Abstract
Introduction
Group-based cognitive stimulation is the only nonpharmacologic intervention recommended by the UK National Institute for Clinical and Health Excellence for people with dementia. The potential of technology to extend the availability of group-based cognitive stimulation has not been tested.
Methods
One hundred sixty-one people with dementia participated in an eight-session group activity using Computer Interactive Reminiscence and Conversation Aid (CIRCA). Cognition, quality of life, and general health were assessed before intervention, postintervention, and 3 months later.
Results
There was a significant improvement in cognition and quality of life at the end of the CIRCA group intervention, which was further improved at 3-month follow-up.
Discussion
CIRCA group sessions improved cognition and quality of life similar to group-based cognitive stimulation approved by the National Institute for Clinical and Health Excellence. These benefits were maintained at 3-month follow-up. The data confirm the potential of CIRCA, which can be populated with different cultural and language contents for different user groups.
Keywords: Nonpharmacologic, Cognition, Quality of life, Technology, Groups
1. Introduction
Nonpharmacologic interventions that can improve the lives of people with dementia are keenly sought across the world. To date only one nonpharmacologic intervention—group-based cognitive stimulation—has been recommended for dementia by the UK National Institute for Health and Care Excellence. This is based on the work of Spector et al. [1] who demonstrated that cognitive stimulation therapy (CST) brought an improvement in cognitive function and quality of life comparable to existing dementia drugs in a study with 97 people with dementia living in care homes. In their study they reported a 0.9 increase in the Mini-Mental State Examination (MMSE) score, with 50% of participants showing improvement in cognitive function. The success of CST has created a demand for scalable nonpharmacologic approaches targeting cognitive function.
Computer Interactive Reminiscence and Conversation Aid (CIRCA) is an interactive multimedia (photographs, video, and music) application developed to support and promote communication between people with dementia and caregivers [2]. CIRCA was developed to enhance speech production and recall from long-term memory, while minimizing the impact of working memory impairment [3]. It is delivered on a touch screen to avoid the need for a mouse, keyboard, prior experience, or intensive training. In dyads comprising a person with dementia and a caregiver, CIRCA provides the opportunity for people with dementia to make independent choices, lead the conversation, and engage as an equal partner [4]. The validation of CIRCA–British Columbia demonstrated the utility of this approach across cultural and geographic boundaries [5].
Shared CIRCA sessions encourage caregivers to view people with dementia in a new light [6] and increase their feelings of competence in caregiving [7], while improving caregiving relationships [8].
This study set out to test the impact of CIRCA as a group-based activity for people with dementia. As the only current recommended nonpharmacologic intervention for dementia, a key aim was to compare the effectiveness of CIRCA as a group intervention with CST. The design [9] was therefore informed by the Spector et al. [1] study of CST, which used a program of 14 × 45-minute sessions, twice per week for 7 weeks. A subsequent cost-effectiveness review of CST suggested it could be more cost-effective than treatment as usual [10]. In addition to the systematic study of CIRCA as a group intervention, the present study was also designed to compare a new, web-based version, CIRCA-WB, against the existing stand-alone version.
2. Method
2.1. Design
The study used a within-participants design where all participants received the intervention.
2.2. Setting
Data collection took place across 11 sites in Sheffield, UK, offered by the local branch of the Alzheimer's Society and Sheffcare, a not-for-profit care provider. The sites included one Alzheimer's Society day program, one Sheffcare day program, and nine Sheffcare care homes.
2.3. Participants
2.3.1. Inclusion criteria
Participants were eligible if they
-
•
met the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition criteria for a neurocognitive disorder of any type [11];
-
•
could speak English and had some ability to communicate and understand verbal communication;
-
•
attended a day program for people with dementia or lived in a care home;
-
•
were able to see and hear well enough to participate in the group;
-
•
did not have any major behavioral difficulties that would affect group participation;
-
•
could engage in group activity for at least 60 minutes, and
-
•
did not have major physical illness or disability, which could affect participation.
All people with dementia who were attending the Sheffield branch of the Alzheimer's Society day programs, Sheffcare day programs, and Sheffcare care homes who met the inclusion criteria were invited to take part.
2.4. Power analysis
Power calculation before the study suggested that, with power set at 0.90 and 5% significance with two-tails, 170 participants with dementia would be required to detect an effect size of 0.25 or greater. To allow for attrition it was decided to aim for 180 participants.
2.5. Procedure
2.5.1. Recruitment
Participants who met the eligibility criteria were contacted to discuss the study. Participant information sheets were distributed to people who expressed an interest in participating through the Sheffield branch of the Alzheimer's Society and Sheffcare. For those who agreed to participate, written informed consent was obtained, in accordance with the Mental Capacity Act [12].
2.5.2. Intervention
CIRCA comprises a multimedia database containing generic photographs, music, and video clips accessed through a touch screen interface. The original stand-alone version contains approximately 2000 generic items across six categories: childhood, entertainment, everyday life, people and events, recreation, and sport. Three themes are available at any time and the presentation of the contents is randomized to maintain variety. The contents are preloaded into a large touch screen computer and a CIRCA session loads when the computer is started.
CIRCA-WB is a replication of CIRCA developed as a web-based service and as such has the same interaction and navigation with the contents. CIRCA-WB has a centralized database, which allows designated curators in different locales to relabel existing content so as to make it relevant to their users (by, for instance, translating captions into the local language) or else to insert entirely new content. Photographs are uploaded to CIRCA-WB and music and video clips are stored as links to third-party streaming services.
To examine the effectiveness of CIRCA as a group intervention, the participants were allocated to groups of three to six participants. The first wave of groups all used stand-alone CIRCA as CIRCA-WB was being completed, whereas groups in waves 2 and 3 were assigned to CIRCA or CIRCA-WB. The group intervention ran twice a week for 60 minutes per session over 4 weeks, and each session consisted of a group plus one facilitator from the research team, interacting with CIRCA(-WB). Sessions were designed to be inclusive and consisted of the facilitator launching CIRCA(-WB), asking the group to make selections of what they would like to see or listen to, and using the content displayed as a prompt for group interactions. Each group session was video-recorded.
2.6. Assessments
Three assessments were planned: baseline (1 week before the intervention), 1 week after the final group session, and 3 months later.
2.7. Outcome measures
2.7.1. Cognition
The primary outcome measure was the Addenbrooke's Cognitive Examination-III (ACE-III; [13]). The ACE-III covers five cognitive domains—attention, memory, fluency, language, and visuospatial—and is scored out of 100. The ACE-III cognitive domains have been found to correlate significantly with standardized neuropsychological tests illustrating high levels of sensitivity and validity [13]. To compare the impact of CIRCA groups with CST (which used MMSE) it is possible to derive an MMSE equivalent score using the algorithm [ACE-4.56]/2.72 [14].
2.7.2. Quality of life
The Quality of Life in Alzheimer's disease (QOL-AD) [15] scale was used as a secondary outcome measure. The QOL-AD comprises 13 items covering physical health, energy, mood, living situation, memory, family, marriage, friends, chores, fun, money, self, and life as a whole. QOL-AD can be completed by people with a range of dementia severity. QOL-AD has good internal consistency, validity, and reliability [1] and is recommended by the European consensus on outcome measures for psychosocial interventions in dementia [16].
2.7.3. Health status
The EuroQol five-dimensions scale (EQ-5D) [17], a self-report, brief health and quality of life measure, was included as a further secondary measure. EQ-5D has been widely used, including with people who have dementia [18].
Two researchers were involved in completing the formal assessment and conducting the sessions. Researcher 1 undertook the assessments at baseline, post-CIRCA(-WB), and 3 months later, and researcher 2 facilitated the eight-group sessions and vice versa to ensure the facilitators were blind to the participants' performance on the formal measures.
2.7.4. Ethics
Health Research Authority approval was received and a favorable opinion received from the Sheffield Research Ethics Committee, reference 16/YH/0354.
2.8. Analysis
The performance of CIRCA-WB was evaluated by comparing participant's scores at baseline and postgroup sessions with those from the participants using CIRCA. Repeated measures analysis of variance (RM ANOVA) with pairwise comparisons was used to examine the impact of CIRCA(-WB) groups on the primary outcome measure—ACE-III—by comparing scores at baseline, postintervention, and 3 months later. RM ANOVA with post hoc analyses was also used to examine the impact of CIRCA(-WB) groups on the two secondary outcome measures—QOL-AD and EQ-5D.
3. Results
3.1. Recruitment and attrition
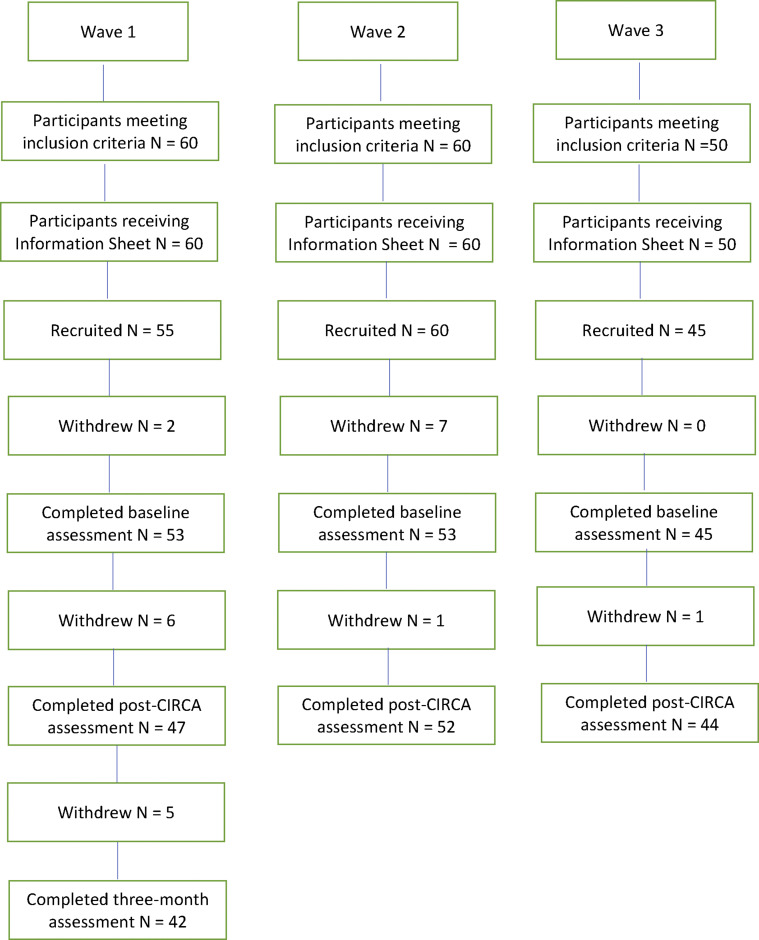
Recruitment took place in three waves resulting in 55 participants in round 1, 60 participants in round 2, and 45 in round 3, giving a total of 160. Of these, 143 were assessed at the end of the CIRCA(-WB) intervention. The other 17 were lost to ill health or death. Because of time and resource constraints it was only possible to complete the 3-month follow-up with the wave 1 participants. Of these, 42 were available for the 3-month follow-up. Fig. 1 displays details of the flow of participants through the research.
Fig. 1.
Flow of participants through the project. Abbreviation: CIRCA, Computer Interactive Reminiscence and Conversation Aid.
To evaluate CIRCA-WB the 143 participants who completed the baseline and postintervention assessment were separated into two groups: CIRCA or CIRCA-WB. Their mean scores are displayed in Table 1. The average age of both groups was more than 80 years, although the participants who used stand-alone CIRCA were significantly older than those who used CIRCA-WB. Both groups contained far more females than males, which is typical among older adults with dementia. There was no significant difference between the two groups on any of the three baseline measures (P > .05) or after the CIRCA(-WB) intervention (P > .05).
Table 1.
Mean scores on three outcome measures at baseline and postintervention for the CIRCA and CIRCA-WB groups
| Measure | CIRCA (N = 58) | CIRCA-WB (N = 85) | Significance |
|---|---|---|---|
| Age | 87.14 | 82.76 | .005 |
| Sex | |||
| Male (%) | 7 (12%) | 18 (21%) | |
| Female (%) | 51 (88%) | 67 (79%) | |
| Baseline | |||
| ACE-III | 46.55 | 46.9 | ns |
| QOL-AD | 31.12 | 32.4 | ns |
| EQ-5D | 62.41 | 64.96 | ns |
| Postintervention | |||
| ACE-III | 49.58 | 48.6 | ns |
| QOL-AD | 32.41 | 32.4 | ns |
| EQ-5D | 64.6 | 64.2 | ns |
Abbreviations: ACE-III, Addenbrooke's Cognitive Examination-III; CIRCA, Computer Interactive Reminiscence and Conversation Aid; CIRCA-WB, web-based version of CIRCA; QOL-AD, Quality of Life in Alzheimer's disease; EQ-5D, EuroQol five-dimensions scale.
As there was no difference between the data from the two CIRCAs, the data were combined for analysis of the impact on cognition, quality of life, and general health. Baseline characteristics of the 143 participants who received the CIRCA(-WB) intervention can be found in Table 2. Very few had previous technology experience and the most were living in care homes.
Table 2.
Baseline scores of 143 participants who completed the intervention
| Characteristics | Total 143 |
|---|---|
| Mean age (y) | 84.43 |
| Sex | |
| Female (%) | 118 (82.5%) |
| Male (%) | 25 (17.5%) |
| Living situation | |
| At home (%) | 23 (16%) |
| Care home (%) | 120 (84%) |
| Previous information and communication technologies experience (%) | 6 (4%) |
3.2. Analysis of outcomes
Mean scores at baseline and at the end of intervention can be found in Table 3. There was a significant improvement in cognition after the CIRCA(-WB) intervention as assessed by an RM ANOVA (F [1, 142] = 7.37, P = .007). The average improvement was 2.24 on ACE-III (for comparison this is equivalent to 0.82 on MMSE calculated using ACE-III to MMSE conversion algorithm [14]). Fifty-nine percent of participants showed an improvement in cognitive function and a further 1.4% maintained their scores. There was a significant increase (+1.0) in the QOL-AD scores post-CIRCA(-WB), (F [1, 142] = 5.19, P = .024), with 58% of the 143 participants improving and a further 5.6% participants maintaining their scores. There was no significant change in general health as measured by the EQ-5D (F [1, 142] = 0.17, P = ns), although 44% had a higher score and 19.5% maintained their score (Table 3).
Table 3.
Mean scores for 143 participants at baseline and postintervention
| Measure | Baseline ACE-III/100 (N = 143) | MMSE equivalent/30 | Post-CIRCA/100 (N = 143) | MMSE equivalent/30 | Change ACE-III | Change MMSE equivalent | % Improve |
Significance |
|---|---|---|---|---|---|---|---|---|
| or same | ||||||||
| ACE-III | 46.76 | 15.51 | 49.0 | 16.33 | +2.24 | +0.82 | 59% | P < .005 |
| 1.4% | ||||||||
| QOL-AD | 31.41 | 32.4 | +1 | 58% | P < .05 | |||
| 5.6% | ||||||||
| EQ-5D | 63.67 | 64.36 | +0.69 | 44% | ns | |||
| 19.5% |
Abbreviations: ACE-III, Addenbrooke's Cognitive Examination-III; CIRCA, Computer Interactive Reminiscence and Conversation Aid; MMSE, Mini-Mental State Examination; EQ-5D, EuroQol five-dimensions scale; QoL-AD, Quality of Life in Alzheimer's disease.
To examine the impact of CIRCA after 3 months, data from the 42 participants from wave 1 who were assessed at all three time points were compared (Table 4). Mauchly's test indicated that the assumption of sphericity was not violated, χ2 (2) = 2.434, P > .05, therefore RM ANOVA was conducted, which revealed that there was a significant difference in cognition across the three assessments (F [2, 41] = 4.66, P = .01). Pairwise comparisons with the Bonferroni correction revealed that there was a significant improvement in cognition from baseline to 3-months follow-up (P = −.02), but not between baseline and postintervention, or postintervention and 3-month follow-up (Table 4). Overall, 25 (59.5%) of the 42 improved their cognitive score and two maintained their cognitive score.
Table 4.
Mean (standard deviation) scores for 42 participants tested at baseline, postintervention, and 3 months later
| Measure | Baseline | Post-CIRCA | 3-mo later |
|---|---|---|---|
| ACE-III | 48.73 | 50.66 | 52.35 |
| QOL-AD | 31.4 | 32.85 | 33.88 |
| EQ-5D | 64.16 | 65.92 | 68.61 |
Abbreviations: ACE-III, Addenbrooke's Cognitive Examination-III; CIRCA, Computer Interactive Reminiscence and Conversation Aid; EQ-5D, EuroQol five-dimensions scale; QoL-AD, Quality of Life in Alzheimer's disease.
Comparison of quality of life as assessed by QOL-AD across the three time points was examined using RM ANOVA, with pairwise comparisons as Mauchly's test indicated that the assumption of sphericity had not been violated, χ2(2) = 0.407, P > .05, revealed a significant difference across the three time points (F [2, 41] = 4.133, P = .01). Post hoc t tests revealed that there was a significant improvement in quality of life from baseline to 3-month follow-up (P = .015) but not between baseline and the end of the groups, and end of groups and 3-month follow-up (Table 4). This was seen in an average improvement in QOL-AD score of 2.47 from baseline to 3-month follow-up.
An RM ANOVA of the EQ-5D scores revealed that there was no significant difference across the three assessments (F [2, 41] = 1.16, P = .31 = ns), although there was an upward trend in the scores (Table 4). By the 3-month follow-up these scores had increased by an average of 4.45 points with 24 (57%) participants showing either improved [19] or maintained [5] scores, respectively.
4. Discussion
An eight-session group activity using CIRCA(-WB) significantly improved both cognitive function and quality of life of people with dementia. There was a nonsignificant improvement in self-reported health, with 63% reporting higher scores. The findings of improved cognition and quality of life compare favorably with those found with CST [1], a group-based intervention with a similar impact to existing dementia drugs. These benefits were achieved with an 8 × 60-minute CIRCA group intervention compared with the 14 × 45-minute group sessions for CST. In addition, the benefits from the CIRCA group intervention were maintained at 3 months after the intervention, which was not measured with CST.
In respect of implementing CIRCA, this activity has previously been shown to be an effective conversation support for one-to-one interactions [4], [20]. Previous research has also demonstrated that CIRCA is easy for caregivers to set up and utilize [3], which engenders their feelings of competence and satisfaction when working with people with dementia [8]. Using CIRCA has also previously demonstrated benefit to caregiving relationships [8] and this may be why the improvements in cognition and quality of life were maintained after 3 months. Most participants (96%) had no previous information and communication technologies experience, but this was not a barrier to their interaction with CIRCA. Analysis of the video-recordings of each group session is currently underway to further examine enjoyment, engagement, and group interaction during the CIRCA(-WB) group activity.
These findings confirm the potential of CIRCA(-WB) as a group activity for people with dementia. This is important for real-world implementation as group interventions have the advantage of one facilitator being able to deliver the intervention to multiple individuals. The amount of human resource an intervention requires is a key factor in calculating its cost-effectiveness alongside other variables including the benefit to participants. In long-term care there is a particular need for engaging people with dementia in meaningful activities [19], which CIRCA(-WB), could address.
The findings of the present study are very promising but have limitations in respect of the number of participants who completed the assessments 3 months postintervention. This may reflect the age and health status of the participants. In addition, most participants were living in long-term care, which may limit the generalizability of the findings to other settings.
In conclusion, CIRCA-WB performed comparably with the original stand-alone version and has the added functionality of being more readily able to incorporate contents from different countries and cultural groups and labeling with multiple languages. This is being tested with local contents and labels by partners in the Netherlands, Spain, and Sweden as another element of the Independent Living Functions in the Elderly (In-LIFE) project.
Research in Context.
-
1.
Systematic review: The authors reviewed the literature using traditional (e.g., PubMed) sources and meeting abstracts and presentations. Recent publications related to cognitive stimulation and technological interventions were identified. These relevant citations are appropriately cited.
-
2.
Interpretation: The manuscript describes the evaluation of Computer Interactive Reminiscence and Conversation Aid as an easy to use, technology-based group activity. Participation in the group activity delivered benefits to cognition and quality of life equivalent to those found in cognitive stimulation therapy and existing dementia drugs.
-
3.
Future directions: The web-based version can be easily populated with materials from different cultures and labeled in different languages. Further testing with different ethnic groups would advance the field of implementing nonpharmacologic interventions and address some of the current ethnoracial disparities in this area.
Acknowledgments
This project was funded by grant number 643442 (IN-LIFE) from the European Commission, as part of the Horizon 2020 program to A.J.A. and supported by the Ontario Shores Foundation through the Research Chair in Dementia Wellbeing at the University of Toronto. The authors are grateful to our partners at Sheffield branch of the Alzheimer's Society and Sheffcare without whom this work would not have been possible.
Footnotes
Conflicts of interest: The authors have declared that no conflict of interest exists.
References
- 1.Spector A., Thorgrimsen L., Woods B.O.B., Royan L., Davies S., Butterworth M. Efficacy of an evidence-based cognitive stimulation therapy programme for people with dementia: randomised controlled trial. Br J Psychiatry. 2003;183:248–254. doi: 10.1192/bjp.183.3.248. [DOI] [PubMed] [Google Scholar]
- 2.Alm N., Astell A., Ellis M., Dye R., Gowans G., Campbell J. A cognitive prosthesis and communication support for people with dementia. Neuropsychol Rehabil. 2004;14:117–134. [Google Scholar]
- 3.Astell A., Ellis M., Alm N., Dye R., Gowans G., Campbell J. Foundations of Augmented Cognition. Lawrence Erlbaum Associates; Mahwah, NJ: 2005. Using hypermedia to support communication in Alzheimer’s disease: the CIRCA project; pp. 758–767. [Google Scholar]
- 4.Astell A.J., Alm N., Gowans G., Ellis M.P., Dye R., Campbell J. CIRCA: a communication prosthesis for dementia. Technol Aging. 2008:67–76. IOS Press Ebooks. [Google Scholar]
- 5.Purves B.A., Phinney A., Hulko W., Puurveen G., Astell A.J. Developing CIRCA-BC and exploring the role of the computer as a third participant in conversation. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring. 2015;30:101–107. doi: 10.1177/1533317514539031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Astell A., Alm N., Gowans G., Ellis M., Dye R., Vaughan P. Involving older people with dementia and their carers in designing computer based support systems: some methodological considerations. Universal Access Inf Soc. 2009;8:49. [Google Scholar]
- 7.Astell A.J., Ellis M., Bernardi L., Alm N., Dye R., Gowans G. 22nd Conference of Alzheimer's Disease International. 2007. Developing technology to support the relationship between people with dementia and caregivers. Berlin. [Google Scholar]
- 8.Astell A.J., Ellis M.P., Bernardi L., Alm N., Dye R., Gowans G. Using a touch screen computer to support relationships between people with dementia and caregivers. Interact Comput. 2010;22:267–275. [Google Scholar]
- 9.Smith S., Astell A.J. Technology-supported group activity to promote communication in dementia: a protocol for a within-participants study. Technologies. 2018;6:33. [Google Scholar]
- 10.Knapp M., Thorgrimsen L., Patel A., Spector A., Hallam A., Woods B. Cognitive stimulation therapy for people with dementia: cost-effectiveness analysis. Br J Psychiatry. 2006;188:574–580. doi: 10.1192/bjp.bp.105.010561. [DOI] [PubMed] [Google Scholar]
- 11.American Psychiatric Association . 5th ed. American Psychiatric Association; Philadelphia, PA: 2013. Diagnostic and Statistical Manual of Mental Disorders. DSM-5. [Google Scholar]
- 12.Anon. Mental Capacity Act 2005. MCA 2005 [Internet]. 2005;(2):2–5. Available at: http://www.opsi.gov.uk/acts/acts2005/pdf/ukpga_20050009_en.pdf%5Cnhttp://www.mca2005.co.uk/
- 13.Hsieh S., Schubert S., Hoon C., Mioshi E., Hodges J.R. Validation of the Addenbrooke's Cognitive Examination III in frontotemporal dementia and alzheimer’s disease. Dement Geriatr Cogn Disord. 2013;36:242–250. doi: 10.1159/000351671. [DOI] [PubMed] [Google Scholar]
- 14.Giebel C.M., Challis D. Sensitivity of the Mini-Mental State Examination, Montreal Cognitive Assessment and the Addenbrooke's Cognitive Examination III to everyday activity impairments in dementia: an exploratory study. Int J Geriatr Psychiatry. 2017;32:1085–1093. doi: 10.1002/gps.4570. [DOI] [PubMed] [Google Scholar]
- 15.Logsdon R.G., Gibbons L.E., McCurry S.M., Teri L.P. Assessing quality of life in older adults with cognitive impairment. Psychosom Med. 2002;64:510–519. doi: 10.1097/00006842-200205000-00016. [DOI] [PubMed] [Google Scholar]
- 16.Moniz-Cook E., Vernooij-Dassen M., Woods R., Verhey F., Chattat R., De Vugt M. A European consensus on outcome measures for psychosocial intervention research in dementia care. Aging Ment Health. 2008;12:14–29. doi: 10.1080/13607860801919850. [DOI] [PubMed] [Google Scholar]
- 17.Euroqol Group EuroQol—a new facility for the measurement of health-related quality of life. Health Policy (New York) 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 18.Orgeta V., Edwards R.T., Hounsome B., Orrell M., Woods B. The use of the EQ-5D as a measure of health-related quality of life in people with dementia and their carers. Qual Life Res. 2015;24:315–324. doi: 10.1007/s11136-014-0770-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trahan M.A., Kuo J., Carlson M.C., Gitlin L.N. A systematic review of strategies to foster activity engagement in persons with dementia. Heal Educ Behav. 2014;41:70S–83S. doi: 10.1177/1090198114531782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ekström A., Ferm U., Samuelsson C. Digital communication support and Alzheimer's disease. Dementia. 2017;16:711–731. doi: 10.1177/1471301215615456. [DOI] [PubMed] [Google Scholar]