenotypic switching
Because TNFα treatment resulted in phenotypic switching of VSMCs due to miR-155-5p–mediated down-regulation of PKG1 (Fig. 4), we examined whether inhibition or knockdown of PKG1 directly promotes SMC phenotypic switching. Knockdown or inhibition of PKG1 in 8-Br-cGMP–exposed HASMCs by treatment with PKG1 siRNA, KT 5823, or Rp-8-pCPT-cGMP promoted cell cycle progression from the G0/G1 to S phase, with subsequent entry to the G2/M phase (Fig. 5A). Furthermore, these PKG1 inhibitors effectively suppressed mRNA and protein levels of VSMC-specific genes, such as α-SMA, SM22α, SM-MHC11, and calponin (Fig. 5, B and C). Consistent with this, PKG1 inhibitors stimulated VSMC proliferation (Fig. 5D). All of the cellular events induced by PKG1 inhibitors were similar to those observed in HASMCs treated with TNFα or miR-155 mimic (Fig. 4). These results suggest that PKG1, which is a target of TNFα-induced miR-155-5p, is a crucial mediator for NO/cGMP-dependent maintenance of the contractile phenotype of VSMCs.
Figure 5.
PKG1 inhibition induces SMC phenotypic switching. HASMCs were transfected with control siRNA (C) or PKG1 siRNA (siPKG1) and treated with KT 5823 (K; 1 μm), Rp-8-pCPT-cGMP (R; 20 μm), or TNFα (T; 10 ng/ml) for 48 h, followed by further stimulation with 8-Br-cGMP (100 μm) for 24 h, except for cell cycle analysis, which was performed at 12 h. A, cells were stained with PI, and cell cycle distribution was analyzed by flow cytometry. B and C, target gene mRNA and protein levels were determined by qRT-PCR and Western blotting. D, cell proliferation was determined by a [3H]thymidine incorporation assay. **, p < 0.01; ***, p < 0.001. Error bars, S.D.
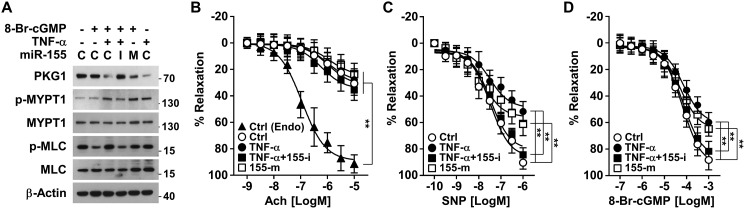
TNFα-induced miR-155-5p impairs cGMP-mediated vasodilation via PKG1 down-regulation
The cGMP/PKG1 pathway plays an important role in not only maintaining the VSMC contractile phenotype but also regulating vascular relaxation (16). We examined the functional role of TNFα-induced miR-155-5p in cGMP/PKG1-mediated vasorelaxation. 8-Br-cGMP treatment maintained low phosphorylation of myosin light-chain phosphatase 1 (MYPT1) at Thr-696 and myosin light chain (MLC), which are signal mediators downstream of PKG1 (17); however, their phosphorylation levels were elevated by co-treatment with either TNFα or miR-155-5p mimic, and TNFα-induced phosphorylation of MYPT1 and MLC was blocked by an miR-155-5p inhibitor (Fig. 6A). Based on these results, we further examined the effect of the miR-155-5p/PKG1 axis on the ex vivo vasorelaxant responses of de-endothelialized mouse aortic vessels to NO and cGMP. Treatment with TNFα, TNFα plus miR-155-5p inhibitor, and miR-155-5p mimic did not induce a vasodilatory response of de-endothelialized vessels to the endothelium-dependent vasodilator acetylcholine, in contrast to the vasorelaxation activity of intact vessels (Fig. 6B). However, treatment of de-endothelialized aortic vessels with TNFα or miR-155-5p mimic significantly decreased the vasodilatory responses to the NO donor sodium nitroprusside (SNP) and 8-Br-cGMP, and the TNFα-induced decrease in vasodilation was reversed by an miR-155-5p inhibitor (Fig. 6, C and D). These results suggest that TNFα-induced miR-155-5p inhibits cGMP-dependent vasorelaxation by silencing PKG1.
Figure 6.
TNFα-induced miR-155-5p impairs NO/cGMP-induced vasorelaxation. A, HASMCs were transfected with control miRNA (C), miR-155-5p mimic (M), or miR-155-5p inhibitor (I) and stimulated with TNFα (10 ng/ml) for 48 h, followed by treatment with 8-Br-cGMP (100 μm) for 1 h. Target protein levels were determined by Western blotting. B–D, intact (Endo) and de-endothelialized mouse aortic rings were stimulated with or without TNFα (10 ng/ml) alone or in combination with miR-155-5p mimic (155-m) or miR-155-5p inhibitor (155-i) for 48 h. B, concentration-dependent vasorelaxant responses of the aortic rings to acetylcholine (Ach) were assessed by myography (n = 6). C and D, vasorelaxant responses of the aortic rings to SNP or 8-Br-cGMP were also determined by myography (n = 6). **, p < 0.01. Error bars, S.E.
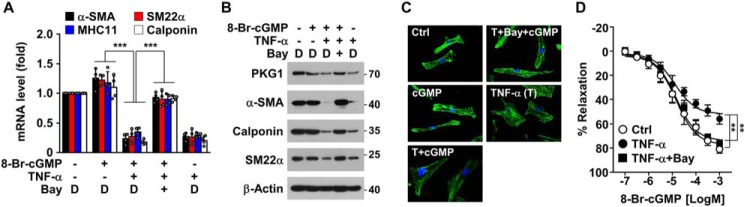
NF-κB activation is essential for TNFα-induced VSMC dysfunction
As the NF-κB pathway plays a key role in expression of various inflammatory genes in response to TNFα (18), we examined whether NF-κB contributes to VSMC phenotypic switching and vasodilatory dysfunction induced by TNFα. The NF-κB inhibitor Bay 11-7082 blocked TNFα-mediated down-regulation of VSMC-specific contractile phenotype marker genes, such as α-SMA, SM22α, SM-MHC11, and calponin (Fig. 7, A and B). In addition, Bay 11-7082 reversed the TNFα-mediated morphological changes of HASMCs to the synthetic phenotype with reduced actin polymerization (Fig. 7C). Moreover, we also found that Bay 11-7082 prevented the inhibitory effect of TNFα on the vasorelaxant response of de-endothelialized mouse aortic vessels to 8-Br-cGMP (Fig. 7D). These findings suggest that the NF-κB pathway is crucially involved in VSMC phenotypic switching and vasodilatory dysfunction, probably through miR-155-5p–mediated PKG1 down-regulation. Thus, activation of the NF-κB signaling pathway is likely to be a key characteristic of many cardiovascular diseases caused by inflammatory states.
Figure 7.
NF-κB inhibition prevents TNFα-induced VSMC phenotypic alteration and vasodilatory dysfunction. HASMCs or de-endothelialized mouse aortic rings were stimulated with TNFα (T; 10 ng/ml) in the presence or absence of Bay 11-7082 (5 μm) or DMSO (D) as a vehicle for 48 h, followed by treatment with 8-Br-cGMP (100 μm) for 24 h. A and B, target gene mRNA and protein levels were determined by qRT-PCR and Western blotting, respectively. C, after staining with Alexa Fluor 488 phalloidin and DAPI, cell morphology and actin polymerization were determined by confocal microscopy. D, vasorelaxant responses of de-endothelialized mouse aortic rings to 8-Br-cGMP were determined following treatment with TNFα alone or in combination with Bay 11-7082 by myography (n = 6). **, p < 0.01; ***, p < 0.001. Error bars, S.D.
Discussion
The pro-inflammatory cytokines, TNFα and IL-1β, are significantly elevated in various disease states and considered as pathogenic risk factors for several vascular diseases, including atherosclerosis and preeclamptic hypertension (19–21). These cytokines cause either endothelial or VSMC dysfunction through impairment of the NO/cGMP pathway that plays a crucial role in vascular function, leading to intimal hyperplasia in atherosclerosis and hypertension in preeclampsia (7, 13). We have previously reported that inflammatory cytokines inhibit endothelial cell-dependent vascular remodeling and vasorelaxation through down-regulation of eNOS expression by promoting NF-κB–dependent biogenesis of miR-155-5p (13, 14). However, little is known about the molecular mechanism by which inflammatory cytokines induce VSMC dysfunction associated with cardiovascular diseases. In this study, we found that TNFα promoted VSMC phenotypic switching and impaired vasorelaxation through inhibition of PKG1, a downstream signaling target of cGMP, by inducing NF-κB–responsive miR-155-5p biogenesis. Our results suggest that NF-κB–responsive miR-155-5p is a pathological determinant for intimal hyperplasia and hypertension by decreasing PKG1 expression in inflammatory disease states.
Immune cell infiltration and cytokine production are crucial pathogenic processes in the early stage of cardiovascular diseases. Persistent increases in circulating or local levels of the cytokines are associated with the pathogenesis of vascular dysfunction and cardiovascular diseases, such as atherosclerosis, aortic aneurysm, and hypertension (22–24). Although cytokines are important mediators of vascular immune responses, they also promote endothelial cell dysfunction and alter VSMC phenotype, which contribute to vascular pathologies (13, 25). Among cytokines, TNFα is a typical risk factor for cardiovascular disorders through the induction of inflammation-associated genes by activating NF-κB. Advanced studies showed that blocking NF-κB activation is beneficial in a number of inflammatory vascular diseases, including atherosclerosis (26) and hypertension (27), suggesting that NF-κB is a key pathogenic factor in the pathogenesis of cardiovascular diseases. Recent studies have demonstrated that NF-κB impairs endothelial function by stimulating biogenesis of miR-155-5p, which is a negative regulator of eNOS expression (13). This suggests that NF-κB–responsive miR-155-5p indirectly impairs VSMC function through inhibition of eNOS-derived NO production. However, the molecular mechanism by which TNFα-dependent activation of NF-κB directly induces VSMC dysfunction has not been clearly elucidated. Our data demonstrate that NF-κB–responsive miR-155-5p is crucially involved in TNFα-induced VSMC dysfunction by targeting the PKG1 mRNA 3′-UTR.
Similar to the determinant role of eNOS/NO in endothelial cell function, the cGMP/PKG pathway is also essential for maintenance of the contractile phenotype and relaxation of VSMCs. There are two types of PKG, PKG1 and PKG2. PKG1 is predominantly expressed in cardiovascular tissues, whereas the expression of PKG2 is generally restricted to the brain, intestine, and kidneys (28), indicating that PKG1 may be more important than PKG2 in VSMC function associated with cardiovascular homeostasis. It has been demonstrated that PKG1 was down-regulated in pathological conditions of various cardiovascular disorders (4, 7). Indeed, PKG1 expression was decreased in neointimal smooth muscle cells of swine and rat coronary arteries after balloon catheter injury (29, 30), and this pathological process was blunted by adenoviral PKG1 gene delivery (30). In addition, SMC-specific deletion of PKG1 has been shown to abolish NO/cGMP-dependent vascular relaxation, resulting in a significant elevation of arterial blood pressure; thus, it was not altered by administration of the exogenous NO donor DEA-NO (31). Therefore, pathological inhibition of PKG1 expression directly dysregulates VSMC-dependent vascular homeostasis, which is normally controlled by the NO/cGMP pathway. Accordingly, our results demonstrated that TNFα-mediated PKG1 down-regulation stimulates VSMC phenotypic modulation and impairs vascular relaxation.
Circulating cytokine levels are directly associated with NF-κB activation in the pathogenic conditions of cardiovascular disorders, including atherosclerosis and preeclamptic hypertension (19–21). TNFα and IL-1β not only cause endothelial dysfunction via eNOS down-regulation (14) but also inhibit VSMC phenotypic modulation and intimal hyperplasia after vascular injury, although the mechanism was unclear (32). However, it has been shown previously that these cytokines result in a significant decrease in PKG1 expression in primary cultured bovine aortic VSMCs (7), which, in agreement with data, suggests that inflammatory down-regulation of PKG1 is likely to be associated with VSMC dysfunction. Another previous study nicely demonstrated that TNFα stimulates VSMC migration, which can be abolished by overexpression of a dominant-negative IκBα mutant (33). These findings provide evidence that NF-κB is crucially involved in VSMC phenotypic switching. Moreover, SMC-selective mutant IκB-expressing mice prevented VSMC phenotypic switching and neointima formation after arterial injury (34), strongly suggesting that activation of the NF-κB pathway contributes to inflammatory cardiovascular diseases. Consistent with these observations, our data demonstrate that NF-κB–responsive miR-155-5p is essential for TNFα-mediated VSMC phenotypic modulation by targeting the PKG1 transcript, further providing new evidence that PKG1 is a negative target gene of NF-κB.
There are several proposed mechanisms in PKG1-dependent regulation of vascular function. Activation of PKG1 by cGMP preferentially phosphorylates MYPT1 at Ser-695, with subsequent prevention of inhibitory phosphorylation at the adjacent Thr-696 (35). This event leads to stimulation of VSMC relaxation by increasing dephosphorylation of MLC. In addition, PKG1 suppresses VSMC proliferation by negatively regulating cell cycle progression through p53-independent up-regulation of the cyclin-dependent kinase inhibitors p21 and p27 (36) or inhibitory hyperphosphorylation of the G1/S transcription inhibitor Rb (37). Elevated levels of p21, p27, and hyperphospho-Rb are critical for negatively regulating cell cycle progression not only from G1 to S phase, but also through S phase. On the other hand, PKG1 also induces inhibitory phosphorylation of VASP at Ser-239 (15). VASP normally localizes at focal adhesion sites, binds to F-actin and G-actin, and facilitates locally constrained actin polymerization and lamellipodial protrusions (38), leading to stimulation of cell spreading and migration. However, PKG1-mediated inhibitory phosphorylation of VASP at Ser-239 inhibits VSMC migration and proliferation by disassembly of focal adhesions (15). This evidence suggests that impaired PKG1 function stimulates VSMC phenotypic switching from the contractile to synthetic state. Accordingly, our data show that TNFα increased VSMC phenotypic switching by down-regulating PKG1 through NF-κB–responsive biogenesis of miR-55-5p, implying that miR-155-5p can be used as a therapeutic target for inflammatory vascular diseases.
Accumulating evidence indicates that nonphysiological expression of miRNAs contributes to the pathogenesis of various diseases, including cardiovascular disorders. In particular, a growing number of miRNAs, such as miR-1 (39), miR-21 (40), miR-22 (9), miR-34a (41), miR-133 (10), miR-145 (11), miR-214 (42), and miR-663 (43), have been implicated in phenotypic modulation of VSMCs, leading to neointima formation in a mouse model of vascular injury or atherosclerosis. Although their target genes associated with VSMC dysfunction have been identified, biogenic mechanisms of these miRNAs have not been clearly elucidated under pathological conditions. We previously showed that NF-κB is crucially involved in expression of the miR-155-5p host gene MIR155HG by binding to its promoter at the 1150-nt region upstream of the transcription start site (14). Data from this study demonstrate that miR-155-5p inhibited PKG1 expression and subsequently stimulated VSMC phenotypic switching and vascular dysfunction. Collectively, these findings suggest that NF-κB plays an important role in the pathogenesis of inflammatory cardiovascular diseases by negatively regulating PKG1 expression through biogenesis of miR-155-5p. However, miR-155-5p appears to play a conflicting role, either as pro- or anti-atherogenic, in the pathogenesis of atherosclerosis (44). Recently, advanced studies have clearly demonstrated that this miRNA is pro-atherogenic in a high fat–induced atherosclerosis model and a vascular injury model using miR-155−/− mice (45, 46), although they did not identify the target genes. Although miR-155-15 can target multiple mRNAs, we propose that it may play an important role in VSMC dysfunction by suppressing PKG1 expression in inflammatory conditions.
Taken together, this study puts forward compelling evidence that NF-κB–responsive miR-155-5p is a novel regulator of TNFα-induced VSMC phenotypic switching and vascular dysregulation by down-regulating expression levels of PKG1, a major downstream target of the NO/cGMP pathway. Our data provide a possible mechanistic explanation for the pathogenic role of inflammatory cytokines, including TNFα, in the pathogenesis of atherosclerosis and hypertension, which is likely due to VSMC dysfunction through the NF-κB/miR-155-5p/PKG1 signaling pathway. Furthermore, these findings strongly suggest that NF-κB–responsive miR-155-5p may be a new therapeutic target for human inflammatory vascular diseases, including atherosclerosis, hypertension, and preeclampsia.
Experimental procedures
Materials
Smooth muscle cell culture media, supplements, and poly-l-lysine were purchased from ScienCell Research Laboratories (San Diego, CA). Lipofectamine RNAiMAX and Lipofectamine 3000 were obtained from Invitrogen. The following antibodies were used in this study: antibodies against α-SMA (sc-130616; 1:3000), p21 (sc-6246; 1:1000), and Rb (sc-102; 1:1000) from Santa Cruz Biotechnology, Inc.; PKG1 (ADI-KAP-PK005; 1:1000) from Enzo Life Sciences (Farmingdale, NY); calponin (ab46794; 1:5000), SM22α (ab14106; 1:5000), and IKKβ (ab32135; 1:1000) from Abcam (Cambridge, MA); MYPT1 (catalog no. 8574; 1:1000), p-MYPT (Thr-696, catalog no. 5163; 1:1000), VASP (catalog no. 3132; 1:1000), p-VASP (Ser-239, catalog no. 3114; 1:1000), p-Rb (Ser-807/811, catalog no. 9308; 1:1000), MLC (catalog no. 8505; 1:1000), and p-MLC (catalog no. 3674; 1:1000) from Cell Signaling Technology (Danvers, MA); and PECAM-1 (catalog no. 550274; 1:200) from BD Bioscience (San Jose, CA). miR-155-5p mimic (MSY0000646), control miRNA (1027281), miR-155-5p inhibitor (MIN0000646), miRNA inhibitor control (1027271), miR-155-5p PCR primers (MS00031486), miScript SYBR Green PCR kit, and miRNeasy minikit were purchased from Qiagen (Hilden, Germany). Collagenase type 2, elastase, and soybean trypsin inhibitor were obtained from Worthington, and TNFα, IL-1β, and IL-6 were purchased from R&D Systems (Minneapolis, MN). Hanks' balanced salt solution (HBSS) was purchased from Gibco. Control siRNA (sc-37007), NF-κB p65 siRNA (sc-29410), IKKβ siRNA (sc-35644), control siRNA (SN-1003), and PKG1 siRNA (mixture containing equal amounts of 1121990, 1121993, and 1121997) were obtained from Santa Cruz Biotechnology or Bioneer (Daejeon, Korea). KT 5823 and Rp-8-pCPT-cGMP were purchased from Tocris (Bristol, UK). SNP, acetylcholine, 8-Br-cGMP, propidium iodide (PI), and RNase A were obtained from Sigma-Aldrich.
VSMC culture and treatment
All animal studies were approved and performed in accordance with the guidelines of the institutional animal care and use ethics committee of Kangwon National University (approval KW-171227-1). MASMCs were isolated from aortic vessels of male C57BL/6J mice as described previously (47). In brief, mouse aortic vessels were placed into cold PBS, and fatty tissues were carefully removed under a dissecting microscope. After washing three times with cold PBS, the aortas were incubated with HBSS containing collagenase type 2 (1 mg/ml), elastase (0.744 units/ml), and soybean trypsin inhibitor (1 mg/ml) at 37 °C for 10 min to remove endothelial cells and extracellular matrix proteins. The vessels were washed three times with cold PBS, and the adventitia was removed using sterile forceps under a dissecting microscope. Adventitia-eliminated aortas were washed with cold PBS and incubated again in HBSS containing collagenase type 2, elastase, and soybean trypsin inhibitor at 37 °C for 1 h. After the aortas were dissolved, supernatants were centrifuged at 900 × g for 5 min. The pellets were resuspended in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum and 1% penicillin/streptomycin and then seeded onto 5% gelatin-coated culture dishes. MASMCs were identified by their “spindle-shaped” pattern and further confirmed by double staining using PECAM-1, a specific marker for endothelial cells, and α-SMA, a specific marker for SMCs. All cells stained positive for α-SMA, but not for PECAM-1. HASMCs (catalog no. 6110, ScienCell, San Diego, CA) were grown in smooth muscle cell culture medium supplemented with 10 ml of fetal bovine serum, 5 ml of smooth muscle cell growth supplement, 5 ml of penicillin/streptomycin solution at 37 °C in a humidified CO2 incubator and used at passages 3–5. VSMCs were seeded into 6-well plates coated with poly-l-lysine (15 μg/ml) at a density of 2 × 105 cells/well and maintained for 1 day. Subsequently, the cells were further cultured in serum-free medium for 24 h and transfected with 80 nm control miRNA, miR-155 mimic, or miR-155 inhibitor and 80 nm siRNAs in Opti-MEM reduced serum medium using Lipofectamine RNAiMAX according to the manufacturer's instructions. After a 24-h incubation, cells were stimulated with TNFα (10 ng/ml) for 48 h.
Reporter gene assay
HASMCs were transfected with 1 μg of psiCHECK-2-human PRKG1 3′-UTR reporter constructs containing either complementary sequence (WT, ∼0.8 kb; nt 2909–3746) for miR-155-5p or its mutant (Fig. S2B) or empty vectors using Lipofectamine 3000. After a 24-h incubation, cells were stimulated with TNFα (10 ng/ml) for 48 h. Reporter gene activity was assayed by a Dual-Luciferase reporter assay kit (Promega, Madison, WI).
Quantitative real-time PCR (qRT-PCR) analysis
Total miRNAs were isolated from HASMCs using a miRNeasy minikit. cDNAs were prepared from 1 μg of miRNAs using a miScript II RT Kit. qRT-PCR was performed with the miScript SYBR Green PCR kit according to the manufacturer's instructions. miR-155-5p levels were analyzed by the miScript primer assay with miR-155-5p–specific and universal primers and normalized to SNORD-95. Total mRNAs were also isolated from cells or tissues using TRIzol reagent, and the mRNA levels of target genes were determined and quantitated by qRT-PCR using their specific primers and normalized to glyceraldehyde-3-phosphate dehydrogenase (48). The primers used in this study are presented in Table S1.
Western blot analysis
HASMCs were suspended in radioimmune precipitation assay buffer (50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 1% Nonidet P-40, 0.5% deoxycholic acid, 0.1% SDS) and incubated on ice for 30 min for complete cell lysis. Aortic vessels were homogenized in radioimmune precipitation assay buffer using a BioMasher-II homogenizer (Optima, Tokyo, Japan). Cell and tissue debris was removed by centrifugation at 12,000 × g for 15 min. Lysates (30 μg of protein) were separated by SDS-PAGE, and target protein levels were determined by Western blot analysis (49).
FACS analysis
HASMCs were transfected with miRNA analogues and treated with TNFα (10 ng/ml) for 48 h, followed by stimulation with or without 8-Br-cGMP (100 μm) for 12 h. Cells were harvested with 1 ml of trypsin-EDTA and centrifuged at 900 × g for 5 min at room temperature. Cell pellets were fixed with 70% ethanol for 12 h at 4 °C and washed with PBS at 900 × g for 5 min. Cells were resuspended in 0.3 ml of PBS containing 20 μg/ml RNase A and mixed with 3 μl of PI (5 mg/ml). The solution was incubated at 37 °C for 30 min. DNA fluorescence of nuclei was measured with a FACScan flow cytometer (17).
Phalloidin immunocytochemistry
HASMCs were transfected with miRNA analogues and stimulated with or without TNFα (10 ng/ml) for 48 h, followed by treatment with or without 8-Br-cGMP (100 μm) for 24 h. The cells were fixed in 3.7% formaldehyde for 30 min at room temperature, washed gently, and permeabilized with Triton X-100, followed by incubation with Alexa Fluor 488 phalloidin (Thermo Fisher Scientific). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) dihydrochloride (Thermo Fisher Scientific). Images were obtained using a confocal laser microscope.
VSMC proliferation assay
HASMCs were transfected with miRNA analogues and stimulated with TNFα (10 ng/ml) for 48 h, followed by stimulation with 8-Br-cGMP (100 μm) for 12 h. Cells were incubated with [3H]thymidine (1 μCi/ml; Amersham Biosciences, Alyesbury, UK) for 6 h. The level of 3H-labeled DNA was determined after TCA precipitation using a liquid scintillation counter (50).
VSMC wound migration assay
After HASMCs were stimulated TNFα (10 ng/ml) for 48 h, a linear wound was then gently introduced in the center of the cell monolayer using a SPLScarTM scratcher (SPL Life Sciences, Pocheon, Korea). Cells were then subjected to stimulation with or without 8-Br-cGMP at a final concentration of 100 μm for an additional 24 h, and images were captured using an Olympus IX71 microscope equipped with a digital camera (Canon Inc., Tokyo, Japan). The wound width was calculated as the average distance between the edges of the scratch using ImageJ software (National Institutes of Health, Bethesda, MD).
Vascular tension assay
Male C57BL/6J mice were anesthetized via inhalation of isoflurane, and the thoracic aortic vessels were rapidly removed. Each aorta was placed on ice-cold oxygenated Krebs–Ringer bicarbonate solution (118.3 mm NaCl, 4.7 mm KCl, 1.2 mm MgSO4, 1.2 mm KH2PO4, 1.6 mm CaCl2, 25 mm NaHCO3, 11.1 mm glucose), and the endothelial layers of vessels were removed physically by gentle rubbing as described previously (13). The vessels were cut into 1.5-mm rings and transfected with miRNA analogues (80 nm) in Opti-MEM reduced serum medium using Lipofectamine RNAiMAX. The vessel rings were incubated in Dulbecco's modified Eagle's medium with or without TNFα (10 ng/ml) for 48 h, suspended between two wire stirrups (150 μm) in a myograph (Multi Myograph System DMT-620) containing Krebs Ringer solution (95% O2, 5% CO2, pH 7.4, 37 °C). One stirrup was connected to a three-dimensional micromanipulator, and the other stirrup was connected to a force transducer. Relaxant responses of intact (as a positive control) and de-endothelialized vessel rings to different concentrations of acetylcholine, SNP, and 8-Br-cGMP were assessed after preconstriction with a single dose of phenylephrine (10−5 m).
Statistical analysis
All quantitative data are expressed as mean ± S.D. from three independent experiments performed in triplicate, except for vascular tone which is presented as mean ± S.E. Statistical analyses were performed using GraphPad Prism version 6.0 for Windows (GraphPad Software). Statistical significance was determined using one-way analysis of variance or a two-tailed Student's t test (paired), depending on the number of experimental groups analyzed. Significance was established at a p value < 0.05.
Author contributions
S. C. designed and performed experiments, analyzed the data, and wrote the paper. M. P., J. K., W. P., S. K., and D.-K. L. isolated and cultured MASMCs, performed experiments, and analyzed the data. J. Y. H., J. C., M.-H. W., S. R., K.-S. H., and Y.-G. K. provided either experimental ideas or technical support. Y.-M. K. designed and supervised the study and wrote the paper. All authors reviewed and approved the final version of the manuscript.
Supplementary Material
Acknowledgment
We thank the Central Research Laboratory of Kangwon National University for use of a liquid scintillation counter (Packard, Tri-Carb 2810TR).
This work was supported by the National Research Foundation of Korea (NRF) Grant 2016M3A9B6903103 funded by the Korea government (MSIP) and also a 2017 Research Grant from Kangwon National University (Grant 520170431). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Table S1 and Figs. S1 and S2.
Please note that the JBC is not responsible for the long-term archiving and maintenance of this site or any other third party hosted site.
- eNOS
- endothelial nitric-oxide synthase
- DAPI
- 4′,6-diamidino-2-phenylindole
- HASMC
- human aortic smooth muscle cell
- IL
- interleukin
- MASMC
- mouse aortic smooth muscle cell
- miR
- microRNA
- MLC
- myosin light chain
- MYPT1
- myosin light chain phosphatase 1
- NF-κB
- nuclear factor-κB
- PKG
- cGMP-dependent protein kinase
- qRT-PCR
- quantitative real-time polymerase chain reaction
- Rb
- retinoblastoma protein
- SNP
- sodium nitroprusside
- TNFα
- tumor necrosis factor-α
- VASP
- vasodilator-stimulated phosphoprotein
- VSMC
- vascular smooth muscle cell
- p-
- phosphorylated
- HBSS
- Hanks' balanced salt solution
- PI
- propidium iodide
- nt
- nucleotide(s)
- IKKβ
- IκB kinase β.
References
- 1. Vanhoutte P. M., Zhao Y., Xu A., and Leung S. W. (2016) Thirty years of saying NO: sources, fate, actions, and misfortunes of the endothelium-derived vasodilator mediator. Circ. Res. 119, 375–396 10.1161/CIRCRESAHA.116.306531 [DOI] [PubMed] [Google Scholar]
- 2. Martín-Sanchez P., Luengo A., Griera M., Orea M. J., López-Olaneta M., Chiloeches A., Lara-Pezzi E., de Frutos S., Rodríguez-Puyol M., Calleros L., and Rodríguez-Puyol D. (2018) H-ras deletion protects against angiotensin II-induced arterial hypertension and cardiac remodeling through protein kinase G-Iβ pathway activation. FASEB J. 32, 920–934 10.1096/fj.201700134RRRR [DOI] [PubMed] [Google Scholar]
- 3. Kim J. Y., Yang H. M., Lee J. E., Kim B. K., Jin S., Lee J., Park K. W., Cho H. J., Kwon Y. W., Lee H. Y., Kang H. J., Oh B. H., Park Y. B., and Kim H. S. (2016) Activation of protein kinase G (PKG) reduces neointimal hyperplasia, inhibits platelet aggregation, and facilitates re-endothelialization. Sci. Rep. 6, 36979 10.1038/srep36979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nguyen T., Lin S., Pantho A. F., Kohl-Thomas B. M., Beeram M. R., Zawieja D. C., Kuehl T. J., and Uddin M. N. (2015) Hyperglycemia down-regulates cGMP-dependent protein kinase I expression in first trimester cytotrophoblast cells. Mol. Cell Biochem. 405, 81–88 10.1007/s11010-015-2398-y [DOI] [PubMed] [Google Scholar]
- 5. Rzucidlo E. M., Martin K. A., and Powell R. J. (2007) Regulation of vascular smooth muscle cell differentiation. J. Vasc. Surg. 45, A25–A32 10.1016/j.jvs.2007.03.001 [DOI] [PubMed] [Google Scholar]
- 6. Davis-Dusenbery B. N., Wu C., and Hata A. (2011) Micromanaging vascular smooth muscle cell differentiation and phenotypic modulation. Arterioscler. Thromb. Vasc. Biol. 31, 2370–2377 10.1161/ATVBAHA.111.226670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Browner N. C., Sellak H., and Lincoln T. M. (2004) Downregulation of cGMP-dependent protein kinase expression by inflammatory cytokines in vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 287, C88–C96 10.1152/ajpcell.00039.2004 [DOI] [PubMed] [Google Scholar]
- 8. Small E. M., Frost R. J., and Olson E. N. (2010) MicroRNAs add a new dimension to cardiovascular disease. Circulation 121, 1022–1032 10.1161/CIRCULATIONAHA.109.889048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang F., Chen Q., He S., Yang M., Maguire E. M., An W., Afzal T. A., Luong L. A., Zhang L., and Xiao Q. (2018) miR-22 is a novel mediator of vascular smooth muscle cell phenotypic modulation and neointima formation. Circulation 137, 1824–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Torella D., Iaconetti C., Catalucci D., Ellison G. M., Leone A., Waring C. D., Bochicchio A., Vicinanza C., Aquila I., Curcio A., Condorelli G., and Indolfi C. (2011) MicroRNA-133 controls vascular smooth muscle cell phenotypic switch in vitro and vascular remodeling in vivo. Circ. Res. 109, 880–893 10.1161/CIRCRESAHA.111.240150 [DOI] [PubMed] [Google Scholar]
- 11. Cheng Y., Liu X., Yang J., Lin Y., Xu D. Z., Lu Q., Deitch E. A., Huo Y., Delphin E. S., and Zhang C. (2009) MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ. Res. 105, 158–166 10.1161/CIRCRESAHA.109.197517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang J., Yan C. H., Li Y., Xu K., Tian X. X., Peng C. F., Tao J., Sun M. Y., and Han Y. L. (2013) MicroRNA-31 controls phenotypic modulation of human vascular smooth muscle cells by regulating its target gene cellular repressor of E1A-stimulated genes. Exp. Cell Res. 319, 1165–1175 10.1016/j.yexcr.2013.03.010 [DOI] [PubMed] [Google Scholar]
- 13. Kim J., Lee K. S., Kim J. H., Lee D. K., Park M., Choi S., Park W., Kim S., Choi Y. K., Hwang J. Y., Choe J., Won M. H., Jeoung D., Lee H., Ryoo S., et al. (2017) Aspirin prevents TNF-α-induced endothelial cell dysfunction by regulating the NF-κB-dependent miR-155/eNOS pathway: role of a miR-155/eNOS axis in preeclampsia. Free Radic. Biol. Med. 104, 185–198 10.1016/j.freeradbiomed.2017.01.010 [DOI] [PubMed] [Google Scholar]
- 14. Lee K. S., Kim J., Kwak S. N., Lee K. S., Lee D. K., Ha K. S., Won M. H., Jeoung D., Lee H., Kwon Y. G., and Kim Y. M. (2014) Functional role of NF-κB in expression of human endothelial nitric oxide synthase. Biochem. Biophys. Res. Commun. 448, 101–107 10.1016/j.bbrc.2014.04.079 [DOI] [PubMed] [Google Scholar]
- 15. Chen L., Daum G., Chitaley K., Coats S. A., Bowen-Pope D. F., Eigenthaler M., Thumati N. R., Walter U., and Clowes A. W. (2004) Vasodilator-stimulated phosphoprotein regulates proliferation and growth inhibition by nitric oxide in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 24, 1403–1408 10.1161/01.ATV.0000134705.39654.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kitazawa T., Semba S., Huh Y. H., Kitazawa K., and Eto M. (2009) Nitric oxide-induced biphasic mechanism of vascular relaxation via dephosphorylation of CPI-17 and MYPT1. J. Physiol. 587, 3587–3603 10.1113/jphysiol.2009.172189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gao Y., Portugal A. D., Liu J., Negash S., Zhou W., Tian J., Xiang R., Longo L. D., and Raj J. U. (2008) Preservation of cGMP-induced relaxation of pulmonary veins of fetal lambs exposed to chronic high altitude hypoxia: role of PKG and Rho kinase. Am. J. Physiol. Lung Cell Mol. Physiol. 295, L889–L896 10.1152/ajplung.00463.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Winther M. P., Kanters E., Kraal G., and Hofker M. H. (2005) Nuclear factor κB signaling in atherogenesis. Arterioscler. Thromb. Vasc. Biol. 25, 904–914 10.1161/01.ATV.0000160340.72641.87 [DOI] [PubMed] [Google Scholar]
- 19. Shaw J., Tang Z., Schneider H., Saljé K., Hansson S. R., and Guller S. (2016) Inflammatory processes are specifically enhanced in endothelial cells by placental-derived TNF-α: implications in preeclampsia (PE). Placenta 43, 1–8 10.1016/j.placenta.2016.04.015 [DOI] [PubMed] [Google Scholar]
- 20. Naya M., Tsukamoto T., Morita K., Katoh C., Furumoto T., Fujii S., Tamaki N., and Tsutsui H. (2007) Plasma interleukin-6 and tumor necrosis factor-α can predict coronary endothelial dysfunction in hypertensive patients. Hypertens. Res. 30, 541–548 10.1291/hypres.30.541 [DOI] [PubMed] [Google Scholar]
- 21. Brand K., Page S., Rogler G., Bartsch A., Brandl R., Knuechel R., Page M., Kaltschmidt C., Baeuerle P. A., and Neumeier D. (1996) Activated transcription factor nuclear factor-κB is present in the atherosclerotic lesion. J. Clin. Invest. 97, 1715–1722 10.1172/JCI118598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ren J., Liu Z., Wang Q., Giles J., Greenberg J., Sheibani N., Kent K. C., and Liu B. (2016) Andrographolide ameliorates abdominal aortic aneurysm progression by inhibiting inflammatory cell infiltration through downregulation of cytokine and integrin expression. J. Pharmacol. Exp. Ther. 356, 137–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peeters A. C., Netea M. G., Janssen M. C., Kullberg B. J., Van der Meer J. W., and Thien T. (2001) Pro-inflammatory cytokines in patients with essential hypertension. Eur. J. Clin. Invest. 31, 31–36 10.1046/j.1365-2362.2001.00743.x [DOI] [PubMed] [Google Scholar]
- 24. Ait-Oufella H., Taleb S., Mallat Z., and Tedgui A. (2011) Recent advances on the role of cytokines in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 31, 969–979 10.1161/ATVBAHA.110.207415 [DOI] [PubMed] [Google Scholar]
- 25. Morisaki N., Xu Q. P., Koshikawa T., Saito Y., Yoshida S., and Ueda S. (1993) Tumour necrosis factor-α can modulate the phenotype of aortic smooth muscle cells. Scand. J. Clin. Lab. Invest. 53, 347–352 10.3109/00365519309086626 [DOI] [PubMed] [Google Scholar]
- 26. Brånén L., Hovgaard L., Nitulescu M., Bengtsson E., Nilsson J., and Jovinge S. (2004) Inhibition of tumor necrosis factor-α reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol. 24, 2137–2142 10.1161/01.ATV.0000143933.20616.1b [DOI] [PubMed] [Google Scholar]
- 27. Elks C. M., Mariappan N., Haque M., Guggilam A., Majid D. S., and Francis J. (2009) Chronic NF-{κ}5 blockade reduces cytosolic and mitochondrial oxidative stress and attenuates renal injury and hypertension in SHR. Am. J. Physiol. Renal Physiol. 296, F298–F305 10.1152/ajprenal.90628.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Feil R., Lohmann S. M., de Jonge H., Walter U., and Hofmann F. (2003) Cyclic GMP-dependent protein kinases and the cardiovascular system: insights from genetically modified mice. Circ. Res. 93, 907–916 10.1161/01.RES.0000100390.68771.CC [DOI] [PubMed] [Google Scholar]
- 29. Anderson P. G., Boerth N. J., Liu M., McNamara D. B., Cornwell T. L., and Lincoln T. M. (2000) Cyclic GMP-dependent protein kinase expression in coronary arterial smooth muscle in response to balloon catheter injury. Arterioscler. Thromb. Vasc. Biol. 20, 2192–2197 10.1161/01.ATV.20.10.2192 [DOI] [PubMed] [Google Scholar]
- 30. Sinnaeve P., Chiche J. D., Gillijns H., Van Pelt N., Wirthlin D., Van De Werf F., Collen D., Bloch K. D., and Janssens S. (2002) Overexpression of a constitutively active protein kinase G mutant reduces neointima formation and in-stent restenosis. Circulation 105, 2911–2916 10.1161/01.CIR.0000018169.59205.CA [DOI] [PubMed] [Google Scholar]
- 31. Pfeifer A., Klatt P., Massberg S., Ny L., Sausbier M., Hirneiss C., Wang G. X., Korth M., Aszódi A., Andersson K. E., Krombach F., Mayerhofer A., Ruth P., Fässler R., and Hofmann F. (1998) Defective smooth muscle regulation in cGMP kinase I-deficient mice. EMBO J. 17, 3045–3051 10.1093/emboj/17.11.3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jovinge S., Hultgårdh-Nilsson A., Regnström J., and Nilsson J. (1997) Tumor necrosis factor-α activates smooth muscle cell migration in culture and is expressed in the balloon-injured rat aorta. Arterioscler. Thromb. Vasc. Biol. 17, 490–497 10.1161/01.ATV.17.3.490 [DOI] [PubMed] [Google Scholar]
- 33. Peppel K., Zhang L., Orman E. S., Hagen P. O., Amalfitano A., Brian L., and Freedman N. J. (2005) Activation of vascular smooth muscle cells by TNF and PDGF: overlapping and complementary signal transduction mechanisms. Cardiovasc. Res. 65, 674–682 10.1016/j.cardiores.2004.10.031 [DOI] [PubMed] [Google Scholar]
- 34. Yoshida T., Yamashita M., Horimai C., and Hayashi M. (2013) Smooth muscle-selective inhibition of nuclear factor-κB attenuates smooth muscle phenotypic switching and neointima formation following vascular injury. J. Am. Heart Assoc. 2, e000230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wooldridge A. A., MacDonald J. A., Erdodi F., Ma C., Borman M. A., Hartshorne D. J., and Haystead T. A. (2004) Smooth muscle phosphatase is regulated in vivo by exclusion of phosphorylation of threonine 696 of MYPT1 by phosphorylation of serine 695 in response to cyclic nucleotides. J. Biol. Chem. 279, 34496–34504 10.1074/jbc.M405957200 [DOI] [PubMed] [Google Scholar]
- 36. Ishida A., Sasaguri T., Miwa Y., Kosaka C., Taba Y., and Abumiya T. (1999) Tumor suppressor p53 but not cGMP mediates NO-induced expression of p21(Waf1/Cip1/Sdi1) in vascular smooth muscle cells. Mol. Pharmacol. 56, 938–946 10.1124/mol.56.5.938 [DOI] [PubMed] [Google Scholar]
- 37. Wang S., and Li Y. (2009) Expression of constitutively active cGMP-dependent protein kinase inhibits glucose-induced vascular smooth muscle cell proliferation. Am. J. Physiol. Heart Circ. Physiol. 297, H2075–H2083 10.1152/ajpheart.00521.2009 [DOI] [PubMed] [Google Scholar]
- 38. Reinhard M., Jarchau T., and Walter U. (2001) Actin-based motility: stop and go with Ena/VASP proteins. Trends Biochem. Sci. 26, 243–249 10.1016/S0968-0004(00)01785-0 [DOI] [PubMed] [Google Scholar]
- 39. Chen J., Yin H., Jiang Y., Radhakrishnan S. K., Huang Z. P., Li J., Shi Z., Kilsdonk E. P., Gui Y., Wang D. Z., and Zheng X. L. (2011) Induction of microRNA-1 by myocardin in smooth muscle cells inhibits cell proliferation. Arterioscler. Thromb. Vasc. Biol. 31, 368–375 10.1161/ATVBAHA.110.218149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ji R., Cheng Y., Yue J., Yang J., Liu X., Chen H., Dean D. B., and Zhang C. (2007) MicroRNA expression signature and antisense-mediated depletion reveal an essential role of microRNA in vascular neointimal lesion formation. Circ. Res. 100, 1579–1588 10.1161/CIRCRESAHA.106.141986 [DOI] [PubMed] [Google Scholar]
- 41. Chen Q., Yang F., Guo M., Wen G., Zhang C., Luong le A., Zhu J., Xiao Q., and Zhang L. (2015) miRNA-34a reduces neointima formation through inhibiting smooth muscle cell proliferation and migration. J. Mol. Cell Cardiol. 89, 75–86 10.1016/j.yjmcc.2015.10.017 [DOI] [PubMed] [Google Scholar]
- 42. Afzal T. A., Luong L. A., Chen D., Zhang C., Yang F., Chen Q., An W., Wilkes E., Yashiro K., Cutillas P. R., Zhang L., and Xiao Q. (2016) NCK associated protein 1 modulated by miRNA-214 determines vascular smooth muscle cell migration, proliferation, and neointima hyperplasia. J. Am. Heart Assoc. 5, e004629 10.1161/JAHA.116.004629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li P., Zhu N., Yi B., Wang N., Chen M., You X., Zhao X., Solomides C. C., Qin Y., and Sun J. (2013) MicroRNA-663 regulates human vascular smooth muscle cell phenotypic switch and vascular neointimal formation. Circ. Res. 113, 1117–1127 10.1161/CIRCRESAHA.113.301306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ma X., Ma C., and Zheng X. (2013) MicroRNA-155 in the pathogenesis of atherosclerosis: a conflicting role? Heart Lung Circ. 22, 811–818 10.1016/j.hlc.2013.05.651 [DOI] [PubMed] [Google Scholar]
- 45. Virtue A., Johnson C., Lopez-Pastraña J., Shao Y., Fu H., Li X., Li Y. F., Yin Y., Mai J., Rizzo V., Tordoff M., Bagi Z., Shan H., Jiang X., Wang H., and Yang X. F. (2017) MicroRNA-155 deficiency leads to decreased atherosclerosis, increased white adipose tissue obesity, and non-alcoholic fatty liver disease: a novel mouse model of obesity paradox. J. Biol. Chem. 292, 1267–1287 10.1074/jbc.M116.739839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sun Y., Wang K., Ye P., Wu J., Ren L., Zhang A., Huang X., Deng P., Wu C., Yue Z., Chen Z., Ding X., Chen J., and Xia J. (2016) MicroRNA-155 promotes the directional migration of resident smooth muscle progenitor cells by regulating monocyte chemoattractant protein 1 in transplant arteriosclerosis. Arterioscler. Thromb. Vasc. Biol. 36, 1230–1239 10.1161/ATVBAHA.115.306691 [DOI] [PubMed] [Google Scholar]
- 47. Cherepanova O. A., Gomez D., Shankman L. S., Swiatlowska P., Williams J., Sarmento O. F., Alencar G. F., Hess D. L., Bevard M. H., Greene E. S., Murgai M., Turner S. D., Geng Y. J., Bekiranov S., Connelly J. J., et al. (2016) Activation of the pluripotency factor OCT4 in smooth muscle cells is atheroprotective. Nat. Med. 22, 657–665 10.1038/nm.4109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yang L., Gao L., Nickel T., Yang J., Zhou J., Gilbertsen A., Geng Z., Johnson C., Young B., Henke C., Gourley G. R., and Zhang J. (2017) Lactate promotes synthetic phenotype in vascular smooth muscle cells. Circ. Res. 121, 1251–1262 10.1161/CIRCRESAHA.117.311819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Choi S., Kim J., Kim J. H., Lee D. K., Park W., Park M., Kim S., Hwang J. Y., Won M. H., Choi Y. K., Ryoo S., Ha K. S., Kwon Y. G., and Kim Y. M. (2017) Carbon monoxide prevents TNF-α-induced eNOS downregulation by inhibiting NF-κB-responsive miR-155-5p biogenesis. Exp. Mol. Med. 49, e403 10.1038/emm.2017.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Baek Y. Y., Cho D. H., Choe J., Lee H., Jeoung D., Ha K. S., Won M. H., Kwon Y. G., and Kim Y. M. (2012) Extracellular taurine induces angiogenesis by activating ERK-, Akt-, and FAK-dependent signal pathways. Eur. J. Pharmacol. 674, 188–199 10.1016/j.ejphar.2011.11.022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.